Abstract
Although insect herbivores are known to evolve resistance to insecticides through multiple genetic mechanisms, resistance in individual species has been assumed to follow the same mechanism. While both mutations in the target site insensitivity and increased amplification are known to contribute to insecticide resistance, little is known about the degree to which geographic populations of the same species differ at the target site in a response to insecticides. We tested structural (e.g., mutation profiles) and regulatory (e.g., the gene expression of Ldace1 and Ldace2, AChE activity) differences between two populations (Vermont, USA and Belchow, Poland) of the Colorado potato beetle, Leptinotarsa decemlineata in their resistance to two commonly used groups of insecticides, organophosphates, and carbamates. We established that Vermont beetles were more resistant to azinphos‐methyl and carbaryl insecticides than Belchow beetles, despite a similar frequency of resistance‐associated alleles (i.e., S291G) in the Ldace2 gene. However, the Vermont population had two additional amino acid replacements (G192S and F402Y) in the Ldace1 gene, which were absent in the Belchow population. Moreover, the Vermont population showed higher expression of Ldace1 and was less sensitive to AChE inhibition by azinphos‐methyl oxon than the Belchow population. Therefore, the two populations have evolved different genetic mechanisms to adapt to organophosphate and carbamate insecticides.
Keywords: carbamate, gene expression, insecticide resistance, organophosphate, target site mutation
We tested structural and regulatory differences between two populations of the Colorado potato beetle in their resistance to two commonly used groups of insecticides. We show that despite the similar frequency of resistance‐associated mutations in the target site gene, the more resistant populations had additional mutations in another target site gene, higher expression of the target site genes and had less sensitive to enzyme to insecticide inhibition. Therefore, the two populations have evolved different genetic mechanisms to adapt to organophosphate and carbamate insecticides.
1. INTRODUCTION
Resistance to insecticides is a serious global problem that can negatively affect human health, food production, and agriculture (Gould et al., 2018). Since 1914, up to 597 arthropod species have become resistant to more than 300 insecticides (Roush & Tabashnik, 2012; Sparks & Nauen, 2015). The evolution of insecticide resistance is a process of genetically based decrease in population susceptibility to insecticides (IRAC, 2020). Although insecticides can have a specific selective pressure, where selection acts on a specific target, there is still considerable variation in the magnitude of insecticide resistance among the populations of the same insect species (Dively et al., 2020; Ryan et al., 2019; Wu et al., 1999). These differences have often been linked to the genetic differences at the target site (Ilias et al., 2014; Weill et al., 2003). Despite increasing knowledge of the biochemical basis of insecticide resistance, relatively little is known about the geographic difference in insecticide target site gene sequences or their regulation (Hawkins et al., 2019; Ryan et al., 2019).
Pest species can become resistant to insecticides via various structural and regulatory mechanisms (Feyereisen et al., 2015). Structural mechanisms are better characterized and involve target site mutations in an enzyme that make the insecticide ineffective. These can be produced with genetic differences such as nonsynonymous nucleotide variation at target site genes (Li & Han, 2004; Malekmohammadi & Galehdari, 2016; Weill et al., 2004; Zhu et al., 1996). Regulatory mechanisms have been less studied and can be related, for example, to biochemical processes such as the overexpression of the target site or enhanced metabolism or excretion of the insecticide (Barres et al., 2016; Ffrench‐Constant, 2013). In general, we know less about the variation among populations at both the nucleotide and regulatory levels of the target site genes. By understanding population‐level variation also at the regulatory level, we may be able to explain better why the same insecticide might cause different outcomes among populations. This is because our predictions based on gene sequence variation alone might bias our estimates of the overall pesticide resistance (see discussion in Hawkins et al., 2019).
Organophosphates (OPs) and carbamates are both widely known as acetylcholinesterase (AChE; EC 3.1.1.7) inhibitors, which have been heavily used as insecticides since the 1970s. The AChE enzyme functions at the synapses of cholinergic neurons in the central and peripheral nervous system (Fukuto, 1990; Taylor, 2011; Taylor et al., 2009). The enzyme terminates neurotransmission at cholinergic synapses in the synaptic cleft by hydrolyzing the neurotransmitter acetylcholine (Taylor et al., 2009). OP and carbamate insecticides inhibit AChE, thus interfering with neurotransmission, leading to paralysis and death (Colovic et al., 2013). In many invertebrates, there are two distinct acetylcholinesterases (AChE1 and AChE2), which are encoded by two ace genes (i.e., ace1 and ace2; Kim & Lee, 2013). The two AChEs are probably homologous, derived from an ancient duplication event that occurred long before the differentiation of insects (Weill et al., 2002). In 67 insect species out of 100, AChE1 accounts for most of the AChE activity and thus is considered as the main catalytic enzyme (Kim & Lee, 2013) responsible for neuronal functions (Weill et al., 2002). However, the role of AChE2 differs between species. It can act as the main catalytic activity, be equally active to AChE1, or show little catalytic activity (Kim & Lee, 2013). For example, in Bombyx mori, Bm‐ace2 is more highly expressed and thus likely the main catalytic enzyme, while Bm‐ace1 may contribute to metamorphosis (Chen et al., 2009). However, more functional studies are needed to demonstrate the exact functions of these two genes (Jiang et al., 2018).
The Colorado potato beetle (CPB), Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae), is a model organism for studying the evolution of insecticide resistance (Schoville et al., 2018). CPB has played an important role in the modern pesticide industry. Since it was first targeted by insecticides in 1864, it has been heavily managed with insecticides (Alyokhin et al., 2008; Gauthier et al., 1981). CPB has currently developed resistance to more than 50 different active ingredients used in insecticides, including OPs (16 active ingredients) and carbamates (five active ingredients; Alyokhin et al., 2008; Brevik et al., 2018; Mota‐Sanchez & Wise, 2020). CPB populations in North America and Europe have developed resistance to both classes of insecticides (Mota‐Sanchez & Wise, 2020). Until recently, only one ace gene, Ldace2, orthologous to the Drosophila melanogaster ace2 gene (Zhu & Clark, 1995), has been associated with the resistance to OP and carbamate insecticides in CPB (Zhu et al., 1996). Three mutations (S291G, R30K, and Y45H) in Ldace2 gene have been described and linked to OP and/or carbamate resistance (Kim et al., 2007; Zhu & Clark, 1997; Zhu et al., 1996), with the S291G mutation being the main target site conferring resistance (Zhu et al., 1996). Revuelta et al. (2011) described Ldace1 for the CPB which is an orthologous to the Anopheles gambiae ace1 gene. The authors suggested that the 2‐ to 11‐fold higher expression of Ldace1 compared with Ldace2 indicated that Ldace1 rather than Ldace2 was the main contributor to the AChE activity and may have been the primary target of the OP insecticides (Revuelta et al., 2011).
We set to study whether the differences among populations in mortality are linked to sequence variation and/or regulatory resistance mechanisms. We hypothesized that sequence and regulatory variation in Ldace1 and Ldace2 genes (but see Piiroinen et al., 2013) might contribute to differences in OP and carbamate resistance development in different geographic populations. We tested how the Ldace1 and Ldace2 genes contribute to resistance to OP and carbamate insecticides by comparing CPB populations that differ in their resistance to these insecticides. Using bioassays, we started by estimating the resistance level of six different populations to the organophosphate azinphos‐methyl (AZ) and the carbamate carbaryl (CAR) insecticides. Based on the resistance status, we selected two populations, Vermont (from the USA) as the most resistant, and Belchow (from Poland) as the less resistant population, for further investigation of Ldace1 and Ldace2 genes. To test for population‐level differences, we first measured the efficacy of AChE inhibition by azinphos‐methyl oxon (AZoxon) and CAR insecticides. Then, we measured Ldace1 and Ldace2 gene expression, both before exposure and after exposure to both insecticides. Finally, we sequenced the Ldace1 and Ldace2 genes and investigated the frequency of amino acid replacements (mutations) in the two populations. We predicted that the more resistant population (i) would be less sensitive to AChE insecticide inhibition, (ii) demonstrate higher target site expression levels, and (iii) show higher frequency of mutations at target sites.
