Supplemental Digital Content is available in the text.
Keywords: adult, antibodies, monoclonal, complement C5a, humans, sepsis/*drug therapy/immunology, treatment outcome
IMPORTANCE:
Anaphylatoxin C5a, a proinflammatory complement split product, plays a central role in mediating organ dysfunction.
OBJECTIVES:
This phase II clinical trial was conducted to study safety, tolerability, pharmacokinetics, and pharmacodynamics of vilobelimab, a recombinant monoclonal antibody against C5a, in patients with severe sepsis or septic shock.
DESIGN:
Multicenter, randomized, and placebo-controlled study.
SETTING AND PARTICIPANTS:
Eleven multidisciplinary ICUs across Germany. Adult patients with severe sepsis or septic shock and with early onset of infection-associated organ dysfunction.
MAIN OUTCOMES AND MEASURES:
Patients were randomly assigned in a ratio of 2:1 to three subsequent dosing cohorts for IV vilobelimab or placebo receiving either 2 × 2 mg/kg (0 and 12 hr), 2 × 4 mg/kg (0 and 24 hr), and 3 × 4 mg/kg (0, 24, and 72 hr). Co-primary endpoints were pharmacodynamics (assessed by C5a concentrations), pharmacokinetics (assessed by vilobelimab concentrations), and safety of vilobelimab. Preliminary efficacy was evaluated by secondary objectives.
RESULTS:
Seventy-two patients were randomized (16 patients for each vilobelimab dosing cohort and eight patients for each placebo dosing cohort). Vilobelimab application was associated with dosing dependent decrease in C5a compared with baseline (p < 0.001). Duration of C5a decrease increased with more frequent dosing. Membrane attack complex lysis capacity measured by 50% hemolytic complement was not affected. Vilobelimab was well tolerated with similar safety findings in all dose cohorts. No vilobelimab-specific adverse events emerged. For vilobelimab-treated patients, investigators attributed less treatment-emergent adverse events as related compared with placebo. Dosing cohorts 2 and 3 had the highest ICU-free and ventilator-free days. There was no difference in mortality, vasopressor-free days, or renal replacement therapy-free days between the groups.
CONCLUSIONS AND RELEVANCE:
Administration of vilobelimab in patients with severe sepsis and septic shock selectively neutralizes C5a in a dose-dependent manner without blocking formation of the membrane attack complex and without resulting in detected safety issues. The data warrant further investigation of C5a inhibition in sepsis.
Sepsis is defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection” and represents a major medical burden associated with a high mortality (1–3). Despite huge efforts to modify the host response, past randomized controlled trials failed to improve survival in sepsis (4).
Septic shock is associated with extensive complement activation (5). The complement system is an important pathway of host defense against pathogens. Activation of the complement cascade results in proteolytic cleavage of the complement factor C5 resulting in the generation of C5b and the anaphylatoxin C5a (6). C5b and subsequent membrane attack complex (MAC) formation is important for bacterial host defense and its inhibition by approved C5 inhibitors such as eculizumab increases susceptibility to infections (7), especially with encapsulated bacteria such as Neisseria (8, 9). In contrast, the recently developed monoclonal antibody vilobelimab (previously known as IFX-1) specifically binds to the soluble human complement split product C5a leaving generation of C5b and the MAC formation intact (10, 11). C5a is a 74 amino acid protein that attracts neutrophils, triggers a systemic inflammatory response, and neutrophil-driven tissue damage (6) associated with adverse outcome (12–14). C5a, among other biomarkers of infection, starts to peak early after sepsis onset (14). Experimental C5a blockade reduces the rate of organ dysfunction and mortality (15–17). Thus, C5a might be a useful target in the early onset of sepsis (18). Vilobelimab, was tested to be safe in a first-in-human, healthy volunteers trial (unpublished data).
The co-primary objectives of this early developmental study were to investigate pharmacodynamics in terms of C5a decrease, pharmacokinetics, and to describe the safety and tolerability of vilobelimab in patients with early severe sepsis and septic shock. Secondary objective was the preliminary assessment of clinical activity of vilobelimab measured by several clinical surrogate endpoints.
METHODS
The “Studying Complement Inhibition in Early, Newly developing Septic organ dysfunction” study (“SCIENS”; ClinicalTrials.gov: NCT02246595) was designed as prospective, randomized, double-blind, placebo-controlled, multicenter, and dose-finding trial. It was conducted in 11 multidisciplinary ICUs across Germany from April 2014 to December 2015.
Study Patients
Patients greater than or equal to 18 years old with severe sepsis—now comparable to the term sepsis according to Sepsis-3 definition (1)—or septic shock (19) with the clinical evidence of pulmonary or abdominal infection as the most frequent foci in sepsis (3) were eligible for this study. Only patients with onset of infection-related organ dysfunction of less than 6 hours or onset of infection-related vasopressor therapy of less than 3 hours before enrollment were considered. A complete list of the eligibility criteria is included in eTable 1 (http://links.lww.com/CCX/A851). All patients were treated according to the Guidelines of the German Sepsis Society (20).
Informed consent was handled as described before (21). Briefly, written informed consent was obtained from all patients or their legal or authorized representatives. In cases where such consent could not be obtained before enrollment, the ethics committee approved a delayed consent process involving an independent physician. As soon as the legal representative was available, or the patient was able to provide consent, written informed consent was immediately obtained; otherwise, the patient was withdrawn from the study and all study procedures were ended. The ethics board of the Jena University Hospital reviewed and approved the trial (file number 3858-08/13). An independent data and safety monitoring board monitored individual safety events during conduct of the trial.
Study Interventions
Patients were randomly assigned by an internet-based randomization tool in a ratio of 2:1 to three subsequent dosing cohorts to receive IV vilobelimab or placebo. Randomization was stratified by focus of infection allowing a distribution between abdominal and pulmonary focus ranging from 3:5 to 5:3. Blinding was maintained by similar appearance of placebo and vilobelimab bottled in neutral containers as provided by the drug manufacturer (InflaRx GmbH, Jena, Germany).
