Abstract
This study analyzed the risk factors for patients with COVID-19 developing severe illnesses and explored the value of applying the logistic model combined with ROC curve analysis to predict the risk of severe illnesses at COVID-19 patients' admissions. The clinical data of 1046 COVID-19 patients admitted to a designated hospital in a certain city from July to September 2020 were retrospectively analyzed, the clinical characteristics of the patients were collected, and a multivariate unconditional logistic regression analysis was used to determine the risk factors for severe illnesses in COVID-19 patients during hospitalization. Based on the analysis results, a prediction model for severe conditions and the ROC curve were constructed, and the predictive value of the model was assessed. Logistic regression analysis showed that age (OR = 3.257, 95% CI 10.466–18.584), complications with chronic obstructive pulmonary disease (OR = 7.337, 95% CI 0.227–87.021), cough (OR = 5517, 95% CI 0.258–65.024), and venous thrombosis (OR = 7322, 95% CI 0.278–95.020) were risk factors for COVID-19 patients developing severe conditions during hospitalization. When complications were not taken into consideration, COVID-19 patients' ages, number of diseases, and underlying diseases were risk factors influencing the development of severe illnesses. The ROC curve analysis results showed that the AUC that predicted the severity of COVID-19 patients at admission was 0.943, the optimal threshold was −3.24, and the specificity was 0.824, while the sensitivity was 0.827. The changes in the condition of severe COVID-19 patients are related to many factors such as age, clinical symptoms, and underlying diseases. This study has a certain value in predicting COVID-19 patients that develop from mild to severe conditions, and this prediction model is a useful tool in the quick prediction of the changes in patients' conditions and providing early intervention for those with risk factors.
1. Introduction
The new coronavirus pneumonia (COVID-19) is an acute respiratory infectious disease caused by the new type of coronavirus (SARS-CoV-2) [1, 2]. The disease occurs insidiously and develops rapidly. In severe cases, it can quickly develop into acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis that is difficult to correct, coagulation dysfunction, etc. [3]. Therefore, it is very important to know the risk factors for COVID-19 that develops from mild to severe, identify those potentially severe cases early, and give active intervention, in order to improve the survival rate of patients. Its clinical classification [4] mainly includes the mild type (with mild clinical symptoms, no pneumonia on imaging), common type (with clinical symptoms such as fever and respiratory diseases and with findings of pneumonia on imaging), severe type, and critical type; asymptomatic carriers [5, 6] refer to those individuals who have no clinical symptoms (such as fever, cough, and sore throat) but have positive pathogenic or serum specific IgM antibodies in the respiratory tract or other origin samples, and asymptomatic carriers can also be sources of infection.
At present, the early warning model for severe/critical type of COVID-19 is mainly based on laboratory test indicators, and the predictive value of clinical symptoms for warning signs of severe COVID-19 is less researched. For example, the study by Luo et al. [7] included many laboratory indicators (routine blood tests, liver and kidney function, etc.) in the prediction model for COVID-19, while fewer clinical symptom indicators were included, which did not predict COVID-19 at earlier phases; therefore, the clinical application value of that study is limited. Studies from Poggiali and others [8] found that the predictive model using age, LDH, and CD4+ ([age × LDH]/CD4) showed 81% sensitivity and 93% specificity for early prediction. Both the sensitivity and specificity of this predictive model were not high, and the predictive evaluation could not be made at patients' admission. Therefore, its clinical predictive value needs to be evaluated. A multicenter study conducted in China [9] was developed and internally verified a nomogram predicting COVID-19 based on the symptoms, vital signs, and comorbidities of 366 laboratory-confirmed COVID-19 patients in the emergency departments. The Harrel concordance index (C-Index) of this model was 0.863 (95% CI, 0.801–0.925). Although the AUC was high and a 95% CI was sufficient, there were a few severe cases, which led to a certain deviation in the prediction results. Therefore, it is important to develop new methods to evaluate at early stages which cases may become clinically severe.
This study was performed in a designated hospital for COVID-19 in one city. It retrospectively collected the clinical data of all the 1,046 cases of confirmed and asymptomatic COVID-19 patients in this hospital from July to September in 2020, analyzed the related risk factors of developing severe illnesses, and explored the predictive value of clinical symptoms in the change from mild COVID-19 to severe conditions. This study may help in predicting the severity and prognosis of COVID-19, thereby providing early interventions for those with risk factors.
2. Data and Methods
2.1. Subjects
Data from all the 1,046 adult patients with confirmed or asymptomatic COVID-19 from July to September in 2020 in a designated hospital were retrospectively collected, and their diagnoses and classifications were in accordance with the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th Edition) [4]. All subjects were grouped into the nonsevere group or the severe group: the former included asymptomatic, mild, and common COVID-19 patients, while the latter included the severe and critical ones.
2.2. Ethics
This study has been approved by the Medical Ethics Committee of the Xinjiang Uiger Municipal People's Hospital (Approval number: 2020056). Written informed consent was obtained from each participant.
2.3. Methods
The clinical data of 1046 COVID-19 patients were retrospectively analyzed. According to the needs of this research, the total included 25 clinical characteristic indicators were as follows: sex, age, fever, cough, fatigue, gastrointestinal symptoms, upper respiratory tract symptoms, muscle aches, headache, chest tightness, hypertension, chronic kidney disease, diabetes, coronary heart disease, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), bronchial asthma, tumor, chronic hepatitis, immune deficiency, venous thrombosis, pneumothorax, acute liver injury, acute kidney injury, and electrolyte imbalance. First, the t-test was used to analyze the age difference between the groups, and the chi-square test was used to analyze the differences between the remaining indicators. Then, the selected indicators were further screened by multivariate unconditional logistic regression, and the factors affecting the severity of COVID-19 patients were analyzed and further classified and modeled. Finally, the predictive value of the model was evaluated through indicators such as receiver operating characteristic (ROC) curve, AUC value, sensitivity, and specificity. Based on this, a model for predicting COVID-19 patients developing severe conditions at admission was constructed.
2.4. Statistical Analysis
Statistical analysis was performed using SPSS 25.0 software. Quantitative data were presented as , and comparisons between groups were made by t-test, while qualitative data or categorical variables were expressed by the number of cases (proportion) and processed by chi-square test. The analysis of the risk predictive factors of COVID-19 developing into severe conditions was performed by binary logistic regression analysis, and ROC curves were plotted. P < 0.05 was considered a statistically significant difference.
