Abstract
Weight gain is a common side-effect of medications used to treat major depressive disorder (MDD). We sought to estimate the frequency of weight gain for obesogenic medications prescribed for MDD and to evaluate if bupropion mitigated risk for weight gain. We analyzed a prospective cohort of patients with weight available at baseline and 12 weeks (n = 1,032) or 24 weeks (n = 871) in a post hoc analysis of the Genomics Used to Improve DEpression Decisions (GUIDED) study of patients with MDD who failed at least one medication trial. We compared weight gain between those on versus not on medications with high risk for weight gain, including a subgroup receiving combination treatment with bupropion. A second analysis evaluated weight gain across traditional medication classes, adjusting for potential confounding variables. Those on medications identified as high risk for weight gain were significantly more likely to experience clinically significant weight gain (≥3%) at 12 weeks (29.3% vs. 16.3%, p < .001) and 24 weeks (33.5% vs. 23.5%, p = .015). No protection from clinically significant weight gain was observed among patients treated with a high-risk medication concomitantly with bupropion (N = 31, 35% and 52% with clinically significant weight gain at 12 and 24 weeks). Antipsychotic medications and tricyclic antidepressants were most often associated with clinically significant weight gain. This study helps quantify the real-world risk of weight gain for patients with MDD on medications with high risk for weight gain, especially for patients taking antipsychotics. Concurrent treatment with bupropion does not appear to mitigate the weight gain risk.
Keywords: antipsychotics, antidepressants, major depressive disorder, obesity, observational study, weight gain
Introduction
Major depressive disorder (MDD) is associated with a higher risk of obesity, particularly in women and those with recurrent episodes.1–3 Longitudinal studies suggest that the temporal relations are bidirectional with stronger associations observed for the direction of depression preceding obesity. In a systematic review of such longitudinal studies ranging from 2–28 years, those who were depressed had a 37% increased risk of developing obesity and those with obesity had an 18% increased risk of becoming depressed.4 One potential contributor to the increase risk of obesity in those with depression relates to obesogenic effects of medications. Among the many of medications now used to treat MDD, a striking majority are associated with some risk for weight gain.5 Many antipsychotic medications are especially well-known for their obesogenic potential. Although their use for MDD is declining, antipsychotics are still prescribed to nearly one in five patients with MDD, typically in combination with antidepressants as an augmentation strategy.6 While increased awareness of side-effects may be one contributor of decreasing use, antipsychotics are not the only medications that can increase weight. Antidepressants also have been associated with varied amounts of weight gain, particularly with long-term use.7 It is therefore important to view risk for weight gain broadly and across traditional medication classes. Such risk for weight gain can continue over years8 and can therefore impact obesity-related comorbidities such as diabetes mellitus and cardiovascular disease.9
Unlike other medications used to treat MDD, bupropion has been associated with modest weight loss in the treatment of depression in both short-term (8 week)10 and long-term (52 week) studies.11 As a consequence, bupropion is often prescribed as an antidepressant either as monotherapy or in combination with another agent for the treatment of MDD when weight gain is deemed a clinical concern.12,13 Bupropion has been studied in combination with other antidepressants in open label studies14,15 and STAR*D16 and differences in weight gain have not been reported or were not assessed. Over the 36-week acute and continuation treatment in the VAST-D trial, the bupropion arms (change or augmentation) showed weight loss of ≥7% in 18.6–21.6% of participants compared to weight gain of ≥7% in 7.5–8.5%, much lower than the 30.6% frequency of ≥7% weight gain with aripiprazole.17 While a combination of bupropion and naltrexone has been approved by the FDA for the treatment of obesity,18 bupropion monotherapy has not been approved for this indication.19
Utilizing 24-week, prospective data from an initially randomized clinical trial in which clinically-driven, real-world treatment decisions were being made for patients with MDD who were not responsive to at least one medication trial, we sought to estimate the magnitude of weight gain risk for users of high risk obesogenic medications. We also sought to assess whether the common practice of combining bupropion, which is not associated with risk of weight gain as a monotherapy, with these medications had any impact on attenuating weight gain risk. We additionally assessed weight gain with more traditionally defined medication classes and explored potential moderators of weight gain.
Methods
The Genomics Used to Improve DEpression Decisions (GUIDED) study involved a randomized controlled trial of 1,167 patients with MDD who did not respond to at least one medication trial. Participants were randomized to pharmacogenomic testing with the GeneSight Combinatorial Pharmacogenomic test from Assurex Health, Inc. (Mason, OH) versus treatment as usual. Results have been published elsewhere.20 The study was conducted between April 2014 and February 2017. While the blind was broken at 12 weeks, treatment continued in an open, naturalistic manner for another 12 weeks. The study was approved by the Copernicus Group Independent Review Board and all patients provided written informed consent to participate.
For this secondary, post hoc analysis of GUIDED data, the sample was restricted to the 1,039 patients with baseline weight and a follow-up weight at 12 or 24 weeks. We identified weight change of ≥3% at 12 and 24 weeks as clinically significant weight gain, a threshold that has been used in previous research.21–31 We chose this threshold a priori over the more widely-used ≥5% threshold to improve detection of potentially longer-term clinically deleterious weight gain in this 24 week study.
Medications at “high risk” for weight gain were identified from a recent review of placebo-controlled trials,5 and included clozapine, olanzapine, iloperidone, chlorpromazine, quetiapine, risperidone, paliperidone, mirtazapine, amitriptyline, amisulpride, valproate, clomipramine, desipramine, doxepin, imipramine, nortriptyline. Thioridazine was added based on clinical trial data showing substantial weight gain relative to the active comparator remoxipride.32,33 Bupropion was identified as the sole “no risk” medication for weight gain with some propensity to cause weight loss, particularly in patients with obesity.34,35
This secondary analysis was performed on the per protocol sample. For the week 12 analyses, use of a given medication or class was categorically operationalized based on whether a participant was on the medication or medication class for at least the final 4 weeks of the treatment period (weeks 8–12). For the week 24 analyses, exposure was based on being on the medication or medication class for at least the final 12 weeks (weeks 12–24). This ensured sufficient time on the medication to see effects on weight. Logistic regression models with the dependent variable consisting of a dichotomous, indicator variable for weight gain, defined as gaining ≥3%, were analyzed separately at 12 and 24 weeks. We assessed the more popular ≥5% threshold in a sensitivity analysis. Multivariable models included the potential confounding variables of baseline weight, age (continuous, linear effect), sex, co-occurring generalized anxiety disorder (GAD), co-occurring posttraumatic stress disorder (PTSD), and total number of other medical conditions as covariates. These six covariates were selected from a total of 17 variables considered for the models based on the strength of their association with the primary outcome in logistic regression models through a backward elimination process. Models estimated weight gain by groupings based on propensity for weight gain to test our primary hypothesis, which divided medications into those with and without high risk of weight gain and later by more traditional medication classes: antipsychotics, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and other antidepressants. Estimates were provided for those on any “high risk” medication for weight gain and later stratified for those only on high-risk medications or including a “no risk” medication (bupropion). Differences with a p-value <0.05 were considered statistically significant. Adjustment for multiple comparisons was deferred given the outcome involving an adverse event for which we want to maximize detection, rather than efficacy, where we would prioritize avoiding false claims due to Type I error. In exploratory analyses, we assessed an array of potential moderators of weight gain at week 12 in logistic regression models that included the medication class/grouping of interest, the putative moderator, and a medication class/grouping by moderator interaction with baseline weight as a covariate. Moderators assessed included age (continuous), sex, baseline Hamilton 17-Item Depression Rating Scale (HAM-D17) score, level of gene-drug interactions for the patients prescribed medications (based on GeneSight Psychotropic test report congruence divided into no/moderate (green/yellow) interaction versus significant (red) interaction), polygenic report category of baseline medications (green/yellow/red from GeneSight Psychotropic test), number of medications not responded to at baseline, race (white vs. non-white), ethnicity (Hispanic vs. non-Hispanic), number of comorbidities, number of cardiovascular comorbidities, and Charlson Comorbidity index.
