Abstract
The current Mycobacterium bovis BCG vaccine provides inconsistent protection against pulmonary infection with Mycobacterium tuberculosis. Immunity induced by subcutaneous immunization with BCG wanes and does not promote early recruitment of T cell to the lungs after M. tuberculosis infection. Delivery of Tuberculosis (TB) vaccines to the lungs may increase and prolong immunity at the primary site of M. tuberculosis infection. Pulmonary immunization with recombinant influenza A viruses (rIAVs) expressing an immune-dominant M. tuberculosis CD4+ T cell epitope (PR8-p25 and X31-p25) stimulates protective immunity against lung TB infection. Here, we investigated the potential use of rIAVs to improve the efficacy of BCG using simultaneous immunization (SIM) and prime-boost strategies. SIM with parenteral BCG and intranasal PR8-p25 resulted in equivalent protection to BCG alone against early, acute and chronic M. tuberculosis infection. Boosting BCG with rIAVs increased the frequency of IFN-γ-secreting specific T cells (p<0.001) and polyfunctional CD4+ T cells (p<0.05) in the lungs compared to the BCG alone, however, this did not result in a significant increase in protection against M. tuberculosis compared to BCG alone. Therefore, sequential pulmonary immunization with these rIAVs after BCG increased M. tuberculosis-specific memory T cell responses in the lung, but not protection against M. tuberculosis infection.
Introduction
Tuberculosis (TB) is the commonest cause of death from an infectious disease globally and was responsible for one billion deaths over the last two centuries [1] and 1.4 million death in 2019 [2]. The current TB vaccine Mycobacterium bovis Bacille Calmette-Guérin (BCG) provides reliable protection against severe TB disease in children, but only variable protection against pulmonary TB in adults [3, 4]. In a population at high risk for TB, neonatal BCG vaccination provided protection for only ten years [5, 6], and the waning of BCG efficacy contributes to the increase in TB cases observed in adolescents and young adults [7, 8]. The variability and limited durability of the protective immunity generated by BCG also limit its impact on the transmission of M. tuberculosis. The failure of BCG to provide long-lived protection against M. tuberculosis infection is related to the waning of the BCG-induced immune response. For example, in mice the level of cytokine secreting antigen-specific CD4+ T induced by BCG significantly declines by 16-weeks post-immunization [9]. The long-term efficacy of BCG may be improved by boosting BCG with subunit vaccines to sustain the level of protective immunity [8], and a variety of viral and protein/adjuvant vaccines are being evaluated as boosting vaccines in preclinical models [10, 11].
One approach for enhancing the efficacy of BCG priming is to deliver the boosting vaccine to the lungs, the primary site of encounter with M. tuberculosis. This requires efficient delivery systems. Viral-vectored TB vaccines present one approach for delivery to the lungs [12], and recently protein/adjuvant vaccines with adjuvants approved for human use have also been safely delivered as pulmonary vaccines [13]. Both modified vaccinia virus expressing the M. tuberculosis Ag85A (MVA85A) and replication deficient adenovirus expressing Ag85A boosted the protective efficacy of BCG in mice when delivered by the intranasal (i.n.), but not the parenteral route [10, 14]. The route of delivery was essential for this effect [14], and subsequently, boosting with intramuscular delivery of the MVA85A vaccine failed to increase the protective efficacy of BCG in a Phase IIB clinical trial in infants [15]. Further, boosting BCG-vaccinated infants with intramuscular adenovirus AERAS-402 vaccine failed to generate a strong polyfunctional CD4+ and CD8+ T cell responses [16]. This contrasts with results from preclinical studies where pulmonary delivery of TB vaccines to boost BCG stimulated the strongest antigen-specific T cell responses in the lungs compared to immunization by other routes [17, 18]. These results highlight the need for novel TB vaccines suitable for pulmonary delivery in humans.
Simultaneous immunization (SIM) with vaccines given by the pulmonary and parenteral routes may activate local mucosal immune responses that inhibit early M. tuberculosis infection and systemic immunity provides sustained protection. For example, SIM with subcutaneous BCG and intranasal Adenovirus expressing M. tuberculosis Ag85A increased protection against pulmonary TB infection [19]. The lack of luminal T cells in the airway following parenteral BCG may contribute to delayed protection in the lungs against M. tuberculosis [20].
The tropism of IAV to the respiratory tract [21] and the availability of licensed intranasal influenza vaccines for use in humans [22] render it a promising potential vector to improve BCG-induced immunity in the lungs. Intranasal immunization with recombinant IAV (rIAV) PR8 (H1N1) expressing the p25 CD4+ T cell epitope of M. tuberculosis Ag85B (PR8-p25) was immunogenic and protective against M. tuberculosis infection [23]. Sequential immunization with H1N1 (PR8-p25) and H3N2 (X31-p25) rIAV expressing the same epitope induced stronger cytokine production by p25-specific T cells and improved protective efficacy in the spleen compared to a single rIAV vaccine [24]. Importantly, pulmonary immunization with PR8-p25 and X31-p25 stimulated antigen-specific CD4+ lung-resident memory T cells that could confer protection against M. tuberculosis in the absence of circulating memory T cells [25]. The present study investigated the impact of pulmonary immunization with these rIAV vaccines in both SIM and prime-boost strategies with BCG on the induction of T cell responses in the lung and protection against M. tuberculosis challenge.
Materials and methods
Mice
Six- to eight-week old female C57BL/6 mice were purchased from the Animal Resources Centre (Perth, Australia) or Australian BioResources (Moss Vale, Australia). Mice were maintained under specific pathogen-free conditions at the Centenary Institute Animal Facility. All murine experiments were approved by Royal Prince Alfred Hospital Animal Ethics Committee (2013/075 and 2016/044).
Recombinant influenza A viruses (rIAVs)
The IAV PR8 (H1N1, A/Puerto Rico/8/1934) and the IAV X31 (H3N2, A/HKx31) were engineered to express the M. tuberculosis p25 CD4+ T cell epitope (PR8-p25 and X31-p25 respectively) of Ag85B, the secreted fibronectin binding protein B encoded by rv1886c, as previously described [23]. In summary, the sequence encoding the 15 amino acids of the M. tuberculosis Ag85B240-254 CD4+ T cell epitope (FQDAYNAAGGHNAVF) was inserted into the gene encoding the neuraminidase (NA) stalk of the PR8 or X31 viruses and the recombinant influenza A viruses rescued using an eight-plasmid reverse genetics system [26–28]. Sequence analysis of recovered virus confirmed integrity of the insert.
Bacteria and growth conditions
M. bovis BCG Pasteur and M. tuberculosis H37Rv strain were grown in Middlebrook 7H9 (Difco) medium supplemented with glycerol (0.2% v/v), tyloxapol (0.02% v/v) and albumin-dextrose-catalase (ADC; 10% v/v). The mycobacteria were enumerated by plating serial dilutions of organ homogenates onto 7H11 (Difco) agar supplemented with oleic-acid-albumin-dextrose-catalase (OADC; 10% v/v) and glycerol (0.5% v/v).
Immunization and M. tuberculosis challenge
Mice were immunized by subcutaneous (s.c) injection at the base of the tail with 5x105 colony forming units (CFU) BCG Pasteur in 200 μl of phosphate buffered saline (PBS) following brief isoflurane anaesthesia. For intranasal (i.n.) immunization, mice were anaesthetized by intraperitoneal (i.p.) injection with ketamine/xylazine solution (125 mg and 8 mg/Kg) and received 20 plaque forming units (PFU) PR8-p25 or 104 PFU X31-p25 [23, 29] in 50 μl PBS. For SIM, parenteral BCG and i.n. PR8-p25 vaccines were delivered at the same time. For the prime-boost strategy, mice were primed with BCG 12 weeks prior to sequential immunization with rIAVs delivered at a six-week interval. Mice were challenged four weeks after the last vaccination with aerosol M. tuberculosis H37Rv with approximately 100 CFU using an inhalation exposure system (Glas-Col, Terre Haute, IN) [23]. Four weeks later, the mice were euthanized by CO2 narcosis and the bacterial loads in the lungs and spleens were enumerated. Following rIAV immunization, mice were monitored daily between 5–15 days post-immunization (d.p.i.) and otherwise twice weekly. If mice lost more than 30% of their starting body weight or met defined criteria, they were euthanized. Following M. tuberculosis infection, mice were monitored twice weekly and if they lost more than 15% of their starting body weight or met defined criteria, they were euthanized.
