Summary
Background
The introduction of immunomodulatory agents, proteasome inhibitors, and autologous haematopoietic stem-cell transplantation has improved outcomes for patients with multiple myeloma, but patients with high-risk multiple myeloma have a poor long-term prognosis. We aimed to address optimal treatment for these patients.
Methods
SWOG-1211 is a randomised phase 2 trial comparing eight cycles of lenalidomide (25 mg orally on days 1–14 every 21 days), bortezomib (1·3 mg/m2 subcutaneously on days 1, 4, 8, and 11 every 21 days), and dexamethasone (20 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12 every 21 days; RVd) induction followed by dose-attenuated RVd maintenance (bortezomib 1 mg/m2 subcutaneously on days 1, 8, and 15; lenalidomide 15 mg orally on days 1–21; dexamethasone 12 mg orally on days 1, 18, and 15 every 28 days) until disease progression with or without elotuzumab (10 mg/kg intravenously on days 1, 8, and 15 for cycles 1–2, on days 1 and 11 for cycles 3–8, and on days 1 and 15 during maintenance). Patients were randomly assigned (1:1) to either RVd or RVd-elotuzumab. High-risk multiple myeloma was defined by one of the following: gene expression profiling high risk (GEPhi), t(14;16), t(14;20), del(17p) or amp1q21, primary plasma cell leukaemia and elevated serum lactate dehydrogenase (two times the upper limit of normal or more). The primary endpoint was progression-free survival, and all analyses were done on intention-to-treat basis among eligible patients who were evaluable for response. This study is registered with ClinicalTrials.gov, NCT01668719.
Findings
100 (RVd n=52, RVd-elotuzumab n=48) patients were enrolled between Oct 27, 2013, and May 15, 2016, across 26 cooperative group institutions in the USA. Median age was 64 years (IQR 57–70, range 36–85). 74 (75%) of 99 had International Staging System stage II or stage III disease, 47 (47%) of 99 had amp1q21, 37 (37%) of 100 had del17p, 11 (11%) of 100 had t(14;16), eight (9%) of 90 were GEPhi, seven (7%) of 100 had primary plasma cell leukaemia, five (5%) of 100 had t(14;20), four (4%) of 100 had elevated serum lactate dehydrogenase, and 17 (17%) had two or more features. With a median follow-up of 53 months (IQR 46–59), no difference in median progression-free survival was observed (RVd 33·64 months [95% CI 19·55–not reached], RVd-elotuzumab 31·47 months [18·56–53·98]; hazard ratio 0·968 [80% CI 0·697–1·344]; one-sided p=0·45]. 37 (71%) of 52 patients in the RVd group and 37 (77%) of 48 in the RVd-elotuzumab group had grade 3 or worse adverse events. No significant differences in the safety profile were observed, although some notable results included grade 3–5 infections (four [8%] of 52 in the RVd group, eight [17%] of 48 in the RVd-elotuzumab group), sensory neuropathy (four [8%] of 52 in the RVd group, six [13%] of 48 in the RVd-elotuzumab group), and motor neuropathy (one [2%] of 52 in the RVd group, four [8%] of 48 in the RVd-elotuzumab group). There were no treatment-related deaths in the RVd group and one death in the RVd-elotuzumab group for which study treatment was listed as possibly contributing by the investigator.
Interpretation
In the first randomised study of high-risk multiple myeloma reported to date, the addition of elotuzumab to RVd induction and maintenance did not improve patient outcomes. However, progression-free survival in both study groups exceeded the original statistical assumptions and supports the role for continuous proteasome inhibitors and immunomodulatory drug combination maintenance therapy for this patient population.
Funding
National Institutes of Health, National Cancer Institute, Bristol Myers Squibb, Celgene, Leukemia and Lymphoma Society.
Introduction
Multiple myeloma is a neoplasm of plasma cells that is characterised by osteolytic bone lesions and organ damage, such as hypercalcaemia, anaemia, and renal insufficiency.1 Although the introduction of immunomodulatory drugs and proteasome inhibitors and advances in high-dose therapy administration (chemo-therapy requiring stem-cell rescue) have had an effect on progression-free survival (PFS) and overall survival for patients with multiple myeloma in general, patients with high-risk disease still have a poor long-term prognosis.2 Therefore, identification of patients with high-risk disease and development of novel therapeutic regimens that will extend their survival outcomes are imperative. To date, no clinical investigations in multiple myeloma have targeted this specific patient population.
The National Cancer Institute Myeloma Steering Committee convened a session in March, 2011, to reach a consensus on how best to risk-stratify multiple myeloma and to develop therapies targeting high-risk disease. A consensus was reached on the definition of high-risk multiple myeloma on the basis of the best available evidence at the time, and included the following four groups: poor risk score by gene expression profiling,3 fluorescence in-situ hybridisation (FISH) or cytogenetics for t(14;16),4 t(14;20),5 and del(17p)6 and amplification 1q21,7 primary plasma cell leukaemia,8 and elevated serum lactate dehydrogenase.9
The SWOG-1211 randomised phase 2 trial was designed as the first concerted, US effort with an enrichment design to identify optimal management of untreated patients with high-risk multiple myeloma. The three-drug induction regimen combining bortezomib, lenalidomide, and dexamethasone (RVd) was believed to be the most promising front-line induction therapy at the time. In a phase 1/2 trial of RVd that enrolled 66 patients,10 all patients responded, including 44 (67%) who had very good partial response or better, and 26 (39%) who had complete response or near-complete response. Moreover, the patients with poor cytogenetics (defined as t[4;14], t[14;16], or del[17p]) responded in a similar fashion to the patients with standard risk or normal cytogenetics. With a median follow-up of 21 months, the estimated 18-month PFS was 75% and overall survival was 97% with or without with or without haematopoietic stem-cell transplantation (HSCT). Shortly thereafter, the Intergroupe Francophone du Myelome reported the primary results of a phase 2 study investigating three RVd cycles before high-dose therapy followed by autologous HSCT and two RVd cycles for consolidation. After induction and high-dose therapy, 91% of patients were responders, including 68% with very good partial response or better, and 36% with complete response plus stringent complete response.11 The availability of novel drugs had renewed the concept of maintenance, and three large randomised phase 3 trials,12 in which lenalidomide was given at low dose and compared with observation, had shown an improvement of PFS in favour of the intervention group. The meta-analysis of the trials comparing lenalidomide maintenance to observation showed overall survival benefit in favour of the lenalidomide maintenance; however, the benefit in high-risk multiple myeloma has not been clearly delineated even with longer follow-up.
