ABSTRACT
Following advances in blood typing and storage, whole blood transfusion became available for the treatment of casualties during World War I. While substantially utilized during World War II and the Korean War, whole blood transfusion declined during the Vietnam War as civilian centers transitioned to blood component therapies. Little evidence supported this shift, and recent conflicts in Iraq and Afghanistan have renewed interest in military and civilian applications of whole blood transfusion. Within the past two decades, civilian trauma centers have begun to study transfusion protocols based upon cold-stored, low anti-A/B titer type O whole blood for the treatment of severely injured civilian trauma patients. Early data suggests equivalent or improved resuscitation and hemostatic markers with whole blood transfusion when compared to balanced blood component therapy. Additional studies are taking place to define the optimal way to utilize low-titer type O whole blood in both prehospital and trauma center resuscitation of bleeding patients.
Keywords: Hemorrhagic shock, transfusion, trauma, whole blood
INTRODUCTION
Current trauma resuscitation protocols, as outlined by Advance Trauma Life Support guidelines, recommend initial resuscitation of the bleeding trauma patient with a crystalloid bolus followed by balanced blood component transfusion (1). One hundred years ago, injured Allied casualties during World War I had limited resuscitation options including experimental colloid solutions comprised of gutta percha or gum acacia and access to a newly introduced therapy: citrated whole blood (2). These current and historical strategies share a common goal, the resuscitation of the bleeding trauma patient with fluids that closely mimic the patient's lost blood. Although whole blood transfusion was phased out in favor of component transfusion, recent military experience has refocused clinical inquiry into the efficacy of whole blood. Here, we review the history of military and civilian whole blood transfusion and current research into its utility to resuscitate severely injured civilian trauma patients.
THE HISTORY OF WHOLE BLOOD TRANSFUSION
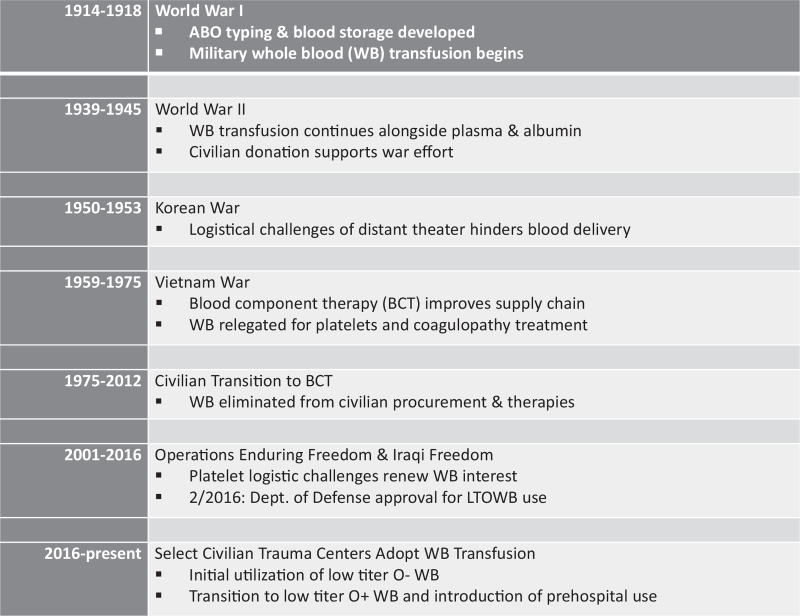
Following the development of blood storage solutions such as citrate and ABO typing in the early twentieth century, whole blood transfusion became a part of combat casualty care in select Allied hospitals during World War I (Fig. 1) (3). At the start of World War II, the British Royal Army Medical Corp utilized stored whole blood for casualty resuscitation; the American military instead chose to focus upon freeze dried plasma and albumin (4). By 1942, however, clinical observations of improved outcomes with whole blood transfusion lead to the adoption of whole blood programs within the US military, a practice that continued through Vietnam (5). While whole blood was used during these initial hostilities, within the first few years of the Vietnam War, advances in fractionation led to the availability of red blood cells (RBC) and fresh frozen plasma. The longer shelf life of these components made them favorable for a conflict so far from home soil. From this point onward, the US military turned to blood components as the primary means of hemorrhage resuscitation, with RBC and saline (which contained none of the clotting factors or platelets contained in whole blood) being the initial and primary products used (6).
