Summary
Whether nonhuman species can change their communicative repertoire in response to socio-ecological environments has critical implications for communicative innovativeness prior to the emergence of human language, with its unparalleled productivity. Here, we use a comparative sample of wild and zoo-housed orangutans of two species (Pongo abelii, Pongo pygmaeus) to assess the effect of the wild-captive contrast on repertoires of gestures and facial expressions. We find that repertoires on both the individual and population levels are larger in captive than in wild settings, regardless of species, age class, or sampling effort. In the more sociable Sumatran species, dominant use of signals toward single outcomes was also higher in captive settings. We thus conclude that orangutans exposed to more sociable and terrestrial conditions evince behavioral plasticity, in that they produce additional innate or innovated signals that are highly functionally specific. These findings suggest a latent capacity for innovativeness in these apes' communicative repertoires.
Subject areas: Wildlife behavior, Zoo animal behavior, Anthropology
Graphical abstract
Highlights
-
•
We studied wild-captive contrasts in gestural repertoires in two orangutan species
-
•
Individual and group-level repertoires were larger in captivity than in wild
-
•
Only in Sumatrans, functional specificity was higher in captivity than in wild
-
•
Some of orangutans’ captivity-specific signals may qualify as weak innovations
Wildlife behavior; Zoo animal behavior; Anthropology
Introduction
Innovativeness is considered the engine of behavioral change in populations and thus pertinent to the study of animal culture and intelligence (Ramsey et al., 2007; Reader and Laland, 2003). Originally defined by Kummer and Goodall (1985) as “a solution to a novel problem or a novel solution to an old one”, there is no consensus on how novel or distinctive from species-typical behavior a trait needs to be to qualify as innovation. Nowadays ethologists widely agree that behaviors exist along a continuum in which the influence of internal (genetic) or external inputs varies in strength (Eibl-Eibesfeldt, 1975; Hinde, 1970). Accordingly, Ramsey et al. (2007) have suggested that there is an innovation gradient: at one end of the gradient, we find fully fledged inventions that tend to be rare, conspicuous, and cognitively demanding. At the other end are weak innovations, that is, behaviors that are partially, but not entirely, environmentally induced or socially learnt. For some groups of large and long-lived animal species, such as great apes, the modification of behavioral repertoires through prior experiences may be more likely, and the extent of innovativeness larger, compared with other animal taxa. This notion is supported by exceptional degrees of behavioral flexibility and ontogenetic plasticity reported for hominid species (Bandini and Harrison, 2020; Manrique et al., 2013; van Schaik et al., 2006).
Does this greater degree of innovativeness also extend to communicative behavior? If this is the case, it would seem less coincidental that language, with its remarkable productivity (Hockett, 1960), emerged in the hominid lineage. For instance, an early study by Kummer and Kurt (1965) showed that, of 68 social signals, 9 were not observed in the wild, providing perhaps the first evidence for innovations or “functional elaborations” in communication (e.g., including a gesture that invites infant to be carried). Nonetheless, the current consensus is that the vast majority of facial, vocal, and gestural expressions in non-human species has evolved through natural selection over long periods of time and have become innate. Thus, the ability to produce them arises spontaneously during ontogeny, and only their use is often fine-tuned by practice (Byrne et al., 2017; Cheney and Seyfarth, 2018; Wegdell et al., 2019). However, accumulating evidence suggests that the socio-ecological environment can profoundly impact the use of communicative signals throughout primate lifetimes (for reviews see Fröhlich and Hobaiter, 2018; Liebal et al., 2013; Snowdon and Hausberger, 1997). For example, recent research on great apes, our closest living relatives, has shown that some gestures and sounds are apparently innovated and maintained over time (Fröhlich et al., 2016; Halina et al., 2013; Hardus et al., 2009b; Hopkins et al., 2007; Tomasello et al., 1997; Wich et al., 2012; Wich et al., 2009), and play the same role in the communication process as evolved signals do—we could thus call them signal innovations (Fröhlich and van Schaik, 2020). This work therefore suggests that it may be timely to distinguish between innate animal signals and those that are acquired developmentally (see Bard et al., 2014 for a similar conclusion, finding that some gestures are clearly genetically predisposed, whereas others are not).
Targeted long-term studies to estimate the extent of ontogenetic plasticity (including “innovativeness”) in great apes' communicative behavior are difficult. Here, we therefore rely on an alternative approach: we systematically compare the same species living in the wild and in man-made, artificial habitats in captivity (Fröhlich and van Schaik, 2020; Kummer and Goodall, 1985). This contrast permits a direct test of how repertoires respond to the differences in the socio-ecological environment. In captivity, individuals face less competition for food, have more spare time, are closer together, are often more visible to each other and more on the ground than in the wild (Fröhlich and van Schaik, 2018; Liebal et al., 2013). Especially in semi-solitary, fission-fusion species such as orangutans (Pongo spp.), interaction rates in contexts such as social play, grooming, conflict situations, and mating are much larger in captive settings (e.g., Fröhlich et al., 2021; Kopp and Liebal, 2018; Maple, 1980; Zucker et al., 1978), which may favor the production of additional signals (including both “signals” in the strict evolutionary sense, and those communicative acts that qualify as weak innovations; see also Fröhlich and van Schaik, 2020) that are completely absent in the wild and thus cause differences in the communicative repertoires of individuals and groups.
The captive-wild contrast also allows us to examine an additional, related issue: the extent to which the functional specificity (i.e., the predominance of particular interaction outcomes) of signals depends on the socio-ecological environment in which they are used (although we must also take into account that the prevalence of certain contexts also varies between research settings). First, signals of any sensory modality can vary in their meaning (function) between individuals and social groups (Boesch, 1996; Fichtel and Van Schaik, 2006; Salmi and Muñoz, 2020). This kind of change may reflect the presence of behavioral plasticity, high levels of which are a prerequisite for the generation of novel signals (i.e., signal innovations). Second, signals specific to one research setting (e.g., captivity) can differ in functional specificity from those present in all settings. Hence, the aim of the present study was to examine repertoires and functional specificity of close-range signals.
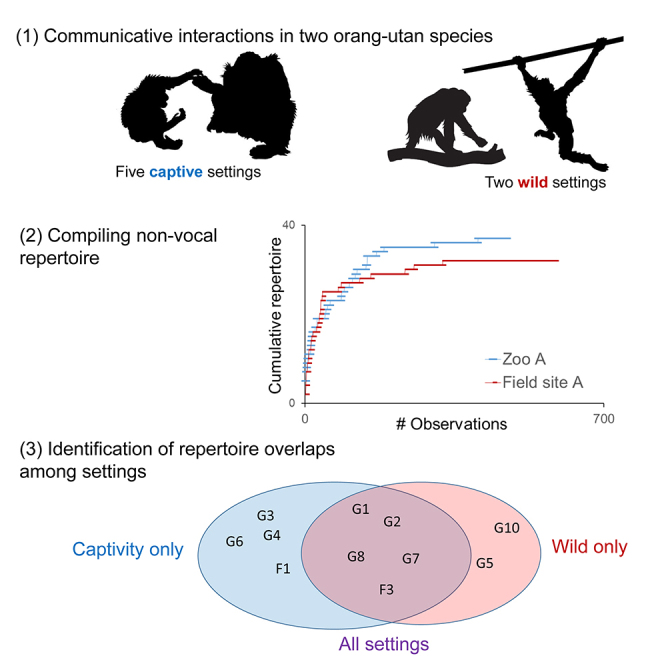
We conducted this study in both wild and captive populations of orangutans, a great ape genus ideal for this avenue of research, because the captive-wild contrast in social behavior is arguably greater than for any other taxon. First, systematic studies on the gestural repertoire of captive orangutans have demonstrated a propensity for elaborate and flexible gesture use that parallels that of other great apes (Cartmill and Byrne, 2010; Cartmill and Byrne, 2007; Liebal et al., 2006). These findings suggest that social propensities may be more fully expressed in captivity, as individuals do not need to avoid associations with conspecifics in order to obtain sufficient food, unlike in the wild, where most close-range communication (i.e., visual, tactile, and audible) is predominantly between mothers and their infants (Bard, 1992; Fröhlich et al., 2019; Knox et al., 2019). This continuous sociality may facilitate the production of more extensive communicative repertoires. Second, visual communication is less hampered by arboreality and obscuring vegetation. We also compared the captivity effect separately for Sumatran and Bornean orangutans. Sociability and interaction rates are reportedly higher in the Northwest Sumatran population than the Bornean populations (Fröhlich et al., 2020; van Schaik, 1999), and studying both species in both research settings (i.e., adopting a 2 x 2 design) permits teasing apart the effect of intrinsic differences in social tolerance from wild-captive contrasts on the production and use of additional innate or innovated signals.
We examined non-vocal (i.e., gestural and facial) signals of Bornean and Sumatran orangutans (Pongo pygmaeus and Pongo abelii) in two wild populations and five zoos. In a preparatory step, we established the repertoires and functions (presumed goals of communicative acts, with outcomes that apparently satisfied the signaler; Cartmill and Byrne, 2010; Hobaiter and Byrne, 2014) of orangutans’ gestural and facial signals separately for wild and captive settings, by pooling our results and previous work conducted on chimpanzees and captive orangutans. We then tested several predictions about how setting affected repertoire sizes and functional specificity of signal types.