Cell preparation
Prior to collecting cells for immunological assays, the lungs were perfused by injecting 10 ml PBS into the right atrium of the heart to remove blood cells from the pulmonary blood vessels prior to. Lung perfusion was not performed in experiments when all lung lobes were used for bacterial counts following M. tuberculosis infection. Single cell suspensions were prepared from lungs and spleen. Lungs were digested by incubation with Collagenase type IV (50 U/ml, Sigma, St Louis, MO) and DNAse I (13 μg/ml, Sigma) in RPMI/10% FCS for 45 minutes at 37 °C. The lungs and spleen were disrupted by passaging the tissues through 70 μm cell strainers, washed and incubated with ACK lysis buffer to remove red blood cells. The washed cells were resuspended in RPMI/10% FCS.
IFN-γ enzyme-linked immunospot (ELISpot) assay
IFN-γ ELISpot assay was performed as previously described [23, 30] using anti-IFN-γ antibody AN18 and biotinylated anti-IFN-γ antibody XMG1.2. The cells (2x105/well) were cultured at 37 °C for 18 hours in the presence of Influenza A NP366-374 peptide (NP; Genscript, Piscataway, NJ), M. tuberculosis Ag85B240-254 peptide (p25; Genscript), M. tuberculosis H37Rv culture filtrate protein (CFP, BEI Resources), and BCG lysate at a final concentration of 10 μg/ml. The spot-forming cells (SFC) were enumerated using an automated ELISpot reader (AID, Strassberg, Germany).
Flow cytometry analysis
Leukocyte populations and cytokine production were analysed as previously described [23, 24] using antibodies to the following cell surface markers: anti- CD3-PerCPCy5.5 (Biolegend, San Diego, CA), anti-CD4 AF700 (BD Pharmingen), anti-CD44 FITC (BD Pharmingen), anti-CD69 PE (BD Pharmingen), anti-KLRG1 PECy7 (eBioscience, San Diego, CA), and live/dead fixable staining (Life Technologies, Carlsbad, CA). For intra-cytoplasmic cytokine staining (ICS), lung and spleen cells were incubated with p25 peptide (10 μg/mL) at 37 °C for 6 hours with addition of Brefeldin A (10 μg/mL, Sigma) for the last 3.5 hours. After surface staining, the cells were washed, fixed and permeabilized with Cytofix (100 μl, BD Biosciences). The cells were then stained with anti-cytokine antibodies: anti-IFN-γ FITC (BD Pharmingen), anti-IL-2 APC (Biolegend), and anti-TNF PE (Biolegend). For analysis of cells following M. tuberculosis infection, the stained cells were re-suspended in 100 μl 4% formalin for at least 2 hours and then transferred into a new plate that was sprayed with disinfectant (3% Vircon) externally for safe transfer and acquisition on the flow cytometer outside the PC3 facility. Cells were acquired using LSR Fortessa cytometer (BD, Franklin Lakes, NJ) and analyzed using FlowJo Macintosh (Tree Star, Ashland, OR). Cytokine expression was determined using the gating strategy in S1 Fig and analyzed using the FlowJo Boolean gating tool.
Statistical analysis
Data analyses were performed using GraphPad Prism 7 software (GraphPad Software, La Jolla, CA). Significant differences between more than two groups were analyzed by analysis of variance (ANOVA) with Tukey’s correction for multiple comparisons if the data were normally distributed or Kruskal-Wallis test with Dunn’s multiple comparison test if the data were not normally distributed based on Saphiro-Wilk test. Statistical differences with p<0.05 were considered significant.
Results
Antigen-specific CD4+ T cell response following simultaneous immunization with BCG and rIAV vaccine
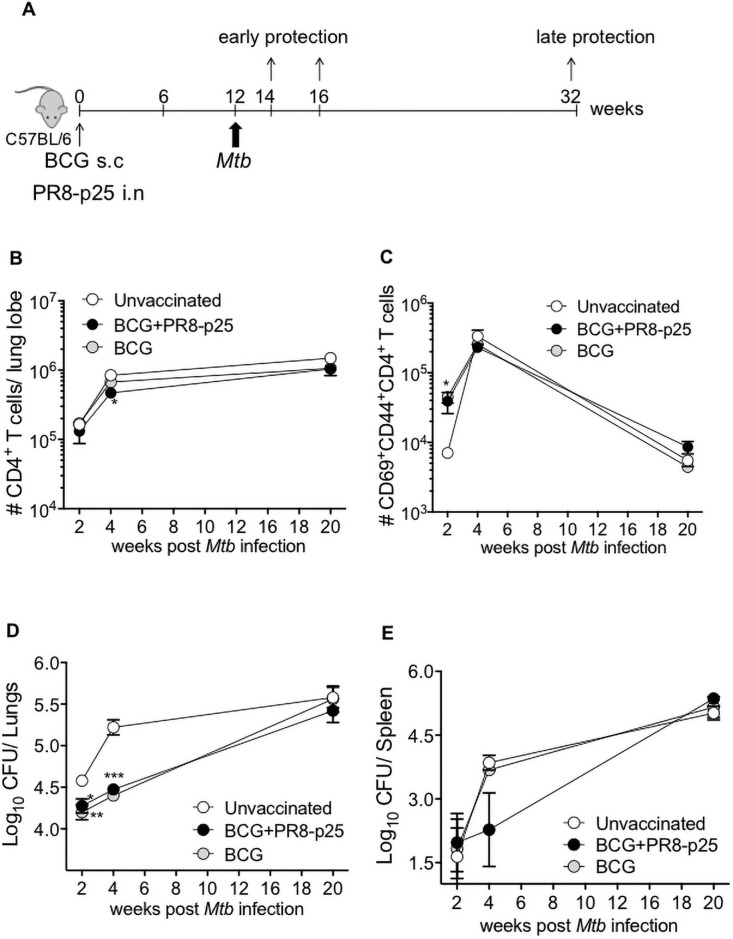
SIM with parenteral and mucosal vaccines has been proposed as a way to improve the protective effect of immunization against M. tuberculosis in the lungs at both early and late phases of infection [14]. To investigate this strategy using BCG and rIAV, C57BL/6 mice were vaccinated with s.c. BCG and i.n. PR8-p25 simultaneously (BCG+PR8-p25) or with s.c. BCG alone. Twelve weeks later, the mice were challenged by aerosol with M. tuberculosis H37Rv, and the cellular immune responses in the lungs and protective efficacy were measured at 2, 4, and 20 weeks after challenge (Fig 1A).
Fig 1. CD4+ T cell influx and protection against M. tuberculosis following SIM BCG and PR8-p25.
(A) Experimental design for simultaneous immunization (SIM) with BCG and PR8-p25 rIAV. C57BL/6 mice (n = 5) were simultaneously vaccinated with 5x105 CFU of s.c. BCG and 20 PFU of i.n. PR8-p25, s.c. BCG alone, or were left unvaccinated. The mice were challenged with M. tuberculosis 12 weeks later. Flow cytometry T cell analysis and enumeration of bacterial load were evaluated at 2, 4, and 20 weeks after M. tuberculosis challenge. (B) The kinetics of the influx of CD4+ T cells and (C) CD69+CD44+ CD4+ T cells in the lungs infected with M. tuberculosis. The kinetics of the bacterial burden are shown for the (D) lungs and (E) spleen. Data are the means ± SEM. Statistically significant differences were determined by one way-ANOVA (*p<0.05;**p<0.01; ***p<0.001).
Following M. tuberculosis infection, there was an influx of CD4+ T cells into the lungs that peaked at 4 weeks post-infection (p.i.) and was sustained for 20 weeks (Fig 1B). In both groups of vaccinated mice there were significantly increased numbers of CD4+ T cells expressing activation markers, CD44 and CD69, as compared to unvaccinated mice (p<0.05, Fig 1C) two weeks after M. tuberculosis infection. The frequency of activated CD4+ T cells peaked at four weeks and fell by 20 weeks (Fig 1C). Chronic M. tuberculosis infection is associated with terminal differentiation of activated T cells characterized by the expression of killer cell lectin-like receptor G1 (KLRG1) [31]. The kinetics of expression of KLRG1 by CD4+ T cells in the lungs was similar to that of the above activation markers, and there were no significant differences between the vaccinated groups (S2 Fig).