2. MATERIALS AND METHODS
2.1. Study species and rearing conditions
Colorado potato beetles used in this study were descendants of beetles collected from potato fields from Vermont, USA (44°43′N, 73°20′W), Belchow, Poland (52°01′N, 20°34′E), Padua, Italy (45°48′N, 12°07′E), Emmen, the Netherlands (52°54′N, 6°51′E) in 2010, near Ufa, Russia (54°47′N, 55°57′E) in 2009, and Petroskoi, Russia (61°49′N, 34°10′E) in 2006. The experiments were performed in 2012, and thus, the populations had been reared without exposure to insecticide for 2–6 generations (one generation/year). In each generation, unrelated parental beetles (i.e., overwintered generation) were mated within the population and each pair (i.e., family) was reared in a petri dish lined with a moisturized filter paper and fed daily with fresh potato leaves (Solanum tuberosum variety Van Gogh). To maintain genetic diversity in the laboratory stock, at least 50 families were reared in each generation. Eggs were collected daily, and larvae were reared in family groups until adulthood. Beetles were maintained at a constant temperature of 23°C under a fluctuating light regime of 18‐h light (16‐h light with 1‐h dim light imitating sunset and sunrise) and 6‐h dark in controlled environmental chambers (Type B1300, Weiss Technic). After rearing the beetles for one generation with an 18:6 L:D photoperiod, we simulated fall conditions to ensure that all populations induced diapause, and we reared newly emerged adult beetles under a 12:12 L:D photoperiod (Lehmann et al., 2014a). Adult beetles of the summer generation overwintered individually at 5°C in environmental chambers.
2.2. Insecticide bioassays
We used bioassays to determine whether the different geographic populations differ in the degree of insecticide resistance to azinphos‐methyl (AZ; organophosphate) and the carbaryl (CAR; carbamate) insecticides (Ovčarenko et al., 2014). For the bioassays, we obtained both AZ and CAR as Pestanal analytical standards from Sigma‐Aldrich, which were dissolved in acetone. Bioassays were performed by applying insecticide treatment to early third instar (5–7 days old, Bointeau & Le Blanc, 1992) larvae using a pipette. Larvae were randomly divided into petri dishes (five individuals/family/petri dish/) and were randomly assigned to an insecticide treatment. Then, we applied a 3 µl drop of AZ, CAR, or acetone (as control) topically to the fifth and sixth dorsal abdominal segments. In order to calculate the median lethal dose (LD50), we treated each of the six populations (N = 160–414 larvae) with 4–8 different insecticide doses ranging from 0.0075 to 30 µg/larvae for AZ and 0.06 to 90 µg/larvae for CAR (3–6 larvae per family, 20–74 larvae per insecticide dose, see Figure S1). After the insecticide application, we provided larvae a standardized potato leaf after 2 h and assessed survival after 22 h (24 h of survival from the application). A larva was considered dead if it was not able to move after being placed on its back.
We analyzed larval survival separately for both insecticides using generalized linear models (GLM; Binary logistic, logit link function) in SPSS (IBM SPSS Statistics 24.0.0). Population and insecticide dose were set as explanatory variables and survival as the dependent variable. We determined the resistance levels (LD50, i.e., lethal dose) and generated the 95% confidence limits for each population and insecticide using Probit analysis. We considered differences in LD50 values between populations and insecticides significant if their 95% confidence limits did not overlap (Ovčarenko et al., 2014). No mortality was observed in any of the populations within the acetone control.
2.3. Sampling AChE enzyme activity and gene expression studies
Based on bioassay results, the Vermont and Belchow populations were selected for further studies to investigate the AChE activity, gene expression, and nonsynonymous point mutations in the two genes (Tables 1, 2; Figure 1). We selected the Vermont population because it showed the highest tolerance to both insecticides, while the Belchow population was less resistant. Since the LD10 dose of Vermont population would have killed all the beetles from Belchow population, we applied population‐specific LD10 doses to cause 10% mortality (LD10) and induce a similar level of insecticidal stress. We used the same protocol described above for applying insecticide doses of AZ (Vermont 0.375 µg/larvae and Belchow 0.0075 µg/larvae), CAR (Vermont 1.5 µg/larvae and Belchow 0.15 µg/larvae), and acetone as control. After the insecticide application, we collected the surviving larvae 2, 4, and 24 h from the insecticide application, froze them in liquid nitrogen, and stored at −80°C until further analysis. In total, we collected 713 larvae from 15 Vermont families and 417 larvae from 15 Belchow families.
TABLE 1.
AZ insecticide toxicity to the Colorado potato beetle larvae
| Population | N | Slope (±SE) | Z | p | LD50 (µg/larvae) (95% confidence limits) | Resistance ratio (RR) a |
|---|---|---|---|---|---|---|
| Ufa (Russia) | 160 | 60.06 (±10.11) | 5.94 | <.001 | 0.02 (0.006–0.045) | 1 |
| Belchow (Poland) | 199 | 26.94 (±4.26) | 6.32 | <.001 | 0.04 (0.017–0.086) | 2.3 |
| Petroskoi (Russia) | 232 | 21.53 (±2.93) | 7.35 | <.001 | 0.05 (0.024–0.113) | 3.0 |
| Emmen (Netherlands) | 205 | 6.20 (±0.79) | 7.81 | <.001 | 0.05 (0.021–0.107) | 2.9 |
| Padua (Italy) | 223 | 0.22 (±0.04) | 5.16 | <.001 | 0.22 (0.099–0.476) | 13.1 |
| Vermont (USA) | 322 | 0.06 (±0.01) | 7.48 | <.001 | 4.14 (1.998–9.416) | 246.1 |
LD50 (i.e., lethal dose that kills 50% of exposed individuals within 24 h since exposure) values for the six Colorado potato beetle populations exposed to the AZ insecticide.
Resistance ratio showing the increase in resistance compared to the least (Ufa) resistant population.
TABLE 2.
CAR insecticide toxicity to the Colorado potato beetle larvae
| Population | N | Slope (±SE) | Z | p | LD50 (µg/larvae) (95% confidence limits) | Resistance ratio (RR) a |
|---|---|---|---|---|---|---|
| Ufa (Russia) | 231 | 0.488 (±0.084) | 5.83 | <.001 | 0.33 (0.155–0.705) | 1 |
| Belchow (Poland) | 267 | 0.438 (±0.056) | 7.83 | <.001 | 0.41 (0.199–0.845) | 1.2 |
| Petroskoi (Russia) | 256 | 0.109 (±0.014) | 7.99 | <.001 | 2.15 (1.065–4.395) | 6.5 |
| Emmen (Netherlands) | 414 | 0.017 (±0.003) | 6.51 | <.001 | 3.70 (2.064–6.974) | 11.2 |
| Padua (Italy) | 211 | 0.012 (±0.003) | 4.29 | <.001 | 71.39 (29.831–187.697) | 215.1 |
| Vermont (USA) | 286 | 0.017 (±0.003) | 6.55 | <.001 | 43.23 (19.496–104.350) | 130.3 |
LD50 values for the six Colorado potato beetle populations exposed to the CAR insecticide.
Resistance ratio showing the increase in resistance compared with the least (Ufa) resistant population.
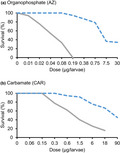
FIGURE 1.
