Abstract:
Necrotizing infundibular crystalline folliculitis is a rare entity, which is a distinctive clinical and histopathological entity. Eruptive yellow waxy umbilicated folliculocentric plugs clinically correspond to pale crystalline filaments embedded in an amorphous sebum-rich material. Remarkably, only the superficial infundibular ostia remain, and the distended cavity is devoid of a follicular or sebaceous gland remnant. The pathogenesis of this enigmatic event remains to be established. The emergence of necrotizing infundibular crystalline folliculitis (NICF) as a paradoxical side effect of antitumor inhibitors epidermal growth factor receptor vascular endothelial growth factor and more recently programmed death-1 represents the expression of altered molecular pathways that underpin the pathogenesis of NICF. To explore these pathways, it is necessary to explore the hierarchy of follicular stem cells, particularly the potential role of committed infundibular stem cells that play a key role in wound healing. Committed infundibular stem cells are closely linked to the sebaceous gland stem cell axis, and this has relevance in the process of homeostatic repair of sebaceous follicles in the wake of folliculitis. The unscheduled modulation of this infundibular homeostatic sebaceous repair axis by epidermal growth factor receptor vascular endothelial growth factor, and programmed death-1 may lead to an aberrant outcome with metaplasia of infundibular keratinocytes to sebocytes. In the absence of sebaceous gland differentiation, these metaplastic infundibular sebocyte cells would lead to the consumption and loss of the infundibulum as a result of holocrine sebum production. This conceptual pathogenic pathway for NICF is constructed by incorporating recent advances in the fields of follicular stem cells, wound repair, follicular homeostasis, regulatory T cells, and molecular pathways linked to the biologicals inducing NICF.
Keywords: necrotizing infundibular crystalline folliculitis, infundibular stem cells, sebaceous stem cells, PD-1, EGFR, VEGF inhibitors
INTRODUCTION
Necrotizing infundibular crystalline folliculitis (NICF) remains an enigmatic process with distinctive clinical and histopathological features. Initially described as an unusual epidermal perforating disorder with urate-like crystals,1 it was subsequently reclassified as a folliculocentric process designated as NICF.2 The pathogenesis of NICF remains elusive. The emergence of NICF as a paradoxical side effect of antitumor inhibitors epidermal growth factor receptor (EGFR) vascular endothelial growth factor (VEGF),3 and more recently programmed death-1 (PD-1)4 represents the expression of altered molecular pathways that underpin the pathogenesis of NICF and are activated by these agents. To explore this aspect, it is necessary to integrate both the clinical and histopathological aspects of NICF and review the complex hierarchy of follicular stem cells5,6,7 and their role in maintaining follicular structural and functional homeostasis including cyclical trichogenesis. A key element in formulating the pathogenesis of NICF is the potential role of committed infundibular stem cells that have the dual capacity of infundibulogenesis and contributing to epidermal wound repair.8,9 This subset of committed stem cells undergoes rapid amplifications as a response to wound healing. This repair process is likely to be pertinent in reconstituting the integrity of the sebaceous infundibular axis in the wake of acute folliculitis. Eruptive NICF is a rare event and is likely to be the result of synchronous combination of multiple cytokine pathways and elements governing follicular repair. The terminal production of polarizable crystalline concretions with the consumption of follicular and epidermal structures may represent an expression of abortive sebaceous folliculogenesis. Ultimately, the rarity of NICF may reflect the strong homeostatic controls linked to the complex interactive cytokine pathways and T-cell subsets governing homeostasis10,11 and a narrow window for the emergence of NICF as a distinctive entity linked to healing follicles.
DEFINING NICF
Characteristically, NICF presents clinically with the sudden appearance of numerous matted pale waxy filamentous follicular plugs (Fig. 1A) that are localized to the seborrheic areas, particularly the forehead and upper back.
FIGURE 1.

A, Clinical presentation with matted waxy fibrillar follicular plugs on the forehead. B, Epidermal parakeratosis, dilated follicular cavity, superficial infundibular ostial remnant, and bare interface of crystalline contents with dermis (H&E, ×40). C, Base and sides of bare cavity lacking a lining as a result of infundibular necrosis (H&E, ×100). D, Details of cavity with minimal dermal inflammation at the base and no granulomatous response to vertically orientated crystalline filaments (H&E, ×250).