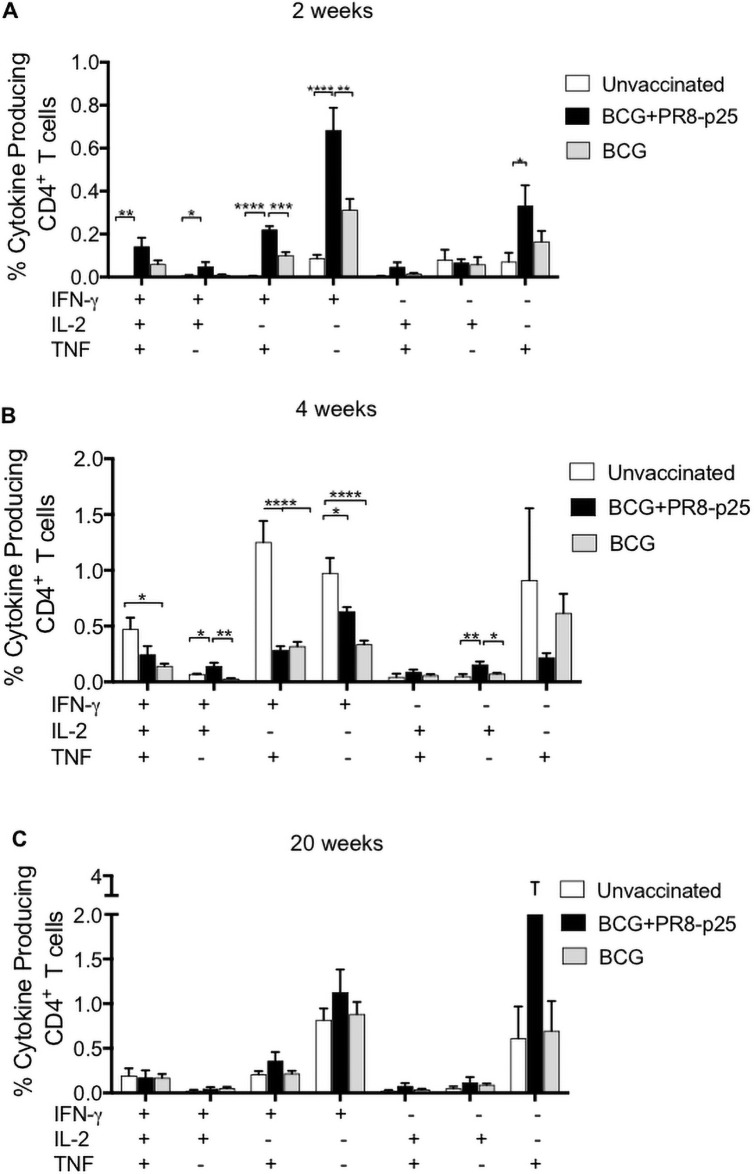
The Ag85B240-254 CD4+ T cell epitope (p25) encoded by PR8-p25 is present in BCG as well as M. tuberculosis. Cytokine production by p25-specific CD4+ T cells in the lungs was analyzed at 2, 4, and 20 weeks after M. tuberculosis challenge. At two weeks, the BCG and BCG+PR8-p25 immunized mice produced early p25-specific CD4+ T cell cytokine responses that were dominated by IFN-γ and TNF, but included smaller, significant increases in the frequencies of IFN-γ/TNF/IL-2 and IFN-γ/TNF secreting CD4+ T cells (Fig 2A). The p25-specific IFN-γ (p<0.01) response was significantly higher in the mice receiving PR8-p25 and BCG than BCG alone. A similar pattern with a lower magnitude of cytokine responses was observed in the spleen (S3A Fig). Therefore, SIM led to an early and stronger recruitment of antigen-specific T cells to the lungs following M. tuberculosis infection [32–34].
Fig 2. Cytokine production by p25-specific CD4+ T cells in the lungs after M. tuberculosis challenge.
C57BL/6 mice (n = 5) were vaccinated simultaneously with s.c. BCG and i.n. PR8-p25, and challenged with M. tuberculosis as in Fig 1A. The lung cells from the same mice were stimulated with p25 antigen and then analyzed for intra-cellular cytokine production by flow cytometry. The frequency of p25-specific CD4+ T cells secreting IFN-γ, IL-2 and TNF at (A) 2, (B) 4, and (C) 20 weeks after challenge. Data are the means ± SEM. Statistically significant differences between groups were determined by one-way ANOVA (*) or Kruskal-Wallis (#) (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001).
By four weeks p.i. unvaccinated mice had the highest frequency of CD4+ T cells secreting IFN-γ and IFN-γ/TNF compared to either vaccinated group, and higher polyfunctional CD4+ T cells compared to the BCG alone (Fig 2B), probably driven by the higher bacterial load in unvaccinated mice (Fig 1D). A similar pattern of cytokine-producing CD4+ T cells was observed in the spleens of unvaccinated mice (S3B Fig). At 20 weeks p.i., there were no differences in the pattern of cytokine production of p25-specific CD4+ T cells between unvaccinated and vaccinated groups (Fig 2C). IFN-γ and TNF responses were the dominant responses in each group in both the lungs (Fig 2C) and spleen (S3C Fig).
Protective efficacy following simultaneous immunization with BCG and rIAV vaccine
The protective efficacy of SIM with BCG and i.n. PR8-p25 against M. tuberculosis aerosol infection was determined at 2, 4, and 20 weeks p.i. Vaccination with BCG or BCG+PR8-p25 resulted in a significant reduction in the lungs at two and four weeks after challenge, but this effect was lost by 20 weeks p.i. (Fig 1D). There was no difference in protective efficacy between SIM BCG+PR8-p25 and BCG at 2 or 4 weeks (Fig 1D). There was a trend towards increased protection in the spleen of mice vaccinated with BCG+PR8-p25 at 4 weeks p.i., but this was not statistically significant (Fig 1E). Therefore, simultaneous immunization with rIAV-p25 did not improve the protective efficacy of BCG against acute M. tuberculosis infection and did not prevent the loss of BCG-induced protective efficacy late in infection.
Antigen-specific CD4+ T cell responses following BCG and boosting rIAV vaccines
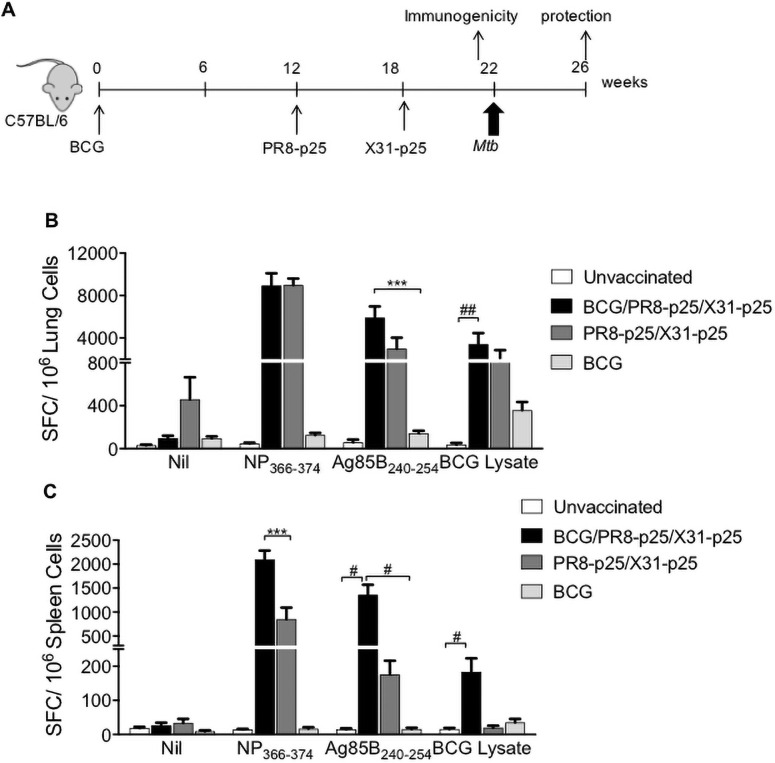
To investigate if the rIAV vaccines could boost the waning protective immunity induced by BCG, BCG-vaccinated mice were sequentially immunized with HIN1 and H3N2 rIAVs expressing p25. (Fig 3A). Control mice were immunized with BCG or rIAVs alone, or were unimmunized. T cell responses in the lungs and spleen were analyzed by ELISpot and flow cytometry four weeks after the last vaccination, when the rIAVs have been cleared [35].