SLAMF7, previously known as CS1, is a cell surface glycoprotein that is universally highly expressed on multiple myeloma cells, but not on normal cells.13 The role of SLAMF7 in the disease is not well characterised, but it appears to have a crucial role in interactions between multiple myeloma cells and the bone marrow stromal cells. Elotuzumab is a fully humanised monoclonal antibody against SLAMF7 that has shown significant in-vitro activity against human multiple myeloma cell lines and in-vivo activity in mouse multiple myeloma xenograft models.14 Elotuzumab has been evaluated in phase 1–3 trials in patients with relapsed or refractory multiple myeloma15–17 and has shown promise in combination with lenalidomide plus dexamethasone and with bortezomib. In a preliminary analysis of an ongoing phase 1 study of elotuzumab plus bortezomib,15 the overall response rate (partial response or better) was 48% among 27 evaluable patients, and responses were seen in several patients with bortezomib-refractory disease. In a preliminary analysis of an ongoing phase 1b combination study16 with lenalidomide and dexamethasone (Rd), the overall response rate was 82% for all treated patients (n=28), 96% for lenalidomide-naive patients (n=22), and 82% for patients who had been refractory to their most recent treatment (n=11). In a phase 2 study of the elotuzumab-Rd combination,16 the overall response rate was 85% for evaluable patients (22 of 26), the remaining four (15%) patients had stable disease, and eight (31%) had either a complete response or a very good partial response. This study was followed by the ELOQUENT-2 randomised phase 3 trial17 that showed superiority of elotuzumab-Rd over Rd for relapsed or refractory multiple myeloma in terms of median PFS. For all studies of elotuzumab, adverse events were primarily infusion-related and were readily manageable using adequate premedication. From the available data, elotuzumab in combination with either lenalidomide or bortezomib had comparable response rates in patients with high-risk multiple myeloma and poor cytogenetics (t[4;14], t[14;16], or del[17p]) compared with those with standard or normal cytogenetics.
On the basis of these encouraging results and its safety profile, the SWOG-1211 study was designed to evaluate the efficacy of adding elotuzumab to RVd as induction and in maintenance for newly diagnosed high-risk multiple myeloma. We report the primary analysis of this study.
Methods
Study design and participants
The SWOG-1211 trial was designed with a phase 1 run-in followed by the randomised phase 2 study. The phase 1 portion of the trial has been previously reported.18 In the randomised, open-label, multicentre phase 2 trial, patients were enrolled across 26 cooperative group institutions in the USA.
Patients were eligible if they had newly diagnosed active multiple myeloma as defined by the International Myeloma Working Group 2009 criteria,19 they had Southwest Oncology Group (SWOG) performance status of 0–2, and they were either ineligible for high-dose chemotherapy with autologous HSCT or deferred transplantation to subsequent relapse. Patients had to have high-risk multiple myeloma on the basis of one or more of the following criteria at the time of initial diagnosis (before any chemotherapy): (1) poor risk genomic signature according to the University of Arkansas 70-gene model (available clinically as MyPRS score; Signal Genetics [San Diego, CA, USA]; here, referred to as GEPhi); (2) translocations t(14;16) or t(14;20) or deletion del(17p) by FISH or cytogenetics, or gain (three copies) or amplification (more than three copies) of chromosome 1q21 by FISH (standard percentage cutoff values for each type of FISH test abnormality were used in local laboratories; typically 5%, but ranging from 1·5% to 7·5%); (3) primary plasma cell leukaemia (defined by either ≥2000 plasma cells per mL of peripheral blood, or 20% on a manual differential count);12 and (4) serum lactate dehydrogenase two times or more the institutional upper limit of normal. Patients on the phase 2 portion could have received one previous cycle of any non-investigational chemotherapy. Stem-cell mobilisation was allowed (not required) after 2–4 cycles of therapy, per local procedures. Full eligibility criteria are shown in the appendix (pp 15–17).
The study was led by the SWOG Barlogie-Salmon Myeloma Committee and supported in participation by ECOG-ACRIN and ALLIANCE Myeloma Committees. Independent ethics or institutional review boards at each site approved the protocol. The trial was done in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written, informed consent. All authors reviewed and approved the manuscript for submission. The authors vouch for the accuracy and completeness of the data and adherence to the trial protocol.
Randomisation and masking
Patients were enrolled at participating institutions and were randomly assigned by Rave and the SWOG Statistics and Data Management Center. Patients were randomly assigned (1:1) to the two treatment groups using a dynamic balancing randomisation algorithm as described by Pocock and Simon20 to ensure that the assignment of treatments was balanced across, the stratification factor, presence of primary plasma cell leukaemia or lactate dehydrogenase of two times or more the upper limit of normal versus everyone else. This procedure balances the marginal distribution of the stratification factors between these treatment regimens. Patients and treating physicians were not masked to treatment allocation.
Procedures
Eligible patients received either eight cycles of RVd (bortezomib, lenalidomide, and dexamethasone) or eight cycles of RVd-elotuzumab (bortezomib, lenalidomide, dexamethasone, and elotuzumab) induction therapy. During the 21-day induction cycles, both groups received bortezomib (1·3 mg/m2 subcutaneously or intravenously on days 1, 4, 8, and 11), lenalidomide (25 mg orally on days 1–14), and dexamethasone (20 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12). In RVd-elotuzumab group, patients also received elotuzumab at the dose determined during the phase 1 portion (10 mg/kg intravenously on days 1, 8, and 15 for cycles 1–2 and days 1 and 11 for cycles 3–8). Patients who had received the permitted one cycle of chemotherapy before registration began protocol therapy on cycle 2 and therefore received a maximum of seven induction chemotherapy cycles. The induction phase was followed by 28-day cycles of dose-attenuated RVd maintenance (bortezomib 1 mg/m2 subcutaneously or intravenously on days 1, 8, and 15; lenalidomide 15 mg orally on days 1–21; dexamethasone 12 mg orally on days 1, 18, and 15) until disease progression for all patients with or without elotuzumab (elotuzumab 10 mg/kg intravenously on days 1 and 15). Patients received the same drugs for induction and maintenance. Patients received deep vein thrombosis prophylaxis and varicella-zoster virus prophylaxis (to include aciclovir) per local institutional standard. The use of bisphosphonates was allowed per local institutional guidelines. Disease assessments were done every cycle. Responses were determined using International Myeloma Working Group Uniform Response Criteria.21
Outcomes
The primary endpoint of this study was PFS in the two treatment groups, RVd and RVd-elotuzumab. PFS was defined as the time from randomisation to the date of first documented progression or death due to any cause, determined centrally. Patients last known to be alive and progression-free were censored at date of last contact. Secondary endpoints were overall survival, overall response rate, and toxicity of the treatment combinations. Overall survival was defined as the time from randomisation until the date of death due to any cause. Patients last known to be alive were censored at the time of last contact. Responses were determined using International Myeloma Working Group Uniform Response Criteria.21 The overall response rate was calculated as the number of patients with a partial response or better among eligible patients who were evaluable for response. Initially, toxicity was evaluated using Common Terminology Criteria for Adverse Events (CTCAE) version 4, which was updated to version 5 during the course of the study.
Exploratory endpoints, which were not prespecified in the protocol, included the evaluation of PFS and overall survival within subgroups that were defined by baseline high-risk features. Data on rescue therapies were not captured as part of the study protocol.
