ABSTRACT
Trauma-induced coagulopathy is associated with very high mortality, and hemorrhage remains the leading preventable cause of death after injury. Directed methods to combat coagulopathy and attain hemostasis are needed. The available literature regarding viscoelastic testing, including thrombelastography (TEG) and rotational thromboelastometry (ROTEM), was reviewed to provide clinically relevant guidance for emergency resuscitation. These tests predict massive transfusion and developing coagulopathy earlier than conventional coagulation testing, within 15 min using rapid testing. They can guide resuscitation after trauma, as well. TEG and ROTEM direct early transfusion of fresh frozen plasma when clinical gestalt has not activated a massive transfusion protocol. Reaction time and clotting time via these tests can also detect clinically significant levels of direct oral anticoagulants. Slowed clot kinetics suggest the need for transfusion of fibrinogen via concentrates or cryoprecipitate. Lowered clot strength can be corrected with platelets and fibrinogen. Finally, viscoelastic tests identify fibrinolysis, a finding associated with significantly increased mortality yet one that no conventional coagulation test can reliably detect. Using these parameters, guided resuscitation begins within minutes of a patient's arrival. A growing body of evidence suggests this approach may improve survival while reducing volumes of blood products transfused.
Keywords: Coagulopathy, hemorrhage, thrombelastography, thromboelastometry, trauma, viscoelastic
INTRODUCTION
Hemorrhage is the leading preventable cause of trauma-related death. Despite advances in care through the last four decades, mortality rates remain consistently high (1–5). Incompressible hemorrhage, anticoagulants, and other coagulopathies can add complexity to an already challenging problem for any individual patient. A particularly deadly coagulopathy directly related to traumatic hemorrhage has been titled “trauma-induced coagulopathy” (TIC), “acute traumatic coagulopathy,” “acute coagulopathy of trauma,” “acute coagulopathy of trauma-shock,” or “early coagulopathy of trauma” (6–9). These various terms for coagulopathy following major trauma all portend death. Present in a third of both civilian and military trauma patients, TIC corresponds to nearly 50% mortality rates (6–8, 10).
Expedient control of ongoing hemorrhage in its myriad forms remains beyond the scope of a single review. Rather, combating TIC with early recognition, identification of compounding factors, and appropriate resuscitation will be discussed here. The basic tenets of damage control resuscitation are transfusion of whole blood (or product ratios approximating whole blood), limited crystalloid use, and permissive hypotension. Beyond this, achieving optimal outcomes in bleeding patients benefits from viscoelastic coagulation testing, which is the focus of this article. Both thrombelastography (TEG) (Haemonetics Corporation, Braintree, Mass) and rotational thromboelastometry (ROTEM) (TEM International GmbH, Munich, Germany) will be reviewed with attention to their clinical uses in the initial resuscitation of the severely injured patient. Topics of review will also include history of viscoelastic testing, interpretation of its results, and guided versus fixed-ratio resuscitation.
METHODS
A systematic search was undertaken to find peer-reviewed literature describing TEG and ROTEM employed for resuscitation of trauma patients. Specific search terms in varying Boolean strings were “thrombelastography,” “TEG,” “rotational thromboelastometry,” “ROTEM,” “viscoelastic,” “trauma” or “traumatic,” “shock,” “injury,” “hemorrhage” or “hemorrhagic,” and “resuscitation.” A combination of PubMed, Ovid MEDLINE, and Google Scholar database searches was employed, and the end date limit was September 15, 2019. Patent descriptions, animal studies, non-clinical in vitro, and elective surgical studies were excluded. Only full-text articles available in the English language were reviewed. Additionally, product manuals and technical information sheets were requested from manufacturers of TEG and ROTEM testing equipment.
RESULTS
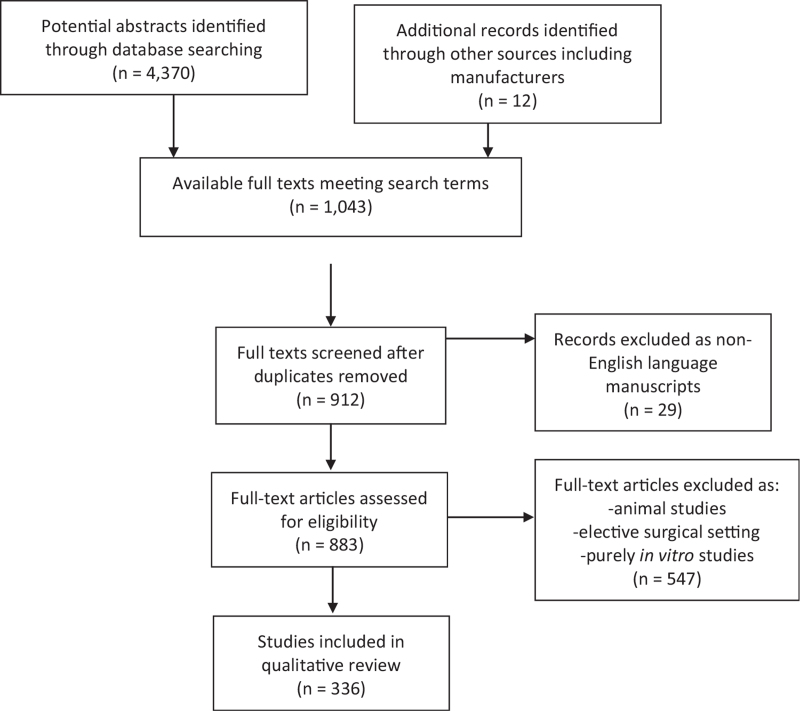
An initial screening search of titles, abstracts, and keyword searches for “‘thromboelastography,’ ‘thrombelastography,’ or ‘rotational thromboelastometry,”’ their abbreviations, or “viscoelastic” yielded 4,370 results. Eligibility searches of those results revealed 883 individual available texts, after removal of duplicates, in the English language with keywords, titles, or abstracts including the terms “trauma/traumatic,” “shock,” “injury,” “hemorrhage/hemorrhagic,” or “resuscitation” with truncation wildcards where appropriate. Full text search followed. Of these texts, 176 described animal studies. A further 371 were excluded as primarily elective surgical studies or purely in vitro/ex vivo studies. The search, therefore, yielded 336 studies comprising the available peer-reviewed literature of TEG and ROTEM in clinical trauma settings on which to base this narrative review (Fig. 1). This body of literature was categorized and summarized by topic heading.