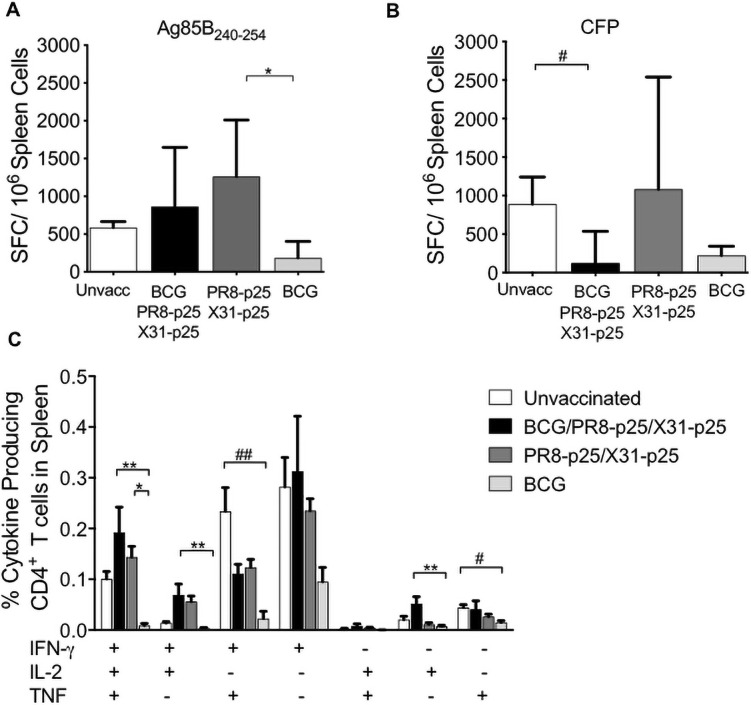
Fig 3. T cells producing IFN-γ in response to M. tuberculosis antigens after boosting BCG with PR8-p25 and X31-p25.
(A) Experimental design for BCG boosting with PR8-p25 and X31-p25 rIAVs. C57BL/6 mice (n = 5) were vaccinated with 5x105 CFU of s.c. BCG and sequentially boosted with 20 PFU of i.n. PR8-p25 and 104 PFU of i.n. X31-p25. Other groups were vaccinated with BCG alone, or the two rIAVs, or were unvaccinated. (B) Four weeks after the last vaccination, the frequency of T cells in the lungs and (C) spleen producing IFN-γ in response to the CD8+ T cell epitope NP366-374 of IAV, the CD4+ T cell epitope Ag85B240-254 of M. tuberculosis, or BCG lysate, were analyzed by ELISpot as SFC/106 cells. Data are the means ± SEM and are representative of two independent experiments. The differences between groups were determined by one-way ANOVA (*) or Kruskal-Wallis (#) (*p<0.05;**p<0.01; ***p<0.001).
Boosting BCG with PR8-p25 followed by X31-p25 (BCG/PR8-p25/X31-p25) elicited a higher frequency of p25-specific T cells producing IFN-γ in the lungs, compared to vaccination with BCG alone (Fig 3B). Increased IFN-γ production was also observed following stimulation with BCG lysate that contains the Ag85B protein (Fig 3B). The response to the endogenous IAV CD8+ T cell epitope NP366-374 in the lungs of mice that received BCG prior to rIAVs was not significantly different from mice that received only the rIAVs (PR8-p25/X31-p25) (Fig 3B). Interestingly, in the spleen, increased IFN-γ T cell responses to both mycobacterial antigens and the IAV peptide antigen were seen following BCG boosting (Fig 3C). Thus, boosting BCG with intranasal rIAVs enhanced local and systemic p25-specific IFN-γ T cell responses.
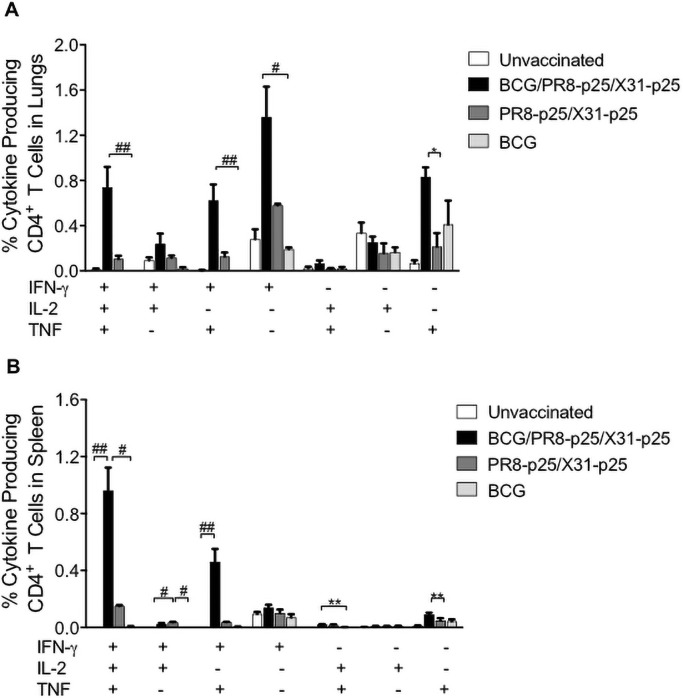
BCG induced a low p25-specific cytokine response in the lungs (Fig 4A). BCG/PR8-p25/X31-p25 immunization stimulated significantly increased proportions of CD4+ T cell producing IFN and IFN-γ/TNF response compared to BCG (Fig 4A). Importantly, immunization with BCG/PR8-p25/X31-p25 induced the highest frequency of polyfunctional CD4+ T cells producing IFN-γ/IL-2/TNF in the lungs (Fig 4A). In the spleen, 1% of total CD4+ T cells produced triple cytokines in response to p25 stimulation, and this was significantly higher than vaccination with the BCG alone (Fig 4B). Reversing the order of the rIAV booster vaccines to BCG/X31-p25/PR8-p25 (S4A Fig) resulted in similar immunogenicity profile as BCG/PR8-p25/X31-p25. BCG/X31-p25/PR8-p25 increased p25-specific T cells secreting IFN-γ in the lungs and spleen (S4B and S4C Fig), and polyfunctional CD4+ T cells (S4D and S4E Fig). Therefore, boosting BCG with rIAVs stimulated stronger polyfunctional CD4+ T cells in the lungs and spleen than vaccination with the rIAVs or BCG alone.
Fig 4. Cytokine production by CD4+ T cells following BCG boosting with rIAVs.
C57BL/6 mice (n = 5) were vaccinated with BCG and sequential PR8-p25 and X31-p25 rIAVs as shown in Fig 3A. Four weeks after the last vaccination, lung and spleen cells were stimulated with p25 antigen and cytokine production was analyzed by flow cytometry using Boolean gating. (A) The frequency of p25-specific CD4+ T cells in the lungs and (B) in the spleen producing individual cytokines or combinations of IFN-γ, IL-2, and TNF. Data are the means ± SEM and are representative of two independent experiments. The differences between groups were determined by one-way ANOVA (*) or Kruskal-Wallis (#) (*p<0.05;**p<0.01).
Antigen-specific CD4+ T cell responses following challenge with M. tuberculosis
To investigate if boosting BCG with rIAVs influenced T cell responses following M. tuberculosis infection, cytokine production by splenocytes was analyzed four weeks after challenge. The frequency of T cells producing IFN-γ in response to stimulation with p25 antigen was similar in the unvaccinated, BCG, or BCG/PR8-p25/X31-p25 (Fig 5A). Following infection, unvaccinated mice also had a high frequency of T cells producing IFN-γ in response to stimulation with M. tuberculosis CFP (Fig 5B).