Patients received two dosages of 2 mg/kg body weight vilobelimab at 0 and 12 hours (cohort 1), 4 mg/kg body weight at 0 and 24 hours (cohort 2) or three dosages of 4 mg/kg body weight vilobelimab at 0, 24, and 72 hours (cohort 3). Placebo was given in equivalent volumes and at equivalent time points. Patients were followed until day 28. Serum for total complement hemolytic activity (CH50) to assess the activation of the classical complement pathway and citrate plasma for C5a, cytokines, and vilobelimab concentrations were taken before dosing of the study drug, and at additional time points throughout the study (eTable 2, http://links.lww.com/CCX/A851). Anti-drug antibodies (ADAs) were measured before first vilobelimab administration, at day 8, and at day 28 or hospital discharge (eTable 2, http://links.lww.com/CCX/A851).
Blood samples for the measurement of vilobelimab levels were analyzed by a validated enzyme-linked immunosorbent assay (ELISA) by the Sponsor (in-house ELISA). In samples with a vilobelimab concentration greater than or equal to 7.3 µg/mL, the blocking activity of vilobelimab on the recombinant human C5a (rhC5a)-induced up-regulation of CD11b on the surface of human granulocytes was measured by flow cytometry as described previously (15). Complement factor C5a was measured by an in-house ELISA (11). CH50 was measured with a commercially available liposome immunoassay (Wako Autokit CH50, FUJIFILM Wako Chemicals Europe GmbH, Germany). The interleukin (IL)-6 (Roche Cobas e411 analyzer, Roche Diagnostics, Switzerland), IL-8, and IL-10 (Siemens Immunolite 1000, Siemens Healthcare GmbH, Germany) were measured with chemiluminescence assays. ADAs were measured using a validated in-house sandwich ELISA for detection and an additional conformational assay in case of positive findings within the first test.
Safety and Efficacy Endpoints
Primary objectives of the study were pharmacodynamics of C5a, pharmacokinetics, and safety and tolerability of three dose regimens of vilobelimab. Safety and tolerability were assessed by descriptive analysis of adverse events (AEs, verbatims coded by the Medical Dictionary for Regulatory Activities Version 17.1) that were reported according to International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guideline E2A. All AEs were reported until hospital discharge but no longer than day 28. In this early development stage, special focus was on potential AE of special interest (AESI) such as acute systemic allergic hypersensitivity, anaphylactic reaction, meningitis, and meningococcal septicemia. Pharmacokinetics was assessed by measuring plasma concentrations of vilobelimab. We calculated the area under the curve of vilobelimab plasma concentrations over time and measured maximum as well as trough concentrations before second- and third-drug infusion. Pharmacodynamics were assessed by C5a, complement factors, and cytokines. Secondary efficacy endpoints included 28-day all-cause mortality, mean Sequential Organ Failure Assessment (SOFA) score until day 10, modified mean SOFA score until day 10 (CNS subscore omitted and renal subscore calculated without considering urine output), ICU-free days until day 28, ventilator-free days until day 14, renal replacement therapy (RRT)-free days until day 14, vasopressor-free days until day 14, and days with antimicrobial therapy until day 14. Only days alive were considered for calculating organ failure and ICU-free days. The mean SOFA score was calculated for an individual patient over 10 days based on the total SOFA score for each study day (22).
Statistical Analysis
Sample size calculation, precision, and power calculations were based on C5a levels measured previously in healthy volunteers and septic patients by the Sponsor. C5a concentrations of 16.4 ± 8.6 and 11.3 ± 2.3 ng/mL were observed in septic patients and healthy volunteers, respectively. The sample size of the study was planned to detect a reduction of 8 ng/mL in serum C5a concentrations with a significance level of 0.05 and a power of 0.8. Assuming a sd of 8 ng/mL in our trial population, 54 evaluable patients were required. To accommodate for an estimated drop-out rate of 15%, the total sample size was set to 72 patients. Efficacy analyses followed the intention-to-treat principle and was based on the full analysis set. Standard descriptive statistics and graphs were used to summarize the data. All data were summarized according to the treatment received. For placebo patients, data were pooled across the cohorts. Absolute and relative changes of C5a compared with baseline were analyzed by Wilcoxon signed rank test per timepoint in order to test for statistical significance of within treatment group dynamics. In addition, differences between the verum groups and the placebo group in C5a distributions per time point were evaluated by Mann-Whitney U test. Safety was analyzed descriptively. Number of events, number and percentage of patients with at least one event per System Organ Class by preferred term category were listed and summarized. Statistical analyses were performed using SAS 9.2 (SAS Institute) or later.