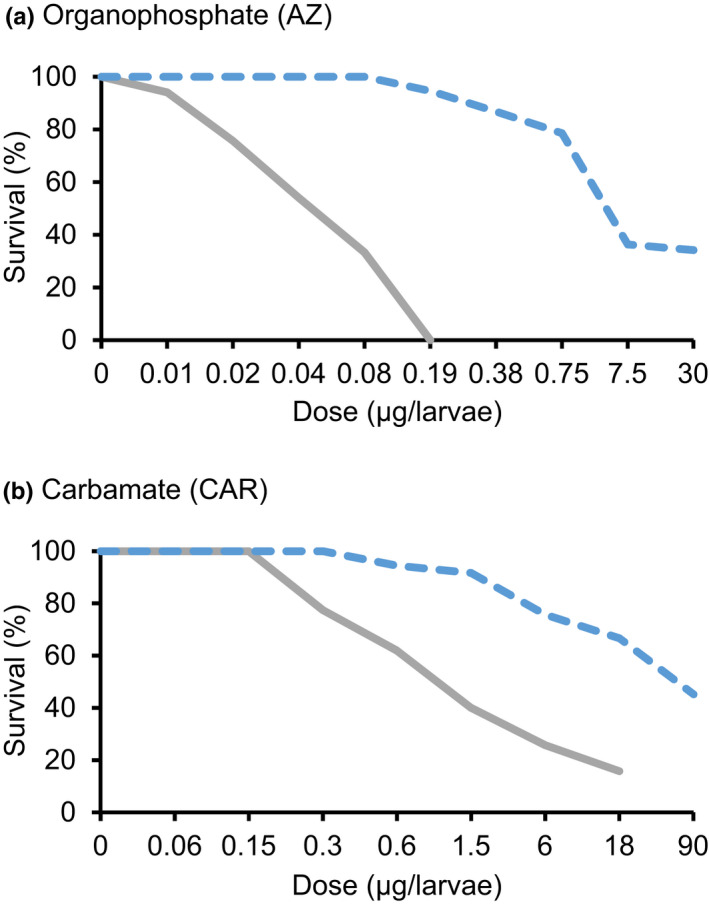
Survival (%) of Colorado potato beetles from Belchow (gray solid line) and Vermont populations (blue dashed line) after exposure to different doses (µg/larvae) of (a) azinphos‐methyl (AZ) and (b) carbaryl (CAR) insecticides
2.4. Measurement of AChE inhibition efficiency
We tested in vitro whether populations that can tolerate higher insecticide doses (see Tables 1, 2) possess an AChE variant that is less sensitive to insecticide inhibition by using a modified Ellman et al. (1961) protocol (see also Anderson & Coats, 2012; Pang et al., 2012; Singh et al., 2017). We assessed AChE enzyme activity inhibition from the control treatment larvae; that is, treated with only acetone, the 2‐ and 4‐h groups (n = 25 and n = 28 for Vermont and Belchow, respectively). Individual larvae were put into 200 µl of ice‐cold 1xPBS buffer (pH 7.5) containing 0.5% (v/v) Triton X‐100, homogenized by crushing for 2–3 min, and sonicated for 40 s before centrifuging at 15,871 g for 20 min at 4 °C. The total protein concentration was measured from the supernatant with a NanoDrop ND‐1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA). Thereafter, samples were normalized to a concentration of 15 mg/ml. The supernatants were subsequently used for the enzyme activity inhibition analysis. Enzyme activity was determined by an Ellman et al. (1961) method with the QuantiChrom™ Acetylcholinesterase Assay Kit (DACE‐100; BioAssay Systems) following the manufacturer’s instructions. The reaction mixture for inhibition consisted of 15 µl of supernatant and 2 µl of the AChE enzyme inhibitor (12.9 and 250 µM for azinphos‐methyl oxon (AZoxon, Sigma‐Aldrich) and CAR, respectively). We used AZoxon in this assay because AZ is metabolized to AZoxon when an insect ingests it. Insecticide stock solutions (AZoxon and CAR in 100% EtOH) were first diluted with water so that the final concentration of EtOH in the inhibition reaction was less than 1%. After the inhibition reaction was incubated for 2 min at RT, the supernatant and inhibitor solution were added to 190 µl of the working reagent provided by the manufacturer, after which the absorbance was measured 2 and 10 min later at 405 nm with a Victor X4 2030 multilabel plate reader (PerkinElmer). Each sample was measured with three technical replicates. AChE enzyme activities were determined following the manufacturer’s instructions. AChE activity after inhibition was calculated as the percentage of enzyme activity after insecticide inhibition divided by enzyme activity without inhibition. We tested whether the populations differed in AChE activity after insecticide inhibition (%) was analyzed using separate one‐way ANOVA tests in SPSS version 26.0 (IBM SPSS Statistics) for each insecticide. We used Levene’s test to test for homogeneity of variance and Shapiro–Wilk test to test for normality.
2.5. Expression of AChE genes
We tested for differences in the gene expression level of Ldace1 and Ldace2 between Vermont and Belchow populations. We sampled larvae 24 h after the treatment applications. Total RNA was extracted from individual larvae with the TriReagent (Sigma‐Aldrich) and the RNeasy Mini RNA Extraction Kit (Qiagen). Extraction was followed by a clean‐up step using RNeasy columns, including DNase I treatment (RNase‐Free DNase Set, Qiagen). The concentration and purity of RNA was measured with a NanoDrop ND‐1000 spectrophotometer. RNA integrity and quality were checked with an Agilent 2100 Bioanalyzer (Agilent). The RNA concentration was normalized to 100 ng/µl before generating complementary DNA (cDNA) using the iScript™ cDNA Synthesis Kit with oligo (dT) and random hexamer primers (Bio‐Rad Laboratories Inc.). The qPCR reaction mix contained 10 µl of 2× SYBR Green Supermix (Bio‐Rad Laboratories Inc.), 0.5 µM of each gene‐specific primer, and 5 µl of cDNA (diluted 1:4) for a total volume of 20 µl. We designed primers used for qPCR (Table S1) amplification of Ldace1 (JF343436.1; (Revuelta et al., 2011)) and Ldace2 (L41180.1; (Zhu & Clark, 1995)) using Primer 3 (http://frodo.wi.mit.edu/primer3/, v. 0.4.0) according to Lehmann, Piiroinen, et al. (2014). The primers were designed based on sequences from the annotated transcriptome of CPB (Kumar et al., 2014) and sequences available in GenBank. The qPCRs were run on a Bio‐Rad CFX96™ instrument with an initial denaturation step of 95°C for 3 min, followed by 39 cycles of 10 s at 95°C, 10 s at 56°C, and 30 s at 72°C. The qPCR was followed by melting curve analysis (65–95°C) to check the purity of qPCR. We analyzed ten biological replicates (individual beetles) with three technical replicates for each treatment group (control, AZ, and CAR) for each population. In order to calibrate the expression across samples, we used two positive control samples with two replicates that were added to each plate. The efficiency of qPCR amplification was calculated for each gene using twofold serial dilutions of pooled cDNA. The amplification efficiencies were between 96% and 113%.
We calculated expression values (mean Cq) for all the samples using the normalized expression (ΔΔCq) method with default threshold values by using the CFX Manager 3.0 software (Bio‐Rad Laboratories Inc.). We used forkhead transcription factor (FOXO) and ribosomal protein L13e (L13e) as reference genes (Table S1; Kumar et al., 2014; Lehmann, Piiroinen, et al., 2014; Yocum et al., 2009). We analyzed the relative expression data with the REST program (http://rest.gene‐quantification.info/; Pfaffl et al., 2002), according to Lehmann, Piiroinen, et al. (2014). The REST program (with 10,000 iterations) was used for pairwise comparisons within and between population and treatment groups.