Fig 5. Cytokine production in the spleen at 4 weeks after aerosol challenge with M. tuberculosis.
Mice were vaccinated with BCG and sequential PR8-p25 and X31-p25 rIAVs and challenged with M. tuberculosis as in Fig 3A. Four weeks after challenge, cytokine production by antigen-specific splenic CD4+ T cells was analyzed. (A) The frequency of T cells producing IFN-γ in response to 16-hour stimulation with p25 peptide, or (B) CFP, derived from M. tuberculosis, was analyzed by ELISpot. (C) The frequency of p25-specific CD4+ T cells producing IFN-γ, IL-2, and TNF following stimulation with p25 was analyzed by flow cytometry. Data are shown as the median and interquartile range (A, B) or the means ± SEM (C) and are representative of two independent experiments. Statistically significant differences were determined by one-way ANOVA (*) or Kruskal-Wallis (#) (*p<0.05;**p<0.01; ***p<0.001).
Analysis of intra-cellular cytokine expression showed unvaccinated and vaccinated mice had similar frequencies of CD4+ T cells producing IFN-γ alone (Fig 5C). The unvaccinated group had a higher frequency of CD4+ T cells producing IFN-γ/TNF compared to the BCG alone. The BCG/PR8-p25/X31-p25 group had a higher frequency of CD4+ T cells producing IL-2 either as an individual cytokine or in combination with IFN-γ. Interestingly, the frequency of polyfunctional CD4+ T cells was significantly higher in the spleens of mice receiving PR8-p25/X31-p25 or BCG/PR8-p25/X31-p25 compared to BCG alone (Fig 5C). Thus, boosting BCG with rIAVs maintained higher frequency of polyfunctional p25-specific CD4+ T cells than BCG alone in the spleen following four weeks M. tuberculosis infection.
Protective efficacy following BCG and boosting rIAV vaccine
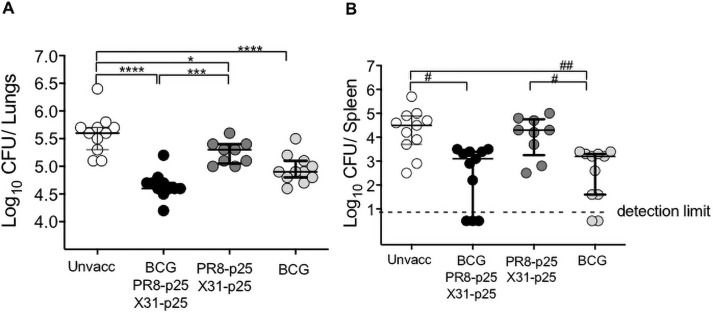
To determine if boosting with rIAVs improved the protection mediated by BCG, mice were vaccinated with BCG alone or BCG followed by the rIAV vaccines (Fig 3A) were challenged with M. tuberculosis H37Rv and bacterial loads determined four weeks later. Immunization with BCG afforded protective efficacy in the lungs as compared to unvaccinated mice (p<0.0001) (Fig 6A). Mice vaccinated with rIAVs alone also had reduced bacterial counts compared to the unvaccinated group (p<0.05) (Fig 6A). Boosting BCG with the rIAVs resulted in a significant reduction of the bacterial load in the lungs compared to the unvaccinated (p<0.0001) and rIAVs (p<0.01) vaccinated groups. There was a trend towards increased protection against M. tuberculosis in the lungs of mice that received BCG boosted with rIAVs compared BCG alone in two independent experiments, but this did not reach statistical significance (Fig 6A). Mice immunized with BCG and BCG/PR8-p25/X31-p25 showed significant reductions in M. tuberculosis load in the spleen, with greater variation in the level of dissemination in the BCG-boosted mice (Fig 6B). Therefore, boosting BCG by mucosal immunization with rIAV vaccines expressing the immunodominant p25 epitope increased specific CD4+ T cell responses in the lung, but this was not associated with a significant increase in protection against M. tuberculosis.
Fig 6. Protective efficacy of BCG boosting with PR8-p25 and X31-p25 against M. tuberculosis infection.
C57BL/6 mice (n = 5–6) were vaccinated with BCG and sequentially boosted i.n. with PR8-p25 and X31-p23 as shown in Fig 3A. Four weeks after the last vaccination, mice were aerosol challenged with M. tuberculosis H37Rv, and the bacterial loads in (A) the lungs and (B) spleen were measured four weeks later. The data are pooled of two independent experiments and are shown as individual values in median and interquartile range. The differences between groups were determined by one-way ANOVA for panel A (*) or Kruskal-Wallis for panel B (#) (*p<0.05;**p<0.01; ***p<0.001;****p<0.0001).
Discussion
Vaccination with BCG at birth will be retained in regions with high burden of TB because BCG protects infants from severe TB disease [4, 36]. The lack of long-term immunity following peripheral BCG vaccination, however, requires new approaches to overcome the waning of BCG-induced immunity. Developing a novel vaccine delivery mechanism, such as a viral vector administered by the pulmonary route, has been recognized as a promising approach in preclinical TB vaccine research [37]. Here, we have investigated the ability of the intranasal rIAV vaccines in SIM and prime-boost strategies to improve BCG-induced immunity.
Multiple studies show that peripheral TB vaccination induces a low frequency of T cells in the airway lumen [38]. SIM with parenteral and mucosal vaccines is proposed to induce effective protective immunity against early and late phase of M. tuberculosis infection owing to the synergistic effect of both local and systemic immune responses [14]. The current study showed that SIM with parenteral BCG and intranasal PR8-p25 did result in the early induction of higher frequency of p25-specific CD4+ T cells than BCG (Fig 2A), but the vaccine protection efficacy was equivalent to that provided by BCG alone (Fig 1D). This result is different to a previous finding that SIM with BCG and intranasal adjuvanted vaccine containing 85A protein, TB10.4 protein or ESAT 6 peptide, induced stronger protection M. tuberculosis than parenteral BCG [19]. Further, when intranasal BCG was combined with parenteral BCG, this provided the highest protective efficacy compared to single route of BCG [19]. The current rIAV vaccines expressed a single CD4+ T cell epitope, and more diverse M. tuberculosis antigens may be critical for achieving additional protection with SIM. Although SIM with BCG and PR8-p25 was able to inhibit the rapid growth of M. tuberculosis during the first four weeks of infection, this inhibitory effect waned by the chronic stage of infection, assessed at 20 weeks after challenge. Further, in cattle SIM with subcutaneous BCG and endobronchial BCG or with subcutaneous BCG and endobronchial Ad85A significantly reduced the lung pathology score, but did not decrease the bacterial load as compared to unvaccinated cattle after M. bovis challenge [39]. Thus, the potential benefit of SIM strategy compared to a single BCG vaccination will require more consistent evidence before clinical implementation.
Prime-boost immunization with BCG and the rIAVs significantly increased M tuberculosis-specific T cell responses compared to BCG alone or SIM with BCG/rIAV. Antigen-specific IFN-γ T cell responses in the lungs peaked at 8 weeks and contracted by 16 weeks after subcutaneous BCG immunization [9]. By contrast, intranasal boosting with PR8-p25 followed by X31-p25, or with the reverse order, prevented waning of BCG-induced immunity T cell and significantly enhanced lung and systemic p25-specific IFN-γ T cell responses for 22 weeks after the BCG. Intranasal boosting with AdHu5 expressing Ag85A also enhanced antigen-specific responses in the lung [9]. TNF production in the lung from macrophages and T cells is essential for the control of TB infection, however, the excessive TNF may contribute to immunopathology [40, 41]. The choice of viral vector may be an important factor in eliciting strong immune responses in the lungs. For instance, aerosol delivery of MVA85A vaccine failed to induce stronger Ag85A-specific responses in the lungs of BCG primed-macaques as compared to the responses following intradermal MVA85A [18]. The recombinant Influenza A virus vector utilized in this study may offer an advantage because of its tropism for the respiratory tract.
