Abstract
Objectives:
To evaluate the effects of low-intensity pulsed ultrasound (LIPUS) on orthodontically induced tooth root resorption caused by torque in human subjects.
Materials and Methods:
Ten healthy patients (12–35 years of age) who required extraction of all first premolars as a part of their routine orthodontic treatment were recruited. A 15° twist was applied in the arch wire using 0.019 × 0.025-inch TMA in a 0.022-inch bracket system (Synergy R) that produced a buccal root torque of approximately 5 N/mm at the bracket level. Using a split mouth design, randomization, and blinding, one side of the arch received LIPUS for 20 minutes per day for 4 weeks at an incident intensity of 30 mW/cm2 of the transducers’ surface area. The other side served as a self-control, which received a sham transducer. After 4 weeks, all first premolars were extracted and micro–computed tomographic analysis was performed on these extracted teeth. A linear mixed-model statistical analysis was used.
Results:
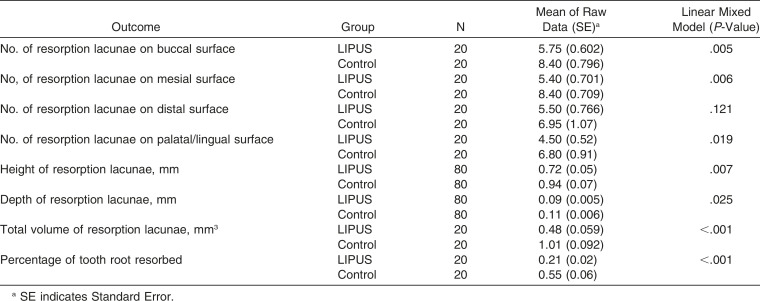
LIPUS-treated teeth showed significantly less total volume of resorption lacunae compared to control teeth by a mean difference of (0.54 ± 0.09 mm3) (P < .001) and percentage of root resorption by a mean difference of (0.33 ± 0.05 mm3) (P < .001). In addition, significantly fewer resorption lacunae were found on all root surfaces in the LIPUS group compared to the control except in the instance of the distal surface.
Limitations:
This study was performed on limited number of cases during a 4-week period.
Conclusions:
LIPUS minimizes root resorption when applied during torque tooth movement over a 4-week period.
Keywords: Orthodontic root resorption, Torque, Low-intensity pulsed ultrasound, LIPUS, Human
INTRODUCTION
Orthodontically induced root resorption (OIRR) is an unavoidable consequence of orthodontic treatment.1 The incidence of OIRR is greater than 90%,2 and the prevalence of minor to severe OIRR ranges from 94% to 6.6%, respectively.3 It has been reported that 6.6% of the orthodontic patients in one study3 had at least one tooth with OIRR greater than 4 mm. Micro–computed tomography (micro-CT) analysis is the current gold standard for the quantification of RR.4 OIRR is a multifactorial problem that can occur as the result of a combination of individuals’ biological variabilities, genetic predisposition, and orthodontic mechanical factors.5 Torque force induces bucco-lingual rotation of the tooth,6 and it has been identified as one of the major risk factors for OIRR.7–10 Several studies explored potential treatments for OIIRR11–14 but the only reported clinically acceptable modality is low-intensity pulsed ultrasound (LIPUS).15 LIPUS is acoustic pressure waves that can stimulate a variety of cells including cementoblasts,16 odontoblasts,17 osteoblasts,18 chondrocytes,19 gingival cells,20,21 and periodontal ligament cells.22 Previous studies investigating the effects of LIPUS on OIRR have reported that LIPUS promotes cementogenesis by increasing the Alkaline phosphate (ALP) activity,16,23 collagen-I synthesis,16 and Runx-2 protein16 in cementoblasts and inhibits cementoclastogenesis24,25 In addition, a previous study15 reported that LIPUS reduced the severity of OIRR due to tipping-type orthodontic tooth movement and promoted cementum repair. The effect of LIPUS on OIRR during other types of orthodontic tooth movement remains unknown. Therefore, the aim of the present clinical trial was to evaluate the effects of LIPUS on OIRR caused by torque tooth movement.