Results
The sociodemographic and clinical characteristics of the sample are detailed in Table 1, broken down by overlapping medication classes. The full sample analyzed at 12 weeks (N = 1032) had a median age of about 50 years and was predominantly (70.5%) female. Those on “high risk” medications were more likely to fall in the very severe depression category (46.2% vs. 34.4% in rest of sample), although mean baseline HAM-D-17 scores were similar. They also had more unsuccessful medication trials (mean = 3.9, SD = 2.9 vs. mean = 3.0, SD = 2.3 in entire sample) and weighed less at baseline (mean = 183.2, SD = 40.6 pounds vs. mean = 193.3, SD = 49.8 pounds in entire sample). The sample broken down by overlapping medication classes is shown in Supplemental Table 1.
Table 1. Clinical and Sociodemographic Characteristics for Subsample Prescribed Obesogenic Medications.
| Weight Gain Risk | ||||||||
| Any High Risk (N = 130) |
High Risk + Bupropion (N = 31) |
High Risk Only (N = 35) |
Total (N = 1032) |
|||||
| Age (years) | ||||||||
| Mean (SD) | 49.8 (14.4) | 48.3 (12.6) | 50.4 (14.3) | 48.0 (14.5) | ||||
| Median | 52.0 | 52.0 | 53.0 | 50.0 | ||||
| Min, Max | 18, 85 | 23, 74 | 24, 74 | 18, 90 | ||||
| Age Group, N(%) | ||||||||
| 18 to 34 | 26 (20.0) | 4 (12.9) | 9 (25.7) | 227 (22.0) | ||||
| 35 to 49 | 28 (21.5) | 10 (32.3) | 5 (14.3) | 285 (27.6) | ||||
| 50 to 64 | 53 (40.8) | 14 (45.2) | 12 (34.3) | 379 (36.7) | ||||
| 65 and Over | 23 (17.7) | 3 (9.7) | 9 (25.7) | 141 (13.7) | ||||
| Sex, N(%) | ||||||||
| Female | 83 (63.8) | 21 (67.7) | 23 (65.7) | 728 (70.5) | ||||
| Male | 47 (36.2) | 10 (32.3) | 12 (34.3) | 304 (29.5) | ||||
| Ethnicity, N(%) | ||||||||
| Hispanic or Latino | 8 (6.2) | 2 (6.5) | 4 (11.4) | 80 (7.8) | ||||
| Not Hispanic or Latino | 122 (93.8) | 29 (93.5) | 31 (88.6) | 952 (92.2) | ||||
| Race, N(%) | ||||||||
| White | 111 (85.4) | 25 (80.6) | 29 (82.9) | 838 (81.2) | ||||
| Black | 16 (12.3) | 5 (16.1) | 5 (14.3) | 151 (14.6) | ||||
| Asian | 2 (1.5) | 1 (3.2) | 0 | 19 (1.8) | ||||
| Other or Multiple | 1 (0.8) | 0 | 1 (2.9) | 24 (2.3) | ||||
| Annual Income ($), N(%) | ||||||||
| 0–25,000 | 47 (36.2) | 13 (41.9) | 10 (28.6) | 442 (42.8) | ||||
| 25,000–50,000 | 42 (32.3) | 11 (35.5) | 12 (34.3) | 253 (24.5) | ||||
| 50,000–75,000 | 12 (9.2) | 3 (9.7) | 5 (14.3) | 117 (11.3) | ||||
| 75,000–100,000 | 7 (5.4) | 1 (3.2) | 2 (5.7) | 52 (5.0) | ||||
| 100,000 and above | 5 (3.8) | 1 (3.2) | 2 (5.7) | 35 (3.4) | ||||
| Refused to answer | 17 (13.1) | 2 (6.5) | 4 (11.4) | 133 (12.9) | ||||
| Highest Level of Education, N(%) | ||||||||
| Less than high school | 6 (4.7) | 3 (10.0) | 0 | 34 (3.3) | ||||
| High school diploma or equivalent | 23 (18.0) | 3 (10.0) | 6 (17.6) | 212 (20.9) | ||||
| Some college or postsecondary, no degree | 32 (25.0) | 11 (36.7) | 6 (17.6) | 252 (24.8) | ||||
| Associate’s degree | 18 (14.1) | 4 (13.3) | 4 (11.8) | 128 (12.6) | ||||
| Bachelor’s degree | 32 (25.0) | 7 (23.3) | 11 (32.4) | 246 (24.2) | ||||
| Master’s degree | 15 (11.7) | 2 (6.7) | 7 (20.6) | 109 (10.7) | ||||
| Doctoral or professional degree | 2 (1.6) | 0 | 0 | 34 (3.3) | ||||
| Smoker, N(%) | ||||||||
| No | 100 (76.9) | 27 (87.1) | 28 (80.0) | 872 (84.5) | ||||
| Yes | 30 (23.1) | 4 (12.9) | 7 (20.0) | 160 (15.5) | ||||
| Generalized Anxiety Disorder Diagnosis, N(%) | ||||||||
| 0 | 99 (76.2) | 22 (71.0) | 28 (80.0) | 864 (83.8) | ||||
| 1 | 31 (23.8) | 9 (29.0) | 7 (20.0) | 167 (16.2) | ||||
| Panic Disorder Diagnosis, N(%) | ||||||||
| 0 | 100 (76.9) | 22 (71.0) | 32 (91.4) | 873 (84.7) | ||||
| 1 | 30 (23.1) | 9 (29.0) | 3 (8.6) | 158 (15.3) | ||||
| Posttraumatic Stress Disorder Diagnosis, N(%) | ||||||||
| 0 | 119 (91.5) | 27 (87.1) | 34 (97.1) | 980 (95.1) | ||||
| 1 | 11 (8.5) | 4 (12.9) | 1 (2.9) | 51 (4.9) | ||||
| Depression Category, N(%) | ||||||||
| Moderate (HAM-D17 14–18) | 25 (19.2) | 8 (25.8) | 5 (14.3) | 289 (28.0) | ||||
| Severe (HAM-D17 19–22) | 45 (34.6) | 12 (38.7) | 10 (28.6) | 373 (36.1) | ||||
| Very Severe (HAM-D17 > 23) | 60 (46.2) | 11 (35.5) | 20 (57.1) | 370 (35.9) | ||||
| Baseline HAM-D17 | ||||||||
| Mean (SD) | 22.0 (4.0) | 21.2 (3.5) | 23.1 (4.5) | 21.2 (4.2) | ||||
| Median | 22.0 | 20.0 | 24.0 | 21.0 | ||||
| Min, Max | 14, 33 | 15, 27 | 14, 33 | 14, 37 | ||||
| Prior Medication Trials Without Response | ||||||||
| Mean (SD) | 3.9 (2.9) | 4.5 (3.3) | 3.8 (3.4) | 3.0 (2.3) | ||||
| Median | 3.0 | 4.0 | 3.0 | 2.0 | ||||
| Min, Max | 1, 18 | 1, 18 | 1, 13 | 1, 18 | ||||
| Number of Comorbidities | ||||||||
| Mean (SD) | 7.8 (6.0) | 8.5 (5.1) | 7.6 (7.9) | 7.0 (5.5) | ||||
| Median | 7.0 | 9.0 | 6.0 | 6.0 | ||||
| Min, Max | 0, 45 | 2, 24 | 0, 45 | 0, 47 | ||||
| Baseline Weight (pounds) | ||||||||
| Mean (SD) | 183.2 (40.6) | 188.8 (36.0) | 171.8 (37.0) | 193.3 (49.8) | ||||
| Median | 185.2 | 190.0 | 176.0 | 186.0 | ||||
| Min, Max | 103, 300 | 130, 271 | 108, 280 | 95, 387 | ||||
The following table details features for patients with mdd on “high risk” medications, alone, or with adjunctive “no risk” bupropion to test the hypothesis of whether bupropion appears to mitigate risk of weight gain. As illustrated in figure 1, these groupings are not mutually exclusive as those in the “high risk only” and “high risk + no risk” categories are also included in the “any high risk” group.