Statistical analysis
The study was designed to accrue 100 eligible patients (50 per group). An additional 30 patients (15 per group) were to be accrued to account for ineligible patients and patients withdrawing consent. The median expected PFS in the RVd control group was based on two University of Arkansas studies.22,23 High-risk patients in those studies were defined based on the GEPhi risk score. Median PFS in that patient group was 2·2 years. Around 20% of newly diagnosed patients with multiple myeloma were anticipated to be high risk as defined in the study protocol and that this study would accrue approximately 25 patients with high-risk multiple myeloma per year. An assumption of uniform accrual of 25 patients per year, 4 years of accrual, and 2 years of follow-up yielded a study with 82% power and a one-sided significance level of 0·1 to detect a hazard ratio (HR) of 1·75 between the two treatment groups, which translates to an increase in median PFS from 2·0 years to 3·5 years in the RVd-elotuzumab group compared with the RVd group. Assuming exponential survival, 64 PFS events were expected to occur within 6 years on the basis of the alternative hypothesis. An interim analysis for futility was scheduled for when around 32 PFS events had occurred. The analysis was done centrally per protocol, with a data cutoff of March 6, 2017. Given that no boundaries were crossed at that time, the study continued as planned.
All analyses were done on an intention-to-treat basis. Patients who met all eligibility criteria and who provided valid informed consent were included in all survival analyses. Of these patients, those who received at least one dose of study drug were considered evaluable for toxicity assessments. Response analyses included all patients who were eligible, who provided valid consent, and who were assessable for response. Patients who did not have an adequate follow-up response assessment were deemed non-assessable for response. PFS and overall survival were evaluated using a one-sided, stratified log-rank test to compare differences in outcome between the treatment groups. HRs were calculated and 80% Wald CIs were constructed. These tests were done at a one-sided significance level of 0·1. Kaplan-Meier was used to estimate and display the distribution of the endpoints over time. Response was compared between treatment groups using Cochran-Mantel-Haenszel tests, stratified by the prespecified randomisation stratification factor, presence of primary plasma cell leukaemia or lactate dehydrogenase of two times or more the upper limit of normal versus all others. All eligible patients that initiated treatment were considered evaluable for response and toxicity analyses.
Additionally, Cox proportional hazards analyses were used in unplanned, exploratory analyses on small subgroups to assess the association between certain high-risk features and PFS and overall survival outcomes.
All analyses were done using SAS version 9.4.
This trial was registered with ClinicalTrials.gov, NCT01668719.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The study team had full access to all data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
134 patients were enrolled between Oct 27, 2013, and May 15, 2016, across 26 cooperative group institutions in the USA (see appendix pp 11–14 for list of participating institutions). 100 patients were deemed eligible and analysable for survival outcomes (figure 1). 16 patients were deemed ineligible in the RVd group, and 17 were ineligible and one withdrew consent in the RVd-elotuzumab group. The RVd group included 52 eligible and analysable patients, whereas the RVd-elotuzumab group included 48. The median age of patients was 66 years (IQR 56–71, range 36–85) in the RVd group and 62 years (58–69, 40–79) in the RVd-elotuzumab group (table 1). 48 (48%) of the 100 patients in the study group were aged 65 years or older, and 24 (24%) were 70 years or older.
Figure 1:
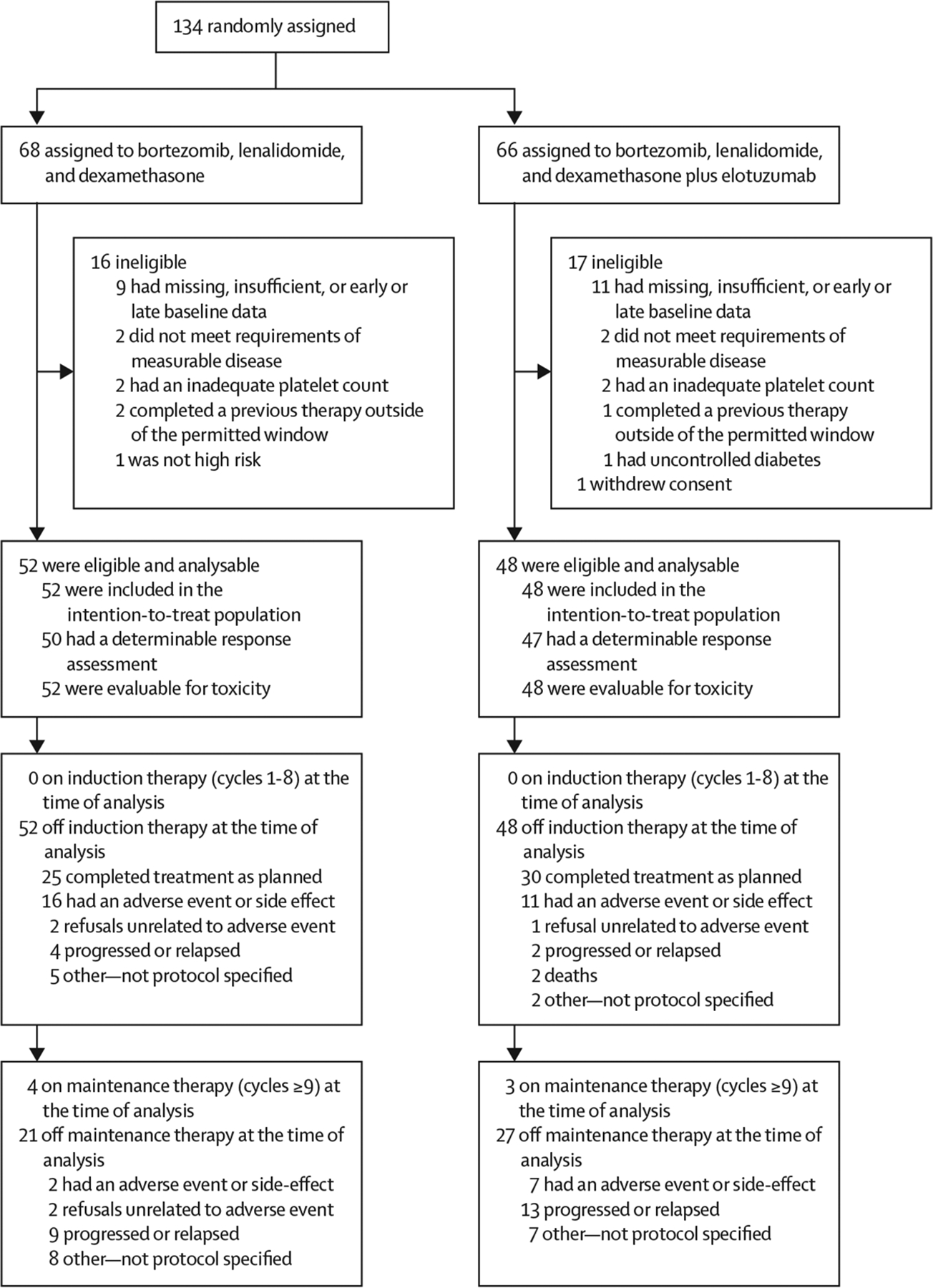
Study profile
Table 1:
Patient characteristics
| Bortezomib, lenalidomide, and dexamethasone (N=52) | Bortezomib, lenalidomide, and dexamethasone plus elotuzumab (N=48) | |
|---|---|---|
| Age | ||
| Median age, years (IQR) | 66 (56–71) | 62 (58–69) |
| Range | 36–85 | 40–79 |
| ≥65 years | 27 (52%) | 21 (44%) |
| ≥70 years | 13 (25%) | 11 (23%) |
| Sex | ||
| Female | 21 (40%) | 19 (40%) |
| Male | 31 (60%) | 29 (60%) |
| Southwest Oncology Group Performance Status >1 | 9/51 (18%) | 7/46 (15%) |
| β2 microglobulin ≥3·5 mg/L | 33 (63%) | 32 (67%) |
| C-reactive protein ≥8 mg/L | 11/49 (22%) | 10/43 (23%) |
| Creatinine ≥2 mg/dL | 2 (4%) | 1/47 (2%) |
| Lactate dehydrogenase ≥190 U/L | 27 (52%) | 24 (50%) |
| Albumin <3·5 g/dL | 20 (38%) | 20 (42%) |
| Haemoglobin <10 g/dL | 25 (48%) | 21 (44%) |
| Platelet count <150000 cells per μL | 9 (17%) | 7 (15%) |