Fig. 1.
Flowchart of the review design and results of literature search.
Coagulopathy following injury
Bleeding begets more bleeding. After injury and major hemorrhage, multiple mechanisms contribute to ongoing hemorrhage, beyond the classic triad of acidosis, hypothermia, and coagulopathy. Acidosis, hypothermia, endothelial injury, hypocalcemia, dilutional coagulopathy, pre-injury medication-related coagulopathy, and TIC can act synergistically and often appear in combinations (11, 12). Most of the enzymes critical to the coagulation cascade, as well as platelets, function most efficiently at physiologic to slightly alkalotic pH (13). Hypothermia, besides its direct effect on decreasing protease and platelet activity (14, 15), negatively alters calcium ion receptor binding in calcium-dependent pro-coagulants (16). Component blood transfusion, the most common approach to replace blood volume and combat coagulopathy, carries its own deleterious effects including diluted coagulation factors, calcium chelation, and immunoinflammatory effects (17–22).
TIC is defined as an independent contributor to hemorrhage after trauma via activated protein C (aPC) (23–25), thrombomodulin (23, 26), plasminogen activator inhibitor-1 (PAI-1), tissue plasminogen activator (tPA), hyperfibrinolysis (27, 28), qualitative platelet dysfunction (29), and release of thrombogenic microparticles (30). In other words, correction of acidosis, hypothermia, and other contributors to coagulopathy assist with hemorrhage control, but TIC can persist despite their control. Moreover, TIC is apparent early and is highly lethal. In the civilian setting, at least a quarter of trauma patients arriving from the scene are coagulopathic and the presence of coagulopathy increases mortality 4-fold (23). Among military casualties in Iraq and Afghanistan, Niles et al. noted that almost 40% presented with evidence of TIC. Those with TIC on admission had a 6-fold higher mortality than their non-coagulopathic cohorts (10).
Because of its lethality, a critical point in combating TIC becomes apparent: it must be predicted or identified early. Several retrospective studies from the civilian arena identified that the median time to death from hemorrhage is within 3 h of injury (31, 32). This time to achieving hemostasis and hemorrhage control, or not, was the same regardless of penetrating or blunt mechanism of injury. In a randomized trial of civilian transfusion ratios, the time to death or hemostasis was the same at 2.6 h after injury (31). The inflection point beyond which bleeding patients suffer significantly worse outcomes has been repeatedly under 3 h in multiple large studies (33, 34). Even brief delays in acting upon hemorrhage carry significant mortality. In a multicenter study including 12 Level I US trauma centers, every minute in delay of activation of a massive transfusion protocol was associated with a 5% incremental increase in mortality (35).
The detection of TIC, therefore, depends on rapid or point of care testing. Even experienced clinicians predict poorly the need for massive transfusion based on judgment alone (36), and clinical scoring systems for predicting transfusion requirements are not designed to predict coagulopathy. Initially, TIC was often defined using conventional coagulation testing (CCT) such as prothrombin time (PT) > 18 s (6, 37), international normalized ratio (INR) > 1.5–1.6 (38–40), active partial thromboplastin time (PTT) > 60 s (6, 37), and decreased platelet (typically < 50,000–100,000/μL) and fibrinogen counts (such as < 100 mg/dL) (39, 41, 42). Unfortunately, CCT carries several disadvantages making it less than ideal for diagnosing TIC. Tests are run on spun-down plasma samples (rather than whole blood specimens), focusing on single parts of the complex clotting cascade. The tests themselves were never designed to diagnose coagulopathy in trauma, but rather, were developed to follow isolated lesions seen in disease such as hemophilia. Moreover, PT and PTT tests are concluded when approximately 5% of the total available thrombin is generated, providing an incomplete representation of fibrin formation (43). Fibrinogen counts have no ability to account for functional versus nonfunctional proteins. CCT also take time: as an example, a multicenter prospective study including actively bleeding patients during major surgical procedures reported a median turnaround time of 88 min, with a range that extended up to 235 min (44).
This is precisely where the advantages of viscoelastic testing have been shown to identify TIC with increased sensitivity and decreased time to interpretation (45–47). In a large prospective cohort, rapid TEG predicted substantial bleeding and red blood cell transfusion requirements better than PT or PTT, α-angle was superior to fibrinogen for predicting plasma transfusion, and maximum amplitude (MA) was superior to platelet count for predicting platelet transfusion (48). Multiple other authors have validated the utility of TEG and ROTEM for predicting coagulopathy and massive transfusion requirement in a wide variety of civilian and military settings (49–60). Most importantly, the figures of clot activation, kinetics, and strength are available within 15 min using rapid testing (61).
History, clot parameter generation, and interpretation of viscoelastic testing
Hartert (62) first reported a method to evaluate whole blood coagulation in 1948. This early version of TEG was relegated to research purposes only, until clinical use was developed for liver transplantation in the 1960s (63). Hyperfibrinolysis after implantation of the donor liver was accurately identified by TEG, a feat not achievable by CCT. It should be noted that viscoelastic testing still provides early detection of fibrinolysis better than CCT, one of the persisting justifications of its use (64, 65). In the 1980s, TEG showed promise in reducing individual blood component transfusion in both liver transplant and later cardiac surgery (66). Kaufmann et al. (67) reported TEG's predictive use in blunt trauma in 1997, one of the first descriptions of viscoelastic testing following trauma. Although a small series, hypocoagulable results predicted early transfusion.
TEG has become the primary version of viscoelastic testing in North America, while ROTEM is seen more commonly in Europe. While they are similar in concept, they rely on different reagents and are not equivalent (68–72). Both provide information on clot initiation, progression, strength, and lysis from whole blood samples run at 37°C in a plastic cylindrical cup with a central wire or pin suspended in the sample. In TEG, the cup rotates while the central pin senses torque via an electromagnetic inducer as the sample coagulates and eventually lyses to varying degrees. In ROTEM, the pin is the rotating piece.
A conventional TEG tracing from a TEG 5000 machine plots time on the X axis with resistance to oscillation on the Y axis, resulting in a symmetric set of diverging curves as the cup rotates clockwise then counterclockwise. Samples are typically run in paired chambers to minimize measurement errors. Citrated blood samples are first reversed with calcium. Kaolin (a type of clay primarily containing hydrated aluminum silicate) and cephalins (a class of phospholipids) start the clotting process. The entire process can be accelerated by adding both kaolin and tissue factor, a process trademarked as Rapid TEG, which reveals early parameters of coagulation initiation in under 5 min. Additional subtypes of TEG include PlateletMapping, which describes the inhibitory effects of antiplatelet agents, and hTEG, in which heparinase is added to the sample. A functional fibrinogren assay is also available (73).