Pulmonary delivery of other non-viral TB vaccines has also been found to induce protective immunity against TB. For example, intratracheal delivery of conjugate vaccines of the Toll-like Receptor (TLR)-2 ligand, Pam2Cys, bound to either CD4+ and CD8+ T cell epitopes of M. tuberculosis [42] or to the secreted proteins ESAT-6 or MTP83 [43, 44] stimulated IL-17 and IFN-γ secreting T cell responses and protection against M. tuberculosis. Pulmonary immunization promotes development of M. tuberculosis-specific IL-17 CD4+ T cell responses, which in some instances are essential for the protection. For example, pulmonary, but not subcutaneous, immunization with BCG stimulated protection in the highly susceptible DBA/2 strain of mice, and this effect was IL-17 dependent [45]. Similarly, the protection conferred by pulmonary delivery of the CysVac2 protein/Advax vaccine against TB infection was also IL-17-dependent [13].
The location of memory T cells in the respiratory tract may impact the capability of T cells to respond rapidly against pulmonary M. tuberculosis infection. Early protection against M. tuberculosis infection is dependent on T cell activation and proliferation in lung-associated lymph nodes [46]. Substantial recruitment of T cells to the airway lumen could generate a suitable T cell pool for early protection [14, 38]. In addition, there is increasing evidence of the protective efficacy of tissue resident memory T cells (TRM) located in the lung parenchyma against M. tuberculosis infection following mucosal immunization with BCG [47], rIAV [25] and a protein vaccine [13] contributes to protection. However, mucosal homing of TB vaccine-induced T cells may also occur without direct mucosal immunization with some TB vaccines. For example, both mucosal and peripheral immunization with the H56:CAF01 TB vaccine that contains the adjuvant, trehalose dibenalate, stimulated vaccine-specific Th1 and Th17 T cells that homed to the lung [48]. Recently, intravenous delivery of BCG in the non-human primate, Macaca mulatta, was demonstrated to stimulate the highest numbers of BCG-specific CD4+ and CD8+ memory T cells in the bronchoalveolar lavage (BAL) and lung lymph nodes and significantly enhanced protection against M. tuberculosis compared to aerosol or intradermal immunization with BCG [49]. Therefore, comprehensive investigation of the immune response in the lung compartments, BAL, and lung-associated lymph nodes is essential to elucidate the mechanism of protective immunity induced by mucosal or non-mucosal immunization with individual TB vaccines and when combined with BCG in prime-boost regimens.
Protective biomarkers for TB vaccines have proved difficult to define (reviewed in [50]). Prime-boost vaccination with BCG and rIAVs generated a high frequency of CD4+ T cells producing IFN-γ, IL-2, and TNF in the lungs and spleen. An increase in polyfunctional CD4+ T cells following BCG boosting with viral TB vaccines has been previously correlated with protection against M. tuberculosis with some [17, 51], but not all, TB vaccines [50]. For example, the increased level of polyfunctional CD4+ T cells following BCG boosting with the Nano-FP1 mucosal vaccine correlated with an early but not long-term increase in protective efficacy compared to BCG alone [52]. Although the mechanism is unclear, polyfunctional cells are proposed to be long-lived cells with potent anti-bacterial activity [53, 54]. In the current study, the significant increase in the frequency of p25-specific T cell producing multiple cytokines in the lungs generated by boosting BCG with rIAVs did not correlate with a significant increase in the protection of lungs from TB compared to the BCG alone. Improving the magnitude and the quality of T cell responses that are specific to a single antigen by mucosal boosting may not be sufficient to stimulate significant additional protection to BCG. The responses to BCG vaccine are complex and it is possible that BCG could induce “decoy immune” responses to dominant BCG antigens that prevent the induction of preventive immune response to boosting vaccines [55]. Therefore, the selection of antigens for boosting vaccines is important, and could include M. tuberculosis proteins that are either missing from BCG or are non-dominant antigens in BCG. For example, immunization with the M72 fusion protein vaccine containing the M. tuberculosis 32A and 39A antigens was found to provide 50% protection against developing active pulmonary TB in adults with latent TB infection [56]. The inclusion of additional M. tuberculosis antigens in the rIAV vaccines can be developed by manipulation of HA, NA and NS segment of rIAV to carry other M. tuberculosis antigens [23, 57, 58]. As a feasible model for an intranasal vaccine, further investigation on the cellular immune responses developed in the lungs following immunization with rIAV expressing multiple antigens may provide insight into the advantages of pulmonary TB vaccine.
Conclusion
This study demonstrates that mucosal vaccination with rIAV expressing single M. tuberculosis epitope could increase cytokine production by antigen-specific T cells following co-delivery or priming with parenteral BCG. Prime-boost immunization has greater potential as a strategy to improve BCG-induced protective immunity than simultaneous immunization. The inclusion of additional M. tuberculosis antigens in the rIAV may result in significant improvement on boosting the protective efficacy of BCG.
Supporting information
Single events were gated using forward scatter (FSC-A/FSC-H) and side scatter (SSC-A/SSC-H) flow plots. Dead cells were excluded and viable cells were gated for the lymphocyte population. The CD4+ T cell population was gated as CD3+CD4+ lymphocytes. For surface staining, T cell and differentiation activation markers were gated as CD44+CD69+ or CD4+KLRG1+ within the CD4+ T cell population. For ICS, the antigen-specific CD4+ T cells secreting IFN-γ, IL-2, or TNF were identified and analyzed using the FlowJo Bolean gating tool.
(TIFF)
C57BL/6 mice (n = 5) were simultaneously vaccinated with 5x105 CFU of s.c. BCG and 20 PFU of i.n. PR8-p25, s.c. BCG alone, or were left unvaccinated. The mice were challenged with M. tuberculosis 12 weeks later. KLRG1+CD44+CD4+ T cells in the lungs at two, four, and twenty weeks after M. tuberculosis challenge. Data are the means ± SEM. Statistically significant differences were determined by one way-ANOVA (p<0.05).
(TIFF)
C57BL/6 mice (n = 5) were vaccinated simultaneously with BCG s.c. and PR8-p25 i.n., and challenged with M. tuberculosis as in Fig 1A. The splenocytes were stimulated with p25 antigen and then analysed for intra-cellular cytokine production by flow cytometry. The frequency of p25-specific CD4+ T cells secreting IFN-γ, IL-2 and TNF were detrermined at (A) two, (B) four, and (C) twenty weeks after challenge. Data are the means ± SEM. Statistically significant differences between groups were determined by one-way ANOVA (*) or Kruskal-Wallis (#) (*p<0.05; **p<0.01).
(TIFF)
(A) Experimental design for BCG boosting with X31-p25 and PR8-p25 rIAVs. C57BL/6 mice (n = 4–6) were vaccinated with 5x105 CFU BCG s.c., vaccinated 12 weeks later with 104 PFU X31-p25 i.n. and then were boosted a second time at 18 weeks with 20 PFU PR8-p25 i.n. Other groups were vaccinated with BCG alone, the two rIAVs alone, or were left unvaccinated and antigen-specific T cell responses were assessed four weeks after the last vaccination. ELISpot analysis of IFN-γ producing T cells in the (B) lungs and (C) spleen following 18 hour stimulation with the relevant antigens. (A) The frequency of p25-specific CD4+ T cells in the (D) lungs and (E) spleen producing individual cytokines or combinations of IFN-γ, IL-2, and TNF were analysed by ICS flow cytometry using Boolean gating. Data are the means ± SEM. Statistically significant differences between groups were determined by one-way ANOVA (*) or Kruskal-Wallis (#) (*p<0.05;**p<0.01; ***p<0.001; ****p<0.0001).
(TIFF)
Acknowledgments
We thank staff in the Animal Facility and Advanced Flow Cytometry at the Centenary Institute for their assistance in these studies, and BEI Resources for provision of reagents.
Data Availability
The minimal datasets for manuscript and supporting files are available in a public repository with DOI: https://doi.org/10.6084/m9.figshare.16922908.v2.
Funding Statement
The National and Medical Research Council of Australia (Project Grant APP1044343), the NHMRC Centre for Research Excellence in Tuberculosis Control (APP1043225, APP1153493), and the NSW Government through its infrastructure grant to the Centenary Institute. https://www.nhmrc.gov.au. HM was a recipient of an Australia Award Scholarship from the Australian Department of Foreign Affairs and Trade, https://www.dfat.gov.au/people-to-people/australia-awards/Pages/australia-awards-scholarships.
References
- 1.Paulson T. A mortal foe. Nature. 2013;502:S2+. doi: 10.1038/502S2a [DOI] [PubMed] [Google Scholar]
- 2.WHO. Tuberculosis Global Report. 2020.