| Serum monoclonal spike >3 g/dL | 21 (40%) | 25/47 (53%) |
| Bone marrow plasma cells >60% | 24 (46%) | 20/47 (43%) |
| Serum free light chain ratio (involved:uninvolved) ≥100 | 21/43 (49%) | 18/43 (42%) |
| One cycle of previous treatment received | 13 (25%) | 14 (29%) |
| High risk by 70-gene gene expression profiling | 2/48 (4%) | 6/42 (14%) |
| Primary plasma cell leukaemia | 4 (8%) | 3 (6%) |
| Lactate dehydrogenase ≥2 times the upper limit of normal | 2 (4%) | 2 (4%) |
| High-risk cytogenetic features | ||
| t(14;16) | 4 (8%) | 7 (15%) |
| t(14;20) | 3 (6%) | 2 (4%) |
| del(17p) | 22 (42%) | 15 (31%) |
| Gain or amp(1q21) | 23/51 (45%) | 24/48 (50%) |
| ≥2 high-risk features | 8 (15%) | 9 (19%) |
| International Staging System | ||
| Stage I | 13 (25%) | 13 (27%) |
| Stage II | 24 (46%) | 20 (42%) |
| Stage III | 15 (29%) | 15 (31%) |
| Revised International Staging System | ||
| Stage I | 2 (4%) | 6 (13%) |
| Stage II | 39 (75%) | 35 (73%) |
| Stage III | 11 (21%) | 7 (15%) |
Data are n (%) or n/N (%), unless otherwise specified.
44 (44%) of 99 patients in the study population had bone marrow plasmacytosis of 60% or higher at diagnosis, 26 (26%) of 100 had International Staging System (ISS) stage I disease, 44 (44%) had stage II disease, and 30 (30%) had stage III disease. High-risk multiple myeloma features included chromosome 1q21 abnormalities in 47 (47%) of 99 patients, del17p in 37 (37%) of 100, t(14;16) in 11 (11%) of 100, GEPhi in eight (9%) of 90, primary plasma cell leukaemia in seven (7%) of 100, t(14;20) in five (5%) of 100, elevated serum lactate dehydrogenase in four (4%) of 100, and two or more features in 17 (17%) of 100. The proportion of patients with two or more high-risk multiple myeloma features was similar between the two groups, as was the distribution of ISS staging. In terms of disease burden, the degree of anaemia and thrombocytopenia and bone marrow plasmacytosis above 60% were similar in the two groups of the study. The median percentage of del(17p)-positive cells in FISH studies in evaluable patients (n=28) was 47%. The distribution of chromosome 1q21 abnormalities (n=47) is provided in the appendix (p 1); this subgroup included 28 patients with three copies and 19 patients with more than three copies.
The median number of cycles received was eight (IQR 6–16) for the RVd group compared with 14 (8–30) for the RVd-elotuzumab group. The median induction dose of bortezomib was 1·3 mg/mg2 (1·3–1·3), of lenalidomide was 25 mg (25–25), and of dexamethasone was 20 mg (20–20) for both groups. The median induction elotuzumab dose for patients in the RVd-elotuzumab group was 10 mg/kg (10–10). The median maintenance dose of bortezomib was 1 mg/mg2 (1–1), of lenalidomide was 15 mg (15–15), and of dexamethasone was 12 mg (12–12) for both groups. The median maintenance elotuzumab dose for patients in the RVd-elotuzumab group was 10 mg/kg (10–10).
At the time of analysis, 62 PFS events had occurred; 31 (60%) of 52 patients in the RVd group and 31 (65%) of 48 in the RVd-elotuzumab group had a PFS event. Median follow-up was 53 months (IQR 46–59). The unstratified median PFS in the RVd group was 33·64 months (95% CI 19·55–not reached) compared with 31·47 months (18·56–53·98) in the RVd-elotuzumab group (figure 2). The stratified HR comparing RVd versus RVd-elotuzumab was 0·968 (80% Wald CI 0·697–1·344) with a one-sided stratified log-rank p value of 0·45 (two-sided p=0·90). The data from the study provided no evidence to support the hypothesis that PFS is improved in patients assigned to RVd-elotuzumab as compared with those assigned to RVd. An exploratory analysis evaluating PFS outcomes for the different high-risk multiple myeloma subsets by study groups (appendix p 2) revealed no statistically significant differences, although the median PFS was numerically higher for patients with del(17p) in the RVd-elotuzumab group than those in the RVd group (54 months [95% CI 22–64] vs 30 months [15–not reached]) and for patients with amp(1q21) in the RVd group than those in the RVd-elotuzumab group (41 months [22–not reached] vs 32 months [18–not reached]). Similarly, no difference was observed in median overall survival (figure 3), when comparing RVd with RVd-elotuzumab; 19 (37%) of 52 patients died in the RVd group and 16 (33%) of 48 patients died in the RVd-elotuzumab group. The median overall survival was not reached in the RVd group, whereas it was reached at 68 months (95% CI 61–68) in the RVd-elotuzumab group. The stratified HR was 1·279 (80% CI 0·819–2·000) with a two-sided log-rank p value of 0·48. An exploratory analysis revealed no statistically significant differences in overall survival by treatment group within the different high-risk multiple myeloma categories (appendix p 3), although patient subgroups were small.
