Epithelial ovarian cancer (EOC) is the most lethal gynecologic malignancy, and although surgery and platinum-based chemotherapy effectively induce initial remission,1 most women will ultimately succumb to recurrent and therapy-resistant disease. High-grade serous histology is the most common EOC pathology and is molecularly characterized by defects in DNA repair, copy number alterations, microsatellite stable status, and low tumor mutational burden.2,3 Platinum-resistant ovarian cancer (PROC), defined as cancer that has progressed within 6 months of last platinum exposure, has a median overall survival (OS) of < 16 months,4 and new treatment strategies are exigently needed.
THE TAKEAWAY
In the article that accompanies this editorial, Hamanishi et al7 report a multicenter, randomized, open-label phase III trial (NINJA) that evaluated the safety and efficacy of nivolumab compared with chemotherapy in platinum-resistant epithelial ovarian cancer. Nivolumab demonstrated no improvement in overall survival and significantly worse progression-free survival compared with nonplatinum chemotherapy, adding to the growing body of evidence that single-agent immune checkpoint blockade should not be used in epithelial ovarian cancer without first defining reliable biomarkers to identify subpopulations likely to benefit from these agents.
Results of multiple studies of immune checkpoint blockade (ICB) therapy in recurrent EOC have been disappointing, and EOC remains a cancer with no ICB-specific approvals. In both the KEYNOTE-1005 and JAVELIN6 trials of pembrolizumab and avelumab in recurrent EOC, respectively, the objective response rate (ORR) of these agents in recurrent EOC was in single digits. The NINJA trial7 was launched in response to promising single-agent nivolumab activity observed in a small phase II study of women with PROC showing an ORR of 15%, including two durable complete responses.8 The NINJA study was designed to evaluate the efficacy and safety of nivolumab monotherapy compared with chemotherapy in women with PROC, reported by Hamanishi et al7 in the article that accompanies this editorial. Eligible patients had PROC, fallopian tube or peritoneal (collectively referred to as ovarian) cancer, and could have received no more than one prior line of therapy in the platinum-resistant setting and no prior exposure to ICB or gemcitabine (GEM) or pegylated liposomal doxorubicin (PLD). Patients were stratified by histology (clear-cell [CC] v non-CC carcinoma) and by number of prior lines of therapy in the PROC setting (0 or 1) and randomly assigned 1:1 to nivolumab monotherapy versus GEM or PLD chemotherapy. The predominant histology of enrolled patients was serous, either high grade or unknown. The primary outcome of the study was OS, and key secondary outcomes were progression-free survival (PFS), ORR, duration of response (DOR), time to response, and safety.
The NINJA investigators are to be congratulated for conducting this randomized phase III study, which gives oncologists a definitive answer to the question: Should single-agent ICB be used for women with PROC, especially given that the predominant EOC histology is high-grade serous histology with low tumor mutational burden (TMB) and microsatellite stable? The results of the NINJA study support that there is no justification for the use of ICB in recurrent EOC, unless the cancer is found to be TMB high or microsatellite instable thus qualifying for pembrolizumab on the basis of the cancer site agnostic approval. ICB performed inferiorly to chemotherapy, and the NINJA trial demonstrated significantly worse PFS for nivolumab compared with nonplatinum chemotherapy. The median PFS was 2.0 months in the nivolumab cohort versus 3.8 months in the GEM/PLD group (hazard ratio [HR], 1.5; 95% CI, 1.2 to 1.9; P = .002). No significant difference in OS was observed between the nivolumab and GEM/PLD treatment groups with a median OS worse in the nivolumab arm compared with the GEM/PLD group (10.1 v 12.1 months). In addition, ORR was nonsignificantly lower in the nivolumab group (7.6% v 13.2%; P = .191). For patients who did respond to treatment, a longer median DOR of 18.7 months (95% CI, 2.5 to not evaluable) versus 7.4 months (95% CI, 3.0 to 10.3) was seen with nivolumab. Fewer grade 3 of 4 and any grade treatment-related adverse events occurred in the nivolumab arm.