First, captivity should result in larger communicative repertoires because of increased terrestriality, sociability, and interaction rates. These conditions may enable the production of signals not feasible or useful in natural environments (Marler, 1965), resulting in the use of “weak innovations” (Ramsey et al., 2007), which are easily reproduced by different individuals under suitable conditions (Lehner et al., 2010; Tennie et al., 2020). Thus, wild repertoires should be largely a subset of the captive ones, but there also may be signal types that are not expressed in captivity (“wild-only”), e.g., due to environmental constraints, but we expect that this effect is modest for the gestures and facial expressions examined here.
Second, we expect that the form of signal types expressed only in captivity should be tightly linked to the more terrestrial lifestyle or the increased sociability (Becker, 1984; Jantschke, 1972; Maple, 1980; Perkins, 1992; Wilson, 1982; Zucker et al., 1978), especially in the less terrestrial Sumatran orangutans. Specifically, we would expect a proliferation of signals whose expression requires flat substrates and involves mobile objects, and in communicative contexts that do not occur on a daily basis in wild settings (e.g., social play and conflict beyond the mother-offspring dyad). We would thus predict that gestural repertoires of two individuals of the same species living in the same research settings (i.e., either captivity or wild, within-setting similarity) exhibit a larger degree of overlap than those of two individuals living in different settings (i.e., captivity versus wild, between-setting similarity). This should be a direct consequence of adaptation to the specific socio-ecological environments individuals interact (i.e., immediate responses, behavioral flexibility) and grow up in (i.e., developmental responses, ontogenetic plasticity).
Third, this greater variety of signals in captivity may be accompanied by higher functional specificity in this setting (estimated by a preponderance of specific outcomes for a given signal, in contrast to multiple outcomes), whereas we may find more flexible (or redundant) use of signals (i.e., one and the same act for several different functions) in natural environments. This is because the additional acts (“weak innovations”) observed in captivity may be tightly linked to the specific interactions we expect to see more often in this artificial environment, especially social play (see above).
In all comparisons, we controlled for confounding variables such as age-sex class (young apes regularly use a larger communicative set than adults, particularly for soliciting social play and food transfers; Call and Tomasello, 2007; Liebal et al., 2013) and sampling effort (an obvious driver in the estimation of individual repertoire sizes). The effect of the setting-species interaction on repertoires and functional specificity will allow us to draw important conclusions regarding the extent of communicative plasticity and flexibility in the Pongo genus.
Results
Production of gestures and facial expressions across settings
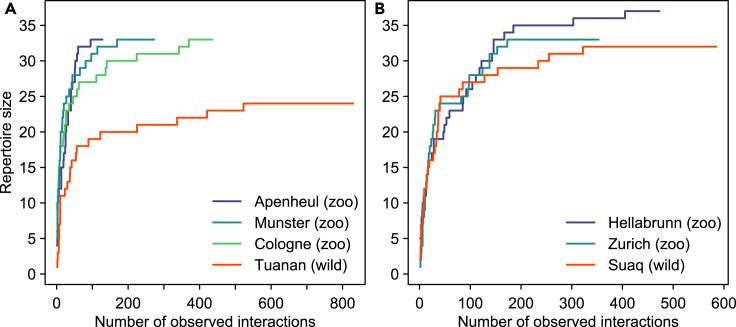
Among 11,035 coded gestural and facial signals (see Table 1 for numbers in relation to species and setting), we identified 41 distinct signal types across all settings, of which 39 were observed in Bornean (captive: N = 38, wild: N = 24) and 39 in Sumatran orangutans (captive: N = 37, wild: N = 32). In Table S1 we provide definitions for all coded behaviors and their relation to previous work on orangutans’ gestural repertoires. Plotting the cumulative number of identified signals over the course of the observation period indicated that study groups have been sufficiently sampled to capture complete site-specific repertoires (Figure 1). The majority of non-vocal signal types consisted of manual (total: N = 20; captive: N = 18; wild: N = 18) and bodily gestures (total: N = 18; captive: N = 18; wild: N = 12), whereas considerably fewer facial expressions (total: N = 3; captive: N = 3; wild: N = 2) were observed (see Table 1 for detailed overview of signals in relation to settings, species, subjects, and age classes). The relatively small repertoire of facial signals may be partly due to our strict criteria of inclusion into the repertoire (see STAR methods), so these findings should be viewed with caution.
Table 1.
Overview of produced signal types in relation to setting, species, and age class
| Signal | Signal type | No subjects | Age class | Dominant outcome | Bornean |
Sumatran |
Total | ||
|---|---|---|---|---|---|---|---|---|---|
| Captive | Wild | Captive | Wild | ||||||
| Beg hand-hand | Manual | 30 | Ad, Oi, Yi | FS | 45 | 152 | 55 | 98 | 350 |
| Beg hand-mouth | Manual | 27 | Ad, Oi, Yi | FS | 27 | 82 | 64 | 73 | 246 |
| Beg mouth-hand | Bodily | 27 | Ad, Oi, Yi | FS | 26 | 69 | 76 | 28 | 199 |
| Beg mouth-mouth | Bodily | 26 | Ad, Oi, Yi | FS | 45 | 38 | 136 | 24 | 243 |
| Bite | Bodily | 39 | Ad, Oi, Yi | PL | 117 | 264 | 54 | 8 | 443 |
| Bite attempt | Bodily | 41 | Ad, Oi, Yi | PL | 83 | 141 | 61 | 24 | 309 |
| Dangle | Bodily | 24 | Ad, Oi, Yi | PL | 307 | 36 | 49 | 65 | 457 |
| Embrace | Manual | 14 | Ad, Oi, Yi | PL | 9 | 8 | 23 | 18 | 58 |
| Flap lip | Facial | 3 | Ad, Oi, Yi | ST | 11 | 11 | |||
| Fling | Manual | 20 | Ad, Oi, Yi | PL | 20 | 4 | 31 | 37 | 92 |
| Grab/hold | Manual | 64 | Ad, Oi, Yi | PL | 795 | 787 | 178 | 348 | 2,108 |
| Hand on | Manual | 43 | Ad, Oi, Yi | PL | 215 | 177 | 29 | 148 | 569 |
| Head-butt | Bodily | 6 | Ad, Oi, Yi | PL | (1) | 29 | 3 | 32 | |
| Head-stand | Bodily | 5 | Ad, Oi, Yi | PL | 16 | 4 | (1) | 20 | |
| Hit | Manual | 16 | Ad, Oi, Yi | PL | 78 | 83 | 4 | 165 | |
| Hit ground/object | Manual | 3 | Ad, Oi, Yi | PL | 3 | 3 | |||
| Kiss | Bodily | 24 | Ad, Oi, Yi | PL | 29 | 57 | 62 | 2 | 150 |
| Look at | Bodily | 46 | Ad, Oi, Yi | PL | 175 | 40 | 72 | 210 | 497 |
| Look back at | Bodily | 14 | Ad, Oi, Yi | PL | 19 | 26 | 2 | 47 | |
| Loud scratch | Manual | 7 | Ad, Oi, Yi | JT | 5 | 29 | 34 | ||
| Peer | Bodily | 34 | Ad, Oi, Yi | FS | 211 | 39 | 223 | 88 | 561 |
| Play face | Facial | 18 | Ad, Oi, Yi | PL | 64 | 10 | 46 | 20 | 140 |
| Poke | Manual | 25 | Ad, Oi, Yi | PL | 124 | 36 | 87 | 3 | 250 |
| Pout face | Facial | 3 | Ad, Oi, Yi | PL | 4 | 10 | 6 | 20 | |
| Present body part | Manual | 29 | Ad, Oi, Yi | GR, JT | 7 | 87 | 28 | 26 | 148 |
| Present object | Manual | 10 | Ad, Oi, Yi | PL | 40 | (1) | 14 | 4 | 58 |
| Pull | Manual | 53 | Ad, Oi, Yi | PL, FS | 178 | 110 | 222 | 161 | 671 |
| Push | Manual | 46 | Ad, Oi, Yi | ST, PL | 164 | 42 | 95 | 115 | 416 |
| Raise limb | Manual | 18 | Ad, Oi, Yi | PL | 187 | 14 | 10 | 211 | |
| Reach | Manual | 41 | Ad, Oi, Yi | PL, FS | 202 | 18 | 88 | 107 | 415 |
| Rise up | Bodily | 8 | Ad, Oi, Yi | PL, JT | 64 | 3 | 67 | ||
| Roll on back | Bodily | 11 | Ad, Oi, Yi | PL | 119 | 2 | 121 | ||
| Rub body | Bodily | 12 | Ad, Oi, Yi | PL, SX | 2 | 60 | 27 | 89 | |
| Shake object | Manual | 1 | Ad, Oi, Yi | PL | 5 | 5 | |||
| Somersault | Bodily | 5 | Ad, Oi | PL | 56 | 4 | 60 | ||
| Spin | Bodily | 3 | Oi, Yi | PL | 8 | 8 | |||
| Spit | Bodily | 2 | Oi, Yi | PL | (1) | 5 | 5 | ||
| Stroke | Manual | 8 | Ad, Oi | PL | (1) | 13 | 5 | 18 | |
| Throw object | Manual | 10 | Ad, Oi, Yi | PL | 65 | 10 | 75 | ||
| Throw self | Bodily | 14 | Ad, Oi | PL | 135 | 58 | 2 | 195 | |
| Touch | Manual | 60 | Ad, Oi | PL | 503 | 598 | 156 | 214 | 1,471 |
| Total | 4,142 | 2,810 | 2,177 | 1,906 | 11,035 | ||||
Ad, adult; Oi, older immature; Yi, younger immature; FS, Share food/object; GR, Groom; JT, Co-locomote; PL, Play/affiliate; SX, Sexual contact; ST, stop action; blue: captivity only; orange: wild only. See also Table S1.
