Abstract
A novel neutralization assay for human group C rotavirus (CHRV) was developed by using a reverse passive hemagglutination (RPHA) test for endpoint determination. In this assay, the neutralization (N)-RPHA test, serial twofold dilutions of sera were mixed with a solution of CHRV that yielded an RPHA test titer of 8 at 3 days after infection. The mixtures were incubated at 37°C for 1 h and were inoculated onto CaCo-2 cell monolayers in a 96-well microplate. Maintenance medium containing 100 μg of pancreatin per ml was placed in each well. The plate was sealed with sticky plastic film and was incubated at 37°C for 3 days under continuous rotation. Then, the RPHA test titer of each well was determined. The neutralization titer was expressed as the reciprocal of the maximum dilution of the serum that exhibited a fourfold (75%) or greater reduction in the RPHA test titer (8 to 2 or less). Seroconversion of neutralizing antibody was demonstrated by this method in four sets of paired serum specimens from patients with diarrheal disease caused by CHRV. The seroprevalence of CHRV in the general population in Okayama Prefecture was 26.8% by immunofluorescence and 25.5% by the N-RPHA test. The N-RPHA test described here is the first system used to assay for a neutralization antibody against CHRV and is applicable in both clinical and epidemiological settings.
Rotaviruses are members of the Reoviridae family and are characterized by their segmented, double-stranded RNA genome and their nonenveloped icosahedral structure (29). Group A rotaviruses are the principal cause of severe dehydrating gastroenteritis in young children (29). Two other antigenically and genetically distinct groups (groups B and C) also infect humans. Group B rotaviruses have caused very large epidemics of diarrheal disease in adults in China (6) but have only rarely been identified elsewhere.
Group C rotaviruses were first recognized as a causative agent of gastroenteritis in piglets (2, 24). Bridger et al. (4) characterized them as a definite human pathogen in 1986. Since then, human group C rotavirus (CHRV) infections have been associated with several outbreaks of acute diarrhea in Asia (22, 23), Europe (3, 5, 7, 10, 11, 17), South America (9), and the United States (12). Thus, CHRV is globally distributed and is thought to be one of the emerging pathogens of medical importance.
CHRV infection in Japan was first recognized by Oseto et al. (21) in 1985. Since then, CHRV infections have been reported sporadically or in the form of epidemics at various areas in Japan (8, 16, 18, 19). Recently, a large-scale outbreak of diarrhea caused by CHRV was reported in schoolchildren in Chiba Prefecture (26). We conducted an epidemiological survey that covered 10 prefectures in Japan during the winter of 1992 and 1993 and first described the molecular epidemiology of CHRV in Japan (14).
Considerable progress has been made in identifying and characterizing the proteins of group A rotaviruses that are the targets of neutralization antibody (29). VP4 and VP7 are the two surface proteins on the outer capsid. VP7 is primarily responsible for determining the viral serotype. VP4 is also responsible for inducing neutralizing antibodies. Serologic classification of rotavirus based on both VP7- and VP4-specific immunity has recently been adopted. In this system, the VP7 and VP4 serotypes are classified as G types and P types, respectively (29). To date, at least 10 distinct G types of human group A rotaviruses have been identified, although the majority of infections appear to be caused by four common serotypes (serotypes 1 to 4).
Several methods for measurement of neutralizing antibody have been developed for group A rotaviruses. These include a classical plaque reduction neutralization assay (30), a fluorescent-focus neutralization test (1), and an enzyme-linked immunosorbent assay (ELISA)-based neutralization test (32). All of these methods were based on the establishment of efficient growth conditions for group A rotaviruses in vitro. Human as well as animal group A rotaviruses grow well in MA104 cells in the presence of trypsin (25, 31). In contrast, efficient growth conditions for group C rotaviruses, especially CHRV, had been difficult to achieve until Oseto et al. (20) first demonstrated the growth of CHRV in CaCo-2 cells in the presence of pancreatin. Even using these optimal conditions, however, neutralization tests by the classical plaque reduction or fluorescent-focus reduction test were difficult to perform because the infected cells grow unevenly without forming a complete monolayer and are prone to detachment from the surface of the plates.
