Dear Editor,
In Italy, after 27th December 2020, the vaccine program against SARS-CoV-2 has been started (using mRNA vaccine encoding spike protein; i.e., Pfizer-BioNTech and Moderna). Initially, the program recruited healthcare professionals and people older than eighty according to their higher risk COVID-related exitus. Later in the year, people with higher infection risk according to SARS-CoV-2 potential exposure were included in this campaign also using ChAdOx1 nCoV-19 a non replicating chimpanzee adenovirus vector expressing spike protein (Oxford-AstraZeneca).
On 25th February 2021, a 45-year old Italian policeman received the first dose of ChAdOx1 nCoV-19 vaccine. He had a history of allergic asthma triggered by pollen, which was well controlled by inhaled corticosteroids plus short acting beta2 agonist drugs. Familiar and personal history was negative for neurological diseases. On December 2019, he had an isolated episode of objective vertigo lasting several hours. Neurological examination and brain MRI performed at that time were both normal excluding a neurological origin of symptoms.
Within 12 h after vaccination, he developed fever associated with diffuse myalgia up to 36 h [1]. Seven days after vaccination (4th of March), he started to complain a feeling of burning on the back followed in a couple of hours by backpain. These symptoms increased in intensity with a sensation of numbness and hypoesthesia symmetrically involving knees, thighs and perineum. On the 5th of March, he was admitted to hospital for urinary retention and evaluated only by a urologist being catheterized and treated with tamsulosin.
On the 7th of March, he was admitted to our emergency department for a deterioration of clinical status, which was characterized by a loss of feet’s vibration sensation accompanied by gait difficulties and febrile status (37.3°). He underwent urological examination that confirmed urinary retention and neurological examination showed an alteration of sensibility on abdominal region associated with diffused myalgia. A cranial-TC was performed excluding any alteration and the patient was discharged.
On the 9th of March, according to gait disorder worsening, he was admitted to our neurological department. Upon admission, blood pressure was 120/50 mmHg, heart rate was 80/min; he was afebrile and saturating well on room air. Neurological examination showed paresis of the lower limbs, reflexes were present and symmetric with a prevalence of left Achillei. Hypoesthesia/hypopallestesia up to D5 and urinary retention were observed.
Laboratory workup including a complete blood count, metabolic panel, thyroid testing and inflammatory markers did not show any pathological data except neutrophile-dominant leukocytosis at 80.7% (cut off 74%), mild increase of C-reactive protein (CRP): 8.44 mg/l (cut off ≤ 5 mg/l). An extensive serological panel was negative for recent infections (e.g., Borrelia, syphilis, HIV, listeriosis, herpes virus, VZV, EBV, HBV, HCV, brucella, toxoplasma, CMV, and adenovirus). A positivity of IgG was found for adenovirus, herpes simplex1, HHV6, and cytomegalovirus, EBV VCA, EBNA, parvovirus B19, toxoplasma, and VZV. Spinal cord MRI revealed a central non expansive short tau iversion recovery (STIR) signal lesions extended to spinal cord from D10 until conus without enhancement after administration of gadolinium (Fig. 1A, B), although a brain scan was not performed. The cerebrospinal fluid (CSF) showed 43 cells (cut off < 25) associated with mild hyperproteinorachia (406 mg/l; cut off 305) and normal glycorrhachia and oligoclonal bands. In the context of an infectious myelitis, an empirical antibiotic treatment was immediately started with ceftriaxone and later with piperacillin/tazobactam. Which was stopped on the basis of negativity both of PCR and cultural CSF microbiological panel (including E. coli, Listeria monocytogenes, Haemophilus influenzae, Streptococcus pneumoniae and S. agalactiae, Cytomegalovirus, Enterovirus, herpes simplex1,2,6, parvovirus, echovirus, varicella zoster virus and Cryptococcus, and JCV quantitative). Autoimmune screening was normal too. Acquaporin-4 antibodies was negative. A positivity to anti-MOG was confirmed with a titer 1:2560 (positive ≥ 1:160). The screening test anti-SARS-CoV2 IgG and IgM, performed to exclude a transverse myelitis COVID 19-related (2), was also negative. Based on vaccination history, we searched and did not detect neutralizing antibodies and antigen-specific T-cell against SARS-CoV2 spike protein.