Figure 1.
Cumulative number of identified signal types in (A) Bornean and (A) Sumatran orangutans over consecutively observed interactions
(A and B) Asymptotes are depicted separately for the captive groups (A: Apenheul, Cologne, Munster, B: Hellabrunn, Zurich) and wild populations (A: Tuanan, B: Suaq) in this study. See also Figure S1.
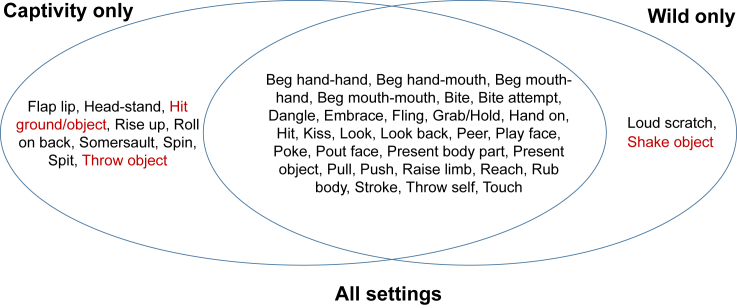
The first prediction was that captivity should result in enlarged communicative repertoires at the aggregate level owing to boosted sociability and interaction rates. The majority of signal types (N = 30) was observed (i.e., coded more than once) in both research settings. However, nine gesture types were restricted to captivity (e.g., “roll on back,” “throw object,” “somersault”), and two to the wild (“loud scratch,” “shake object,” see Table 1 and Figure 2 for overview of gestures and facial expressions used across settings), thus confirming the prediction. In addition, another five gesture types were observed very rarely (i.e., less than five times) in wild compared with captive settings (“head-butt,” “hit,” “look back at,” present object,” “throw self,” Table 1). We thus find that gestural communication repertoires expressed by zoo-housed orangutans are about 20% greater than those of their wild counterparts.
Figure 2.
Overview of signal types observed across research settings
Gesture types observed in the contrasting setting by other studies are marked in red. See also Table S2.
A more conservative way of testing the prediction is by producing a list based on all previous studies. Our own findings regarding zoo repertoires (i.e., 38 different types of non-vocal signal types in captive Borneans and 37 in captive Sumatrans, as compared with 24 and 32 signal types in the wild, respectively) are broadly consistent with the available systematic studies in single settings. Liebal and colleagues (2006), studying two captive groups of Sumatran orangutans, reported a repertoire of 34 signal types (29 gestures, and 5 facial expressions). Like them, we found that the majority of communicative acts were used to solicit social play and food transfers. Cartmill and Byrne (2010), examining two zoo groups of Bornean and one group of Sumatran orangutans, identified 38 types of gesture and facial expressions that allowed the analysis of “intentional meaning.” For wild settings, a recent systematic study on mother-offspring gesture use among wild orangutans, conducted at the Bornean population of Sabangau Forest, identified 21 gesture types that met the criteria for inclusion into the repertoire (Knox et al., 2019). With 24 different observed signal types observed in our study population at Tuanan, it thus seems like communicative interactions outside the mother-offspring bond do not result in a substantially larger repertoire size. Three of the gesture types we found only in captive (“hit ground/object, “throw object”) or wild settings (“shake object”) were also observed in other species-setting combinations in other studies, which leaves at least seven captivity-only acts and one wild-only act (see Table S2; note that Cartmill and Byrne (2010) do not specify which gestures were observed in which orangutan species). However, it should be noted that our study was restricted to intra-specific interactions, whereas other studies in the wild presumably included displays directed at humans (MacKinnon, 1974; Rijksen, 1978). This more conservative comparison, assessing whether the captivity-only acts of our study were observed in the wild in previous studies and vice versa, thus also confirms the prediction that gestural/facial repertoires in captivity should be larger.
To ensure that differences between captivity and the wild do not merely reflect differences in social opportunities (e.g., with regard to the availability of same-age play partners), we compiled separate play repertoires for mother-offspring, for which there is no change in partner availability between natural and captive settings, versus same-aged interactions (Table S3). For both orangutan species, we found that play solicitation repertoires observed in mother-offspring and peer interactions did not significantly differ depending on research setting (Fisher's exact test, Borneans: P = 0.072, Sumatrans: P = 0.408). This shows that differences in repertoire sizes between captive and wild settings are not mainly driven by partner availability.
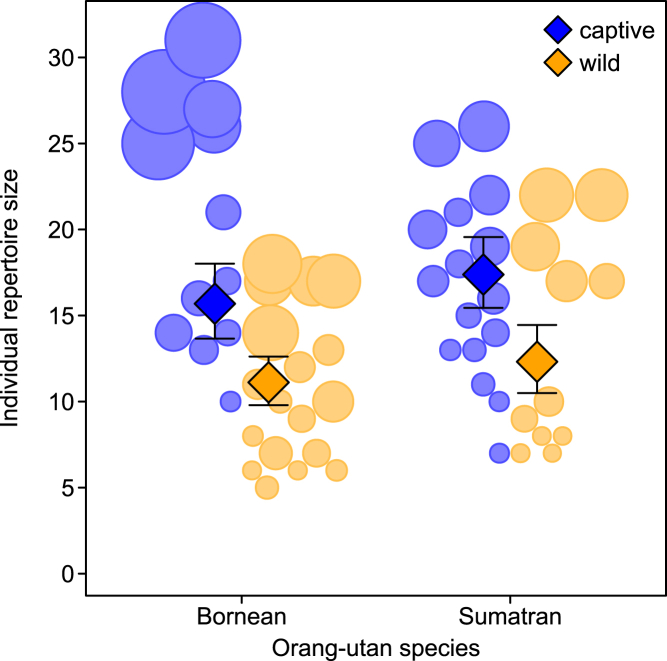
Individual repertoire sizes
The repertoire differences should also hold at the individual level, even if sampling issues might weaken the effect of setting and species. Using a generalized linear mixed model (GLMM), we tested how setting, species, and important confounding variables such as age class and sampling effort affected the repertoire size of gestures and facial expressions in individuals recorded during the study (for details see STAR methods). The full model including the key test predictors (i.e., setting and species) fitted the data better than the null models irrespective of the subsets of individuals included (likelihood ratio test [LRT] only highly sampled individuals: χ23 = 26.011, P < 0.001, N = 57; LRT all individuals: χ23 = 25.007, P < 0.001, N = 71). As expected, individual repertoire sizes were strongly affected by the number of samples contributed to the dataset (see Table 1). After removing the non-significant interaction term, we found that captive individuals exhibited a significantly larger variety of gestural and facial signals than their wild counterparts regardless of individual sample size (Table 2, Figure 3), again confirming our prediction. We also found that both immature age classes produced significantly more signal types than adults (Tables 2, S4, Figure S2). These results are consistent with previous work showing that immature apes regularly use a larger gestural set than adults (e.g., Liebal et al., 2006). For effects of non-significant predictors see Table 2. A less conservative analysis, including all individuals regardless of contributed samples, yielded essentially the same results (Table S4). Descriptive results indicated no difference between the sexes: female orangutans used an average of 12.3 different signal types (SD = 6.7, N = 42), whereas males had 12.9 different types (SD = 8.9, N = 29). Our descriptive results on presumed goals of signal types broken up by species and setting (see Table S5) suggest that the higher rate of interactions in both affiliative and conflict situations rather than co-locomotion or food-sharing underlies the proliferation of signal types in captive settings. We thus conclude that captive Borneans and Sumatrans also have larger individual repertoire sizes, even if broken down by age classes (see Table S6).
Table 2.
Effects of research setting, orangutan species, and control variables on (a) repertoire size of individuals (N = 57) and (b) functional specificity of signal types (N = 114), derived using GLMMs
| Estimate | SE | χ21 | P | |
|---|---|---|---|---|
| (a) Repertoire size | ||||
| Intercept | 2.519 | 0.085 | – | – |
| Setting [wild] | −0.344 | 0.077 | 20.430 | <0.001 |
| Species [Sumatran] | 0.103 | 0.087 | 1.350 | 0.245 |
| Age class [young imm.] | 0.37 | 0.098 | 14.051 | <0.001 |
| Age class [old imm.] | 0.308 | 0.108 | 5.875 | 0.015 |
| No. observations | 0.178 | 0.037 | 22.813 | <0.001 |
| (b) Functional specificity | ||||
| Intercept | 2.691 | 0.272 | – | – |
| Setting [wild] | 0.299 | 0.135 | – | – |
| Species [Sumatran] | −0.407 | 0.152 | – | – |
| Dominant outcome [play] | −0.304 | 0.157 | 3.721 | 0.054 |
| No. subjects | −0.182 | 0.085 | 4.563 | 0.033 |
| No. observations | −0.004 | 0.037 | 0.012 | 0.911 |
| Setting x species | −1.083 | 0.189 | 33.210 | <0.001 |
Significant effects (p < 0.05) are depicted in italics. See also Table S4.
