Abstract
We compared lesion-based sensitivity of dual-time-point FDG-PET/CT, bone scintigraphy (BS), and low-dose CT (LDCT) for detection of various types of bone metastases in patients with metastatic breast cancer. Prospectively, we included 18 patients with recurrent breast cancer who underwent dual-time-point FDG-PET/CT with LDCT and BS within a median time interval of three days. A total of 488 bone lesions were detected on any of the modalities and were categorized by the LDCT into osteolytic, osteosclerotic, mixed morphologic, and CT-negative lesions. Lesion-based sensitivity was 98.2% (95.4–99.3) and 98.8% (96.8–99.5) for early and delayed FDG-PET/CT, respectively, compared with 79.9% (51.1–93.8) for LDCT, 76.0% (36.3–94.6) for BS, and 98.6% (95.4–99.6) for the combined BS+LDCT. BS detected only 51.2% of osteolytic lesions which was significantly lower than other metastatic types. SUVs were significantly higher for all lesion types on delayed scans than on early scans (P<0.0001). Osteolytic and mixed-type lesions had higher SUVs than osteosclerotic and CT-negative metastases at both time-points. FDG-PET/CT had significantly higher lesion-based sensitivity than LDCT and BS, while a combination of the two yielded sensitivity comparable to that of FDG-PET/CT. Therefore, FDG-PET/CT could be considered as a sensitive one-stop-shop in case of clinical suspicion of bone metastases in breast cancer patients.
Introduction
Breast cancer mortality is almost exclusively a result of distant metastatic disease [1] with survival rates of 99% for patients with localized disease and only 25% for patients with metastatic disease [2]. Bone is the most common site of metastasis in patients with breast cancer, occurring in up to 70% of patients with advanced disease [1,3]. This leads to chronic metastatic bone disease in many, since relevant treatment can often delay progression [4]. Bone metastases seem to originate in bone marrow, and structural changes in the bone will occur in a postponed phase [5]. Structural changes in the bone can be detected and classified as osteolytic, osteosclerotic, or mixed (osteolytic/osteosclerotic) metastases [6]. Planar bone scintigraphy (BS) reflects osteoblastic activity and is probably superior in detecting osteosclerotic and mixed metastases than other types of bone metastases [7]. BS and computed tomography (CT) are the most often used modalities of conventional imaging and are recommended in current guidelines for the detection of bone metastases in breast cancer [8,9].
[18F]-fluorodeoxyglucose-Positron Emission Tomography with integrated computed-tomography (FDG-PET/CT) reflects glucose metabolism, and thus this modality may facilitate detection of all types of bone metastases including bone marrow metastases [10]. It is well known that breast cancer patients represent various types of bone metastatic lesions, but the literature is equivocal regarding whether FDG-PET/CT or conventional imaging is superior in detection of bone metastases [11–13]. However, it has often been pointed out that FDG-PET/CT may be superior in detecting osteolytic rather than osteosclerotic bone lesions in breast cancer patients [13–17].
The ability to distinguish malignant from benign lesions with FDG-PET/CT may be improved by delayed imaging [18,19]. Also, delayed imaging using FDG may therefore be particularly useful for diagnosing low metabolic malignancies such as breast cancer and especially for the less detectable lesions on regular FDG-PET/CT such as osteosclerotic bone metastases [19].
Considering the fact that bone involvement is the predilection site for metastasis in breast cancer patients, and when the progression of bone metastasis is not detected and taken care of, the risk of developing skeletal-related events increases and result in higher risk of mortality [20,21]. We hypothesized that delayed FDG-PET/CT scan would act more accurately regarding the detection of bone metastatic lesions than on early FDG-PET/CT and conventional imaging. Therefore, we aimed to investigate the lesion-based sensitivity of dual-time-point FDG-PET/CT compared with BS and low-dose CT (LDCT) for the detection of bone metastases in breast cancer patients. Furthermore, we aimed to determine FDG standardized uptake values (SUVs) in different types of bone lesions at early and delayed images; however the small sample size is a critical limitation to this specific aim.
Materials and methods
Study design and subjects
This prospective study was carried out at the Department of Nuclear Medicine of Odense University Hospital (Odense, Denmark). A written informed consent form was obtained from all included patients and the study protocol was approved by the ethics committee (S-20110138) at the University of Southern Denmark (Odense, Denmark), which was in compliance with good clinical practice and the Declaration of Helsinki (Registration code at ClinicalTrials.gov: NCT01552655).