3. Results
3.1. Baseline Data
3.1.1. Characteristics of the Age Distribution of Different Types of COVID-19
This study collected 1,046 asymptomatic and confirmed COVID-19 patients, including 226 asymptomatic carriers (21.6%), with an average age of 24.56 ± 14.83 years, and 820 confirmed cases (78.4%), with an average age of 37.67 ± 18.34 years. Among the confirmed subjects, 56 (5.4%) were of a severe type and aged 63.18 ± 12.2 years, 529 (50.6%) were of common type and aged 52.02 ± 15.62 years, and 235 (20.7%) were of a mild type and aged 27.24 ± 15.88)) years. There was no significant difference in either age or sex between the two groups (P > 0.05).
3.1.2. Symptomatic Characteristics of Confirmed COVID-19 Patients
Among the 820 confirmed patients with COVID-19, 568 (69.27%) had clinical manifestations such as fever, fatigue, and cough, and 426 (75%) had single symptoms, of whom 143 (33.57%) had a fever, 109 (25.59%) had asthenia, 65 (15.26%) had a cough, 59 (10.52%) had a sore throat, 23 (3.15%) had nausea, 13 (2.87%) had a headache, 10 (2.29%) had muscle aches, and 4 (1.24%) had shortness of breath (Figure 1). There were 142 cases (25%) with 2 or more clinical symptoms, including 89 cases (62.68%) with 2 symptoms, 41 cases (28.87%) with 3 symptoms, and 1 case with the most symptoms of 6.
Figure 1.
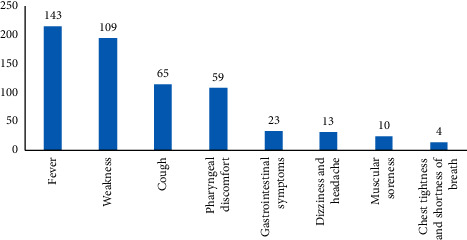
Analysis of the proportion of single symptoms in confirmed COVID-19 patients.
3.1.3. Underlying Conditions of COVID-19 Patients
There were 197 cases among the 1046 COVID-19 patients (18.83%) in this study that had underlying diseases, including 97 (9.27%) with hypertension, 67 (6.41%) with diabetes, 41 (3.92%) with coronary heart disease, 25 (2.39%) with COPD, 24 (2.29%) with hepatitis B, 17 (1.63%) with cerebrovascular disease, 12 (1.15%) with chronic kidney disease, 10 (0.96%) with malignant tumor, 5 (0.48%) with bronchial asthma, and 4 (0.38%) with HIV infection (Figure 2).
Figure 2.
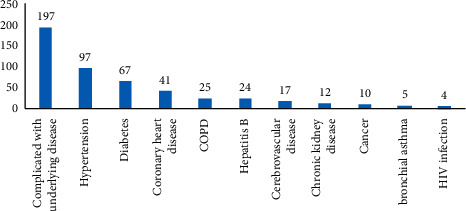
Underlying diseases in COVID-19 patients.
Of the severe and critically severe patients, 62% had complications with underlying diseases, which was higher than those of the mild and moderate cases (Figure 3).
Figure 3.
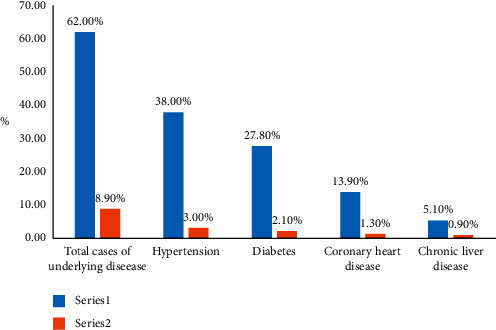
Underlying diseases in confirmed COVID-19 patients of different severities (62% of the severe and critical cases had underlying diseases, compared to 8.9% of the mild and common cases).
3.1.4. Clinical Characteristics of Severe and Critical COVID-19 Patients
Advanced Age. The average age of patients with severe cases was 63.18 ± 18.21 years, with a median age of 66 years, and the average age of patients with critical cases was 66.37 ± 13.69 years, with a median age of 68 years, which were significantly higher than those in other types of cases. Among the 75 severe and critical cases, 26 were 70–80 years old (accounting for 34.67%), 7 were over 80 years old (accounting for 9.33%), and cases over 70 years old accounted for 44% of the severe and critical cases. These proportions were significantly higher than those in other types of cases.
With More Underlying Diseases. Among the 56 severe cases, 38 (67.86%) had underlying diseases, and 14 (73.68%) of the 19 critically ill patients had underlying diseases. The main underlying diseases were hypertension, coronary heart disease, diabetes, chronic liver disease, chronic kidney disease, and chronic respiratory diseases. These proportions were significantly higher than those in other types of cases.
3.1.5. Severe Organic Dysfunction and Complications in Severe and Critical COVID-19 Patients
During the diagnosis and treatment of 1046 cases, 105 (10.04%) developed hypokalemia, 39 (3.73%) developed an acute liver injury, 17 (1.63%) developed an acute kidney injury, 5 (0.48%) developed venous thrombosis, and 4 (0.38%) developed pneumothorax.
A respiratory failure occurred in both severe and critical cases; 49 cases with severe or critical COVID-19 (65.33%) had hypokalemia, 22 cases (29.33%) had an abnormal liver function, 6 cases (8.00%) had an abnormal renal function, 5 cases (6.67%) developed venous thrombosis, and 4 cases (5.33%) had a pneumothorax.
3.2. Analysis of Characteristic Clinical Indicators of the Patients in the Severe and Nonsevere Groups at Admission
A total of 25 indicators were included. Compared with nonsevere patients, severe COVID-19 patients showed significant differences (P < 0.05) in 17 items, including age, fever, cough, fatigue, digestive tract symptoms, chest tightness, hypertension, chronic kidney disease, diabetes, cerebrovascular disease, COPD, tumor, venous thrombosis, pneumothorax, acute liver injury, and electrolyte imbalance (Table 1).
Table 1.