Figure 2:
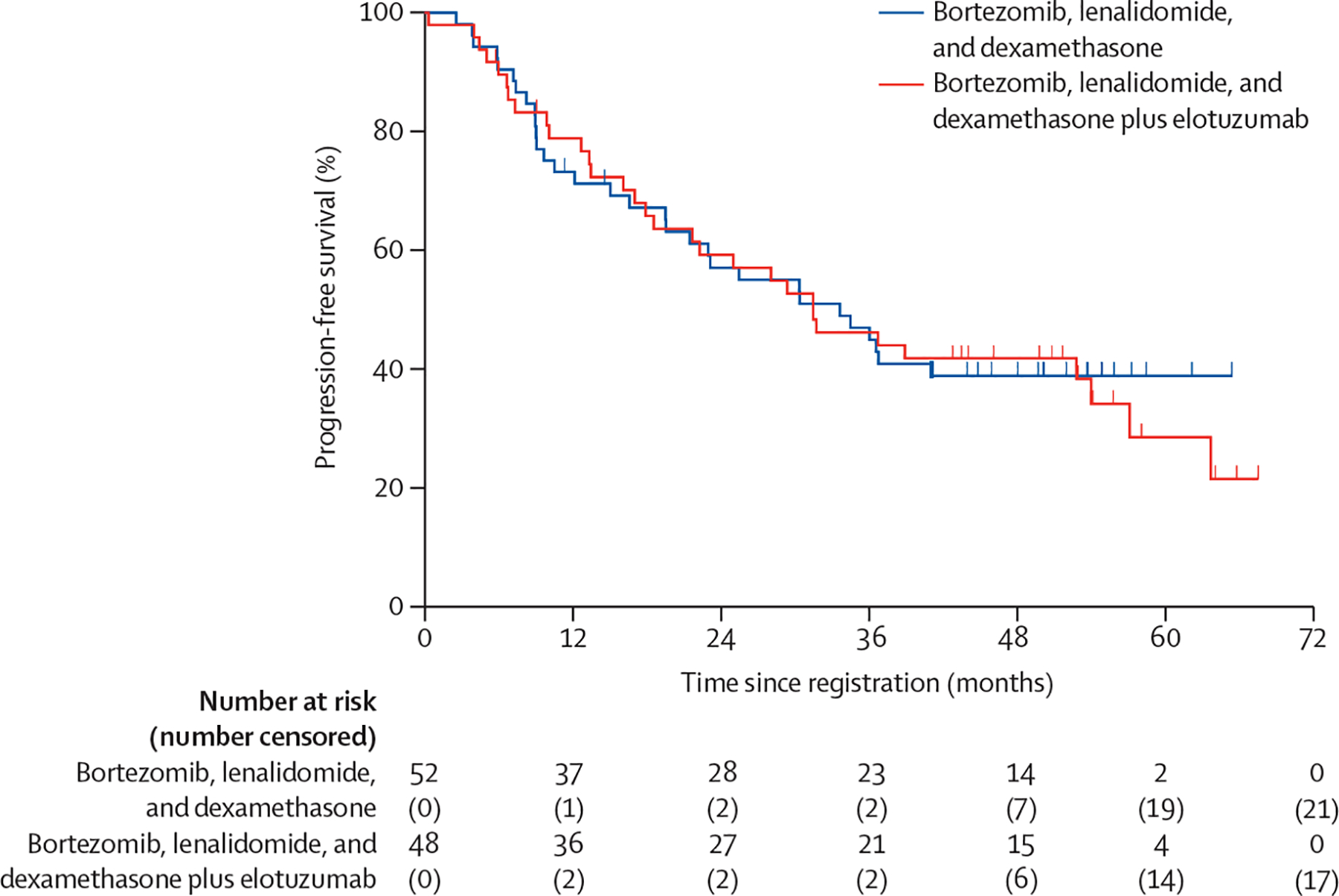
Progression-free survival by treatment group
Figure 3:
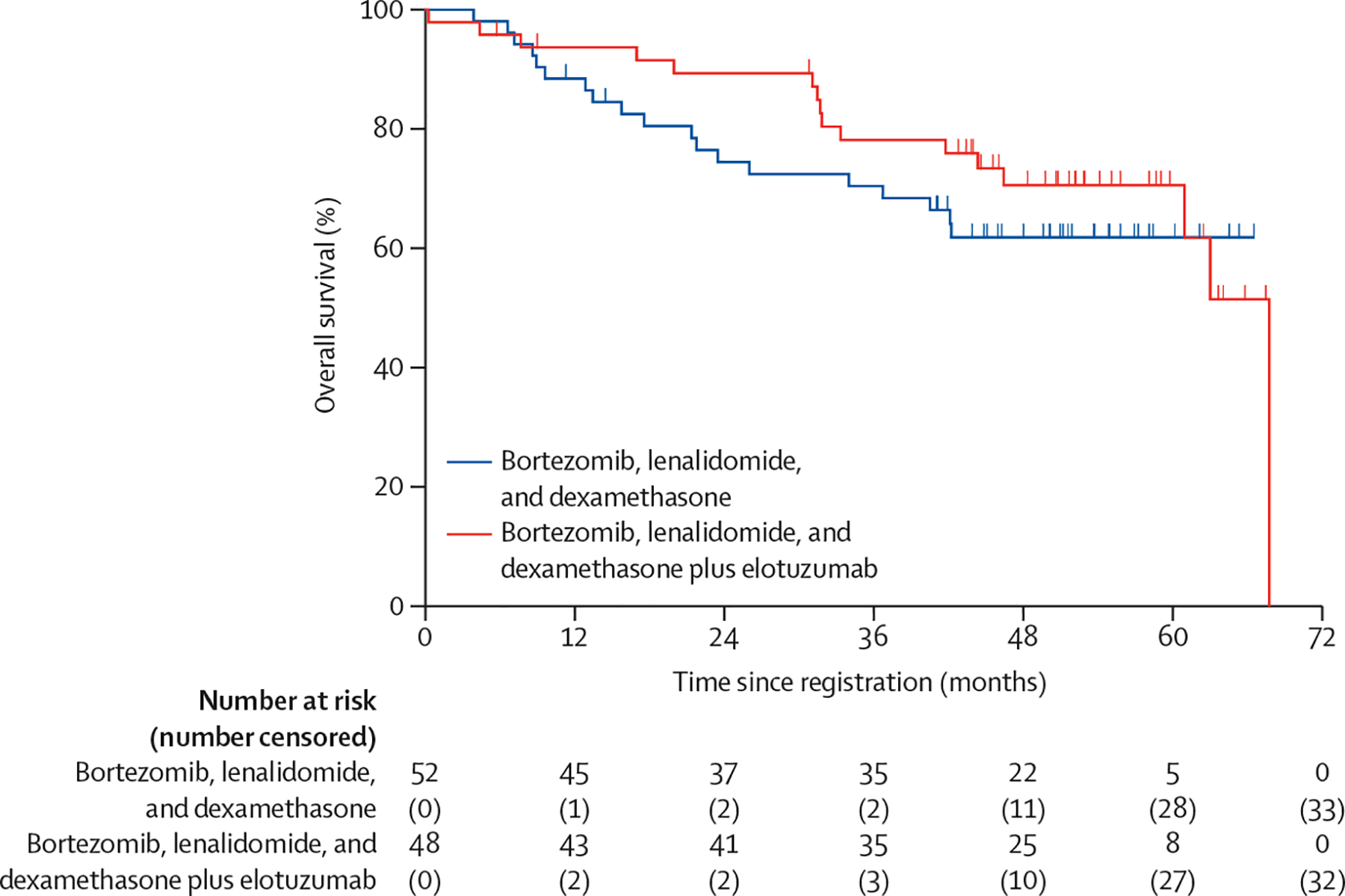
Overall survival by treatment group
97 patients were eligible, analysable, and assessable for response. There was no improvement in overall response rate of partial response or better in the RVd-elotuzumab group (44 [83%] of 50) compared with the RVd group (39 [88%] of 47) based on a Cochran-Mantel-Haenszel test (two-sided p=0·29; appendix p 4). Similarly, there was no evidence of improved responses when evaluating very good partial response or better (two-sided p=0·52) and complete response or better (two-sided p=0·19). There were no statistically significant differences observed between the two groups among the different high-risk multiple myeloma subsets (appendix p 5).