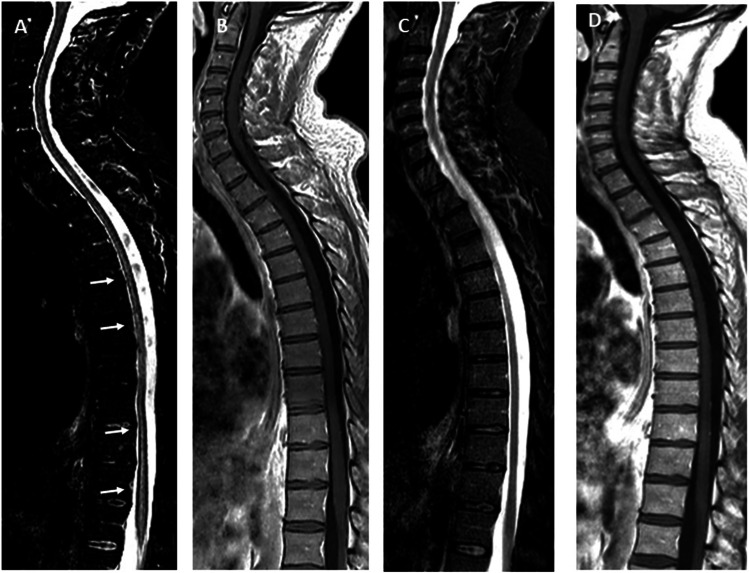
Fig. 1.
MRI of spinal cord at onset on short tau inversion recovery (STIR) images showed large confluent lesions extending over multiple segments until conus (white arrows) (A) without cord swelling and without enhancement after gadolinium on T1-weighted image with gadolinium (B). Three months later on STIR images disappeared completely hyperintense steak (C), none enhancement after gadolinium on T1 weighted image (D)
Intravenous methylprednisolone (1 g/day for 5 days) was started. After 4 days, the patient improved on sensibility gait symptoms while bladder dysfunction was still maintained.
We decided to perform a brain MRI on the 13th of March in order to exclude the potential risk of an erroneously diagnosed ChAdOx1nCoV-19-related transverse myelitis as reported in clinical trials (e.g., a pre-existing unrecognized multiple sclerosis) [1].
Brain MRI showed multiple poorly defined margins hyperintense T2-weighted and FLAIR bilateral subcortical/cortical gray-white matter lesions, and a frontal venous malformation (Fig. 2A, B, C, D, E, F). Spinal cord involvement was stable. None of the brain and spinal lesions showed gadolinium-enhancement and a subsequent EEG was normal.
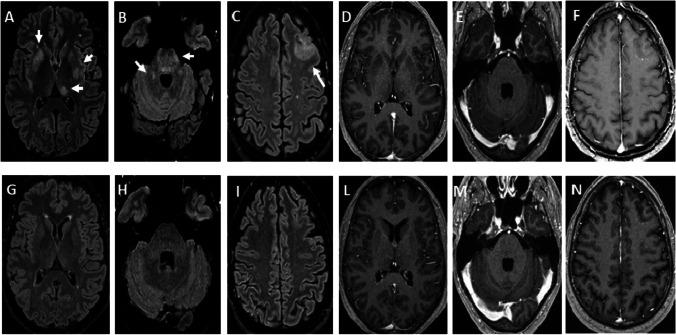
Fig. 2.