At 12 weeks, a total of 130 (12.6%) patients were on medications classified as “high risk” of weight gain, 24% of those 130 were also on the “no risk” bupropion. At 24 weeks, 132 (15.2%) were on “high risk” medications with a similar proportion on the “no risk” medication as illustrated in Figure 1. The “no risk” medication, bupropion, was used alone or in any combination by 237 patients at 12 weeks and 217 patients at 24 weeks.
Figure 1.
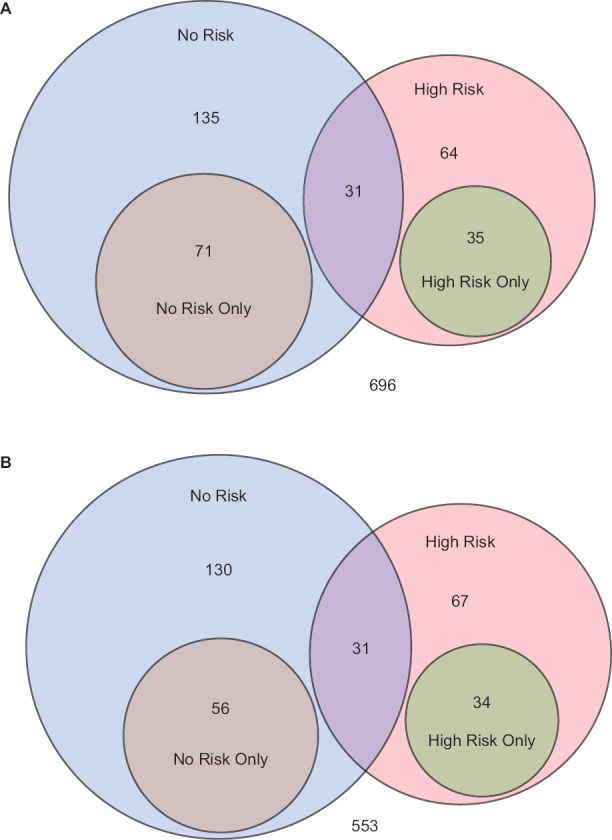
Patient Groupings Based on Classifications of Medications by Weight Gain Liability
The venn diagrams (a 12 weeks, b 24 weeks) show the frequency at which “no risk” (bupropion) and “high risk” medications were used with the degree of overlap between groups. The remainder of patients (696 at week 12 and 553 at week 24) are on medications that are neither classified as high or no risk.
Across the entire sample, a total of 186/1032 (18.0%) of patients experienced ≥3% weight gain by 12 weeks and 220/871 (25.3%) did so at 24 weeks. As shown in Table 2 and consistent with the study hypothesis, those taking “high risk” medications were significantly more likely to experience clinically significant weight gain. Those on any “high risk” medications were more likely to have weight gain at 12 weeks (29.3% vs. 16.3%, p < .001) and 24 weeks (33.5% vs. 23.5%, p = .015). Those who concurrently used bupropion (high risk and no risk) remained significantly more likely to experience weight gain at 12 weeks (35.1% vs. 17.4%, p = .014) and 24 weeks (51.6% vs. 24.0%, p = .001). Compared to those on high medications without bupropion, those on high risk medications with bupropion were significantly more likely to experience weigh gain at week 24 (χ2 = 6.09, p = .01), but not at week 12 (χ2 = .77, p = .38). Figure 2 illustrates how these frequencies varied by medication class prescribed. The highest frequency of weight gain was evident for those on antipsychotics and tricyclic antidepressants. Those prescribed antipsychotic medications were significantly more likely to demonstrate weight gain at both 12 weeks (n = 96, 28.3% vs. 16.8%, p = .006) and 24 weeks (n = 101, 39.4% vs. 23.1%, p < .001). Patients taking tricyclic antidepressants were also more likely to show weight gain at 12 weeks (n = 38, 28.1% vs. 17.5%, p = .10) and 24 weeks (n = 39, 34.9% vs. 24.6%, p = .15), although these differences were not statistically significant in this smaller subsample.
Table 2. Frequency of Weight Gain by Weight Gain Liability Medication Category.
| Taking Medication Grouping | Not Taking Medication Grouping | |||||||||||||
| Medication Category | Week | N | % of Patients with Wt. Gain | N | % of Patients with Wt. Gain | ≥ | P-value | |||||||
| Any High Risk | 12 | 130 | 29.3 | 902 | 16.3 | 13.1 | 0.001 | |||||||
| 24 | 132 | 33.5 | 739 | 23.5 | 10.0 | 0.015 | ||||||||
| High Risk Only | 12 | 35 | 32.8 | 997 | 17.4 | 15.4 | 0.022 | |||||||
| 24 | 34 | 31.0 | 837 | 24.8 | 6.3 | 0.41 | ||||||||
| High Risk & No Risk | 12 | 31 | 35.1 | 1001 | 17.4 | 17.7 | 0.014 | |||||||
| 24 | 31 | 51.6 | 840 | 24.0 | 27.6 | 0.001 | ||||||||
This table highlights the relative frequencies by which clinically significant weight gain (≥3%) was observed for those taking “high risk” medications compared to those who were not. The subgroups taking “only high risk” medication or taking a “high risk” medication with a “no risk” medication (i.E., Bupropion) are also shown. The statistical reporting is based on univariable models.
Figure 2.
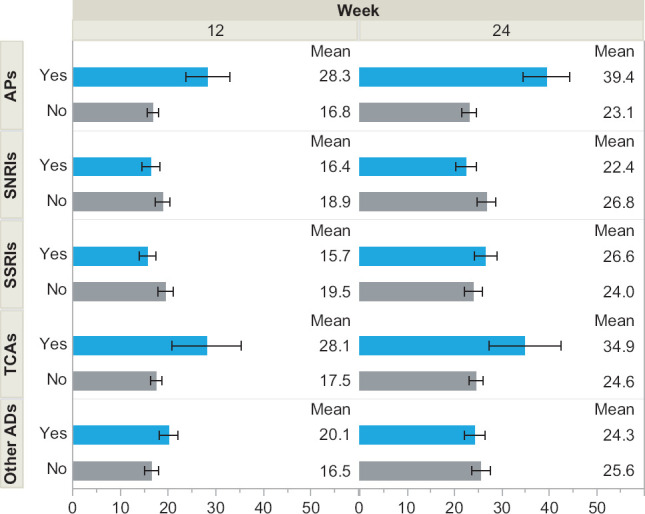
Percent of Patients Experiencing Weight Gain by Medication Class
The proportion of patients experiencing clinically significant weight gain (≥3%) is illustrated by common medication classes. The estimates are based on univariable models. AD = Antidepressant, AP = antipsychotic, SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor, TCA = tricyclic antidepressant.