Comparison of clinical symptom indices between common and severe COVID-19 patients at admission.
| Score | Degree of freedom | Significance | |||
|
| |||||
| Step 0 | Variate | Sex | 0.557 | 1 | 0.456 |
| Age | 201.977 | 1 | <0.001 | ||
| Fever | 31.379 | 1 | <0.001 | ||
| Cough | 57.544 | 1 | <0.001 | ||
| Fatigue | 16.094 | 1 | <0.001 | ||
| Gastrointestinal symptoms | 4.094 | 1 | 0.043 | ||
| Respiratory symptoms | 0.353 | 1 | 0.553 | ||
| Muscle aches | 1.175 | 1 | 0.278 | ||
| Headache | 1.763 | 1 | 0.184 | ||
| Chest tightness | 27.151 | 1 | <0.001 | ||
| Hypertension | 17.225 | 1 | <0.001 | ||
| Chronic kidney disease | 12.484 | 1 | <0.001 | ||
| Diabetes | 30.032 | 1 | <0.001 | ||
| Coronary artery disease | 1.619 | 1 | 0.203 | ||
| Cerebrovascular disease | 6.948 | 1 | 0.008 | ||
| COPD | 58.400 | 1 | <0.001 | ||
| Bronchial asthma | 1.242 | 1 | 0.265 | ||
| Tumor | 7.906 | 1 | 0.005 | ||
| Chronic hepatitis | 3.328 | 1 | 0.068 | ||
| Immune deficiency | 1.918 | 1 | 0.166 | ||
| Venous thrombosis | 40.036 | 1 | <0.001 | ||
| Pneumothorax | 38.952 | 1 | <0.001 | ||
| Acute renal injury | 2.850 | 1 | 0.091 | ||
| Acute liver injury | 7.071 | 1 | 0.008 | ||
| Electrolyte imbalance | 48.550 | 1 | <0.001 | ||
| Total statistics | 338.201 | 25 | <0.001 | ||
COPD, chronic obstructive pulmonary disease.
3.3. Analysis of Predictors for COVID-19 Patients Developing Severe Illnesses
Logistic regression analysis was employed, the above 17 indicators with differences were included in the study, and the correlation between the above indicators and the occurrence of severe illnesses in the two groups was analyzed. The results showed that age, cough, COPD, and venous thrombosis of COVID-19 patients were the influencing factors for developing severe illnesses, and P < 0.05 indicated that the difference was statistically significant (Table 2).
Table 2.
Influencing factors for COVID-19 patients developing severe illnesses.
| B | Standard error | Wald | Degree of freedom | Significance | Exp (B) | 95% CI of Exp (B) | |||
| Lower limit | Upper limit | ||||||||
|
| |||||||||
| Step 1a | Age | 0.116 | 0.013 | 83.385 | 1 | <0.001 | 1.123 | 1.096 | 1.152 |
| Fever | 0.632 | 0.435 | 2.108 | 1 | 0.147 | 1.881 | 0.802 | 4.415 | |
| Cough | 1.627 | 0.450 | 13.067 | 1 | <0.001 | 5.088 | 2.106 | 12.292 | |
| Fatigue | 0.415 | 0.507 | 0.671 | 1 | 0.413 | 1.515 | 0.561 | 4.090 | |
| Gastrointestinal symptoms | 0.080 | 0.934 | 0.007 | 1 | 0.932 | 1.083 | 0.174 | 6.753 | |
| Chest tightness | 0.041 | 0.868 | 0.002 | 1 | 0.962 | 1.042 | 0.190 | 5.709 | |
| Hypertension | −0.130 | 0.469 | 0.076 | 1 | 0.782 | 0.879 | 0.351 | 2.201 | |
| Chronic kidney disease | −0.560 | 0.874 | 0.411 | 1 | 0.522 | 0.571 | 0.103 | 3.167 | |
| Diabetes | 0.558 | 0.484 | 1.332 | 1 | 0.248 | 1.747 | 0.677 | 4.509 | |
| Cerebrovascular disease | 0.797 | 0.983 | 0.657 | 1 | 0.418 | 2.219 | 0.323 | 15.242 | |
| COPD | 1.971 | 0.779 | 6.398 | 1 | 0.011 | 7.178 | 1.559 | 33.055 | |
| Tumor | 0.895 | 1.086 | 0.679 | 1 | 0.410 | 2.448 | 0.291 | 20.579 | |
| Venous thrombosis | 5.092 | 2.227 | 5.231 | 1 | 0.022 | 162.753 | 2.071 | 12787.552 | |
| Pneumothorax | 19.951 | 20619.154 | <0.001 | 1 | 0.999 | 461834480.538 | <0.001 | . | |
| Acute liver injury | 0.511 | 0.630 | 0.658 | 1 | 0.417 | 1.667 | 0.485 | 5.733 | |
| Electrolyte imbalance | 0.418 | 0.381 | 1.206 | 1 | 0.272 | 1.519 | 0.720 | 3.204 | |
| Constant | −9.153 | 0.781 | 137.240 | 1 | <0.001 | <0.001 | |||
Regression equation: Logit(1)=0.016∗Age+1.627∗Cough+1.971∗COPD+5.902∗Venous thrombosis − 9.153.
The incidence of COVID-19 patients with an advanced age to develop severe illnesses was 1.123 times that of nonadvanced aged patients; the incidence of COVID-19 patients with a cough to develop serious illnesses was 5.088 times that of patients without cough; the incidence of COVID-19 patients with COPD to develop serious illnesses was 7.018 times that of non-COPD patients; the incidence of patients with venous thrombosis to develop serious illnesses was 162.753 times that of those without venous thrombosis.
Since Logit (1) regression analysis may eliminate meaningless indicators, to avoid errors, after the aforementioned 17 indicators with significant differences were classified into 4 dimensions and each indicator was assigned 1 point, the analysis was carried out. The four dimensions were age, clinical symptoms (fever, cough, fatigue, gastrointestinal symptoms, and chest tightness), underlying diseases (hypertension, chronic kidney disease, diabetes, cerebrovascular disease, COPD, and tumor), and complications (venous thrombosis, pneumothorax, acute liver injury, and electrolyte imbalance). Univariate analysis found that age, complications, underlying diseases, and the number of symptoms were significantly different (P < 0.05) (Table 3). Further logistic regression analysis found that the age of COVID-19 patients (OR = 1.115, P < 0.001), number of complications (OR = 2.034, P=0.010), number of underlying diseases (OR = 1.623, P=0.012), and number of symptoms (OR = 2.206, P < 0.001) were risk factors influencing the development of severe illnesses. P < 0.05 indicated a statistical significance (Table 4).