2.6. Identification of sequence variation in the Ldace1 and Ldace2 genes
To identify and compare mutations in Ldace1 and Ldace2 between populations, we sequenced the genes from the experimental larvae. We extracted total RNA from 38 (Belchow n = 19, Vermont n = 19) whole larvae and synthesized cDNA using the same procedures described earlier. Primers for Ldace1 and Ldace2 (Table S2) were designed using the CPB sequences available in GenBank [Ldace1: JF343436.1, (Revuelta et al., 2011); Ldace2: L41180.1, (Zhu & Clark, 1995)] similarly as described before. PCRs were performed in a 25 µl reaction containing 4 µl of cDNA, 1× of Dream buffer (Thermo Fisher Scientific), 1 µM of forward and reverse primer, 0.2 mM of dNTPs, and 0.2 U of Dream Taq DNA polymerase (Thermo Fisher Scientific). The cycling conditions for the PCR were 94°C for 3 min, then 35 cycles at 94°C for 45 s, 56°C for 1 min 30 s, and 72°C for 1 min 30 s. PCR products were purified using Exonuclease (Exonuclease I, Thermo Fisher Scientific) and Shrimp Alkaline Phosphatase (SAP, Thermo Fisher Scientific). Sequencing reactions were performed using BigDye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific). Sequencing reactions were performed in a 20 µl reaction containing 3.75 µl of 5× BigDye Sequencing buffer, 0.5 µl of 2.5× Ready Reaction Premix, 1 µl of 3.2 µM of the primer, and 3–10 µl of the purified PCR product. Thereafter, samples were run on an ABI Prism 3130xl genetic analyzer (Thermo Fisher Scientific). Chromatograms were analyzed using Geneious version 8.1.9 (Biomatters, Ltd, New Zealand, http://geneious.com (Kearse et al., 2012)). The 1036 bp of Ldace1 and 1890 bp of Ldace2 were aligned by codons with the reference sequence from GenBank, Ldace1 (JF343436.1; Revuelta et al., 2011) and Ldace2 (L41180.1; Zhu & Clark, 1995) using Muscle in Geneious with default parameters. In total, we analyzed the sequences of 36 (Belchow n = 17, Vermont n = 19) and 37 (Belchow n = 19, Vermont n = 18) individuals for Ldace1 and Ldace2, respectively. Frequency of amino acid replacements was compared between populations by a chi‐square test in SPSS.
3. RESULTS
3.1. Insecticide bioassays
Beetle survival (i.e., insecticide resistance) varied among all six populations for both insecticides, AZ (Wald χ2 = 742.1, df = 5, p < .001; Table 1) and CAR (Wald χ2 = 965.3, df = 5, p < .001; Table 2, Figures 1 and S1). Survival decreased with increasing dose of AZ (Wald χ2 = 930.1, df = 8, p < .001) and CAR insecticides (Wald χ2 = 1650.0, df = 7, p < .001). There was a significant interaction between the insecticide dose and population: AZ (Wald χ2 = 8408.0, df = 15, p < .001) and CAR (Wald χ2 = 5253.8, df = 20, p < .001). These significant interactions indicated that different populations tolerated different doses of insecticides, which can be seen as differences in the LD50 values among populations (Tables 1, 2). The lowest LD50 values to AZ insecticide were recorded for Ufa, Belchow, Petroskoi, and Emmen, whereas the highest values were recorded for Vermont (Table 1). The lowest LD50 values for the CAR insecticide were recorded in Ufa and Petroskoi, whereas the highest values were in Vermont and Padua (Table 2). The Vermont population was 107—times more resistant to AZ and 20—times more resistant to CAR insecticide than the Belchow population when comparing the LD50 levels.
3.2. Insecticide inhibition efficiency
Both insecticides inhibited AChE activity by more than 30% (CAR: t test =8.6, df = 24, p < .001, AZoxon: t test =10.8, df = 36, p < .001; Figure 2). However, the Belchow population was more sensitive to AZoxon inhibition than the Vermont population (F 1,35 = 11.6, p = .002). The AChE activity in Belchow beetles was 12% more inhibited than those in Vermont beetles (F 1,35 = 4.6, p = .039; Figure 2). In contrast, there were no differences between the two populations in AChE activity after inhibition by CAR (F 1,23 = 0.034, p = .855; Figure 2).
FIGURE 2.
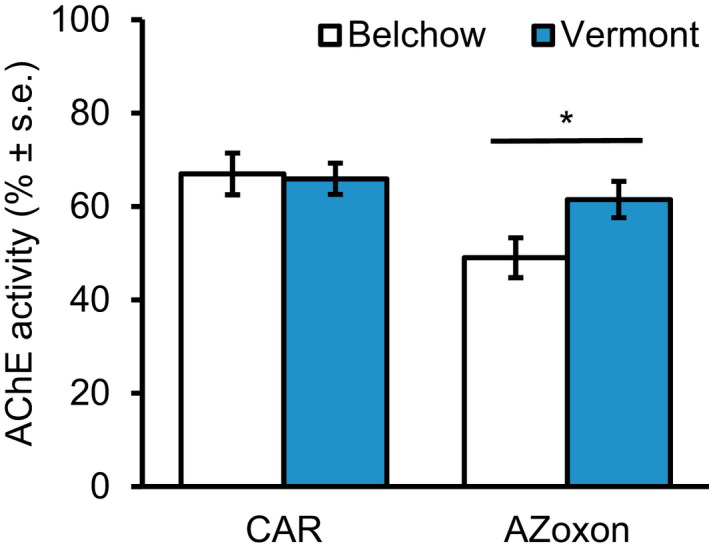
AChE activity (% ± s.e.) after inhibition with either a) 250 µM of carbaryl (CAR) or (b) 12.9 µM of azinphos‐methyl oxon (AZoxon) of Colorado potato beetles from Belchow (white) and Vermont (blue) populations. * indicates significant difference (p < .05) between the populations
3.3. Expression of AChE genes
The Ldace1 gene was expressed at a higher level in the beetles than the Ldace2 gene in both Belchow (Fold change (FC = 49, p < .001) and Vermont (FC = 145, p < .001) populations (Figure 3). The expression of Ldace1 significantly differed between populations (Figure 3a). Ldace1 was significantly upregulated in all treatment groups in the Vermont population compared to the Belchow population (Figure 3a). Averaged across all treatment groups, Ldace1 expression was 2.4‐fold higher in the Vermont population than in the Belchow population. The differences in expression varied significantly between populations for each treatment group: control group (FC = 2.3, p < .001), AZ group (FC = 2.5, p < .001), and CAR group (FC = 2.4, p = .01; Figure 3a). Within‐population comparisons revealed that exposure to insecticide (both to AZ and CAR) did not induce changes in Ldace1 expression levels when compared to the control group (Figure 3a).
FIGURE 3.
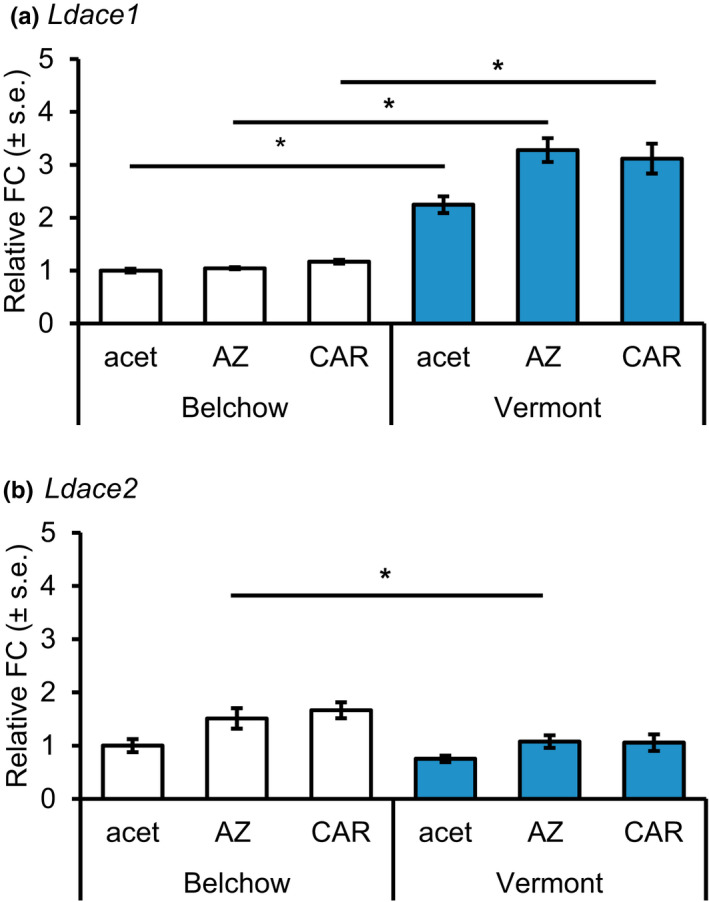
Relative fold change (FC ± s.e.) to Belchow control (acet) group of (a) Ldace1 and (b) Ldace2 expression levels between different treatments azinphos‐methyl (AZ), carbaryl (CAR), and control (acet) for Colorado potato beetles from Belchow (white) and Vermont (blue) populations. The fold change difference between Ldace1 and Ldace2 transcripts was 49 in the Belchow population and 145 in the Vermont population. * indicates a significant difference (p < .05) between populations
Ldace2 was not statistically differently expressed in the Vermont and Belchow populations in both the control and CAR insecticide groups (Figure 3b.). However, exposure to the AZ insecticide resulted in downregulation of Ldace2 gene in Vermont compared with the Belchow population (FC = 0.85, p = .04). Within the Belchow population, Ldace2 expression was marginally non‐significantly (FC = 1.7, p = .059) upregulated in the AZ exposed group when compared to the control group. This suggested that individuals in the Belchow population increased Ldace2 expression in response to AZ insecticide exposure. We did not identify any between‐ or within‐population effects on Ldace2 expression when comparing the CAR insecticide treatment group to the control group.