RESULTS
Study Population
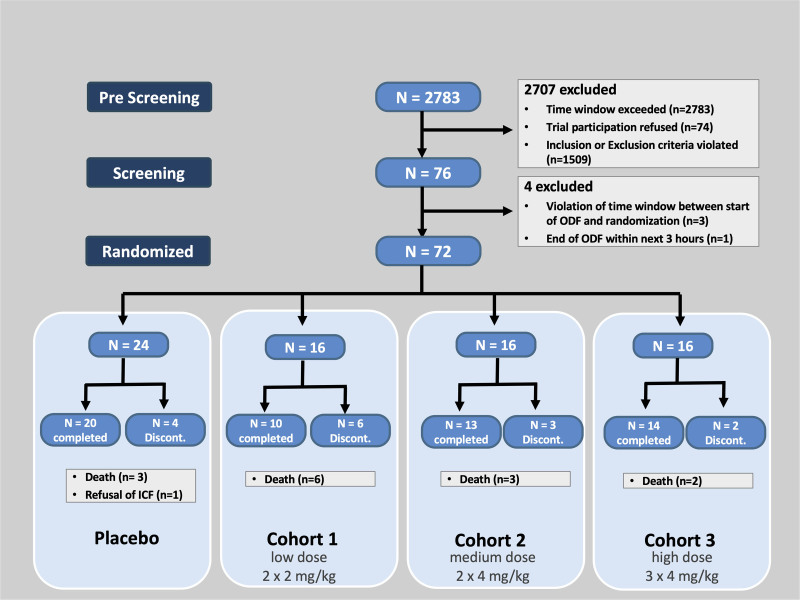
Two thousand seven hundred thirty-eight patients were prescreened resulting in 76 eligible patients, of which 72 patients were enrolled (Fig. 1; and eTable 9, http://links.lww.com/CCX/A851). All patients were dosed and included into the intention-to-treat dataset. Twenty-two major protocol deviations were documented in 15 subjects (violation of eligibility criteria in eight subjects, deviation from dose schedule in four subjects, prohibited medication in six patients, deviation from visit schedule in four patients; eTable 3, http://links.lww.com/CCX/A851). For 14 subjects, an insufficient number of pharmacokinetics samples were collected resulting in 58 subjects included in pharmacokinetics analyses. Patient characteristics are summarized in Table 1. Vilobelimab-treated patients in dosing cohort 1 (the lowest vilobelimab dosing cohort) were all mechanically ventilated at baseline, vilobelimab-treated patients in dosing cohort 3 (the highest dose cohort) had higher median lactate levels at baseline when compared with placebo. Placebo-treated patients had a slightly higher Acute Physiology and Chronic Health Evaluation-II-score, while baseline SOFA was similar in all groups.
Figure 1.
Patient flow in the SCIENS-trial. A patient could have failed prescreening for more than one reason. Discont = discontinued, ICF = informed consent, ODF = organ dysfunction.
TABLE 1.
Patient Characteristics
| Parameter | Placebo (n = 24) | Cohort 1 (n = 16) | Cohort 2 (n = 16) | Cohort 3 (n = 16) |
|---|---|---|---|---|
| Male sex | 16 (66.7%) | 10 (62.5%) | 9 (56.3%) | 11 (68.8%) |
| Age (yr) | 65 (53–75) | 65 (61–81) | 70 (51–83) | 78 (66–80) |
| Body mass index (kg/m2) | 25.3 (22–27) | 26.4 (24–32) | 26.6 (24–31) | 27.3 (24–30) |
| Acute Physiology And Chronic Health Evaluation-II score | 22.5 (17–27) | 19.5 (18–22) | 17.5 (14–26) | 20.0 (18–27) |
| SOFA score | 9.0 (8–11) | 8.5 (7–10) | 9.0 (7–11) | 8.5 (7–11) |
| Modified mean SOFA until day 10 with CNS subscore omitted and calculating renal subscore without considering urine output | 8.0 (7–10) | 7.0 (7–10) | 7.0 (6–8) | 7.5 (7–10) |
| Acute kidney injury | 6 (25.0%) | 2 (12.5%) | 6 (37.5%) | 6 (37.5%) |
| Vasopressor use | 22 (91.7%) | 15 (93.8%) | 13 (81.3%) | 14 (87.5%) |
| Mechanical ventilation | 20 (83.3%) | 16 (100%) | 12 (75.0%) | 10 (62.5%) |
| Surgery | 18 (75.0%) | 16 (100%) | 12 (75.0%) | 11 (68.8%) |
| Primary focus | ||||
| Pulmonary | 9 (37.5%) | 6 (37.5%) | 6 (37.5%) | 7 (43.8%) |
| Abdominal | 15 (62.5%) | 10 (62.5%) | 10 (62.5%) | 9 (56.3%) |
| Coexisting diseases | ||||
| Coronary artery disease | 5 (20.8%) | 6 (37.5%) | 1 (6.3%) | 2 (12.5%) |
| Diabetes mellitus | 2 (8.3%) | 3 (18.8%) | 1 (6.3%) | 2 (12.5%) |
| Arterial hypertension | 10 (41.7%) | 9 (56.3%) | 8 (50.0%) | 13 (81.3%) |
| Chronic obstructive pulmonary disease | 3 (12.5%) | 1 (6.3%) | 5 (31.3%) | 3 (18.8%) |
| Lactate (mmol/L) | 1.9 (1.6–3.7) | 2.6 (1.7–4.2) | 2.75 (1.6–4.4) | 3.89 (2.6–6.6) |
| Time to IP (CD only) (hr) | 4.5 (4–6) | 4.5 (3–6) | 3.3 (2–6) | 4.0 (1–5) |
| Time to IP (non-CD only) (hr) | 8.1 (5–10) | 6.2 (4–10) | 6.8 (5–9) | 6.8 (3–10) |
CD = cardiovascular dysfunction, IP = first infusion of investigational product (vilobelimab or placebo), SOFA = Sequential Organ Failure Assessment.
Data are presented as median and 25% and 75% percentiles or absolute numbers (percentage).
