Abstract
Hydrophobic components of the germ tube of the dimorphic pathogenic fungus Candida albicans were used as immunogens to prepare monoclonal antibodies (MAbs). Among the resulting MAbs, one (MAb 16B1-F10) was shown by indirect immunofluorescence to be specific to the surface of the mycelium phase of the C. albicans and C. stellatoidea species. No labeling of any other genera and Candida species tested was observed, including C. dubliniensis, a newly described species which has many phenotypic similarities to C. albicans. This phase-specific epitope resides on a protein moiety. The molecular mass of the antigen released by Zymolyase digestion was determined by gel filtration and ranges from 25 to 166 kDa. The antigen was also shown to be highly hydrophobic. This anti-C. albicans cell wall surface-specific MAb may be a good candidate for use in tests for the rapid differentiation of the two closely related species C. albicans and C. dubliniensis.
Candida albicans is a commensal dimorphic yeast of the oral cavity and digestive tract which can cause severe infections, especially in immunocompromised patients. A hypothetical set of virulence factors has been proposed and supported by various studies. These fungal attributes include the production of secreted hydrolytic enzymes, dimorphic transition (morphogenetic conversion from budding yeast to the filamentous growth form or hyphae), the ability to switch between different cell phenotypes, adhesion to inert and biological substrates, immunomodulation of host defense mechanisms, and antigenic variability (for a review, see reference 11). Although blastoconidia are commonly present in infected tissue, there is substantial evidence that the filamentous growth form plays a more critical role in the pathogenesis of candidiasis (1).
One critical step in the pathogenic process is adherence to host tissues. Adherence is achieved by a combination of specific and nonspecific mechanisms. Specific mechanisms involve the ability of the yeast to recognize a variety of host cell receptors with cell surface adhesins (4, 5, 7, 9, 18, 20, 32). Nonspecific mechanisms include electrostatic forces (23, 24), aggregation (3), and cell surface hydrophobicity (16).
The morphological transition of C. albicans from the yeast to the mycelial form is an important area of study. Surface-specific molecules of C. albicans germ tubes have been identified by biochemical and immunological approaches. To our knowledge, four monoclonal antibodies (MAbs) have been demonstrated by indirect immunofluorescence assay (IFA) to be specific for the C. albicans mycelial phase (8, 21, 22, 26, 28); however, only two of these components that are recognized by Western blotting have been recovered exclusively from C. albicans hyphae (8, 21). Concerning species specificity, two MAbs have been isolated; the first of these is specific to C. krusei (30) and the second of these is specific to C. albicans blastoconidia (31). In this paper we present the characteristics of a novel C. albicans germ tube-specific MAb (MAb 16B1-F10) that allows differentiation of C. albicans from the recently identified species C. dubliniensis (10, 37).
MATERIALS AND METHODS
Organisms and culture conditions.
C. albicans ATCC 66369 (serotype A), originally isolated from a patient with Candida septicemia, was used throughout this work.
Clinical isolates of C. albicans, Candida spp., and other fungi including Saccharomyces, Rhodotorula, and Cryptococcus were obtained from the Mycological Laboratory of the Medical School Angers. These isolates were identified by using the ID 32C system (bioMérieux, Marcy l'Étoile, France). Other strains of Candida spp. were purchased from the American Type Culture Collection or were from the Dublin University strain collection. C. dubliniensis was initially isolated in diverse geographically regions (Norway, Holland, France, and Ireland) and from patients with oral and blood C. dubliniensis infections. All have been identified by a number of techniques, including a PCR test based on the intron sequence of the ACT1 gene.
Cultures were maintained on Sabouraud dextrose agar (SDA) slants (Merck, Darmstadt, Germany) at 22°C for 48 h. The cells were inoculated at a concentration of 2 × 106 per ml in 1.7‰ yeast nitrogen base (YNB; Difco Laboratories, Detroit, Mich.) containing 2% glucose and 5‰ ammonium sulfate (YNB-G-SA) for (i) 48 h at 22°C and pH 5, (ii) 48 h at 37°C and pH 4 or pH 7, or (iii) 3 h at 37°C and pH 7. In some experiments, the cells were also prepared by incubation of blastoconidia for (i) 48 h at 22 or 37°C in medium 199 (pH 6.7; Gibco Laboratories, Grand Island, N.Y.) or in Sabouraud dextrose broth (Merck) or (ii) 3 h in medium 199 at 37°C. Then, the cells were washed with distilled water and were harvested by centrifugation (2,000 × g, 10 min) and used immediately or stored at −20°C.
Cells (5 × 107) were also inoculated intraperitoneally into mice. Two days later, the mice were killed and peritoneal abscesses were recovered, disrupted in phosphate-buffered saline (PBS), and placed on Teflon-coated immunofluorescence slides.
Preparation of MAbs. (i) Immunogen preparation.
Germ tubes were prepared by incubation of blastoconidia for 3 h at 37°C in medium 199. Cells were recovered by filtration through a membrane filter (pore size, 0.45 μm). Cell components were extracted by enzymatic digestion. Briefly, 320 mg of freeze-dried germ tubes were digested with 10 ml of Zymolyase 20T (2 mg per ml) (Arthrobacter luteus; Seikagaku, Kogyu Co., Tokyo, Japan) containing 1 mM phenylmethylsulfonyl fluoride for 1 h and 30 min at 37°C with shaking at 1,500 rpm on a titer plate shaker (Heidolph; Bioblock Scientific, Strasbourg, France). Solubilized antigenic components were recovered by centrifugation at 12,000 × g for 5 min. These were then stored at −20°C.