3.4. Identification of sequence variation in the Ldace1 and Ldace2 genes
Sequencing analyses revealed five nonsynonymous mutations in the AChE genes: G192S and Y402F in the Ldace1 gene and R30K, Y54H, and S291G in the Ldace2 gene. In the Ldace1 gene, the G192S and Y402F mutations were only present in the Vermont population (Figure 4a). Allele frequencies differed significantly (G192S: χ2 = 7.8, df = 2, p = .020; Y402F: χ2 = 10.7, df = 2, p = .005; Figure 4b) between populations. Homozygotes for the G192S and Y402F mutations represented 31% and 8% of the individuals from the Vermont population, respectively. The two alleles never co‐occurred in the same individual.
FIGURE 4.
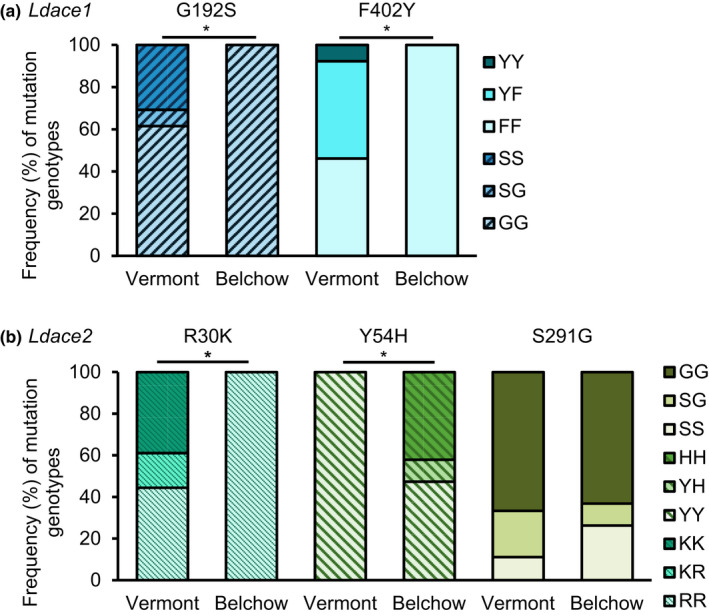
Frequency (%) of different genotypes for alleles in the (a) Ldace1 and (b) Ldace2 gene in the Colorado potato beetle. Light shades show the frequency of homozygous non‐mutated sites, intermediate shades show the frequency of heterozygous sites, and dark shades show homozygous mutated sites. * indicates significant difference (p < .05) between populations. SS‐homozygous for G192S allele, SG‐heterozygous, GG‐homozygous, lacking G192S allele; YY‐homozygous for the F402 allele, YF‐heterozygous, FF‐homozygous, lacking the F402 allele in the Ldace1 gene. KK‐homozygous for R30K allele, RK‐heterozygous, RR‐homozygous lacking R30K allele; HH‐homozygous for Y54H allele, YH‐heterozygous, YY‐lacking the Y54H allele; GG‐homozygous for the OP resistance‐associated allele S291G, SG‐heterozygous, SS‐homozygous, lacking the S291G allele in the Ldace2 gene
In the Ldace2 gene, the R30K mutation was only present in the individuals from the Vermont population, whereas the Y54H mutation was only present in the Belchow population. The S291G mutation was identified in both populations (Table 2; Figure 4b). Allele frequencies of the R30K and Y54H mutations differed significantly between the populations (R30K: χ2 = 14.5, df = 2, p = .001; Y54H: χ2 = 13.0, df = 2, p = .002; Figure 4b). In the Vermont population, the R30K and S291G mutations were present in 39% and 67% of the individuals, respectively. In the Belchow population, the frequency of the Y54H mutation was 42%, whereas that of the S291G mutation was 63% (Figure 4b). The frequency of the S291G mutation was similar in both populations (S291G: χ2 = 1.9, df = 2, p = .382). Both the R30K (in Vermont) and the Y54H (in Belchow) alleles occurred together with the S291G in 39% and 42% of individuals, respectively.
4. DISCUSSION
We investigated the population‐level sequence variation of resistance‐associated Ldace1 and Ldace2 genes together with their responses to two commonly used pesticides against the Colorado potato beetle to understand the role of these genes on insecticide resistance. We found that geographic populations differ in their resistance to two commonly used insecticides due to multiple differences in the insecticide target site. We demonstrated that the North American population (Vermont, USA) had higher resistance compared with a European population (Belchow, Poland), which was associated with higher occurrence of mutations (Figure 4), higher baseline expression of the Ldace1 gene (Figure 3a), and an AChE enzyme less sensitive to AZoxon insecticide inhibition (Figure 2). In addition, the Vermont population had two mutations in the Ldace1 gene that were absent from Belchow (Figure 4a). Therefore, it is likely that repeated application of insecticides (Dively et al., 2020) or different insecticide intensities (Crossley et al., 2018) together with invasion history (Grapputo et al., 2005) has resulted in the evolution of sequence variation and regulatory changes in the target genes that probably contributed to the overall higher resistance of the Vermont population (Figure 1).
We identified two novel mutations (G192S and F402Y) in the Ldace1 gene in the Vermont population. The absence of these mutations in the Belchow population could be due to the loss of genetic variation when the beetle invaded Europe (Grapputo et al., 2005). Although the mutation in the Ldace2 S291G site has been previously described as the main mutation contributing to organophosphate insecticide resistance in the CPB (Zhu & Clark, 1997; Zhu et al., 1996), the fact that it was equally common in both Vermont and Belchow populations suggests that it is unlikely to be the main factor explaining explains the 107‐time difference in survival between the populations (Figure 1). It may be possible that the ancestral state of the resistance mutation in the Ldace2 gene has been G291S rather than S291G. This is because individuals with the resistance mutation are more susceptible to the host plant alkaloids (Wierenga & Hollingworth, 1992). This mutation might be related to the beetles’ adaptation to the lower concentrations of steroidal alkaloids in agricultural solanaceous plants rather than insecticide resistance (Piiroinen et al., 2013; Wierenga & Hollingworth, 1992; Zhu & Clark, 1995). Therefore, it is possible that the novel mutations in the Ldace1 gene might play a more important role in the insecticide resistance than the resistance‐associated mutation S291G in the Ldace2 gene.
The two novel mutations (see Figure 4a) described here could contribute more to the insecticide resistance in the Vermont population than the previously identified mutations in the Ldace2 gene. Similar substitutions in the ace1 gene (glycine to serine (G192S) and phenylalanine to tyrosine (F402Y)) have been associated with insecticide resistance (e.g., insensitivity) in other insects. Different substitutions in the ace‐1 gene occur frequently across multiple species, for example, the glycine to serine substitution (i.e., G119S) in Anopheles gambiae (Weetman et al., 2015) and Culex pipiens (Weill et al., 2003, 2004), phenylalanine to tyrosine (i.e., F327Y, F331Y, and F445Y) in Musca domestica, Bemisia tabaci, and Culex tritaeniorhnchus (Alon et al., 2008; Nabeshima et al., 2004; Oh et al., 2006; Walsh et al., 2001). Some of the mutations in the ace1 seemed to confer high levels of resistance in combination with other mutations in other species (Mutero et al., 1994; Vontas et al., 2002) but interestingly, in our sample, the two mutations (G192S and F402Y) never co‐occurred in Ldace1. We would need more functional studies to confirm the role these mutations play the insecticide resistance of the Colorado potato beetle.