MATERIALS AND METHODS
This study was approved by the Health Research Ethics Board at the University of Alberta, Canada (Pro 00001454). All of the study participants signed a written informed consent document before the start of the experiment. Sample size was calculated based on data published by El-Bialy et al.15 using the following formula26:
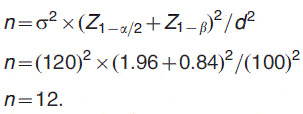 |
The statistical significance level and power of the test were set at .5 and .8, respectively.
The study participants consisted of 12 healthy individuals, two males and 10 females, with an average age of 15.5 ± 5.48 years at the beginning of the study, who were scheduled to receive orthodontic treatment and extraction of all of their first premolars as a part of their orthodontic treatment. During the experiment, two patients were eliminated as a result of noncompliance. Ultimately, 10 patients, two males and eight females, completed the study.
This study design was a prospective, split-mouth, double-blinde, randomized, controlled clinical trial. For each patient, Nance holding arch and mandibular lingual arch were delivered to support the first molars (anchorage) (Figure 1). All first premolars were bonded with Synergy-R brackets (0.022-inchg slot size) (RMO, Denver, Colo) in a passive position using 0.021 × 0.025-inch wires to ensure that they were at zero torque at the bonding. The thickness of bonding material between the bracket base and tooth surface varied to accommodate variations in the slope of the buccal aspect of the teeth. Next, a 15° twist was applied in the sectional 0.019 × 0.025-inch wire (Titanium Molybdenum Alloy, RMO) connecting the first premolar and first molar on each side, producing clinically appropriate buccal root torque at the premolar bracket level of approximately 5 N/mm. The torque value was calibrated in the biomechanics lab at the University of Alberta, Canada.
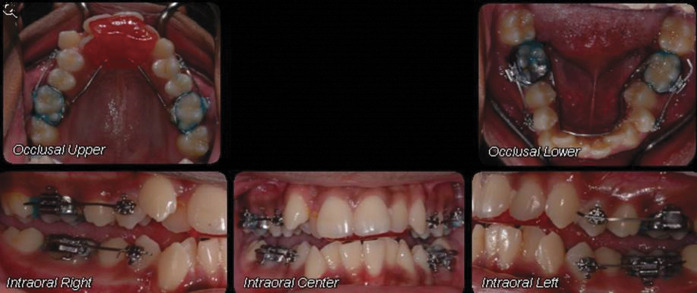
Figure 1.
Intraoral pictures of the patient showing orthodontic appliances and arch wire.
For each patient, both the upper and lower first premolars on one side were randomly assigned to receive active LIPUS transducers, while the other side’s premolars were used as a self-control measure that received sham transducers. Before the start of the study, inactive and active LIPUS transducers were blinded and coded and they were indistinguishable in appearance but were marked “right” or “left”. The clinician, the study coordinator, the patient, and the outcome assessor all were blinded to the active and control sides. LIPUS was applied using a custom-made ultrasound device (SmileSonica Inc, Edmonton, Alberta, Canada) that was started on the same day the orthodontic force was applied—for 20 minutes per day for 4 weeks. The patients were instructed to apply the ultrasound transducer to the assigned first premolars in the muco-buccal fold. Intraoral ultrasound gel was used to couple the ultrasound energy between the transducer and the gum. The LIPUS parameters used in this study were 200-µs-wide pulses that each had a frequency of 1.5 MHz and a pulse repetition frequency of 1 kHz and that delivers an intensity of 30 mW/cm2 of the transducer’s surface area. After 4 weeks all of the first premolars were extracted, disinfected in 70% alcohol for 30 minutes, and stored in Milli-Q Millipore (Bedford, Mass),8,27 and then they were prepared for micro-CT scanning, as described previously.8,28 The teeth were scanned in a SkyScan® (1076) micro-CT scanner at a resolution of 9 µm. Analysis of the reconstructed images was performed as described before.28 Resorption lacunae (RL) were analyzed over the whole length of the roots starting at the cemento-enamel junction and proceeded to the root apex. Location, number, height, and volume of RL were calculated.28 The percentage of tooth root resorbed was calculated by adding the volume of all RL to the estimated tooth root volume, as determined by the micro-CT software.28 The tooth roots were divided into cervical, middle, and apical thirds to analyze the distribution of RL along the entire root length. When all the measurements were completed, the blinding code was broken, and the variables were analyzed for the ultrasound and control teeth.