No differences in incidence of grade 3 or worse adverse events were observed between the two study groups across most CTCAE categories (37 [71%] of 52 patients in the RVd group and 37 [77%] of 48 in the RVd-elotuzumab group; table 2; appendix pp 6–10). The safety profile was as expected for the RVd regimen for the most part. Numbers of patients with the skin and subcutaneous disorder (any grade) usually seen with lenalidomide were similar across the two groups (22 [42%] of 52 in the RVd group, 21 [44%] of 48 in the RVd-elotuzumab group), as were numbers of patients with gastrointestinal symptoms (41 [79%] in the RVd group, 38 [79%] in the RVd-elotuzumab group) and thromboembolic events (six [12%] in the RVd group, seven [15%] in the RVd-elotuzumab group). However, larger proportions of patients had grade 3 or worse infections (eight [17%] of 48 vs four [8%] of 52), sensory neuropathy (six [13%] vs four [8%]), and motor neuropathy (four [8%] vs one [2%]) in the RVd-elotuzumab group than in the RVd group. There was one grade 5 event in the RVd-elotuzumab group, which was associated with multi-organ failure and underlying multiple myeloma, for which study treatment was listed as possibly contributing by the investigator.
Table 2:
Adverse events at least possibly related to study treatment by Common Terminology Criteria for Adverse Events category
| Bortezomib, lenalidomide, and dexamethasone (N=52) | Bortezomib, lenalidomide, and dexamethasone plus elotuzumab (N=48) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Blood and lymphatic system disorders | 8 (15%) | 16 (31%) | 8 (15%) | 1 (2%) | 0 | 6 (13%) | 8 (17%) | 7 (15%) | 0 | 0 |
| Cardiac disorders | 3 (6%) | 0 | 3 (6%) | 0 | 0 | 4 (8%) | 0 | 1 (2%) | 0 | 0 |
| Ear and labyrinth disorders | 1 (2%) | 0 | 0 | 0 | 0 | 1 (2%) | 1 (2%) | 0 | 0 | 0 |
| Endocrine disorders | 2 (4%) | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 |
| Eye disorders | 8 (15%) | 2 (4%) | 0 | 0 | 0 | 12 (25%) | 2 (4%) | 2 (4%) | 0 | 0 |
| Gastrointestinal disorders | 15 (29%) | 19 (37%) | 7 (13%) | 0 | 0 | 12 (25%) | 24 (50%) | 2 (4%) | 0 | 0 |
| General disorders and administration site conditions | 15 (29%) | 17 (33%) | 8 (15%) | 0 | 0 | 11 (23%) | 20 (42%) | 7 (15%) | 2 (4%) | 1 (2%) |
| Hepatobiliary disorders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 |
| Immune system disorders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 |
| Infections and infestations | 0 | 8 (15%) | 2 (4%) | 2 (4%) | 0 | 0 | 7 (15%) | 7 (15%) | 1 (2%) | 0 |
| Injury, poisoning, and procedural complications | 5 (10%) | 0 | 1 (2%) | 0 | 0 | 2 (4%) | 0 | 1 (2%) | 0 | 0 |
| Investigations | 8 (15%) | 10 (19%) | 20 (38%) | 4 (8%) | 0 | 5 (10%) | 8 (17%) | 16 (33%) | 8 (17%) | 0 |
| Metabolism and nutrition disorders | 13 (25%) | 14 (27%) | 5 (10%) | 0 | 0 | 6 (13%) | 16 (33%) | 8 (17%) | 2 (4%) | 0 |
| Musculoskeletal and connective tissue disorders | 9 (17%) | 16 (31%) | 3 (6%) | 1 (2%) | 0 | 11 (23%) | 8 (17%) | 6 (13%) | 0 | 0 |
| Nervous system disorders | 19 (37%) | 21 (40%) | 6 (12%) | 0 | 0 | 14 (29%) | 16 (33%) | 9 (19%) | 1 (2%) | 0 |
| Psychiatric disorders | 11 (21%) | 2 (4%) | 0 | 0 | 0 | 9 (19%) | 5 (10%) | 3 (6%) | 0 | 0 |
| Renal and urinary disorders | 1 (2%) | 0 | 1 (2%) | 0 | 0 | 3 (6%) | 2 (4%) | 0 | 0 | 0 |
| Reproductive system and breast disorders | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 11 (21%) | 6 (12%) | 1 (2%) | 2 (4%) | 0 | 13 (27%) | 6 (13%) | 3 (6%) | 1 (2%) | 0 |
| Skin and subcutaneous tissue disorders | 15 (29%) | 4 (8%) | 3 (6%) | 0 | 0 | 15 (31%) | 3 (6%) | 3 (6%) | 0 | 0 |
| Vascular disorders | 3 (6%) | 9 (17%) | 5 (10%) | 3 (6%) | 0 | 5 (10%) | 14 (29 | 6 (13%) | 0 | 0 |
| Maximum grade any adverse event | 1 (2%) | 14 (27%) | 28 (54%) | 9 (17%) | 0 | 2 (4%) | 9 (19 | 26 (54%) | 10 (21%) | 1 (2%) |
Date are n (%).
Discussion
To our knowledge, the SWOG-1211 is the first randomised trial for newly diagnosed high-risk multiple myeloma. Although the trial did not meet its primary endpoint, several lessons can be learned. The study used a consensus high-risk multiple myeloma definition developed by the National Cancer Institute Myeloma Steering Committee in 2011, which incorporates clinical, karyotypic, and gene expression profiling features. Elevated serum lactate dehydrogenase was a recognised high-risk feature that usually accompanied a proliferative, extramedullary multiple myeloma phenotype.24 This abnormality is incorporated in the revised ISS staging as a high-risk feature, as is presence of del(17p) by cytogenetics or FISH.25 At the time of study design, there were conflicting reports on prognosis associated with t(14;16) and t(14;20), with more data leaning towards poor outcomes, prompting inclusion of these features. Subsequent publications2 have corroborated the poor prognosis of these translocations. Similarly, three or more copies of chromosome 1q21 is associated with worse PFS or overall survival and bortezomib resistance.2,3,19 Primary plasma cell leukaemia is recognised as a poor prognostic clinical phenotype of multiple myeloma, with a median overall survival of 1·3 years even with tandem transplantation-based approaches.8 Although historically excluded from clinical trials owing to poor prognosis, the SWOG-1211 trial was able to incorporate and enrol patients with primary plasma cell leukaemia.