Table 3.
Analysis of clinical symptoms in common and severe types of COVID-19 in classification analysis.
| Score | Degree of freedom | Significance | |||
|
| |||||
| Step 0 | Variate | Age | 201.977 | 1 | <0.001 |
| Complications | 77.084 | 1 | <0.001 | ||
| Underlying diseases | 62.608 | 1 | <0.001 | ||
| Number of symptoms | 88.775 | 1 | <0.001 | ||
| Total statistics | 268.215 | 4 | <0.001 | ||
Table 4.
Influencing factors for COVID-19 patients to develop severe illnesses in classification analysis.
| B | Standard error | Wald | Degree of freedom | Significance | Exp (B) | 95% CI of Exp (B) | |||
| Lower limit | Upper limit | ||||||||
|
| |||||||||
| Step 1a | Age | 0.109 | 0.012 | 89.053 | 1 | <0.001 | 1.115 | 1.090 | 1.141 |
| Complications | 0.710 | 0.275 | 6.662 | 1 | 0.010 | 2.034 | 1.186 | 3.488 | |
| Underlying diseases | 0.485 | 0.194 | 6.265 | 1 | 0.012 | 1.623 | 1.111 | 2.373 | |
| Number of symptoms | 0.791 | 0.183 | 18.755 | 1 | <0.001 | 2.206 | 1.542 | 3.155 | |
| Constant | −8.818 | 0.720 | 150.063 | 1 | <0.001 | <0.001 | |||
Regression equation: Logit(2)=0.109∗Age+0.710∗Number of complications+0.485∗Number of underlying diseases+0.791∗Number of symptoms − 8.818.
The incidence of COVID-19 patients with an advanced age to develop severe illnesses was 1.115 times that of nonadvanced aged patients (95% CI, 1.090–1.141); the incidence of COVID-19 patients with more complications to develop serious illnesses was 2.034 times that of patients with less complications (95% CI, 1.186–3.488); the incidence of COVID-19 patients with more underlying diseases to develop serious illnesses was 1.623 times that of those with less underlying diseases (95% CI, 1.111–2.373); the incidence of patients with more clinical symptoms to develop serious illnesses was 2.206 times that of those with less symptoms (95% CI, 1.542–3.155).
3.4. Analysis of the ROC Curve for Predicting Risk Factors for Severe COVID-19 Patients
In the single prediction, the area under the curve (AUC) of patients with severe COVID-19 was 0.910, the cutoff value (cutoff) was −8.329, the sensitivity of diagnosing severe COVID-19 was 92%, and the specificity was 79.4% (Table 5).
Table 5.
Index analysis of Logit (1) and Logit (2).
| Indices | Logistic 1 ROC curve | Logistic 2 ROC curve |
|
| ||
| AUC | 0.910 | 0.944 |
| Youden index | 0.714 | 0.767 |
| Sensitivity | 0.92 | 0.867 |
| Specificity | 0.794 | 0.90 |
| Cutoff | −8.329 | −2.2255 |
In the classification prediction, the AUC of patients with severe COVID-19 was 0.944, the cutoff was −2.2255, the sensitivity of diagnosing severe COVID-19 was 86.7%, and the specificity was 90% (Table 5).
Both AUC values were greater than 0.9, indicating high accuracy. Logistic 1 showed higher sensitivity, and Logistic 2 showed higher specificity (Figure 4). The AUC of Logistic 2 was greater than that of Logistic 1, indicating that the Logistic 2 ROC curve had more diagnostic value (Table 5).
Figure 4.
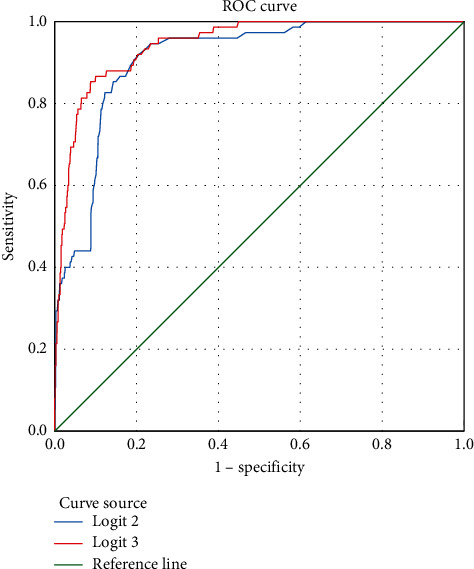
ROC curve analysis of risk factors predicting severe COVID-19.
3.5. Analysis of Predictors of COVID-19 Patients Developing Severe Illnesses at Admission
3.5.1. Predictors of COVID-19 Patients Developing Severe Illnesses at Admission
Since complications are late symptoms of COVID-19, the aforementioned prediction model may have delayed prediction. To achieve timely and early prediction of a patient's severe risk at admission, after complications were removed, univariate analysis was employed to screen factors with differences (Table 6). Then, logistic regression analysis was applied to analyze the correlation between the above indicators and developing severe illnesses. The results showed that the age, number of symptoms, and underlying diseases of COVID-19 patients were the influencing risk factors for developing severe illnesses (Table 7). P < 0.05 indicated a statistically significant difference.
Table 6.
Comparison of clinical symptom indices between common and severe COVID-19 patients after removing complications.
| Score | Degree of freedom | Significance | |||
|
| |||||
| Step 0 | Variate | Age | 201.977 | 1 | <0.001 |
| Fever | 31.379 | 1 | <0.001 | ||
| Cough | 57.544 | 1 | <0.001 | ||
| Fatigue | 16.094 | 1 | <0.001 | ||
| Gastrointestinal symptoms | 4.094 | 1 | 0.043 | ||
| Chest tightness | 27.151 | 1 | <0.001 | ||
| Hypertension | 17.225 | 1 | <0.001 | ||
| Chronic kidney disease | 12.484 | 1 | <0.001 | ||
| Diabetes | 30.032 | 1 | <0.001 | ||
| Cerebrovascular disease | 6.948 | 1 | 0.008 | ||
| COPD | 58.400 | 1 | <0.001 | ||
| Tumor | 7.906 | 1 | 0.005 | ||
| Total statistics | 289.142 | 12 | <0.001 | ||
Regression equation: Logit(3)0.119∗Age+1.807∗Cough+2.017∗COPD − 9.082.