Intrarater Reliability Measurements
The number and location, height, depth, and volume of RL as well as the percentage of tooth root resorbed were measured by the same investigator twice at a 1-week interval to test the intrarater reliability.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences software (SPSS version 20, Chicago, Ill). Because of the split-mouth design, the data had matched-pair structure, and the data sets were not independent of each other. In addition, the data were collected repeatedly from the same subject in whom the repeated factor is upper/lower and left/right, and so we had four measurements for each subject. As a result, measurements might have had correlated structure within the subject. To analyze data measured on the same subject, a linear mixed model was recommended by the biostatistician, assuming a specific covariance (or correlation) structure of the outcome variable (eg, compound symmetry, etc). Intrarater reliability for all of the variables was determined using the intraclass correlation coefficient (ICC).
RESULTS
The reliability test showed absolute agreement in the number of RL. The reproducibility of other variables was also very high where the ICC values ranged from 0.981 to 0.997.
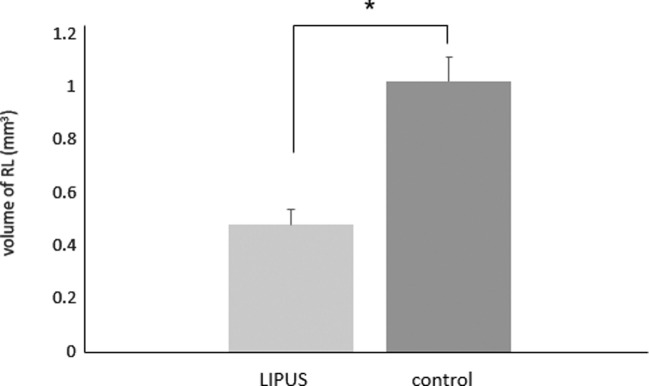
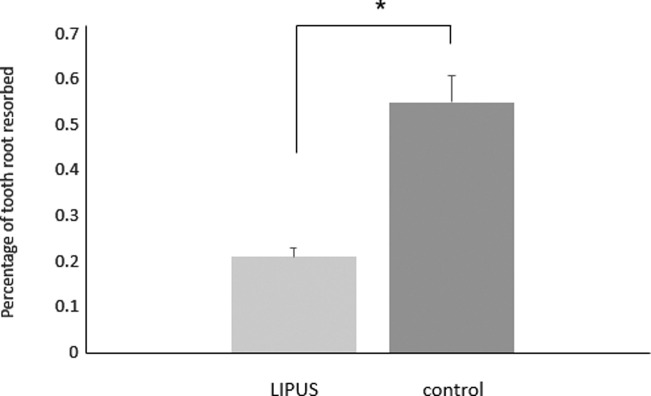
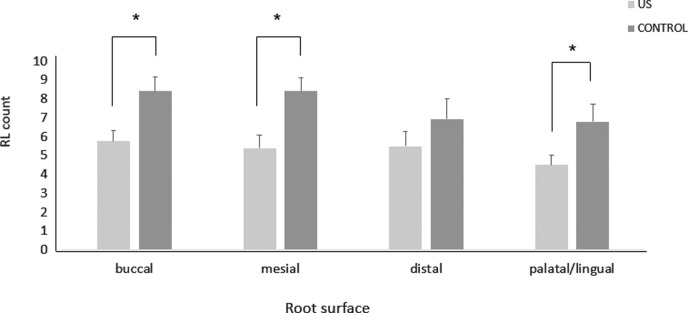
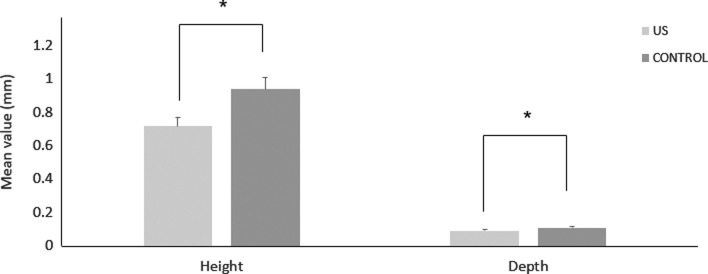
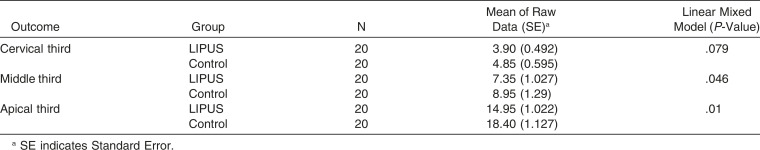
Overall, the result of the study demonstrated that LIPUS significantly decreased root damage compared to the control group (Figure 2). The RL volume of the LIPUS group was significantly less than that of the control (P < .001) (Figure 3; Table 1). In addition, the mean percentage of root resorption of the LIPUS group was significantly less than that of the control (P < .001) (Figure 4; Table 1). LIPUS decreased the number of RL on the mesial, buccal, and palatal root surfaces compared to the control group (P < .05), while it was decreased on the distal surface but not statistically significant (Figure 5; Table 1). The corono-apical height and depth of RL were also significantly less in the LIPUS group compared to the control group (Figure 6; Table 1) (P < .05).
Figure 2.
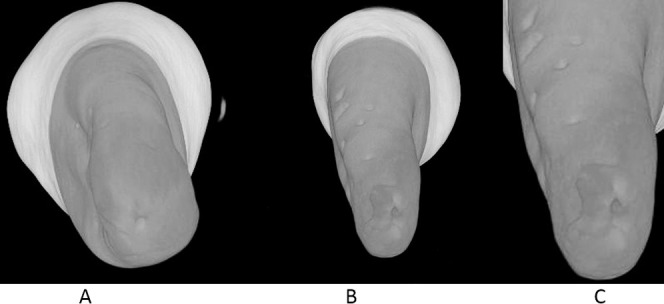
Three-dimensional illustration of the micro-CT image of the mandibular premolar showing root resorption craters (A), LIPUS-treated premolar (B), Control premolar (C), and Root resorption crater in detail. Torque application.
Figure 3.
Total volume of RL (mm3) (mean ± standard error) in LIPUS and control group (* P < .05).
Table 1.
Comparison of Root Resorption Measured Variables Between Low-Intensity Pulsed Ultrasound (LIPUS) and Control Groups Along with Statistical Analysis Results
Figure 4.
Percentage of tooth root resorbed (mean ± standard error) in LIPUS and control group (* P < .05).
Figure 5.
RL count (mean ± standard error) on different root surfaces in LIPUS and control group (* P < .05).
Figure 6.
