INTRODUCTION
Achalasia is an esophageal motility disorder of unknown etiology1. Case-control analysis has revealed that antecedent viral infections are significantly more prevalent in achalasia than in control patients (p < 0.0001); moreover, diseases caused by varicella zoster virus (VZV; the cause of varicella and zoster) are by far the most prevalent of viral infections (77.4%; p < 0.0001)2. Antibody titers to VZV in patients with achalasia are significantly higher than those of age- and sex-matched controls and in-situ hybridization has revealed VZV DNA in neurons of the achalasia esophagus3. VZV establishes latency in peripheral neurons after varicella or administration of live attenuated varicella vaccine4. Cutaneous zoster results from VZV reactivation in skin-projecting neurons, however, VZV reactivation in enteric neurons, which lack cutaneous projections, causes enteric zoster, which may lack a rash and thus escape suspicion4, 5. We therefore investigated the possibility that VZV (enteric zoster) might be linked to achalasia.
METHODS
Upper endoscopy and high-resolution manometry were used to diagnose achalasia according to the Chicago Classification v4.01. Nine male and 6 female patients with the following phenotypes were analyzed: Type I achalasia (n=3), Type II Achalasia (n=8), Type III Achalasia (n=4) (Supplemental Table 1). No patient had received a varicella vaccine. To determine whether DNA, transcripts, and proteins encoded by late VZV genes are present in the esophagus in achalasia, we used nested PCR, RT-PCR, and immunocytochemistry to analyze lower esophageal sphincter (LES) musculature resected during clinically indicated Heller myotomies5, 6. We also determined whether salivary VZV DNA, a marker of active VZV infections that is absent from control saliva5, 6, was present prior to surgery in the same patients. Control saliva was obtained from 2 male and 3 female healthy adults (age 70.5–81).
RESULTS
A significant proportion of patients (12/15, 80%) was found to have detectable salivary VZV DNA, whereas no salivary VZV was detected in the current controls (0/5, p < 0.004); or in those published previously (0/139, p < 0.001)5, 7. A large proportion of the cohort of patients with achalasia was found to have transcripts encoding VZV late gene products (ORF 40 or ORF67) in surgically excised esophageal tissue (Supplemental Table 2; 13/15, 87%). This proportion was similar to that with salivary VZV DNA. Transcripts encoding VZV gene products were found in the esophagus of all but one of the patients with salivary VZV DNA and salivary VZV DNA was found in all but one of the patients in whom transcripts were detected. The proportion of patients in whom VZV DNA was detected in resected esophageal tissue was not significantly different (Fisher’s exact test) from that of VZV transcripts (Supplemental Table 2; 7/15, 44%).
Antibodies to neuronal markers (β3-tubulin and peripherin) were combined with those to VZV late proteins to test immunocytochemically the idea that esophageal neurons or nerve fibers are the site of VZV infection in achalasia. Although, as anticipated, many specimens lacked neuronal cell bodies, all contained nerve fibers. Neuronal cell bodies, surrounded by abundant nerve fibers, were found in sections from 4/15 patients (Fig. 1A, B). The immunoreactivity of gE, the most abundant VZV protein, was found in cells, confirmed as neurons with antibodies to β3-tubulin (Fig. 1A). The immunoreactivities of gH (Fig. 1B) and ORF40p (not illustrated), like that of gE, was also observed in esophageal neurons identified with antibodies to peripherin. Many nerve fiber bundles appeared to be abnormal with bulbous swellings and strictures. Examination at high resolution revealed small gE-fluorescent particles within these nerve fibers (Fig. 1C). The diameters of these particles were 0.5–0.6 μm, which is greater than that of a single varicella virion (~0.2 μm), but consistent with that expected for a small cluster of 2–3 virions. Multinucleated giant cells, which displayed coincident gE and β3-tubulin immunoreactivities, were also observed (Fig. 1D). Similar multinucleated VZV-immunoreactive giant cells have been seen previously in low grade chronic VZV infection of an immunocompromised patient6.
Figure 1.