Brain axial fluid-attenuated inversion recovery weighted (FLAIR) images at onset showed bilateral diffuse multifocal poorly marginated, large asymmetric lesions of white matter, basal ganglia and cortical gray matter with asymmetric involvement of thalami (A, B see white arrows). A tumefactive lesion on left frontal lobe was detected too (C). Brain axial T1-weighted image did not show enhancement after gadolinium (from D to F). Three months later, axial FLAIR images showed a complete resolution of the lesions (from G to L) and no enhancement after gadolinium on T1-weighted image was detected too (M, N)
A follow-up brain MRI study, performed three months later, showed a resolution of a monophasic demyelinating event previously seen on initial presentation (Fig. 2 from G to N). Spinal cord status was also improved, the hyperintense streak in STIR has almost completely disappeared with concomitant clinical resolution of neurological deficits except for urinary retention that was persisting (Fig. 1C, D). Anti MOG titer was stable.
Due to MRI features, CSF findings, and negativity of CNS infections, a diagnosis of acute disseminated encephalomyelitis (ADEM) was made.
ADEM is a monophasic inflammatory demyelinating disorder clinically characterized by new onset of polyfocal neurological symptoms preceded by prodromal symptoms such as fever; notably, symptomatology may vary from mild to severe and life-threatening issues. The disease course is rapidly progressive responding to corticosteroids. Although ADEM causes and mechanisms are currently not completely understood, a close relationship between ADEM and infections or vaccines is known. Infectious agents or vaccines, as postulated, may share the same pathogenic mechanism triggering an autoimmune response against CNS-antigens which leads to ADEM. This may occur at any age with an overall incidence between 0.1 and 0.4 out of 100.000 cases following vaccination also depending on the vaccine [2]. Infectious agents or vaccines may share the same pathogenic mechanism triggering an autoimmune response against CNS-antigens such as anti-MOG (a glycoprotein expressed only in central nervous system) antibodies leading to ADEM. Anti-MOG antibodies are identified in 33–66% of pediatric ADEM and are closely linked to transverse myelitis. The role of anti MOG antibodies remains unclear, while the titer is not correlated with severity. Furthermore, the presence of anti-MOG antibodies at presentation is prognostically linked to a better recovery and MRI resolution compared to negative patients [2]. The close temporal relationship with vaccination and onset of clinical and neuroradiological features is highly suggestive of anti-MOG ADEM related to ChAdOx1nCov19 vaccine. Indeed, the complete resolution of lesions at 3-month follow-up MRI (not typically seen in MS), the aspect of lesions at onset (poorly defined margins), bilateral and symmetrical involvement of brainstem lesions (asymmetrical or unilateral in MS) support ADEM diagnosis. To our knowledge, this is the first case reported in literature. The two major studies performed on the safety of ChAdOx1nCov19 published do not mention any case of ADEM [1, 3]. A very recent publication reported the case of an ADEM following the inactivated SARS-CoV-2 vaccine [4]. Only 6 cases of ADEM have been reported in the USA Vaccine Adverse Event Reporting System (VAERS) after mRNA SARS-CoV2 vaccines, but the cases are not published. Vogrig and colleagues have described the first case of ADEM following the first dose of mRNA in an adult female with a previous history of post infectious rhombencephalitis [5]. However, it is difficult to assess whether in our case the vaccination is causal or coincidental, although we have excluded many potential other causes and the increasing number of vaccinated subjects may support its occurrence. ADEM may occur several weeks after vaccine administration, and therefore, patients manifesting symptoms suggestive of ADEM may be promptly evaluated and treated to avoid unnecessary associated problems. Finally, it remains unclear whether ADEM may be linked to the type of vaccine. On the other hand, neurological adverse events related to COVID-19 vaccine might occur in the longer term and might be discovered during vaccine administration in a large population with a longer follow up.
Declarations
Ethical approval
None.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of pediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatric. 2014;86:265–272. doi: 10.1136/jnnp-2014-308346. [DOI] [PubMed] [Google Scholar]
- 3.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao L, Ren L (2021) Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Bel. 10.1007/s13760-021-01608-2 [DOI] [PMC free article] [PubMed]
- 5.Vogrig A, Janes F. Gian Luigi Gigli, Francesco Curcio, Ilaria Del Negro, Serena D'Agostini, Martina Fabris, Mariarosaria Valente Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]