The freeze-dried germ tube extract (45 mg) containing 7.5 to 8 mg of protein (6) and 34 to 35 mg of carbohydrate (13) was solubilized in 2 M ammonium sulfate by slowly adding a 50 mM phosphate–2 M ammonium sulfate buffer (pH 7.2). After incubation for 1 h at 4°C, insoluble residues were removed by centrifugation at 12,000 × g for 5 min. The supernatant was then applied to a phenyl-Superose column (HR 5/5, containing 1 ml of gel; Pharmacia, LKB-Biotechnology, Uppsala, Sweden) that was equilibrated with the same buffer. The column was then rinsed with this buffer until no absorbance at 280 nm was detected in the effluent. Elutions were carried out at a flow rate of 0.5 ml · min−1 with a stepwise decrease in the concentration of ammonium sulfate and maintenance of the concentration of phosphate at 50 mM throughout the elution until an ammonium sulfate concentration of 0.1 M was achieved. The remaining bound material was eluted with 50 mM phosphate buffer and then with 20% ethanol and, finally, distilled water (fraction sizes, 12 ml). By applying this method, the most hydrophilic components were eluted in the first fractions. Increasingly hydrophobic molecules were eluted in subsequent fractions. The 20% ethanol fraction was dialyzed against distilled water and freeze-dried. Analysis by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and concanavalin A staining indicates that this fraction contains mainly 42-, 39-, and 27-kDa glycoprotein components.
(ii) Immunization.
Adult, 8-week-old, female BALB/c mice (Iffa-Credo, L'Arbresle, France) received four subcutaneous injections (100 μl each) of a 20% ethanol fraction (50 μg [dry weight]) emulsified in complete Freund's adjuvant (Sigma, St. Louis, Mo.) for the first injection and in incomplete Freund's adjuvant (Sigma) for the three following injections at 1-week intervals. One week after the last booster, samples of blood were obtained and checked by IFA on blastoconidia bearing germ tubes for germ tube-specific antibodies. The final injection (50 μg/100 μl) was given intravenously 3 days before the mice were killed.
(iii) Production of hybridomas.
Cell fusion (spleen cells from immunized mice and mouse plasmocytoma X63/Ag8.653) and selection of hybrids were performed essentially as described previously (21) by Dippold et al. (12), with minor modifications. Ten days after fusion, aliquots of medium from wells with growing hybridomas were screened for the production of antibodies directed to the germ tubes of C. albicans by IFA. Positive hybrids were immediately subcloned twice by limiting dilution in 96-well plates and were stored in liquid nitrogen. MAbs were obtained from confluent hybridoma cultures. Isotypes were determined by IFA with class-specific antisera (Nordic, Tilburg, The Netherlands).
(iv) Screening.
IFA was carried out on germ tubes. Cells obtained in medium 199 (3 h at 37°C, pH 6.7) were rinsed twice in PBS; their concentration was adjusted to 106 cells per ml by the addition of PBS, and drops (10 μl) from this suspension were placed in the wells of microscope slides (PolyLabo Paul Block, Strasbourg, France), and the slides were allowed to dry at 37°C for 1 h. The microscope slides were stored at −20°C until they were used. Twenty microliters of undiluted culture supernatants was dropped over the cells. The microscope slides were placed in a moist chamber at 37°C for 1 h and were then rinsed twice with PBS. Twenty microliters of fluorescein isothiocyanate-conjugated, goat anti-mouse immunoglobulin G (IgG; γ-chain specific) or IgM (μ-chain specific) (Caltag, Burlingame, Calif.) diluted 1:150 in PBS was added to slides, which were then reincubated at 37°C for 30 min in a moist chamber and then rinsed twice with PBS. Then, the slides were mounted in PBS containing 90% glycerol. The preparations were examined and photographed with a Nikon microscope equipped with reflected light fluorescence, and pictures were taken with ILFORD HP 5 400 ASA slide film.
Characterization of MAb 16B1-F10. (i) IFA.
Antigen expression within fungal genera was studied by IFA on cells grown under various conditions as described above.
In some experiments, C. albicans cells were treated with several agents. (i) Cells (109) were incubated for 1 h and 30 min with 1 ml of either 2% (wt/vol) sodium dodecyl sulfate (SDS; Gerbu, Gaiberg, Germany), 70 mM dithiothreitol (DTT; Sigma), 0.35 M 2-mercaptoethanol (2ME; Merck), EDTA-2ME (at 50 mM and 0.35 M, respectively; pH 9), or 20 mM sodium periodate in acetoacetate buffer (0.15 M, pH 4.5). For the latter, after rinsing in this buffer, the cells were incubated for 30 min with 1 ml of 1% (wt/vol) glycine to block the aldehyde groups generated by the periodate treatment. (ii) The cells were digested at 37°C for 1 h and 30 min with 2.5 mg of pronase E (Merck) per ml in distilled water or with 2 mg of Zymolyase 20T per ml in distilled water or in 0.6 M KCl. Control cells were incubated with buffer alone.
After treatment, the organisms were rinsed twice with PBS and were fixed to the wells of Teflon-coated microscope slides. Solubilized antigenic components were stored at −20°C until they were used for enzyme-linked immunosorbent assay (ELISA) or dot blotting.
(ii) ELISA.