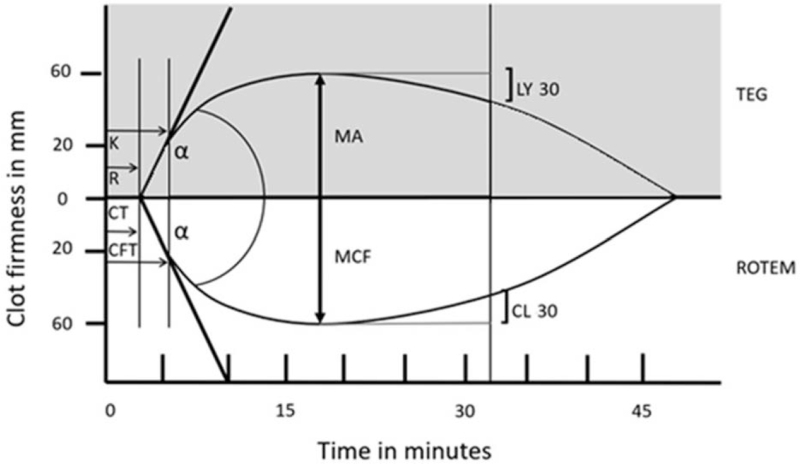
ROTEM produces five tracings, many of which correspond to TEG subtypes: intrinsic activation (INTEM), extrinsic activation (EXTEM), fibrin component of the clot (FIBTEM), aprotinin addition (APTEM) to show the effect of fibrinolysis inhibition, and heparinase addition (HEPTEM) to remove the effect of heparin on the sample (74). The similarities in interpretation of TEG and EXTEM tracings are shown in Figure 2. Normal values for each component of the tests are shown in Table 1, along with the primary contributors of the coagulation and clot formation web reflected in the individual components of the tracings.
Fig. 2.
Comparison of TEG and ROTEM tracings to demonstrate their similarities. TEG indicates thrombelastography; ROTEM, rotational thromboelastometry; R, reaction time; CT, clotting time; k, k-time or kinetics; CFT, clot formation time; α, alpha-angle; MA, maximum amplitude; MCF, maximum clot firmness; LY30, lysis at 30 min; CL30, clot lysis at 30 min.
Table 1.
Viscoelastic testing parameters and their reference ranges for citrated samples according to manufacturer labeling
| Kaolin TEG | ROTEM EXTEM | Description | Main contributor |
| R: 5–10 min rTEG ACT: 86–118 s | CT: 43–82 s | Activation: time from start of test to first detectable clot formation, defined as an amplitude of 2 mm | Coagulation factors initiating thrombin generation |
| k: 1–3 min rTEG k: 34–138 s | CFT: 48–127 s | Amplification: time to 20 mm clot strength due to fibrin deposition and cross-linking | Concentration of fibrinogen and activation by thrombin |
| α: 53–72° rTEG α: 64–80° | α: 65–80° | Propagation: slope of tracing indicating speed of thrombin generation and fibrin deposition and cross-linking | Concentration of functioning fibrinogen, and to a lesser extent platelets |
| MA: 50–70 mm rTEG MA: 52–71 mm | MCF: 52–70 mm | Termination: maximal amplitude of clot strength | Platelet count and activity, and to a lesser extent fibrinogen |
| G: 4,500–11,000 kilodynes/cm2 | MCE: no provided range (dimensionless value) | Calculated value (logarithmic computation based on MA and MCF) | MA and MCF |
| LY30: -2.3–5.77% rTEG LY30: < 7.5% | LY30 or CL30: < 18% | Fibrinolysis: percentage decrease in amplitude at 30 min after MA, indicating clot lysis | Speed of fibrinolysis (concentration of plasminogen and its activators) |
α indicates alpha-angle; ACT, activated clotting time; CFT, clot formation time; CL30, clot lysis at 30 min; CT, clotting time; EXTEM, extrinsic thromboelastometry; G, G-value; k, k-time or kinetics; LY30, lysis at 30 min; MA, maximum amplitude; MCE, maximum clot elasticity; MCF, maximum clot firmness; R, reaction time; ROTEM, rotational thromboelastometry; rTEG, rapid thrombelastography; TEG, thrombelastography.
Correct interpretation of these laboratory tests relies on cognizance of their limitations as much as their strengths. While TEG and ROTEM are relatively rapid global assessments in comparison to CCT, they suffer from clinically significant shortcomings. Plastic cylinders do not mimic the complex endovascular environment of coagulation, a contributor to the coagulation cascade gaining increasing attention (75, 76). Hypothermia, especially core body temperature below 34°C, is common after major injury and significantly reduces platelet and factor activity (11, 77, 78). However, standard protocol runs the sample at 37°C, introducing mismatch between in vivo and in vitro coagulability profiles. Addition of reagents requires a high degree of precision in viscoelastic testing, introducing inter-technician and inter-facility variability (79). As a non-citrated whole blood sample must be run in under 4 min, citrated samples are more widely employed. Storage and stability time, pipetting of reagents, vibration artifacts, and frequent quality control checks are all sources of concern (73, 80–82). United Kingdom National External Quality Assessment Scheme (NEQAS) data have revealed coefficients of variances of 7.1% to 39.9% for TEG and 7.0% to 83.6% for ROTEM (81). It should also be noted that a newer generation of both TEG (6s, employing resonance-based measurements rather than rotation resistance) and ROTEM (Sigma) equipment is now available, both of which are cartridge-based. These more automated devices may reduce inter-test variability and increase correlation between the two technologies (83, 84). Finally, as with all laboratory testing, TEG and ROTEM require clinical judgment for safe and appropriate interpretation. Moreover, if you can “hear the bleeding,” the patient does not need viscoelastic testing to guide the need for blood products, suture, and a surgeon.
Employing viscoelastic testing to guide resuscitation
As discussed previously, TEG and ROTEM, guided by sound clinical judgment, can efficiently identify patients likely to require massive transfusion. Taken as a whole, a hypocoagulable tracing should prompt immediate efforts to correct the modifiable contributors to TIC: obtaining early control of massive hemorrhage, addressing acidosis, preventing hypothermia, treating hypocalcemia, and avoiding dilutional coagulopathy. Individual components of the TEG or ROTEM tracing can further guide resuscitation strategies. A summary of the measures describes below is shown in Table 2.