- 3.Fine PEM. Variation in protection by BCG: implications of and for heterologous immunity. The Lancet. 1995;346(8986):1339–45. [DOI] [PubMed] [Google Scholar]
- 4.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, et al. Protection by BCG Vaccine Against Tuberculosis: A Systematic Review of Randomized Controlled Trials. Clin Infect Dis. 2014;58(4):470–80. doi: 10.1093/cid/cit790 [DOI] [PubMed] [Google Scholar]
- 5.Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne JAC, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assesst. 2013;17(37):1–vi. doi: 10.3310/hta17370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangtani P, Nguipdop-Djomo P, Keogh RH, Trinder L, Smith PG, Fine PE, et al. Observational study to estimate the changes in the effectiveness of bacillus Calmette-Guerin (BCG) vaccination with time since vaccination for preventing tuberculosis in the UK. Health Technol Assess. 2017;21(39):1–54. doi: 10.3310/hta21390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snow K, Yadav R, Denholm J, Sawyer S, Graham S. Tuberculosis among children, adolescents and young adults in the Philippines: a surveillance report. Western Pac Surveill Response J. 2018;9(4):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen P, Doherty TM. The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3(8):656–62. doi: 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- 9.Damjanovic D, Khera A, Afkhami S, Lai R, Zganiacz A, Jeyanathan M, et al. Age at Mycobacterium bovis BCG Priming Has Limited Impact on Anti-Tuberculosis Immunity Boosted by Respiratory Mucosal AdHu5Ag85A Immunization in a Murine Model. PLoS One. 2015;10(6):e0131175. doi: 10.1371/journal.pone.0131175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171(3):1602–9. doi: 10.4049/jimmunol.171.3.1602 [DOI] [PubMed] [Google Scholar]
- 11.Counoupas C, Pinto R, Nagalingam G, Britton WJ, Petrovsky N, Triccas JA. Delta inulin-based adjuvants promote the generation of polyfunctional CD4+ T cell responses and protection against Mycobacterium tuberculosis infection. Sci Rep. 2017;7(1):8582. doi: 10.1038/s41598-017-09119-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stylianou E, Paul MJ, Reljic R, McShane H. Mucosal delivery of tuberculosis vaccines: a review of current approaches and challenges. Expert Rev Vaccines. 2019;18(12):1271–84. doi: 10.1080/14760584.2019.1692657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Counoupas C, Ferrell K, Ashhurst A, Bhattacharyya N, Nagalingam G, Feng CG, et al. Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependant protection against pulmonary tuberculosis. NPJ Vaccines.2020:5(1):105 doi: 10.1038/s41541-020-00255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beverley PCL, Sridhar S, Lalvani A, Tchilian EZ. Harnessing local and systemic immunity for vaccines against tuberculosis. Mucosal Immunol. 2014;7(1):20–6. doi: 10.1038/mi.2013.99 [DOI] [PubMed] [Google Scholar]
- 15.Tameris M, Hatherill M, Landry B, Scriba T, Snowden M, Lockhart S, et al. Safety and effi cacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. The Lancet. 2013; 381(9871):1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tameris M, Hokey DA, Nduba V, Sacarlal J, Laher F, Kiringa G, et al. A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants. Vaccine. 2015;33(25):2944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forbes EK, Sander C, Ronan EO, McShane H, Hill AVS, Beverley PCL, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181(7):4955–64. doi: 10.4049/jimmunol.181.7.4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White AD, Sibley L, Dennis MJ, Gooch K, Betts G, Edwards N, et al. Evaluation of the safety and immunogenicity of a candidate tuberculosis vaccine, MVA85A, delivered by aerosol to the lungs of macaques. Clin Vaccine Immunol. 2013;20(5):663–72. doi: 10.1128/CVI.00690-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tchilian EZ, Ronan EO, de Lara C, Lee LN, Franken KL, Vordermeier MH, et al. Simultaneous immunization against tuberculosis. PLoS One. 2011;6(11):e27477. doi: 10.1371/journal.pone.0027477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath CN, Shaler CR, Jeyanathan M, Zganiacz A, Xing Z. Mechanisms of delayed anti-tuberculosis protection in the lung of parenteral BCG-vaccinated hosts: A critical role of airway luminal T cells. Mucosal Immunol. 2012;5(4):420–31. doi: 10.1038/mi.2012.19 [DOI] [PubMed] [Google Scholar]
- 21.Zeng H, Goldsmith CS, Maines TR, Belser JA, Gustin KM, Pekosz A, et al. Tropism and infectivity of influenza virus, including highly pathogenic Avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures. J Virol. 2013;87(5):2597–607. doi: 10.1128/JVI.02885-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71(12):1591–622. doi: 10.2165/11206860-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 23.Flórido M, Pillay R, Gillis CM, Xia Y, Turner SJ, Triccas JA, et al. Epitope-specific CD4+, but not CD8+, T-cell responses induced by recombinant influenza A viruses protect against Mycobacterium tuberculosis infection. Eur J Immunol. 2015;45(3):780–93. doi: 10.1002/eji.201444954 [DOI] [PubMed] [Google Scholar]
- 24.Muflihah H, Flórido M, Lin LCW, Xia Y, Triccas JA, Stambas J, et al. Sequential pulmonary immunization with heterologous recombinant influenza A virus tuberculosis vaccines protects against murine M. tuberculosis infection. Vaccine. 2018;36(18):2462–70. doi: 10.1016/j.vaccine.2018.03.037 [DOI] [PubMed] [Google Scholar]
- 25.Flórido M, Muflihah H, Lin LCW, Xia Y, Sierro F, Palendira M, et al. Pulmonary immunization with a recombinant influenza A virus vaccine induces lung-resident CD4+ memory T cells that are associated with protection against tuberculosis. Mucosal Immunol. 2018;11:1743–52. doi: 10.1038/s41385-018-0065-9 [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci USA. 2000;97(11):6108–13. doi: 10.1073/pnas.100133697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cukalac T, Moffat JM, Venturi V, Davenport MP, Doherty PC, Turner SJ, et al. Narrowed TCR diversity for immunised mice challenged with recombinant influenza A-HIV Env311–320 virus. Vaccine. 2009;27(48):6755–61. doi: 10.1016/j.vaccine.2009.08.079 [DOI] [PubMed] [Google Scholar]
- 28.La Gruta NL, Kedzierska K, Pang K, Davenport M, Chen WS, Turner SJ, et al. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc Natl Acad Sci USA. 2006;103(4):994–9. doi: 10.1073/pnas.0510429103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Kumar A, Miao H, Holden-Wiltse J, Mosmann TR, Livingstone AM, et al. Modeling of influenza-specific CD8+ T cells during the primary response indicates that the spleen is a major source of effectors. J Immunol. 2011;187(9):4474–82. doi: 10.4049/jimmunol.1101443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao FF, Mahmuda S, Pinto R, Triccas JA, West NP, Britton WJ. The secreted lipoprotein, MPT83, of Mycobacterium tuberculosis is recognized during human tuberculosis and stimulates protective immunity in mice. PLoS One. 2012;7(5):e34991. doi: 10.1371/journal.pone.0034991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard Gs, Moon JJ, Jenkins MK, et al. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci. 2010;107(45):19408–13. doi: 10.1073/pnas.1006298107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70(8):4501–9. doi: 10.1128/IAI.70.8.4501-4509.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper AM. Cell-Mediated Immune Responses in Tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamath AT, Groat NL, Bean AG, Britton WJ. Protective effect of DNA immunization against mycobacterial infection is associated with the early emergence of interferon-gamma (IFN-gamma)-secreting lymphocytes. Clin Exp Immunol. 2000;120(3):476–82. doi: 10.1046/j.1365-2249.2000.01240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim TS, Hufford MM, Sun J, Fu Y-X, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207(6):1161–72. doi: 10.1084/jem.20092017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker KB, Guo M, Guo Y, Poecheim J, Velmurugan K, Schrager LK. Novel approaches to preclinical research and TB vaccine development. Tuberculosis. 2016;99, Supplement 1:S12–S5. [DOI] [PubMed] [Google Scholar]