Height and depth of RL (mm) (mean ± standard error) in LIPUS and control group (* P < .05).
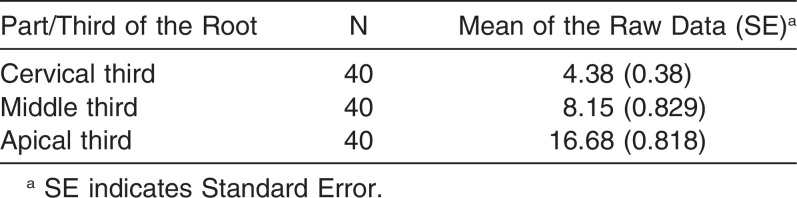
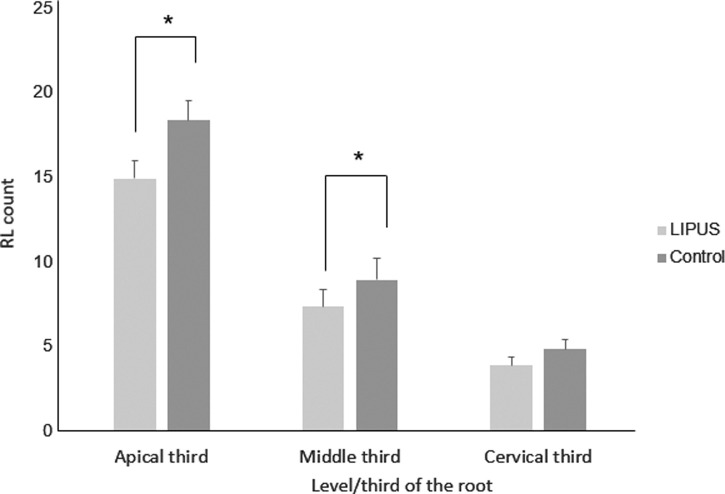
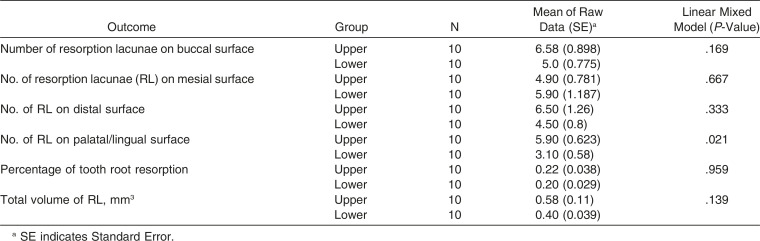
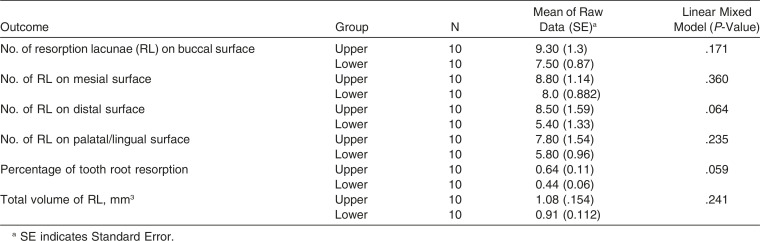
In general, the apical region showed the highest number of RL, followed by the middle third and cervical third (Table 2). LIPUS significantly reduced the number of RL at the apical level and middle level of the root compared to the control (Figure 7; Table 3) (P < .05). However, no significant differences were found in the number of RL at the cervical level between the LIPUS and control groups (Figure 7; Table 3) (P > .05). No significant differences were found in the severity of OIRR between the upper and the lower teeth in both groups (P > .05) (Tables 4 and 5).
Table 2.
The Mean Number of Resorption Lacunae at Each Level/Third of the Root
Figure 7.
RL count (mean ± standard error) at different level/third of the root in LIPUS and control group (* P < .05).
Table 3.
Comparison of Resorption Lacunae Count at Each Level/Third of the Root Between Low-Intensity Pulsed Ultrasound (LIPUS) and Control Group Along with Statistical Analysis Results
Table 4.
Comparison of Outcomes of Root Resorption Measured Variables Between the Upper and Lower Teeth in the Low-Intensity Pulsed Ultrasound (LIPUS) Group
Table 5.
Comparison of Outcomes of Root Resorption Measured Variables Between the Upper and Lower Teeth in the Control Group
DISCUSSION
The results of this study demonstrated that LIPUS reduced the total volume of RL and percentage of root resorption by more than 50% during clinically relevant torque tooth movement. This may be due to the anabolic effect of LIPUS on cementoblasts that either helped repair RL or stimulated secretion of a protective cementum layer against OIRR.16,23,24,29 In addition, it has been demonstrated that LIPUS can inhibit osteoclast activity by decreasing the Receptor activator of nuclear factor kappa-B ligand/Osteoprotegerin (RANKL/OPG) ratio.23,24,30 This could also be due to the anti-inflammatory effect of LIPUS,31 as it has been reported to decrease the tumor necrosis factor–α or interleukin-1β31 that are also involved in the proliferation and differentiation of odontoclast cells.32 Our findings are in accordance with previous reports15,25,30,31 that showed significantly fewer RL in LIPUS-treated teeth compared to control teeth, except for at the distal surface. The distal surface in the LIPUS group showed a lower number of RL compared to the control; however, this difference was not significant. Most of the patients in this experiment had their first premolars rotated disto-palatally, making ultrasound penetration less toward the distal surface. Utilizing finite element analysis, Vafaeian et al.33 demonstrated the quantitative relationship between the thicknesses of regenerated cementum and ultrasound power. They also observed greater cementum thickness in areas of the root that received greater ultrasound pressure, and vice versa.33