ELISA was performed by coating the wells of microtitration plates with antigenic extracts that were obtained as described above and that were diluted in PBS, and the plates were incubated for 2 h at 37°C. The plates were rinsed twice with PBS and blocked by adding 250 μl of PBS containing 10% (wt/vol) nonfat dry milk (Régilait, Saint-Martin-Belle-Roche, France) to each well overnight at 4°C. The plates were rinsed with 250 μl of 0.05% (vol/vol) Tween 20 in PBS (PBST) and were then incubated with 100 μl of diluted (1:2) culture supernatant for 1 h at 37°C. The wells were then rinsed twice with 250 μl of PBST. One hundred microliters of goat anti-mouse IgG1 coupled to alkaline phosphatase (Caltag) at a 1:2,000 dilution in PBST containing 1% (wt/vol) nonfat dry milk (PBSTM) was added to each well. The plates were incubated again for 1 h at 37°C. After extensive rinsings, 200 μl of substrate mixture (containing 1 mg of p-nitrophenyl phosphate per ml in 1 M diethanolamine buffer [pH 9.8]) was added. The plates were then incubated at room temperature in the dark for 30 min. The reaction was stopped by the addition of 50 μl of 3 N NaOH to each well. The color density was determined at 405 nm on a Titertek multiscan instrument (Flow Laboratories, Puteaux, France). To evaluate nonspecific adsorption of MAbs and conjugates, negative control reactions were carried out by using hybridoma medium culture or by omitting the antigenic extracts.
(iii) Native electrophoresis and SDS-PAGE.
Electrophoreses were carried out as described previously (21) on a gel electrophoresis apparatus (2/4; Pharmacia, LKB-Biotechnology) on isotropic 5 to 15% (wt/vol) acrylamide slab gels (17 by 14 by 0.15 cm) by using the discontinuous buffer system of Laemmli (19). When samples were electrophoresed under native conditions, no boiling step was included and SDS was omitted from all buffers.
(iv) Electrophoretic blotting procedures and immunoblotting.
After SDS-PAGE, the proteins were transferred to polyvinylidene difluoride sheets (Immobilon; pore size, 0.45 μm; Millipore Corp., Bedford, Mass.) at 300 mA for 40 min in the Milliblot-SDE System (Millipore) (39). Electrotransfer to Optitran BA-S reinforced nitrocellulose sheets (Schleicher & Schuell, Dassel, Germany) following native electrophoresis was performed at 4°C overnight in a transblot cell (Hoefer Scientific Instruments, San Francisco, Calif.) at 30 V with a Tris-glycine-methanol (25 mM, 192 mM, and 20%, respectively) buffer. The sheets were then preadsorbed with 10% (wt/vol) nonfat dry milk in PBS at 4°C overnight and were incubated with undiluted culture supernatant at room temperature for 1 h. After rinsing in PBST, the sheets were incubated with a dilution (1:300) of goat anti-mouse IgG (γ-chain specific) coupled to horseradish peroxidase (Caltag) in PBSTM. After further rinsings, the bound antibodies were revealed by submersion of the sheets in 0.1 M Tris buffer (pH 7.6) containing 0.5 mg of 3,3′-diaminobenzidine (Sigma) ml−1 and 0.1% (vol/vol) hydrogen peroxide. The development of a color reaction was arrested by rinsing in a solution of 5% (vol/vol) acetic acid.
(v) Dot blotting.
The components released by chemical and physical agents from blastoconidia and germ tubes were checked by dot blotting. Briefly, 10 μl of solubilized material was dropped onto Immobilon sheets and was allowed to dry. The subsequent steps were performed as described above for immunoblotting.
(vi) Gel filtration.
The solubilized antigenic components obtained following Zymolyase treatment in distilled water were recovered by centrifugation at 12,000 × g for 5 min to remove any remaining cell debris. Then, the extract was applied to a HiLoad 16/60 Superdex 200 column (Pharmacia) that had previously been equilibrated with PBS. Elution was carried out at room temperature and at a flow rate of 1 ml · min−1, and 2-ml-volume fractions were collected. Each fraction was assayed for 16B1-F10 antigenic activity by ELISA (as described above). Concurrently, fractions were checked with MAb 3D9.3, which was previously produced in our laboratory and which is directed to a C. albicans germ tube-specific epitope. Antigenic fractions reactive with MAb 16B1-F10 were pooled and were stored at −20°C until use.
(vii) HIC.
The antigenic fractions that were reactive with MAb 16B1-F10 and that were obtained by gel filtration were submitted to hydrophobic interaction chromatography (HIC) as described above for immunogen preparation.
RESULTS
MAb isolation.
Hybrids resulting from the fusion of X63/Ag8.653 myeloma cells and lymphocytes from BALB/c mice that had been immunized with the fraction that was obtained by HIC with 20% ethanol were obtained as described in Materials and Methods. Eighty antibody-producing hybridoma cell lines were identified by IFA with germ tubes of C. albicans ATCC 66369. Two patterns of reactivity were observed. Group 1 MAbs reacted with both blastospores and germ tubes. The immunofluorescence of the germinating cells was typically intense on the mother yeast cells compared to that on hyphal cells. Group 2 MAbs (73 cell lines) reacted exclusively with germ tubes. MAb 16B1-F10 belonging to group 2 was found to be IgG1.
Cell surface antigen expression by C. albicans ATCC 66369 in different growth states and culture conditions.
The variability of the antigen expression was examined by observing the surface reactivities of cells grown under various conditions. Incubation with MAb 16B1-F10 revealed that the antigen expression was not detectable on blastoconidia, whatever the culture conditions (medium, pH, temperature) were. When filamentation occurred, the parent yeast cells were still not stained, but the germ tubes and hyphae that had emerged exhibited a strong fluorescence. On germ tubes, fluorescence was in all cases homogeneous on the surface (Fig. 1A and B), whereas it became heterogeneous on the surface of the mycelium. For a given fungal filament, the intensity of labeling varied from some of the cells to the others; some of them were negative and some were intensely stained. Mother blastoconidia were not stained as well as the lateral blastoconidia emanating from the mycelium (Fig. 1C and D).
