Abstract
Objectives:
Fibromyalgia (FM) patients have an increased risk for glucose metabolism disturbances, and impaired glucose tolerance may be associated with symptom severity. Elevated levels of plasma lactate have been detected in FM patients. Both pyruvate and lactate are produced in glucose metabolism and reflect oxidative metabolism. The objective of our study was to analyse disturbances in glucose, pyruvate, or lactate metabolism in FM patients.
Methods:
We measured plasma levels of glucose, pyruvate, and lactate during an oral glucose tolerance test in 40 non-diabetic, female FM patients and 30 age- and gender-matched healthy controls.
Results:
FM patients showed a higher glycaemic response to the glucose load at 1 hour (F [1,68] = 10.4, P = .006) and 2 hours (F [1,68] = 7.80, P = .02), and higher glucose area under the curve (13.8 [SD 2.92] vs 11.6 [SD 2.31], P < .01), than healthy controls. Group differences were explained by higher body mass index and percentage of smokers among the FM patients. Pyruvate and lactate levels were similar in both groups.
Discussion:
Impaired glucose regulation in FM patients is likely not due to FM itself, but to associated lifestyle factors. Our results highlight the importance of assessing the glucose regulation status and the lifestyle factors affecting glucose regulation in FM patients for prevention or early treatment of diabetes and associated complications.
Trial Registration:
ClinicalTrials.gov (NCT03300635)
Keywords: diabetes, fibromyalgia, glucose tolerance, lactate, pyruvate, widespread pain
KEY MESSAGES
Fibromyalgia is associated with an increased risk of metabolic syndrome or diabetes
Fibromyalgia patients have a greater glycaemic response to a glucose load than do healthy controls
The difference in the glycaemic response is explained by overweight and smoking, both are modifiable risk factors
1. Introduction
Fibromyalgia (FM) is a common pain condition, defined as chronic widespread pain in combination with either widespread tenderness to palpation,[1] or by the severity of comorbid symptoms such as fatigue, sleep, and mood disturbance,[2] thus presenting a multifaceted health burden.[1,2]
While some predictors for the development of FM are known, such as local chronic or recurrent pain and sleep disturbances,[3] the exact pathophysiological mechanisms remain unclear. The diffuse nature of FM symptoms suggests a systemic cause or mediator.
Disturbances of glucose metabolism are among the possible pathophysiological factors associated with FM or chronic widespread pain (CWP). Metabolic syndrome and type 2 diabetes mellitus (DM) are common comorbidities of FM.[4–7] Disturbances of glucose metabolism may also be associated with severity of FM. Higher glucose levels raise the level of glycated haemoglobin (HbA1c) and higher HbA1c has been reported to be associated with a greater number of tender points in patients with both FM and DM.[4] A connection between impaired glucose regulation and memory impairment in FM has also been reported.[8]
Glucose is the main source of energy for human cells and an indispensable energy source for the brain.[9] Glucose is converted into pyruvate through glycolysis. Pyruvate is then further metabolized either anaerobically by lactase hydrogenase into lactate, or aerobically in the mitochondrial respiratory chain. Aerobic metabolism makes far more energy available to the cell than does anaerobic. However, aerobic metabolism is limited by the availability of oxygen and thus by blood flow, as well as by the number and functioning of mitochondria. The conversion of pyruvate into lactate therefore increases in anaerobic conditions. During heavy physical activity lactate can supplement some of the energy requirements of the brain.[10] Glycolysis and the lactate hydrogenase reaction are reversible, and pyruvate and lactate can be converted to glucose in the liver and stored as glycogen or transported out to be used by other tissues.[11]
Several studies have used microdialysis to analyse interstitial metabolite concentrations in muscles. They have shown elevated levels of pyruvate and lactate in the muscles of FM and CWP patients, compared with healthy controls.[12,13] Elevated lactate and pyruvate levels have also been detected during microdialysis of painful muscles in other musculoskeletal pain conditions.[14,15] Elevated lactate levels have also been measured in the ventricular cerebrospinal fluid (CSF) of FM and chronic fatigue syndrome patients, compared with controls.[16]
Pyruvate and lactate levels in the circulating blood of FM patients have been less studied. However, Gerdle et al. found elevated plasma lactate levels in FM patients compared with controls, but no difference in plasma pyruvate levels.[17]
FM patients have also shown altered responses to hyperinsulinaemia and hypoglycaemia, as the adrenocorticotrophin, adrenalin, cortisol, and growth hormone responses are reduced compared with healthy controls, suggesting insulin resistance and thus possibly decreased glucose tolerance.[18–20]
We hypothesized that, compared with healthy controls, FM patients would 1) have impaired glucose tolerance in an oral glucose tolerance test, 2) show elevated plasma pyruvate and lactate levels in response to the glucose load due to abnormalities in glucose metabolism, and 3) exhibit FM symptom severity correlating with impaired glucose regulation and pyruvate and lactate levels.
2. Materials and methods
2.1. Study subjects
We conducted this study as part of a project comprising three elements: a mental stress test, an oral glucose tolerance test, and bicycle ergometry. The recruitment process has been described previously.[21]
The exclusion criteria were diabetes, heart disease, peripheral atherosclerotic disease, uncontrolled hypertension, neurological, neuromuscular, or muscle disease, severe psychiatric disorders, continuous use of beta-blockers, beta-agonists, or statins, any musculoskeletal condition that would prevent performing bicycle ergometry, and poor command of Finnish.
Fifty-one female FM patients aged 18 to 65 years and 31 age- and gender-matched healthy controls, who had entered our study, were invited to undertake the oral glucose tolerance test. As we were losing patients to attrition after the first study visit, four additional patients were recruited from the Helsinki University Pain Clinic and primary health care in the Helsinki metropolitan area, to ensure a minimum number of 40 FM patients. This resulted in a total of 55 patients being invited. The sample size was based on recruiting the maximum number of patients and matched volunteers available during the funding period of the study.