In addition to substitution differences, we also identified gene expression differences between Ldace1 and Ldace2 in the two populations (see also Dively et al., 2020). The Ldace1 gene was 49‐ to 145‐fold more expressed than Ldace2 under control conditions, suggesting that ace1 encoding ACHE1 is the major catalytic enzyme also in the CPB. These results are consistent with previous data, which indicated that in 66 insect species out of 100, AChE1 is more highly expressed than AChE2 and therefore the major catalytic enzyme of acetylcholine (Kim & Lee, 2013). Compared to a previous study (Revuelta et al., 2011), the difference between the Ldace1 and Ldace2 gene expression within Belchow (49‐fold difference) and Vermont (145‐fold difference) populations was higher than the reported difference between the developmental stages (i.e., from embryos to adults, 2‐ to 11‐fold difference). The increased expression in the Vermont beetles suggests that the Ldace1 gene could also play a role in insecticide resistance. Indeed, regulatory changes in the target gene (i.e., increased expression of acetylcholinesterase gene) have been previously shown to increase OP resistance in the greenbug (Shizaphis graminum; Gao & Zhu, 2002). The lack of differences in the Ldace2 expression between populations in the control groups further suggests a greater role for Ldace1 than Ldace2 in conferring resistance to insecticides in the CPB.
The Vermont population (see Figure 1) was less sensitive to AZoxon inhibition than the Belchow population. These differences could be explained either by regulatory resistance, that is, the lower gene expression of the Belchow populations compared with the Vermont population, or alternatively by structural resistance, that is, the mutation profile differences between the populations. Unfortunately, our sampling does not allow us to separate these two hypotheses. Previously, AChE sensitivity in the CPB has been associated with S291G and R30K mutations in the Ldace2 gene (Kim et al., 2006), but they were not aware of the presence of the Ldace1 gene. Therefore, it would be interesting to test the sensitivity differences related to the two mutations in the Ldace1 (G192S and F402Y) instead. Although the CAR insecticide inhibited AChE, there were no differences between populations. This suggests that alternative resistance mechanisms are present since there was still 20‐time survival difference between the two populations. Alternatively, the differences between populations could be due to differences in OP and carbamate insecticide‐induced inhibitory actions (Colovic et al., 2013). Finally, we cannot entirely exclude an insufficient dose in the enzyme inhibition assay.
Our results demonstrate that CPB resistance to commonly used OP and carbamate insecticides is due to multiple mechanisms acting at one target site that are not mutually exclusive. Besides changes at these target sites, it is likely that other resistance mechanisms have also been under selection (see Barres et al., 2016; Mutero et al., 1994), so we cannot expect that geographically separated populations have similar mechanisms of resistance. Therefore, the same management strategy in vast areas may not be equally successful across different populations and may potentially select for further differences among populations. Differences in the resistance mechanism can also challenge the development of new application strategies if the resistance of a population differs more in structural (has higher genetic variation) or regulatory (is better able to deal with xenobiotics) mechanisms. Further research is clearly needed to uncover which mechanisms confer the resistance in different populations. Understanding the mechanisms behind the rapid evolution of insecticide resistance will help us in making better insecticide risk assessment and management strategies, in this and other pest species.
5. CONCLUSIONS
Our results indicate that the differences we observe in insecticide resistance among populations are a result of multiple factors acting at the same time. We demonstrated that within the target site, we need to incorporate both sequence variation and regulatory changes in AChE biochemistry and physiology to understand resistance evolution. The fact that populations differ in multiple levels even within one target site means that populations can respond to the same selection pressure in different ways. From a management perspective, it is important to understand that insecticides select not only for resistance genes but also for their function at the same time. Beetles from the Vermont population had (1) an AChE that is less sensitive to insecticide inhibition, (2) higher Ldace1 gene expression (which suggests that AChE1 is the major catalytic enzyme in the CPB), and (3) more target site mutations (G192S and F402Y) in the Ldace1 gene than the Belchow population. At the same time, our results underlined that studying changes at the target sites were not sufficient in explaining the observed resistance differences between populations and that there are other pathways to achieve the resistance besides changes at the target sites (see Barres et al., 2016). Therefore, to develop more efficient pest management strategies, we need more studies to lighter the geographical variation in resistance to insecticides.
CONFLICT OF INTEREST
The authors declare no competing interest.
AUTHOR CONTRIBUTION
Aigi Margus: Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (equal); Visualization (equal); Writing‐original draft (lead); Writing‐review & editing (equal). Saija Piiroinen: Conceptualization (lead); Formal analysis (equal); Investigation (equal); Methodology (equal); Validation (equal); Visualization (equal); Writing‐review & editing (equal). Philipp Lehmann: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Validation (equal); Visualization (equal); Writing‐review & editing (equal). Alessandro Grapputo: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Supervision (equal); Writing‐review & editing (equal). Leona Gilbert: Methodology (supporting); Validation (supporting); Writing‐review & editing (supporting). Yolanda Chen: Resources (equal); Writing‐review & editing (equal). Leena Lindström: Conceptualization (lead); Data curation (supporting); Funding acquisition (lead); Investigation (supporting); Methodology (equal); Project administration (equal); Resources (equal); Supervision (equal); Writing‐review & editing (equal).
Supporting information
Supinfo S1
ACKNOWLEDGEMENTS
The authors would like to thank Andreas Plischke and Maxim Udalov for the beetles collected from Holland and Ufa. We thank Sari Viinikainen and Emily Knott for their advice during the data sequencing and Sara Calhim for fruitful discussions on survival data analysis. We thank Kati Kivisaari and Joel Rahkonen for their help of rearing the beetles. This work was financed by the Academy of Finland (Grant to LL: 308302, 250248) and Centre of Excellence in Biological interactions research (252411). Since the CPB is a quarantine species in Finland, this research was carried out under a permission from Evira (3861/541/2007).
Margus, A. , Piiroinen, S. , Lehmann, P. , Grapputo, A. , Gilbert, L. , Chen, Y. H. , & Lindström, L. (2021). Sequence variation and regulatory variation in acetylcholinesterase genes contribute to insecticide resistance in different populations of Leptinotarsa decemlineata . Ecology and Evolution, 11, 15995–16005. 10.1002/ece3.8269
DATA AVAILABILITY STATEMENT
Bioassay, AChE enzyme activity, gene expression, and gene sequence data for this study are available at University of Jyväskylä Digital Repository (JYX https://jyx.jyu.fi/handle/123456789/78053). https://doi.org/10.17011/jyx/dataset/78053.