In the present study, a novel neutralization assay for CHRV was developed by using a reverse passive hemagglutination (RPHA) test for endpoint determination. Some seroepidemiological data obtained by this assay are also presented.
MATERIALS AND METHODS
Cells.
CaCo-2 cells were kindly provided by K. Shinozaki (27). The cells were grown and maintained in Eagle's minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal calf serum (27). For large-scale virus stock culture, the cells were cultured in 1-liter roller bottles coated with collagen (Cosmo Bio, Tokyo, Japan).
Viruses.
The OK118 and OK450 strains of CHRV were propagated more than five times in CaCo-2 cells and were adapted to in vitro culture. These two strains were the representatives of two electropherotypes (patterns I and II) observed in the epidemic in Okayama Prefecture from 1988 to 1990 (8, 13). For preparing a virus stock, CaCo-2 cells in 1-liter roller bottles were infected with either of these two strains and were cultivated at 37°C for 4 days in the presence of 100 μg of pancreatin per ml under continuous rotation. After one freeze-thaw cycle, the culture supernatant was clarified by centrifugation at 9,000 × g for 20 min. One-milliliter aliquots of the virus sample were stored at −80°C until use.
Sera.
Sera that had been obtained from four patients in the acute and convalescent stages of diarrheal disease caused by CHRV in 1989 were kindly provided by S. Nakata. In addition, a total of 231 serum samples were collected from healthy people (125 males and 106 females; age range, 0 to 73 years) who lived in three cities (Okayama, Kurashiki, and Tsuyama) located in Okayama Prefecture. Sera were collected randomly from each age group during medical examinations performed from November 1994 to February 1995. Hyperimmune sera against two strains of CHRV were prepared as follows. A culture supernatant of CHRV was purified by 20 to 50% sucrose density gradient centrifugation. The virus band was collected and dialyzed with phosphate-buffered saline (PBS). The purified virus sample was mixed with the same amount of Freund's complete adjuvant and was inoculated into mice. Three weeks later, a booster injection was administered. One week after the final injection, the animals were killed and blood was collected.
Indirect immunofluorescence (IF) test.
CaCo-2 cells infected with the OK450 strain of CHRV, as well as mock-infected cells, were spotted on a multiwell glass slide. The slide was air-dried and fixed with acetone. The slide was covered with a 1:10 dilution of human or mouse sera. After washing with PBS, the second antibody, fluorescein isothiocyanate-labeled anti-human or anti-mouse immunoglobulin (DAKO, Glostrup, Denmark) was applied. After washing with PBS and mounting with glycerol, the slide was observed under a fluorescence microscope (Axioskop; Carl Zeiss, Jena GmbH, Germany).
Titration of infectious virus by monitoring viral antigens by RPHA test.
The reagents for the RPHA test are described in our previous paper (15). The method was originally developed for direct detection of CHRV antigen in fecal samples by using erythrocytes coated with a mouse monoclonal antibody (MAb), MAb 13A3, which is directed to inner capsid protein VP6, and was applied to the titration of infectious virus in 96-well microplates by monitoring viral antigens.
CaCo-2 cells (2 × 105/well) were cultivated in collagen-coated 96-well microplates (FALCON, Bedford, England) at 37°C for 5 days. After washing twice with MEM, the CaCo-2 cells in each well were inoculated with 25 μl of viral sample that was serially diluted in twofold steps in MEM containing pancreatin (300 μg/ml). The microplates were incubated at 37°C for 60 min for adsorption and washed twice with MEM, each well was given 200 μl of MEM containing pancreatin (100 μg/ml), and plates were sealed with sticky plastic film (Plate Seal; Sumitomo Bakelite, Tokyo, Japan) and incubated at 37°C for the appropriate number of days under continuous rotation at a speed of 0.1 rpm by using a roller-bottle rotator (RT-550; TAITEC, Tokyo, Japan). After one cycle of freezing-thawing, they were centrifuged at 350 × g for 10 min in a microplate centrifuge. Then the supernatant in each well was subjected to the RPHA test to monitor the amount of viral antigen.