Histopathology reveals pale follicular-based plugs consisting of crystalloid urate-like filaments embedded within a pale sebaceous and at times mucinous amorphous concretion. Strikingly, only the superficial remnants of infundibular epithelium with parakeratotic epithelial lips are recognizable and the remaining histopathological features defining sebaceous follicles are usually no longer apparent (Figs. 1B, C). A distinctive feature is the lack of epithelium at the base of the sebaceous concretion containing upward streaming crystalline filamentous material that abuts a naked dermal interface (Fig. 1D). Minimal dermal inflammation is present at the naked base, and a granulomatous reaction is absent despite the crystalline contents.
Multiple case reports of NICF have been published10–13 including a series of 9 cases by Denisjuk et al14 who analyzed the clinicopathological features and found that there was a predilection for lesions to be localized to the seborrheic-rich forehead and back. Birefringent crystalline sebaceous deposits were evident within follicular ostia and were bounded by parakeratotic columns and apparent necrosis of the follicular epithelium. Both yeasts and gram-positive bacteria were found in 56% of their NICF cases. NICF was considered to be pathogenically linked to these organisms and the birefringent crystals to be related to keratin tonofilaments rather than cholesterol.
Although NICF seemed to be an uncommon event, in this study, the same crystalline structured sebum-rich material admixed with organisms, particularly yeast, was observed as an incidental feature and as a more frequent feature in intact sebaceous follicles. This incidental finding usually occurs within cystically dilated fully differentiated sebaceous follicles (Fig. 2) or as limited crystalline plugs at the orifice of intact sebaceous follicles. The necrotizing aspects of eruptive NICF with the loss of both infundibular and mature sebaceous gland differentiation are absent.
FIGURE 2.
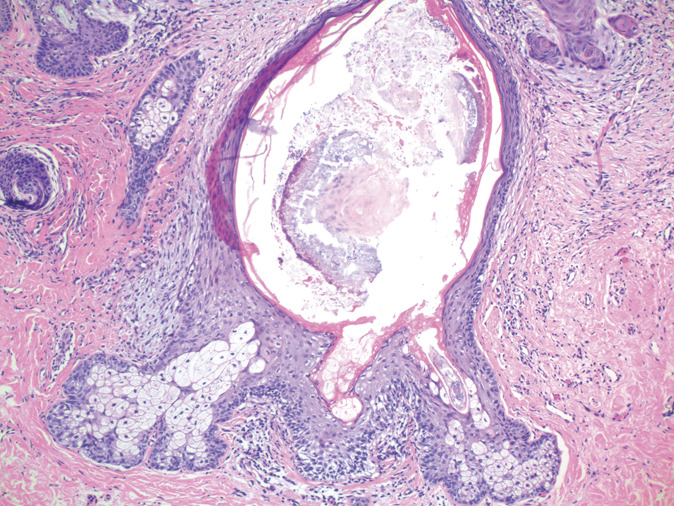
Dilated intact cystic infundibular canal with crystalline deposits and surrounding arcuate strands of keratin with scant pityrosporum and bacterial organisms (H&E, ×100).
STRUCTURAL AND FUNCTIONAL HOMEOSTASIS OF HAIR FOLLICLES AND HIERARCHICAL COMMITTED STEM CELLS
Although follicular stem cells are pluripotential with respect to regenerative medicine,15 for the purpose of homeostasis, a hierarchy of committed stem cells exist and are ultimately identified by their terminal differentiation both as a single element such as the infundibulum or as a complex structured array linked to trichogenesis. The follicular bulge region houses the major niche for progenitor stem cells dedicated to generating committed stem cells in regulated pathways.6,7,16 Hair follicles represent a complex model for committed stem cells because follicles have a regenerative cycling trichogenic component linked to a permanent component comprising the isthmus, bulge, infundibulum, and sebaceous glands. This division serves to highlight the hierarchy of committed stem cells.