Table 2.
A protocol for guided resuscitation based on rTEG values with normal and abnormal tracings
Prolonged reaction time (R) in TEG and clotting time (CT) in ROTEM indicates delayed initiation of the coagulation web. A surrogate in rTEG is the activated clotting time (ACT). As plasma contains the greatest concentration of clotting factors among fractionated blood products, many protocols start with infusion of plasma while starting red blood cells (RBC) during the initial phases of a resuscitation. While plasma use in patients who eventually required fewer than 10 units of RBC in 24 h may be considered undesirable, early administration of plasma in a massive transfusion event of more than 10 units improves survival (32, 85–87). R, ACT, and CT are the first values to return in viscoelastic testing. Thus, they sound the alarm for early activation of massive transfusion protocols (if not already ordered) and early transfusion of plasma.
Anticoagulant-associated hemorrhage can also often be identified via early viscoelastic measures, a critical finding in a patient with unknown medication history. R, ACT, and CT are usually prolonged in the presence of clinically significant bleeding due to direct thrombin inhibitors and Xa inhibitors such as dabigatran, rivaroxaban, and apixaban use (88, 89). R-time in the presence of dabigatran correlates linearly to gold-standard assays (90). It should be noted that warfarin does not similarly affect these values, and INR remains the standard for monitoring this anticoagulant. Although consensus is lacking regarding plasma and factor concentrate use, targeted methods do exist to treat anticoagulant-associated hemorrhage. Dabigatran can be reversed with idarucizumab (91), and andexanet alfa is now available for reversal of rivaroxaban and apixaban (92). Retrospective data have associated ROTEM-guided prothrombin complex concentrate use with increased survival and decreased transfusion requirements (93, 94), although prospective trials are still needed to validate these results.
The next resulting values in TEG and ROTEM describe clot kinetics and indicate the need for additional plasma, cryoprecipitate, and/or fibrinogen concentrate. The alpha angle in both TEG and ROTEM represents the early rate of fibrin polymerization and can be thought of as the slope of the curve as clot forms. The kinetic or “k-time” for TEG or clot formation time (CFT) for ROTEM measure the time from initiation of clotting (at the point of separation of the resistance curves) to a resistance level of 20 mm. The clot amplitude at 10 min (CA10) for ROTEM EXTEM, more specific for fibrinogen with FIBTEM, also describes early polymerization. Cryoprecipitate and fibrinogen concentrates, and FFP to a lesser degree, can maintain or increase the circulating fibrinogen to increase the rate of polymerization (95, 96). A clinical trial is underway to compare fibrinogen concentrate versus cryoprecipitate for this purpose (97).
Fibrinogen and platelets contribute to maximum amplitude (MA) for TEG and maximum clot firmness (MCF) for ROTEM. These values occur at the peak of the resistance curve and indicate the clot strength. Fibrinogen contributes between approximately 20% and 30% of the amplitude of MA and MCF, the rest belonging to platelet count and activity (98–100). While platelet transfusions are the obvious choice to correct a low MA or MCF, prospective data show that platelets may not restore function despite rise in platelet counts (22). Correction of MA and MCF should therefore take into consideration both platelet and fibrinogen replacement, especially if derangements in clot kinetics are apparent.
Fibrinolysis, based upon the percentage lysis at 30 mi (LY30) or clot lysis at 30 min (CL-30) after maximum amplitude (MA), has gained the most attention perhaps because viscoelastic testing identifies this issue whereas no other CCT reliably can. Many centers use a threshold of 3% lysis as the upper limit of normal, given the 10-fold increase in hemorrhagic death associated with LY30 > 3% (64), although consensus on the exact percentage is lacking. The Clinical Randomisation of Anti-fibrinolytic in Severe Hemorrhage trial (CRASH-2) (101) and Military Application of Tranexamic Acid in Trauma Emergency Resuscitation Study (MATTERs) (102) showed decrease in mortality with tranexamic acid (TXA) as an antifibrinolytic, but concerns abound regarding its use in certain settings (103), particularly given the increase in mortality and thromboembolic events seen if TXA was administered after 3 h postinjury. More selective use of TXA in only those patients demonstrating fibrinolysis has gained increasing support (104–106). The other end of the fibrinolytic spectrum also carries increased mortality risk. Often defined as LY30 < 0.8%, fibrinolysis shutdown was seen in 46% of severely injured patients in a large multicenter observational study. This phenotype, while not associated with as high a mortality as hyperfibrinolysis, shutdown remained an independent risk factor for mortality (OR 1.6 when compared to physiologic LY30 of 0.8–2.9%) (107). Given its high incidence, shutdown has also been used as an argument against nonselective use of TXA (108). It should be noted that this topic continues to be controversial, with ongoing debate regarding implications of shutdown, such as overdiagnosis, differences between TEG and ROTEM lysis values, and whether it actually represents a pathophysiologic phenomenon (109).
Guided versus fixed ratio resuscitation
Multiple algorithms have been published describing viscoelastic testing-guided resuscitation (57, 61, 110–115). Military experience, where judicious use of limited resources such as blood products becomes particularly essential, has shown ROTEM-guided resuscitation to be feasible (116, 117). A remaining question is whether this type of guided resuscitation is superior to CCT-guided or fixed ratio transfusion of red blood cells, plasma, and platelets, a standard fairly well established in clinical trials (33, 118). Three systematic reviews found a paucity of studies with low risk of bias regarding viscoelastic-guided identification and management of TIC (119–121), although some findings were encouraging. Veigas et al. found 13 observational studies after trauma, including nine prospective, showing ROTEM CA and MCF consistently predicted coagulopathy, massive transfusion, and mortality. Da Luz et al., with more relaxed search criteria, identified 55 studies and concluded viscoelastic testing “may also be used to direct blood and blood-product transfusion; effects on patient-important outcomes are uncertain.” Whiting et al. (122) pooled 31 studies to demonstrate substantial cost savings for viscoelastic testing of trauma patients. More recently, Wikkelsø et al. (123) performed a meta-analysis on 17 trials covering bleeding patients in both elective and trauma settings, finding that blood product use and mortality were decreased with TEG and ROTEM use, compared to historical controls. Unfortunately, risk of bias was generally high and the majority of patients underwent elective cardiac procedures. The most recent meta-analysis by Dias et al. (124) confirmed these findings in elective settings. The Denver group has recently published the only randomized trial in trauma patients comparing TEG to CCT-guided resuscitation in patients predicted to receive a massive transfusion. The Goal-Directed Hemostatic Resuscitation of Trauma-Induced Coagulopathy Trial showed a survival benefit at 28 days postinjury, as well as less transfused plasma and platelets, in patients receiving TEG-guided resuscitation (125). Additional clinical trials are underway to compare directly patient outcomes and transfusions with viscoelastic testing versus conventional management (126, 127).