Table 7.
Influential factors of aggravation to severe COVID-19 after removing complications.
| B | Standard error | Wald | Degree of freedom | Significance | Exp (B) | 95% CI of Exp (B) | |||
| Lower limit | Upper limit | ||||||||
|
| |||||||||
| Step 1a | Age | 0.119 | 0.012 | 93.249 | 1 | <0.001 | 1.126 | 1.099 | 1.153 |
| Fever | 0.456 | 0.424 | 1.156 | 1 | 0.282 | 1.577 | 0.687 | 3.622 | |
| Cough | 1.807 | 0.421 | 18.389 | 1 | <0.001 | 6.092 | 2.667 | 13.915 | |
| Fatigue | 0.390 | 0.499 | 0.611 | 1 | 0.434 | 1.477 | 0.556 | 3.927 | |
| Gastrointestinal symptoms | 0.023 | 0.886 | 0.001 | 1 | 0.979 | 1.024 | 0.180 | 5.816 | |
| Chest tightness | 0.247 | 0.810 | 0.093 | 1 | 0.760 | 1.281 | 0.262 | 6.268 | |
| Hypertension | −0.153 | 0.461 | 0.110 | 1 | 0.740 | 0.858 | 0.348 | 2.117 | |
| Chronic kidney disease | −0.044 | 0.838 | 0.003 | 1 | 0.958 | 0.957 | 0.185 | 4.946 | |
| Diabetes | 0.436 | 0.484 | 0.813 | 1 | 0.367 | 1.547 | 0.599 | 3.993 | |
| Cerebrovascular disease | 0.931 | 0.995 | 0.875 | 1 | 0.350 | 2.536 | 0.361 | 17.815 | |
| COPD | 2.017 | 0.792 | 6.480 | 1 | 0.011 | 7.513 | 1.590 | 35.491 | |
| Tumor | 0.731 | 1.101 | 0.441 | 1 | 0.507 | 2.078 | 0.240 | 17.998 | |
| Constant | −9.082 | 0.755 | 144.843 | 1 | <0.001 | <0.001 | |||
3.5.2. Predictors of COVID-19 Patients Developing Severe Illnesses at Admission
Since Logit (3) regression analysis may eliminate meaningless indicators, to avoid errors, after eliminating the complication factor among the aforementioned 17 indicators with a significant difference, the remaining were classified into 3 dimensions, each indicator was assigned 1 point, and the analysis was carried out. The 3 dimensions were age, clinical symptoms (fever, cough, fatigue, gastrointestinal symptoms, and chest tightness), and underlying diseases (hypertension, chronic kidney disease, diabetes, cerebrovascular disease, COPD, and tumor). Univariate analysis found that age, underlying diseases, and the number of symptoms were significantly different (P < 0.05) (Table 8). Further logistic regression analysis found that the age of the COVID-19 patients (OR = 1.120, P < 0.001), underlying diseases (OR = 1.579, P=0.020), and the number of symptoms (OR = 2.268, P < 0.001) were risk factors influencing the development of severe illnesses. P < 0.05 indicated a statistical significance (Table 9).
Table 8.
Comparison of factors affecting the COVID-19 patients developing severe illnesses after assignment.
| Score | Degree of freedom | Significance | |||
|
| |||||
| Step 0 | Variate | Age | 201.977 | 1 | <0.001 |
| Underlying diseases | 62.608 | 1 | <0.001 | ||
| Number of symptoms | 88.775 | 1 | <0.001 | ||
| Total statistics | 253.824 | 3 | <0.001 | ||
Table 9.
Factors affecting the COVID-19 patients developing severe illnesses after assignment.
| B | Standard error | Wald | Degree of freedom | Significance | Exp (B) | 95% CI of Exp (B) | |||
| Lower limit | Upper limit | ||||||||
|
| |||||||||
| Step 1a | Age | 0.113 | 0.011 | 98.200 | 1 | <0.001 | 1.120 | 1.095 | 1.145 |
| Underlying diseases | 0.457 | 0.196 | 5.447 | 1 | 0.020 | 1.579 | 1.076 | 2.318 | |
| Number of symptoms | 0.819 | 0.181 | 20.505 | 1 | <0.001 | 2.268 | 1.591 | 3.232 | |
| Constant | −8.811 | 0.708 | 154.838 | 1 | <0.001 | <0.001 | |||
Logit(4)=0.113∗Age+0.457∗Number of underlying diseases+0.819∗Number of symptoms − 8.811.
3.5.3. Analysis of the ROC Curve for Predicting COVID-19 Patients Developing Severe Illnesses
Before removing the complications factor, the AUC for predicting COVID-19 patients developing severe illnesses was 0.940, the cutoff was −3.24, the sensitivity of diagnosing the severity of COVID-19 was 94.7%, and the specificity was 80.8% (Figure 5).
Figure 5.
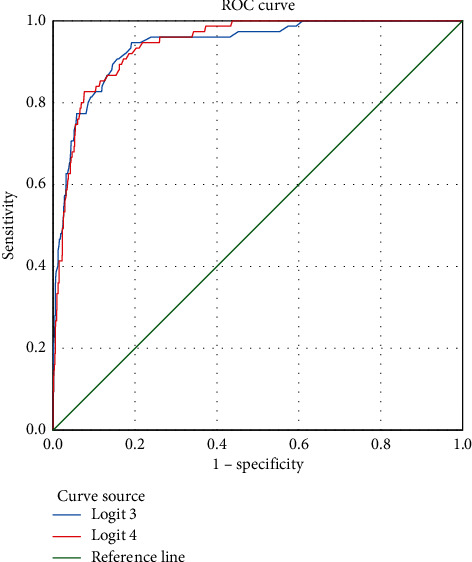
ROC curve of risk factors for predicting COVID-19 patients developing severe illnesses.
After removing the complications factor, the AUC for predicting COVID-19 patients developing severe illnesses was 0.943, and the cutoff was −1.9465. The sensitivity of diagnosing the severity of COVID-19 was 82.7%, and the specificity was 92.4% (Figure 5).