Results that were similar to the univariable models also were observed in multivariable models. These were adjusted for baseline weight, age (continuous, linear effect), sex, co-occurring GAD, co-occurring PTSD, and number of other medical conditions as covariates. All statistically significant findings from univariable models remained statistically significant in multivariable models. Those on “any high risk” medication were more likely to experience weight gain at 12 weeks (29.4% vs. 16.5%, p < .001) and 24 weeks (36.0% vs. 24.5%, p = .009). Individuals on a” high risk” medication with “no risk” bupropion similarly gained significant weight at 12 weeks (35.2% vs. 18.0%, p = .02) and 24 weeks (55.3% vs. 25.4%, p < .001). Antipsychotic medications were still significantly associated with weight gain at 12 weeks (29.3% vs. 17.3%, p = .006) and 24 weeks (43.1% vs. 23.7%, p < .001). For the aforementioned multivariable models of “any high risk” medications, baseline weight, age, and number of comorbidities, and sex were found to be statistically significant at week 12. Participants with higher weight at baseline were less likely to gain weight (OR per pound: 0.996, 95% CI: 0.992–0.999); older subjects were less likely to gain weight (OR per year: 0.98, 95% CI: 0.97–0.99); women were less likely to gain weight than men (OR: 0.67, 95% CI: 0.49–0.99); participants with higher number of comorbidities were more likely to gain weight (OR: 1.04, 95% CI: 1.01–1.07 per comorbidity). Baseline weight, age, and number of comorbidities remained statistically significant in models for week 24 while sex was no longer significant. These three variables were also significant for the models including “high risk” medications and “high risk” medication with “no risk” bupropion, and of any individual medication class (antipsychotics, SNRIs, SSRIs, TCAs, other antidepressants). In all of these models, female sex was protective for weight gain at 12 weeks, but not at 24 weeks.
Exploratory analyses looked at interactions between 11 moderator variables and medication groupings/classes in separate models. There was no evidence of moderation for any of the 11 moderator variables with the “high risk” medication grouping, antipsychotics, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, or tricyclic antidepressants. There was evidence of significant moderation by age (p = .02) and Charlson Comorbidity Index (p = .04) for the disjunctive “other antidepressant” category, which predominantly involved bupropion (54%), vilazodone (13%), mirtazapine (11%), trazodone (11%), and vortioxetine (10%). This moderation was in the direction of greater weight gain with in those taking other antidepressants with more advanced age and more medical comorbidities relative to those taking other antidepressants. In these moderation models with the interaction term, the main effects of age (p = .79) and number of comorbidities (p = .19) were not significant.
In the sensitivity analysis, using the more conventional ≥5% threshold, relative differences between groups were similar with, as would be expected, lower frequencies of categorical weight gain. One exception was that those taking SSRIs were significantly less likely to experience ≥5% weight gain at 12 weeks (4.8% with vs. 9.9% without, p = .003). No differences in frequency of weight gain were seen for SSRIs at 24 weeks (13.5% with vs. 14.8% without, p = .59). Supplemental Table S2 presents the results from the sensitivity analysis related to the medication groupings based on weight gain risk. Supplemental Figure S1 presents the results for more traditional medication classes.
Table S2. Frequency of Weight Gain by Weight Gain Liability Medication Category.
| Taking Medication Grouping | Not Taking Medication Grouping | |||||||||||||
| Medication Category | Week | N | % of Patients with Wt. Gain | N | % of Patients with Wt. Gain | Δ | P-value | |||||||
| Any High Risk | 12 | 130 | 16.8 | 902 | 6.4 | 10.4 | 0.001 | |||||||
| 24 | 132 | 22.5 | 739 | 12.8 | 9.7 | 0.004 | ||||||||
| High Risk Only | 12 | 35 | 17.0 | 997 | 7.4 | 9.6 | 0.034 | |||||||
| 24 | 34 | 24.4 | 837 | 13.8 | 10.6 | 0.084 | ||||||||
| High Risk & No Risk | 12 | 31 | 18.4 | 1001 | 7.4 | 10.9 | 0.029 | |||||||
| 24 | 31 | 28.7 | 840 | 13.7 | 15.0 | 0.024 | ||||||||
This table highlights the relative frequencies from sensitivity analyses, wherein clinically significant weight gain was based on the ≥5% threshold, was observed for those taking “high risk” medications compared to those who were not. The subgroups taking “only high risk” medication or taking a “high risk” medication with a “no risk” medication (i.E., Bupropion) are also shown. The statistical reporting is based on univariable models. This table is the sensitivity analysis version of table 2.
Figure S1.
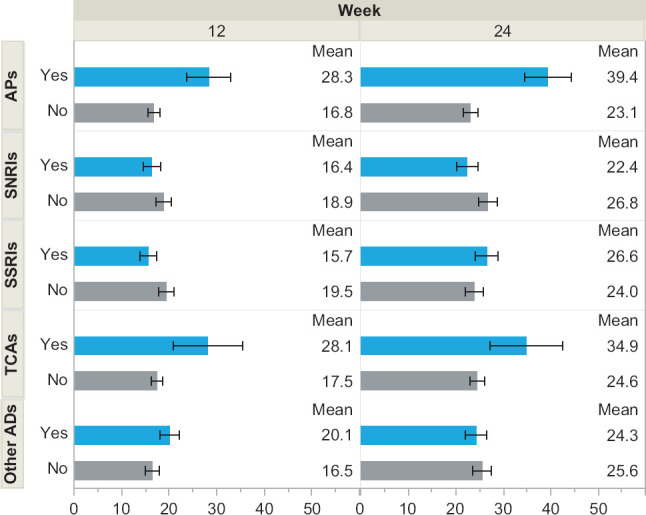
Percent of Patients Experiencing Weight Gain by Medication Class
The proportion of patients experiencing clinically significant weight gain from the sensitivity analysis, wherein clinically significant weight gain was based on the ≥5% threshold, is illustrated by common medication classes. The estimates are based on univariable models. AD = Antidepressant, AP = antipsychotic, SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor, TCA = tricyclic antidepressant.
Discussion
In this analysis from the GUIDED study, participants in this trial were prescribed medications with varying weight gain liability. As expected, those on a medication identified as high risk were significantly more likely to experience weight gain at weeks 12 and 24. Importantly, those taking bupropion and high-risk medications had even higher rates of weight gain, in both univariable and multivariable models, and significantly more than those on high-risk medications without bupropion at 24 weeks. Higher frequencies of weight perhaps could have been observed if bupropion was a maker for treatment resistant depression (confounding) or through inhibition of CYP 2D6 metabolism for select high risk medications (iatrogenic weight gain). Regardless, there was certainly no evidence that the addition of bupropion mitigated weight gains. We found that patients taking antipsychotics for at least 4 weeks had significant weight gain compared to those not on antipsychotics.
Overall, this analysis lends further support to the principle that medication treatment selection matters with regard to risk of weight gain. It also fails to lend support for the common, inadequately studied, practice of using bupropion to mitigate risk. While the study was not focused on identifying risk factors for weight gain, multivariate models found older age to generally be protective for weight gain and medical comorbidities to be a risk factor for weight gain. A higher baseline weight was protective although it is worth noting that a greater absolute weight gain was required for those who were higher weight to cross the threshold of our outcome with ≥3%. Similar results were seen on sensitivity analysis using the ≥5% weight gain threshold. Interestingly, female gender was protective for weight gain at 12, but not 24 weeks in all models. We did not find evidence of moderation by sex and prior studies have had conflicting results on sex differences in iatrogenic weight gain for these medications.