These negative results from the NINJA trial add to the growing list of trials demonstrating lack of activity of ICB in EOC; compared with other ICB monotherapy trials in EOC, the ORR of nivolumab is comparable with that of pembrolizumab (8.0%)5 and avelumab (9.6%).6 The significance of the worse PFS and nonsignificantly lowered ORR compared with chemotherapy in this population of women with PROC raises the question of whether anti–programmed cell death-1 (PD-1) therapy may be even less effective in the platinum-resistant setting, but KEYNOTE-1005 showed that degree of platinum sensitivity was not predictive of response to ICB. Other clinical biomarkers such as number of prior lines of treatment have also not predicted response to ICB,5 and this is clear in the NINJA study in which dismal results of ICB are observed in patients with minimally treated PROC. CC histology has also been identified as a potential biomarker of response,5,8 but in the NINJA study, CC histology was not predictive of response to nivolumab.7 Although the data were negative for the overall population, the median DOR with nivolumab doubled indicated that the few patients who did respond to nivolumab had a durable response. Conversely, the response to chemotherapy in PROC tends to be short-lived, underscoring the importance of identifying the small subset of patients who have a higher chance of benefiting from immunotherapy. Overall, this study further supports what is becoming increasingly clear—that ICB has the potential to induce durable responses in only a very limited subset of patients in PROC, reliable biomarkers to select responders have not been identified, and ICB should not be used as single agents in EOC without further defining appropriate subpopulations, clinical settings, and/or combinations that allow for improved activity.
Given the modest activity of single-agent ICBs in ovarian cancer, combinations of anti-PD-1/programmed cell death ligand 1 (PD-L1) therapy with chemotherapy or targeted therapy have been reported while some trials are currently ongoing. The editorial by Konstantinopoulos and Cannistra9 highlighting the results of the IMagyn050 trial10 also reviewed trials several testing ICB-containing chemotherapy combinations in the newly diagnosed10,11 and recurrent12 EOC, all of which reported discouraging results; thus, triplet combinations including ICB, poly(ADP-ribose) polymerase inhibitors, antiangiogenics, and chemotherapy are now being tested. The ongoing MEDIOLA trial reported promising initial results with the triplet combination of olaparib, durvalumab, and bevacizumab in recurrent platinum-sensitive ovarian cancer generating an ORR of 77%.13 The combination of pembrolizumab, bevacizumab, and oral cyclophosphamide in recurrent EOC showed an ORR of 47.5% and a durable response in 25%.14 Taken together, the results of the trials to date suggest distinct biological differences between ovarian and other solid tumors that must be identified and addressed for successful immunotherapy implementation in this lethal disease.
An evolving understanding of the mechanisms driving low response rates to ICB in EOC, including tumor cell intrinsic features such as low TMB and lack of inflamed gene expression profile,15 and suppressed major histocompatibility complex protein expression,16 as well as a generalized immunosuppressive tumor immune microenvironment (TIME),17-25 has catalyzed the development of predictive biomarkers for response to ICB. PD-L1 expression in tumor cells and the TIME remains the most well-studied potential biomarker in EOC, given its correlation with therapeutic response across diverse cancer types.26,27 This correlation has been observed in EOC, although not uniformly across all studies. KEYNOTE-100 reported an ORR to pembrolizumab of 5% and 17.1% with combined positive score (CPS) ≥ 1 and ≥ 10, respectively,5 with all seven complete responses in the CPS ≥ 10 subgroup; however, the incidence of cancers demonstrating CPS ≥ 10 was very low. Stratification by PD-L1 staining in immune cells of ≥ 1% in IMagyn050 did not correlate with response to atezolizumab10; however, a potential benefit from the addition of atezolizumab was seen in prespecified exploratory analysis of two populations, one exhibiting immune cell PD-L1 expression of ≥ 5% (HR, 0.66; 95% CI, 0.44 to 0.98) and another with tumor cell PD-L1 expression of ≥ 1% (HR, 0.45; 95% CI, 0.19 to 1.02). No differences were seen in OS by PD-L1 expression in the NINJA study, although stratification was by tumor cell (tumor-positive score) PD-L1 ≥ 1%, potentially limiting the sensitivity.7 Overall, several challenges limit the utility of PD-L1 as a biomarker,28,29 and optimization of PD-L1 testing and/or combining it with other methods of assessing the immune-inflamed phenotype of a tumor30 will likely be necessary for its clinical use in EOC.