No significant differences were found in the severity of OIRR caused by torque between the upper and lower premolars in both groups. This could be due to the relatively lower torque magnitude used in this experiment (approximately 5 N/mm) that might not be enough to produce contact of the roots of these premolars with their respective cortical plate.7,10
In our study, increased root resorption was observed at the apical third, followed by the middle third and cervical third. This is likely because torque results in compressive forces being concentrated at the apex,10,34 which is more susceptible to root resorption.35,36 Schwarz37 reported that orthodontic force should not exceed the capillary blood pressure, and areas in which the orthodontic force exceeds the capillary blood pressure show root resorption.37 Hohmann et al.10 studied the effect of torque on Periodontal ligament (PDL) hydrostatic pressure using finite element analysis. They observed maximum root resorption at the apical region where orthodontic force exceeded the capillary blood pressure.10 Bartley et al.8 applied 2.85 N/mm (285 g/mm) of torque and observed more resorption at the apical level than at the middle and cervical level. Casa et al.9 applied 6 N/mm of torque and reported severe root resorption at the apex. Apical RR is clinically significant, as RL can accumulate at the apical region and can lead to permanent root shortening and a reduced crown/root ratio.38,39 Our study also highlighted the deleterious effect of torque on OIRR, as previously reported8,9 Since this 4-week period corresponds to the usual time interval between two orthodontic appointments, further activation of orthodontic force or orthodontic appliances might greatly increase the risk of OIRR. LIPUS application, however, was not able to completely heal the resorption craters during this time. Further long-term clinical trials evaluating the effect of LIPUS on OIRR will be helpful in determining the efficacy of LIPUS in accelerating cementum repair over extended periods of time. In addition, future studies would benefit from evaluating tooth movement and the level of cytokines, which can be evaluated by collecting it from the crevicular fluid concurrent with LIPUS application.
CONCLUSIONS
From the present clinical trial, it can be concluded that daily application of LIPUS for 20 min/d for 4 weeks significantly reduced the severity of OIRR caused by torque in humans.
REFERENCES
- 1.Tsesis I, Fuss Z, Rosenberg E, Taicher S. Radiographic evaluation of the prevalence of root resorption in a Middle Eastern population. Quintessence Int Berl Ger 1985. 2008;39:e40–e44. [PubMed] [Google Scholar]