There has been limited study of weight gain related to treatment of MDD in large clinical research and real-world samples. In an analysis of the Canadian National Population Health Survey, those under age 65 on antidepressants were found to have significantly more weight gain over the 12-year follow-up. The weight gain attributed to antidepressants was modest (1.1 kg/12 years) although this estimate should be interpreted cautiously given the limitations in assessment of exposure to medications (recorded use for past two days in interviews conducted every two years) and outcome (self-reported height and weight).36 Risk for weight gain with antidepressants may persist over many years and was found especially prominent in years two and three,8 beyond the follow-up length of this analysis. In a large, prospective occupational cohort in Finland followed for a mean of 4.8 years, incident antidepressant use, defined as at least 200 daily doses, was significantly associated with self-reported weight gain. In this study, incident users of any antidepressants (n = 910) experienced significantly greater relative weight change of 4.5% vs. 2.2% in propensity-score matched controls. This difference was most pronounced for incident users of tricyclic antidepressants compared to propensity score matched controls (n = 27, 7.9% vs. 1.5%, p = .0004).37 In a slightly larger subsample on tricyclic antidepressants, our analysis similarly showed a higher frequency of clinically significant weight gain for users of tricyclic antidepressants although this difference was not statistically significant. In the Netherlands Study of Depression and Anxiety, after one year of follow-up, self-reported weight gain was 29% in users of mirtazapine, 22% in users of tricyclic antidepressants, and 19% in users of selective serotonin reuptake inhibitors.38 Using electronic medical record data in the Northeastern United States, weight change was assessed in 19,244 patients receiving an antidepressant for varied indications. As expected, bupropion was associated with significantly less weight gain than the referent comparator citalopram although unexpectedly so too were the tricyclic antidepressants nortriptyline and amitriptyline, although these may have been used at lower doses (e.g., for pain) and with less frequent concomitant antipsychotic use39 or used in lower doses for indications such as pain. Clinical trials have shown greater weight gain with tricyclic antidepressants over comparator selective serotonin reuptake inhibitors.40,41
Weight gain can occur quickly and dramatically for some patients with mood disorders.42 Better understanding and consideration of the factors that influence weight gain will improve precision-medicine decisions. With antipsychotic medications, weight gain of ≥5% in the first month was found to be the best predictor of intermediate (3 month) and long-term (1 year) weight gain.43 Early changes in weight (1 week) also have been associated with intermediate (6 week) weight gain with antidepressants.44 The mortality risks associated with weight gain likely are most relevant for those with obesity. Increases in body mass index of 3–5 kg/m2 over 2 years in those with Class II or greater obesity are associated with a 33–53% increase in mortality.45 It is therefore important for clinicians to recognize weight gain early and intervene appropriately for at risk patients. Clinicians should also be aware of the considerable individual differences in vulnerability to weight gain with obesogenic psychotropic medications.5
Strengths of the study included the large and reasonably generalizable sample with MDD. The results are also generally consistent with existing evidence and are biologically plausible. One notable exception to generalizability is an underrepresentation of Asian participants, which may be of particular importance given a tendency for more adiposity and related metabolic factors at a given body mass index in this population.46
This prospective cohort study has several limitations. While this was a randomized controlled trial, participants were randomized to whether their provider would have an additional tool for clinical decision making. Participants were not randomized to specific medications or medication groupings such as the “high risk” grouping used in our analysis or to adjuvant “low risk” bupropion. The analyses therefore effectively rely on an observational study design and weight gain was not a pre-specified outcome. The study design dictated that our analyses had to use generic, previously-established medication groupings that did not fully take dose or duration (beyond four weeks) of medications into consideration. We also did not take specific prior medication trials into account. Those prescribed antipsychotics had more prior medication trials without response. In schizophrenia, lack of prior obesogenic medication exposure, is associated with greater weight gain.47 Assuming this also holds for MDD, our results would be biased toward the null and away from the observed weight gain seen with antipsychotics. It is possible that providers discontinued medications that were causing weight gain prior to a given assessment. If anything, this reasonable practice might bias our results toward the null hypothesis and diminish our assay sensitivity. Our thresholds for medication exposure also carry some risk of misclassification with, for example, those being on medications less than 4 week for the 12-week analysis not being included. Any resultant bias from this exposure misclassification would again be in the direction of the null hypothesis. With data being collected as part of a clinical trial and weights regularly collected, it is also likely that providers may have displayed less therapeutic inertia than is commonly seen in real-world.48,49 and even trial settings.50 As noted, greater changes in medications would be expected to bias this analysis toward not finding differences between medication groups and some clear differences were nonetheless observed. This study also was not designed to assess weight changes; thus, some predictors of medication-induced weight gain were not available. For instance, family history of obesity has also been found to a be a risk factor for weight gain with both antidepressants51 and antipsychotics.52 Our multivariable models adjusted for comorbidities, but not the severity of the comorbidities or depression. None of the Genesight-associated genes in the combinatorial test have been demonstrated to be strongly associated with weight gain, so were not evaluated in this analysis. Nevertheless, this is an area that could be explored in future studies. For example, the T allele of the 102T/C polymorphism of HRT2A has been associated with weight gain,53 but was not tested in Genesight.
Conclusions