In a prospective comparative design, patients with suspected breast cancer recurrence or with verified local recurrence and potential distant disease, referred from the Department of Oncology between 2011 (Dec) and 2014 (Sep), were considered eligible for the inclusion. Exclusion criteria were history of concurrent malignancy, age younger than 18 years, pregnancy or breast-feeding, diagnosed diabetes mellitus, or considered unable to cooperate. All of the patients who accepted participation were asked to undergo dual-time-point FDG-PET/CT and whole-body BS, within a median time interval of three days (range: 0–24). The patients with histopathologically confirmed metastatic breast cancer with approved bone involvement were included in analysis. All patients initiated systemic therapy based on the biopsy-verified diagnosis and according to national oncologic guidelines for metastatic breast cancer [22]. Overall patient-based accuracy results of this study have been published previously [23], and the current analysis considered lesion-based sensitivity focusing on various types of bone metastases along with respective quantification measures reflecting FDG-uptake.
FDG-PET/CT protocol
Before the FDG-PET/CT scan, patients were required to fast for at least 6 h, after which their blood sugar levels were measured. PET/CT was considered acceptable at levels up to 144 mg/dL. The 18F-FDG tracer was administered intravenously with an activity of 4 MBq per kg of body weight. The patients were requested to rest for 60 min (±5 min) p.i. before PET/CT imaging was performed from the top of the skull to the proximal femur [24]. The second scan was performed in the same manner after 180 min (±5 min) [25]. The total examination time was approximately 210 min for each patient. All scans were performed using either the Discovery STE (VCT) equipped with BGO crystals or the Discovery RX equipped with LYSO(Ce) crystals (GE Healthcare Systems, Chicago, IL, USA). PET was performed over 7–9 bed positions in 3D, with a scan time of 2.5 min per bed position for 1-h images and 3.5 min per bed position for 3-h images. PET images were reconstructed iteratively, with ordered subset expectation maximization, 2 iterations, and 21 or 28 subsets.
LDCT protocol
Low-dose CT imaging, with two scout views for both exams, was performed using either GE Discovery STE or Discovery RX (GE Medical Systems, Milwaukee, WI), at 140 kV with SmartmA tube current modulation (noise index 35, 0.8 seconds per rotation, slice thickness 3.75 mm) and used for attenuation correction and anatomic orientation followed by a 3D PET scan (OSEM iterative reconstruction, slice thickness 3.75 mm) [26].
Bone scintigraphy
The patients were injected with 700 MBq (0.019 Ci) Technetium-99m-3,3-disphosphono-1,2-propanodicarboxylic acid (Tc-99m-DPD) three to four hours prior to whole-body imaging. In the waiting period, the patients were asked to drink approximately 1 liter of clear liquids. The scan was performed on a Skylight or PRISM XP2000 gamma camera (Philips Medical, Surrey, UK) with the following parameters: LEHR collimator, energy window 140 keV ± 20%, matrix 256 x 1024, scan speed 14 cm/min.
Reference standard
Suspected recurrence was verified by biopsy as the reference standard. All patients treated explicitly for bone metastasis, typically with bisphosphonates, were categorized as having bone metastases. Follow-up time was defined as the time interval between the date of the first scan and the date of the latest registered clinical contact to the Departments of Clinical Oncology or Breast Surgery.
Image interpretation
All FDG-positive bone lesions present on 1h or 3h FDG-PET/CT scans were counted by single group of nuclear medicine specialists through daily practice. BS studies were examined to identify the FDG-positive lesions and potential additional lesions. An experienced radiologist categorized metastatic bone lesions into osteolytic (partially ill-defined margin with pattern of bone resorption and focal bone destruction), osteosclerotic (dense and often well-defined margin with pattern of bone formation and ossification), and mixed subtypes based on radiographic features of the LDCT. FDG-positive lesions without changes on LDCT were designated as “CT-negative metastases” [27,28]. All bone lesions were categorized as positive in patients with confluent FDG-uptake in bone on FDG-PET/CT or confluent Tc-99m-DPD uptake on BS (super scan). All bone lesions detected by FDG-PET/CT, LDCT, or BS were considered positive, although degenerative lesions in large joints were not included. The radiologist had the LDCT and BS scans in two separate screens (side by side) for the lesion categorization through LDCT+CT.
Lesion-based sensitivity and quantification
Lesion-based sensitivity with 95% confidence intervals (95% CIs) was calculated for all three modalities and for the combined LDCT+BS. FDG-avid bone lesions were quantified using dedicated software (ROVER, ABX, Radeberg, Germany) to determine maximum and mean SUVs and the latter corrected for partial volume effect (SUVmax, SUVmean, cSUVmean). Segmentation of bone lesions was obtained by manually placing a three-dimensional mask on all suspected lesions and delineating the region of interest (ROI) by using a threshold of 40% of the maximum value of the three-dimensional mask. We included a minimum ROI volume of one cubic centimeter and excluded ROI intersections [29]. The software then automatically calculated metabolically active volume (MAV) for each ROI [30]. The retention index of each lesion was calculated as follows [19]:
Statistical analyses
Descriptive statistics were performed according to the data type (continuous: median and range; categorical: frequencies and percentages). Simple linear regression was used to test for differences in SUVs and MAV between different bone lesion types with 3h and 1h FDG-PET/CT imaging. Clustered sandwich estimators were used in both linear regression and derivation of 95% CIs to account for clustered data. P-values of <0.05 were considered significant. All statistical analyses were conducted with STATA/MP 16 (StataCorp, College Station, 77845 Texas, USA).