Fourteen FM patients did not attend this part of the study for one or more of the following reasons: schedule conflicts with work, travel, etc. (n = 5); worsening of FM symptoms (n = 2); other health complaints (n = 6); could not be contacted (n = 2); other (n = 2). One control subject did not attend due to health complaints. In total, samples were collected from 40 FM patients and 30 healthy controls.
We used the American College of Rheumatology 1990 diagnostic criteria (ACR1990) for the diagnosis of FM.[1] The 2016 modified diagnostic criteria (ACR2016) were also assessed to improve comparability between our work and other studies using these criteria.[2] Thirty-five of the 40 FM patients (87.5%) fulfilled both ACR1990 and ACR2016 criteria. One patient did not complete the ACR2016 questionnaire.
We recorded medical history, height, and weight and calculated the body mass index (BMI). The FM patients completed the Finnish version of the Fibromyalgia Impact Questionnaire (Finn-FIQ), which measures both the severity of FM symptoms and the impact of symptoms on daily functioning on a scale of 0 to 100.[22,23] Subjects completed a questionnaire detailing, among others: subjective physical fitness rated as “1: Worse than average,” “2: Average,” or “3: Better than average;” leisure time physical activity habits with a scale for frequency rated from “1: None” to “4: Several times per week;” and a scale for leisure time activity intensity rated from “1: Walking” to “4: Brisk running.” A physical activity score ranging from 3 to 11 was calculated as a sum of these three items. Sleep quality was rated with two questions, “Do you have trouble sleeping?” (yes/no), and “Do you wake during sleep?” rated as “1: Not usually,” “2: 1 to 2 times per night,” “3: 3 to 4 times night,” and “4: At least 5 times per night.”
The study complied with the Declaration of Helsinki. All study subjects gave written informed consent. The study protocol was approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District (229/13/03/02/2015) and registered in ClinicalTrials.gov (NCT03300635).
2.2. Blood samples and oral glucose tolerance tests
The oral glucose tolerance tests and blood sample collection were performed at the Helsinki University Hospital laboratories Meilahti facility by Helsinki University Hospital laboratories staff.
Study subjects were instructed to fast for 10 to 12 hours before the visit and to avoid any non-essential medication and strenuous exercise during the previous day and on the morning of the visit, and to cancel the visit if they were sick.
The visit began between 7 and 9 a.m. First, venous blood samples were taken from the cubital vein. Fasting blood glucose (Glucose0h, mmol/L) and pyruvate (Pyruvate0h, μmol/L), fasting serum lactate (Lactate0h, mmol/L) were analysed from this sample. Starting from the ninth FM patient and the eighth control, serum glutamate decarboxylase antibodies (GADAb, IU/mL) were also analysed. The presence of GADAb is a risk factor for the onset of type 1 diabetes mellitus.[24]
Glucose samples were taken in fluoride citrate tubes, centrifugated, and measured photometrically using the hexokinase method. One mL of venous blood was drawn without stasis for the Pyruvate samples into EDTA tubes, then 0.5 mL was pipetted into two pre-chilled tubes containing 1 mL of 8% perchloric acid each. Then the contents of the two tubes were carefully mixed and centrifugated and measured enzymatically by photometry. Lactate samples were drawn without a stasis into fluoride oxalate tubes and measured photometrically. GADAb samples were drawn into serum gel tubes and measured with an accredited enzyme immunoassay using recombinant human Gad65 protein as antigen.
Then the subjects drank a water solution containing 75 g of glucose within five minutes or less. One hour after drinking the glucose solution, the second venous blood samples were taken for the measurement of glucose (Glucose1h). After two hours, the final samples were taken for the measurement of glucose (Glucose2h), lactate (Lactate2h), and pyruvate (Pyruvate2h).
We calculated the area under the curve (AUC) for glucose (AUCgluc), pyruvate (AUCpyru), and lactate (AUClact), using the linear trapezoidal method.
The samples were collected between December 2015 and February 2019.
2.3. Assessment of glucose regulation
The subject was judged as having impaired glucose regulation (IGR) if Glucose0h > 6.0 mmol/L, Glucose1h > 8.6 mmol/L, or Glucose2h > 7.7 mmol/L.[25] Glucose2h of > 11.0 mmol/L is diagnostic for diabetes.
2.4. Statistical analysis
We compared the values between the groups with Student t-test for numerical variables and χ2-test for categorical variables.
To compare changes over time in the glucose, pyruvate, and lactate levels between the groups, we conducted two-way mixed analysis of variance (ANOVA) testing. We handled missing data with the k-nearest-neighbours imputation. We checked for extreme outliers using the boxplot method, and for normality of distribution using the Shapiro-Wilk test (P > .05). We used a logarithmic transformation on any non-normally distributed data for further analysis. We tested for homogeneity of variance using the Levene's test (P > .05).
We analysed the effect of lifestyle factors on AUCgluc, AUCpyru, and AUClact with factorial ANOVA using type II sum of squares. We considered BMI, smoking, physical activity, and sleep disturbance as likely covariates. We treated these lifestyle factors as dichotomous variables as elaborated below. We conducted post hoc testing with Tukey's honest significant difference test.
BMI was dichotomized as normal weight (BMI 18.5 – 25) or overweight (BMI > 25). Physical activity was dichotomized by physical activity score of < 7 as inactive and ≥ 7 as active. This cut-off level also produced the most balanced category sizes. Sleep disturbance was defined by the subject reporting both sleep problems and waking from sleep at least three times per night. Smoking was a dichotomous categorical variable (non-smoker or smoker).