- 38.Jeyanathan M, Heriazon A, Xing Z. Airway luminal T cells: A newcomer on the stage of TB vaccination strategies. Trends in Immunology. 2010;31(7):247–52. doi: 10.1016/j.it.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 39.Dean GS, Clifford D, Whelan AO, Tchilian EZ, Beverley PC, Salguero FJ, et al. Protection Induced by Simultaneous Subcutaneous and Endobronchial Vaccination with BCG/BCG and BCG/Adenovirus Expressing Antigen 85A against Mycobacterium bovis in Cattle. PLoS One. 2015;10(11):e0142270. doi: 10.1371/journal.pone.0142270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect Immun. 2000;68(12):6954–61. doi: 10.1128/IAI.68.12.6954-6961.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 2007;85(2):103–11. doi: 10.1038/sj.icb.7100027 [DOI] [PubMed] [Google Scholar]
- 42.Ashhurst AS, McDonald DM, Hanna CC, Stanojevic VA, Britton WJ, Payne RJ. Mucosal Vaccination with a Self-Adjuvanted Lipopeptide Is Immunogenic and Protective against Mycobacterium tuberculosis. Journal of Medicinal Chemistry. 2019;62(17):8080–9. doi: 10.1021/acs.jmedchem.9b00832 [DOI] [PubMed] [Google Scholar]
- 43.Hanna CC, Ashhurst AS, Quan D, Maxwell J, Britton WJ, Payne RJ. Synthetic protein conjugate vaccines provide protection against Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2021;118 (4):e2013730118. doi: 10.1073/pnas.2013730118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyne AS, Chan JGY, Shanahan ER, Atmosukarto I, Chan H-K, Britton WJ, et al. TLR2-targeted secreted proteins from Mycobacterium tuberculosis are protective as powdered pulmonary vaccines. Vaccine. 2013;31(40):4322–9. doi: 10.1016/j.vaccine.2013.07.022 [DOI] [PubMed] [Google Scholar]
- 45.Aguilo N, Alvarez-Arguedas S, Uranga S, Marinova D, Monzon M, Badiola J, et al. Pulmonary but Not Subcutaneous Delivery of BCG Vaccine Confers Protection to Tuberculosis-Susceptible Mice by an Interleukin 17-Dependent Mechanism. J Infect Dis. 2016;213(5):831–9. doi: 10.1093/infdis/jiv503 [DOI] [PubMed] [Google Scholar]
- 46.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205(1):105–15. doi: 10.1084/jem.20071367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perdomo C, Zedler U, Kühl AA, Lozza L, Saikali P, Sander LE, et al. Mucosal BCG Vaccination Induces Protective Lung-Resident Memory T Cell Populations against Tuberculosis. mBio. 2016;7(6):e01686–16. doi: 10.1128/mBio.01686-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodworth JS, Christensen D, Cassidy JP, Agger EM, Mortensen R, Andersen P. Mucosal boosting of H56:CAF01 immunization promotes lung-localized T cells and an accelerated pulmonary response to Mycobacterium tuberculosis infection without enhancing vaccine protection. Mucosal Immunol. 2019;12(3):816–26. doi: 10.1038/s41385-019-0145-5 [DOI] [PubMed] [Google Scholar]
- 49.Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH, Hughes TK, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577(7788):95–102. doi: 10.1038/s41586-019-1817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Counoupas C, Triccas JA, Britton WJ. Deciphering protective immunity against tuberculosis: implications for vaccine development. Expert Rev Vaccines. 2019;18(4):353–64. doi: 10.1080/14760584.2019.1585246 [DOI] [PubMed] [Google Scholar]
- 51.Derrick SC, Yabe IM, Yang A, Morris SL. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine. 2011;29(16):2902–9. doi: 10.1016/j.vaccine.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 52.Martínez-Pérez A, Igea A, Estévez O, Ferreira CM, Torrado E, Castro AG, et al. Changes in the Immune Phenotype and Gene Expression Profile Driven by a Novel Tuberculosis Nanovaccine: Short and Long-Term Post-immunization. Front Immunol. 2021;11:3521. doi: 10.3389/fimmu.2020.589863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindenstrom T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182(12):8047–55. doi: 10.4049/jimmunol.0801592 [DOI] [PubMed] [Google Scholar]
- 54.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13(7):843–50. doi: 10.1038/nm1592 [DOI] [PubMed] [Google Scholar]
- 55.Ivanyi J. Tuberculosis vaccination needs to avoid ‘decoy’ immune reactions. Tuberculosis. 2021;126:102021. doi: 10.1016/j.tube.2020.102021 [DOI] [PubMed] [Google Scholar]
- 56.Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N Engl J Med. 2019;381(25):2429–39. doi: 10.1056/NEJMoa1909953 [DOI] [PubMed] [Google Scholar]
- 57.Muster T, Ferko B, Klima A, Purtscher M, Trkola A, Schulz P, et al. Mucosal model of immunization against human immunodeficiency virus type 1 with a chimeric influenza virus. J Virol. 1995;69(11):6678–86. doi: 10.1128/JVI.69.11.6678-6686.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stukova MA, Sereinig S, Zabolotnyh NV, Ferko B, Kittel C, Romanova J, et al. Vaccine potential of influenza vectors expressing Mycobacterium tuberculosis ESAT-6 protein. Tuberculosis. 2006;86(3–4):236–46. doi: 10.1016/j.tube.2006.01.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Single events were gated using forward scatter (FSC-A/FSC-H) and side scatter (SSC-A/SSC-H) flow plots. Dead cells were excluded and viable cells were gated for the lymphocyte population. The CD4+ T cell population was gated as CD3+CD4+ lymphocytes. For surface staining, T cell and differentiation activation markers were gated as CD44+CD69+ or CD4+KLRG1+ within the CD4+ T cell population. For ICS, the antigen-specific CD4+ T cells secreting IFN-γ, IL-2, or TNF were identified and analyzed using the FlowJo Bolean gating tool.
(TIFF)
C57BL/6 mice (n = 5) were simultaneously vaccinated with 5x105 CFU of s.c. BCG and 20 PFU of i.n. PR8-p25, s.c. BCG alone, or were left unvaccinated. The mice were challenged with M. tuberculosis 12 weeks later. KLRG1+CD44+CD4+ T cells in the lungs at two, four, and twenty weeks after M. tuberculosis challenge. Data are the means ± SEM. Statistically significant differences were determined by one way-ANOVA (p<0.05).
(TIFF)
C57BL/6 mice (n = 5) were vaccinated simultaneously with BCG s.c. and PR8-p25 i.n., and challenged with M. tuberculosis as in Fig 1A. The splenocytes were stimulated with p25 antigen and then analysed for intra-cellular cytokine production by flow cytometry. The frequency of p25-specific CD4+ T cells secreting IFN-γ, IL-2 and TNF were detrermined at (A) two, (B) four, and (C) twenty weeks after challenge. Data are the means ± SEM. Statistically significant differences between groups were determined by one-way ANOVA (*) or Kruskal-Wallis (#) (*p<0.05; **p<0.01).
(TIFF)
(A) Experimental design for BCG boosting with X31-p25 and PR8-p25 rIAVs. C57BL/6 mice (n = 4–6) were vaccinated with 5x105 CFU BCG s.c., vaccinated 12 weeks later with 104 PFU X31-p25 i.n. and then were boosted a second time at 18 weeks with 20 PFU PR8-p25 i.n. Other groups were vaccinated with BCG alone, the two rIAVs alone, or were left unvaccinated and antigen-specific T cell responses were assessed four weeks after the last vaccination. ELISpot analysis of IFN-γ producing T cells in the (B) lungs and (C) spleen following 18 hour stimulation with the relevant antigens. (A) The frequency of p25-specific CD4+ T cells in the (D) lungs and (E) spleen producing individual cytokines or combinations of IFN-γ, IL-2, and TNF were analysed by ICS flow cytometry using Boolean gating. Data are the means ± SEM. Statistically significant differences between groups were determined by one-way ANOVA (*) or Kruskal-Wallis (#) (*p<0.05;**p<0.01; ***p<0.001; ****p<0.0001).
(TIFF)
Data Availability Statement
The minimal datasets for manuscript and supporting files are available in a public repository with DOI: https://doi.org/10.6084/m9.figshare.16922908.v2.