For trichogenesis, the process is complex because a spatially grouped set of committed stem cells must be integrated within the hair bulb complex under the control of mesenchymal mediators and cytokines linked to modulating hair follicle regulatory T cells17–19 and other mesenchymal signals. This gestational process takes up to 3 months and corresponds to the telogen phase of the hair cycle. Regulatory T cells interact with stem cells during the hair cycle, and studies have shown increased regulatory T cells during telogen and a marked decrease of regulatory T cells in anagen.18 Although hair growth is quiescent during telogen, the increased regulatory T cells reflect their importance during this gestational phase in which committed stem cells are assembled for anagen. Anagen heralds the activation of the assembled committed stem cells and the onset of the transitory amplifying cell phase in which committed stem cells are clonally expanded before terminal differentiation.
The creation of a structured complex of committed stem cell elements forms the basis for both trichogenesis and a new follicular root sheath complex integrated within the anagen phase. This rapid amplifying phase has been recognized as the fast lane from stem cells toward terminal differentiation and the anagen phase.20
By contrast, the permanent portion of the hair follicle does not undergo involution but is maintained by committed stem cells that are constantly active in maintaining structural and functional homeostasis. Sebaceous glands are complex structures, and their genesis and homeostasis are dependent on mesenchymal signals, including EGFR and VEGF, and regulatory T cells that are also capable of inducing follicular-derived and epidermal-derived sebaceous glands from resident stem cells.21–24 The sebaceous glands are directly connected to the infundibular canal through cuticular-lined sebaceous ducts forming an axis representing a complex homeostatically controlled structure. Sebum production is more prominent than trichogenesis in sebaceous follicles.
Committed infundibular stem cells are not only recognized by terminal infundibulogenesis but also have an important role in wound healing. The latter function distinguishes committed infundibular stem cells as a special subset because wound healing requires rapid amplification of keratinocytes without passing through the complex induction of progenitor cells required for sebaceous gland induction and trichogenesis.
NICF AND COMMITTED INFUNDIBULAR STEM CELLS
NICF has been linked both clinically and histopathologically to the follicular infundibulum. Infundibulogenesis is dependent on the activation of committed infundibular stem cells, and importantly, the infundibulum has an epidermoid lining integrated to the interfollicular epidermis with an indistinguishable pattern of differentiation. This aspect is key for the committed infundibular stem cells in wound repair and their entry into a rapidly amplifying phase with clonal expansion and production of keratinocytes to re-establish epidermal integrity. The same process is likely to apply to the healing phase of a folliculitis that damages or disrupts the infundibular lining requiring subsequent repair.
The histopathology of NICF is dominated by sebum production but an absence of recognizable structured sebaceous glands. There is a close relationship of the sebaceous glands to the infundibulum because the sebaceous glands are directly linked to the infundibulum. This is relevant in structural and functional homeostasis because infundibular committed stem cells have a close spatial relationship to the committed sebaceous gland pathways that may lead to metaplasia of infundibular keratinocytes to aberrant sebum production. As a result of sudden unscheduled rapid amplification inherent in the infundibular committed stem cell pathway, there is a potential for failure to form differentiated sebaceous glands. The resulting aberrant sebum producing infundibular stem cells may undergo clonal expansion and disintegrate as a result of holocrine sebum production leading to a total loss of the integrity of the infundibulum rather than true necrosis. The resolution of NICF is enhanced by the presence of open expanded infundibular orifices providing a passage for the elimination of sebaceous concretions that are the product of terminal sebum production.
The rare phenomenon of NICF may represent an aberrant pathway with disrupted sebaceous follicular histogenesis due to a loss of homeostatic control. A series of synchronized events inherent in the committed infundibular stem pathway linked to the healing phase of a folliculitis, as well as temporary activating triggers, such as a mix of organisms in the sebum-rich follicles may be factors in activating NICF. An important element in NICF is the key roles of regulatory T cells in maintaining homeostatic integrity of sebaceous follicles and also in establishing immune tolerance to skin commensal microbes.17
The reported emergence of unexpected eruptive NICF in patients with metastatic adenocarcinomas receiving EGFR, VEGF, or PD-1 inhibitors as biological antitumor inhibitory agents provides an opportunity to further explore the pathogenesis of NICF. Ultimately, these inhibitors block or activate biological and immunological pathways that are integral to the pathogenesis of NICF.