Both AUC values were greater than 0.9, indicating that the accuracy was very high. The AUC of the Logistic 4 ROC curve was greater than that of the Logistic 3 ROC curve, meaning that the Logistic 4 ROC curve had more diagnostic value. The Logistic 4 ROC curve had the highest prediction specificity (Table 10).
Table 10.
Index analysis of Logit (3) and Logit (4).
| Indices | Logistic 3 ROC curve | Logistic 4 ROC curve |
|
| ||
| AUC | 0.940 | 0.943 |
| Youden index | 0.755 | 0.751 |
| Sensitivity | 0.947 | 0.827 |
| Specificity | 0.808 | 0.924 |
| Cutoff | −3.24 | −1.9465 |
3.6. Risk Prediction Model for Patients with COVID-19 Developing Severe Illnesses
Based on the above research, we established a risk prediction model for patients with COVID-19 developing severe illnesses at admission. This model has a certain value in predicting the transition of COVID-19 patients from mild conditions to severe illnesses and can be used as an indicator for the rapid prediction of changes in COVID-19 patients' conditions and administering early interventions. The link for this prediction model is https://www.healthy.vip/mode/005/index.html.
4. Discussion
In this study, according to the clinical data of 1046 patients with COVID-19, we developed a risk prediction model based on the age, clinical symptoms, and underlying diseases of newly admitted patients with COVID-19 to predict their development into severe conditions and further programmed a predictor on the Internet. The study screened 25 clinical factors and established a ROC regression model with an AUC score of 0.943. Clinicians can use the predictor on the web to assess the risk of newly admitted patients with COVID-19 at their admission and predict the trend of their condition to provide early intervention and treatment for those with a high risk for the occurrence of severe illnesses. Since the proportion of critically ill patients directly determines patient mortality and the cost and difficulty of medical treatment, recognizing risk and intervening early has important clinical significance for the treatment and improvement of the prognosis of patients with COVID-19 [10]. In areas with more COVID-19 patients but limited medical resources, for patients with lower risks of developing severe illnesses, early interventions can be implemented accordingly, while patients with higher risks can be provided with active diagnosis and treatment to the greatest extent. How to formulate an objective, accurate, simple, and easy-to-obtain early warning model has a certain reference value for guiding clinical work.
In some studies, the clinical symptoms of fever, cough, chest tightness, fatigue, and gastrointestinal symptoms and the underlying diseases of hypertension, COPD, chronic kidney disease, diabetes, cerebrovascular disease, and malignant tumors are important factors affecting COVID-19 development into severe illnesses [11–13]. These findings are similar to the results of this study. In this study, we found that the average age of the patients with common COVID-19 cases was 52.02 ± 15.62 years, while the average age of patients with severe cases was 63.18 ± 12.21 years. Multivariate analysis showed that age was a risk factor for patients with the common type of COVID-19 for developing severe conditions (OR = 3.257, 95% CI 1.095~1.145). This may be related to the decline in physical function, poor body resistance, and chronic diseases in the elderly. This is consistent with the findings from Zhang et al. [14, 15]. Meanwhile, the decline in ACE2 expression with age is related to the higher morbidity and mortality of older people [10].
Fever is the most common clinical manifestation of COVID-19. The peak and duration of fever in severe/critically ill patients are higher than those in common type patients. This finding may be related to the continuous stimulation of the hypothalamus by endogenous pyrogenic substances, which is the same as the characteristics of SARS patients in 2003. The body temperature of the common type of COVID-19 patients gradually decreases after treatment. When a patient continues to have a high fever after receiving active treatment (the highest body temperature exceeds 39.0°C for 2 consecutive days), we should be vigilant to whether it has a tendency to develop severe disease or has progressed to a critical condition [16]. The principal early clinical manifestations of COVID-19 in patients are fever, cough, and fatigue, which often mislead a patient to think they have a common cold and therefore lead to a delay in patients seeking medical attention and the rapid development of the disease. The clinical symptoms of severe COVID-19 patients are mainly fatigue and chest tightness, and the incubation period of severe patients is longer than that of common patients. These results are consistent with the results from Wang et al. [17]. In this study, patients with COVID-19 who had gastrointestinal symptoms had a higher chance of developing severe illnesses. This may be related to the expression of the receptors of the COVID-19 virus in almost all tissues of the body, especially in the gastrointestinal tract. Ignorance of gastrointestinal symptoms may hinder the diagnosis of some patients and exacerbate the effects of the virus through fecal or oral transmission [18–20].
In this study, 62% of severe and critical COVID-19 cases had underlying diseases, which was significantly higher than the 8.9% of mild and common cases with underlying diseases. In severe and critical cases, the proportion of patients with coronary heart disease or hypertension complications was significantly higher than that of patients with other chronic diseases. The results of multivariate analysis showed that COVID-19 complicated with coronary heart disease or hypertension was an independent risk factor for developing severe and critical illnesses. This may be related to the widespread expression of ACE2 receptors in the cardiovascular system, and patients with coronary heart disease or hypertension complications have a high expression of ACE2 [21]. Patients with coronary heart disease have poor cardiac reserve function due to myocardial ischemia or necrosis. Once they develop COVID-19, they are prone to ARDS and hypoxemia, their body's tolerance becomes low, and cardiac insufficiency is more likely to occur, leading to a sharp decrease in their condition [22]. Yang et al. [11] found that some COVID-19 patients with coronary heart disease complications had serious conditions and extremely high mortality, which is consistent with the results of our study. Studies [11, 23] have shown that ACE2, the key receptor of the new coronavirus, is expressed at a high level in the human kidney (nearly 100 times higher than that of the lungs), and ACE2 is one of the main receptors that mediate the entry of the new coronavirus into human cells. The new coronavirus can enter renal tubular cells by binding to ACE2, causing cytotoxicity and abnormal renal function. In patients with chronic kidney disease, this process has been strengthened and further aggravated the transformation of common COVID-19 to severe COVID-19. COVID-19 has a long incubation period and insidious onset. When it occurs in patients with COPD, the clinical manifestations may be more atypical and may be confused with the existing symptoms of COPD. Patients with COPD have poor basic lung function and poor tolerance to hypoxia. Once infected with COVID-19, lung function may deteriorate rapidly, and respiratory failure may easily occur [24–26]. In this study, the proportion of severe and critical COVID-19 patients with diabetes was 27.8%, which was much higher than 6.41% of patients with the common type of COVID-19 who had diabetes. This may be related to the fact that high blood sugar can inhibit the release of interleukin-10 from lymphocytes and macrophages and weaken the flow, chemotaxis, and phagocytosis of polymorphonuclear leukocytes, which further leads to disorder of the humoral immune response [17]. Patients with malignant tumors experience physical weakness and are susceptible to COVID-19. After getting COVID-19, they are more likely to develop severe cases. After receiving immunosuppressive agents, surgery, radiotherapy, or chemotherapy, the body's ability to resist pathogens may be weaker and more likely to develop severe cases of COVID-19. One study suggested that infection with SARS-CoV-2 reduced the expression and destroyed the function of ACE2, which increased the patient's blood pressure and the risk of stroke [27]. For patients with underlying cerebrovascular disease who are infected with the coronavirus, the risk of stroke will be further aggravated, and for patients with the common type, the risk of developing a severe case of COVID-19 will be increased.