Fig. 1.
Timeline of military and civilian whole blood transfusion.
After Vietnam, considerations for civilian transfusion became primary as military utilization of blood products declined. Separation of donated blood into components maximized the potential utilization of each unit (7). Non-surgical specialties such as pediatrics and hematology utilized far more blood components than specialties treating surgical hemorrhage with whole blood (8). Treatment of disorders such as thrombocytopenia and hemophilia with platelet concentrates and plasma favored the streamlining of component production and storage. Red Cross Regional Blood Centers, established during World War II to buttress military transfusion with civilian donors, chose to focus upon the more widely used and applicable blood components, abandoning whole blood transfusion for civilians, even those in hemorrhagic shock (5). Most concerning is that it was done with complete absence of data demonstrating noninferiority of component therapy.
During the late 1970s and early 1980s, resuscitation practices also changed with literature supporting the use of crystalloid as the initial product of choice for civilian trauma resuscitation (9, 10). The civilian shift to component transfusion and the popularization of crystalloid resuscitation cemented the decline of whole blood transfusion. Often cited studies found that the addition of platelets and fresh frozen plasma to resuscitations did little to alter the incidence of coagulopathy in severely injured trauma patients (11). However, these studies were only partially read and repeatedly misinterpreted. The investigators’ actual papers stated that crystalloid solutions were safe while awaiting arrival type-specific whole blood and additional plasma and platelets were unnecessary when using whole blood to resuscitate hemorrhagic shock in trauma patients. What was heard and practiced, however, was that crystalloids were the preferred resuscitation product and that plasma and platelets were unnecessary when using red blood cells.
RECENT MILITARY WHOLE BLOOD EXPERIENCE
Recent military conflicts in Iraq and Afghanistan renewed interest in the use of whole blood transfusion. The distance of theaters of operation from the United States made maintaining sufficient quantities of platelets difficult due to their relatively short shelf life compared to RBCs and plasma. As a result, and combined with exhaustion of available component therapy, military hospitals turned to fresh whole blood from “walking blood banks.” The low resource environment of many combat support hospitals made refrigerated storage of adequate blood components insufficient for the care of mass casualties (12, 13). Current military doctrine guided the use of fresh whole blood, rapidly screened for ABO compatibility and blood-borne disease, when appropriate blood components were not available or had failed to correct traumatic coagulopathy.
With over 8,000 units of fresh whole blood transfused during these conflicts, outcomes data emerged suggesting equivalent if not improved outcomes of combat casualties that received fresh whole blood versus component-only transfusion (14, 15). In the austere environments encountered by US Forward Surgical Teams in Afghanistan, resuscitation including fresh whole blood demonstrated a survival benefit over stored RBC and plasma alone (16). This benefit existed for both type-specific and un-crossmatched group O fresh whole blood. Comparisons between resuscitation including fresh whole blood and component therapy alone with apheresis platelets demonstrated 24-h and 30-day survival benefits for patients receiving fresh whole blood (17).
Military Combat Support Hospitals were uniquely positioned during these conflicts to provide rapid availability of fresh whole blood from “walking blood banks.” Hospital and on-base personnel were prescreened for eligibility to serve as fresh whole blood donors and, by foregoing leukoreduction or irradiation, could provide a donated unit to a causality in as little as 30 min. Although trauma care providers have proposed establishing similar systems for fresh whole blood at civilian hospitals, most recent studies of civilian whole blood use have focused upon cold-stored whole blood as an alternative to blood components during massive transfusion (MT). Based on evolving data and recent military experience, in February of 2016, the US military implemented a cold-stored low-titer whole blood program for transfusion in austere settings, beginning with the US Army Ranger Program.
CIVILIAN CLINICAL TRIALS
This positive military experience renewed interest in whole blood for civilian trauma patients. Initial resistance to this practice focused upon the logistical challenges of ABO compatibility to avoid hemolytic transfusion reactions and the absence of leukoreduction methods that did not result in dramatic reductions in platelet count (18). Many civilian centers were both unfamiliar and uncomfortable with whole blood procurement and providing fresh whole blood similar to that performed in the military. In addition, early attempts at establishing donors who could be contacted on an urgent basis to provide fresh whole blood failed to show reductions in blood product utilization or mortality when compared with component therapy.