CONCLUSION
Trauma causes 973 million people a year to seek care for injury worldwide (128). Among these casualties, hemorrhage remains a major cause of mortality, both in the immediate phase of care as well as later contributing to multi-organ system failure. TIC is associated with a 10-fold increase in mortality for the bleeding patient. Viscoelastic testing, including TEG and ROTEM, has been shown to predict massive transfusion requirement and identify TIC with greater sensitivity than clinical judgment or CCT. Further, TEG and ROTEM can guide resuscitation to combat specific derangements of physiologic coagulation within minutes of a patient's arrival. When minutes count, immediate control of hemorrhage and prevention of fulminant coagulopathy are keys to increasing survival. Serial reassessment of coagulation can then proceed. While viscoelastic testing has its limitations, it expediently provides a global assessment of clotting. In elective surgical settings, viscoelastic testing decreases blood product consumption, and the available trauma literature suggests the same may be true in emergency resuscitation. Ongoing trials may reinforce the growing evidence promoting guided resuscitation.
Footnotes
No funding was received for this work.
Dr BAC is on the Scientific Advisory Council for Haemonetics Corporation. There are no other relevant financial relationships or any sources of support in the form of grants, equipment, or drugs. For Dr JBB, no conflicts, actual or potential, are declared. The views expressed in this presentation are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
Dr.Cotton has served as a conultant for Haemonetics Corp (Braintree, MA), the makers of TEG. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Geeraedts L, Jr, Kaasjager H, Van Vugt A, Frölke J. Exsanguination in trauma: a review of diagnostics and treatment options. Injury 40 (1):11–20, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Neuman TS, Bockman MA, Moody P, Dunford JV, Griffith LD, Guber SL, Guss DA, Baxt WG. An autopsy study of traumatic deaths; San Diego County, 1979. Am J Surg 144 (6):722–727, 1982. [DOI] [PubMed] [Google Scholar]
- 3.Wright CS, McMurtry RY, Hoyle M, Pickard J. Preventable deaths in multiple trauma: review of deaths at Sunnybrook Medical Centre Trauma Unit. Can J Surg 26 (1):20–23, 1983. [PubMed] [Google Scholar]
- 4.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma 38 (2):185–193, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Curry N, Hopewell S, Doree C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care 15 (2):R92, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma 54 (6):1127–1130, 2003. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma 55 (1):39–44, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, Simanski C, Neugebauer E, Bouillon B. Society AGPotGT. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury 38 (3):298–304, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Spivey M, Parr MJ. Therapeutic approaches in trauma-induced coagulopathy. Minerva Anestesiol 71 (6):281–289, 2005. [PubMed] [Google Scholar]
- 10.Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, Holcomb JB. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma 64 (6):1459–1463, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma 65 (4):951–960, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman M. The cellular basis of traumatic bleeding. Mil Med 169: (12 suppl): 5–7, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Meng ZH, Wolberg AS, Monroe DM, 3rd, Hoffman M. The effect of temperature and pH on the activity of factor VIIa: implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J Trauma 55 (5):886–891, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Valeri CR, Feingold H, Cassidy G, Ragno G, Khuri S, Altschule MD. Hypothermia-induced reversible platelet dysfunction. Ann Surg 205 (2):175–181, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med 20 (10):1402–1405, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki N, Fujimoto Z, Morita T, Fukamizu A, Mizuno H. pH-Dependent structural changes at Ca(2+)-binding sites of coagulation factor IX-binding protein. J Mol Biol 353 (1):80–87, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth 95 (2):130–139, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care 11 (6):590–597, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Elmongy H, Sims C, Diamond SL. Ex vivo recapitulation of trauma-induced coagulopathy and preliminary assessment of trauma patient platelet function under flow using microfluidic technology. J Trauma Acute Care Surg 80 (3):440–449, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan S, Brohi K, Chana M, Raza I, Stanworth S, Gaarder C, Davenport R. Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg 76 (3):561–567, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Khan S, Davenport R, Raza I, Glasgow S, De’Ath HD, Johansson PI, Curry N, Stanworth S, Gaarder C, Brohi K. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med 41 (2):239–247, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Vulliamy P, Gillespie S, Gall LS, Green L, Brohi K, Davenport RA. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J Trauma Acute Care Surg 83 (3):388–397, 2017. [DOI] [PubMed] [Google Scholar]
- 23.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg 245 (5):812–818, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davenport RA, Guerreiro M, Frith D, Rourke C, Platton S, Cohen M, Pearse R, Thiemermann C, Brohi K. Activated protein C drives the hyperfibrinolysis of acute traumatic coagulopathy. Anesthesiology 126 (1):115–127, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, Frith D, Pittet JF, Cohen MJ. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock 32 (6):659–665, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cadroy Y, Diquelou A, Dupouy D, Bossavy JP, Sakariassen KS, Sie P, Boneu B. The thrombomodulin/protein C/protein S anticoagulant pathway modulates the thrombogenic properties of the normal resting and stimulated endothelium. Arterioscler Thromb Vasc Biol 17 (3):520–527, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, Mitra S, Ghasabyan A, Chin TL, Sauaia A, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg 80 (1):16–23, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gall LS, Vulliamy P, Gillespie S, Jones TF, Pierre RSJ, Breukers SE, Gaarder C, Juffermans NP, Maegele M, Stensballe J, et al. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann Surg 269:1184–1191, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Brown LM, Call MS, Knudson M, Cohen MJ, Trauma Outcomes G, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G, MacLeod J, et al. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma 71: (2 suppl 3): S337–342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matijevic N, Wang YW, Wade CE, Holcomb JB, Cotton BA, Schreiber MA, Muskat P, Fox EE, Del Junco DJ, Cardenas JC, et al. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thromb Res 134 (3):652–658, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg 248 (3):447–458, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg 197 (5):565–570, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 313 (5):471–482, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma 66: (4 suppl): S69–76, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer DE, Vincent LE, Fox EE, O’Keeffe T, Inaba K, Bulger E, Holcomb JB, Cotton BA. Every minute counts: time to delivery of initial massive transfusion cooler and its impact on mortality. J Trauma Acute Care Surg 83 (1):19–24, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pommerening MJ, Goodman MD, Holcomb JB, Wade CE, Fox EE, Del Junco DJ, Brasel KJ, Bulger EM, Cohen MJ, Alarcon LH, et al. Clinical gestalt and the prediction of massive transfusion after trauma. Injury 46 (5):807–813, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 13 (6):680–685, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Stahel PF, Moore EE, Schreier SL, Flierl MA, Kashuk JL. Transfusion strategies in postinjury coagulopathy. Curr Opin Anaesthesiol 22 (2):289–298, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Hoyt DB, Dutton RP, Hauser CJ, Hess JR, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, et al. Management of coagulopathy in the patients with multiple injuries: results from an international survey of clinical practice. J Trauma 65 (4):755–764, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, Allaouchiche B, Negrier C. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost 5 (2):289–295, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Spahn DR, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Komadina R, Neugebauer E, et al. Management of bleeding following major trauma: a European guideline. Crit Care 11 (1):R17, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossaint R, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Neugebauer E, Spahn DR. Key issues in advanced bleeding care in trauma. Shock 26 (4):322–331, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Mann KG, Brummel K, Butenas S. What is all that thrombin for? J Thromb Haemost 1 (7):1504–1514, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Toulon P, Ozier Y, Ankri A, Fleron MH, Leroux G, Samama CM. Point-of-care versus central laboratory coagulation testing during haemorrhagic surgery. A multicenter study. Thromb Haemost 101 (2):394–401, 2009. [PubMed] [Google Scholar]
- 45.Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, Hart D, Pearse R, Pasi KJ, MacCallum P, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med 39 (12):2652–2658, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frith D, Davenport R, Brohi K. Acute traumatic coagulopathy. Curr Opin Anaesthesiol 25 (2):229–234, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Hagemo JS, Christiaans SC, Stanworth SJ, Brohi K, Johansson PI, Goslings JC, Naess PA, Gaarder C. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care 19:97, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cotton BA, Minei KM, Radwan ZA, Matijevic N, Pivalizza E, Podbielski J, Wade CE, Kozar RA, Holcomb JB. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg 72 (6):1470–1475, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, Kozar RA, Holcomb JB. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma 71 (2):407–414, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Folkerson LE, Sloan D, Cotton BA, Holcomb JB, Tomasek JS, Wade CE. Predicting progressive hemorrhagic injury from isolated traumatic brain injury and coagulation. Surgery 158 (3):655–661, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Meyer AS, Meyer MA, Sorensen AM, Rasmussen LS, Hansen MB, Holcomb JB, Cotton BA, Wade CE, Ostrowski SR, Johansson PI. Thrombelastography and rotational thromboelastometry early amplitudes in 182 trauma patients with clinical suspicion of severe injury. J Trauma Acute Care Surg 76 (3):682–690, 2014. [DOI] [PubMed] [Google Scholar]
- 52.Schochl H, Cotton B, Inaba K, Nienaber U, Fischer H, Voelckel W, Solomon C. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care 15 (6):R265, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogel AM, Radwan ZA, Cox CS, Jr, Cotton BA. Admission rapid thrombelastography delivers real-time “actionable” data in pediatric trauma. J Pediatr Surg 48 (6):1371–1376, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Bouzat P, Guerin R, Boussat B, Nicolas J, Lambert A, Greze J, Maegele M, David JS. Diagnostic performance of thromboelastometry in trauma-induced coagulopathy: a comparison between two level I trauma centres using two different devices. Eur J Trauma Emerg Surg 2019; Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Carroll RC, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, Dakin PA, Lawson CM, Enderson BL, Kurek SJ. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res 154 (1):34–39, 2009. [DOI] [PubMed] [Google Scholar]
- 56.Cohen J, Scorer T, Wright Z, Stewart IJ, Sosnov J, Pidcoke H, Fedyk C, Kwan H, Chung KK, Heegard K, et al. A prospective evaluation of thromboelastometry (ROTEM) to identify acute traumatic coagulopathy and predict massive transfusion in military trauma patients in Afghanistan. Transfusion 59 (S2):1601–1607, 2019. [DOI] [PubMed] [Google Scholar]
- 57.Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence J, Biffl WL, Burlew CC, Barnett C, Sawyer M, et al. Initial experiences with point-of-care rapid thrombelastography for management of life-threatening postinjury coagulopathy. Transfusion 52 (1):23–33, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Liou DZ, Shafi H, Bloom MB, Chung R, Ley EJ, Salim A, Tcherniantchouk O, Margulies DR. Defining early trauma-induced coagulopathy using thromboelastography. Am Surg 80 (10):994–998, 2014. [PubMed] [Google Scholar]
- 59.Ostrowski SR, Sorensen AM, Larsen CF, Johansson PI. Thrombelastography and biomarker profiles in acute coagulopathy of trauma: a prospective study. Scand J Trauma Resusc Emerg Med 19:64, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tauber H, Innerhofer P, Breitkopf R, Westermann I, Beer R, El Attal R, Strasak A, Mittermayr M. Prevalence and impact of abnormal ROTEM(R) assays in severe blunt trauma: results of the ’Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br J Anaesth 107 (3):378–387, 2011. [DOI] [PubMed] [Google Scholar]
- 61.Pezold M, Moore EE, Wohlauer M, Sauaia A, Gonzalez E, Banerjee A, Silliman CC. Viscoelastic clot strength predicts coagulation-related mortality within 15 minutes. Surgery 151 (1):48–54, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartert H. Blood coagulation studies using thromboelastography, a new evaluation technique. Klin Wochenschr 26:577–583, 1948. [DOI] [PubMed] [Google Scholar]