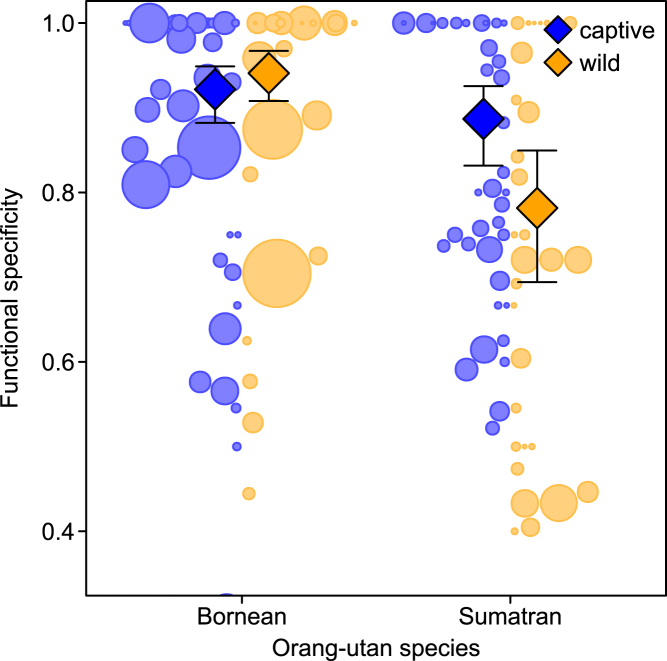
Figure 3.
Number of observed signal types per individual as a function of research setting and orangutan species, restricted to subjects with >40 samples
Circles represent different individuals with area corresponding to sample size; diamonds depict model estimates with 95% confidence intervals (all other variables centered to a mean of zero). See also Figure S2.
Repertoire similarity within and between settings
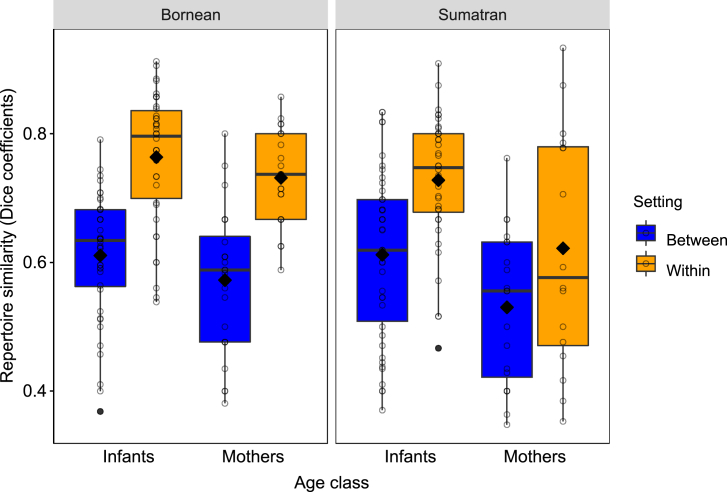
Next, we investigated whether the composition of individual repertoires systematically differed between captivity and the wild. To this end, we calculated Dice coefficients and conducted matrix permutations tests to analyze whether within-setting repertoire similarity (i.e., similarity of repertoires between two individuals living in the same research setting, e.g., captivity) differs from between-setting repertoire similarity, separately for mothers and young immatures and separately for Bornean and Sumatran orangutans. For both age classes in both species, we found that within-setting similarity of communicative repertoires was significantly larger than between-setting similarity (Bornean mothers: mean Within-Dc = 0.73, Between-Dc = 0.57, P < 0.001; Bornean immatures: Within-Dc = 0.76, Between-Dc = 0.61, P < 0.001; Sumatran mothers: Within-Dc = 0.62, Between-Dc = 0.53, P = 0.025; Sumatran immatures: Within-Dc = 0.73, Between-Dc = 0.61, P < 0.001; see Figure 4). In contrast, degrees of repertoire overlaps within captivity and within the wild were largely similar, except in Sumatran mothers (Bornean mothers: mean Within-Dc (wild) = 0.72, Within-Dc (captive) = 0.76, P = 0.662; Bornean immatures: Within-Dc (wild) = 0.73, Within-Dc (captive) = 0.85, P = 0.959; Sumatran mothers: Within-Dc (wild) = 0.81, Within-Dc (captive) = 0.51, P < 0.001; Sumatran immatures: Within-Dc (wild) = 0.72, Within-Dc (captive) = 0.73, P = 0.595). This provides further support for the notion that communicative repertoires used in the wild and in captivity systematically differ in composition and that the additional signal types produced in captivity were often the same ones.
Figure 4.
Repertoire similarity of two individuals living in different (“between”) and the same (“within”) research settings, separately for two age classes and both orangutan species
Indicated are dyadic coefficients (circles), population means (filled diamonds), medians (horizontal lines), quartiles (boxes), percentiles (2.5% and 97.5%, vertical lines), and outliers (filled dots). Individuals have contributed to multiple data points.
Functional specificity of signal types
Finally, we tested the second prediction that the functional specificity of signal types (i.e., proportion specifying the dominance of single interaction outcomes, see STAR methods) depends on research setting and orangutan species. On average, functional specificity of signal types was 0.82 ± 0.19 across all settings and both species. It was somewhat lower in captive than in wild individuals among Borneans (captive: 0.85 ± 0.18; wild: 0.88 ± 0.18) and higher among Sumatrans (captive: 0.82 ± 0.16; wild: 0.72 ± 0.22). Using a GLMM, the full model including the key test predictors (i.e., setting, species) fitted the data better than the null models (LRT: χ23 = 126.511, P < 0.001, N = 128). Specifically, there was a significant interaction between research setting and orangutan species: post hoc Sidak tests revealed that functional specificity of signal types in wild Sumatrans was significantly lower compared with their captive counterparts (estimate ± standard error = 0.784 ± 0.12, Z = 6.556, P < 0.001) and compared with wild Borneans (1.49 ± 0.144, Z = 10.348, P < 0.001; see Figure 5). In contrast, functional specificity was significantly lower in captive Bornean orangutans compared with their wild counterparts (−0.299 ± 0.135, Z = −2.224, P = 0.026) but higher compared with captive Sumatrans (0.407 ± 0.152, Z = 2.678, P = 0.007), although these effects were smaller (see Figure 5). Regardless of the species-setting effects, specificity was significantly higher for signal types observed in a larger number of subjects (Table 2).
Figure 5.
Functional specificity measures of signal types as a function of research setting and orangutan species
Circles represent different signal types with area corresponding to sample size; diamonds depict model estimates with 95% confidence intervals (all other variables centered to a mean of zero).
In Sumatrans, functional specificity of captivity-only acts (N = 7) was on average 0.9 ± 0.16, as opposed to 0.76 ± 0.19 for signal types used in both wild and captive settings (N = 30). In Borneans, functional specificity of captivity-only acts (N = 7) was also higher than for acts used in both settings (N = 30; 0.97 ± 0.06 versus 0.85 ± 0.19). These descriptive results (the sample size prevents meaningful inferential analyses) also suggest that both species use signal types with higher functional specificity in captive than in wild settings, which seems to be driven by those acts used only in captivity. We also note that functional specificity seems to be on average higher in Borneans versus Sumatrans, which may be due to fewer social opportunities both in the wild and in captivity, and thus less exposure to the full range of social contexts.
Our findings thus support the prediction that larger repertoire sizes in captivity should be accompanied by greater average functional specificity compared with wild settings. However, the slightly lower specificity for captive Borneans than their wild counterparts was not expected and may have been caused by contextual differences in the respective research environments.
Discussion
To explore the extent of communicative plasticity and innovativeness in gestural and facial signals, we here adopted a 2 x 2 design, investigating repertoire sizes and functional specificity of signal types in zoo-housed and wild groups of two different orangutan species diverging in sociability. By examining the captive-wild contrast in these two related species we found that captive environments favor larger and different repertoires of signals (including additional signal types that may represent “weak innovations”) among orangutans. We also found that this difference was reflected in larger within- versus between-setting repertoire overlaps between individuals. Moreover, considering species differences related to differential sociability and terrestriality on one hand, and setting on the other, we found that Sumatran orangutans, but not Borneans, showed a pronounced wild-captive contrast in the functional specificity of signals, offering insights into the degrees of “communicative innovativeness” (i.e., additional signals) versus behavioral flexibility (i.e., using the same signal type for different social goals and vice versa) underlying the communicative repertoires of orangutans.
Consistent with our first prediction, communicative repertoires on both the aggregate and individual levels were larger in captivity as compared with the wild, even after controlling for the expected effects of age class and sampling effort (i.e., irrespective of whether all or only highly sampled individuals are included in the analysis). There may be some doubt that a single study can exhaustively sample signal repertoires. We therefore also compared the captive-wild contrast for each species using all available studies. This comparison corroborated the conclusions based on our study, in that the actual repertoire composition found in previous studies in the same setting and species revealed no major differences (Table S2). From the results of this and previous studies, we can conclude that moving wild orangutans into captivity leads to a 20% to 25% increase (i.e., 7–9 acts “gained,” 1–2 acts “lost”) in their non-vocal communicative repertoire.