REFERENCES
- Alon, M. , Alon, F. , Nauen, R. , & Morin, S. (2008). Organophosphates' resistance in the B‐biotype of Bemisia tabaci (Hemiptera: Aleyrodidae) is associated with a point mutation in an ace1‐type acetylcholinesterase and overexpression of carboxylesterase. Insect Biochemistry and Molecular Biology, 38(10), 940–949. [DOI] [PubMed] [Google Scholar]
- Alyokhin, A. , Baker, M. , Mota‐Sanchez, D. , Dively, G. , & Grafius, E. (2008). Colorado potato beetle resistance to insecticides. American Journal of Potato Research, 85, 395–413. [Google Scholar]
- Anderson, J. A. , & Coats, J. R. (2012). Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pesticide Biochemistry and Physiology, 102(2), 124–128. [Google Scholar]
- Barres, B. , Corio‐Costet, M. F. , Debieu, D. , Délye, C. , Fillinger‐David, S. , Grosman, J. , Micoud, A. , Siegwart, M. , & Walker, A. S. (2016). Trends and challenges in pesticide resistance detection. Trends in Plant Science, 21(10), 834–853. [DOI] [PubMed] [Google Scholar]
- Bointeau, G. , & Le Blanc, J. P. R. (1992). Colorado potato beetle LIFE STAGES. Agriculture Canada Publication 1878/E; [Google Scholar]
- Brevik, K. , Schoville, S. D. , Mota‐Sanchez, D. , & Chen, Y. H. (2018). Pesticide durability and the evolution of resistance: A novel application of survival analysis. Pest Management Science, 74, 1953–1963. [DOI] [PubMed] [Google Scholar]
- Chen, H. J. , Liao, Z. , Hui, X. M. , Li, G. Q. , Li, F. , & Han, Z. J. (2009). Ace2, rather than ace1, is the major acetylcholinesterase in the silkworm, Bombyx mori . Insect Science, 16(4), 297–303. [Google Scholar]
- Colovic, M. B. , Krstic, D. Z. , Lazarevic‐Pasti, T. D. , Bondzic, A. M. , & Vasic, V. M. (2013). Acetylcholinesterase inhibitors: Pharmacology and toxicology. Current Neuropharmacology, 11(3), 315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley, M. S. , Rondon, S. I. , & Schoville, S. D. (2018). A comparison of resistance to imidacloprid in Colorado potato beetle (Leptinotarsa decemlineata Say) populations collected in the Northwest and Midwest US. American Journal of Potato Research, 95(5), 495–503. [Google Scholar]
- Dively, G. P. , Crossley, M. S. , Schoville, S. D. , Steinhauer, N. , & Hawthorne, D. J. (2020). Regional differences in gene regulation may underlie patterns of sensitivity to novel insecticides in Leptinotarsa decemlineata . Pest Management Science, 12, 4278–4285. [DOI] [PubMed] [Google Scholar]
- Ellman, G. L. , Courtney, K. D. , Andres, V. Jr. , & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemistry and Pharmacology, 7, 88–95. [DOI] [PubMed] [Google Scholar]
- Feyereisen, R. , Dermauw, W. , & Van Leeuwen, T. (2015). Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pesticide Biochemistry and Physiology, 121, 61–77. 10.1016/j.pestbp.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Ffrench‐Constant, R. H. (2013). The molecular genetics of insecticide resistance. Genetics, 194, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto, T. R. (1990). Mechanism of action of organophosphorus and carbamate insecticides. Environmental Health Perspectives, 87, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J. , & Zhu, K. Y. (2002). Increased expression of an acetylcholinesterase gene may confer organophosphate resistance in the greenbug, Schizaphis graminum (Homoptera: Aphididae). Pesticide Biochemistry and Physiology, 73, 164–173. [Google Scholar]
- Gauthier, N. L. , Hofmaster, R. N. , & Semel, M. (1981). History of Colorado potato beetle control. In Lashomb J. H. & Casagrande R. A. (Eds.), Advances in potato pest management (pp. 13–33). Hutchinson Ross. [Google Scholar]
- Gould, F. , Brown, Z. S. , & Kuzma, J. (2018). Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science, 360, 728–732. 10.1126/science.aar3780 [DOI] [PubMed] [Google Scholar]
- Grapputo, A. , Boman, S. , Lindström, L. , Lyytinen, A. , & Mappes, J. (2005). The voyage of an invasive species across continents: Genetic diversity of North American and European Colorado potato beetle populations. Molecular Ecology, 14, 4207–4219. [DOI] [PubMed] [Google Scholar]
- Hawkins, N. J. , Bass, C. , Dixon, A. , & Neve, P. (2019). The evolutionary origins of pesticide resistance. Biological Reviews, 94, 135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilias, A. , Vontas, J. , & Tsagkarakou, A. (2014). Global distribution and origin of target site insecticide resistance mutations in Tetranychus urticae . Insect Biochemistry and Molecular Biology, 48, 17–28. [DOI] [PubMed] [Google Scholar]
- IRAC . (2020). Insecticide Resistance Action Committee, IRAC. Available at http://www.irac‐online.org (Accessed 2.2.2020). [Google Scholar]
- Jiang, X. C. , Jiang, X. Y. , & Liu, S. (2018). Molecular characterization and expression analysis of two acetylcholinesterase genes from the small white butterfly Pieris rapae (Lepidoptera: Pieridae). Journal of Insect Science, 18, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. , Buxton, S. , Cooper, A. , Markowitz, S. , Duran, C. , & Thierer, T. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J. , Dunn, J. B. , Yoon, K. S. , & Clark, J. M. (2006). Target site insensitivity and mutational analysis of acetylcholinesterase from a carbofuran‐resistant population of Colorado potato beetle, Leptinotarsa decemlineata (Say). Pesticide Biochemistry and Physiology, 84, 165–179. [Google Scholar]
- Kim, H. J. , Yoon, K. S. , & Clark, J. M. (2007). Functional analysis of mutations in expressed acetylcholinesterase that result in azinphosmethyl and carbofuran resistance in Colorado potato beetle. Pesticide Biochemistry and Physiology, 88, 181–190. [Google Scholar]
- Kim, Y. H. , & Lee, S. H. (2013). Which acetylcholinesterase functions as the main catalytic enzyme in the Class Insecta? Insect Biochemistry and Molecular Biology, 43, 47–53. 10.1016/j.ibmb.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Congiu, L. , Lindström, L. , Piiroinen, S. , Vidotto, M. , & Grapputo, A. (2014). Sequencing, de novo assembly and annotation of the Colorado potato beetle, Leptinotarsa decemlineata, transcriptome. PLoS One, 9, e86012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, P. , Lyytinen, A. , Piiroinen, S. , & Lindström, L. (2014). Northward range expansion requires synchronization of both overwintering behaviour and physiology with photoperiod in the invasive Colorado potato beetle (Leptinotarsa decemlineata). Oecologia, 176, 57–68. [DOI] [PubMed] [Google Scholar]
- Lehmann, P. , Piiroinen, S. , Kankare, M. , Lyytinen, A. , Paljakka, M. , & Lindström, L. (2014). Photoperiodic effects on diapause‐associated gene expression trajectories in European Leptinotarsa decemlineata populations. Insect Molecular Biology, 23, 566–578. [DOI] [PubMed] [Google Scholar]
- Li, F. , & Han, Z. (2004). Mutations in acetylcholinesterase associated with insecticide resistance in the cotton aphid, Aphis gossypii Glover. Insect Biochemistry and Molecular Biology, 34, 397–405. [DOI] [PubMed] [Google Scholar]
- Malekmohammadi, M. , & Galehdari, H. (2016). Target site insensitivity mutations in the AChE enzyme confer resistance to organophosphorous insecticides in Leptinotarsa decemlineata (Say). Pesticide Biochemistry and Physiology, 126, 85–91. [DOI] [PubMed] [Google Scholar]
- Mota‐Sanchez, D. , & Wise, J. (2020). Arthropod pesticide resistance database (APRD). Available at http://www.pesticideresistance.org/. (Accessed 2.2.2020). [Google Scholar]
- Mutero, A. , Pralavorio, M. , Bride, J. M. , & Fournier, D. (1994). Resistance‐associated point mutations in insecticide‐insensitive acetylcholinesterase. Proceedings of the National Academy of Sciences, 91, 5922–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima, T. , Mori, A. , Kozaki, T. , Iwata, Y. , Hidoh, O. , Harada, S. , Kasai, S. , Severson, D. W. , Kono, Y. , & Tomita, T. (2004). An amino acid substitution attributable to insecticide‐insensitivity of acetylcholinesterase in a Japanese encephalitis vector mosquito, Culex tritaeniorhynchus . Biochemical and Biophysical Research Communications, 313, 794–801. [DOI] [PubMed] [Google Scholar]