We used the Pearson correlation coefficient to evaluate whether symptom severity (FIQ score) correlated with fasting levels of glucose, pyruvate, or lactate, or with their AUCs (AUCgluc, AUCpyru, and AUClact). The significance level was set to P < .05 and the data were analysed using R version 4.0.0 (The R Foundation for Statistical Computing 2020).
3. Results
FM patients and controls did not differ in age, as this was matched in the study design. FM patients had significantly higher BMIs and lower physical activity scores and were more likely to smoke and to have sleep disturbance, than the controls. None of the subjects were underweight. GADAbs were not detected in any of the subjects (Table 1).
Table 1.
Descriptive data.
| FM (n = 40) | Control (n = 30) | P | |
| Age (yr) | |||
| Mean (SD) | 45.9 (10.9) | 45.6 (11.7) | .923 |
| Median [Min, Max] | 47.0 [23.0, 65.0] | 48.0 [22.0, 61.0] | |
| BMI (kg/m2) | |||
| Mean (SD) | 27.8 (5.87) | 24.7 (3.27) | .007 |
| Median [Min, Max] | 26.5 [19.3, 45.4] | 24.5 [19.1, 32.2] | |
| Missing | 1 (2.5%) | 1 (3.3%) | |
| BMI class | |||
| Normal weight | 14 (35.0%) | 16 (53.3%) | .012 |
| Overweight | 25 (62.5%) | 13 (43.4%) | |
| Missing | 1 (2.5%) | 1 (3.3%) | |
| Smoking | |||
| Non-smoker | 30 (75.0%) | 28 (93.3%) | .27 |
| Smoker | 7 (17.5%) | 2 (6.7%) | |
| Missing | 3 (7.5%) | 0 (0%) | |
| Physical Activity Score (3–11) | |||
| Mean (SD) | 6.82 (1.67) | 8.23 (1.83) | .002 |
| Median [Min, Max] | 7.00 [4.00, 11.0] | 9.00 [4.00, 11.0] | |
| Missing | 1 (2.5%) | 0 (0%) | |
| Physically Active (Physical Activity Score ≥ 7) | |||
| Active | 14 (35.0%) | 20 (66.7%) | .017 |
| Inactive | 26 (65.0%) | 10 (33.3%) | |
| Sleep disturbance | |||
| No | 9 (22.5%) | 27 (90.0%) | <.001 |
| Yes | 30 (75.0%) | 3 (10.0%) | |
| Missing | 1 (2.5%) | 0 (0.0%) | |
| Glutamate decarboxylase antibodies (IU/mL) | |||
| < 10 | 31 (77.5%) | 22 (73.3%) | .216 |
| Missing | 9 (22.5%) | 8 (26.7%) | |
FM patients had higher glucose1h, glucose2h, as well as higher AUCgluc than the controls. However, the groups did not differ in fasting glucose and pyruvate and lactate levels or AUCpyru and AUClact. (Table 2)
Table 2.
Result data.
| FM (n = 40) | Control (n = 30) | P | |
| Impaired glucose regulation | |||
| Yes | 16 (40.0%) | 6 (20.0%) | .128 |
| No | 24 (60.0%) | 24 (80.0%) | |
| Glucose at 0h (mmol/L) | |||
| Mean (SD) | 5.49 (0.554) | 5.31 (0.493) | .177 |
| Median [Min, Max] | 5.40 [4.50, 6.80] | 5.30 [4.30, 6.40] | |
| Glucose at 1h (mmol/L) | |||
| Mean (SD) | 7.94 (2.23) | 6.38 (1.77) | .003 |
| Median [Min, Max] | 7.80 [3.90, 12.5] | 6.50 [3.70, 9.70] | |
| Missing | 3 (7.5%) | 2 (6.7%) | |
| Glucose at 2h (mmol/L) | |||
| Mean (SD) | 6.20 (1.44) | 5.27 (1.27) | .006 |
| Median [Min, Max] | 6.30 [3.70, 10.3] | 5.00 [3.40, 7.90] | |
| Missing | 0 (0%) | 1 (3.3%) | |
| Glucose AUC (mmol × h/L) | |||
| Mean (SD) | 13.8 (2.92) | 11.6 (2.31) | .002 |
| Median [Min, Max] | 13.2 [8.70, 20.6] | 11.3 [7.55, 16.3] | |
| Missing | 3 (7.5%) | 3 (10.0%) | |
| Pyruvate at 0h (μmol/L) | |||
| Mean (SD) | 91.8 (13.5) | 92.4 (15.8) | .869 |
| Median [Min, Max] | 91.0 [66.0, 122] | 92.0 [61.0, 133] | |
| Missing | 1 (2.5%) | 1 (3.3%) | |
| Pyruvate at 2h (μmol/L) | |||
| Mean (SD) | 99.9 (12.6) | 102 (15.1) | .487 |
| Median [Min, Max] | 99.0 [81.0, 139] | 102 [73.0, 134] | |
| Missing | 5 (12.5%) | 3 (10.0%) | |
| Pyruvate AUC (μmol × h/L) | |||
| Mean (SD) | 95.8 (11.3) | 97.7 (14.0) | .581 |
| Median [Min, Max] | 94.5 [73.5, 124] | 96.8 [67.0, 134] | |
| Missing | 5 (12.5%) | 4 (13.3%) | |
| Lactate at 0h (mmol/L) | |||
| Mean (SD) | 0.929 (0.351) | 0.890 (0.420) | .685 |
| Median [Min, Max] | 0.900 [0.500, 2.20] | 0.800 [0.500, 2.10] | |
| Missing | 2 (5.0%) | 0 (0%) | |
| Lactate at 2h (mmol/L) | |||
| Mean (SD) | 1.35 (0.353) | 1.37 (0.280) | .842 |
| Median [Min, Max] | 1.40 [0.700, 2.20] | 1.40 [0.900, 2.00] | |
| Missing | 5 (12.5%) | 3 (10.0%) | |
| Lactate AUC (mmol × h/L) | |||
| Mean (SD) | 1.11 (0.242) | 1.09 (0.222) | .689 |
| Median [Min, Max] | 1.10 [0.700, 1.70] | 1.05 [0.750, 1.60] | |
| Missing | 6 (15.0%) | 3 (10.0%) | |
Seventeen FM patients (41%) and six controls (20%) were found to have IGR or DM, though this tendency was not significant at the < 0.05 significance level (χ2 = 2.73, P = .10). One FM patient had a two-hour glucose level of > 11.0 mmol/L, was diagnosed with DM and excluded from further analyses.