This study conveys three important messages for clinical providers. First, it illustrates the real-world risk of weight gain with obesogenic medications used to treat MDD. Findings largely replicate those seen in clinical trials with these medications and hopefully call attention to this known, but still under-studied and under-appreciated phenomenon. Second, our findings do not support the practice of adding bupropion to mitigate any risk of high-risk medications although the overall study was not directly designed to test this question. The combination of bupropion and naltrexone is well-established for weight loss54–56 and may be better suited for any such study using a randomized controlled trial design. Third, the high observed frequencies of weight gain underscore the importance of monitoring weight in patients being treated for MDD, addressing weight gain early in treatment, and undertaking evidence-based strategies to manage this risk.
Table S1. Clinical and Sociodemographic Characteristics of Full Sample.
| Drug Class | ||||||||||||
| Antipsychotic (N = 96) |
SNRI (N = 409) |
SSRI (N = 430) |
TCA (N = 38) |
Other AD (N = 399) |
Total (N = 1032) |
|||||||
| Age (years) | ||||||||||||
| Mean (SD) | 49.1 (12.8) | 49.1 (14.3) | 47.2 (14.9) | 51.8 (14.1) | 47.8 (14.4) | 48.0 (14.5) | ||||||
| Median | 53.0 | 51.0 | 49.0 | 54.0 | 50.0 | 50.0 | ||||||
| Min, Max | 18, 74 | 18, 83 | 18, 90 | 23, 73 | 18, 85 | 18, 90 | ||||||
| Age Group, N(%) | ||||||||||||
| 18 to 34 | 15 (15.6) | 80 (19.6) | 103 (24.0) | 7 (18.4) | 90 (22.6) | 227 (22.0) | ||||||
| 35 to 49 | 23 (24.0) | 115 (28.1) | 119 (27.7) | 6 (15.8) | 109 (27.3) | 285 (27.6) | ||||||
| 50 to 64 | 49 (51.0) | 154 (37.7) | 151 (35.1) | 16 (42.1) | 146 (36.6) | 379 (36.7) | ||||||
| 65 and Over | 9 (9.4) | 60 (14.7) | 57 (13.3) | 9 (23.7) | 54 (13.5) | 141 (13.7) | ||||||
| Sex, N(%) | ||||||||||||
| Female | 71 (74.0) | 296 (72.4) | 297 (69.1) | 26 (68.4) | 277 (69.4) | 728 (70.5) | ||||||
| Male | 25 (26.0) | 113 (27.6) | 133 (30.9) | 12 (31.6) | 122 (30.6) | 304 (29.5) | ||||||
| Ethnicity, N(%) | ||||||||||||
| Hispanic or Latino | 8 (8.3) | 33 (8.1) | 39 (9.1) | 2 (5.3) | 19 (4.8) | 80 (7.8) | ||||||
| Not Hispanic or Latino | 88 (91.7) | 376 (91.9) | 391 (90.9) | 36 (94.7) | 380 (95.2) | 952 (92.2) | ||||||
| Race, N(%) | ||||||||||||
| White | 78 (81.3) | 347 (84.8) | 333 (77.4) | 34 (89.5) | 327 (82.0) | 838 (81.2) | ||||||
| Black | 16 (16.7) | 45 (11.0) | 78 (18.1) | 2 (5.3) | 57 (14.3) | 151 (14.6) | ||||||
| Asian | 0 | 7 (1.7) | 9 (2.1) | 1 (2.6) | 9 (2.3) | 19 (1.8) | ||||||
| Other or Multiple | 2 (2.1) | 10 (2.4) | 10 (2.3) | 1 (2.6) | 6 (1.5) | 24 (2.3) | ||||||
| Annual Income ($), N(%) | ||||||||||||
| 0–25,000 | 46 (47.9) | 174 (42.5) | 198 (46.0) | 7 (18.4) | 176 (44.1) | 442 (42.8) | ||||||
| 25,000–50,000 | 21 (21.9) | 94 (23.0) | 105 (24.4) | 17 (44.7) | 104 (26.1) | 253 (24.5) | ||||||
| 50,000–75,000 | 8 (8.3) | 53 (13.0) | 45 (10.5) | 6 (15.8) | 38 (9.5) | 117 (11.3) | ||||||
| 75,000–100,000 | 10 (10.4) | 20 (4.9) | 7 (4.0) | 1 (2.6) | 26 (6.5) | 52 (5.0) | ||||||
| 100,000 and above | 2 (2.1) | 19 (4.6) | 7 (1.6) | 3 (7.9) | 13 (3.3) | 35 (3.4) | ||||||
| Refused to answer | 9 (9.4) | 49 (12.0) | 58 (13.5) | 4 (10.5) | 42 (10.5) | 133 (12.9) | ||||||
| Highest Level of Education, N(%) | ||||||||||||
| Less than high school | 5 (5.2) | 13 (3.3) | 18 (4.2) | 1 (2.7) | 14 (3.5) | 34 (3.3) | ||||||
| High school diploma or equivalent | 15 (15.6) | 66 (16.5) | 103 (24.3) | 6 (16.2) | 80 (20.3) | 212 (20.9) | ||||||
| Some college or postsecondary, no degree | 25 (26.0) | 100 (25.0) | 106 (25.0) | 8 (21.6) | 110 (27.8) | 252 (24.8) | ||||||
| Associate’s degree | 14 (14.6) | 60 (15.0) | 42 (9.9) | 2 (5.4) | 50 (12.7) | 128 (12.6) | ||||||
| Bachelor’s degree | 20 (20.8) | 99 (24.8) | 103 (24.3) | 12 (32.4) | 94 (23.8) | 246 (24.2) | ||||||
| Master’s degree | 12 (12.5) | 47 (11.8) | 43 (10.1) | 7 (18.9) | 30 (7.6) | 109 (10.7) | ||||||
| Doctoral or professional degree | 5 (5.2) | 15 (3.8) | 9 (2.1) | 1 (2.7) | 17 (4.3) | 34 (3.3) | ||||||
| Smoker, N(%) | ||||||||||||
| No | 79 (82.3) | 347 (84.8) | 356 (82.8) | 31 (81.6) | 344 (86.2) | 872 (84.5) | ||||||
| Yes | 17 (17.7) | 62 (15.2) | 74 (17.2) | 7 (18.4) | 55 (13.8) | 160 (15.5) | ||||||
| Generalized Anxiety Disorder Diagnosis, N(%) | ||||||||||||
| 0 | 71 (74.0) | 326 (79.7) | 367 (85.5) | 31 (81.6) | 330 (82.9) | 864 (83.8) | ||||||
| 1 | 25 (26.0) | 83 (20.3) | 62 (14.5) | 7 (18.4) | 68 (17.1) | 167 (16.2) | ||||||
| Panic Disorder, Diagnosis, N(%) | ||||||||||||
| 0 | 68 (70.8) | 337 (82.4) | 366 (85.3) | 33 (86.8) | 334 (83.9) | 873 (84.7) | ||||||
| 1 | 28 (29.2) | 72 (17.6) | 63 (14.7) | 5 (13.2) | 64 (16.1) | 158 (15.3) | ||||||
| Posttraumatic Stress Disorder Diagnosis, N(%) | ||||||||||||
| 0 | 87 (90.6) | 382 (93.4) | 413 (96.3) | 36 (94.7) | 379 (95.2) | 980 (95.1) | ||||||
| 1 | 9 (9.4) | 27 (6.6) | 16 (3.7) | 2 (5.3) | 19 (4.8) | 51 (4.9) | ||||||
| Drug Class | ||||||||||||
| Antipsychotic (N = 96) |
SNRI (N = 409) |
SSRI (N = 430) |
TCA (N = 38) |
Other AD (N = 399) |
Total (N = 1032) |
|||||||
| Depression Category, N (%) | ||||||||||||
| Moderate (HAM-D17 14–18) | 16 (16.7) | 111 (27.1) | 134 (31.2) | 9 (23.7) | 102 (25.6) | 289 (28.0) | ||||||
| Severe (HAM-D17 19–22) | 43 (44.8) | 145 (35.5) | 155 (36.0) | 13 (34.2) | 152 (38.1) | 373 (36.1) | ||||||
| Very Severe (HAM-D17 > 23) | 37 (38.5) | 153 (37.4) | 141 (32.8) | 16 (42.1) | 145 (36.3) | 370 (35.9) | ||||||
| Baseline HAM-D17 | ||||||||||||
| Mean (SD) | 21.9 (3.7) | 21.3 (4.2) | 21.0 (4.3) | 21.4 (4.0) | 21.3 (4.1) | 21.2 (4.2) | ||||||
| Median | 21.0 | 21.0 | 21.0 | 21.0 | 21.0 | 21.0 | ||||||
| Min, Max | 15, 33 | 14, 35 | 14, 37 | 14, 30 | 14, 35 | 14, 37 | ||||||
| Number of Medication Trials Without Response | ||||||||||||
| Mean (SD) | 4.5 (3.0) | 3.1 (2.2) | 2.8 (2.1) | 4.2 (3.1) | 3.3 (2.5) | 3.0 (2.3) | ||||||
| Median | 4.0 | 2.0 | 2.0 | 3.0 | 3.0 | 2.0 | ||||||
| Min, Max | 1, 18 | 1, 16 | 1, 18 | 1, 13 | 1, 18 | 1, 18 | ||||||
| Number of Comorbidities | ||||||||||||
| Mean (SD) | 8.3 (6.4) | 7.5 (5.6) | 7.0 (5.4) | 8.2 (4.5) | 6.8 (5.5) | 7.0 (5.5) | ||||||
| Median | 7.0 | 6.0 | 6.0 | 8.0 | 6.0 | 6.0 | ||||||
| Min, Max | 1, 45 | 0, 47 | 0, 38 | 0, 21 | 0, 45 | 0, 47 | ||||||
| Baseline Weight (pounds) | ||||||||||||
| Mean (SD) | 197.2 (41.8) | 196.3 (49.8) | 193.2 (49.1) | 182.4 (48.1) | 192.4 (48.4) | 193.3 (49.8) | ||||||
| Median | 191.2 | 191.8 | 185.2 | 177.9 | 185.5 | 186.0 | ||||||
| Min, Max | 112, 320 | 98, 363 | 102, 387 | 108, 300 | 95, 380 | 95, 387 | ||||||
The following table details a variety of features for the full sample used in this analysis stratified by five broad medication classes (n = 1032). These strata are not mutually exclusive. For example, someone on both a selective serotonin reuptake inhibitor and an antipsychotic would be included in both relevant columns.
Snri = serotonin-norepinephrine reuptake inhibitor, ssri = selective serotonin reuptake inhibitor, tca = tricyclic antidepressant, ad = antidepressan.
Acknowledgments
This study was supported by Myriad Neuroscience and its parent company, Myriad Genetics, Inc. Testing was provided in-kind by Assurex Health (now Myriad Neuroscience) as part of the original GUIDED trial. Individuals currently or previously associated with Myriad Neuroscience and Myriad Genetics, Inc. are included as authors and were involved in the study design, data collection and analysis, writing of the report, and the decision to submit the article for publication.