Results
Demographic information
Eighteen patients with a median age of 61.5 years (range: 38–76) had confirmed bone recurrence; 7 by bone biopsies and 11 by biopsies from other sites with confirmation of bone involvement by further imaging, or retrospectively observed progression in bone lesions on later scans. The patients were followed-up for a median period of 19 months (range: 1–35 months). Baseline characteristics of included patients are summarized in Table 1.
Table 1. Baseline characteristics of included patients with metastatic breast cancer.
| Variable | Results* | Variable | Results* | ||
|---|---|---|---|---|---|
| Primary tumor size (mm) | 21 (10–70) | Estrogen receptor status | Positive | 15 (83.3) | |
| Time until relapse** (month) | 60 (0–324) | Negative | 2 (11.1) | ||
| Histopathology | Invasive ductal carcinoma | 15 (83.3) | Unknown | 1 (5.6) | |
| Invasive lobular carcinoma | 3 (16.7) | Herceptin-2 receptor status | Positive | 3 (16.7) | |
| Surgery type | Lumpectomy | 7 (38.9) | Negative | 14 (77.8) | |
| Mastectomy | 11 (61.1) | Unknown | 1 (5.6) | ||
| Treatment protocol | Chemotherapy | 13 (72.2) | Malignancy Grade | 1 | 3 (16.7) |
| Hormone therapy | 12 (66.7) | 2 | 7 (38.9) | ||
| Radiotherapy | 15 (83.3) | 3 | 8 (34.4) | ||
*Data are shown as frequency (%) and median (interquartile range).
**Time period between primary breast cancer and diagnosis of metastasis.
Lesion-based sensitivity
A total of 488 bone lesions were detected by any modality with a median of five lesions per patient (range: 1–99). Three FDG-PET/CT studies did not include the head by technical mistake. Four patients had a super scan on BS, and seven patients (39.9%) had more than ten bone lesions. FDG-PET did not identify five osteolytic skull lesions, four of which were detected by both LDCT and BS, one by LDCT only.
The lesion-based sensitivity for each modality is presented in Table 2. Early and delayed FDG-PET/CT images had higher sensitivity compared with BS and LDCT separately, while they showed almost the same sensitivity when compared with the combined BS+LDCT. Sixty-two of 98 (63%) CT-negative lesions on LDCT were located in the ribs, humerus, scapula, or clavicles.
Table 2. Types of detected lesions and lesion-based sensitivity by each modality.
|
Detected lesions Modality |
Lesion type | Lesion-based sensitivity(95% CI) | ||||
|---|---|---|---|---|---|---|
| Osteolytic | Osteosclerotic | Mixed | CT-negative | All lesions | ||
| LDCT | 213 | 80 | 97 | 0 | 390 | 79.9 (51.1–93.8) |
| BS | 104 | 79 | 97 | 91 | 371 | 76.0 (36.3–94.6) |
| FDG-PET/CT (1h) | 206 | 78 | 97 | 98 | 479 | 98.2 (95.4–99.3) |
| FDG-PET/CT (3h) | 208 | 79 | 97 | 98 | 482 | 98.8 (96.8–99.5) |
| LDCT+ BS | 213 | 80 | 97 | 91 | 481 | 98.6 (95.4–99.6) |
CI: Confidence interval; LDCT: Low-dose computed tomography; BS: Bone scintigraphy; FDG-PET/CT, Fluorodeoxyglucose positron emission tomography with integrated computed-tomography.
BS detected significantly fewer osteolytic lesions (104/213) than other bone metastatic lesions (267/275). Also, BS could not identify any lesion in three patients and detected only a few of several lesions (1/17 and 8/87) in two patients (Figs 1 and 2).
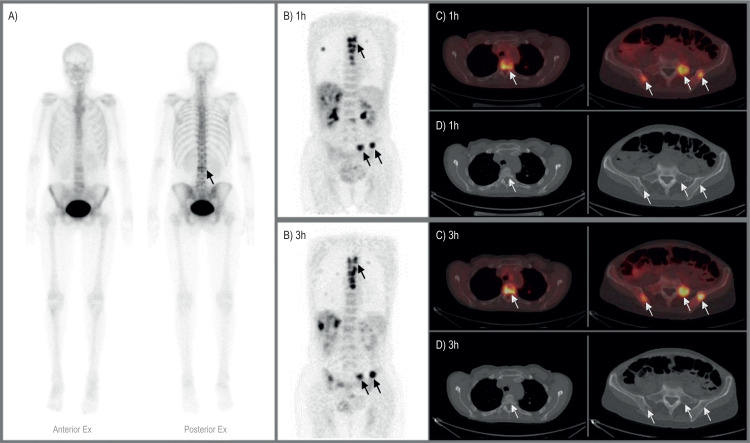
Fig 1. A 54-year-old woman with true-positive bone metastases.