TMB is another leading biomarker candidate based on the assumption that a high mutational load fosters enhanced immunogenicity.31,32 Pembrolizumab is now approved for use in all unresectable or metastatic tumors with high TMB as determined using the FoundationOne CDx assay; however, these are rare in EOC.33,34 No association between TMB and response to pembrolizumab was seen in a prespecified exploratory analysis in KEYNOTE-100, although two tumors with high TMB did exhibit an ORR and PFS double that of low TMB tumors.34 In IMagyn050, most tumors had a low TMB regardless of BRCA1/2 mutation or homologous recombination deficiency status, and neither was associated with response to atezolizumab,33 underscoring the likely low utility of TMB assessment in EOC. BRCA mutations have also not been shown to be predictive of cancer responsiveness to ICB.5,6,11 The quest for more sensitive and precise biomarkers to predict ICB response in EOC is robust and includes transcriptomic analyses to identify tumors with an inflamed phenotype or other signatures related to response,2,34,35 single-cell spatially resolved analyses of the TIME,36 liquid biopsy−based approaches,37-41 and assessment of the host microbiome42,43 (Fig 1).
FIG 1.
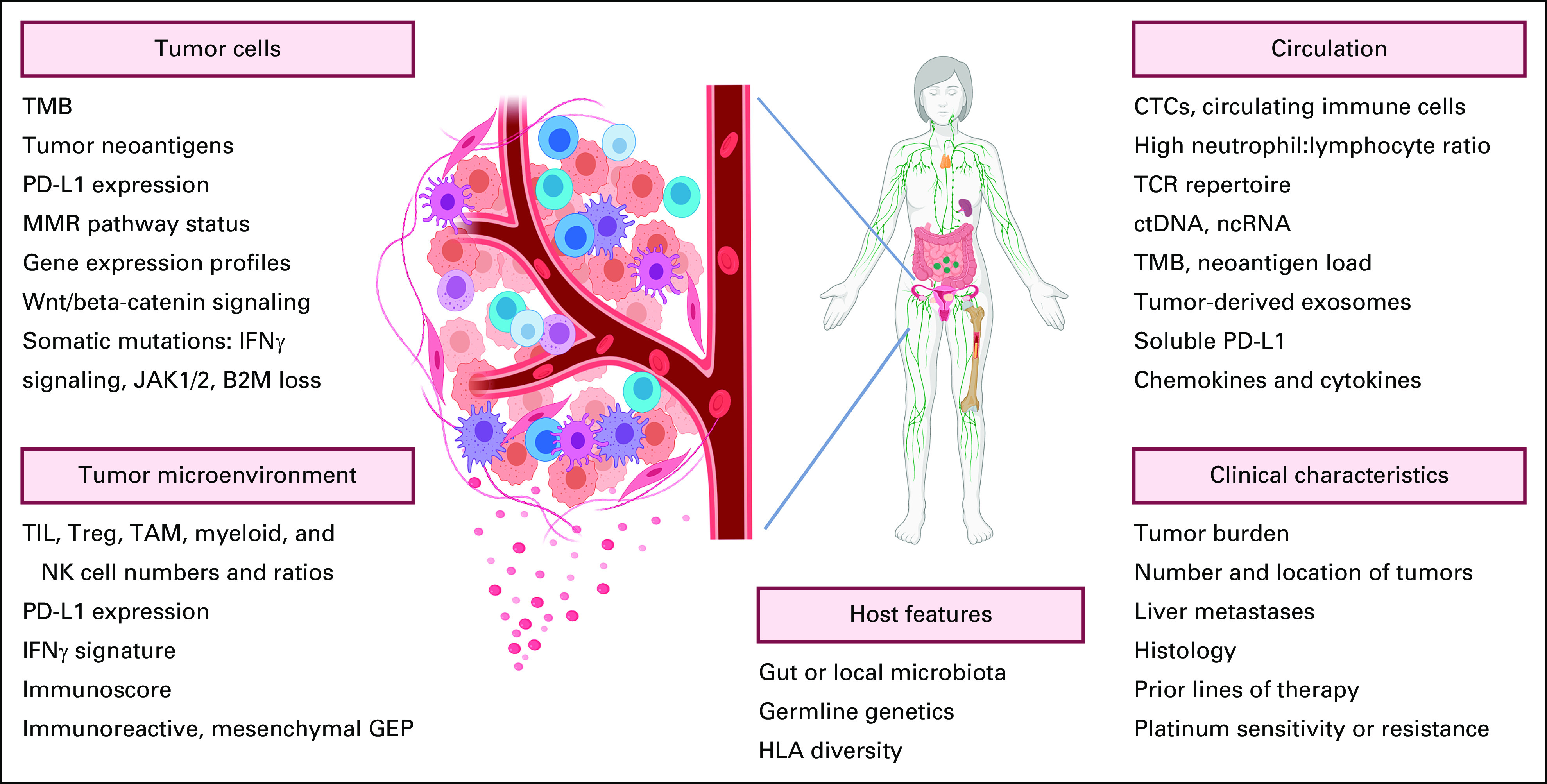
Summary of potential biomarkers under investigation for predicting response to therapy with immune checkpoint inhibitors. Representative candidates for biomarkers in tumor cells, the tumor microenvironment, and the circulation are depicted. In addition, clinical and host factors under investigation are included. B2M, beta-2 microglobulin; CTC, circulating tumor cell; ctDNA, circulating tumor DNA; GEP, gene expression profile; IFN, interferon; JAK1/2, Janus kinase 1/2; MMR, mismatch repair; ncRNA, noncoding RNA; NK, natural killer; PD-L1, programmed cell death ligand; TAM, tumor-associated macrophage; TCR, T-cell receptor; TMB, tumor mutational burden; TIL, tumor-infiltrating lymphocyte; Treg, regulatory T cell. Created with BioRender.44
Despite the low response rates thus far to ICBs in EOC, studies such as the NINJA trial highlight their capability to produce durable responses in a very small subset of patients, challenging the medical and scientific community to uncover the specific barriers limiting the activity of ICB and apply novel technologies to develop predictive strategies but also to develop immunotherapies that extend beyond ICB or help these agents work better. The identification of transformative biomarkers for predicting response to immunotherapy—which ultimately requires a method of testing if a patient has a tumor-reactive T-cell repertoire with the ability to both access the TME and also effectively eliminate cancer cells once there—remains challenging and will likely require incorporation of multiple parameters. Combining patient data from across ICB trials with low numbers of responders may prove useful for discovery of new candidates, which could then be validated in prospective trials. Building on what has already been learned and applying emerging technologies that can characterize tumors and their microenvironment at the single-cell level will undoubtedly lead to improved biomarkers that will not only allow for optimal patient selection for these therapies but will also improve our ability to test novel ICB combinations and sequences in the most appropriate populations.
Ursula A. Matulonis
Honoraria: Advaxis
Consulting or Advisory Role: Merck, Novartis, NextCure
Research Funding: Merck, Novartis, Tesaro, Syndax, Immunogen, Mersana, Leap Therapeutics, Fujifilm, SQZ Biotech
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
Footnotes
See accompanying article on page 3671
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Ursula A. Matulonis
Data analysis and interpretation: Ursula A. Matulonis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ursula A. Matulonis
Honoraria: Advaxis
Consulting or Advisory Role: Merck, Novartis, NextCure