Primary Outcomes
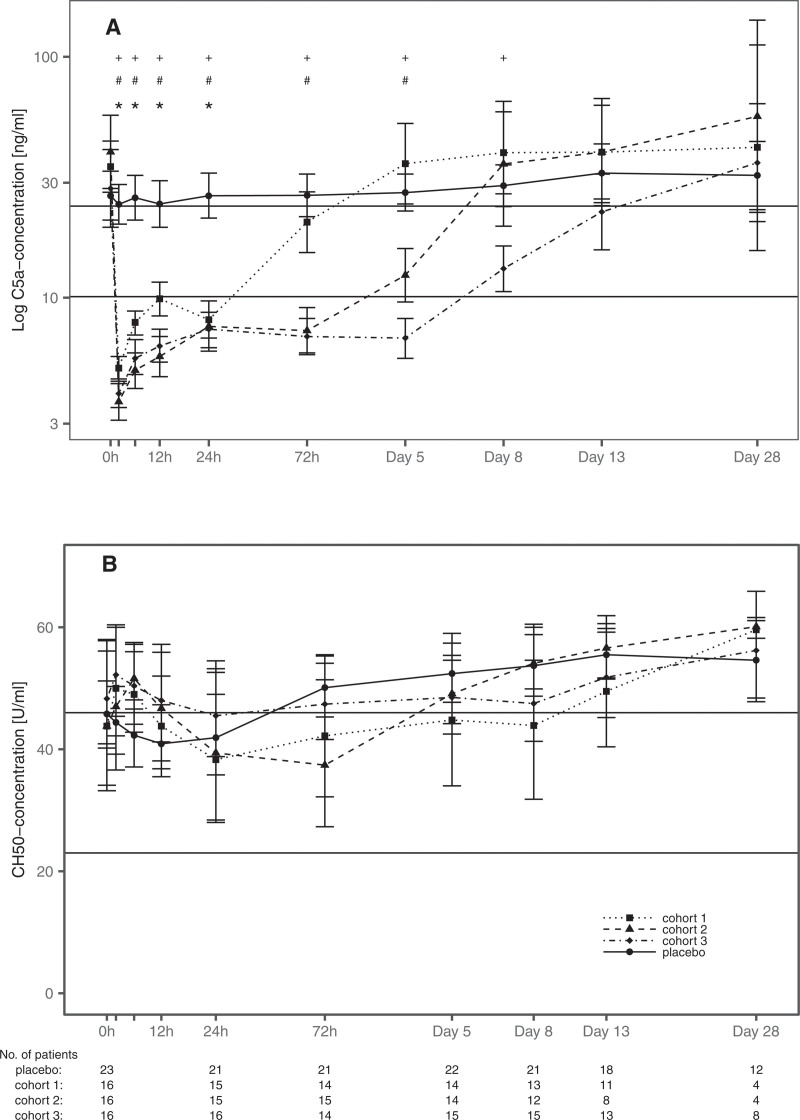
Application of vilobelimab resulted in a statistically significant drop of plasma C5a concentration within 2 hours in all three cohorts. C5a concentrations remained statistically significantly depressed depending on the applied dosage and schedule ranging from 24 hours (recovery time 72 hr) in cohort 1, 72 hours (recovery time 5 d) in cohort 2, until 5 d (recovery time > 8 d) in cohort 3 with p value of less than 0.001 (Fig. 2A). Mean plasma concentrations of vilobelimab increased with dosage (Table 2; and eFig. 1, http://links.lww.com/CCX/A851). As typical for monoclonal antibodies, the drug elimination curve showed a linear and nonlinear component, that is, the half-life is concentration dependent.
Figure 2.
Effects of vilobelimab on C5a plasma concentrations and total complement acticity. Mean C5a concentrations (A, black lines: normal range 10.1–24.0 ng/mL) and total complement activity (B, black lines: reference range of healthy subjects 23–46 U/mL) of patients alive. Data are shown as geometric mean ± 95% CIs. Statistically significant difference compared with baseline is displayed with p < 0.001 for cohort 1 (*), cohort 2 (#), and cohort 3 (+); detailed p values are given in eTable 4 (http://links.lww.com/CCX/A851). CH50 = 50% hemolytic complement..
TABLE 2.
Summary of Pharmacokinetic Parameters of Vilobelimab
| Parameter | Cohort 1 (n = 13) | Cohort 2 (n = 12) | Cohort 3 (n = 13) |
|---|---|---|---|
| Maximum concentration (µg/mL) | 47.7 (40.2–56.7) | 103.3 (87.1–122.4) | 103.6 (92.2–116.5) |
| Plasma concentration measured before drug infusion (µg/mL) | |||
| Second infusion | 19.4 (16.1–23.2) | 33.4 (27.4–40.7) | 30.1 (25.0–36.3) |
| Third infusion | — | — | 35.8 (26.8–47.7)a |
| Area under the curve (hr × µg/mL) | 2,101 (1,690–2,613) | 6,346 (5,321–7,568)b | 10,800 (9,233–12,632) |
aOnly 12 evaluable subjects for third infusion.
bEleven valid area under the curve measurements only.
Data are presented as geometric mean ± 95% CIs (statistical difference with p < 0.05 is given when the CIs do not overlap). Dashes indicate no third infusion was given in cohort 1 and cohort 2.
Vilobelimab was well tolerated. AEs are shown in eTable 5 (http://links.lww.com/CCX/A851). As expected in this severely ill patient population, the proportion of subjects with at least one AE was high and ranged between 75% and 94% within the treatment groups. Neither specific nor dose-related AE emerged with increasing exposure to vilobelimab. The majority of the AEs were classified by the investigator as not related to the study medication. The proportion of patients with serious AEs was highest in patients treated with placebo (13/24, 54%) and cohort 1 (10/16, 63%). Fatal AEs occurred in 12.5% (2/16 patients) to 37.5% (6/16 patients) being numerically highest in cohort 1. However, more patients were mechanically ventilated at baseline in cohort 1 than in all other cohorts. One patient of cohort 1 died of acute liver failure that was assessed by the investigator as possibly related to the study medication. However, this patient was diagnosed shortly after enrollment with nonocclusive mesenteric ischemia offering a likely cause for acute liver failure. No AESI was reported during the study. Similar frequencies and similar AE profiles across placebo and vilobelimab groups suggested a good safety profile of vilobelimab.
Secondary Outcomes
Secondary efficacy outcomes are shown in Table 3. There was no dose-dependent effect of vilobelimab administration on secondary clinical outcomes. Twenty-eight-day mortality was low in the placebo cohort with 12.5% (3/24 patients). The same mortality (2/16 patients) could be detected in the high dose cohort 3 in which patients were significantly older and with two-fold elevated baseline lactate levels. Dosing cohort 1, in which only a very short-term C5a blockade could be achieved, demonstrated a mortality rate at 37.5% (6/16 patients) and dosing cohort 2 also demonstrated a low mortality rate with 18.8% (3/16 patients). Dosing cohorts 2 and 3 had the highest ICU-free and ventilator-free days. There was no difference in the mean SOFA score, vasopressor-free days, or RRT-free days between the groups. Vilobelimab administration had no negative impact on antimicrobial treatment-free days.