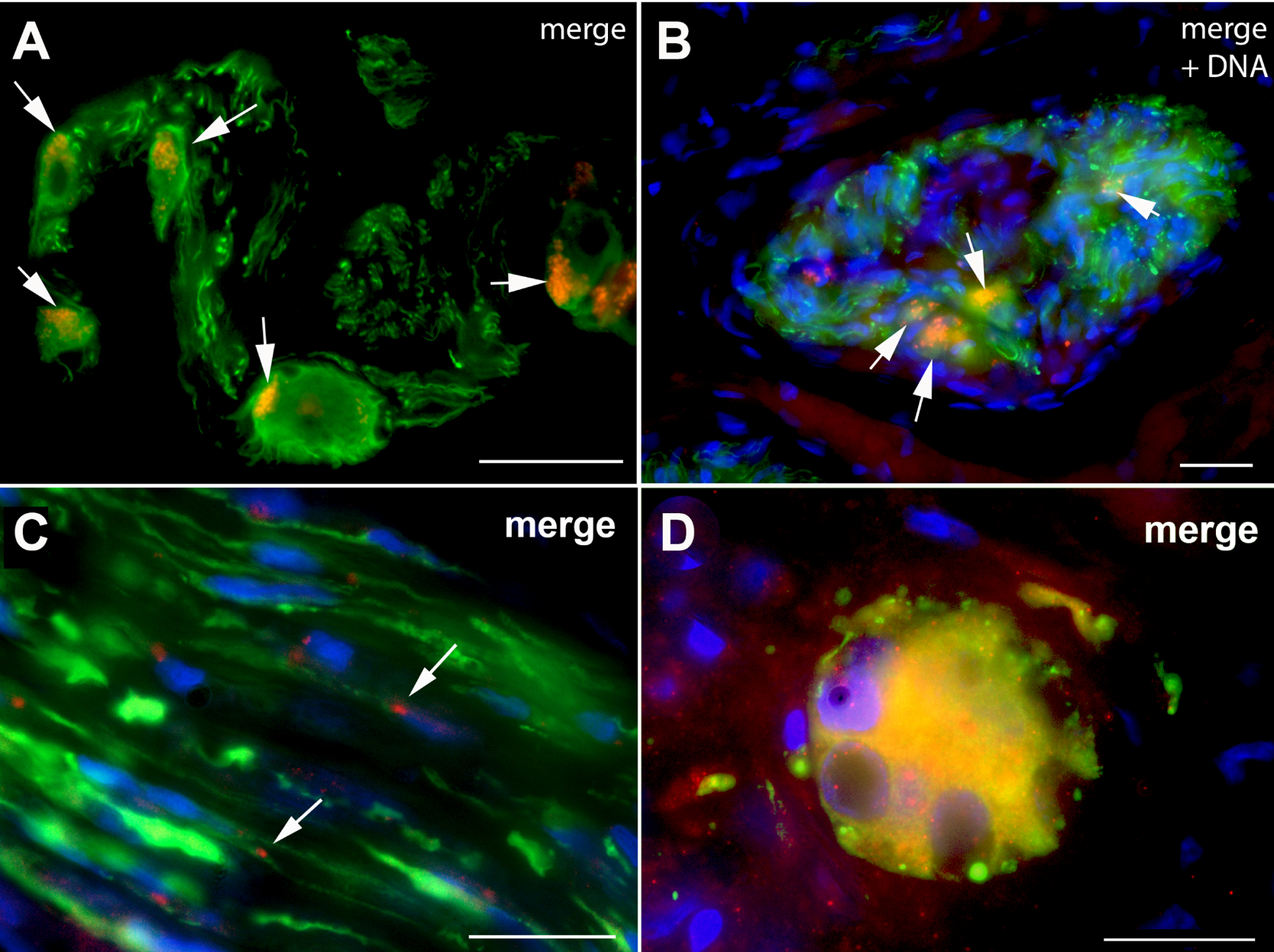
Immunoreactivity of VZV late proteins can be detected in intrinsic esophageal neurons, nerve fibers and multinucleated giant cells. A: A small intra-esophageal ganglion is shown. The wave length of the light used for excitation and the dichroic mirror/filter set were adjusted to show the immunoreactivity of the neuronal marker, β3-tubulin (green) and the immunoreactivity of VZV gE (red); the merged image is shown (coincident fluorescence is yellow). The arrows point to neurons with gE immunoreactivity. The neurons within the ganglion are surrounded by bundles of nerve fibers. B: Another small intra-esophageal ganglion in a different patient is shown, illuminated to show the immunoreactivity of the neuronal marker, peripherin (green), the immunoreactivity of VZV gH (red); the merged image is shown (coincident fluorescence is yellow). The fluorescence of DNA (blue) stained with bisbenzimide is also illustrated in the merged image to show the locations of nuclei. The arrows point to the neurons with gH immunoreactivity. The neurons within the ganglion are again surrounded by bundles of nerve fibers. The markers = 25 μm. C: A small bundle of nerve fibers with intermittent irregular bulbous swellings and narrowings can be seen in longitudinal section. The nerve is immunostained with antibodies to the neuronal marker, β3-tubulin (green) and VZV gE (red). The locations of Schwann cell and fibroblast nuclei are illustrated with the fluorescence of DNA (bisbenzimide stain; blue). The merged image is shown. The arrows show the locations of gE-immunofluoescent particles. The marker = 10 μm. D: The immunofluorescence of a neuronal marker and that pf VZV gE can be detected in multinucleated giant cells in the esophagus of patients with achalasia. A multinucleated giant cell displays the immunoreactivity of the neuronal marker, β3-tubulin (green) and the immunoreactivity of VZV gE (red). The nuclei are visualized by the fluorescence of DNA (bisbenzimide stain; blue). Note that the fluorescence of the cytoplasm is yellow due to the superimposition in the merged image, respectively, of the green and red fluorescence of β3-tubulin and VZV gE. The marker = 10 μm.