A) Whole-body bone scintigraphy shows only one area with increased uptake of 99mTc-DPD (arrow). B) FDG-PET 1h and 3h images show multiple osseous metastases in the spine and the pelvis. C) Axial FDG-PET/CT images demonstrating FDG-avid lesions in the spine, sacrum, and iliac bones. D) Axial CT images at the same level as C show osteolytic changes.
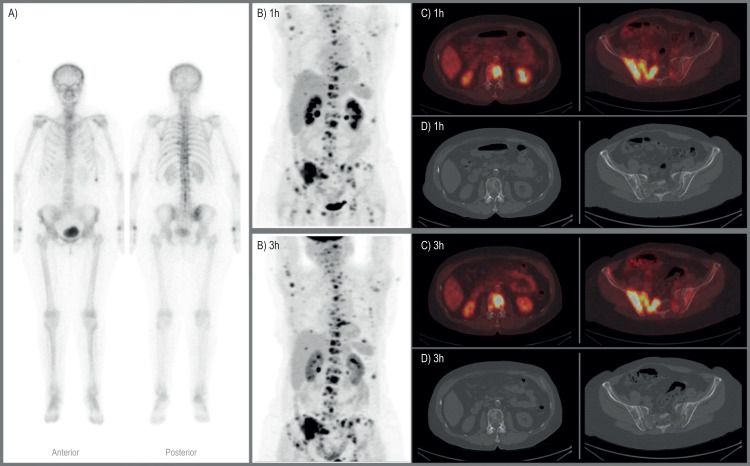
Fig 2. A 71-year-old woman with true-positive bone metastases.
A) Whole-body bone scintigraphy shows few areas with increased uptake of 99mTc-DPD osteolytic lesions. B) FDG-PET images show multiple osseous metastases in the skeleton and metastases in other organs on 1h and 3h images. C) Axial 1h and 3h FDG-PET/CT images showing FDG-avid lesions in the spine, sacrum, and iliac bones. D) Axial CT images at the same level as C show osteolytic changes.
One patient with bone metastatic lobular carcinoma presented with diffuse osteosclerotic changes in the skeleton that did not take up FDG. The diffuse appearance made the lesions uncountable. She had seven lytic lesions that were FDG-avid and therefore counted as true positive on FDG-PET/CT.
Quantification findings
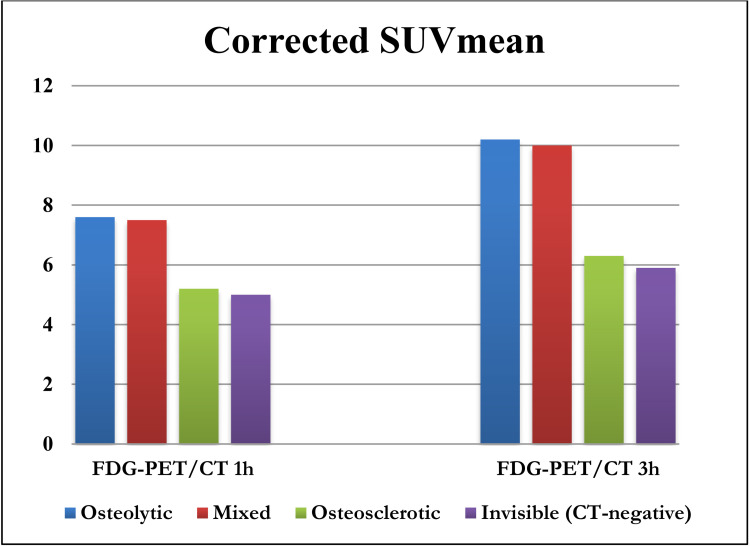
Seven lesions were located in the skull and were excluded from quantification analyses due to scatter from high FDG-uptake in the cerebrum. The remaining 481 lesions showed a statistically significant 1h to 3h increase in SUVmax, SUVmean, and cSUVmean for all lesion types (P<0.0001, Table 3). Osteolytic and mixed-type lesions had higher SUVs than osteosclerotic and CT-negative metastases at both time-points. The 1h to 3h increase in SUVs was lower for osteosclerotic than other lesion types. The median retention index was significantly lower in osteosclerotic lesions compared with other types of lesions (P = 0.006). Comparison of early and delayed cSUVmean through different lesion types is shown in Fig 3.