BIOLOGICAL ANTITUMOR INHIBITORS AND THE PARADOXICAL DEVELOPMENT OF ERUPTIVE NICF
Two patients receiving either VEGF or EGFR inhibitors for metastatic adenocarcinomas developed eruptive NICF with the emergence of clinically typical waxy crystalline folliculocentric plugs and the distinct histopathological features of NICF.3 The eruption in both cases was particularly localized to acne prone seborrheic skin areas. In one case, the lesions settled and recurred cyclically every 3 weeks after each injection of VEGF inhibitor and recurred cyclically even when treatment was changed to an EGFR inhibitor. In the second patient receiving oral EGFR inhibitor, the NICF persisted over an 8-month period until the drug was discontinued.
Destruction and repair to the follicular infundibulum itself is likely to be integral to activating the committed infundibular committed stem cell pathway for NICF. Both VEGF and EGFR inhibitors have pustular and necrotizing folliculitis as a common side effect.25,26
Eruptive NICF has also been subsequently reported in a patient receiving a PD-1 inhibitor for metastatic renal carcinoma.4 The eruptive lesions appeared in a dermatomal distribution over the upper trunk in the site of healing herpes zoster. The histopathology in PD-1 inhibitor herpes zoster case revealed massive sebaceous concretions that differed from those usually restricted to individual infundibular canals and clearly destroyed the interfollicular epidermis connecting multiple follicles. This difference may reflect the fact that HZV-induced necrosis is not restricted to follicles and involves the interfollicular epidermis. The rapid reparative amplification of clonally altered sebum producing infundibular stem cells may lead to a form of consumptive destruction of the interfollicular epidermis through terminal sebum production.
Both EGFR and VEGF have been shown to be important in sebaceous gland physiology, homeostasis, and stem cell modulation as well as sebum production.17–19 The introduction of these inhibitors during the critical healing phase of sebaceous folliculitis may play a key role in the aberrant sebaceous metaplasia of infundibular keratinocytes by abrogating the normal differentiation pathway of the infundibulum to directly produce sebum without glandular differentiation.
Regulator T cells have been recognized as a heterogenous group of cells including a key role in many aspects of homeostasis. This includes follicular-related regulatory T cells that have many functions including modulating follicular stem cells, maintaining follicular homeostasis, immune privilege, tolerance, and also participating in the process of wound healing.9,10,21
Subsets of regulatory T cells have also been identified that have nonimmunological roles in relationship to stem cell modulation. Hair follicles have a subset of regulatory T cells that express high levels of the Notch pathway, Jagged 1 (Jag1) that stimulate stem cells critical to trichogenesis, follicular differentiation, and homeostasis. The PD-1/PD-L1, VEGF/VEGFR, and EGFR/amphiregulin axes govern multiple pathways of signal transduction.22–25 These pathways are widely expressed and are key in maintaining skin homeostasis, including those related to the follicular infundibulum and sebaceous glands. Repair Tregs have been identified that are integrated to these signal transduction pathways forming a complex wound healing and follicular repair system encompassing infundibular committed stem cell.
The concept that regulatory T cells play an integral role in both immune and nonimmune homeostasis is important.17 This seems pertinent to the pathogenesis of NICF that would represent an example of nonimmune homeostatic disorder. NICF may ultimately represent a breakdown of the sebaceous follicular repair pathway dependent on alteration of regulatory T cells and their homeostatic role in restoring sebaceous follicular integrity.
Footnotes
The author declares no conflicts of interest.