3.1. Glucose, pyruvate, and lactate levels
The glucose, pyruvate, or lactate data contained no extreme outliers. The glucose and pyruvate levels were normally distributed, but the lactate levels were left-skewed and we therefore used logarithmic transformation for their further analysis. The variance of the data was homogeneous.
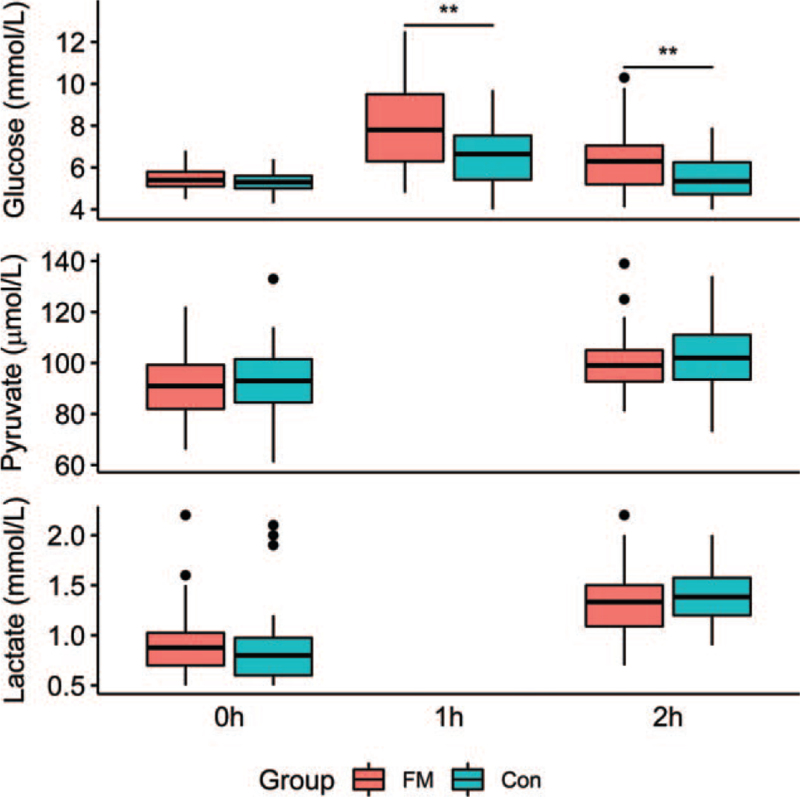
In the ANOVA, glucose was affected by group (F[1,68] = 10.9, P < .01) and time (F[1.7,115.29] = 54.6, P < .01). There was also a significant group-time interaction (F[1.7,115.29103.81] = 6.6, P < .01) (Fig. 1). In post hoc testing after Bonferroni correction, the baseline glucose levels at 0 hour were similar between the groups, but the glucose levels of the FM patients were significantly higher at 1 hour (F[1,68] = 10.4, P < .01) and 2 h (F[1,68] = 7.80, P = .02).
Figure 1.
Blood glucose (mmol/L), pyruvate (μmol/L), and lactate (mmol/L) levels for fibromyalgia patients (red) and healthy controls at baseline and 1 h and 2 h after an oral glucose test. ∗∗significant group difference P < .01 (Tukey's HSD).
Both the pyruvate and lactate levels rose significantly from 0 hour to 2 hour in response to the glucose tolerance test (F[1,68] = 28.2, P < .01 and F[1,68] = 83.5, P < .01), but there was no difference between the groups (F[1,68] = 0.32, p = 0.58 and F[1,68] = 0.08, P = .78) (Fig. 1).
3.2. Glucose, lifestyle factors, and symptoms
We further analysed the effect of lifestyle factors (BMI, physical activity, smoking, and sleep disturbance) on AUCgluc.
The distribution of subjects by BMI category and smoking status is shown in Table 2. Due to the unbalanced sample, we used unbalanced factorial ANOVA with type II sum of squares. There were no extreme outliers in the AUCgluc data, and the data were normally distributed and homogeneous. We found no significant interactions between the groups (patients vs controls) or any of the lifestyle factors. Thus, we only considered the main effects.
Significant main effects were found for BMI category (F[1, 64] = 6.63, P = .01, η2 = 0.071, partial-η2 = 0.094), and smoking (F[1, 64] = 4.28, P = .04, η2 = 0.046, partial-η2 = 0.063). The effects were not significant for physical activity (F[1, 64] = 1.24, P = .27, η2 = 0.013, partial-η2 = 0.019) or sleep disturbance (F[1, 64] = 0.168, P = .68, η2 = 0.002, partial-η2 = 0.003). The effect of group was also non-significant in the model accounting for lifestyle factors (F[1, 64] = 1.89, P = .17, η2 = 0.020, partial-η2 = 0.029).