TABLE 3.
Secondary Efficacy Outcomes
| Parameter | Placebo (n = 24) | Cohort 1 (n = 16) | Cohort 2 (n = 16) | Cohort 3 (n = 16) |
|---|---|---|---|---|
| 28-d mortality | 3 (12.5%) | 6 (37.5%) | 3 (18.8%) | 2 (12.5%) |
| ICU-free days until day 28 | 2.5 (0–20) | 5.0 (0–18) | 19.5 (6–24) | 12.5 (0–23) |
| Mean SOFA score | 5.7 (4.0–8.5) | 6.0 (3.8–8.1) | 5.1 (3.7–6.5) | 5.2 (4.3–8.0) |
| Mean modified SOFA score | 5.1 (3.7–7.9) | 4.8 (3.5–7.3) | 3.8 (3.2–5.6) | 4.9 (4.1–6.5) |
| Ventilator-free days until day 14 | 10.5 (1–13) | 8 (0–13) | 12.5 (5–13) | 10.5 (1–14) |
| Vasopressor-free days until day 14 | 9.5 (4–13) | 8.5 (0–13) | 13.0 (8–14) | 9.0 (5–12) |
| RRT-free days until day 14 | 14 (14–14) | 14 (7–14) | 14 (14–14) | 14 (10–14) |
| AT-free days until day 14 | 0 (0–4) | 0 (0–2) | 4.5 (2–7) | 0.5 (0–3) |
| Ventilator-free days until day 28 | 24 (6–26) | 10 (0–27) | 26.5 (13–27) | 24.5 (3–28) |
| Vasopressor-free days until day 28 | 23 (8–27) | 20.5 (0–27) | 27 (21–28) | 23 (7–26) |
| RRT-free days until day 28 | 28 (28–28) | 28 (0–28) | 28 (28–28) | 28.0 (10–28) |
| AT-free days until day 28 | 9.5 (2–14) | 4 (0–16) | 18 (7–20) | 6 (0–16) |
AT = antimicrobial therapy, RRT = renal replacement therapy, SOFA = Sequential Organ Failure Assessment.
Data are presented as median and 25% and 75% percentiles or absolute numbers (percentage). The mean SOFA score was calculated for each individual patient over 10 d based on the SOFA score for each study day.
Mean bioactivity of vilobelimab was 96.5%. No ADAs were detected. Vilobelimab was able to block the rhC5a-induced up-regulation of CD11b on the surface of human granulocytes with at least 92% for all measured time points. The majority of patients showed elevated CH50 at baseline in line with their early septic condition. During treatment, no pattern could be observed that would suggest a vilobelimab-induced change of CH50 (Fig. 2B). There was a marked difference in predosing plasma concentrations of IL 6 and IL 8 with cohort 3 having the highest and cohort 2 having the lowest concentrations. All groups reached the nadir of IL-6 and IL-8 concentrations at 72 hours after dosing (eFig. 2, http://links.lww.com/CCX/A851). Time course of IL-10 was similar among the placebo group, cohort 1, and cohort 2, while cohort 3 showed a trend of a faster decline in IL-10 plasma concentrations during the first 12 hours after dosing.
Post Hoc Analysis
According to the higher and more frequent vilobelimab dosing, cohort 2 and cohort 3 achieved significantly longer neutralization of C5a. Therefore, we compared placebo with the combined cohort 2 and 3 as an unplanned sensitivity analysis (patients characteristics: eTable 6, http://links.lww.com/CCX/A851 and C5a plasma concentrations: eFig. 4, http://links.lww.com/CCX/A851). We observed a faster decline in IL-8 and IL-10 concentrations during the first 24 hours after vilobelimab administration when compared with placebo (eFig. 3, http://links.lww.com/CCX/A851). Patients receiving higher dosages of vilobelimab had more ICU-, vasopressor-, and ventilator-free days (eTable 7, http://links.lww.com/CCX/A851). High dosages of vilobelimab were not associated with safety issues (eTable 8, http://links.lww.com/CCX/A851).
DISCUSSION
In this multicenter, double-blinded, randomized clinical trial of patients with early sepsis or septic shock caused by pneumonia or peritonitis, the monoclonal antibody vilobelimab was able to significantly reduce C5a levels by about 90% in all dose cohorts. The duration of C5a blockade was dependent on dosing frequency and lasted more than 5 d in the highest dose regimen with most vilobelimab administrations. The total complement hemolytic activity CH50 is a screening assay for the activation of the classical complement pathway and compilation of the MAC as result of the terminal complement activation and generation of complement split factor C5b. It is sensitive to an impairment of the activity of any component of the pathway (23). CH50 was not impacted, confirming the selective blockade of C5a by vilobelimab, which did not affect C5b and left the MAC generation intact. Vilobelimab was biologically active in line with preclinical results (10, 11). Administration of vilobelimab was well tolerated and did not result in safety concerns, which confirms the safety profile of vilobelimab reported in critically ill coronavirus disease 2019 (COVID-19) patients (24).
C5a blockade by vilobelimab at 4 mg/kg given two or three times achieved a trend in a faster drop in IL-8 and IL-10 concentrations when compared with placebo. This is in line with findings in animal experiments where pretreatment with a C5a antibody resulted in a decrease of cytokine concentrations (10, 25–27). We did not see a clear association of IL-6 concentrations with increasing dose and frequency of vilobelimab administration. However, the trial was not powered to show difference in these highly variable inflammatory mediators, and thus, conclusions should be drawn with care.