The 2-year PFS in high-risk multiple myeloma is approximately 50%, even with tandem autologous HSCT, as observed in the Total Therapy protocols,22,23 and the historic control assumption of 2·2 years was based on this experience. Because autologous HSCT is associated with toxicity and morbidity, the SWOG-1211 trial only allowed use of this therapeutic option for patients at the time of progression or relapse. Deferring autologous HSCT also allowed inclusion of patients with high-risk multiple myeloma, who might not have been candidates for autologous HSCT in this trial. Despite the deferral of autologous HSCT, the median PFS and overall survival seen in both groups of the study exceeded the original statistical assumptions. However, SWOG-1211 was not designed to answer the question on the role of autologous HSCT in the high-risk multiple myeloma setting. It is also the first randomised trial to prospectively examine induction using a protea some inhibitor and immuno modulatory drug-based regimen followed by maintenance with the same combination in this patient population and to examine whether the addition of a monoclonal antibody improves outcomes. The study did not achieve its primary endpoint—the addition of elotuzumab to RVd induction and maintenance did not improve patient outcomes. There could have been an antagonistic effect between bortezomib and elotuzumab, because of elotuzumab’s natural killer cell-mediated anti-multiple myeloma activity.26 Examining the different high-risk multiple myeloma subsets, there was non-significantly longer PFS for the del(17p) group in the RVd-elotuzumab group, which corroborates similar observations made with Rd-elotuzumab in the relapsed multiple myeloma setting.17 However, the small number of patients makes this observation hypothesis-generating at best in the newly diagnosed high-risk multiple myeloma setting.
The high-risk multiple myeloma definition is not uniform across the contemporary randomised phase 3 trials27–32 involving newly diagnosed multiple myeloma, and high-risk multiple myeloma generally accounts for a small subset of the overall study populations. The SWOG-0777 phase 3 trial27 compared RVd induction with Rd induction for newly diagnosed multiple myeloma and showed that RVd was superior in terms of PFS and overall survival. However, only 44 (8%) of 525 patients had high-risk multiple myeloma, defined as having either t(4;14), t(14;16), or del(17p), and no difference in PFS or overall survival between the two groups was shown. ALCYONE28 studied addition of the anti-CD38 monoclonal antibody daratumumab to Rd, MAIA29 studied its addition to bortezomib, melphalan, and prednisone, and CASSIOPEIA30 studied its addition to bortezomib, thalidomide, and dexamethasone. Although each of these trials met their primary endpoints, no outcome benefit was observed in the small high-risk multiple myeloma subsets (defined by t[4;14], t[14;16], or del[17p]). A meta-analysis31 suggested that addition of daratumumab across these frontline trials does improve PFS for patients with protocol-defined high-risk multiple myeloma when compared with control groups, but not to the same degree as patients with standard-risk multiple myeloma. The BMT-CTN-0702 (Stamina) three-arm phase 3 study,32 which compared maintenance with autologous HSCT plus lenalidomide, autologous HSCT plus RVd consolidation and lenalidomide maintenance, and tandem autologous HSCT plus lenalidomide maintenance, did not meet its primary endpoint, and to date does not show improvement in high-risk multiple myeloma (defined as having either ISS stage III disease, del[13], t[4;14], t[14;16], or del[17p]) outcomes for the intention-to-treat patient population.33
The EMN-02/HO-95 trial33 was a randomised phase 2 trial that included 1197 patients aged 18–65 years who had received 3–4 cycles of bortezomib, cyclophosphamide, dexamethasone induction followed by first randomisation to either bortezomib, melphalan, and prednisone or autologous HSCT (single or tandem based on institutional preferences). The EMN-02/HO-95 trial defined high risk as presence of either del(17p), t(4;14), or t(14;16) and clinical high-risk features, such as elevated lactate dehydrogenase and primary plasma cell leukaemia, were not included. A post-hoc analysis was done in a small subset of high-risk patients (39 participants who received tandem transplants vs 42 participants who received a single transplant) and showed that PFS was 46·0 months for the tandem approach and 26·7 months for patients who had a single transplantation (HR 0·59, 95% CI 0·34–1·03; p=0·062). The median age of this population was younger (58 years) than in the SWOG-1211 trial, making any meaningful comparisons challenging.
Of note, studies of retrospective data from a single institution34 and single-group studies22,23 have been done, with small numbers of patients showing the benefit of proteasome inhibitor plus immunomodulatory drug maintenance strategies for high-risk multiple myeloma following single or tandem autologous HSCT. The SWOG-1211 study, however, shows for the first time, in a prospectively designed clinic trial, the benefit of induction with a proteasome inhibitor and immunomodulatory drug-based regimen followed by maintenance with the same combination for a population of patients with high-risk multiple myeloma without autologous HSCT in the therapy schema. Both groups in the SWOG-1211 trial exceeded the median expected PFS calculated on the basis of the Total Therapy protocols used for the sample size calculation and appear comparable to published single institution data that have reported post-autologous HSCT proteasome inhibitor and immunomodulatory drug-based maintenance.
In summary, the SWOG-1211 data support the role for maintenance therapy with a continuous proteasome inhibitor and immunomodulatory drug combination for patients with high-risk multiple myeloma. The PFS and overall survival data from this study will serve as a benchmark for future enrichment design, randomised trials for high-risk multiple myeloma.
Supplementary Material
Research in context.
Evidence before the study
We searched PubMed, Ovid, and the Cochrane Library for papers published between 1960 and 2011 with the terms “multiple myeloma”, “high risk”, “newly diagnosed”, and “treatment”. There was no standard of care approach for untreated high-risk multiple myeloma when the SWOG-1211 study was designed, and the role of high-dose melphalan and autologous haematopoietic stem-cell transplantation was unclear. In Arkansas Total Therapy, the median progression-free survival for tandem transplantations for patients with genomically defined, newly diagnosed, high-risk multiple myeloma was 2·2 years. No enrichment design clinical trials for untreated high-risk multiple myeloma have previously been reported.
Added value of this study
Both groups of the SWOG-1211 study showed improved progression-free survival compared with historic values, supporting a role for induction based on proteasome inhibitors and immunomodulatory drugs followed by maintenance with the same combination in patients with newly diagnosed high-risk multiple myeloma.
Implications of all available evidence
The findings from the SWOG-1211 trial show no added benefit of elotuzumab to bortezomib, lenalidomide, and dexamethasone for patients with high-risk multiple myeloma. This study supports the use of a proteasome inhibitor and immunomodulatory drug-based induction and maintenance strategy for patients with untreated high-risk multiple myeloma, and the outcomes from the trial could serve as a benchmark for future trials including patients with untreated high-risk multiple myeloma.
Acknowledgments
This study was supported by funding support from the National Institutes of Health and National Cancer Institute (grants U10CA180888-01 and U10CA180819-01), and in part by Bristol Myers Squibb and Celgene (a Bristol-Myers company). SZU is supported by the Leukemia and Lymphoma Society Scholar in Clinical Research Grant, Carolinas Myeloma Research Fund, and the William Britton Family Fund. RZO is a Florence Maude Thomas Cancer Research Professor and acknowledges support from the National Cancer Institute (R01 CA194264 and R01 CA184464), the Leukemia and Lymphoma Society, the Dr Miriam and Sheldon G Adelson Medical Research Foundation, and the MD Anderson Cancer Center High Risk Multiple Myeloma Moon Shot.