- Oh, S. , Kozaki, T. , Mizuno, H. , Tomita, T. , & Kono, Y. (2006). Expression of Ace‐paralogous acetylcholinesterase of Culex tritaeniorhynchus with an amino acid substitution conferring insecticide insensitivity in baculovirus‐insect cell system. Pesticide Biochemistry and Physiology, 85, 46–51. [Google Scholar]
- Ovčarenko, I. , Kapantaidaki, D. E. , Lindström, L. , Gauthier, N. , Tsagkarakou, A. , Knott, K. E. , & Vänninen, I. (2014). Agroecosystems shape population genetic structure of the greenhouse whitefly in Northern and Southern Europe. BMC Evolutionary Biology, 14, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, Y. P. , Brimijoin, S. , Ragsdale, D. W. , Xhu, K. V. , & Suranyi, R. (2012). Novel and viable acetylcholinesterase target site for developing effective and environmentally safe insecticides. Current Drug Targets, 13, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. W. , Horgan, G. W. , & Dempfle, L. (2002). Relative expression software tool (REST©) for group‐wise comparison and statistical analysis of relative expression results in real‐time PCR. Nucleic Acids Research, 30(9), e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piiroinen, S. , Lindström, L. , Lyytinen, A. , Mappes, J. , Chen, Y. H. , Izzo, V. , & Grapputo, A. (2013). Pre‐invasion history and demography shape the genetic variation in the insecticide resistance‐related acetylcholinesterase 2 gene in the invasive Colorado potato beetle. BMC Evolutionary Biology, 13, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revuelta, L. , Ortego, F. , Díaz‐Ruíz, J. R. , Castañera, P. , Tenllado, F. , & Hernández‐Crespo, P. (2011). Contribution of Ldace1 gene to acetylcholinesterase activity in Colorado potato beetle. Insect Biochemistry Molecular Biology, 41, 795–803. [DOI] [PubMed] [Google Scholar]
- Roush, R. , & Tabashnik, B. E. (2012). Pesticide resistance in arthropods. Springer Science & Business Media. [Google Scholar]
- Ryan, S. J. , Mundis, S. J. , Aguirre, A. , Lippi, C. A. , Beltrán, E. , Heras, F. , Sanchez, V. , Borbor‐Cordova, M. J. , Sippy, R. , Stewart‐Ibarra, A. M. , & Neira, M. (2019). Seasonal and geographic variation in insecticide resistance in Aedes aegypti in southern Ecuador. PLoS Neglected Tropical Diseases, 13, e0007448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoville, S. D. , Chen, Y. H. , Andersson, M. N. , Benoit, J. B. , Bhandari, A. , Bowsher, J. H. , Brevik, K. , Cappelle, K. , Chen, M. M. , Childers, A. K. , Childers, C. , Christiaens, O. , Clements, J. , Didion, E. M. , Elpidina, E. N. , Engsontia, P. , Friedrich, M. , García‐Robles, I. , Gibbs, R. A. , … Richards, S. (2018). A model species for agricultural pest genomics: The genome of the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Scientific Reports, 8, 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, K. D. , Labala, R. K. , Devi, T. B. , Singh, N. I. , Chanu, H. D. , Sougrakpam, S. , Nameirakpam, B. S. , Sahoo, D. , & Rajashekar, Y. (2017). Biochemical efficacy, molecular docking and inhibitory effect of 2, 3‐dimethylmaleic anhydride on insect acetylcholinesterase. Scientific Reports, 7, 12483. 10.1038/s41598-017-12932-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks, T. C. , & Nauen, R. (2015). IRAC: Mode of action classification and insecticide resistance management. Pesticide Biochemistry and Physiology, 121, 122–128. [DOI] [PubMed] [Google Scholar]
- Taylor, P. (2011). Anticholinesterase agents. In Brunton L. L., Chabner B., & Knollman B. (Eds.), The pharmacological basis of therapeutics, 13th ed. (pp. 239–254). McGraw‐Hill. [Google Scholar]
- Taylor, P. , Camp, S. , & Radić, Z. (2009). Acetylcholinesterase. In Squire L. R. (Ed.), Encyclopedia of neuroscience (pp. 5–7). Academic Press. [Google Scholar]
- Vontas, J. G. , Hejazi, M. J. , Hawkes, N. J. , Cosmidis, N. , Loukas, M. , & Hemingway, J. (2002). Resistance‐associated point mutations of organophosphate insensitive acetylcholinesterase, in the olive fruit fly Bactrocera oleae . Insect Molecular Biology, 11, 329–336. [DOI] [PubMed] [Google Scholar]
- Walsh, S. B. , Dolden, T. A. , Moores, G. D. , Kristensen, M. , Lewis, T. , Devonshire, A. L. , & Williamson, M. S. (2001). Identification and characterization of mutations in housefly (Musca domestica) acetylcholinesterase involved in insecticide resistance. Biochemical Journal, 359, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman, D. , Mitchell, S. N. , Wilding, C. S. , Birks, D. P. , Yawson, A. E. , Essandoh, J. , Mawejje, H. D. , Djogbenou, L. S. , Steen, K. , Rippon, E. J. , & Clarkson, C. S. (2015). Contemporary evolution of resistance at the major insecticide target site gene Ace‐1 by mutation and copy number variation in the malaria mosquito Anopheles gambiae . Molecular Ecology, 24(11), 2656–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill, M. , Fort, P. , Berthomieu, A. , Dubois, M. P. , Pasteur, N. , & Raymond, M. (2002). A novel acetylcholinesterase gene in mosquitoes codes for the insecticide target and is non‐homologous to the ace gene Drosophila. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269(1504), 2007–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill, M. , Lutfalla, G. , Mogensen, K. , Chandre, F. , Berthomieu, A. , Berticat, C. , Pasteur, N. , Philips, A. , Fort, P. , & Raymond, M. (2003). Comparative genomics: Insecticide resistance in mosquito vectors. Nature, 423, 136. [DOI] [PubMed] [Google Scholar]
- Weill, M. , Malcolm, C. , Chandre, F. , Mogensen, K. , Berthomieu, A. , Marquine, M. , & Raymond, M. (2004). The unique mutation in ace‐1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Molecular Biology, 13, 1–7. [DOI] [PubMed] [Google Scholar]
- Wierenga, J. M. , & Hollingworth, R. M. (1992). Inhibition of insect acetylcholinesterase by the potato glycoalkaloid α‐chaconine. Natural Toxins, 1, 96–99. [DOI] [PubMed] [Google Scholar]
- Wu, K. , Guo, Y. , & Lv, N. (1999). Geographic variation in susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis insecticidal protein in China. Journal of Economic Entomology, 92, 273–278. [DOI] [PubMed] [Google Scholar]
- Yocum, G. D. , Rinehart, J. P. , Chirumamilla‐Chapara, A. , & Larson, M. L. (2009). Characterization of gene expression patterns during the initiation and maintenance phases of diapause in the Colorado potato beetle, Leptinotarsa decemlineata . Journal of Insect Physiology, 55, 32–39. 10.1016/j.jinsphys.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Zhu, K. Y. , & Clark, J. M. (1995). Cloning and sequencing of a cDNA encoding acetylcholinesterase in Colorado potato beetle, Leptinotarsa decemlineata (say). Insect Biochemistry and Molecular Biology, 25, 1129–1138. [DOI] [PubMed] [Google Scholar]
- Zhu, K. Y. , & Clark, J. M. (1997). Validation of a point mutation of acetylcholinesterase in Colorado potato beetle by polymerase chain reaction coupled in enzyme inhibition assay. Pesticide Biochemistry and Physiology, 57(1), 28–35. [Google Scholar]
- Zhu, K. Y. , Lee, S. H. , & Clark, J. M. (1996). A point mutation of acetylcholinesterase associated with azinphosmethyl resistance and reduced fitness in Colorado potato beetle. Pesticide Biochemistry and Physiology, 55, 100–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo S1
Data Availability Statement
Bioassay, AChE enzyme activity, gene expression, and gene sequence data for this study are available at University of Jyväskylä Digital Repository (JYX https://jyx.jyu.fi/handle/123456789/78053). https://doi.org/10.17011/jyx/dataset/78053.