A single-center randomized control trial of blood component versus modified whole blood transfusion assessed the primary outcome of 24-h and total transfusion volumes as well as other secondary outcomes in severely injured patients precited to receive a MT (19). Due to study design restrictions, investigators were forced to use leukoreduced whole blood with resultant platelet filtration (and destruction of platelets). This was due to the absence at the time of any platelet-sparing leukoreduction techniques. As a result, both treatment arms required apheresis platelet supplementation in balanced ratios. While the component and whole blood treatment arms did not demonstrate significant differences in mortality, the primary aim was achieved in those patients without non-survivable TBI. The authors demonstrated a significant reduction in transfusion requirements, where the WB group received less 24-h RBC, plasma, platelets, and total products. Moreover, it was the first study to demonstrate that the median time to death in hemorrhagic shock could be moved beyond the 3-h mark. While patients in the component group had a median time to death of less than 2 h, the whole blood patients had a time to death of almost 12 h.
Building on this, attention turned to cold-stored whole blood as a means of providing a reliable supply for civilian trauma patients. Data suggesting cold-stored platelets retained their hemostatic potential supported trials of refrigerated whole blood in place of component therapy (20). In vitro studies also demonstrated adequate concentrations of coagulation factors in cold-stored whole blood when compared to conventionally obtained fresh frozen plasma (20). The need to provide the safest universal whole blood led to studies of civilian trauma using group O, low anti-A, and anti-B titer (<1:100) whole blood from male donors, to mitigate the risk of transfusion-related acute lung injury. Units were leukoreduced with newly developed platelet-sparing filtration systems. Units of whole blood were preserved with CPD solution and refrigerated at 1 to 6°C and were transfused up to 10 days following collection (21). To reduce the perceived risk of alloimmunization to Rhesus (Rh) factor, whole blood transfusion was limited to male trauma patients. This series reported no hemolytic transfusion reactions related to incompatible plasma and established the feasibility of cold-stored whole blood transfusion for civilian trauma.
While multiple civilian studies compared clinical outcomes of whole blood transfusion, efforts were also made to characterize the in vitro properties of whole blood compared to balanced component transfusion. Due to the need for appropriate anticoagulation and preservation of stored blood products, a single unit of whole blood (approximately 450–500 mL) has less dilution from added solutions compared to a reconstituted unit of blood from 1:1:1 components (approximately 660 mL). Donated whole blood contains a higher hematocrit (33–43% vs 29%), higher platelet count (130–350 vs 88 × 109/L) and higher coagulation factor concentration (80–100% vs 65%) when compared to a reconstituted unit of blood from individual components (Table 1) (22–24). Cold-stored whole blood, refrigerated at 1 to 6°C without agitation, remains viable for up to 35 days in citrate phosphate dextrose adenine-1 (CPDA-1) solution and 21 days in citrate phosphate dextrose (CPD) solution. While notably shorter than RBC stored up to 42 days (in AS-1), these shelf lives represent longer storage times compared to the 5-day shelf life of both apheresis platelets and thawed plasma (25).
Table 1.
Composition of whole blood versus 1:1:1 balanced blood component units
| Whole blood | Blood component (1:1:1) | |
| Hematocrit (%) | 33–44 | 29 |
| Platelet count (k/mm3) | 150–350 | 88 |
| Coagulation factor (%) | 80–90 | 65 |
| Volume (mL) | 450–600 | 650 |
| Shelf life | 21–35 d | RBC 21–42 days Cryo/FFP 12 months Thawed plasma and PLT 5 days |
With this extended storage time, concerns were raised that hemostatic function of whole blood would decline due to platelet inactivation. One study utilizing rotational thromboelastometry (ROTEM) demonstrated preserved platelet-dependent coagulation function at 2 weeks and preserved fibrinogen-dependent coagulation function at 3.5 weeks (26). Another study utilizing thrombelastography demonstrated normal hemostatic profiles for refrigerated whole blood at 11 days shelf life with platelet aggregation assays preserved to 21 days (27). These data support preserved hemostatic function of cold-stored whole blood beyond the current 5-day shelf life of apheresis platelets. Cold-stored whole blood not only offers a new source of platelets for transfusion in austere environments (where reliable supplies of apheresis platelets are not available), but also has the potential to offload civilian trauma massive transfusion protocol (MTP) dependence on limited supplies of apheresis platelets in civilian blood banks.