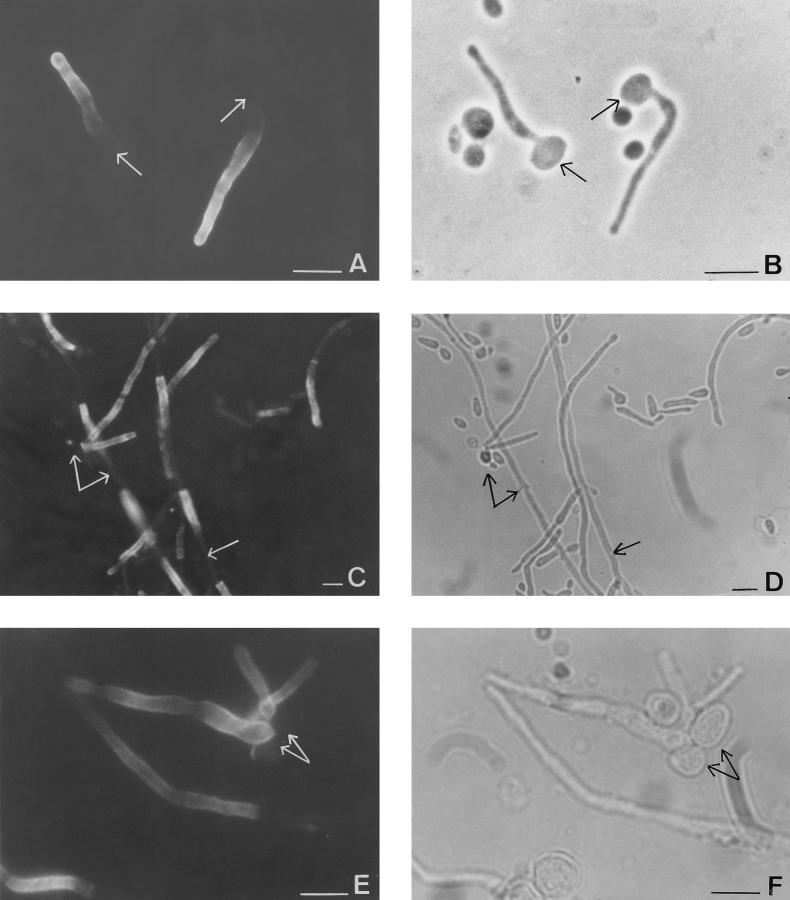
FIG. 1.
Phase-contrast (B, D, and F) and immunofluorescent (A, C, and E) photomicrographs of the same microscopic fields of C. albicans ATCC 66369 grown in medium 199 for 3 h at pH 6.7 (A and B) or 48 h (C and D) or C. albicans that originated from mouse abscesses (E and F) stained with MAb 16B1-F10. Note the homogeneous fluorescent labeling located solely on the filamentous cells; arrows point to the location of blastoconidia and cells that exhibited no fluorescence under UV illumination. Bars, 10 μm.
Cell surface antigen expression by C. albicans ATCC 66369 recovered from abscesses.
The cell surface expression of antigen 16B1-F10 on yeast cells arising from abscesses was investigated by IFA. A result similar to that from the in vitro study was obtained: no fluorescence was observed on blastoconidia, whereas filamentous forms exhibited a constant homogeneous staining on their lateral walls (Fig. 1E and F).
Cell surface antigen expression by other isolates and species.
To investigate the strain and species specificity of expression of the antigen recognized by MAb 16B1-F10, other isolates of C. albicans, other Candida species, and representatives of other fungal genera were examined by IFA. For these species, cells were grown in YNB-G-SA medium as described in Materials and Methods. The antigen recognized by MAb 16B1-F10 was not detectable in genera other than Candida. In the genus Candida, no binding was noticed for other species of yeast tested except C. albicans and C. stellatoidea under conditions that support cell development. It is important that no fluorescence was observed on the germ tubes of C. dubliniensis. Concerning C. albicans, fluorescence was noticed on mycelial phase cells (hyphae or germ tube). No significant differences were noticed among the strains that gave positive responses (Table 1). Regardless of the conditions (pH and culture temperature) used, cell surface expression on blastoconidia and on mycelial phase cells was not modified.
TABLE 1.