FIQ did not differ between FM patients with IGR and patients without IGR (45.4 [14.1] and 40.0 [14.1] respectively, P = .24). There was also no correlation with FIQ and the fasting levels of glucose (r[38] = 0.03, P = .88), pyruvate (r[38] = 0.19, P = .24), or lactate (r[38] = 0.03, P = .87), nor with AUCgluc (r[38] = 0.08, P = .63), AUCpyru (r[38] = 0.12, P = .45), or AUClact (r[38] = -0.09, P = .57).
4. Discussion
In accordance with our hypothesis, the FM patients had higher blood glucose levels after the glucose load. Forty percent of the FM patients and only 20% of the controls had glucose levels diagnostic of impaired glucose regulation (IGR) but, because of the small sample size, the difference was not significant. Our findings are in line with previous reports of IGR being more prevalent in FM patients than healthy controls. There was also a non-significant tendency for more smoking among the FM patients, which is likely to influence their risk of IGR.[26]
Contrary to our hypothesis, we found no difference in systemic pyruvate or lactate levels between the FM patients and the controls. Blood levels of pyruvate or lactate do not necessarily reflect levels in other fluid compartments of the body, such as the muscle interstitium or CSF. Therefore, our findings do not rule out a role for pyruvate and lactate metabolism in the pathophysiology of FM. Blood samples are easier to obtain than, for example, CSF samples but our results do cast doubt on the usefulness of measuring blood levels of pyruvate or lactate in FM patients.
While the FM patients had higher blood glucose levels, these were greatly influenced by lifestyle factors. High BMI is known to disturb glucose regulation, as is smoking.[26,27] As expected, we found overweight and smoking to significantly increase glucose levels and AUC of glucose in the factorial analysis.
Physical activity is known to reduce the risk of type 2 DM,[28] while several studies have shown insufficient sleep to cause obesity, insulin resistance, glucose metabolism disturbance and risk of type 2 diabetes, likely through increased levels of cortisol and appetite-promoting ghrelin, and decreased levels of satiety-promoting leptin.[29] Not only too little sleep, but also poor sleep quality, is a risk factor for type 2 diabetes.[29,30]
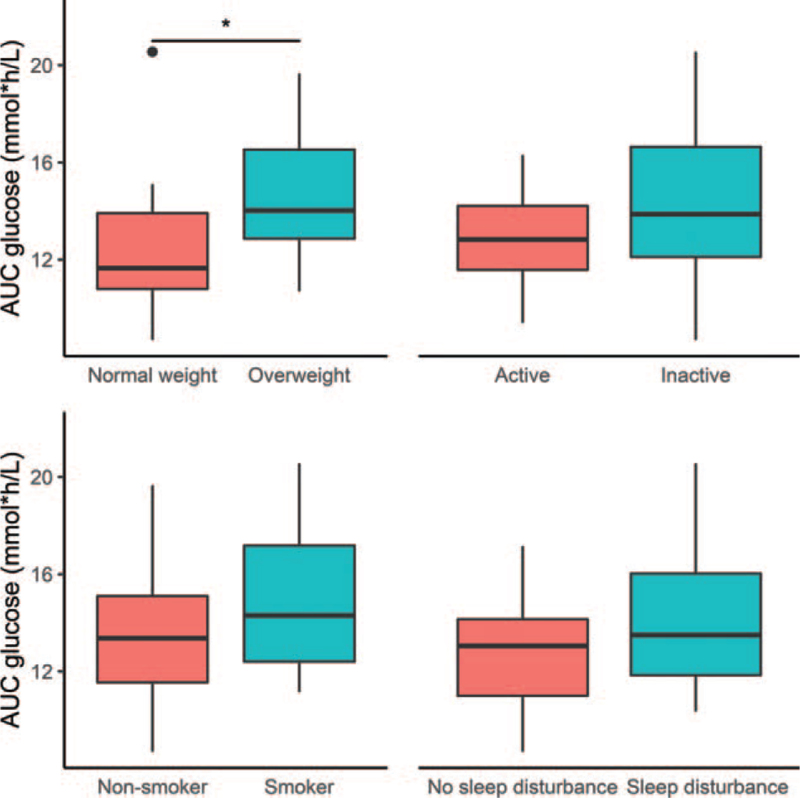
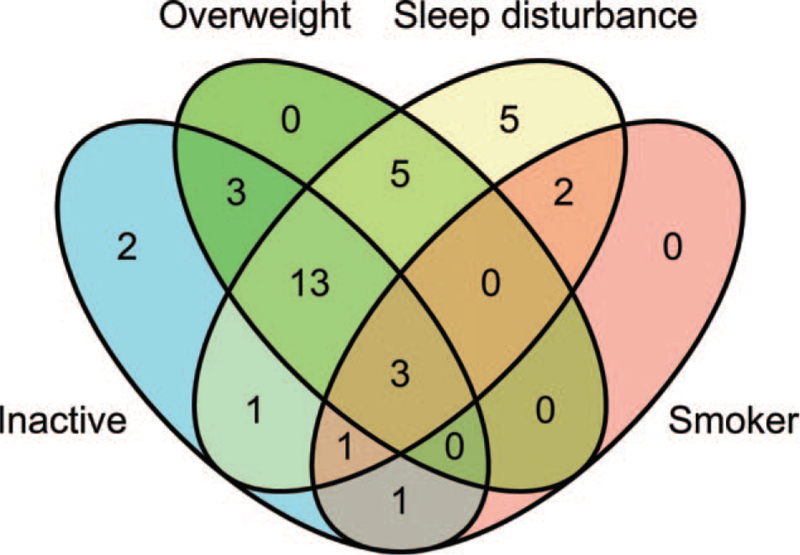
In the factorial analysis of our data, physical activity and sleep disturbance did not influence glucose levels significantly. Nonetheless, physical inactivity and sleep disturbance suggested poorer glucose control (Fig. 2). In our sample, the lifestyle factors overlapped greatly, as shown in Figure 3. Therefore, bigger samples would be needed to evaluate the effect of each lifestyle factor more precisely.