The supernatant (25 μl) was serially diluted in twofold steps in PBS containing 1% heat-inactivated normal rabbit serum in V-shaped microplates. The same amount of MAb-coated sheep erythrocytes was added to each well. After shaking, the plates were allowed to stand at room temperature. The hemagglutination pattern of each well was observed after 1 h. The RPHA test titer was expressed as the reciprocal of the endpoint dilution of hemagglutination.
Neutralization assay of CHRV by RPHA test.
The heat-inactivated sera (56°C, 30 min) were serially diluted in twofold steps in MEM. The initial serum dilution used for the neutralization test was 1:32 or 1:64, because some sera showed nonspecific background reactions at lower dilutions. The stock of CHRV was diluted with MEM containing pancreatin (300 μg/ml) to obtain virus samples that would yield an RPHA test titer of 8 at 3 days after infection. The same amounts (25 μl) of diluted sera and virus samples were mixed, and the mixture was incubated for 1 h at 37°C. Residual infectivity was measured by inoculating the mixture into CaCo-2 cells prepared in 96-well microplates, and the amount of viral antigen at 3 days after infection was measured by the RPHA test. The maximum dilution of serum that exhibited a fourfold (75%) or more reduction in the RPHA test titer was defined as the endpoint of neutralization. We named this assay the neutralization (N)-(RPHA) test.
RESULTS
Establishment of optimal growth conditions for CHRV in a 96-well microplate and monitoring growth of CHRV by RPHA test.
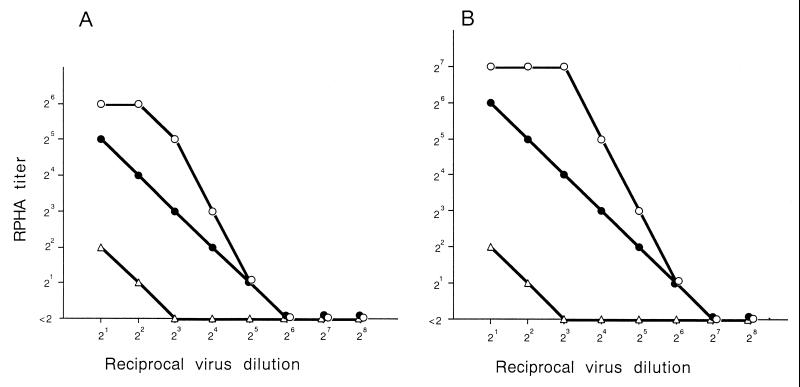
It was shown that rotation of roller tubes during incubation of cells infected with rotaviruses enhances replication of the virus (27). To apply this methodology to a microplate culture, 96-well microplates were covered with sticky plastic film. Rotation culture was continued for 2, 3, and 4 days after infection with twofold dilutions of the viral sample, and the amounts of viral antigens were quantitated by the RPHA test (Fig. 1). Ideally, it was desirable that the culture could be maintained until the endpoint of infection so that the tissue culture infectious dose could be definitely determined. However, the holding power of the sticky film was not strong enough to allow the plates to stand for 4 days or more. In fact, it was not always possible to incubate the plates for 4 days without leakage. Alternatively, we decided to use the RPHA test titer obtained at 3 days after infection (RPHA value) as an indicator of the infectious titer of the inoculum because the RPHA value correlated with the amount of virus in the inoculum over a very wide range. For the two representative viral samples shown in Fig. 1, strains OK118 and OK450, an RPHA value of 8 corresponded to approximately 8 to 16 tissue culture infectous doses.
FIG. 1.
Amount of CHRV antigen quantitated by the RHPA test after rotation culture for 2 days (▵), 3 days (●), and 4 days (○). CaCo-2 cells were infected with twofold dilutions of two virus samples, the OK118 (A) and OK450 (B) strains.
Establishment of neutralization test for CHRV.