The second prediction we made was that the form of these communicative acts expressed only in captivity should be tightly linked to the increased sociability and more terrestrial lifestyle that comes with it. As predicted, differences in repertoire size were particularly pronounced for presumed goals related to seeking body contact (e.g., soliciting “Play/affiliate,” “Groom,” “Sexual contact”) and social conflict (e.g., with the apparent aim to have the recipient “Move away” or “Stop action”). Interaction rates involving these outcomes are greatly boosted in captivity, where a more differentiated use of bodily communication is both enabled (owing to the availability of even substrate) and required (to navigate social contexts uncommon in the wild; Fröhlich and van Schaik, 2020). This setting effect is not attributable to the presence of certain social partners alone: we demonstrated that wild-captive contrasts were strong for both mother-offspring and peer play interactions. Captive facilities are stable, plentiful, and predator-free environments that may provide opportunities for, and even require (e.g., due to increasing conflict with limited space), weak signal innovations and modifications, just like they foster innovations in general (Lehner et al., 2010; van Schaik, 2016).
Our findings thus provide indirect evidence that the new environments we have created lead to the production of larger gestural and facial repertoires in orangutans, which may be linked to at least some degree of communicative innovativeness. Nonetheless, we cannot entirely rule out that a few of these additional signal types (e.g., rise up, flapped lip) may also be observed in wild individuals after more extensive research efforts. We may also have missed a specific part of the wild repertoire that is only produced in poor-visibility conditions such as nesting and copulation, but since these signals were also not recorded in captivity this does not affect the above conclusion. Other studies on captive apes have generated convincing evidence for specific, invented (“species-atypical”) signals, encompassing novel pant-hoot variants (Marshall et al., 1999), and “whistling” (Wich et al., 2009), as well as pointing with hands and fingers (Leavens and Hopkins, 1998; Leavens et al., 2010), “raspberries” and “extended grunts” (Hopkins et al., 2007). However, these novel signals were predominantly observed in direct interaction with human caretakers and thus potentially imitated from them. In conclusion, our results support the notion that the socio-ecological conditions linked to captivity may lead to the production of additional innate or learned communicative utterances.
We also expected that the form of communicative acts expressed only in captivity are linked to the novel element of a more terrestrial lifestyle. Accordingly, repertoires of individuals living in the same setting should overlap more than those of individuals living in different settings. Indeed, we found that the signal types that are exclusively (or overwhelmingly) produced in captive settings are strongly linked to the more terrestrial nature of their artificial habitat: “somersaults”, “hit object”, “head-stands”; signal types that involve either the ground or objects obtained from the ground, would be very difficult or even impossible to produce by wild orangutans with their purely (Sumatra) or predominantly (Borneo: Ashbury et al., 2015) arboreal lifestyle. Moreover, we found a strikingly higher similarity of gestural and facial communication repertoires within settings (comparing dyads of the same setting) than between them (comparing dyads of different settings), for both age classes considered (mothers versus offspring) and both orangutan species (Bornean versus Sumatran). It thus appears that the new affordances of captive settings, on top of the elevated exposure to certain social contexts, enabled orangutans to better exploit their (communicative) motion spectrum, resulting in novel communicative movements that may independently and predictably be produced in several captive colonies and species (such as spitting as an attention-getter, see Jantschke, 1972). The wild-captive contrast in signal use also confirms earlier reports making the case that the complex individual-based fission fusion structure of orangutans and their sophisticated social-cognitive skills seem to be reflected in a highly variable communicative repertoire (Liebal et al., 2006; Maestripieri, 1999).
Advocates of phylogenetic gesture origins may argue that those orangutan gestures observed only in captivity may in fact be “family-typical”, in that they are also found in the African apes (e.g., Byrne et al., 2017; Kersken et al., 2018). If so, the absence of certain signals in wild orangutans would not mean that captivity-only signals are not part of the innate species repertoire, and thus zoo-housed orangutans would just exhibit gestures inherited from previous, less arboreal ancestral species. To us, however, this is a less parsimonious explanation for at least some of the terrestrial behavior patterns we have observed here, because a signal is defined not only by its looks or morphology but also (or even more so) by its function in communication or “meaning” (Fröhlich and Hobaiter, 2018). Somersaulting or spitting, for instance, may be found in playful contexts of many social mammals, but its successful use as a play solicitation gesture may still be largely learned during ontogeny.
Finally, we predicted that the functional specificity of signals should also be larger in captivity compared with wild settings. In line with our predictions, we found that Sumatrans in the wild exhibited strikingly lower functional specificity scores compared with their captive counterparts, but the opposite effect was found for Bornean orangutans. When comparing Borneans' and Sumatrans' signal types used only in captivity with those used in both research settings, captivity-only acts were more functionally specific in both species (although the small sample and unbalanced data prevented inferential analyses). In other words, wild Sumatrans seem to use their signal types much more flexibly (and thus redundantly) across presumed goals than in captivity, which appears to be largely due to captive Sumatrans' use of additional functionally specific acts not present in the wild repertoire. In contrast, the relatively low interaction rates and fewer social opportunities of wild Bornean orangutans (Fröhlich et al., 2020; Mitra Setia et al., 2009; van Schaik, 1999) may be reflected in a lesser need to use signals flexibly across contexts (although social opportunities are not necessarily tightly linked to repertoire size). Thus, the species difference is mainly related to differences in functional specificity in the wild, not in captivity. Together, these results corroborate our expectation that average functional specificity increased with repertoire size in captive Sumatran, although not in Bornean, orangutans.
Our results and previous findings regarding communicative flexibility and development (e.g., Cheney and Seyfarth, 2018; Fröhlich and Hobaiter, 2018; Liebal et al., 2013) provide evidence for two forms of phenotypic plasticity in great ape communication: behavioral flexibility (immediate responses) and ontogenetic plasticity (developmental responses). On the immediate level, an intentional agent can flexibly communicate by using one and the same communicative act for several different functions (i.e., means-end dissociation), relying on situational context (possibly age difference or sex relative to recipient; Graham et al., 2020) to disambiguate between signals. This behavioral flexibility produces at least some redundancy in the communicative repertoire (Byrne et al., 2017), as we can see in our sample of wild Sumatran orangutans. On the developmental level, the intentional agent may either start to produce a new communicative behavior, from their species-specific repertoire not previously expressed due to environmental constraints, or, if not available, produce one for which naïve recipients (over repeated instances of interaction, e.g. through ontogenetic ritualization; Halina et al., 2013) infer their meaning from context and reactions. This ontogenetic plasticity may well produce higher functional specificity because novel signals may be more readily understood and thus maintained in the repertoire when they are highly functionally specific (Wheeler and Fischer, 2012). However, it could be argued that context is nearly always present in every-day usage of signals, and future studies may show that differences in functional specificity, in practice, need not correspond to varying levels of ambiguity. Moreover, we cannot rule out that our functional specificity measures also reflect differences in the socio-ecological environments to which the subjects in this study were exposed, so these findings have to be viewed with caution. If the signal types unique to captive settings qualify as innovations, they are surely of a “weak” nature (i.e., easily reinvented by individuals in the proper context; Ramsey et al., 2007). However, these weak innovations seem to be widespread in the great ape behavioral repertoire: experiments have shown repeatedly that many great ape innovations in the wild can readily be reproduced by captive ones when suitable conditions are offered (Lehner et al., 2010; Tennie et al., 2020). Future research needs to look more thoroughly at between-individual versus within-individual variation in signal use, in order to provide more direct evidence that the captivity- or wild-specific gestures indeed qualify as innovations (as opposed to elements of a latent repertoire shared by all hominids).
Answering the question of whether our primate relatives possess the behavioral plasticity, or communicative creativity, to complement their species-typical repertoires by creating signals from scratch is highly relevant to theories of language evolution (see also Fröhlich and van Schaik, 2020), given that the productivity feature of language (the ability to create and understand novel utterances with novel meanings; Hockett, 1960) reflects extreme communicative plasticity. Although this study cannot provide direct evidence for productivity, our findings regarding behavioral plasticity (“weak inventions” sensu Ramsey et al., 2007) are nonetheless consistent with the view that apes possess a latent capacity for communicative innovativeness. So far, no study had explicitly and systematically examined communication systems of apes exposed to novel socioecological conditions relative to the wild baseline situation. Although our knowledge of the taxonomic distribution of signal innovation is still incomplete, this phenomenon is so far reported almost exclusively for great apes. Ongoing work supports this pattern, in that great apes are increasingly documented to make up new vocal (see above, and Taglialatela et al., 2012) and gestural (e.g., Halina et al., 2013; Tomasello et al., 1994) signals in the novel conditions of captivity, whereas reports from other taxa are rare (but see, e.g., Garland et al., 2011; Grant and Grant, 1996; Moura, 2007; Perry et al., 2003). These findings imply that once the conditions were in place that favored the open-ended use of invented expressions, our hominin ancestors readily responded to this opportunity, because they could build on a long evolutionary history of communicative plasticity. This might explain why language evolved in the hominin lineage and not others that found themselves in similar conditions (e.g., reliance on interdependent foraging and cooperative breeding).