Figure 2.
Blood glucose area under the curve (AUC, mmol × h/L) values of the fibromyalgia patients by lifestyle factors. ∗significant at P < .05 (Student t-test).
Figure 3.
Venn diagram of the evaluated lifestyle factors among the fibromyalgia patients (total with complete data n = 36).
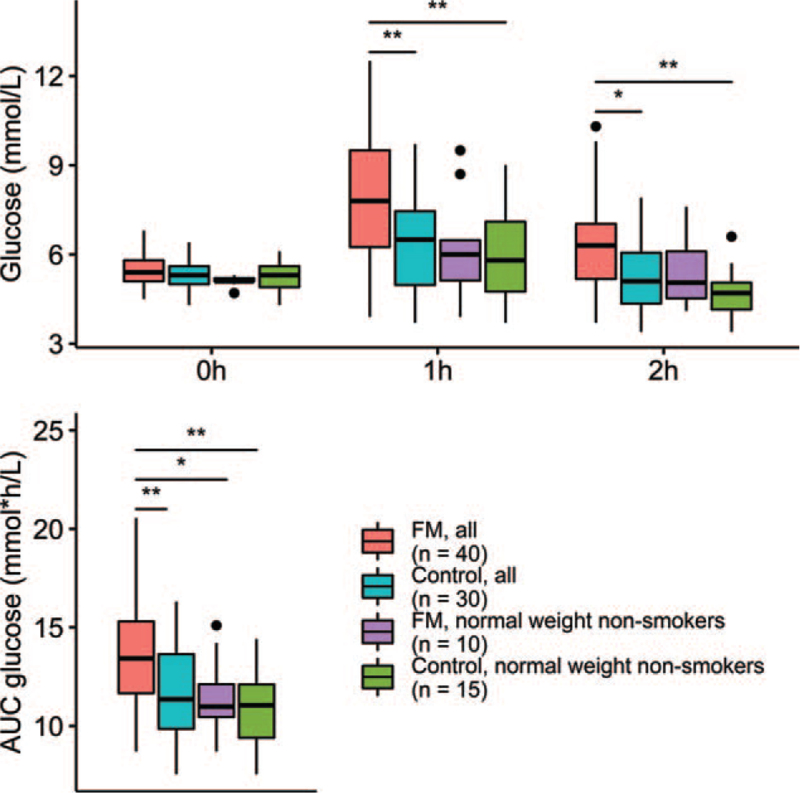
Importantly, FM patients who were of normal weight and did not smoke had lower glucose levels and glucose AUC, comparable with controls (Fig. 4). This suggests that the glucose metabolism disturbance seen in FM patients is not due to FM itself, but to the accumulation of detrimental lifestyle factors. This contrasts with the findings of Mäntyselkä et al., where higher glucose levels were associated with chronic pain, even after adjusting for BMI.[6] Loevinger et al. found higher glycated haemoglobin (HbA1c) in FM patients than in healthy controls with similar BMIs. However, in their study, FM patients had greater waist circumferences than controls, suggesting higher percentage of body fat or central adiposity which could affect glucose metabolism.[5]
Figure 4.
Visualization of the effect of overweight and smoking on blood glucose levels and area under the curve for the fibromyalgia patients and controls. Significant differences at ∗P < .05 and ∗∗P < .01 (Tukey's HSD).
FM patients have a higher prevalence of obesity and overweight than healthy controls.[31–33] Obesity is also a predictor for developing FM.[3,34] In several studies, higher BMI is correlated with higher symptom severity, pain, tender point count, fatigue, stiffness, anxiety, depression, physical disability, and decreased quality of life. Possible mediators for high BMI and FM symptoms include decreased physical activity, disruption of growth hormone metabolism, and low-grade inflammation.[31,32]
Weight loss interventions have resulted in improvement of FM symptoms,[31,32] a weight loss of at least 10% being associated with greater improvement of symptoms as well as increase in immunoregulatory interleukin IL-10.[35] Of the 12 FM patients in the study by Hooper et al., 11 could no longer be diagnosed with FM after bariatric surgery resulting in mean BMI decrease of 15 kg/m2.[36] The beneficial effects seen with various dietary interventions in FM may, at least partially, be due to associated weight loss.[37] However, these results are based on a few, mostly uncontrolled, intervention studies with limited population sizes. Thus, more research is needed.
In previous studies, the prevalence of smoking has been similar among FM patients and among general population, but higher among FM patients than rheumatoid arthritis patients.[33,38] Smoking is unlikely to predict the onset of FM,[3] but several studies have shown that FM patients who smoke have more severe and interfering symptoms and higher levels of pain.[39–42] Suggested mediators between smoking and FM pain include neuropeptide Y and leptin.[43] Some studies have associated smoking in FM patients with higher anxiety[38] and sleep problems,[41] while others have found no such association.[40,44] Weingarten et al. found that, subjectively, FM patients often find smoking reduces anxiety and other negative emotional symptoms, and helps them cope with pain, even though smoking has no effect on the intensity of pain.[45] While smoking is clearly correlated with more severe FM symptoms, smoking cessation in FM, or in chronic pain in general, has not been studied sufficiently to draw conclusions on its effectiveness in pain management.[46]
While some studies have suggested a correlation between blood glucose levels or IGR status and FM symptom severity, we found no such connection.[4,8] Nevertheless, our work highlights the importance of assessing the IGR status of FM patients, as disturbances of glucose regulation are an important risk factor for cardiovascular morbidity.[47] Weight loss and cessation of smoking are recognized as particularly important strategies in the management of glucose regulation in FM patients, with weight loss also known to improve FM symptoms. These are important considerations, as successful treatment of FM is multifaceted and a multidisciplinary approach is often needed.