The first step of the neutralization test was similar to that of the classical neutralization test with plaque reduction. Twofold dilutions of the test serum were mixed with CHRV samples that would yield an RPHA value of 8. The mixtures were then incubated for 1 h at 37°C and inoculated onto CaCo-2 cells, and the mixture was incubated at 37°C for 3 days with continuous rotation. The amount of viral antigens in each well was measured by the RPHA test. Table 1 summarizes the neutralization antibody titers of murine sera against CHRV. A significant rise in the neutralization antibody titer against CHRV was observed after immunization with CHRV. A cross-neutralization test between the two clinical isolates OK118 and OK450 revealed that these two strains belonged to the same serotype. No cross-reactive neutralizing antibody was detected in the sera of mice hyperimmunized with group A rotaviruses.
TABLE 1.
Neutralization antibody titers of murine sera hyperimmunized with two strains of CHRV and group A rotaviruses
| Serum sample no. | Strain used for immunization | Neutralization antibody titera against:
|
|||
|---|---|---|---|---|---|
| OK118
|
OK450
|
||||
| Preimmunization | Postimmunization | Preimmunization | Postimmunization | ||
| 1 | OK118b | <32 | 256 | <32 | 256 |
| 2 | OK118b | <32 | 128 | <32 | 128 |
| 3 | OK450b | <32 | 128 | <32 | 128 |
| 4 | OK450b | <32 | 256 | <32 | 256 |
| 5 | OK450c | <32 | 256 | <32 | 512 |
| 6 | OK450c | <32 | 256 | <32 | 256 |
| 7 | Wab | <32 | <32 | <32 | <32 |
| 8 | Hochib | <32 | <32 | <32 | <32 |
Neutralization antibody titers in sera of mice immunized with CHRV (OK118 and OK450 strains) and human group A rotaviruses (Wa and Hochi strains) were determined against OK118 or OK450.
For the immunization viral antigens purified from culture fluid were used.
For the immunization viral antigens directly purified from feces were used.
Seroepidemiology of CHRV by the N-RPHA test.
By the N-RPHA test, seroconversion of neutralizing antibody against CHRV was demonstrated in four sets of paired serum specimens obtained from patients with acute gastroenteritis symptoms during epidemics of CHRV in 1989 in Hokkaido (Table 2). The CHRV genome was detected in fecal samples from all these patients by polyacrylamide gel electrophoresis. In patient 4, the antibody titer was already elevated at 10 days after the onset of the disease, suggesting that seroconversion might have occurred within 2 weeks of the onset.
TABLE 2.
Neutralization antibody titer of paired serum specimens obtained from patients with diarrheal disease caused by CHRV
| Patient no. | Serum neutralization antibody titera
|
|
|---|---|---|
| Acute phase | Convalescent phase | |
| 1 | <64 (2) | 1,024 (21) |
| 2 | <64 (2) | 1,024 (15) |
| 3 | <64 (3) | 1,024 (18) |
| 4 | 128 (10) | 1,024 (20) |
Antibody titers were determined against CHRV strain OK118. Values in parentheses are the intervals (in days) after the onset of the disease.
Seroprevalence of CHRV determined by indirect IF and the N-RPHA test.
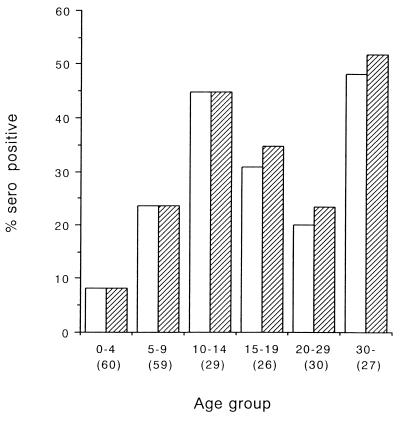
A total of 231 serum samples were tested for CHRV antibodies. The overall seroprevalence of CHRV in the general population of Okayama Prefecture was 26.8% by the indirect IF test and 25.5% by the N-RPHA test. The correlation between these assays was 98.1% (228 of 231) (Table 3). The seroprevalence of CHRV increased with age during the first 0 to 14 years and reached a level of 30 to 50% at about adolescence, although the seroprevalence varied thereafter (Fig. 2).