Table 3. FDG uptake and metabolically active volume in types of bone metastasis*.
| Lesion type Quantitative measure |
Osteolytic (n = 207) | Osteosclerotic (n = 79) | Mixed (n = 97) | CT-negative (n = 98) | All lesions (n = 481) | |
|---|---|---|---|---|---|---|
| SUVmax | 1h | 6.0 (1.2–16.6) | 4.4 (1.5–11.8) | 6.5 (3.1–14.9) | 3.8 (1.7–11.5) | 5.3 (1.2–16.6) |
| 3h | 7.7 (1.8–21.2) | 5.5 (2.3–15.5) | 8.4 (2.7–21.1) | 5.1 (2.1–14.4) | 6.6 (1.8–21.2) | |
| Δ | 1.5 (-1.4–7.1) | 0.9 (-0.6–5.2) | 2.2 (-4.8–7.3) | 1.2 (-0.8–5.0) | 1.4 (-4.8–7.3) | |
| SUVmean | 1h | 4.0 (0.9–10.0) | 3.1 (1.1–6.3) | 4.2 (1.8–9.2) | 2.6 (1.0–7.4) | 3.6 (0.9–10.0) |
| 3h | 5.1 (1.0–13.1) | 3.8 (1.4–8.1) | 5.1 (2.0–11.7) | 3.1 (1.3–9.6) | 4.5 (1.0–13.1) | |
| Δ | 0.9 (-0.9–4.7) | 0.6 (-0.6–2.6) | 1.2 (-2.7–4.1) | 0.7 (-0.2–4.6) | 0.9 (-2.7–4.7) | |
| cSUVmean | 1h | 7.6 (0.9–36.4) | 5.2 (1.6–17.0) | 7.5 (2.1–19.9) | 5.0 (1.2–15.9) | 6.7 (0.9–36.4) |
| 3h | 10.2 (1.1–26.0) | 6.3 (2.0–25.8) | 10.0 (2.9–36.3) | 5.9 (1.7–19.1) | 4.5 (1.0–13.1) | |
| Δ | 2.1 (-18.5–14.1) | 1.2 (-5.9–15.5) | 3.0 (-12.7–21.7) | 1.2 (-10.5–11.9) | 1.8 (-18.5–21.7) | |
| Metabolically active volume (cm 3 ) | 1h | 1.8 (0.1–65.1) | 4.2 (0.2–31.0) | 3.6 (0.4–61.9) | 2.4 (0.3–26.6) | 2.5 (0.1–65.1) |
| 3h | 1.9 (0.2–71.7) | 3.9 (0.3–35.5) | 3.4 (0.4–52.3) | 2.0 (0.5–21.7) | 2.3 (0.2–71.7) | |
| Δ | -0.1 (-34.6–6.6) | 0.0 (-5.6–8.3) | -0.4 (-18.2–4.3) | -0.1 (-14.5–2.8) | -0.1 (-34.6–8.3) | |
| Retention index (%) | 25.0 (-28.0–125.0) | 20.0 (-14.6–81.0) | 34.5 (-57.1.102.1) | 29.4 (-13.6–166.7) | 27.7 (-57.1–166.7) | |
SUV: Standardized uptake value; cSUVmean: Corrected SUVmean.
*Data was shown as median (interquartile range).
Fig 3. Comparison of early and delayed corrected standardized uptake value for partial volume within different lesion types (FDG-PET/CT: Fluorodeoxyglucose positron emission tomography with integrated computed-tomography; Corrected-SUVmean: Corrected standardized uptake value for partial volume).
Discussion
FDG-PET/CT was superior to BS and LDCT regarding the detection of bone metastases in patients with recurrent metastatic breast cancer. This modality had significantly higher lesion-based sensitivity for bone recurrence than LDCT or BS alone, in particular, because it was much better than BS for the detection of osteolytic lesions and superior to LDCT in the detection of lesions which were deemed invisible by LDCT (CT-negative metastases). Early and delayed FDG-PET/CT images showed almost the same sensitivity (98.2% vs. 98.8%). Although all types of bone metastases showed increased FDG-uptake and were equally detectable at 1h and 3h images and with high respective lesion-based sensitivities, FDG-PET/CT parameters (SUVs and retention index) were significantly lower in osteosclerotic lesions compared with the others.
Strengths of our study were the prospective design, which all patients were treatment-naive concerning bone metastases, and that patients acted as their own controls during the follow-up time. Besides, the short time interval between imaging procedures, using of the experienced readers for each specific modality, the implication of dedicated software for PET quantification, and LDCT of the same field of view as with FDG-PET/CT could count as the advantages of the current study. Limitations were a relatively small sample size with a skewed range of lesions per patient, that image modalities could not be blinded for the lesion-based analysis that osteosclerotic lesions were more difficult to characterize due to their more diffuse appearance and that only a single biopsy from each patient dictated the origin of the majority of lesions. Furthermore, BS was without SPECT/CT and that 18F-Sodium Fluoride PET/CT and contrast-enhanced CT were not included in the comparison.
In a retrospective Japanese study of 88 breast cancer patients with bone metastasis, they found higher lesion-based sensitivity (94%) for FDG-PET/CT than for CT and BS (77% and 89%, respectively), which were in line with the results of our study. However, they found a relatively lower detection of osteosclerotic lesions for FDG-PET/CT than other lesion types [13], which was not confirmed by our study. Additional to the results of previous studies regarding the superiority of FDG-PET/CT in detection of bone metastases compared to BS [11,12,15], our study showed that LDCT and BS combined could provide sensitivity equal to that of FDG-PET/CT in detection of skeletal metastases.