There is evidence from experimental Neisseria meningitidis sepsis that the C5a receptors C5aR1 and C5aR2 augment disease pathology and are both interesting targets for treatment (28). Similarly, in sepsis models, both receptors have been suggested to have a proinflammatory role within knockout experiments (29). Importantly, C5aR2 has been suggested to have several ligands other than C5a such as C3a, the acylation-stimulating protein C3adesArg, C4a, and others that bind to a different pocket of this receptor when compared with C5a (30). In addition, C5a signaling through C5aR2 has been linked to activation of High-Mobility Group Box 1 and, thus, inflammasome activation, underlining the proinflammatory role of C5a-induced C5aR2 signaling (31).
Vilobelimab administered two or three times at 4 mg/kg suggests more ICU-free days and more ventilator-free days until day 28 compared with placebo administration. In baboons with Escherichia coli sepsis, pretreatment with the C5 cleavage inhibitor RA101295 (27) significantly reduced organ damage and mortality. Similarly, vilobelimab-treated African green monkeys with H7N9 infection showed less lung damage and less inflammatory cell infiltration compared with the control group (10). In these studies, the immediate response to infection was attenuated by pretreatment or very early treatment, stressing again the necessity to treat early when using anti-inflammatory agents such as vilobelimab. C5a blockade with vilobelimab may also be a promising target in COVID-19 (32) and is currently investigated in a phase-III trial (33) (ClinicalTrials.gov: NCT 04333420).
Vilobelimab-induced C5a blockade was present in a dosing schedule dependent manner up to 13 days, while C5a concentrations remained elevated in the placebo group throughout the whole study period. The general approach of C5a blockade has been identified as an important target for approaching sepsis-induced development of the multiple organ dysfunction syndrome (34). Animal experiments identified several possible beneficial roles of C5a blockade that might be responsible for effects beyond the initial impact on the cytokine response such as beneficial effects on neutrophil function (35, 36), decreased neutrophil adherence to lung endothelial (26), prevention of septic cardiomyopathy and apoptosis of immune cells, and prevention of disseminated intravascular coagulation (6). Furthermore, C5aR1 signaling can interact with other danger sensing systems such as the Toll-like receptors (37, 38).
This study has strengths and limitations. Strengths of this trial include the randomized, double-blinded, placebo-controlled design (internal validity), its multicenter approach (external validity), the inclusion of sepsis patients very early in the course of the disease, and studying a novel study drug addressing a novel target. Noteworthy, we achieved a median time from sepsis onset until first infusion of the study drug of 8.1 hours. Our trial design therefore had the potential to minimize the contribution of the time window on trial failure (4). Limitations of this trial include a low sample size that was sufficient to address the primary endpoints but did not allow conclusive statements about clinical efficacy. Thus, any observed differences in clinical endpoints need confirmation by larger trials. We observed baseline imbalances in various important parameters despite randomization. We cannot exclude that these differences in disease severity diminished possible effects of vilobelimab on 28-day mortality and were responsible for the differences in predosing plasma concentrations of IL-6 and IL-8. We have only measured IL-6, IL-8, and IL-10 concentrations but effects of C5a on immune function are very complex. Therefore, we cannot conclude whether the detected differences in ventilator-free days or ICU-free days were attributable to the impact of vilobelimab on other immune functions aside from the early cytokine response (39). We observed similar numbers of patients experiencing infections in placebo and vilobelimab-treated patients during the trial. However, we did not monitor clinical or microbiological cure of the underlying infection causing sepsis. Antibiotic-free days served as a surrogate for infectious complications during the trial. We decided to only study patients with peritonitis or pneumonia to avoid too much variability in a study with low sample size (4). This resulted into a mainly surgical study population and might reduce external validity of our trial, although peritonitis and pneumonia are the most frequent foci of sepsis on German ICUs (3). We observed major protocol deviations in 15 patients (21% of the study population). In our opinion, this does not impact the validity of our trial as appropriate pharmacodynamic and pharmacokinetic effects were observed.
CONCLUSIONS
Administration of vilobelimab in patients with severe sepsis or septic shock selectively neutralized C5a in a dose-dependent manner without affecting activation of the MAC. No safety issues with vilobelimab treatment were identified. Our data support studying the highly selective neutralization of anaphylatoxin C5a by vilobelimab to prevent infection-associated organ dysfunction in future clinical studies.
ACKNOWLEDGMENTS
Contributing sites and site investigators for the “Studying Complement Inhibition in Early, Newly developing Septic organ dysfunction (SCIENS)”-trial, alphabetical by city: Department of Intensive and Intermediate Care Medicine, University Hospital RWTH Aachen, Aachen, Germany: Thorben Beeker, Jessica Pezechk, Bianca Meier. Department of Anesthesiology and Surgical Intensive Care Medicine, Augsburg University Hospital, Augsburg, Germany: Ulrich Jaschinski, Philipp Deetjen, Marlene Zanquila, Ilse Kummer. Department of Anesthesiology and Intensive Care Medicine, Greifswald University Hospital, Greifswald, Germany: Christian Fuchs, Matthias Gründling, Sven-Olaf Kuhn, Christian Scheer. Department of Anesthesiology and Intensive Care Medicine, Jena University Hospital, Jena, Germany: Michael Bauer, Ole Bayer, Frank Bloos, Martin Brauer, Carsten Herzog, Michael Hofmann, Stefan Russwurm, Yasser Sakr, Florian Setzer, Mark Simon, Helga Skupin, Daniel Thomas-Rüddel, Isabella Westermann, Johannes Winning. Department of Anesthesiology and Intensive Care Medicine, University Medical Center Kiel, Kiel, Germany: Tobias Becher, Gunnar Elke, Matthias Lindner, Dirk Schädler, Günther Zick. Department of Anaesthesiology and Intensive Care Medicine, University of Leipzig Medical Centre, Leipzig, Germany: Diana Becker-Rux, Sven Bercker, Udo X. Kaisers, Christian Schlegel, Philipp Simon. Department of Anesthesiology/Intensive Care Medicine/Emergency Medicine/Pain Therapy, Oldenburg University Hospital, Oldenburg, Germany: Oliver Djuren, Florian Jelschen, Heinrich Klingler, Georg Rohe, Andreas Weyland, Jörg Zundel. Clinical Data Collection and Management, Pharmacovigilance (alphabetical): Clinical Trial Centre Leipzig, University Leipzig, Leipzig, Germany: Anja Blaser, Holger Bogatsch, Yasmine Breitenstein, Matthias Collier, Madlen Dörschmann, Dagmar Fiedler, Thekla Haage, Silke Hauer, Thomas Junge, Monika Rohwedder, André Rothe, Reinhild Schnabel, Anja Schneider, Bianca Scholze, Annett Schrock, Katja Sommer, Anke Stolle, Evelyn Trips. ACOMED statistik, Leipzig, Germany: T. Keller was responsible for the data analysis and S. Weber conducted the data analysis. Independent Data and Safety Monitoring Board: Andreas Weyland (Research Center Neurosensory Science, Carl von Ossietzky Universität Oldenburg, Oldenburg, Germany), Herwig Gerlach (Department of Anesthesiology, operative Intensive Care Medicine, and Pain Therapy, Vivantes Klinikum Neukölln, Berlin, Germany), Dorothea Wessiepe (Metronomia Clinical Research GmbH, Munich, Germany).