Declaration of interests
SZU reports grants and personal fees from Amgen, AbbVie, and MundiPharma, grants from Bristol Myers Squibb and Pharmacyclics, and grants and personal fees from Celgene, Sanofi, Seattle Genetics, Janssen, Takeda, SkylineDX, and Merck, outside the submitted work. PGR reports grants from Bristol Myers Squibb, grants and honoraria (advisory committee member) from Oncopeptides, Celgene, Takeda, and Karyopharm and honoraria (advisory committee member) from Janssen, Sanofi, and SecuraBio, outside the submitted work. JAZ reports grants from Bristol Myers Squibb and personal fees from Amgen, Regeneron, and Caelum, outside the submitted work. BD reports personal fees from Amgen, Janssen, Celgene-Bristol Myers Squibb, and Takeda, outside the submitted work. PMV reports personal fees from Bristol Myers Squibb, Novartis, Oncopeptides TeneoBio, Janssen, GlaxoSmithKline, Adaptive Biotechnologies, and Takeda Pharmaceuticals, outside the submitted work. JV reports personal fees from Takeda Pharmaceuticals, Amgen, and Celgene, outside the submitted work. RZO reports grants from BioTheryX, CARsgen Therapeutics, and Exelixis, grants and personal fees from Celgene-Bristol Myers Squibb, Janssen Biotech, Sanofi-Aventis, and Takeda Pharmaceuticals North America, and personal fees from Amgen, EcoR1 Capital, Forma Therapeutics, Genzyme, GlaxoSmithKline, Ionis Pharmaceuticals, Juno Therapeutics, Kite Pharma, Legend Biotech USA, Molecular Partners, Regeneron Pharmaceuticals, and STATinMED Research, outside of the submitted work. RZO is also Founder of Asylia Therapeutics with associated patents and an equity interest, outside of the submitted work. MD reports personal fees from Janssen, Amgen, Lava Therapeutics, and Roche-Genentech, outside the submitted work. MZ reports personal fees for Speaker Bureau from Celgene-Bristol Myers Squibb, Takeda, and Janssen, outside the submitted work. SA reports grants from Amgen, Cellectar, Pharmacyclics, Janssen, Bristol Myers Squibb, and AstraZeneca and personal fees from Takeda, Celgene, GlaxoSmithKline, Sanofi-Genzyme, and Oncopeptides, outside the submitted work. TZ reports stocks in AbbVie and BeiGene, outside the submitted work. All other authors declare no competing interests.
Footnotes
Data sharing
All publication-related data will be uploaded to the National Clinical Trials Network data upload site. There will be two datasets, one analysis dataset with one row per patient, and another toxicity dataset, allowing for multiple rows per patient. A data dictionary will be provided for each dataset.
For National Clinical Trials Network data archive see https://nctn-data-archive.nci.nih.gov/
References
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15: e538–48. [DOI] [PubMed] [Google Scholar]
- 2.Usmani SZ, Rodriguez-Otero P, Bhutani M, Mateos MV, Miguel JS. Defining and treating high-risk multiple myeloma. Leukemia 2015; 29: 2119–25. [DOI] [PubMed] [Google Scholar]
- 3.Shaughnessy JD Jr, Qu P, Usmani S, et al. Pharmacogenomics of bortezomib test-dosing identifies hyperexpression of proteasome genes, especially PSMD4, as novel high-risk feature in myeloma treated with Total Therapy 3. Blood 2011; 118: 3512–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca R, Blood E, Rue M, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 2003; 101: 4569–75. [DOI] [PubMed] [Google Scholar]
- 5.Boersma-Vreugdenhil GR, Kuipers J, Van Stralen E, et al. The recurrent translocation t(14;20)(q32;q12) in multiple myeloma results in aberrant expression of MAFB: a molecular and genetic analysis of the chromosomal breakpoint. Br J Haematol 2004; 126: 355–63. [DOI] [PubMed] [Google Scholar]
- 6.Klein U, Jauch A, Hielscher T, et al. Chromosomal aberrations +1q21 and del(17p13) predict survival in patients with recurrent multiple myeloma treated with lenalidomide and dexamethasone. Cancer 2011; 117: 2136–44. [DOI] [PubMed] [Google Scholar]
- 7.Avet-Loiseau H, Attal M, Campion L, et al. Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol 2012; 30: 1949–52. [DOI] [PubMed] [Google Scholar]
- 8.Usmani SZ, Nair B, Qu P, et al. Primary plasma cell leukemia: clinical and laboratory presentation, gene-expression profiling and clinical outcome with Total Therapy protocols. Leukemia 2012; 26: 2398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Barlogie B, Smith TL, Alexanian R. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Intern Med 1991; 115: 931–35. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010; 116: 679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017; 376: 1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol 2017; 35: 3279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008; 14: 2775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai YT, Dillon M, Song W, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112: 1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakubowiak AJ, Benson DM, Bensinger W, et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol 2012; 30: 1960–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012; 30: 1953–59. [DOI] [PubMed] [Google Scholar]
- 17.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–31. [DOI] [PubMed] [Google Scholar]
- 18.Usmani SZ, Sexton R, Ailawadhi S, et al. Phase I safety data of lenalidomide, bortezomib, dexamethasone, and elotuzumab as induction therapy for newly diagnosed symptomatic multiple myeloma: SWOG S1211. Blood Cancer J 2015; 5: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt TM, Barwick BG, Joseph N, et al. Gain of chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J 2019; 9: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975; 31: 103–15. [PubMed] [Google Scholar]
- 21.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–73. [DOI] [PubMed] [Google Scholar]
- 22.Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol 2007; 138: 176–85. [DOI] [PubMed] [Google Scholar]
- 23.Nair B, van Rhee F, Shaughnessy JD Jr, et al. Superior results of Total Therapy 3 (2003–33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006–66 with VRD maintenance. Blood 2010; 115: 4168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia 2020; 34: 1–20. [DOI] [PubMed] [Google Scholar]
- 25.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol 2015; 33: 2863–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markasz L, Stuber G, Vanherberghen B, et al. Effect of frequently used chemotherapeutic drugs on the cytotoxic activity of human natural killer cells. Mol Cancer Ther 2007; 6: 644–54. [DOI] [PubMed] [Google Scholar]
- 27.Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J 2020; 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018; 378: 518–28. [DOI] [PubMed] [Google Scholar]
- 29.Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 2019; 380: 2104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 2019; 394: 29–38. [DOI] [PubMed] [Google Scholar]
- 31.Giri S, Grimshaw A, Bal S, et al. Evaluation of daratumumab for the treatment of multiple myeloma in patients with high-risk cytogenetic factors: a systematic review and meta-analysis. JAMA Oncol 2020; published online September 24. 10.1001/jamaoncol.2020.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hari P, Pasquini MC, Stadtmauer EA, et al. Long-term follow-up of BMT CTN 0702 (STaMINA) of postautologous hematopoietic cell transplantation (autoHCT) strategies in the upfront treatment of multiple myeloma (MM). Proc Am Soc Clin Oncol 2020; 38 (suppl): 8506 (abstr). [Google Scholar]
- 33.Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol 2020; 7: e456–68. [DOI] [PubMed] [Google Scholar]
- 34.Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol 2020; 38: 1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