TABLE 3.
Seroprevalence of CHRV determined by indirect IF and N-RPHA tests
| Indirect IF test result | No. of samples with the following N-RPHA test result:
|
||
|---|---|---|---|
| Positivea | Negative | Total | |
| Positiveb | 59 | 3 | 62 |
| Negative | 0 | 169 | 169 |
| Total | 59 | 172 | 231 |
Samples which exhibited neutralization activity at a dilution of 1:64.
Samples which revealed specific immunofluorescence at a dilution of 1:10.
FIG. 2.
Seroprevalence as a function of age by the indirect IF test (≥1:10; ▨) and the N-RPHA test (≥1:64; □). The sample number for each age group is shown in parentheses.
DISCUSSION
Prior to this study, we had attempted to establish a neutralization assay using a classical plaque reduction assay, a fluorescent-focus neutralization test, and an ELISA-based neutralization test. However, neither clear plaques nor fluorescent focuses were observed. Furthermore, the growth of CHRV was not monitored by the ELISA. Because of this, we established the N-RPHA test, in which the endpoint of neutralization was determined by measuring the amount of viral antigen by the RPHA test. The key reagent for the RPHA test was the anti-CHRV MAb. We have established three MAbs against CHRV and have applied them to the direct detection of CHRV antigen in fecal samples by ELISA, the RPHA test, and latex agglutination tests (8, 15). Some advantages of the RPHA test are that MAb-coated erythrocytes can be preserved for a long time, and once the reagent is ready, the assay itself is faster and easier to perform than the ELISA.
It is not clear that the neutralization antibody detected in this assay has a protective role because no animal model of CHRV infection has been established to date. However, by using the Cowden strain of porcine group C rotavirus and the Shintoku strain of bovine group C rotavirus, it was demonstrated that the neutralizing antibody detected by the fluorescent-focus neutralization assay had a protective role in the experimental infections with the respective viruses (28).
As in the group A rotaviruses, the outer capsid glycoprotein VP7 of group C rotavirus is thought to be primarily responsible for determining the viral serotype. We have described two distinct electropherotypes in the epidemic in Okayama from 1988 to 1990 (8, 13). Sequence analysis of the VP7 genes of these two electropherotypes revealed 95.7 and 96.7% homologies at the nucleotide and amino acid levels, respectively, suggesting that these two electropherotypes belong to the same serotype (13–15). In fact, a cross-neutralization test between strains of two electropherotypes, strains OK118 and OK450, revealed that these two strains belonged to the same serotype. To date, there are no data suggesting that there is more than a single serotype of CHRV. The alignments of the VP7 genes of strains isolated in various countries of the world revealed that they are highly homologous (13). However, some evidence supports the presence of multiple serotypes in animal group C rotaviruses (28). Once the novel neutralization assay is established for CHRV, careful and systematic monitoring of new isolates by cross-neutralization tests would help investigators find new serotypes. We are also planning to conduct an experiment to assess cross neutralization between animal group C rotaviruses and CHRV.
The present assay should be beneficial for obtaining information about the neutralization epitopes of CHRV, if it is used in combination with neutralizing MAbs against CHRV. Seroepidemiology based on the neutralization assay should be useful in clinical virology for the evaluation of protective immunity and should be applicable to future development of CHRV vaccines.
ACKNOWLEDGMENTS
We thank Kuniko Shinozaki, Public Health Laboratory of Chiba Prefecture, for providing CaCo-2 cells and Shuji Nakata, Sapporo Medical College, for providing paired serum specimens from patients with diarrhea disease caused by CHRV.