Research Funding: Merck, Novartis, Tesaro, Syndax, Immunogen, Mersana, Leap Therapeutics, Fujifilm, SQZ Biotech
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Matulonis UA, Sood AK, Fallowfield L, et al. : Ovarian cancer. Nat Rev Dis Primers 2:16061, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network : Integrated genomic analyses of ovarian carcinoma. Nature 474:609-615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang SYC, Lheureux S, Karakasis K, et al. : Landscape of genomic alterations in high-grade serous ovarian cancer from exceptional long- and short-term survivors. Genome Med 10:81, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pujade-Lauraine E, Hilpert F, Weber B, et al. : Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 32:1302-1308, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Matulonis UA, Shapira-Frommer R, Santin AD, et al. : Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann Oncol 30:1080-1087, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Disis ML, Taylor MH, Kelly K, et al. : Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 5:393-401, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamanishi J, Takeshima N, Katsumata N, et al. : Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: Open-label, randomized trial in Japan (NINJA). J Clin Oncol 39:3671-3681, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamanishi J, Mandai M, Ikeda T, et al. : Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 33:4015-4022, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Konstantinopoulos PA, Cannistra SA: Immune checkpoint inhibitors in ovarian cancer: Can we bridge the gap between IMagynation and reality? J Clin Oncol 39:1833-1838, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Moore KN, Bookman M, Sehouli J, et al. : Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: Placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol 39:1842-1855, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledermann JAC, Oza N, Fujiwara AM, et al. : Avelumab in combination with and/or following chemotherapy vs chemotherapy alone in patients with previously untreated epithelial ovarian cancer: Results from the phase 3 javelin ovarian 100 trialin. Gynecol Oncol 159:13-14, 2020. 32771275 [Google Scholar]
- 12.Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. : Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): An open-label, three-arm, randomised, phase 3 study. Lancet Oncol 22:1034-1046, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Drew Y, Penson RT, O'Malley DM, et al. : 814MO Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): Initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol 31:S615-S616, 2020 [Google Scholar]
- 14.Zsiros E, Lynam S, Attwood KM, et al. : Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: A phase 2 nonrandomized clinical trial. JAMA Oncol 7:78-85, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarchoan M, Hopkins A, Jaffee EM: Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377:2500-2501, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le YS, Kim TE, Kim BK, et al. : Alterations of HLA class I and class II antigen expressions in borderline, invasive and metastatic ovarian cancers. Exp Mol Med 34:18-26, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Kandalaft LE, Odunsi K, Coukos G: Immune therapy opportunities in ovarian cancer. Am Soc Clin Oncol Ed Book 40:1-13, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Conejo-Garcia JR, Katsaros D, et al. : Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203-213, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Sato E, Olson SH, Ahn J, et al. : Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102:18538-18543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez GM, Galpin KJC, McCloskey CW, et al. : The tumor microenvironment of epithelial ovarian cancer and its influence on response to immunotherapy. Cancers (Basel) 