REFERENCES
- 1.Lucke TW, Fallowfield MF, Evans A, et al. Transepidermal elimination of urate-like crystals: a new perforating disorder?. Br J Dermatol. 1999;141:310–314. [DOI] [PubMed] [Google Scholar]
- 2.Kossard S, Scurry J, Killingsworth M. Necrotizing infundibular crystalline folliculitis. Brit J Dermatol. 2001;145:165–168. [DOI] [PubMed] [Google Scholar]
- 3.Fattouh K, Collet-Benzaquen D, Provensal AM, et al. Necrotizing infundibular crystalline folliculitis (NICF) induced by anti-tumoral therapies: report of 2 cases. Am J Dermatopathol. 2017;39:764–766. [DOI] [PubMed] [Google Scholar]
- 4.Fischer AS, Pei S, Shields BE, et al. Dermatomal necrotizing infundibular crystalline folliculitis following herpes zoster in a patient on PD-1 inhibitor therapy. J Cutan Pathol. 2020;47:501–505. [DOI] [PubMed] [Google Scholar]
- 5.Lavker RM, Sun T-T, Oshima H, et al. Hair follicle stem cells. JID Symposium Proceedings. 2003;8:28–38. [DOI] [PubMed] [Google Scholar]
- 6.Tadeu AMB, Horsley V. Epithelial stem cells in adult skin. Curr Top Dev Biol. 2014;107:109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu Y-C, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Li L, Feng K, et al. “Repair” Treg cells in tissue injury. Cell Physiol Biochem. 2017;43:2155–2169. [DOI] [PubMed] [Google Scholar]
- 9.Kossard S. Keratoacanthoma, committed stem cells and neoplastic aberrant infundibulogenesis integral to formulating a conceptual model for an infundibulocystic pathway to squamous cell carcinoma. J Cutan Pathol. 2021;48:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadoso JC, Leonard J, Carton J, et al. Multiple follicular papules on bac of an elderly man. Necrotizing infundibular crystalline folliculitis (NICF). JAMA Dermatol. 2013;149:1233–1234. [DOI] [PubMed] [Google Scholar]
- 11.Saxer-Sekulic N, Vion-Gauthery B, Gurken K. Necrotizing infundibular crystalline folliculitis: a case report of an exceptional lesion of unknown etiology. Dermatopathology. 2014;1:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraga GR, Gadzia JE, Abraham JL, et al. Necrotizing infundibular crystalline folliculitis manifesting as a perforating mucinosis: a case report. Am J Dermatopathol. 2013;35:757–760. [DOI] [PubMed] [Google Scholar]
- 13.Yim S, Yang J, Nam JH, et al. A case of necrotizing infundibular crystalline folliculitis. Int J Dermatol. 2019;58:846–848. [DOI] [PubMed] [Google Scholar]
- 14.Denisjuk N, Hilty N, Pfaltz M, et al. Necrotizing infundibular crystalline folliculitis: a clinicopathological study. J Am Acad Dermatol. 2012;169:823–826. [DOI] [PubMed] [Google Scholar]
- 15.Dal R, Hua W, Xie H, et al. The human skin- derived precursors for regenerative medicine: current state, challenges and perspectives. Stem Cell Int. 2018;2018:8637812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali N, Zirak B, Rodriguez RS, et al. Regulatory T cells in the skin facilitate epithelial stem cell differentiation. Cell. 2017;169:1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maryanovich M, Frenette PS. T-regulating hair follicle stem cells. Immunity. 2017;46:979–980. [DOI] [PubMed] [Google Scholar]
- 19.Alonso L, Fuchs E. Stem cells in the skin: waste not, WNT not. Genes Dev. 2003; 17:1189–1200. [DOI] [PubMed] [Google Scholar]
- 20.Rangel-Huerta E, Maldonado E. Transit amplifying cells in the fast lane from stem cells towards differentiation. Stem Cell Int. 2017;2017:7602951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanba D, Toki F, Barrandon Y, et al. Recent advances in epidermal growth factor reseptor/ligand system biology on skin homeostasis and keratinocyte stem cell regulation. J Dermatol Sci. 2013;72:81–86. [DOI] [PubMed] [Google Scholar]
- 22.Pierard GE, Pierard-Franchimont C, Humbert P. Bioimpact of EFGR antagonists on the pilosebaceous follicles. Eur J Dermatol. 2012;22:54–57. [DOI] [PubMed] [Google Scholar]
- 23.Wu X-J, Jijg J, Lu Z-F, et al. VEGFR-2 is in a state of activation in hair follicles, sebaceous glands, eccrine glands and epidermis from human scalp: an in situ immunohistochemistry study of phosphorylated VEGFR-2. Med Sci Basic Res. 2019;25:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahlhoff M, Camera E, Ludovici M, et al. EFGR/ERBB receptors differentially modulate sebaceous lipogenesis. FEBS Lett. 2015;589:1376–1382. [DOI] [PubMed] [Google Scholar]
- 25.Nakahara T, Moroi Y, Takayama K, et al. Changes in sebum levels and the development of acneiform rash in patients with non-small lung cancer after treatment with EFGR inhibitors. OncoTargets Ther. 2015;8:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liza A, Agero C, Dusza W, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657–670. [DOI] [PubMed] [Google Scholar]