PURSUIT OF UNIVERSAL WHOLE BLOOD
Initial civilian studies of whole blood transfusion for trauma utilized either type-specific whole blood, group O Rh− whole blood (a very limited resource) or group O Rh+ units with restricted administration to male recipients. During emergencies time is not available to wait for recipient blood type or Rh status prior to emergency transfusion. Given whole blood contains both red blood cells and plasma from the same donor, definition of a universal whole blood donor type is more complex than providing type O, Rh-negative units. In addition, the production of sufficient quantities of type O, Rh− whole blood would impinge against the production of other blood components of this type as such donors represent less than 5% of the general US population. Donors of universally compatible type O red cells may also contribute plasma with restrictively high levels of anti-A and/or anti-B antibodies that could result in recipient hemolysis. As a result, significant research has focused upon defining an optimal form of whole blood to serve as a universal donor unit for civilian trauma patients.
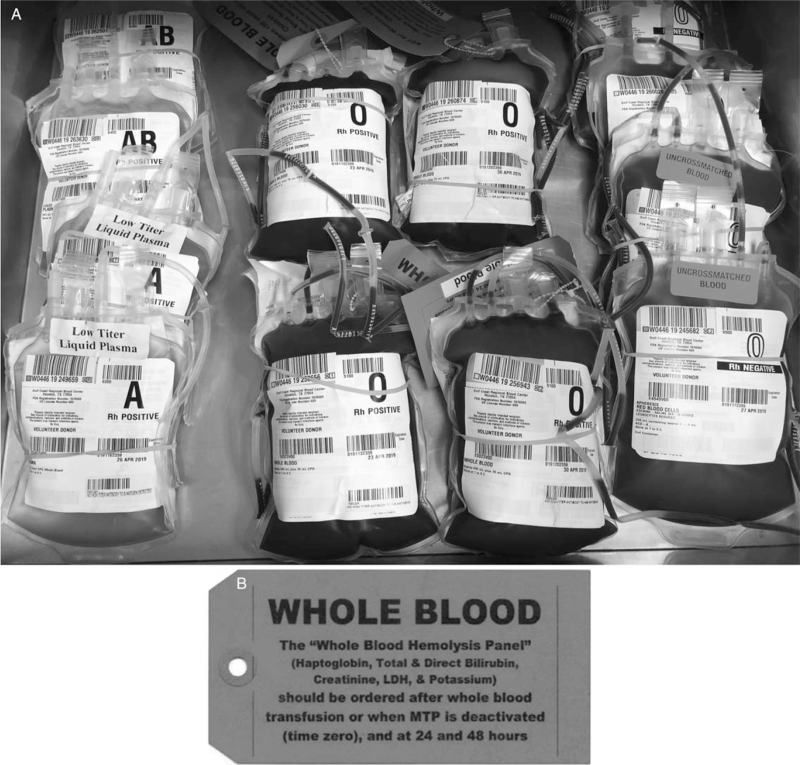
While the provision of type O whole blood assures red cell universal compatibility regardless of recipient ABO type, it does not protect against donor plasma anti-A or anti-B antibodies causing hemolysis of recipient red cells following whole blood transfusion. Taking experience from utilization of type A, low anti-B titer plasma as universal product due to the limited supply of donated AB plasma, blood centers realized type O whole blood could potentially be utilized if it contained low-titer levels of anti-A and anti-B antibodies. A successful whole blood transfusion program is predicated on the assay of these antibody levels in donor units followed by diligent monitoring for hemolysis following transfusion. Multiple civilian trauma centers have reported low to zero rates of recipient hemolysis with iso-hemagglutinin antibody titers of less than 50 to 100 following whole blood transfusion of 2 to 4 units (21, 28). However, military data has shown that an antibody titer of less than 256 is a safe and can be used for emergency release blood product and qualify as low-titer (29). Based on this, our center began using low-titer (<1:256) group O whole blood in both the trauma bay as well as on our helicopters. Patients receiving this non-leukoreduced whole blood product (stored up to 21 days) were recently compared to those receiving emergency release blood component therapy only (RBC, plasma, platelets) (30). In this study, we found that low-titer group O whole blood had similar evidence of laboratory hemolysis and transfusion reaction rates compared to component therapy, with a reduction in overall transfusions and increased likelihood of survival. Many trauma centers providing low-titer group O whole blood follow protocols in the post-transfusion period to monitor for possible hemolysis. Hemolysis panels usually involve the measurement of daily serum lactate dehydrogenase (LDH), total bilirubin, haptoglobin, potassium and creatinine for 72 h following transfusion (Fig. 2).