FDG-PET/CT has also previously been reported to be superior to BS in the detection of osteolytic and less sensitive in detecting osteosclerotic lesions [13,14]. However, in retrospective studies, it may be likely that some patients are not treatment-naive, and in that case, FDG-negative osteosclerotic lesions may represent bone healing. We detected FDG-positive osteosclerotic lesions although we did find higher FDG-uptake in osteolytic and mixed metastases than in osteosclerotic and CT-negative lesions, thus supporting previous findings to some degree. The delayed FDG-PET/CT images had in general better tumor-to-background discrimination and improved image quality in agreement with previous reports on delayed imaging [31]. Nonetheless, the improved image quality and higher SUVs at delayed scans did not translate into significantly higher detection sensitivity.
In a recently published paper, comparing clinical management of metastatic breast cancer patients undergoing BS, contrast-enhanced CT, and FDG-PET/CT regarding the assessment of bone metastasis, it has been shown that FDG-PET/CT resulted in clinically relevant management differences in 16% of patients compared with BS [32]. Since it has already been approved that early detection of bone metastasis plays an important role in the survival of patients with metastatic breast cancer [33], the clinical application of FDG-PET/CT may guide the treatment better than when using conventional imaging [34,35].
Therefore, proper detection of bone metastases is crucial for the choice of proper treatment. Previous studies showed higher patient-based sensitivities with FDG-PET/CT than with conventional imaging when diagnosing bone recurrence [23,36]. These findings suggest that oligometastatic bone disease can be detected earlier by FDG-PET/CT than by conventional imaging. Also, patient-based specificity was improved by FDG-PET/CT, which may significantly benefit patients and reduce management costs in this particular patient group.
Our results indicated that FDG-PET/CT, compared with conventional imaging, could act more sensitive regarding the detection of bone metastasis and distinguishing the different types of bone lesions. However, these results need to be approved by prospective larger studies which include 18F-Sodium Fluoride PET/CT and contrast-enhanced CT to the comparison in order to achieve a firm conclusion about the most sensitive modality to detect bone metastasis. Additional information derived from follow-up scans could provide relevant results on diagnostic accuracy of FDG-PET/CT in response evaluation of skeletal metastases and needs to be considered in future studies.
Conclusions
FDG-PET/CT had significantly higher lesion-based sensitivity than low-dose CT or bone scintigraphy alone and thus, may act more clinically useful as a one-stop-shop for diagnosing bone recurrence in breast cancer patients. FDG-PET/CT had significantly higher sensitivity than BS and LDCT for the detection of osteolytic metastases and lesions appearing in the bone marrow, respectively. Delayed FDG-PET/CT imaging did not improve lesion-based sensitivity significantly.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was partially supported by the Independent Research Fund Denmark (DFF-7016-00359) and the Centre of Personalized Response Monitoring in Oncology at Odense University Hospital (Denmark). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Monteran L, Ershaid N, Sabah I, Fahoum I, Zait Y, Shani O, et al. Bone metastasis is associated with acquisition of mesenchymal phenotype and immune suppression in a model of spontaneous breast cancer metastasis. Sci Rep. 2020;10(1):13838. Epub 2020/08/17. doi: 10.1038/s41598-020-70788-3 ; PubMed Central PMCID: PMC7429866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alteri R, Bertaut T, Brooks D, Chambers W, Chang E, DeSantis C, et al. Cancer facts & figures 2015. American Cancer Society, Atlanta. 2015:58–72. [Google Scholar]
- 3.Fornetti J, Welm AL, Stewart SA. Understanding the Bone in Cancer Metastasis. J Bone Miner Res. 2018;33(12):2099–113. Epub 2018/11/27. doi: 10.1002/jbmr.3618 . [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(20 Pt 2):6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931 . [DOI] [PubMed] [Google Scholar]
- 5.Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J, on behalf of the EGWG. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014. doi: 10.1093/annonc/mdu103 . [DOI] [PubMed] [Google Scholar]