Immunofluorescent reactivities of C. albicans and related yeast strains with MAb 16B1-F10
| Organisma | Reactivityb
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates tested | Yeast grown for 48 h at 22°C (pH 5)
|
Yeast grown for 48 h at 37°C (pH 4)
|
Yeast grown for 48 h at 37°C (pH 7)
|
Yeast grown for 3 h at 37°C (pH 7)
|
|||||||||
| B | PM | M | B | PM | M | B | PM | M | B | PM | GT | ||
| Candida albicans | 22 | −(22) | −(5) | NO | −(22) | −(3) | NO | −(22) | −(5) | +(22) | −(22) | −(22) | +(22) |
| Candida dubliniensis | 14 | −(14) | −(3) | NO | −(14) | −(1) | NO | −(14) | −(14) | −(14) | −(14) | −(14) | −(14) |
| Candida stellatoidea | 5 | −(5) | −(1) | NO | −(5) | −(1) | NO | −(5) | −(2) | +(5) | −(5) | −(1) | +(5) |
| Candida tropicalis | 13 | −(13) | −(5) | −(4) | −(13) | −(5) | −(5) | −(13) | −(5) | −(9) | −(13) | −(5) | NO |
| Candida glabrata | 8 | −(8) | NO | NO | −(8) | NO | NO | −(8) | NO | NO | −(8) | NO | NO |
| Candida kefyr | 10 | −(10) | −(1) | NO | −(10) | NO | NO | −(10) | NO | NO | −(10) | NO | NO |
| Candida krusei | 10 | −(10) | −(1) | NO | −(10) | NO | NO | −(10) | −(7) | NO | −(10) | −(4) | NO |
| Candida parapsilosis | 4 | −(4) | −(2) | NO | −(4) | −(3) | NO | −(4) | −(4) | NO | −(4) | −(4) | NO |
| Candida guilliermondii | 5 | −(5) | −(5) | NO | −(5) | NO | NO | −(5) | NO | NO | −(5) | NO | NO |
| Cryptococcus neoformans | 3 | −(3) | NO | NO | −(3) | NO | NO | −(3) | NO | NO | −(3) | NO | NO |
| Rhodotorula rubra | 1 | −(1) | NO | NO | −(1) | NO | NO | −(1) | NO | NO | −(1) | NO | NO |
| Saccharomyces cerevisiae | 4 | −(4) | NO | NO | −(4) | NO | NO | −(4) | NO | NO | −(4) | NO | NO |
Clinical isolates and reference strains.
B, blastoconidia; PM, pseudomycelium; M, true mycelium; GT, germ tube; −, no fluorescence; +, fluorescent labeling; NO, morphological phase not observed. Numbers in parentheses indicate the number of strains tested displaying the indicated reactivity.
Antigenic extraction procedures.
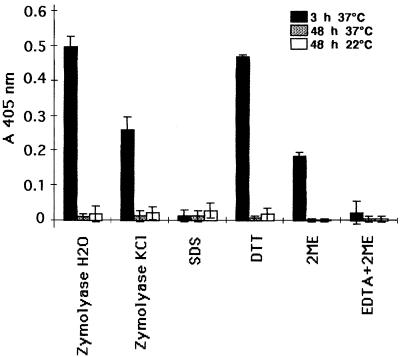
Blastoconidia of C. albicans ATCC 66369 grown on SDA (48 h at 22 or 37°C) and germ tubes of C. albicans ATCC 66369 grown in medium 199 (3 h at 37°C) were first subjected to several treatments. Then, cell surface expression was examined by IFA and the released components were checked for antigenic activity by dot blotting and ELISA. When the treated blastoconidia were incubated with MAb 16B1-F10, no change in reactivity was observed by IFA. Any reactive material was evidenced by dot blotting and ELISA in the homologous extract of blastoconidia. The fluorescent labeling on the germ tubes disappeared after treatment with Zymolyase. A partial decrease in the staining was noticed after incubation of the cells with DTT, EDTA-2ME, and 2ME. For all these treatments, loss of reactivity by IFA correlates with positive signals by ELISA and dot blotting (Fig. 2). Incubation with Zymolyase was shown to be the best method for extraction of the antigen recognized by MAb 16B1-F10. The positive signal observed with the DTT extract was considerably decreased with higher dilutions, whereas no significant differences were observed with the Zymolyase extract. Labeling of germ tubes disappeared after treatment with 2% SDS, and no positive reaction in the homologous extracts was evidenced by ELISA or dot blotting.
FIG. 2.
Reactivity of MAb 16B1-F10 by ELISA against components (dilution, 1:10) released by several methods from blastoconidia and germ tubes grown for 48 h at 37 or 22°C on SDA or for 3 h at 37°C in medium 199, respectively. Results represent the means ± standard deviations for two experiments performed in duplicate.
Antigenic expression in other species and strains.
The antigenic expressions of C. albicans ATCC 66369, C. dubliniensis CD 36, and a C. tropicalis isolate were also studied by ELISA. Since Zymolyase had been shown to achieve the most efficient extraction of the antigen recognized by MAb 16B1-F10 from the germ tube, this enzyme was used for the extraction of further strains. Yeast cells grown in YNB-G-SA for 48 h at 37°C (pH 7) were submitted to digestion. A positive signal was observed only for C. albicans.
Biochemical characterization.
The sensitivity of the antigen to pronase E and periodate was examined. Germ tubes were treated with the agents prior to IFA. Treatment with pronase E strongly reduced the level of fixation of MAb 16B1-F10, whereas preparations incubated with periodate retained the same activity as those treated with acetoacetate buffer.
Molecular mass determination.
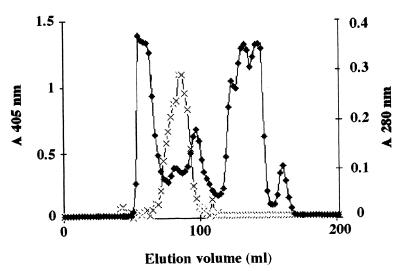
Although the antigen was extracted with Zymolyase from the germ tube, it was not identified by SDS-PAGE due to its sensitivity to heat and SDS. Under native conditions, immunoblot analysis performed with MAb 16B1-F10 revealed a polydispersed material located in the upper portion of the membrane, with three areas exhibiting a more intensive labeling within the smear (Fig. 3). As the molecular mass could not be determinated by this method, it was estimated by gel filtration (Fig. 4). The antigen recognized by MAb 16B1-F10 was eluted in fractions corresponding to components spanning a molecular mass range of 25 to 166 kDa.