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
InflaRx, the sponsor of this trial, was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and the preparation and review of the article, but had no role in the decision to submit the article for publication. The decision to submit the article was made by the principal investigator and the coauthors.
Supported, in part, by grant from the InflaRx GmbH (Jena, Germany), the sponsor of this trial.
Dr. Bauer holds shares of SmartDyeLivery GmbH, Jena, Germany. He has received funding for scientific advisory boards, travel, and speaker honoraria by T2 Biosystems, La Jolla Pharmaceutical Company, SNIPR BIOME Denmark, CytoSorbents GmbH, Thermo Fisher Scientific (B·R·A·H·M·S GmbH), Roche Diagnostics International, Transgene S.A., and sphingotec GmbH. Dr. Bloos received an honorarium for an expert advisory board meeting by Baxter and lecture honoraria from biosyn. Dr. Kluge received research support from Ambu, E.T. View, Fisher & Paykel, Pfizer, and Xenios. He also received lecture honorarium from Arjo-Huntleigh, Astellas, Astra, Basilea, Bard, Baxter, Biotest, CSL Behring, Cytosorbents, Fresenius, Gilead, MSD, Orion, Pfizer, Philips, Sedana, Sorin, Xenios, and Zoll. He received consultant honorarium from AMOMED, Astellas, Baxter, Bayer, Fresenius, Gilead, MSD, Pfizer, and Xenios. Dr. Marx received research support from BBraun, Adrenomed, Biotest, lecture honorarium from BBraun, Biotest, and Philips, and consultant honorarium from Adrenomed, Bbraun, and 4teen4. Dr. Moerer received a research grant from CSL Behring and honoraria for participating as a speaker at workshops on hemodynamic monitoring at the European medical school in Oldenburg, Germany, and for participating at an expert advisory board meeting by Baxter. The Department of Anesthesiology holds courses and workshops on hemodynamic monitoring, partially supported by Getinge. Dr. Reinhart was paid advisor for Adrenomed Berlin Hennigsdorf, Hennigsdorf, Germany, for the development of an antibody to treat shock states until 2019 and holds shares of InflaRx, Jena, Germany. Dr. P. Simon reports lecture fees from InfectoPharm (Heppenheim, Germany). Dr. T. P. Simon received consultancy fees and travel grants from SphingoTec GmbH, BBraun AG, and Biotest AG. Mr. Weber received payments for the statistical analysis from the sponsor of the trial. Dr. Weyland received honoraria for lectures from Pulsion Medical Systems, Teva Pharmaceutical Industries, CytoSorbents Europe, and Bayer Health Care. Drs. Guo and Riedemann are founders, active officers, and executive directors of InflaRx (InflaRx GmbH, InflaRx Pharmaceuticals, and InflaRx N.V.) and hold shares and stock options in InflaRx. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at Department of Intensive and Intermediate Care Medicine, University Hospital RWTH Aachen, Aachen, Germany; Department of Anesthesiology and Surgical Intensive Care Medicine, University Hospital Augsburg, Augsburg, Germany; Department of Nephrology and Medical Intensive Care, Charité-Universitätsmedizin Berlin, Berlin, Germany; Department of Internal Medicine/Cardiology, Angiology, Nephrology, and Conservative Intensive Care Medicine, Vivantes Klinikum Neukölln, Berlin, Germany; Department of Anesthesiology and Intensive Care Medicine, Jena University Hospital, Jena, Germany; Department of Anesthesiology, University Medical Center, Georg-August-University Göttingen, Göttingen, Germany; Department of Anesthesiology and Intensive Care Medicine, University Hospital Greifswald, Greifswald, Germany; Department of Intensive Care Medicine, University Hospital Hamburg-Eppendorf, Hamburg, Germany; Department of Anesthesiology and Intensive Care Medicine, University Medical Center Kiel, Kiel, Germany; Department of Anesthesiology and Intensive Care Medicine, University of Leipzig Medical Centre, Leipzig, Germany; and University Department for Anesthesia, Intensive and Emergency Medicine and Pain Management, Hospital Oldenburg, Oldenburg, Germany.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014; 311:1308–1316 [DOI] [PubMed] [Google Scholar]
- 3.SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: The prospective, multicentre INSEP study. Intensive Care Med. 2016; 42:1980–1989 [DOI] [PubMed] [Google Scholar]
- 4.Mebazaa A, Laterre PF, Russell JA, et al. Designing phase 3 sepsis trials: Application of learned experiences from critical care trials in acute heart failure. J Intensive Care. 2016; 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unnewehr H, Rittirsch D, Sarma JV, et al. Changes and regulation of the C5a receptor on neutrophils during septic shock in humans. J Immunol. 2013; 190:4215–4225 [DOI] [PubMed] [Google Scholar]
- 6.Klos A, Tenner AJ, Johswich KO, et al. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009; 46:2753–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annane D, Heming N, Grimaldi-Bensouda L, et al. ; Garches COVID 19 Collaborative Group. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study. EClinicalMedicine. 2020; 28:100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991; 4:359–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007; 25:1256–1264 [DOI] [PubMed] [Google Scholar]