After patients with COVID-19 are admitted to the hospital, their risk of developing severe or critical illness can be further judged according to their complications, the treatment plan can be adjusted in advance, and early intervention can be given if necessary to reduce the incidence of severe cases. In this study, 29.33% of severe and critically ill COVID-19 patients suffered from abnormal liver function, which was higher than the 3.73% in common cases. This liver damage may be caused by the hypoxic state or shock in COVID-19 patients, which may cause liver ischemia, hypoxia, and hypoperfusion. Studies by Sun Dawei and others [28–30] found that the incidence of liver damage in COVID-19 patients was 56.9%, among which the incidence in critically ill patients was as high as 90.9%, meaning that the incidence of liver damage in COVID-19 patients was higher, which is similar to the results of this study. Zhou et al. [21] found that 30% of COVID-19 patients have shortened prothrombin times, and coagulation dysfunction can lead to thrombosis on the surface of intravenous plaques and induce venous thrombosis, myocardial infarction, and other cardiovascular diseases. When there is venous thrombosis, a clot can easily fall off and form an embolus, which further leads to the occurrence of myocardial infarction or other severe or critical complications.
Multivariate logistic regression analysis in this study showed that age, clinical symptoms (fever, cough, fatigue, gastrointestinal symptoms, and chest tightness), underlying diseases (hypertension, chronic kidney disease, diabetes, cerebrovascular disease, COPD, and tumors), and complications (venous thrombosis, pneumothorax, acute liver injury, and electrolyte imbalance) are risk factors for severe/critical COVID-19. We further used the above factors as indicators of the early warning model through four logistic regression models and the assignment of the OR value of each risk factor. We compared them before and after admission and with or without complications as predictors and more accurately and comprehensively evaluated the value of each risk factor in the development of critical illness. The ROC curve analysis for all the above factors showed that the AUC of this early warning model reached 0.944, and when −2.2255 was used as the critical value, the sensitivity and specificity reached 86.7% and 90.0%, respectively. The efficacy of this early warning model is at a relatively high level in predicting severe/critical COVID-19. To predict the probability of severe/critical illness in patients with COVID-19 as early as possible, we removed complications after admission. Then, we analyzed the ROC curve again based on age, clinical symptoms, and underlying diseases at admission and established the prediction model. Its AUC reached 0.943, and when 1.9465 was used as the critical value, the sensitivity and specificity reached 82.7% and 92.4%, respectively, and the effectiveness of the prediction model was also at a relatively high level.
This study was based on the screening of 25 clinical characteristics of 1046 patients with COVID-19 in China. We found 3 risk factors that affect the development of severe illnesses, which can predict the possibility of COVID-19 patients developing critical illnesses at admission and can serve for triage at the patient's visit, improving the efficiency of medical resource allocation. The prediction model can be used to evaluate the risk for COVID-19 patients to progress to severe conditions.
Statistics found that approximately 20% of COVID-19 patients are seriously ill, and 5%–10% of them are transferred to the ICU and need to be intubated or to use a ventilator. Currently, in areas with scarce medical resources, it is particularly important to identify the risk for COVID-19 patients becoming critically ill in the early stages and strengthen the monitoring of patients with high risks in a timely manner. At present, most of the risk prediction models for severe illnesses are comprehensive predictions of clinical symptoms and laboratory test indicators. For example, the study by the team of Academician Zhong Nanshan [31] was based on 1590 patients with COVID-19 across the country, and “the HNC-LL score for predicting the severity of coronavirus disease 2019” was based on the team of Professor Zhu Hong [32]. Based on the general symptoms and laboratory examination outcomes of 4711 COVID-19 patients, Altschul et al. [33] proposed a model that can be used to predict the mortality of the patients during the surge period. Our model greatly differed from those reported in the literature: (1) in this model, clinical symptoms were the main indicators; (2) it was at the time of admission that this model could be used to predict the risk factors for severe disease based on the patient's age, clinical symptoms, and underlying diseases to strengthen the testing of patients and improve the utilization of medical resources. This model is easy to apply, provides individualized prediction probability for each patient, and can be used for triage during visits, greatly improving the efficiency of medical resource allocation. Early identification of patients who are at risk of becoming severely or critically ill in the early stage of the disease and timely strengthening of monitoring can help improve the prognosis of patients and have important implications for the allocation of medical resources.
This study has some limitations. First, the number of included severely ill COVID-19 patients was small, and the sample size was small. Second, the severe cases of COVID-19 in this region only account for a small part of the country's severely ill cases; therefore, this study is regional and needs to be further verified and evaluated in other parts of the country. In addition, there may be selection bias when determining the factors that affect the severity of the disease. Finally, this study was a retrospective study, so certain biases might have occurred.
5. Conclusions
At the admission of COVID-19 patients, we can predict the probability of severe/critical cases based on the patient's age, clinical symptoms, and underlying diseases by applying this early warning model. The indicators are relatively simple and easy to obtain and have good applicability and operability. This model can be used as a tool in clinical practice, which may help predict changes in disease conditions by dynamic scoring. It has high clinical application value and is worthy of promotion. In the future, we plan to improve this early warning model by including more research indicators, screening the indicators, and classifying each risk factor to improve its prediction accuracy.