Acknowledgments
Thanks to Les Hearn for scientific and English proofreading and editing (les_hearn@yahoo.co.uk).
Author contributions
Conceptualization: Ritva Markkula, Eija Kalso, Teemu Zetterman.
Data curation: Teemu Zetterman, Ritva Markkula.
Formal analysis: Teemu Zetterman.
Funding acquisition: Teemu Zetterman, Eija Kalso.
Investigation: Teemu Zetterman, Ritva Markkula.
Methodology: Teemu Zetterman, Ritva Markkula, Eija Kalso.
Project administration: Teemu Zetterman, Eija Kalso.
Resources: Eija Kalso.
Software: Teemu Zetterman.
Supervision: Ritva Markkula, Eija Kalso.
Validation: Teemu Zetterman, Eija Kalso.
Visualization: Teemu Zetterman.
Writing – original draft: Teemu Zetterman.
Writing – review & editing: Teemu Zetterman, Ritva Markkula, Eija Kalso.
Footnotes
Abbreviations: ACR1990 = American College of Rheumatology 1990 diagnostic criteria for fibromyalgia, ACR2016 = American College of Rheumatology 2016 modified diagnostic criteria for fibromyalgia, ANOVA = analysis of variance, AUC = area under the curve, BMI = body mass index, CSF = cerebrospinal fluid, CWP = chronic widespread pain, DM = diabetes mellitus type 2, FIQ = fibromyalgia impact questionnaire, FM = Fibromyalgia, GADAb = glutamate decarboxylase antibodies, HbA1c = glycated haemoglobin, IGR = impaired glucose regulation.
How to cite this article: Zetterman T, Markkula R, Kalso E. Glucose tolerance in fibromyalgia. Medicine. 2021;100:46(e27803).
This work was supported by Finnish State Research Funding (TYH2017215); The Signe and Ane Gyllenberg Foundation; and personally, by The Emil Aaltonen Foundation to TZ.
Eija Kalso has served on the advisory boards of Orion Pharma and Pfizer.
The other authors have no conflicts of interest to disclose.
The datasets generated and analysed during the current study are not publicly available as consent for this was not asked from the study subjects. The data are available from the corresponding author on reasonable request if also approved by our ethics committee
References
- [1].Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- [2].Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- [3].Markkula RA, Kalso EA, Kaprio JA. Predictors of fibromyalgia: a population-based twin cohort study. BMC Musculoskelet Disord 2016;17:01–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tishler M, Smorodin T, Vazina-Amit M, Ramot Y, Koffler M, Fishel B. Fibromyalgia in diabetes mellitus. Rheumatol Int 2003;23:171–3. [DOI] [PubMed] [Google Scholar]
- [5].Loevinger BL, Muller D, Alonso C, Coe CL. Metabolic syndrome in women with chronic pain. Metabolism 2007;56:87–93. [DOI] [PubMed] [Google Scholar]
- [6].Mäntyselkä P, Miettola J, Niskanen L, Kumpusalo E. Glucose regulation and chronic pain at multiple sites. Rheumatology 2008;47:1235–8. [DOI] [PubMed] [Google Scholar]
- [7].Lichtenstein A, Tiosano S, Comaneshter D, Amital H, Cohen AD, Amital D. Cross-sectional analysis of the associations between fibromyalgia and diabetes mellitus. Reumatologia 2018;56:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fava A, Plastino M, Cristiano D, et al. Insulin resistance possible risk factor for cognitive impairment in fibromialgic [sic] patients. Metab Brain Dis 2013;28:619–27. [DOI] [PubMed] [Google Scholar]
- [9].Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 2013;36:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Van Hall G, Strømstad M, Rasmussen P, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 2009;29:1121–9. [DOI] [PubMed] [Google Scholar]
- [11].Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014;17:76–100. [DOI] [PubMed] [Google Scholar]
- [12].McIver KL, Evans C, Kraus RM, Ispas L, Sciotti VM, Hickner RC. NO-mediated alterations in skeletal muscle nutritive blood flow and lactate metabolism in fibromyalgia. Pain 2006;120:161–9. [DOI] [PubMed] [Google Scholar]
- [13].Gerdle B, Ernberg M, Mannerkorpi K, et al. Increased interstitial concentrations of glutamate and pyruvate in vastus lateralis of women with Fibromyalgia syndrome are normalized after an exercise intervention - A case-control study. PLoS One 2016;11:01–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gerdle B, Ghafouri B, Ernberg M, Larsson B. Chronic musculoskeletal pain: review of mechanisms and biochemical biomarkers as assessed by the microdialysis technique. J Pain Res 2014;7:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gerdle B, Kristiansen J, Larsson B, Saltin B, Søgaard K, Sjøgaard G. Algogenic substances and metabolic status in work-related Trapezius Myalgia: a multivariate explorative study. BMC Musculoskelet Disord 2014;15:01–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Proia P, Amato A, Contrò V, et al. Relevance of lactate level detection in migrane [sic] and fibromyalgia. Eur J Transl Myol 2019;29:01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerdle B, Larsson B, Forsberg F, et al. Chronic widespread pain: increased glutamate and lactate concentrations in the trapezius muscle and plasma. Clin J Pain 2014;30:409–20. [DOI] [PubMed] [Google Scholar]
- [18].Adler GK, Kinsley BT, Hurwitz S, Mossey CJ, Goldenberg DL. Reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Am J Med 1999;106:534–43. [DOI] [PubMed] [Google Scholar]