- 2.Harry MR, Sims MR. Root resorption in bicuspid intrusion. A scanning electron microscope study. Angle Orthod. 1982;52:235–258. doi: 10.1043/0003-3219(1982)052<0235:RRIBI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Lund H, Gröndahl K, Hansen K, Gröndahl H-G. Apical root resorption during orthodontic treatment. A prospective study using cone beam CT. Angle Orthod. 2012;82:480–487. doi: 10.2319/061311-390.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudic A, Giannopoulou C, Martinez M, Montet X, Kiliaridis S. Diagnostic accuracy of digitized periapical radiographs validated against micro-computed tomography scanning in evaluating orthodontically induced apical root resorption. Eur J Oral Sci. 2008;116:467–472. doi: 10.1111/j.1600-0722.2008.00559.x. [DOI] [PubMed] [Google Scholar]
- 5.Weltman B, Vig KWL, Fields HW, Shanker S, Kaizar EE. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop. 2010;137:462–476. doi: 10.1016/j.ajodo.2009.06.021. discussion 12A. [DOI] [PubMed] [Google Scholar]
- 6.Profitt W, Ackerman J. Diagnosis and Treatment Planning in Orthodontics. St Louis, Mo: CV Mosby; 1994. [Google Scholar]
- 7.Kaley J, Phillips C. Factors related to root resorption in edgewise practice. Angle Orthod. 1991;61:125–132. doi: 10.1043/0003-3219(1991)061<0125:FRTRRI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Bartley N, Türk T, Colak C, et al. Physical properties of root cementum: part 17. Root resorption after the application of 2.5° and 15° of buccal root torque for 4 weeks: a microcomputed tomography study. Am J Orthod Dentofacial Orthop. 2011;139:e353–e360. doi: 10.1016/j.ajodo.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Casa MA, Faltin RM, Faltin K, Sander FG, Arana-Chavez VE. Root resorptions in upper first premolars after application of continuous torque moment. Intra-individual study. J Orofac Orthop Fortschritte Kieferorthopädie Organ Official J Dtsch Ges Für Kieferorthopädie. 2001;62:285–295. doi: 10.1007/pl00001936. [DOI] [PubMed] [Google Scholar]
- 10.Hohmann A, Wolfram U, Geiger M, et al. Periodontal ligament hydrostatic pressure with areas of root resorption after application of a continuous torque moment. Angle Orthod. 2007;77:653–659. doi: 10.2319/060806-234. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales C, Hotokezaka H, Karadeniz EI, et al. Effects of fluoride intake on orthodontic tooth movement and orthodontically induced root resorption. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Its Const Soc Am Board Orthod. 2011;139:196–205. doi: 10.1016/j.ajodo.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Seifi M, Atri F, Yazdani MM. Effects of low-level laser therapy on orthodontic tooth movement and root resorption after artificial socket preservation. Dent Res J. 2014;11:61–66. [PMC free article] [PubMed] [Google Scholar]
- 13.Altan AB, Bicakci AA, Mutaf HI, Ozkut M, Inan VS. The effects of low-level laser therapy on orthodontically induced root resorption. Lasers Med Sci. 2015;30(8):2067–2076. doi: 10.1007/s10103-015-1717-6. [DOI] [PubMed] [Google Scholar]
- 14.Ekizer A, Uysal T, Güray E, Akkuş D. Effect of LED-mediated photobiomodulation therapy on orthodontic tooth movement and root resorption in rats. Lasers Med Sci. 2015;30:779–785. doi: 10.1007/s10103-013-1405-3. [DOI] [PubMed] [Google Scholar]