FIG. 3.
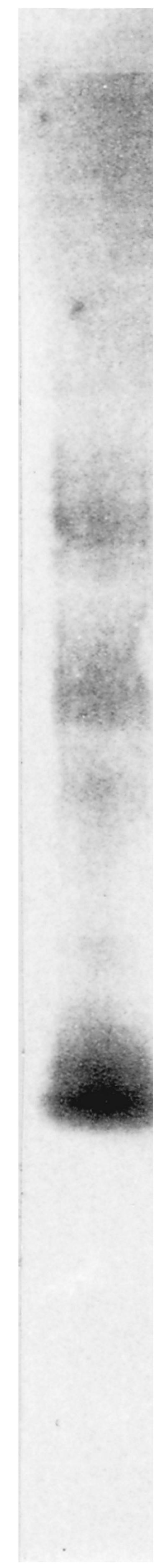
Western blot analysis with MAb 16B1-F10 following PAGE (5 to 15% [wt/vol] polyacrylamide) of a Zymolyase extract of a C. albicans germ tube.
FIG. 4.
Analytical fractionation by gel filtration on a HiLoad 16/60 Superdex 200 column of a crude Zymolyase extract of a C. albicans ATCC 66369 germ tube. The crude extract was applied to the column and was then eluted with PBS (pH 7.2). The optical density at 280 nm (⧫) was monitored during elution, and 10 μl of each fraction was analyzed by ELISA with MAb 16B1-F10 (×) as the probe.
HIC.
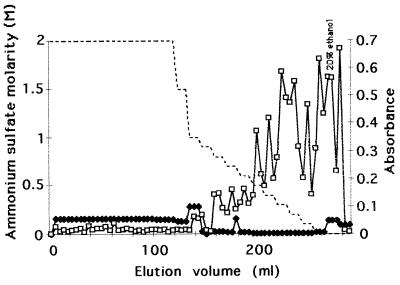
Fractions from the gel filtration enriched with the antigen recognized by MAb 16B1-F10 were applied to an HIC column. After elution of a large peak of unbound UV-absorbing material, the remaining components that were attached to the phenyl-Superose column were eluted with a phosphate buffer containing a stepwise decreasing concentration gradient of ammonium sulfate. For most ammonium sulfate concentrations, a peak of very low intensity was observed at 280 nm. ELISA performed with the relevant antibody displayed the antigen recognized by MAb 16B1-F10 in the most hydrophobic fractions obtained with 0.5 to 0.1 M ammonium sulfate, 50 mM phosphate buffer, and 20% ethanol (Fig. 5).
FIG. 5.
Analytical HIC on a phenyl-Superose column of pooled antigenic reactive fractions from the gel filtration. The sample loaded on the column was eluted with a stepwise decreasing concentration gradient of ammonium sulfate (dotted line). The optical density at 280 nm (⧫) was monitored during elution, and each fraction was analyzed by ELISA with MAb 16B1-F10 (□) as the probe.
DISCUSSION
We report here on the identification of a novel C. albicans germ tube-specific antigen. The strategy for immunization which we followed was different from that used in previous studies. As germ tubes have been shown to be highly and invariably hydrophobic, regardless of whether the mother cell displays cell surface hydrophobicity (15), we have chosen to immunize mice with the most hydrophobic components of germ tubes recovered by HIC. This choice of immunogen gave rise to 50% IgG-secreting hybridomas among the 80 stable cell lines. In the present study we describe an MAb (MAb 16B1-F10) which was first labeled as recognizing only germ tubes by IFA following cell hybridization. This MAb was used to determine, at least partially, the cellular location of the recognized determinant. We also looked for the presence of this epitope in cells of other members of the genus Candida in different growth states, under different growth conditions, and with different morphologies.
MAb 16B1-F10-specific staining of the germ tubes and hyphae of C. albicans was observed by IFA. No fluorescence was observed on blastoconidia, indicating that the epitope is associated with the cell surface of the filamentous growth form. The labeling of the germ tubes was always marked and was generally homogeneous on the lateral walls of the tube. However, the labeling of mycelium became heterogeneous according to the cell observed. Immunofluorescent staining showed that the epitope was expressed by all C. albicans strains and isolates tested. MAb 16B1-F10 was shown to react exclusively with C. albicans and C. stellatoidea mycelial forms but not with the species C. dubliniensis, which is very closely phenotypically and genotypically related to C. albicans. The identification of this antigen on the surface of the mycelial phase of C. albicans cells could be a powerful tool for laboratory diagnostics and especially for C. albicans and C. dubliniensis differentiation. An IFA performed with this MAb would be easier, more rapid, more reliable, cheaper, and applicable to a larger volume of isolates than the several phenotype-based methods including determination of colonial coloration on differential media (25), atypical carbohydrate assimilation profile analysis, determination of the lack of β-glucosidase activity (36), and determination of the ability to grow at 45°C (14, 27, 35, 38, 40), as well as the molecular biology-based techniques used up until now (10, 37). As the antigen is also evidenced on hyphal forms recovered from abscesses, IFA examination could be done directly with tissue samples, allowing laboratories to avoid a primary isolation that requires a further 24 to 48 h of incubation (33). In contrast to the monospecific immune sera obtained following adsorption, MAbs afford lot-to-lots reproducibility and can undergo large-scale production. To our knowledge, the single immune serum that allows the differentiation of C. albicans and C. dubliniensis by IFA cross-reacts with a variety of antigens from other yeast species, such as C. krusei and Rhodotorula rubra (2). Use of a bicolored latex agglutination test, a less time-consuming test than IFA, is also conceivable (29, 31).