- 63.Kang Y. Thromboelastography in liver transplantation. Semin Thromb Hemost 21: (suppl 4): 34–44, 1995. [PubMed] [Google Scholar]
- 64.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg 75 (6):961–967, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walsh M, Thomas SG, Howard JC, Evans E, Guyer K, Medvecz A, Swearingen A, Navari RM, Ploplis V, Castellino FJ. Blood component therapy in trauma guided with the utilization of the perfusionist and thromboelastography. J Extra Corpor Technol 43 (3):162–167, 2011. [PMC free article] [PubMed] [Google Scholar]
- 66.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 88 (2):312–319, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma 42 (4):716–720, 1997. [DOI] [PubMed] [Google Scholar]
- 68.Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth 20 (4):548–553, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Afshari A, Wikkelso A, Brok J, Moller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev 2011; (3):CD007871. [DOI] [PubMed] [Google Scholar]
- 70.Venema LF, Post WJ, Hendriks HG, Huet RC, de Wolf JT, de Vries AJ. An assessment of clinical interchangeability of TEG and RoTEM thromboelastographic variables in cardiac surgical patients. Anesth Analg 111 (2):339–344, 2010. [DOI] [PubMed] [Google Scholar]
- 71.Nielsen VG. A comparison of the thrombelastograph and the ROTEM. Blood Coagul Fibrinolysis 18 (3):247–252, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Jackson GN, Ashpole KJ, Yentis SM. The TEG vs the ROTEM thromboelastography/thromboelastometry systems. Anaesthesia 64 (2):212–215, 2009. [DOI] [PubMed] [Google Scholar]
- 73.Haemonetics Corporation TEG®5000 System User Manual. Niles, IL: Haemonetics Corp., Haemoscope Division; 2011. [Google Scholar]
- 74.ROTEM® delta Manual 2.2.0.01.EN. Munich, Germany: Tem Innovations GmbH; 2012. [Google Scholar]
- 75.Johansson PI, Henriksen HH, Stensballe J, Gybel-Brask M, Cardenas JC, Baer LA, Cotton BA, Holcomb JB, Wade CE, Ostrowski SR. Traumatic endotheliopathy: a prospective observational study of 424 severely injured patients. Ann Surg 265 (3):597–603, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg 73 (1):60–66, 2012. [DOI] [PubMed] [Google Scholar]
- 77.Wolberg AS, Meng ZH, Monroe DM, 3rd, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma 56 (6):1221–1228, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Ramaker AJ, Meyer P, van der Meer J, Struys MM, Lisman T, van Oeveren W, Hendriks HG. Effects of acidosis, alkalosis, hyperthermia and hypothermia on haemostasis: results of point-of-care testing with the thromboelastography analyser. Blood Coagul Fibrinolysis 20 (6):436–439, 2009. [DOI] [PubMed] [Google Scholar]
- 79.Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, Huang H, Pinto R, Speers V, Reis M, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res 42 (12):1210–1217, 2009. [DOI] [PubMed] [Google Scholar]
- 80.Zambruni A, Thalheimer U, Leandro G, Perry D, Burroughs AK. Thromboelastography with citrated blood: comparability with native blood, stability of citrate storage and effect of repeated sampling. Blood Coagul Fibrinolysis 15 (1):103–107, 2004. [DOI] [PubMed] [Google Scholar]
- 81.Kitchen DP, Kitchen S, Jennings I, Woods T, Walker I. Quality assurance and quality control of thrombelastography and rotational Thromboelastometry: the UK NEQAS for blood coagulation experience. Semin Thromb Hemost 36 (7):757–763, 2010. [DOI] [PubMed] [Google Scholar]
- 82.Perry DJ, Fitzmaurice DA, Kitchen S, Mackie IJ, Mallett S. Point-of-care testing in haemostasis. Br J Haematol 150 (5):501–514, 2010. [DOI] [PubMed] [Google Scholar]
- 83.Gill M. The TEG®6 s on Shaky Ground? A novel assessment of the TEG®6 s performance under a challenging condition. J Extra-corporeal Technol 49 (1):26–29, 2017. [PMC free article] [PubMed] [Google Scholar]
- 84.Ziegler B, Voelckel W, Zipperle J, Grottke O, Schöchl H. Comparison between the new fully automated viscoelastic coagulation analysers TEG 6 s and ROTEM Sigma in trauma patients: a prospective observational study. Eur J Anaesthesiol 36:834–842, 2019. [DOI] [PubMed] [Google Scholar]
- 85.Chang R, Folkerson LE, Sloan D, Tomasek JS, Kitagawa RS, Choi HA, Wade CE, Holcomb JB. Early plasma transfusion is associated with improved survival after isolated traumatic brain injury in patients with multifocal intracranial hemorrhage. Surgery 161 (2):538–545, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cardenas JC, Holcomb JB. Time to plasma transfusion: a patient centered approach and modifiable risk factor. Transfusion 57 (4):869–873, 2017. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma 62 (1):112–119, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Dias JD, Norem K, Doorneweerd DD, Thurer RL, Popovsky MA, Omert LA. Use of thromboelastography (TEG) for detection of new oral anticoagulants. Arch Pathol Lab Med 139 (5):665–673, 2015. [DOI] [PubMed] [Google Scholar]
- 89.Benes J, Zatloukal J, Kletecka J. Viscoelastic methods of blood clotting assessment—a multidisciplinary review. Front Med (Lausanne) 2:62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Solbeck S, Ostrowski SR, Stensballe J, Johansson PI. Thrombelastography detects dabigatran at therapeutic concentrations in vitro to the same extent as gold-standard tests. Int J Cardiol 208:14–18, 2016. [DOI] [PubMed] [Google Scholar]
- 91.Pollack CV, Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, et al. Idarucizumab for dabigatran reversal. N Engl J Med 373 (6):511–520, 2015. [DOI] [PubMed] [Google Scholar]
- 92.Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, Mathur VS, Castillo J, Bronson MD, Leeds JM, et al. Andexanet Alfa for the reversal of Factor Xa inhibitor activity. N Engl J Med 373 (25):2413–2424, 2015. [DOI] [PubMed] [Google Scholar]
- 93.Schochl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care 14 (2):R55, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schochl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, Arndt C, Hanke A, Voelckel W, Solomon C. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care 15 (2):R83, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seebold JA, Campbell D, Wake E, Walters K, Ho D, Chan E, Bulmer AC, Wullschleger M, Winearls J. Targeted fibrinogen concentrate use in severe traumatic haemorrhage. Crit Care Resusc 21 (3):171–178, 2019. [PubMed] [Google Scholar]
- 96.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 10 (7):1342–1351, 2012. [DOI] [PubMed] [Google Scholar]