Footnotes
Disclosures
Dr. Fiedorowicz has received research support from the National Institute of Mental Health (NIMH) and National Center for Advancing Translational Sciences (NCATS). He receives support from Elsevier for duties as an Editor-in-Chief, and from the United States Food and Drug Administration (USFDA) for service on the Psychopharmacologic Drugs Advisory Committee.
Dr. Brown owns stock in Myriad Genetics, Inc. where she was an employee from June 2015 to July 2020.
Mr. Li owns stock in Myriad Genetics, Inc. where he was an employee from January 2016 to November 2020. He now works for PTC Therapeutics.
Dr. Parikh has received research funding from the Ontario Brain Institute, the Canadian Institutes of Health Research, the James and Ethel Flinn Foundation; served as a consultant for Assurex Health (now Myriad Neuroscience), has received honoraria from Mensante Corporation, Takeda, Sage, and Janssen, and the Canadian Network for Mood and Anxiety Treatments (CANMAT); and has equity in Mensante.
Dr. Dunlop has received research support from Acadia, Compass Pathways, Aptinyx, NIMH, Sage, and Takeda, and has served as a consultant to Greenwich Biosciences, Myriad Neuroscience, Otsuka, Sage, and Sophren Therapeutics.
Dr. Forester has received grant support from Eli Lily, Biogen, Eisai, National Institute of Aging, Spier family foundation, and Rogers family foundation. He has served as a consultant to Acadia Pharmaceuticals and Biogen.
Dr. Shelton reports grant support from Myriad Genetics, Inc. relevant to the current work. He also reports grant funding from Acadia Pharmaceuticals, Allergan PLC, Intracellular therapies, Neurorx, inc, Novartis International AG, Evecxia Therapeutics, Otsuka Pharmaceuticals, INmuneBIO, LivaNova PLC, Boehringer Ingelheim. He has had personal fees from Acadia Pharmaceuticals, Allergan PLC, Neurorx, Inc, Evecxia Therapeutics, and Seelos Therapeutics.
Dr. Thase reports the following relationships over the past three years: Consultant/Independent Contractor to Acadia, Inc.; Akili, Inc; Alkermes plc; Allergan, Inc.; Cerecor, Inc.; Gerson Lehrman Group, Inc.;Guidepoint Global, LLC; H. Lundbeck A/S; MedAvante, Inc.;moksha8 Pharmaceuticals Inc.; Novartis International AG; Janssen(Johnson & Johnson) Pharmaceuticals, Inc.; Otsuka Pharmaceutical Co., Ltd.; Pfizer Inc.; Sage Pharmaceuticals; Sunovion Pharmaceuticals Inc.; Takeda Pharmaceutical Company Ltd. Grant/Research Support from: Acadia, Inc.; Agency for Healthcare Research and Quality; Alkermes plc; Allergan, Inc.; AssureRx Health, Inc.; Axome, Inc.; Intracellular Inc.; Janssen Pharmaceuticals, Inc.; National Institute of Mental Health; Patient Centered Outcomes Research Instittue; Otsuka Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Company Ltd. Royalties: American Psychiatric Foundation, Guilford Publications, Herald House, W.W. Norton & Company, Inc. Dr. Thase’s spouse, Diane Sloan, PharmD, is a Senior Medical Director of Peloton Advantage, which does business with a number of pharmaceutical companies.
Liva Nova, Otsuka Dr. Macaluso has had research funding for clinical trials from Acadia, Allergan, Alkermes, AssureRx/Myriad, Eisai, Lundbeck, Janssen, Naurex/Aptinyx, Neurim, SAGE pharmaceuticals, and Suven. All clinical trial and study contracts were with and payments made to the Kansas University Medical Center Research Institute, a research institute affiliated with Kanas University School of Medicine-Wichita (KUSM-W). Dr. Macaluso is also a member of the speakers bureau for Janssen pharmaceuticals (Spravato/esketamine).
Mr. Yu owns stock in Myriad Genetics, Inc., where he was employed from May 2017 to April 2021.
Dr. Greden has nothing to disclose.
References
- 1.Fiedorowicz JG, He J, Merikangas KR. The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. J Psychosom Res . 2011;70(2):145–154. doi: 10.1016/j.jpsychores.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res . 2010;178(2):230–235. doi: 10.1016/j.psychres.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Nigatu YT, Bultmann U, Reijneveld SA. The prospective association between obesity and major depression in the general population: does single or recurrent episode matter. BMC Public Health . 2015;15:350. doi: 10.1186/s12889-015-1682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannan M, Mamun A, Doi S, Clavarino A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J Psychiatr . 2016;21:51–66. doi: 10.1016/j.ajp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Abosi O, Lopes S, Schmitz S, Fiedorowicz JG. Cardiometabolic effects of psychotropic medications. Horm Mol Biol Clin Investig . 2018;36(1) doi: 10.1515/hmbci-2017-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee TG, Mohamed S, Rosenheck RA. Antipsychotic Prescriptions Among Adults With Major Depressive Disorder in Office-Based Outpatient Settings: National Trends From 2006 to 2015. J Clin Psychiatry . 2018;79(2) doi: 10.4088/JCP.17m11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry . 2010;71(10):1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 8.Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ . 2018;361(k1951) doi: 10.1136/bmj.k1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol . 2010;316(2):104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Settle EC, Stahl SM, Batey SR, Johnston JA, Ascher JA. Safety profile of sustained-release bupropion in depression: results of three clinical trials. Clin Ther . 1999;21(3):454–463. doi: 10.1016/s0149-2918(00)88301-0. [DOI] [PubMed] [Google Scholar]
- 11.Croft H, Houser TL, Jamerson BD et al. Effect on body weight of bupropion sustained-release in patients with major depression treated for 52 weeks. Clin Ther . 2002;24(4):662–672. doi: 10.1016/s0149-2918(02)85141-4. [DOI] [PubMed] [Google Scholar]
- 12.Clayton AH. Extended-release bupropion: an antidepressant with a broad spectrum of therapeutic activity. Expert Opin Pharmacother . 2007;8(4):457–466. doi: 10.1517/14656566.8.4.457. [DOI] [PubMed] [Google Scholar]
- 13.Fredman SJ, Fava M, Kienke AS, White CN, Nierenberg AA, Rosenbaum JF. Partial response, nonresponse, and relapse with selective serotonin reuptake inhibitors in major depression: a survey of current “next-step” practices. J Clin Psychiatry . 2000;61(6):403–408. doi: 10.4088/jcp.v61n0602. [DOI] [PubMed] [Google Scholar]
- 14.DeBattista C, Solvason HB, Poirier J, Kendrick E, Schatzberg AF. A prospective trial of bupropion SR augmentation of partial and non-responders to serotonergic antidepressants. J Clin Psychopharmacol . 2003;23(1):27–30. doi: 10.1097/00004714-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Lam RW, Hossie H, Solomons K, Yatham LN. Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiatry . 2004;65(3):337–340. [PubMed] [Google Scholar]
- 16.Bech P, Fava M, Trivedi MH, Wisniewski SR, Rush AJ. Outcomes on the pharmacopsychometric triangle in bupropion-SR vs. buspirone augmentation of citalopram in the STAR*D trial. Acta Psychiatr Scand . 2012;125(4):342–348. doi: 10.1111/j.1600-0447.2011.01791.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed S, Johnson GR, Chen P et al. Effect of Antidepressant Switching vs Augmentation on Remission Among Patients With Major Depressive Disorder Unresponsive to Antidepressant Treatment: The VAST-D Randomized Clinical Trial. JAMA . 2017;318(2):132–145. doi: 10.1001/jama.2017.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. CONTRAVE (naltrexone HCl and bupropion HCl) Extended-Release Tablets Prescribing Information. 2020 July 24; https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200063s000lbl.pdf. Accessed. [Google Scholar]
- 19.Food and Drug Administration. WELLBUTRIN (bupropion hydrochloride) Prescribing Information. 2020 July 24; https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/018644s039s040.pdf. Accessed. [Google Scholar]