REFERENCES
- 1.Bernstein D I, Kacica M A, McNeal M M, Schiff G M, Ward R L. Local and systemic antibody response to rotavirus WC3 vaccine in adult volunteers. Antivir Res. 1989;12:293–300. doi: 10.1016/0166-3542(89)90056-9. [DOI] [PubMed] [Google Scholar]
- 2.Bohl E H, Saif L J, Theil K W, Agnes A G, Cross R F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982;15:312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonsdorf C H V, Svensson L. Human serogroup C rotavirus in Finland. Scand J Infect Dis. 1988;20:475–478. doi: 10.3109/00365548809032493. [DOI] [PubMed] [Google Scholar]
- 4.Bridger J C, Pedley S, McCrae M A. Group C rotaviruses in humans. J Clin Microbiol. 1986;23:760–763. doi: 10.1128/jcm.23.4.760-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caul E O, Ashley C R, Darville J M, Bridger J C. Group C rotavirus associated with fatal enteritis in a family outbreak. J Med Virol. 1990;30:201–205. doi: 10.1002/jmv.1890300311. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Hung T, Bridger J C, McCrae M A. Chinese adult rotavirus is a group B rotavirus. Lancet. 1985;ii:1123–1124. doi: 10.1016/s0140-6736(85)90710-x. [DOI] [PubMed] [Google Scholar]
- 7.Espejo R T, Puerto F, Soler C, Gonzales N. Characterization of a human pararotavirus. Infect Immun. 1984;44:112–116. doi: 10.1128/iai.44.1.112-116.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii R, Kuzuya M, Hamano M, Yamada M, Yamazaki S. Detection of human group C rotaviruses by an enzyme-linked immunosorbent assay using monoclonal antibodies. J Clin Microbiol. 1992;30:1307–1311. doi: 10.1128/jcm.30.5.1307-1311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabbay Y B, D'Arc J, Mascarenas P, Linhares A C, Freitas R B. Atypical rotavirus among diarrhoeic children living in Belém, Brazil. Mem Inst Oswaldo Cruz. 1989;84:5–7. doi: 10.1590/s0074-02761989000100002. [DOI] [PubMed] [Google Scholar]
- 10.James V L, Lambden P R, Caul E O, Clarke I N. Enzyme-linked immunosorbent assay based on recombinant human group C rotavirus inner capsid protein (VP6) to detect human group C rotaviruses in fecal samples. J Clin Microbiol. 1998;36:3178–3181. doi: 10.1128/jcm.36.11.3178-3181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James V L, Lambden P R, Caul E O, Cooke S J, Clarke I N. Seroepidemiology of human group C Rotaviruses in the UK. J Med Virol. 1997;52:86–91. doi: 10.1002/(sici)1096-9071(199705)52:1<86::aid-jmv14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Jiang B, Dennehy P H, Spangenberger S, Gentsch J R, Glass R I. First detection of group C rotavirus in fecal specimens of children with diarrhea in the United States. J Infect Dis. 1995;172:45–50. doi: 10.1093/infdis/172.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Kuzuya M, Fujii R, Hamano M, Nakamura J, Yamada M, Nii S, Mori T. Molecular analysis of outer capsid glycoprotein (VP7) genes from two isolates of human group C rotavirus with different genome electropherotypes. J Clin Microbiol. 1996;34:3185–3189. doi: 10.1128/jcm.34.12.3185-3189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuzuya M, Fujii R, Hamano M, Yamada M, Shinozaki K, Sasagawa A, Hasegawa S, Kawamoto H, Matsumoto K, Kawamoto A, Itagaki A, Funatsumaru S, Urasawa S. Survey of human group C rotaviruses in Japan during the winter of 1992 to 1993. J Clin Microbiol. 1998;36:6–10. doi: 10.1128/jcm.36.1.6-10.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzuya M, Fujii R, Hamano M, Nagabayashi T, Tsunemitsu H, Yamada M, Nii S, Mori T. Rapid detection of human group C rotaviruses by reverse passive hemagglutination and latex agglutination tests using monoclonal antibodies. J Clin Microbiol. 1993;31:1308–1311. doi: 10.1128/jcm.31.5.1308-1311.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto K, Hatano M, Kobayashi K, Hasegawa A, Yamazaki S, Nakata S, Chiba S, Kimura Y. An outbreak of gastroenteritis associated with acute rotaviral infection in schoolchildren. J Infect Dis. 1989;160:611–615. doi: 10.1093/infdis/160.4.611. [DOI] [PubMed] [Google Scholar]