The specificity of C. albicans germ tubes established by IFA was reinforced by ELISA. Whatever the extraction procedure used, the antigenic determinant has never been evidenced in C. albicans blastoconidia. Cell surface expression appears to be associated with the germ tube form of growth and not merely with the temperature change, insofar as blastoconidia grown at 37°C did not exhibit the antigen. Moreover, the antigen was rapidly (45 min) detected by IFA on small excrescences on blastoconidia after the latter were incubated in a medium that allows germination and before these buds gave rise to small germ tubes noticeable by examination by phase-contrast microscopy (data not shown).
Several observations suggest that the antigen recognized by MAb 16B1-F10 is protein in nature. (i) IFA staining on germ tubes disappears following treatment with pronase E, and (ii) heating of the zymolyase germ tube extract for 2 min at 100°C leads to a loss of reactivity by dot blotting (data not shown). MAb 16B1-F10 appears to recognize a conformational or a native protein epitope which is present on intact cells and which could be denatured subsequent to incubation with SDS. It cannot be excluded that the antigen recognized by MAb 16B1-F10 is released by SDS but loses its reactivity. The hypothesis that the antigen is sensitive to SDS is strengthened by the results obtained by dot blotting: MAb 16B1-F10 still does not recognize the zymolyase germ tube extract diluted in the presence of SDS (data not shown). These characteristics did not permit molecular mass determination by SDS-PAGE; thus, the molecular mass was determined by gel filtration. By this technique, the molecular mass of the antigen recognized by MAb 16B1-F10 was estimated to range from 25 to 166 kDa, suggesting that the epitope recognized by MAb 16B1-F10 is carried by a component unrelated to the antigen recognized by MAb 3D9.3 described previously (21, 22). Moreover, the polydispersed staining of the antigen recognized by MAb 16B1-F10 under native conditions can be correlated with its chromatographic behavior by gel filtration.
Several germ tube cell surface-specific antigens have been described in the literature. Moreover, most of them have also been recovered from blastoconidia after extraction of cell components (26, 28). By molecular biology techniques, a gene (HWP1) encoding for a germ tube cell surface antigen has been cloned, with adsorbed antiserum reacting only with hyphal surfaces (34). The calculated molecular mass of the encoded protein was 22.750 kDa, and immune serum raised to the recombinant protein reacted only with hyphal surfaces of the 20 C. albicans isolates tested. The antigen recognized by the immune serum was also expressed in vivo, and by Western blotting it was shown to present a molecular mass of 34 kDa. No information concerning the expression by C. dubliniensis was provided. Moreover, Hazen et al. (17) have described several low-molecular-mass components unique to hydrophobic cells subsequent to radiolabeling. Unfortunately, the study was performed with blastoconidia and it is not possible to connect our hydrophobic antigen recognized by MAb 16B1-F10 with the hydrophobic molecular species identified by those investigators.
In conclusion, we provide here the first report of the development of an MAb which differentiates C. albicans and C. stellatoidea from C. dubliniensis. Experiments are now being performed with MAb 16B1-F10 in order to perfect a rapid identification test.
ACKNOWLEDGMENT
This work was supported by Pfizer Laboratorie, Orsay, France.
REFERENCES
- 1.Antley P P, Hazen K C. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect Immun. 1988;56:2884–2890. doi: 10.1128/iai.56.11.2884-2890.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bikandi J, SanMillan R, Moragues M D, Cebas G, Clarke M, Coleman D C, Sullivan D J, Quindos G, Ponton J. Rapid identification of Candida dubliniensis by indirect immunofluorescence based on differential localization of antigens on Candida dubliniensis blastospores and Candida albicans germ tubes. J Clin Microbiol. 1998;36:2428–2433. doi: 10.1128/jcm.36.9.2428-2433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonaly R. Molecules responsible for yeast aggregation. J Mycol Med. 1992;2:26–35. [Google Scholar]
- 4.Bouali A, Robert R, Tronchin G, Senet J M. Characterization of binding of human fibrinogen to the surface of germ-tubes and mycelium of Candida albicans. J Gen Microbiol. 1987;132:545–551. doi: 10.1099/00221287-133-3-545. [DOI] [PubMed] [Google Scholar]
- 5.Bouchara J P, Tronchin G, Annaix V, Robert R, Senet J M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990;58:48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Calderone R A, Linehan L, Wadsworth E, Sandberg A L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988;56:252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casanova M, Martinez J P, Chaffin W L. Fab fragments from a monoclonal antibody against a germ tube mannoprotein block the yeast-to-mycelium transition in Candida albicans. Infect Immun. 1990;58:3810–3812. doi: 10.1128/iai.58.11.3810-3812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova M, Lopez-Ribot J, Monteaugudo C, Llombart-Bosch A, Sentandreu R, Martinez J P. Identification of a 58-kilodalton cell surface fibrinogen-binding mannoprotein from Candida albicans. Infect Immun. 1992;60:4221–4229. doi: 10.1128/iai.60.10.4221-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman D C, Sullivan D J, Bennett D E, Moran G P, Barry H J, Shanley D B. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS. 1997;11:557–567. doi: 10.1097/00002030-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Cutler J. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 12.Dippold W, Lloyd K, Li L, Ikeda H, Oettgen H, Old L. Cell surface antigens of human malignant melanoma; definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci USA. 1980;77:6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois M, Gilles K A, Hamilton J K, Reber P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 14.Gilfillan G D, Sullivan D J, Haynes K, Parkinson T, Coleman D C, Gow N A R. Candida dubliniensis: phylogeny and putative virulence factors. Microbiology UK. 1998;144:829–838. doi: 10.1099/00221287-144-4-829. [DOI] [PubMed] [Google Scholar]