- 10.Sun S, Zhao G, Liu C, et al. Treatment with anti-C5a antibody improves the outcome of H7N9 virus infection in African green monkeys. Clin Infect Dis. 2015; 60:586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riedemann NC, Habel M, Ziereisen J, et al. Controlling the anaphylatoxin C5a in diseases requires a specifically targeted inhibition. Clin Immunol. 2017; 180:25–32 [DOI] [PubMed] [Google Scholar]
- 12.Bengtson A, Heideman M. Anaphylatoxin formation in sepsis. Arch Surg. 1988; 123:645–649 [DOI] [PubMed] [Google Scholar]
- 13.Nakae H, Endo S, Inada K, et al. Serum complement levels and severity of sepsis. Res Commun Chem Pathol Pharmacol. 1994; 84:189–195 [PubMed] [Google Scholar]
- 14.Nakae H, Endo S, Inada K, et al. Chronological changes in the complement system in sepsis. Surg Today. 1996; 26:225–229 [DOI] [PubMed] [Google Scholar]
- 15.Czermak BJ, Sarma V, Pierson CL, et al. Protective effects of C5a blockade in sepsis. Nat Med. 1999; 5:788–792 [DOI] [PubMed] [Google Scholar]
- 16.Hoehlig K, Maasch C, Shushakova N, et al. A novel C5a-neutralizing mirror-image (l-)aptamer prevents organ failure and improves survival in experimental sepsis. Mol Ther. 2013; 21:2236–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber-Lang MS, Younkin EM, Sarma JV, et al. Complement-induced impairment of innate immunity during sepsis. J Immunol. 2002; 169:3223–3231 [DOI] [PubMed] [Google Scholar]
- 18.Rivers EP, Jaehne AK, Nguyen HB, et al. Early biomarker activity in severe sepsis and septic shock and a contemporary review of immunotherapy trials: Not a time to give up, but to give it earlier. Shock. 2013; 39:127–137 [DOI] [PubMed] [Google Scholar]
- 19.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 20.Brunkhorst FM, Reinhart K. [Diagnosis and causal treatment of sepsis]. Internist (Berl). 2009; 50:810–816 [DOI] [PubMed] [Google Scholar]
- 21.Bloos F, Trips E, Nierhaus A, et al. ; for SepNet Critical Care Trials Group. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: A randomized clinical trial. JAMA Intern Med. 2016; 176:1266–1276 [DOI] [PubMed] [Google Scholar]
- 22.Lambden S, Laterre PF, Levy MM, et al. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019; 23:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costabile M. Measuring the 50% haemolytic complement (CH50) activity of serum. J Vis Exp. 2010:1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlaar APJ, de Bruin S, Busch M, et al. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): An exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020; 2:e764–e773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höpken U, Mohr M, Strüber A, et al. Inhibition of interleukin-6 synthesis in an animal model of septic shock by anti-C5a monoclonal antibodies. Eur J Immunol. 1996; 26:1103–1109 [DOI] [PubMed] [Google Scholar]
- 26.Herrmann JB, Muenstermann M, Strobel L, et al. Complement C5a receptor 1 exacerbates the pathophysiology of N. meningitidis sepsis and is a potential target for disease treatment. mBio. 2018; 9:e01755–e01717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keshari RS, Silasi R, Popescu NI, et al. Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proc Natl Acad Sci U S A. 2017; 114:E6390–E6399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muenstermann M, Strobel L, Klos A, et al. Distinct roles of the anaphylatoxin receptors C3aR, C5aR1 and C5aR2 in experimental meningococcal infections. Virulence. 2019; 10:677–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rittirsch D, Flierl MA, Nadeau BA, et al. Functional roles for C5a receptors in sepsis. Nat Med. 2008; 14:551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalant D, Cain SA, Maslowska M, et al. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. J Biol Chem. 2003; 278:11123–11129 [DOI] [PubMed] [Google Scholar]
- 31.Colley CS, Popovic B, Sridharan S, et al. Structure and characterization of a high affinity C5a monoclonal antibody that blocks binding to C5aR1 and C5aR2 receptors. MAbs. 2018; 10:104–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlaar APJ, de Bruin S, Busch M, et al. Anti-C5a antibody (IFX-1) treatment of severe COVID-19: An exploratory phase 2 randomized controlled trial. SSRN J. 2020; 2:E764–E773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao T, Hu M, Zhang X, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. Preprint posted online June 18, 2020. doi: 2020.03.29.20041962 [Google Scholar]
- 34.Karasu E, Nilsson B, Köhl J, et al. Targeting complement pathways in polytrauma- and sepsis-induced multiple-organ dysfunction. Front Immunol. 2019; 10:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo RF, Ward PA. C5a, a therapeutic target in sepsis. Recent Pat Antiinfect Drug Discov. 2006; 1:57–65 [DOI] [PubMed] [Google Scholar]
- 36.Guo RF, Riedemann NC, Bernacki KD, et al. Neutrophil C5a receptor and the outcome in a rat model of sepsis. FASEB J. 2003; 17:1889–1891 [DOI] [PubMed] [Google Scholar]
- 37.Hawlisch H, Belkaid Y, Baelder R, et al. C5a negatively regulates Toll-like receptor 4-induced immune responses. Immunity. 2005; 22:415–426 [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Kimura Y, Fang C, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007; 110:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer M. Critical illness and flat batteries. Crit Care. 2017; 21:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.