- 97.Winearls J, Wullschleger M, Wake E, Hurn C, Furyk J, Ryan G, Trout M, Walsham J, Holley A, Cohen J, et al. Fibrinogen Early In Severe Trauma studY (FEISTY): study protocol for a randomised controlled trial. Trials 18 (1):241, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moore HB, Moore EE, Chapman MP, Gonzalez E, Slaughter AL, Morton AP, D’Alessandro A, Hansen KC, Sauaia A, Banerjee A, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost 13 (10):1878–1887, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peng HT, Nascimento B, Beckett A. Thromboelastography and thromboelastometry in assessment of fibrinogen deficiency and prediction for transfusion requirement: a descriptive review. Biomed Res Int 2018:7020539, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harr JN, Moore EE, Ghasabyan A, Chin TL, Sauaia A, Banerjee A, Silliman CC. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock 39 (1):45–49, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.collaborators C-t. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 376 (9734):23–32, 2010. [DOI] [PubMed] [Google Scholar]
- 102.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg 147 (2):113–119, 2012. [DOI] [PubMed] [Google Scholar]
- 103.Pusateri AE, Weiskopf RB, Bebarta V, Butler F, Cestero RF, Chaudry IH, Deal V, Dorlac WC, Gerhardt RT, Given MB, et al. Tranexamic acid and trauma: current status and knowledge gaps with recommended research priorities. Shock 39 (2):121–126, 2013. [DOI] [PubMed] [Google Scholar]
- 104.Valle EJ, Allen CJ, Van Haren RM, Jouria JM, Li H, Livingstone AS, Namias N, Schulman CI, Proctor KG. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg 76 (6):1373–1378, 2014. [DOI] [PubMed] [Google Scholar]
- 105.Khan M, Jehan F, Bulger EM, O’Keeffe T, Holcomb JB, Wade CE, Schreiber MA, Joseph B. Severely injured trauma patients with admission hyperfibrinolysis: Is there a role of tranexamic acid? Findings from the PROPPR trial. J Trauma Acute Care Surg 85 (5):851–857, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harvin JA, Peirce CA, Mims MM, Hudson JA, Podbielski JM, Wade CE, Holcomb JB, Cotton BA. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg 78 (5):905–909, 2015. [DOI] [PubMed] [Google Scholar]
- 107.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, Sauaia A, Cotton BA. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg 222 (4):347–355, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moore EE, Moore HB, Gonzalez E, Chapman MP, Hansen KC, Sauaia A, Silliman CC, Banerjee A. Postinjury fibrinolysis shutdown: rationale for selective tranexamic acid. J Trauma Acute Care Surg 78: (6 suppl 1): S65–69, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gomez-Builes JC, Acuna SA, Nascimento B, Madotto F, Rizoli SB. Harmful or physiologic: diagnosing fibrinolysis shutdown in a trauma cohort with rotational thromboelastometry. Anesth Analg 127 (4):840–849, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johansson PI, Ostrowski SR, Secher NH. Management of major blood loss: an update. Acta Anaesthesiol Scand 54 (9):1039–1049, 2010. [DOI] [PubMed] [Google Scholar]
- 111.Enriquez LJ, Shore-Lesserson L. Point-of-care coagulation testing and transfusion algorithms. Br J Anaesth 103: (suppl 1): i14–i22, 2009. [DOI] [PubMed] [Google Scholar]
- 112.Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, Spain DA, Brundage SI. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg 209 (2):198–205, 2009. [DOI] [PubMed] [Google Scholar]
- 113.Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, Perkins JG, Holcomb JB. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma 64: (2 suppl): S64–68, 2008. [DOI] [PubMed] [Google Scholar]
- 114.Baksaas-Aasen K, Van Dieren S, Balvers K, Juffermans NP, Naess PA, Rourke C, Eaglestone S, Ostrowski SR, Stensballe J, Stanworth S, et al. Data-driven development of ROTEM and TEG algorithms for the management of trauma hemorrhage: a prospective observational multicenter study. Ann Surg 270:1178–1185, 2019. [DOI] [PubMed] [Google Scholar]
- 115.Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, Komadina R, Maegele M, Nardi G, Riddez L, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care 23 (1):98, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Keene DD, Nordmann GR, Woolley T. Rotational thromboelastometry-guided trauma resuscitation. Curr Opin Crit Care 19 (6):605–612, 2013. [DOI] [PubMed] [Google Scholar]
- 117.Prat NJ, Meyer AD, Ingalls NK, Trichereau J, DuBose JJ, Cap AP. Rotational thromboelastometry significantly optimizes transfusion practices for damage control resuscitation in combat casualties. J Trauma Acute Care Surg 83 (3):373–380, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown LM, Aro SO, Cohen MJ, Trauma Outcomes G, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G, MacLeod J, Dutton RP, et al. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma 71: (2 suppl 3): S358–363, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Da Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NK. Effect of thromboelastography (TEG(R)) and rotational thromboelastometry (ROTEM(R)) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care 18 (5):518, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, Zhelev Z, Hyde C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev 2015; (2):Cd010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM(R)) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med 24 (1):114, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, Misso K, Ross J, Severens J, Kleijnen J. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess 19 (58):1–228, 2015. v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wikkelso A, Wetterslev J, Moller AM, Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta-analysis and trial sequential analysis. Anaesthesia 72 (4):519–531, 2017. [DOI] [PubMed] [Google Scholar]
- 124.Dias JD, Sauaia A, Achneck HE, Hartmann J, Moore EE. Thromboelastography-guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: a systematic review and analysis. J Thromb Haemost 17 (6):984–994, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg 263 (6):1051–1059, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baksaas-Aasen K, Gall L, Eaglestone S, Rourke C, Juffermans NP, Goslings JC, Naess PA, van Dieren S, Ostrowski SR, Stensballe J. iTACTIC–implementing Treatment Algorithms for the Correction of Trauma-Induced Coagulopathy: study protocol for a multicentre, randomised controlled trial. Trials 18 (1):486, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.dos Reis Rodrigues ROR, Lucena L, Paiva H, Cordeiro V, José Carmona M, Costa Auler JO, Massazo Utiyama E, Gorlinger K, Spahn D, Schöchl H. STATA—Strategy of Transfusion in Trauma Patients: A Randomized Trial. J Clin Trials 6:287, 2016. [Google Scholar]
- 128.Haagsma JA, Graetz N, Bolliger I, Naghavi M, Higashi H, Mullany EC, Abera SF, Abraham JP, Adofo K, Alsharif U, et al. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev 22 (1):3–18, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]