- 15.Hazen B W, Hazen K C. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Infect Immun. 1988;56:2521–2525. doi: 10.1128/iai.56.9.2521-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazen K C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989;57:1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazen K C, Lay J G, Hazen B W, Ging Fu R C, Murthy S. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990;58:3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klotz S, Chen R, Smith R, Rouse J. The fibronectin adhesin of Candida albicans. Infect Immun. 1994;62:4679–4681. doi: 10.1128/iai.62.10.4679-4681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Ribot J, Chaffin W. Binding of the extracellular matrix component entactin to Candida albicans. Infect Immun. 1994;62:4564–4571. doi: 10.1128/iai.62.10.4564-4571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marotleblond A, Robert R, Aubry J, Ezcurra P, Senet J M. Identification and immunochemical characterization of a germ tube specific antigen of Candida albicans. FEMS Immunol Med Microbiol. 1993;7:175–186. doi: 10.1111/j.1574-695X.1993.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 22.Marotleblond A, Robert R, Senet J M, Palmer T. Purification of a Candida albicans germ tube specific antigen. FEMS Immunol Med Microbiol. 1995;12:127–136. doi: 10.1111/j.1574-695X.1995.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyahe Y, Tsunoda T, Minagi S, Akagawa Y, Tsuru H, Suginaka H. Antifungal drugs affect adherence of Candida albicans to acrylic surfaces by changing the zeta-potential of fungal cells. FEMS Microbiol Lett. 1990;69:211–214. doi: 10.1016/0378-1097(90)90067-z. [DOI] [PubMed] [Google Scholar]
- 24.Nikawa H, Sadamori S, Hamada T, Satou N, Okuda K. Non-specific adherence of Candida species to surface-modified glass. J Med Vet Mycol. 1989;27:269–271. [PubMed] [Google Scholar]
- 25.Odds F, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;12:1923–1929. doi: 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ollert M W, Calderone R A. A monoclonal antibody that defines a surface antigen on Candida albicans hyphae cross-reacts with yeast cell protoplasts. Infect Immun. 1990;58:625–631. doi: 10.1128/iai.58.3.625-631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinjon E, Sullivan D, Salkin I, Shanley D, Coleman D. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J Clin Microbiol. 1998;36:2093–2095. doi: 10.1128/jcm.36.7.2093-2095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponton J, Marot-Leblond A, Ezkurra P A, Barturen B, Robert R, Senet J M. Characterization of Candida albicans cell wall antigens with monoclonal antibodies. Infect Immun. 1993;61:4842–4847. doi: 10.1128/iai.61.11.4842-4847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quindos G, San Millian R, Robert R, Bernard C, Ponton J. Evaluation of Bichro-latex Albicans, a new method for rapid identification of Candida albicans. J Clin Microbiol. 1997;35:1263–1265. doi: 10.1128/jcm.35.5.1263-1265.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert R, Faure O, Carloti A, Lebeau B, Bernard C, Marot-Leblond A, Grillot R, Senet J M. A monoclonal antibody specific to surface antigen on Candida krusei. Clin Diagn Lab Immunol. 1998;5:121–124. doi: 10.1128/cdli.5.1.121-124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert R, Sentandreu R, Bernard C, Senet J M. Evaluation du réactif Bichrolatex albicans® pour l'identification rapide de colonies de Candida albicans. J Mycol Med. 1994;4:226–229. [Google Scholar]
- 32.Saxena A, Calderone R. Purification and characterization of the extracellular C3d-binding protein of Candida albicans. Infect Immun. 1990;58:309–314. doi: 10.1128/iai.58.2.309-314.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoofs A, Odds F, Colebunders R, Leven M, Goossens H. Use of specialized isolation media for recognition and identification of Candida dubliniensis isolates from HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1997;16:296–300. doi: 10.1007/BF01695634. [DOI] [PubMed] [Google Scholar]
- 34.Staab J F, Ferrer C A, Sundstrom P. Developmental expression of a tandemly repeated, proline and glutamine-rich amino acid motif on hyphal surfaces of Candida albicans. J Biol Chem. 1996;271:6298–6305. doi: 10.1074/jbc.271.11.6298. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan D, Bennett D, Henman M, Harwood P, Flint S, Mulcahy F, Shanley D, Coleman D. Oligonucleotide fingerprinting of isolates of Candida species other than C. albicans and of atypical Candida species from human immunodeficiency virus-positive and AIDS patients. J Clin Microbiol. 1993;31:2124–2133. doi: 10.1128/jcm.31.8.2124-2133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan D, Westerneng T, Haynes K, Bennett D, Coleman D. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan D, Henman M C, Moran G P, Oneill L C, Bennett D E, Shanley D B, Coleman D. Molecular genetic approaches to identification, epidemiology and taxonomy of non-albicans Candida species. J Med Microbiol. 1996;44:399–408. doi: 10.1099/00222615-44-6-399. [DOI] [PubMed] [Google Scholar]
- 39.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velegraki A, Logotheti M. Presumptive identification of an emerging yeast pathogen: Candida dubliniensis (sp. nov.) reduces 2,3,5-triphenyltetrazolium chloride. FEMS Immunol Med Microbiol. 1998;20:239–241. doi: 10.1111/j.1574-695X.1998.tb01132.x. [DOI] [PubMed] [Google Scholar]