DISCUSSION
Observations made in the current study are consistent with the hypothesis that the reactivation of VZV from latency in esophageal neurons gives rise to chronic VZV infection that impairs the functional regulation of esophageal motility and control of the LES in achalasia. Evidence of VZV infection (salivary VZV DNA in 80% [12/15] and transcripts encoding VZV late gene products in myotomy tissue in 87% [13/15]) was present in a surprisingly high proportion of achalasia patients. Salivary VZV DNA was a good predictor of esophageal VZV infection in patients with symptoms of achalasia. The VZV transcripts, furthermore, appear to be translated in esophageal neurons because the immunoreactivities of VZV late proteins (gE, gH, and ORF40p) were detected in enteric neuronal cell bodies and nerve fibers. The presence of small VZV-immunoreactive particles in nerve fibers within the esophageal wall is also consistent with the idea that infected esophageal neurons produce virions that enter axons.
The multinucleated giant cells in the esophagus that contained coincident neuronal and VZV immunoreactivities were probably derived from infected neurons, reflecting either the fusogenic properties of VZV or the phagocytic removal of neurons that VZV killed. The frequent presence of VZV late gene transcripts in the resected esophagus suggests that there is a continuing VZV infection of the esophagus. The persistent zoster in the esophageal ENS is clearly a lower grade of infection than that responsible for the previously reported perforation of the stomach5. Neurogenesis, which has been shown to occur in the adult ENS of mice and humans8 might balance a slow loss of esophageal neurons during VZV infection. The current study has made the hypothesis that VZV plays a causal role in achalasia plausible, but further evidence of causality is needed. Given the large number of ganglia in body that harbor latent VZV, it seems unlikely that a stochastic process could lead to reactivation exclusively in esophageal neurons; therefore, an additional, possibly genetic, factor is probably involved.
Supplementary Material
ACKNOWLEDGEMENTS
Funding:
This work was supported by funds from grants DK093094 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NS15547 from the National Institute of Neurological Disorders and Stroke (NINDS), and TR000445 from the National Center for Advancing Translational Sciences.
We thank Eric Howard, RN, CCRP at the Center for Esophageal Research, Vanderbilt University Medical Center for tissue preparation; Chetan Aher, MD at the Vanderbilt Minimally Invasive Surgery for collection of myotomy tissue, Mary Kay Washington at the Vanderbilt University Medical Center Division of Pathology.
Abbreviations:
- cDNA
complementary DNA
- LES
lower esophageal sphincter
- ENS
Enteric Nervous System
- GERD
Gastro-esophageal reflux disease
- GPS
Glycoproteins
- IRP
Integrated relaxation pressure
- ORF
open reading frame
- PPIs
Proton pump inhibitors
- VZV
Varicella Zoster Virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Nothing to disclose
REFERENCES
- 1.Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. Am J Gastroenterol 2020;115:1393–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker J, Niebisch S, Ricchiuto A, Schaich EJ, Lehmann G, Waltgenbach T, Schafft A, Hess T, Lenze F, Venerito M, Huneburg R, Lingohr P, Matthaei H, Seewald S, Scheuermann U, Kreuser N, Veits L, Wouters MM, Gockel HR, Lang H, Vieth M, Muller M, Eckardt AJ, von Rahden BH, Knapp M, Boeckxstaens GE, Fimmers R, Nothen MM, Schulz HG, Gockel I, Schumacher J. Eur J Gastroenterol Hepatol 2016;28:689–95. [DOI] [PubMed] [Google Scholar]
- 3.Robertson CS, Martin BA, Atkinson M. Gut 1993;34:299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gershon M, Gershon A. J Infect Dis 2018;218:S113–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershon AA, Chen J, Gershon MD. Clin Infect Dis 2015;61:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhalla P, Forrest GN, Gershon M, Zhou Y, Chen J, LaRussa P, Steinberg S, Gershon AA. Clin Infect Dis 2015;60:1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birlea M, Cohrs RJ, Bos N, Mehta SK, Pierson DL, Gilden D. J Med Virol 2014;86:360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iruzubieta P, Cantarero I, Monzon M, Lahoz M, Junquera C. Cell Mol Neurobiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