Limitations of the study
Our study has two important caveats. First, given that orangutans are arboreal primates, wild and captive settings differ profoundly in conditions for behavioral observation. Dense vegetation may have hampered the recording of subtle visual or acoustic communication, whereas the glass barriers of zoo enclosures often prevented the collection of sufficient high-quality data on vocal repertoires. We may also have missed a specific part of the wild repertoire (e.g., signals that are only produced in poor-visibility conditions such as nesting and copulation), but since these were also not recorded in captivity this does not affect the above conclusions. The future development of data recording technology (e.g., silent drones above canopy nests, enclosure-integrated cameras and microphones) may alleviate some of these difficulties. Second, captive orangutans may differ from their wild counterparts not only in general sociability and terrestriality but also in other opportunities and constraints. For instance, the functional specificity measures obtained in this study may also reflect differences in the specific contexts to which the subjects were exposed, so these findings have to be viewed with caution.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Repository data | This paper | https://doi.org/10.5281/zenodo.5561236 |
| Experimental models: organisms/strains | ||
| Pongo abelii | Housed at the zoos of Hellabrunn (Munich) and Zurich; wild population living in Gunung Leuser National Park, Northern Sumatra, Indonesia | N/A |
| Pongo pygmaeus | Housed at the zoos of Apenheul (Apeldoorn), Cologne and Munster; wild population living in Mawas Reserve, Central Kalimantan, Indonesia | N/A |
| Software and algorithms | ||
| R Studio | R Development Core Team, 2020; R Foundation for statistical computing | https://www.r-project.org/ |
| Custom R scripts | This paper | https://doi.org/10.5281/zenodo.5561236 |
Resource availability
Lead contact
Information and requests for resources should be directed to and will be fulfilled by the lead contact, Marlen Fröhlich (marlen.froehlich@uzh.ch).
Materials availability
No new materials were generated in this study.
Experimental model and subject details
Data were collected at two field sites and five captive facilities (zoos). We observed wild orang-utans at the long-term research sites of Suaq Balimbing (03°02’N; 97°25’E, Gunung Leuser National Park, South Aceh, Indonesia) and Tuanan (02°15’S; 114°44’E, Mawas Reserve, Central Kalimantan, Indonesia), in a population of wild Sumatran (Pongo abelii) and Bornean orang-utans (Pongo pygmaeus wurmbii), respectively. Both study sites consist mainly of peat swamp forest and show orang-utan densities of 7 individuals per km2 at Suaq and 4 at Tuanan (Husson et al., 2009; Singleton et al., 2009). Captive Bornean orang-utans were observed at the zoos of Cologne and Munster, and at Apenheul (Apeldoorn), while Sumatran orang-utans were observed at the zoo of Zurich and at Hellabrunn (Munich; see EEP studbook for details on captive groups; Becker 2016). While captive Sumatran orang-utans were housed in groups of nine individuals each, captive Bornean groups were generally smaller and sometimes included only a mother and her offspring (e.g. Apenheul). Subjects (i.e. signallers) included in this study consisted of 33 Bornean (20 wild/13 captive) and 38 Sumatran orang-utans (20 wild/18 captive). Subjects consisted of 33 adults (26 females, 7 males) and 38 immatures (16 females, 22 males). Detailed information on subjects and group compositions is provided in Table S7.
The following institutions granted permission to conduct research on wild orang-utans: the Indonesian State Ministry for Research and Technology (RISTEK, 398/SIP/FRP/E5/Dit.KI/XI/2017), the Indonesian Institute of Science (LIPI), the Directorate General of Natural Resources and Ecosystem Conservation – Ministry of Environment & Forestry of Indonesia (KSDAE-KLHK, SI.70/SET/HKST/Kum.I/II/2017), the Ministry of Internal affairs, the Nature Conservation Agency of Central Kalimantan (BKSDA), the local governments in Central Kalimantan, the Kapuas Protection Forest Management Unit (KPHL), the Bornean Orang-utan Survival Foundation (BOSF) and MAWAS in Palangkaraya.
Method details
Data collection
Focal observations were conducted between November 2017 and October 2018 (Suaq Balimbing: November 2017 – October 2018; Tuanan: January 2018 – July 2018, European zoos: January 2018 – June 2018). At the two field sites, these observations consisted of full (nest-to-nest) or partial follows (e.g. nest-to-lost or found-to-nest) of mother-infant units, whereas in zoos 6-hour focal follows were conducted. Two different behavioural sampling methods were combined: First, presumable intra-specific communicative interactions of all observed social interactions of the focal either as signaller or receiver with all partners, and among other conspecifics present (if the focal was engaged in a non-social activity while still in full sight) were recorded using a digital High-Definition camera (Panasonic HC-VXF 999 or Canon Legria HF M41) with an external directional microphone (Sennheiser MKE600 or ME66/K6). In captive settings with glass barriers, we also used a Zoom H1 Handy recorder that was placed in background areas of the enclosure whenever possible. Second, using instantaneous scan sampling at ten-minute intervals, we recorded complementary data on the activity of the focal individual, the distance and identity of all association partners, and in case of social interactions the interaction partner as well as several other parameters. During ca. 1600 hours of focal observations, we video-recorded more than 6300 communicative interactions which were subsequently screened for good enough quality to ensure video coding.
Coding procedure
A total of 3014 high-quality recordings of orang-utan interactions (wild: 1354, captive: 1660) were coded using the program BORIS version 7.0.4. (Friard and Gamba, 2016). We designed a coding scheme to enable the analysis of presumably communicative acts directed at conspecifics (i.e. close-range social behaviours that apparently served to elicit a behavioural change in the recipient and were mechanically ineffective, thus excluding practical acts such as picking up an object or acts produced with physical force; Call and Tomasello, 2007; Cartmill and Byrne, 2010; Fröhlich et al., 2021). This resulted in the coding of 11,153 non-vocal communicative acts (i.e. potential gestures and facial expressions), of which 4939 (wild: 3476, captive: 1463) were produced within mother-offspring interactions, and 6214 (wild: 1248, captive: 4966) among other interaction dyads (see Table S8 for distribution across settings and species). Although we also coded vocalizations based on field studies (Hardus et al., 2009a), we did not include vocalizations in the analyses of repertoire and functional specificity as we could not equally pick up soft, low-frequency sounds in captive and wild settings, which hampered the fine-grained comparison across settings. Detailed results for sensory modalities and articulators involved, as well as the multisensory and multicomponent use of communicative acts have been reported elsewhere (Fröhlich et al., 2021). All individual gestures and facial expressions were defined and aligned based on previous studies on orang-utan intentional communication in captive (Cartmill and Byrne, 2010; Jantschke, 1972; Liebal et al., 2006; Zucker et al., 1978) and wild settings (Fröhlich et al., 2019; Knox et al., 2019; MacKinnon, 1974; Rijksen, 1978; see Table S1). Comparing our dataset to this literature, we then identified the subset of setting- and species-specific signal types.
For each gesture or facial expression, we also coded the presumed goal (following the distinction of Cartmill and Byrne, 2010) and apparently satisfactory outcome (i.e. whether signaller ceased communication and if it represented the signaller’s plausible social goal, Hobaiter and Byrne, 2014; see Table S9 for levels and definitions), but also several other variables not directly relevant in this study. After an initial training period of two to four weeks, and afterwards in regular intervals (once a month), consistency of coding performance between at least two observers was evaluated with different sets of video recordings (10 to 20 clips each) using the Cohen’s Kappa coefficient to ensure inter-coder reliability (Bakeman and Quera, 2011). Trained coders proceeded with video coding only if at least a ‘good’ level (κ = 0.75) of agreement was found for type of signal type, presumed goal, and interaction outcome.
Data processing
For our repertoire analyses, we plotted the cumulative number of communicative behaviours over the number of coded interactions for each study group (Figures 1 and 2) and for a subset of highly sampled individuals (Figure S1), to estimate how many observations are necessary to grasp the repertoire of these groups/individuals as indicated by an asymptote. Signal types were counted as part of an individual’s repertoire only when observed at least twice per subject.
To analyse functional specificity, we considered only those signal types that were produced at least three times towards a particular interaction outcome (cf. Hobaiter and Byrne, 2014). We defined functional specificity depending on how often a signal type was produced towards a single apparently satisfactory outcome (ASO). For instance, “somersault” was exclusively produced to initiate “Play/affiliate” interactions (specificity value of 1), whereas “touch” was produced towards several different interaction outcomes, e.g. “Play/affiliate”, “Share food/object” and “Co-locomote” (specificity values < 0.7).
Quantification and statistical analysis
We ran two separate generalized linear mixed models (GLMMs; Baayen, 2008) with a Poisson or binomial error structure, respectively, to examine sources of variation in (a) individual communicative repertoires (i.e. number of signal types used at least twice, for two different datasets: one including all individuals and one including only those that contributed at least 40 samples), and (b) signal types’ specificity in function (i.e. the proportion of cases for which a signal type was used towards a single interaction outcome). In both models, we included research setting (2 levels: captive, wild) and orang-utan species (2 levels: Bornean, Sumatran) as our key test predictors. Because we assumed that the effect of research setting might depend on genetic predisposition (i.e. species), we also included the interaction between these two variables into our full model.
In model (a) testing individual repertoire size, we included the following variables as additional fixed effects (control predictors) into the models: subjects’ age class (3 levels: “adult”: females > 15 years, males > 16 years; “old immature”: independent and dependent immature > 5 years of age, “young immature”: dependent immature < 5 years of age), and the number of observations (range = 5–467; z-transformed). To control for repeated measurements within the same sampling unit, group ID was treated as random effect. We also included an observation level random effect, which models the extra-Poisson variation in the response variable using a random effect with a unique level for every data point (Gelman and Hill, 2006). We checked for overdispersion, revealing no issue (dispersion parameters < 1). To keep type 1 error rates at the nominal level of 5% (i.e. accounting for the non-independence of data points that pseudo-replicate slope information), we initially also included all relevant random slopes components within group ID (Schielzeth and Forstmeier, 2009). In model (b) testing functional specificity, we included the dominant outcome (2 levels: play, other; accounting for possibility that social opportunities differ between species and settings), number of subjects contributing to the use of a signal type (range = 1–21; z-transformed) and number of observations (range = 1–626; z-transformed) in the respective setting as control predictors. To control for repeated observations of the same signal types across settings, signal type was treated as random effect (Pinheiro and Bates, 2000).