- 6.Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, et al. Bone Metastases: An Overview. Oncol Rev. 2017;11(1):321. Epub 2017/06/07. doi: 10.4081/oncol.2017.321 ; PubMed Central PMCID: PMC5444408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook GJ, Azad GK, Goh V. Imaging Bone Metastases in Breast Cancer: Staging and Response Assessment. J Nucl Med. 2016;57 Suppl 1:27S–33S. doi: 10.2967/jnumed.115.157867 . [DOI] [PubMed] [Google Scholar]
- 8.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2015;26 Suppl 5:v8–30. doi: 10.1093/annonc/mdv298 . [DOI] [PubMed] [Google Scholar]
- 9.Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(7):961–5. Epub 2012/11/07. doi: 10.1200/JCO.2012.45.9859 . [DOI] [PubMed] [Google Scholar]
- 10.Hoilund-Carlsen PF, Hess S, Werner TJ, Alavi A. Cancer metastasizes to the bone marrow and not to the bone: time for a paradigm shift! Eur J Nucl Med Mol Imaging. 2018;45(6):893–7. Epub 2018/02/23. doi: 10.1007/s00259-018-3959-6 ; PubMed Central PMCID: PMC5915506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shie P, Cardarelli R, Brandon D, Erdman W, Abdulrahim N. Meta-analysis: comparison of F-18 Fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clinical nuclear medicine. 2008;33(2):97–101. Epub 2008/01/23. doi: 10.1097/RLU.0b013e31815f23b7 . [DOI] [PubMed] [Google Scholar]
- 12.Pires AO, Borges US, Lopes-Costa PV, Gebrim LH, da Silva BB. Evaluation of bone metastases from breast cancer by bone scintigraphy and positron emission tomography/computed tomography imaging. European journal of obstetrics, gynecology, and reproductive biology. 2014;180:138–41. Epub 2014/07/20. doi: 10.1016/j.ejogrb.2014.06.021 . [DOI] [PubMed] [Google Scholar]
- 13.Sugihara T, Koizumi M, Koyama M, Terauchi T, Gomi N, Ito Y, et al. Bone metastases from breast cancer: associations between morphologic CT patterns and glycolytic activity on PET and bone scintigraphy as well as explorative search for influential factors. Annals of nuclear medicine. 2017;31(10):719–25. Epub 2017/09/03. doi: 10.1007/s12149-017-1202-3 ; PubMed Central PMCID: PMC5691120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koolen BB, Vegt E, Rutgers EJ, Vogel WV, Stokkel MP, Hoefnagel CA, et al. FDG-avid sclerotic bone metastases in breast cancer patients: a PET/CT case series. Annals of nuclear medicine. 2012;26(1):86–91. Epub 2011/09/29. doi: 10.1007/s12149-011-0538-3 . [DOI] [PubMed] [Google Scholar]
- 15.Hahn S, Heusner T, Kummel S, Koninger A, Nagarajah J, Muller S, et al. Comparison of FDG-PET/CT and bone scintigraphy for detection of bone metastases in breast cancer. Acta radiologica (Stockholm, Sweden: 1987). 2011;52(9):1009–14. Epub 2011/10/05. doi: 10.1258/ar.2011.100507 . [DOI] [PubMed] [Google Scholar]
- 16.Sahin E, Zincirkeser S, Baris Akcan A, Elboga U. Is (99m)Tc-MDP whole body bone scintigraphy adjuvant to (18)F-FDG-PET for the detection of skeletal metastases? Journal of BUON: official journal of the Balkan Union of Oncology. 2014;19(1):291–6. Epub 2014/03/25. . [PubMed] [Google Scholar]
- 17.Caglar M, Kupik O, Karabulut E, Hoilund-Carlsen PF. Detection of bone metastases in breast cancer patients in the PET/CT era: Do we still need the bone scan? Revista espanola de medicina nuclear e imagen molecular. 2015. Epub 2015/10/31. doi: 10.1016/j.remn.2015.08.006 . [DOI] [PubMed] [Google Scholar]
- 18.Houshmand S, Salavati A, Segtnan EA, Grupe P, Hoilund-Carlsen PF, Alavi A. Dual-time-point Imaging and Delayed-time-point Fluorodeoxyglucose-PET/Computed Tomography Imaging in Various Clinical Settings. PET Clin. 2016;11(1):65–84. Epub 2015/11/23. doi: 10.1016/j.cpet.2015.07.003 . [DOI] [PubMed] [Google Scholar]
- 19.Cheng G, Torigian DA, Zhuang H, Alavi A. When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET? Eur J Nucl Med Mol Imaging. 2013;40(5):779–87. doi: 10.1007/s00259-013-2343-9 . [DOI] [PubMed] [Google Scholar]
- 20.Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J, Group EGW. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 Suppl 3:iii124–37. Epub 2014/05/02. doi: 10.1093/annonc/mdu103 . [DOI] [PubMed] [Google Scholar]
- 21.Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Breast Cancer Res Treat. 2012;131(1):231–8. Epub 2011/08/16. doi: 10.1007/s10549-011-1721-x . [DOI] [PubMed] [Google Scholar]