Fig. 2.
Emergency Department Whole Blood Deployment. A: Emergency department storage of universal low-titer group O+ whole blood alongside liquid plasma and RBCs. B: Tag affixed to all low-titer group O+ whole blood for hemolysis monitoring.
In addition to considering the ABO compatibility of donor and recipient, early use of whole blood for civilian trauma has carefully considered Rh status of its donors and recipients. Blood centers are primarily concerned with the alloimmunization of Rh− female recipients of child-bearing age who receive low-titer group O whole blood that is Rh+. This poses the potential risk of future loss of a Rh− pregnancy in a recipient previously sensitized by low-titer group O whole blood transfusion. As a result, early trials required type-specific whole blood administration with the required delay in whole blood transfusion to allow for lab results (22). Currently, centers utilizing Rh− group O whole blood have no age or sex restrictions for their product, while those transfusing Rh+ low-titer group O whole blood allow women greater than 50 years of age to receive whole blood. In the latter centers, females under 50 years of age receive component therapy (31). As trauma is the leading cause of death for male and female individuals <65 years in the United States, increasing availability of low-titer group O whole blood transfusion to all trauma patients has been investigated.
Due to the limited supply of Rh− low-titer group O whole blood for civilian trauma patients, some trauma centers have carried out risk assessments of instituting a Rh+ low-titer group O whole blood transfusion program for severely injured trauma patients (32). As whole blood is currently utilized for patients with life-threatening, large-volume hemorrhage, this study evaluated the demographics and Rh status of a large cohort of trauma patients treated with a MTP at a US level 1 trauma center. While the risk of alloimmunization of a Rh− recipient with Rh+ transfusion is reported as high as 80% in a healthy volunteer not treated with Rh immune globulin, a recent study of emergency transfusion of type O+ blood demonstrated alloimmunization rates of Rh− trauma patients as low as 3% (33). This low rate is hypothesized due to the immunosuppression that can occur during severe traumatic injury and profound shock; the population that would actually be receiving emergency blood products (34). When this large level 1 trauma center reviewed patients treated with their MTP, they found only one Rh− female of child-bearing age received this therapy over a 30-month period of time with a total of 124 protocol activations. However, 15% of their MTP activations were for women of child-bearing age with life-threatening hemorrhage who could potentially benefit from low-titer group O whole blood. Taken together, the low rate of alloimmunization in severely injured trauma patients and the availability of Rh immune globulin therapy for Rh− women of child-bearing age who receive Rh+ low-titer group O whole blood support the use of Rh+ low-titer group O whole blood transfusion for all severely injured trauma patients, male and female. For female patients of child-bearing age considered for Rh immune globulin therapy, those who received greater than 20% circulating volume Rh+ transfusion should be considered for red cell exchange therapy given the elevated risk of splenic sequestration and hemolysis with Rh immune globulin treatment. In light of these data, several centers have begun to use Rh+ group O whole blood on all patients regardless age or sex.
FUTURE CONSIDERATIONS WITH WHOLE BLOOD
Following in the footsteps of prehospital RBCs and plasma administration to initiate hemostatic resuscitation earlier in the care of severely injured trauma patients, multiple US civilian trauma centers are trialing the administration of low-titer group O whole blood by prehospital personnel for the treatment of hemorrhagic shock (Table 2) (30–32, 35, 36). Approval of Rh+ low-titer group O whole blood protocols permits the sustainable production of sufficient quantities of whole blood to stock entire metropolitan helicopter-based Emergency Medical Services and advanced ground unit services that would not be possible with more scarce Rh− whole blood. Current prehospital protocols through our regional providers have allowed the successful hemostatic resuscitation of patients with traumatic hemorrhage as well as non-traumatic hemorrhagic shock.
Table 2.