10:242, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo X, Xu J, Yu J, et al. : Shaping immune responses in the tumor microenvironment of ovarian cancer. Front Immunol 12:692360, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curiel TJ, Coukos G, Zou L, et al. : Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942-949, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Yuan X, Zhang J, Li D, et al. : Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol 147:181-187, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Condamine T, Ramachandran I, Youn JI, et al. : Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 66:97-110, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandalaft LE, Odunsi K, Coukos G: Immunotherapy in ovarian cancer: Are we there yet? J Clin Oncol 37:2460-2471, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Taube JM: Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology 3:e963413, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443-2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taube JM, Anders RA, Young GD, et al. : Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4:127ra37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumeh PC, Harview CL, Yearley JH, et al. : PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568-571, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trujillo JA, Sweis RF, Bao R, et al. : T cell-inflamed versus non-T cell-inflamed tumors: A conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol Res 6:990-1000, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan TA, Yarchoan M, Jaffee E, et al. : Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann Oncol 30:44-56, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cristescu R, Mogg R, Ayers M, et al. : Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362:eaar3593, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landen C, Molinero L, Sehouli J, et al. : Association of BRCA1/2, homologous recombination deficiency, and PD-L1 with clinical outcomes in patients receiving atezolizumab vs placebo combined with carboplatin, paclitaxel, and bevacizumab for newly diagnosed. Gynecol Oncol 162:S37-S38, 2021 [Google Scholar]
- 34.Ledermann JA, Shapira-Frommer R, Santin AD, et al. : 843P Association of gene expression signatures and TMB with response to pembrolizumab in patients with recurrent ovarian cancer (ROC) enrolled in KEYNOTE-100. Ann Oncol 31:S631-S632, 2020 [Google Scholar]
- 35.Ayers M, Lunceford J, Nebozhyn M, et al. : IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 127:2930-2940, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Färkkilä A, Gulhan DC, Casado J, et al. : Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun 11:1459, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilgour E, Rothwell DG, Brady G, et al. : Liquid biopsy-based biomarkers of treatment response and resistance. Cancer Cell 37:485-495, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Snyder A, Morrissey MP, Hellmann MD: Use of circulating tumor DNA for cancer immunotherapy. Clin Cancer Res 25:6909-6915, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Huber V, Vallacchi V, Fleming V, et al. : Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J Clin Invest 128:5505-5516, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Mahoney KM, Giobbie-Hurder A, et al. : Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res 5:480-492, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber JS, Sznol M, Sullivan RJ, et al. : A serum protein signature associated with outcome after anti-PD-1 therapy in metastatic melanoma. Cancer Immunol Res 6:79-86, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Sears CL, Pardoll DM: The intestinal microbiome influences checkpoint blockade. Nat Med 24:254-255, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayase E, Jenq RR: Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med 13:107, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.BioRender : biorender.com