Acknowledgments
This study was supported by the Special Project for the Construction of Innovation Environment (Talent and Base) in the Autonomous Region-Construction of Scientific and Technological Innovation Base (Resource Sharing Platform) (grant no., PT1903) and Special Project for the Research and Development of Key Public Health Technologies and Construction of Epidemic Prevention System in Xinjiang, Construction of Three-Dimensional Prevention and Control System for Major Outbreaks of New (Sudden) Infectious Diseases (grant no., 2020A03004-3).
Contributor Information
Xiaohong Yang, Email: yangxh_16@163.com.
Chao Wu, Email: mail@wu95.com.
Data Availability
The data used for the analysis in this study are available on reasonable request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Zhichuang Lian and Yafang Li contributed equally.
References
- 1.Rubin E. J., Baden L. R., Morrissey S., Campion E. W. Medical Journals and the 2019-nCoV outbreak. New England Journal of Medicine . 2020;382(9):p. 866. doi: 10.1056/nejme2001329. [DOI] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology . 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J. J. Y., Lee K. S., Ang L. W., Leo Y. S., Young B. E. Risk factors for severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis, and meta-regression analysis. Clinical Infectious Diseases . 2020;71(16):2199–2206. doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.China Health Commission Issued. New Coronavirus pneumonia diagnosis and treatment plan. Chinese Medical Information Guide . 2020;35(6):p. 23. [Google Scholar]
- 5.Nogrady B. What the data say about asymptomatic COVID infections. Nature . 2020;587(7835):534–535. doi: 10.1038/d41586-020-03141-3. [DOI] [PubMed] [Google Scholar]
- 6.Sayampanathan A. A., Heng C. S., Pin P. H., Pang J., Leong T. Y., Lee V. J. Infectivity of asymptomatic versus symptomatic COVID-19. The Lancet . 2021;397(10269):93–94. doi: 10.1016/s0140-6736(20)32651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo M., Huang X., Jiang B., Lv K., Yang Q., Sun Q. G. Logistic regression combined with ROC curve model to predict risk of critically ill-patients with COVID-19. Chinese Traditional and Herbal Drugs . 2020;51(20):5287–5292. [Google Scholar]
- 8.Poggiali E., Zaino D., Immovilli P., et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clinica Chimica Acta . 2020;509:135–138. doi: 10.1016/j.cca.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y., He Y., Yang H., et al. Development and validation a nomogram for predicting the risk of severe COVID-19: a multi-center study in Sichuan, China. PLoS One . 2020;15(5) doi: 10.1371/journal.pone.0233328.e0233328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin S., Fuentes S., Sanchez C., et al. Development and validation of a laboratory-based risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. Scandinavian Journal of Clinical and Laboratory Investigation . 2021;81 doi: 10.1080/00365513.2020.1847313. [DOI] [PubMed] [Google Scholar]
- 11.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine . 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai C.-C., Liu Y. H., Wang C.-Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. Journal of Microbiology, Immunology, and Infection . 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.-j., Ni Z.-y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine . 2020;382(18):1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J. J., Dong X., Cao Y. Y., et al. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy . 2020;75 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 15.Ren M., Liu F., Sun J., et al. Research progress on influencing factors of critical and death cases of COVID-19. International Journal of Virology . 2020;27(06):516–520. [Google Scholar]
- 16.Chen Y., Hu F., Zhu W., Ai L. Clinical analysis of fever in 416 patients diagnosed with COVID-19. The Journal of Medical Theory and Practice . 2020;33(21):3553–3555. [Google Scholar]
- 17.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Journal of the American Medical Association . 2020;323 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H., Kang Z., Gong H., et al. The Digestive System Is a Potential Route of 2019-nCov Infection:a Bioinformatics Analysis Based on Single-Cell Transcriptomes . BioRxiv; 2020. [DOI] [Google Scholar]
- 19.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Frontiers of Medicine . 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang W., Feng Z., Rao S., et al. Diarhoea may be underestimated:a missing link in 2019 novel coronavirus. Gut . 2020;69(6):1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 21.Zhou P., Yang X. L., Wang X. G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Naure . 2020;579 doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tajbakhsh A., Gheibi Hayat S. M., Taghizadeh H., et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment and follow up. Expert Review of Anti-infective Therapy . 2021;19(3):345–357. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- 23.de Barros Viana M., Rosário B. d. A., de Fátima Santana de Nazaré M., Estadella D., Ribeiro D. A., Socorro de Barros Viana G. COVID-19 in age-related neurodegenerative diseases: is there a role for vitamin D3 as a possible therapeutic strategy? Reviews in the Neurosciences . 2020;32(2):235–247. doi: 10.1515/revneuro-2020-0074. [DOI] [PubMed] [Google Scholar]
- 24.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of 2019 novel coronavirus infection in China. New England Journal of Medicine . 2020;382 doi: 10.1056/NEJMoa2002032. [DOI] [Google Scholar]
- 25.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet . 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:a descriptive study. The Lancet . 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H. Y., Li X. L., Yan Z. R., Sun X.-P., Han J., Zhang B.-W. Potential neurological symptoms of COVID-19. Therapeutic Advances in Neurological Disorders . 2020;13 doi: 10.1177/1756286420917830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun D., Zhang D., Tian R., et al. Influencing factors and clinical significance of liver function damage in patients diagnosed with COVID-19. Chinese Journal of Digestive Diseases . 2019;19(4):360–365. [Google Scholar]
- 29.Gabarre P., Dumas G., Dupont T., Darmon M., Azoulay E., Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Medicine . 2020;46(7):1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pei G., Zhang Z., Peng J., et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. Journal of the American Society of Nephrology . 2020;31(6):1157–1165. doi: 10.1681/asn.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang W., Liang H., Ou L., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Internal Medicine . 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medical Letter on the CDC & FDA. Coronavirus-COVID-19; Data on COVID-19 Detailed by Researchers at Southern Medical University (Development and Validation of the HNC-LL Score for Predicting the Severity of Coronavirus Disease 2019) Atlanta, GA, USA: Medical Letter on the CDC & FDA; 2020. [Google Scholar]
- 33.Altschul D. J., Unda S. R., Benton J., et al. A novel severity score to predict inpatient mortality in COVID-19 patients. Scientific Reports . 2020;10(1) doi: 10.1038/s41598-020-73962-9.16726 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for the analysis in this study are available on reasonable request from the corresponding authors.