All models were implemented in R (v4.0.3; R Development Core Team, 2020) using the function glmer of the package lme4 (Bates et al., 2014). To control for collinearity, we determined the Variance Inflation Factors (VIF; Field, 2005; Quinn and Keough, 2002) from a model including only the fixed main effects using the function vif of the R package car (Fox and Weisberg, 2011). This revealed no collinearity issues (maximum VIF = 1.6). To test whether the effects of our key test predictors were statistically significant (Forstmeier and Schielzeth, 2011; Mundry, 2014), we compared the full models with the respective null models comprising only the control predictors as well as all random effects using a likelihood ratio test (Dobson, 2002). To adjust for multiple comparisons, we tested interaction effects using pairwise contrasts with the function lsmeans (with argument “adjust” set to “sidak”) of the package lsmeans. When non-significant, these interaction terms were removed before testing the individual fixed effects. Tests of the individual fixed effects were derived using likelihood ratio tests (R function drop1 with argument “test” set to “Chisq”).
To compare repertoire similarity within and between research settings, we calculated Dice coefficients (Dice, 1945) for each pairing of individuals with DC = (2CAB) / (RA + RB), where CAB is the number of signals observed in both individuals (A and B), while RA and RB are the number of signals in the repertoire of two individuals A and B. The values range between 0 and 1: if two individuals have no signal types in common we get DC = 0, whereas two identical signal repertoires correspond to DC = 1 (see Fröhlich et al., 2016; Halina et al., 2013 for similar analyses). Because data points were non-independent as individuals contributed to multiple pairings, we conducted matrix permutation tests (N = 1000 permutations, significance level α = 0.025) in R to analyse whether (i) individuals (i.e. mothers, dependent immatures) of the same settings share more signal types than individuals living in contrasting settings, and (ii) individuals living in captive settings have more dissimilar repertoires than individuals in wild settings. We only included subjects that contributed more than 40 samples, and only considered signal types that were used at least twice by each individual.
Acknowledgments
We thank Erin Vogel and Suci Utami Atmoko (Tuanan), Kerstin Bartesch (Tierpark Hellabrunn), Claudia Rudolf von Rohr (Zoo Zürich), Alexander Sliwa (Kölner Zoo), Simone Sheka (Allwetterzoo Münster), and Thomas Bionda (Apenheul Primate Park) as well as all research staff and zoo keepers for a fruitful collaboration during this study. We gratefully acknowledge Clemens Becker for providing the EEP studbook, the Indonesian State Ministry for Research and Technology (RISTEK), the Indonesian Institute of Science (LIPI), the Directorate General of Natural Resources and Ecosystem Conservation – Ministry of Environment & Forestry of Indonesia (KSDAE-KLHK), the Ministry of Internal affairs, the Nature Conservation Agency of Central Kalimantan (BKSDA), the local governments in Central Kalimantan, the Kapuas Protection Forest Management Unit (KPHL), the Bornean Orangutan Survival Foundation (BOSF), MAWAS in Palangkaraya, as well as the Sumatran Orangutan Conservation Programme (SOCP) and Yayasan Ekosistem Lestari (YEL) in Medan. Moreover, we thank Simone Pika and Eva Luef for providing some of the essential equipment, Santhosh Totagera and Elisa Dore for coding assistance, and Uli Knief and Alex Hausmann for help with matrix permutations and a customized jitter-plot function. M.F. was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grant FR 3986/1-1), the Forschungskredit Postdoc (grant FK-17-106), and the A.H. Schultz Foundation of the University of Zurich, the Sponsorship Society of the German Primate Center (DPZ), the Stiftung Mensch und Tier (Freiburg), and the Christiane Nüsslein-Volhard Foundation. C.P.v.S. acknowledges the support of the NCCR Evolving Language Program (SNF #51NF40_180888).
Author contributions
M.F. and C.P.v.S. conceived the study. M.F. designed the project and collected, coded, and analyzed data. N.B., C.F., C.W., L.M., and M.J. helped to collect, curate, and code data. T.M.S., C.S., M.A.v.N., and C.P.v.S. provided resources. M.F. and C.P.v.S. wrote the manuscript with inputs from C.F., L.M., C.S., and M.A.v.N. All authors approved the submission of the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We worked to ensure sex balance in the selection of non-human subjects. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list. The author list of this paper includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Published: November 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103304.
Supplemental information
9
Data and code availability
-
•
Data have been deposited at Zenodo and is publicly available as of the date of publication. DOI is listed in the key resources table.
-
•
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOI is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request
References
- Ashbury A.M., Posa M.R.C., Dunkel L.P., Spillmann B., Atmoko S.S.U., van Schaik C.P., van Noordwijk M.A. Why do orangutans leave the trees? Terrestrial behavior among wild Bornean orangutans (Pongo pygmaeus wurmbii) at Tuanan, Central Kalimantan. Am. J. Primatol. 2015;77:1216–1229. doi: 10.1002/ajp.22460. [DOI] [PubMed] [Google Scholar]
- Baayen R.H. Cambridge University Press; 2008. Analyzing Linguistic Data. [Google Scholar]
- Bakeman R., Quera V. Cambridge University Press; 2011. Sequential Analysis and Observational Methods for the Behavioral Sciences. [Google Scholar]
- Bandini E., Harrison R.A. Innovation in chimpanzees. Biol. Rev. 2020;95:1167–1197. doi: 10.1111/brv.12604. [DOI] [PubMed] [Google Scholar]
- Bard K.A. Intentional behavior and intentional communication in young free-ranging orangutans. Child Dev. 1992;63:1186–1197. doi: 10.1111/j.1467-8624.1992.tb01688.x. [DOI] [PubMed] [Google Scholar]
- Bard K.A., Dunbar S., Maguire-Herring V., Veira Y., Hayes K.G., McDonald K. Gestures and social-emotional communicative development in chimpanzee infants. Am. J. Primatol. 2014;76:14–29. doi: 10.1002/ajp.22189. [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. 2014. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1.1-7. [Google Scholar]
- Becker C. Profil-Verlag; 1984. Orangutans und Bonobos im Spiel [Play behavior of Orangutans and Bonobos] [Google Scholar]
- Becker C. Orang-Utan: Europäisches Erhaltungszuchtprogramm, Zuchtbuch für Europa. Zoo Karlsruhe; 2016. [Google Scholar]
- Boesch C. The emergence of cultures among wild chimpanzees. Proc. Br. Acad. 1996;88:251–268. [Google Scholar]
- Byrne R.W., Cartmill E., Genty E., Graham K.E., Hobaiter C., Tanner J. Great ape gestures: intentional communication with a rich set of innate signals. Anim. Cogn. 2017;20:755–769. doi: 10.1007/s10071-017-1096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call J., Tomasello M. Lawrence Erlbaum Associates; 2007. The Gestural Communication of Apes and Monkeys. [Google Scholar]
- Cartmill E., Byrne R. Semantics of primate gestures: intentional meanings of orangutan gestures. Anim. Cogn. 2010;13:793–804. doi: 10.1007/s10071-010-0328-7. [DOI] [PubMed] [Google Scholar]
- Cartmill E.A., Byrne R.W. Orangutans modify their gestural signaling according to their audience's comprehension. Curr. Biol. 2007;17:1345–1348. doi: 10.1016/j.cub.2007.06.069. [DOI] [PubMed] [Google Scholar]
- Cheney D.L., Seyfarth R.M. Flexible usage and social function in primate vocalizations. Proc. Natl. Acad. Sci. U S A. 2018;115:1974–1979. doi: 10.1073/pnas.1717572115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice L.R. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. doi: 10.2307/1932409. [DOI] [Google Scholar]
- Dobson A.J. Chapman & Hall/CRC; 2002. An Introduction to Generalized Linear Models. [Google Scholar]
- Eibl-Eibesfeldt I. Holt, Rinehart & Winston; 1975. Ethology, the Biology of Behavior. [Google Scholar]
- Fichtel C., Van Schaik C.P. Semantic differences in sifaka (Propithecus verreauxi) alarm calls: a reflection of genetic or cultural variants? Ethology. 2006;112:839–849. [Google Scholar]
- Field A. Sage publications; 2005. Discovering Statistics Using SPSS. [Google Scholar]
- Forstmeier W., Schielzeth H. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav. Ecol. Sociobiol. 2011;65:47–55. doi: 10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Weisberg S. 2 Edition. Sage; 2011. An R Companion to Applied Regression. [Google Scholar]
- Friard O., Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016;7:1325–1330. doi: 10.1111/2041-210x.12584. [DOI] [Google Scholar]