- 20.Greden JF, Parikh SV, Rothschild AJ et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res . 2019;111:59–67. doi: 10.1016/j.jpsychires.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O’Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med . 2000;342(12):861–867. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asmar KE, Feve B, Colle R et al. Early weight gain predicts later weight gain in depressed patients treated with antidepressants: Findings from the METADAP cohort. J Affect Disord . 2018;241:22–28. doi: 10.1016/j.jad.2018.07.059. [DOI] [PubMed] [Google Scholar]
- 23.Carr KA, Lin H, Fletcher KD, Epstein LH. Food reinforcement, dietary disinhibition and weight gain in nonobese adults. Obesity (Silver Spring) . 2014;22(1):254–259. doi: 10.1002/oby.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiroma EJ, Sesso HD, Lee IM. Physical activity and weight gain prevention in older men. Int J Obes (Lond) . 2012;36(9):1165–1169. doi: 10.1038/ijo.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong HW, Jung KW, Kim SO et al. Early postoperative weight gain is associated with increased risk of graft failure in living donor liver transplant recipients. Sci Rep . 2019;9(1):20096. doi: 10.1038/s41598-019-56543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor BS, Liang Y, Garduno LS et al. High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr . 2014;65(2):e33–40. doi: 10.1097/QAI.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Strien T, Herman CP, Verheijden MW. Eating style, overeating and weight gain. A prospective 2-year follow-up study in a representative Dutch sample. Appetite . 2012;59(3):782–789. doi: 10.1016/j.appet.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Weickert MO, Kaltsas G, Horsch D et al. Changes in Weight Associated With Telotristat Ethyl in the Treatment of Carcinoid Syndrome. Clin Ther . 2018;40(6):952–962 e952. doi: 10.1016/j.clinthera.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Sawada K, Murayama N, Takemi Y, Ishida H. Cohort study examining the association between vegetable consumption and weight gain in a single year among Japanese employees at a manufacturing company. Asia Pac J Clin Nutr . 2015;24(4):633–638. doi: 10.6133/apjcn.2015.24.4.08. [DOI] [PubMed] [Google Scholar]
- 30.Tucker L, Peterson T. Fitness level and risk of weight gain in middle-age women: a prospective cohort study. J Phys Act Health . 2010;7(3):308–315. doi: 10.1123/jpah.7.3.308. [DOI] [PubMed] [Google Scholar]
- 31.Guimaraes JB, Nevitt MC, McCulloch CE et al. Association of weight change with progression of meniscal intrasubstance degeneration over 48 months: Data from the Osteoarthritis Initiative. Eur Radiol . 2018;28(3):953–962. doi: 10.1007/s00330-017-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keks N, McGrath J, Lambert T et al. The Australian multicentre double-blind comparative study of remoxipride and thioridazine in schizophrenia. Acta Psychiatr Scand . 1994;90(5):358–365. doi: 10.1111/j.1600-0447.1994.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 33.McCreadie RG, Todd N, Livingston M et al. A double-blind comparative study of remoxipride and thioridazine in the acute phase of schizophrenia. Acta Psychiatr Scand Suppl . 1990;358:136–137. doi: 10.1111/j.1600-0447.1990.tb05305.x. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind, placebo- controlled trial. Obes Res . 2002;10(7):633–641. doi: 10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- 35.Jain AK, Kaplan RA, Gadde KM et al. Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obes Res . 2002;10(10):1049–1056. doi: 10.1038/oby.2002.142. [DOI] [PubMed] [Google Scholar]
- 36.Patten SB, Williams JV, Lavorato DH, Khaled S, Bulloch AG. Weight gain in relation to major depression and antidepressant medication use. J Affect Disord . 2011;134(1–3):288–293. doi: 10.1016/j.jad.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Kivimaki M, Hamer M, Batty GD et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care . 2010;33(12):2611–2616. doi: 10.2337/dc10-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bet PM, Hugtenburg JG, Penninx BW, Hoogendijk WJ. Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol . 2013;23(11):1443–1451. doi: 10.1016/j.euroneuro.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Blumenthal SR, Castro VM, Clements CC et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry . 2014;71(8):889–896. doi: 10.1001/jamapsychiatry.2014.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uher R, Mors O, Hauser J et al. Changes in body weight during pharmacological treatment of depression. Int J Neuropsychopharmacol . 2011;14(3):367–375. doi: 10.1017/S1461145710000933. [DOI] [PubMed] [Google Scholar]
- 41.Pande AC, Sayler ME. Adverse events and treatment discontinuations in fluoxetine clinical trials. Int Clin Psychopharmacol . 1993;8(4):267–269. doi: 10.1097/00004850-199300840-00010. [DOI] [PubMed] [Google Scholar]
- 42.Fiedorowicz JG, Andersen LE, Persons JE, Calarge C. Rapid adipose deposition with mood disorders. Ann Clin Psychiatry . 2015;27(4):283–288. [PMC free article] [PubMed] [Google Scholar]
- 43.Vandenberghe F, Gholam-Rezaee M, Saigi-Morgui N et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry . 2015;76(11):e1417–1423. doi: 10.4088/JCP.14m09358. [DOI] [PubMed] [Google Scholar]
- 44.Himmerich H, Schuld A, Haack M, Kaufmann C, Pollmacher T. Early prediction of changes in weight during six weeks of treatment with antidepressants. J Psychiatr Res . 2004;38(5):485–489. doi: 10.1016/j.jpsychires.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Myrskyla M, Chang VW. Weight change, initial BMI, and mortality among middle- and older-aged adults. Epidemiology . 2009;20(6):840–848. doi: 10.1097/EDE.0b013e3181b5f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razak F, Anand SS, Shannon H et al. Defining obesity cut points in a multiethnic population. Circulation . 2007;115(16):2111–2118. doi: 10.1161/CIRCULATIONAHA.106.635011. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez-Jimenez M, Gonzalez-Blanch C, Crespo-Facorro B et al. Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs . 2008;22(7):547–562. doi: 10.2165/00023210-200822070-00002. [DOI] [PubMed] [Google Scholar]
- 48.Sowa NA, Jeng P, Bauer AM et al. Psychiatric Case Review and Treatment Intensification in Collaborative Care Management for Depression in Primary Care. Psychiatr Serv . 2018;69(5):549–554. doi: 10.1176/appi.ps.201700243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henke RM, Zaslavsky AM, McGuire TG, Ayanian JZ, Rubenstein LV. Clinical inertia in depression treatment. Med Care . 2009;47(9):959–967. doi: 10.1097/MLR.0b013e31819a5da0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang TE, Jing Y, Yeung AS et al. Effect of communicating depression severity on physician prescribing patterns: findings from the Clinical Outcomes in MEasurement-based Treatment (COMET) trial. Gen Hosp Psychiatry . 2012;34(2):105–112. doi: 10.1016/j.genhosppsych.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Uguz F, Sahingoz M, Gungor B, Aksoy F, Askin R. Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatry . 2015;37(1):46–48. doi: 10.1016/j.genhosppsych.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M et al. Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. J Psychiatr Res . 2009;43(6):620–626. doi: 10.1016/j.jpsychires.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Ujike H, Nomura A, Morita Y et al. Multiple genetic factors in olanzapine-induced weight gain in schizophrenia patients: a cohort study. J Clin Psychiatry . 2008;69(9):1416–1422. doi: 10.4088/jcp.v69n0909. [DOI] [PubMed] [Google Scholar]
- 54.Greenway FL, Dunayevich E, Tollefson G et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab . 2009;94(12):4898–4906. doi: 10.1210/jc.2009-1350. [DOI] [PubMed] [Google Scholar]
- 55.Greenway FL, Fujioka K, Plodkowski RA et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet . 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 56.Apovian CM, Aronne L, Rubino D et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) . 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]