Institutional practices in civilian low-titer O whole blood transfusion
| LTOWB units | Anti A/B titers | Expiration | Eligible patients | |
| UPMC | Prehospital 0 In-hospital 4 | <1:50 | 21 d | Males > 18 years Females > 50 years |
| Mayo Clinic | Prehospital 0 In-hospital 2 | <1:200 | 14 d | Males > 18 years Females > 55 years |
| UT San Antonio | Prehospital 2 In-hospital 4+ | <1:256 | 35 d | Males > 10 years Females > 50 years |
| UT Houston | Prehospital 2 In-hospital 4+ | <1:256 | 21 d | All males Females > 50 years |
Coupled with the concerns for donor plasma ABO incompatibility and Rh alloimmunization, centers initially investigating low-titer group O whole blood restricted transfusion to two to four units in an individual patient. Recent data, however, has demonstrated very low rates of hemolysis and alloimmunization and liberalized whole blood transfusion up to six units per patient (31). A recent case report documents the successful use of a whole blood-based MT for a civilian trauma patient who received 38 units of low-titer group O whole blood and additional component therapy in the first 24 h of care (37). This whole blood resuscitation maintained the patient's hemostatic indices but required platelet and cryoprecipitate supplementation late in the process to aid in hemostasis.
Civilian centers providing prehospital and in-hospital whole blood resuscitation have not reached consensus on optimal whole blood shelf-life (Table 2). Just as blood components develop storage lesion, whole blood units demonstrate age-related alterations in parameters of coagulation (27, 38). While coagulation factor-based clot formation appears stable to 31 days utilizing thrombelastography, platelet and fibrinogen-based clot formation degrades between 14 to 21 days of storage. Conversely, thrombin generation assays demonstrate progressive increases in thrombin generation and peak thrombin levels with increased storage duration. Other factors including the type of storage solution appear to affect the age-based hemostatic potential of cold stored whole blood as well. The most appropriate expiration based upon patient outcomes has not been established.
As trauma care providers increasingly use whole blood as a first-line resuscitative agent, additional logistical challenges will arise with this new intervention. During traditional, component-based MTP resuscitations, rapid infusion devices are frequently used to deliver large volumes of blood product over short periods of time. Current manufacturers’ guidelines do not recommend the administration of platelets through infusers such as the Belmont Rapid Infuser (Belmont Medical Technologies, Billerica, Mass) (39). While administration of platelets through fluid warmers has demonstrated preserved platelet function and counts, the effect of warming and delivery through rapid infusing devices has not been fully characterized. A recent study of in vitro whole blood hemostatic profile prior to and after transit through a pressure infusion device demonstrated the use of rapid infusers is appropriate despite containing platelets and fibrinogen. While platelet count was significantly decreased when whole blood was subjected to a rapid infuser device (as compared to pressure bag and standard blood tubing), platelet function and overall clot strength are preserved. Moreover, when compared to standard transfusion tubing or pressure bag, thrombin generation was accelerated and thrombin potential increased when whole blood was administered through a rapid infuser device (40).
CONCLUSION
The return to whole blood transfusion for trauma resuscitation represents a culmination of research and clinical experience in how to improve upon balanced component resuscitation. Current data suggests that low-titer group O whole blood has an acceptable shelf life and hemostatic profile to serve as a universal blood product to resuscitate severely injured civilian trauma patients. Ongoing research into its safety profile will serve to broaden adoption by blood procurement centers and civilian trauma centers. Widespread incorporation of low-titer group O whole blood into trauma care has the potential to revolutionize hemostatic resuscitation, decrease overall transfusion volumes, and improve patient outcomes.
Footnotes
No other financial support was used for this study.
The authors report no conflicts of interest.