- 17.Maunula L, Svensson L, Bonsdorff C H V. A family outbreak of gastroenteritis caused by group C rotavirus. Arch Virol. 1992;124:269–278. doi: 10.1007/BF01309808. [DOI] [PubMed] [Google Scholar]
- 18.Oishi I, Yamazaki K, Minekawa Y. An occurrence of diarrheal cases associated with group C rotavirus in adults. Microbiol Immunol. 1993;37:505–509. doi: 10.1111/j.1348-0421.1993.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 19.Oseto M. Epidemiological study of group C rotavirus. J Jpn Assoc Infect Dis. 1990;64:1264–1273. doi: 10.11150/kansenshogakuzasshi1970.64.1264. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 20.Oseto M, Yamashita Y, Hattori M, Mori M, Inoue H, Ishimaru Y, Matsuno S. Serial propagation of human group C rotavirus in a continuous cell line (CaCo-2) J Clin Exp Med. 1994;168:177–178. . (In Japanese.) [Google Scholar]
- 21.Oseto M, Yamashita Y, Okuyama M, Kuwabara H, Inoue H. Detection of atypical rotaviruses by polyacrylamide gel electrophoresis. J Clin Exp Med. 1986;136:223–224. . (In Japanese.) [Google Scholar]
- 22.Penaranda M E, Cubitt W D, Sinarachatanant P, Taylor D N, Likanonsakul S, Saif L, Glass R I. Group C rotavirus infection in patients with diarrhea in Thailand, Nepal, and England. J Infect Dis. 1989;160:392–397. doi: 10.1093/infdis/160.3.392. [DOI] [PubMed] [Google Scholar]
- 23.Rasool N B, Hamzah M, Jegathesan M, Wong Y H, Qian Y, Green K Y. Identification of a human group C rotavirus in Malaysia. J Med Virol. 1994;43:209–211. doi: 10.1002/jmv.1890430302. [DOI] [PubMed] [Google Scholar]
- 24.Saif L J, Bohl E H, Theil K W, Cross R F, House J A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol. 1980;12:105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Inaba Y, Shinozaki T, Fujii R, Matsumoto M. Isolation of human rotavirus in cell cultures. Arch Virol. 1981;69:155–160. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- 26.Shinozaki K, Yamanaka T, Kaiho I, Tokieda M. An outbreak of group C rotavirus associated gastroenteritis in a elementary school in Chiba. Bull Pub Health Lab Chiba Pref. 1994;18:10–12. . (In Japanese.) [Google Scholar]
- 27.Shinozaki K, Yamanaka T, Tokieda M, Shirasawa H, Simizu B. Isolation and serial propagation of human group C rotaviruses in a cell line (CaCo-2) J Med Virol. 1996;48:48–52. doi: 10.1002/(SICI)1096-9071(199601)48:1<48::AID-JMV8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Tsunemitsu H, Jiang B, Yamashita Y, Oseto M, Ushijima H, Saif L J. Evidence of serologic diversity within group C rotaviruses. J Clin Microbiol. 1992;30:3009–3012. doi: 10.1128/jcm.30.11.3009-3012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umesh D P, Bresee J S, Gentsch J R, Glass R I. Rotavirus. Emerg Infect Dis. 1998;4:561–570. doi: 10.3201/eid0404.980406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urasawa S, Urasawa T, Taniguchi K. Three human rotavirus serotypes demonstrated by plaque neutralization of isolated strains. Infect Immun. 1982;38:781–784. doi: 10.1128/iai.38.2.781-784.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urasawa T, Urasawa S, Taniguchi K. Sequential passages of human rotavirus in MA-104 cells. Microbiol Immunol. 1981;25:1025–1035. doi: 10.1111/j.1348-0421.1981.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 32.Ward R L, Kapikian A Z, Goldberg K M, Knowlton D R, Watson M W, Rappaport R. Serum rotavirus neutralizing-antibody titers compared by plaque reduction and enzyme-linked immunosorbent assay-based neutralization assays. J Clin Microbiol. 1996;34:983–985. doi: 10.1128/jcm.34.4.983-985.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]