- 22.Jensen MB, Laenkholm AV, Offersen BV, Christiansen P, Kroman N, Mouridsen HT, et al. The clinical database and implementation of treatment guidelines by the Danish Breast Cancer Cooperative Group in 2007–2016. Acta Oncol. 2018;57(1):13–8. Epub 2017/12/06. doi: 10.1080/0284186X.2017.1404638 . [DOI] [PubMed] [Google Scholar]
- 23.Hildebrandt MG, Gerke O, Baun C, Falch K, Hansen JA, Farahani ZA, et al. [18F]Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET)/Computed Tomography (CT) in Suspected Recurrent Breast Cancer: A Prospective Comparative Study of Dual-Time-Point FDG-PET/CT, Contrast-Enhanced CT, and Bone Scintigraphy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(16):1889–97. Epub 2016/03/24. doi: 10.1200/JCO.2015.63.5185 . [DOI] [PubMed] [Google Scholar]
- 24.Abdelmalik AG, Alenezi S, Muzaffar R, Osman MM. The Incremental Added Value of Including the Head in (18)F-FDG PET/CT Imaging for Cancer Patients. Front Oncol. 2013;3:71. Epub 2013/04/12. doi: 10.3389/fonc.2013.00071 ; PubMed Central PMCID: PMC3616260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi WH, Yoo IR, O JH, Kim SH, Chung SK. The value of dual-time-point 18F-FDG PET/CT for identifying axillary lymph node metastasis in breast cancer patients. Br J Radiol. 2011;84(1003):593–9. Epub 2010/11/18. doi: 10.1259/bjr/56324742 ; PubMed Central PMCID: PMC3473484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54. Epub 2014/12/03. doi: 10.1007/s00259-014-2961-x ; PubMed Central PMCID: PMC4315529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard S, Walker E, Raghavan M. An Approach to the Evaluation of Incidentally Identified Bone Lesions Encountered on Imaging Studies. AJR Am J Roentgenol. 2017;208(5):960–70. Epub 2017/04/25. doi: 10.2214/AJR.16.17434 . [DOI] [PubMed] [Google Scholar]
- 28.Kitajima K, Fukushima K, Yamamoto S, Kato T, Odawara S, Takaki H, et al. Diagnostic performance of (11)C-choline PET/CT and bone scintigraphy in the detection of bone metastases in patients with prostate cancer. Nagoya J Med Sci. 2017;79(3):387–99. Epub 2017/09/08. doi: 10.18999/nagjms.79.3.387 ; PubMed Central PMCID: PMC5577024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baun C, Falch K, Gerke O, Hansen J, Nguyen T, Alavi A, et al. Quantification of FDG-PET/CT with delayed imaging in patients with newly diagnosed recurrent breast cancer. BMC Med Imaging. 2018;18(1):11. Epub 2018/05/11. doi: 10.1186/s12880-018-0254-8 ; PubMed Central PMCID: PMC5943993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torigian DA, Lopez RF, Alapati S, Bodapati G, Hofheinz F, van den Hoff J, et al. Feasibility and performance of novel software to quantify metabolically active volumes and 3D partial volume corrected SUV and metabolic volumetric products of spinal bone marrow metastases on 18F-FDG-PET/CT. Hell J Nucl Med. 2011;14(1):8–14. Epub 2011/04/23. . [PubMed] [Google Scholar]
- 31.Cheng G, Alavi A, Lim E, Werner TJ, Del Bello CV, Akers SR. Dynamic changes of FDG uptake and clearance in normal tissues. Mol Imaging Biol. 2013;15(3):345–52. Epub 2012/10/24. doi: 10.1007/s11307-012-0600-0 . [DOI] [PubMed] [Google Scholar]
- 32.van Es SC, Velleman T, Elias SG, Bensch F, Brouwers AH, Glaudemans A, et al. Assessment of Bone Lesions with (18)F-FDG-PET Compared to (99m)Technetium Bone Scintigraphy Leads to Clinically Relevant Differences in Metastatic Breast Cancer Management. J Nucl Med. 2020. Epub 2020/08/21. doi: 10.2967/jnumed.120.244640 . [DOI] [PubMed] [Google Scholar]
- 33.Al-Muqbel KM. Bone Marrow Metastasis Is an Early Stage of Bone Metastasis in Breast Cancer Detected Clinically by F18-FDG-PET/CT Imaging. Biomed Res Int. 2017;2017:1–7. Epub 2017/09/09. doi: 10.1155/2017/9852632 ; PubMed Central PMCID: PMC5572575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avril S, Muzic RF Jr, Plecha D, Traughber BJ, Vinayak S, Avril N. 18F-FDG PET/CT for monitoring of treatment response in breast cancer. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2016;57(Suppl 1):34S. doi: 10.2967/jnumed.115.157875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park S, Yoon J-K, Lee SJ, Kang SY, Yim H, An Y-S. Prognostic utility of FDG PET/CT and bone scintigraphy in breast cancer patients with bone-only metastasis. Medicine. 2017;96(50):85–9. doi: 10.1097/MD.0000000000008985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rong J, Wang S, Ding Q, Yun M, Zheng Z, Ye S. Comparison of 18 FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients. A meta-analysis. Surg Oncol. 2013;22(2):86–91. Epub 2013/06/04. doi: 10.1016/j.suronc.2013.01.002 . [DOI] [PubMed] [Google Scholar]