- 15.El-Bialy T, El-Shamy I, Graber TM. Repair of orthodontically induced root resorption by ultrasound in humans. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Its Const Soc Am Board Orthod. 2004;126:186–193. doi: 10.1016/j.ajodo.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Inubushi T, Tanaka E, Rego EB, et al. Effects of ultrasound on the proliferation and differentiation of cementoblast lineage cells. J Periodontol. 2008;79:1984–1990. doi: 10.1902/jop.2008.080081. [DOI] [PubMed] [Google Scholar]
- 17.Scheven BA, Man J, Millard JL, et al. VEGF and odontoblast-like cells: stimulation by low frequency ultrasound. Arch Oral Biol. 2009;54:185–191. doi: 10.1016/j.archoralbio.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Hasuike A, Sato S, Udagawa A, Ando K, Arai Y, Ito K. In vivo bone regenerative effect of low-intensity pulsed ultrasound in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e12–e20. doi: 10.1016/j.tripleo.2010.09.061. [DOI] [PubMed] [Google Scholar]
- 19.Iwabuchi Y, Tanimoto K, Tanne Y, et al. Effects of low-intensity pulsed ultrasound on the expression of cyclooxygenase-2 in mandibular condylar chondrocytes. J Oral Facial Pain Headache. 2014;28:261–268. doi: 10.11607/ofph.1156. [DOI] [PubMed] [Google Scholar]
- 20.Shiraishi R, Masaki C, Toshinaga A, et al. The effects of low-intensity pulsed ultrasound exposure on gingival cells. J Periodontol. 2011;82:1498–1503. doi: 10.1902/jop.2011.100627. [DOI] [PubMed] [Google Scholar]
- 21.Mostafa NZ, Uludağ H, Dederich DN, Doschak MR, El-Bialy TH. Anabolic effects of low-intensity pulsed ultrasound on human gingival fibroblasts. Arch Oral Biol. 2009;54:743–748. doi: 10.1016/j.archoralbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Zhang Y, Zhou J, et al. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PloS One. 2014;9:e95168. doi: 10.1371/journal.pone.0095168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalla-Bona DA, Tanaka E, Oka H, et al. Effects of ultrasound on cementoblast metabolism in vitro. Ultrasound Med Biol. 2006;32:943–948. doi: 10.1016/j.ultrasmedbio.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Dalla-Bona DA, Tanaka E, Inubushi T, et al. Cementoblast response to low- and high-intensity ultrasound. Arch Oral Biol. 2008;53:318–323. doi: 10.1016/j.archoralbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Xu J, Lingling E, Wang D. Ultrasound enhances the healing of orthodontically induced root resorption in rats. Angle Orthod. 2012;82:48–55. doi: 10.2319/030711-164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosner B. Fundamentals of Biostatistics. Ed. Boston, Mass: Brooks/Cole; 2011. 232 p 7th. [Google Scholar]
- 27.Malek S, Darendeliler MA, Rex T, et al. Physical properties of root cementum: part 2. Effect of different storage methods. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Its Const Soc Am Board Orthod. 2003;124:561–570. doi: 10.1016/s0889-5406(03)00398-6. [DOI] [PubMed] [Google Scholar]