- [19].McCall-Hosenfeld JS, Goldenberg DL, Hurwitz S, Adler GK. Growth hormone and insulin-like growth factor-1 concentrations in women with fibromyalgia. J Rheumatol 2003;30:809–14. [PubMed] [Google Scholar]
- [20].Kirnap M, Çolak R, Eser C, Özsoy O, Tutus A, Kelestimur F. A comparison between low-dose (1 (g), standard-dose (250 (g) ACTH stimulation tests and insulin tolerance test in the evaluation of hypothalamo-pituitary-adrenal axis in primary fibromyalgia syndrome. Clin Endocrinol (Oxf) 2001;55:455–9. [DOI] [PubMed] [Google Scholar]
- [21].Zetterman T, Markkula R, Partanen JV, Miettinen T, Estlander A-M, Kalso E. Muscle activity and acute stress in fibromyalgia. BMC Musculoskelet Disord 2021;22:01–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Burckhardt CS, Clark SR, B R. The fibromyalgia impact questionnaire: development and validation. J Rheumatol 1991;18:728–33. [PubMed] [Google Scholar]
- [23].Gauffin J, Hankama T, Kautiainen H, Arkela-Kautiainen M, Hannonen P, Haanpää M. Validation of a Finnish version of the Fibromyalgia Impact Questionnaire (Finn-FIQ). Scand J Pain 2012;3:15–20. [DOI] [PubMed] [Google Scholar]
- [24].Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 2017;60:1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Joshipura KJ, Andriankaja MO, Hu FB, Ritchie CS. Relative utility of 1-hr oral glucose tolerance test as a measure of abnormal glucose homeostasis. Diabetes Res Clin Pract 2011;93:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tonstad S. Cigarette smoking, smoking cessation, and diabetes. Diabetes Res Clin Pract 2009;85:04–13. [DOI] [PubMed] [Google Scholar]
- [27].Yuan S, Larsson SC. An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia 2020;63:2359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 2015;30:529–42. [DOI] [PubMed] [Google Scholar]
- [29].Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev 2010;17:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis 2009;51:381–91. [DOI] [PubMed] [Google Scholar]
- [31].Ursini F, Naty S, Grembiale RD. Fibromyalgia and obesity: the hidden link. Rheumatol Int 2011;31:1403–8. [DOI] [PubMed] [Google Scholar]
- [32].D’Onghia M, Ciaffi J, Lisi L, et al. Fibromyalgia and obesity: a comprehensive systematic review and meta-analysis. Semin Arthritis Rheum 2021;51:409–24. [DOI] [PubMed] [Google Scholar]
- [33].Font Gayà T, Bordoy Ferrer C, Juan Mas A, et al. Prevalence of fibromyalgia and associated factors in Spain. Clin Exp Rheumatol 2020;123:47–52. [PubMed] [Google Scholar]
- [34].Mork PJ, Vasseljen O, Nilsen TIL. Association between physical exercise, body mass index, and risk of fibromyalgia: Longitudinal data from the Norwegian Nord-Trøndelag health study. Arthritis Care Res 2010;62:611–7. [DOI] [PubMed] [Google Scholar]
- [35].Schrepf A, Harte SE, Miller N, et al. Improvement in the spatial distribution of pain, somatic symptoms, and depression following a weight-loss intervention. J Pain 2017;18:1542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes 2007;31:114–20. [DOI] [PubMed] [Google Scholar]
- [37].Pagliai G, Giangrandi I, Dinu M, Sofi F, Colombini B. Nutritional interventions in the management of fibromyalgia syndrome. Nutrients 2020;12:01–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pamuk ÖN, Dönmez S, Çakir N. The frequency of smoking in fibromyalgia patients and its association with symptoms. Rheumatol Int 2009;29:1311–4. [DOI] [PubMed] [Google Scholar]
- [39].Goesling J, Brummett CM, Meraj TS, Moser SE, Hassett AL, Ditre JW. Associations between pain, current tobacco smoking, depression, and fibromyalgia status among treatment-seeking chronic pain patients. Pain Med 2015;16:1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yunus MB, Arslan S, Aldag JC. Relationship between fibromyalgia features and smoking. Scand J Rheumatol 2002;31:301–5. [DOI] [PubMed] [Google Scholar]
- [41].Ge L, D'Souza RS, Oh T, et al. Tobacco use in fibromyalgia is associated with cognitive dysfunction. Mayo Clin Proc Innov Qual Outcomes 2019;3:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee SS, Kim SH, Nah SS, et al. Smoking habits influence pain and functional and psychiatric features in fibromyalgia. Joint Bone Spine 2011;78:259–65. [DOI] [PubMed] [Google Scholar]
- [43].Bokarewa MI, Erlandsson MC, Bjersing J, Dehlin M, Mannerkorpi K. Smoking is associated with reduced leptin and neuropeptide Y levels and higher pain experience in patients with fibromyalgia. Mediators Inflamm 2014;2014: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lee C, Liptan G, Kantorovich S, Sharma M, Brenton A. Association of catechol-O-methyltransferase single nucleotide polymorphisms, ethnicity, and sex in a large cohort of fibromyalgia patients. BMC Rheumatol 2018;2:38.doi: 10.1186/s41927-018-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weingarten TN, Vincent A, Luedtke CA, et al. The perception of female smokers with fibromyalgia on the effects of smoking on fibromyalgia symptoms. Pain Pract 2016;16:1054–63. [DOI] [PubMed] [Google Scholar]
- [46].Saragiotto BT, Kamper SJ, Hodder R, et al. Interventions targeting smoking cessation for patients with chronic pain: an evidence synthesis. Nicotine Tob Res 2020;22:135–40. [DOI] [PubMed] [Google Scholar]
- [47].Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]