REFERENCES
- 1.Subcommittee A. American College of Surgeons’ Committee on T, International Awg: Advanced trauma life support (ATLS(R)): the ninth edition. J Trauma Acute Care Surg 74 (5):1363–1366, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Pope A, French G, Longnecker DE. Fluid Resuscitation: State of the Science for Treating Combat Casualties and Civilian Injuries. Washington (DC) 1999. [PubMed] [Google Scholar]
- 3.Hess JR, Thomas MJ. Blood use in war and disaster: lessons from the past century. Transfusion 43 (11):1622–1633, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Hardaway RM. Wound shock: a history of its study and treatment by military surgeons. Mil Med 169 (4):265–269, 2004. [PubMed] [Google Scholar]
- 5.Diamond LK. History of Blood Banking in the United States. JAMA 193:40–44, 1965. [DOI] [PubMed] [Google Scholar]
- 6.Giangrande PL. The history of blood transfusion. Br J Haematol 110 (4):758–767, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Beal RW. The rational use of blood. Aust N Z J Surg 46 (4):309–313, 1976. [DOI] [PubMed] [Google Scholar]
- 8.Moore FD. Should blood be whole or in parts? N Engl J Med 280 (6):327–328, 1969. [DOI] [PubMed] [Google Scholar]
- 9.Carrico CJ, Canizaro PC, Shires GT. Fluid resuscitation following injury: rationale for the use of balanced salt solutions. Crit Care Med 4 (2):46–54, 1976. [PubMed] [Google Scholar]
- 10.Shires T, Coln D, Carrico J, Lightfoot S. Fluid therapy in hemorrhagic shock. Arch Surg 88:688–693, 1964. [DOI] [PubMed] [Google Scholar]
- 11.Reed RL, 2nd, Ciavarella D, Heimbach DM, Baron L, Pavlin E, Counts RB, Carrico CJ. Prophylactic platelet administration during massive transfusion. A prospective, randomized, double-blind clinical study. Ann Surg 203 (1):40–48, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Repine TB, Perkins JG, Kauvar DS, Blackborne L. The use of fresh whole blood in massive transfusion. J Trauma 60: (6 suppl): S59–S69, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Azarow K, Holcomb JB. 31st CSH Research Working Group. Fresh whole blood transfusions in coalition military, foreign national, and enemy combatant patients during Operation Iraqi Freedom at a U.S. combat support hospital. World J Surg 32 (1):2–6, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Spinella PC, Dunne J, Beilman GJ, O’Connell RJ, Borgman MA, Cap AP, Rentas F. Constant challenges and evolution of US military transfusion medicine and blood operations in combat. Transfusion 52 (5):1146–1153, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Perkins JG, Cap AP, Spinella PC, Shorr AF, Beekley AC, Grathwohl KW, Rentas FJ, Wade CE, Holcomb JB. 31st Combat Support Hospital Research Group. Comparison of platelet transfusion as fresh whole blood versus apheresis platelets for massively transfused combat trauma patients (CME). Transfusion 51 (2):242–252, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Nessen SC, Eastridge BJ, Cronk D, Craig RM, Berseus O, Ellison R, Remick K, Seery J, Shah A, Spinella PC. Fresh whole blood use by forward surgical teams in Afghanistan is associated with improved survival compared to component therapy without platelets. Transfusion 53 suppl 1:107S–113S, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma 66: (4 suppl): S69–S76, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho KM, Leonard AD. Lack of effect of unrefrigerated young whole blood transfusion on patient outcomes after massive transfusion in a civilian setting. Transfusion 51 (8):1669–1675, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Cotton BA, Podbielski J, Camp E, Welch T, del Junco D, Bai Y, Hobbs R, Scroggins J, Hartwell B, Kozar RA, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg 258 (4):527–532, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apher Sci 42 (1):63–70, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostrom F, Sjodahl M, Wehlin L, Egberg N, Lundahl J. Coagulation parameters in apheresis and leukodepleted whole-blood plasma during storage. Transfusion 47 (3):460–463, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Yazer MH, Jackson B, Sperry JL, Alarcon L, Triulzi DJ, Murdock AD. Initial safety and feasibility of cold-stored uncrossmatched whole blood transfusion in civilian trauma patients. J Trauma Acute Care Surg 81 (1):21–26, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Spinella PC, Pidcoke HF, Strandenes G, Hervig T, Fisher A, Jenkins D, Yazer M, Stubbs J, Murdock A, Sailliol A, et al. Whole blood for hemostatic resuscitation of major bleeding. Transfusion 56 suppl 2:S190–S202, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Hardy JF, De Moerloose P, Samama M. Groupe d’intérêt en Hémostase Périopératoire. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth 51 (4):293–310, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Pivalizza EG, Stephens CT, Sridhar S, Gumbert SD, Rossmann S, Bertholf MF, Bai Y, Cotton BA. Whole Blood for Resuscitation in Adult Civilian Trauma in 2017: a narrative review. Anesth Analg 127 (1):157–162, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Strandenes G, Austlid I, Apelseth TO, Hervig TA, Sommerfelt-Pettersen J, Herzig MC, Cap AP, Pidcoke HF, Kristoffersen EK. Coagulation function of stored whole blood is preserved for 14 days in austere conditions: A ROTEM feasibility study during a Norwegian antipiracy mission and comparison to equal ratio reconstituted blood. J Trauma Acute Care Surg 78: (6 suppl 1): S31–S38, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Jobes D, Wolfe Y, O’Neill D, Calder J, Jones L, Sesok-Pizzini D, Zheng XL. Toward a definition of “fresh” whole blood: an in vitro characterization of coagulation properties in refrigerated whole blood for transfusion. Transfusion 51 (1):43–51, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seheult JN, Triulzi DJ, Alarcon LH, Sperry JL, Murdock A, Yazer MH. Measurement of haemolysis markers following transfusion of uncrossmatched, low-titre, group O+ whole blood in civilian trauma patients: initial experience at a level 1 trauma centre. Transfus Med 27 (1):30–35, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Armed Services Blood Program. Available at: https://militaryblood.dod.mil, November 26, 2018. [Google Scholar]
- 30.Williams J, Merutka N, Meyer D, Bai Y, Prater S, Cabrera R, Holcomb JB, Wade CE, Love JD, Cotton BA. Safety profile and impact of low-titer group O whole blood for emergency use in trauma. J Trauma Acute Care Surg 88 (1):87–93, 2020. [DOI] [PubMed] [Google Scholar]
- 31.Seheult JN, Bahr M, Anto V, Alarcon LH, Corcos A, Sperry JL, Triulzi DJ, Yazer MH. Safety profile of uncrossmatched, cold-stored, low-titer, group O+ whole blood in civilian trauma patients. Transfusion 58 (10):2280–2288, 2018. [DOI] [PubMed] [Google Scholar]
- 32.McGinity AC, Zhu CS, Greebon L, Xenakis E, Waltman E, Epley E, Cobb D, Jonas R, Nicholson SE, Eastridge BJ, et al. Prehospital low-titer cold-stored whole blood: Philosophy for ubiquitous utilization of O-positive product for emergency use in hemorrhage due to injury. J Trauma Acute Care Surg 84: (6S suppl 1): S115–S119, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Selleng K, Jenichen G, Denker K, Selleng S, Mullejans B, Greinacher A. Emergency transfusion of patients with unknown blood type with blood group O Rhesus D positive red blood cell concentrates: a prospective, single-centre, observational study. Lancet Haematol 4 (5):e218–e224, 2017. [DOI] [PubMed] [Google Scholar]
- 34.Reed W, Lee TH, Norris PJ, Utter GH, Busch MP. Transfusion-associated microchimerism: a new complication of blood transfusions in severely injured patients. Semin Hematol 44 (1):24–31, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Henriksen HH, Rahbar E, Baer LA, Holcomb JB, Cotton BA, Steinmetz J, Ostrowski SR, Stensballe J, Johansson PI, Wade CE. Pre-hospital transfusion of plasma in hemorrhaging trauma patients independently improves hemostatic competence and acidosis. Scand J Trauma Resusc Emerg Med 24 (1):145, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stubbs JR, Zielinski MD, Jenkins D. The state of the science of whole blood: lessons learned at Mayo Clinic. Transfusion 56 suppl 2:S173–S181, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Condron M, Scanlan M, Schreiber M. Massive transfusion of low-titer cold-stored O-positive whole blood in a civilian trauma setting. Transfusion 59 (3):927–930, 2019. [DOI] [PubMed] [Google Scholar]
- 38.Meledeo MA, Peltier GC, McIntosh CS, Bynum JA, Cap AP. Optimizing whole blood storage: hemostatic function of 35-day stored product in CPD, CP2D, and CPDA-1 anticoagulants. Transfusion 59 (S2):1549–1559, 2019. [DOI] [PubMed] [Google Scholar]
- 39.Belmont. Belmont Rapid Infuser, RI-2 Operator's Manual. Boston: Belmont Instrument Corporation. [Google Scholar]
- 40.Zaza M, Meyer DM, Wang YW, George MJ, Wade C, Cardenas JC, Cotton B. Rapid Transfuser Impact on Whole Blood Platelet Count, Platelet Function, and Hemostatic Potential. Houston, TX: Academic Surgical Congress; 2019. [Google Scholar]