- 28.Wierzbicki T, El-Bialy T, Aldaghreer S, Li G, Doschak M. Analysis of orthodontically induced root resorption using micro-computed tomography (micro-CT) Angle Orthod. 2009;79:91–96. doi: 10.2319/112107-546.1. [DOI] [PubMed] [Google Scholar]
- 29.Al-Daghreer S, Doschak M, Sloan AJ, et al. Effect of low-intensity pulsed ultrasound on orthodontically induced root resorption in beagle dogs. Ultrasound Med Biol. 2014;40:1187–1196. doi: 10.1016/j.ultrasmedbio.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Inubushi T, Tanaka E, Rego EB, et al. Ultrasound stimulation attenuates resorption of tooth root induced by experimental force application. Bone. 2013;53:497–506. doi: 10.1016/j.bone.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Fujihara S, Yamamoto-Nagata K, Katsura T, Inubushi T, Tanaka E. Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis. Ann Biomed Eng. 2011;39:2964–2971. doi: 10.1007/s10439-011-0408-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Goetz W, Braumann B, Bourauel C, Jaeger A. Effect of soluble receptors to interleukin-1 and tumor necrosis factor alpha on experimentally induced root resorption in rats. J Periodont Res. 2003;38:324–332. doi: 10.1034/j.1600-0765.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 33.Vafaeian B, Al-Daghreer S, El-Rich M, Adeeb S, El-Bialy T. Simulation of low-intensity ultrasound propagating in a beagle dog dentoalveolar structure to investigate the relations between ultrasonic parameters and cementum regeneration. Ultrasound Med Biol. 2015;41:2173–2190. doi: 10.1016/j.ultrasmedbio.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Puente MI, Galbán L, Cobo JM. Initial stress differences between tipping and torque movements. A three-dimensional finite element analysis. Eur J Orthod. 1996;18:329–339. doi: 10.1093/ejo/18.4.329. [DOI] [PubMed] [Google Scholar]
- 35.Henry JL, Weinmann JP. The pattern of resorption and repair of human cementum. J Am Dental Assoc. 1951;42:270–290. doi: 10.14219/jada.archive.1951.0045. [DOI] [PubMed] [Google Scholar]
- 36.Srivicharnkul P, Kharbanda OP, Swain MV, Petocz P, Darendeliler MA. Physical properties of root cementum: part 3. Hardness and elastic modulus after application of light and heavy forces. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Its Const Soc Am Board Orthod. 2005;127:168–176. doi: 10.1016/j.ajodo.2003.12.021. quiz 260. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz AM. Tissue changes incidental to orthodontic tooth movement. Int J Orthod Oral Surg Radiogr. 1932;18:331–352. [Google Scholar]
- 38.Dorow C, Sander F-G. Development of a model for the simulation of orthodontic load on lower first premolars using the finite element method. J Orofac Orthop Fortschritte Kieferorthopädie Organ Official J Dtsch Ges Für Kieferorthopädie. 2005;66:208–218. doi: 10.1007/s00056-005-0416-5. [DOI] [PubMed] [Google Scholar]
- 39.Proffit W, Feilds H, Sarver D. Contemporary Orthodontics. Philadelphia, Pa: Elsevier; 2006. [Google Scholar]