- Fröhlich M., Bartolotta N., Fryns C., Wagner C., Momon L., Jaffrezic M., Noordwijk M.A., van Schaik C.P. Multicomponent and multisensory communicative acts in orang-utans may serve different functions. Commun. Biol. 2021;4:917. doi: 10.1038/s42003-021-02429-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich M., Hobaiter C. The development of gestural communication in great apes. Behav. Ecol. Sociobiol. 2018;72:194. doi: 10.1007/s00265-018-2619-y. [DOI] [Google Scholar]
- Fröhlich M., Kunz J., Fryns C., Falkner S., Rukmana E., Schuppli M., Knief U., Utami Atmoko S.S., Schuppli C., van Noordwijk M.A. Social interactions and interaction partners in infant orang-utans of two wild populations. Anim. Behav. 2020;166:183–191. doi: 10.1016/j.anbehav.2020.06.008. [DOI] [Google Scholar]
- Fröhlich M., Lee K., Mitra Setia T., Schuppli C., van Schaik C.P. The loud scratch: a newly identified gesture of Sumatran orangutan mothers in the wild. Biol. Lett. 2019;15:20190209. doi: 10.1098/rsbl.2019.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich M., van Schaik C.P. The function of primate multimodal communication. Anim. Cogn. 2018;21:619–629. doi: 10.1007/s10071-018-1197-8. [DOI] [PubMed] [Google Scholar]
- Fröhlich M., van Schaik C.P. Must all signals be evolved? A proposal for a new classification of communicative acts. Wiley Interdiscip. Rev. Cogn. Sci. 2020;11:e1527. doi: 10.1002/wcs.1527. [DOI] [PubMed] [Google Scholar]
- Fröhlich M., Wittig R.M., Pika S. Should I stay or should I go? Initiation of joint travel in mother–infant dyads of two chimpanzee communities in the wild. Anim. Cogn. 2016;19:483–500. doi: 10.1007/s10071-015-0948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland E.C., Goldizen A.W., Rekdahl M.L., Constantine R., Garrigue C., Hauser N.D., Poole M.M., Robbins J., Noad M.J. Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 2011;21:687–691. doi: 10.1016/j.cub.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Gelman A., Hill J. Cambridge University Press; 2006. Data Analysis Using Regression and Multilevel/Hierarchical Models. [Google Scholar]
- Graham K.E., Furuichi T., Byrne R.W. Context, not sequence order, affects the meaning of bonobo (Pan paniscus) gestures. Gesture. 2020;19:336–365. [Google Scholar]
- Grant B.R., Grant P.R. Cultural inheritance of song and its role in the evolution of Darwin's finches. Evolution. 1996;50:2471–2487. doi: 10.1111/j.1558-5646.1996.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Halina M., Rossano F., Tomasello M. The ontogenetic ritualization of bonobo gestures. Anim. Cogn. 2013;16:653–666. doi: 10.1007/s10071-013-0601-7. [DOI] [PubMed] [Google Scholar]
- Hardus M., Lameira A.R., Singleton I., Morrogh-Bernard H.C., Knott C.D., Ancrenaz M., Utami Atmoko S., Wich S.A. In: Orangutans: Geographic Variation in Behavioral Ecology and Conservation. Wich S.A., Utami-Atmoko S.S., Mitra Setia T., van Schaik C.P., editors. Oxford University Press; 2009. A description of the orangutan's vocal and sound repertoire, with a focus on geographical variation; pp. 49–64. [Google Scholar]
- Hardus M.E., Lameira A.R., Van Schaik C.P., Wich S.A. Tool use in wild orang-utans modifies sound production: a functionally deceptive innovation? Proc. R. Soc. Lond. Ser. B: Biol. Sci. 2009;276:3689–3694. doi: 10.1098/rspb.2009.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde R.A. 2nd edition. McGraw-Hill; 1970. Animal Behavior: a Synthesis of Ethology and Comparative Psychology. [Google Scholar]
- Hobaiter C., Byrne R.W. The meanings of chimpanzee gestures. Curr. Biol. 2014;24:1596–1600. doi: 10.1016/j.cub.2014.05.066. [DOI] [PubMed] [Google Scholar]
- Hockett C.F. The origin of speech. Sci. Am. 1960;203:88–97. [PubMed] [Google Scholar]
- Hopkins W.D., Taglialatela J.D., Leavens D. Chimpanzees differentially produce novel vocalizations to capture the attention of a human. Anim. Behav. 2007;73:281–286. doi: 10.1016/j.anbehav.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson S.J., Wich S.A., Marshall A.J., Dennis R.D., Ancrenaz M., Brassey R., Gumal M., Hearn A.J., Meijaard E., Simorangkir T. In: Orangutans: Geographic Variation in Behavioral Ecology and Conservation. Wich S.A., Utami-Atmoko S.S., Mitra Setia T., van Schaik C.P., editors. Oxford University Press; 2009. Orangutan distribution, density, abundance and impacts of disturbance; pp. 77–96. [Google Scholar]
- Jantschke F. Piper; 1972. Orang-Utans in Zoologischen Gärten. [Google Scholar]
- Kersken V., Gómez J.-C., Liszkowski U., Soldati A., Hobaiter C. A gestural repertoire of 1- to 2-year-old human children: in search of the ape gestures. Anim. Cogn. 2018;22:577–595. doi: 10.1007/s10071-018-1213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox A., Markx J., How E., Azis A., Hobaiter C., van Veen F.J.F., Morrogh-Bernard H. Gesture use in communication between mothers and offspring in wild orang-utans (Pongo pygmaeus wurmbii) from the Sabangau Peat-Swamp Forest, Borneo. Int. J. Primatol. 2019;40:393–416. doi: 10.1007/s10764-019-00095-w. [DOI] [Google Scholar]
- Kopp K.S., Liebal K. Conflict resolution in socially housed Sumatran orangutans (Pongo abelii) PeerJ. 2018;6:e5303. doi: 10.7717/peerj.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer H., Goodall J. Conditions of innovative behaviour in primates. Philos. Trans. R. Soc. B. 1985;308:203–214. doi: 10.1098/rstb.1985.0020. [DOI] [Google Scholar]
- Kummer H., Kurt F. In: The Baboon in Medical Research. Vagtborg H., editor. University of Texas Press; 1965. A comparison of social behavior in captive and wild hamadryas baboons; pp. 1–46. [Google Scholar]
- Leavens D.A., Hopkins W.D. Intentional communication by chimpanzees: a cross-sectional study of the use of referential gestures. Dev. Psych. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens D.A., Russell J., Hopkins W. Multimodal communication by captive chimpanzees (Pan troglodytes) Anim. Cogn. 2010;13:33–40. doi: 10.1007/s10071-009-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner S.R., Burkart J.M., van Schaik C.P. An evaluation of the geographic method for recognizing innovations in nature, using zoo orangutans. Primates. 2010;51:101–118. doi: 10.1007/s10329-009-0184-8. [DOI] [PubMed] [Google Scholar]
- Liebal K., Pika S., Tomasello M. Gestural communication of orangutans (Pongo pygmaeus) Gesture. 2006;6:1–38. doi: 10.1075/gest.6.1.02lie. [DOI] [Google Scholar]
- Liebal K., Waller B.M., Burrows A.M., Slocombe K.E. Cambridge University Press; 2013. Primate Communication: A Multimodal Approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon J. The behaviour and ecology of wild orang-utans (Pongo pygmaeus) Anim. Behav. 1974;22:3–74. doi: 10.1016/s0003-3472(74)80054-0. [DOI] [Google Scholar]
- Maestripieri D. In: The Origins of Language: What Nonhuman Primates Can Tell. King B.J., editor. School of American Research Press; 1999. Primate social organization, gestural repertoire size, and communication dynamics; pp. 55–77. [Google Scholar]
- Manrique H.M., Völter C.J., Call J. Repeated innovation in great apes. Anim. Behav. 2013;85:195–202. [Google Scholar]
- Maple T.L. Van Nostrand Reinhold; 1980. Orangutan Behavior. [Google Scholar]
- Marler P. In: Primate Behaviour. Field Studies of Monkeys and Apes. DeVore I., editor. Holt, Rinehart and Winston; 1965. Communication in monkeys and apes; pp. 544–584. [Google Scholar]
- Marshall A.J., Wrangham R.W., Arcadi A.C. Does learning affect the structure of vocalizations in chimpanzees? Anim. Behav. 1999;58:825–830. doi: 10.1006/anbe.1999.1219. [DOI] [PubMed] [Google Scholar]
- Mitra Setia T., Delgado R., Utami Atmoko S., Singleton I., van Schaik C.P. In: Orangutans: Geographic Variation in Behavioral Ecology and Conservation. Wich S.A., Utami Atmoko S., Mitra Setia T., van Schaik C.P., editors. Oxford University Press; 2009. Social organization and male-female relationships; pp. 245–253. [Google Scholar]
- Moura A.C.d.A. Stone banging by wild capuchin monkeys: an unusual auditory display. Folia Primatol. 2007;78:36–45. doi: 10.1159/000095684. [DOI] [PubMed] [Google Scholar]
- Mundry R. In: Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology. Garamszegi L., editor. Springer; 2014. Statistical issues and assumptions of phylogenetic generalized least squares (PGLS) pp. 131–153. [Google Scholar]
- Perkins L.A. Variables that influence the activity of captive orangutans. Zoo Biol. 1992;11:177–186. [Google Scholar]
- Perry S., Panger M., Rose L.M., Baker M., Gros-Louis J., Jack K., MacKinnon K.C., Manson J., Fedigan L., Pyle K. In: The Biology of Traditions: Models and Evidence. Fragaszy D.M., Perry S., editors. Cambridge University Press; 2003. Traditions in wild white-faced capuchin monkeys; pp. 391–425. [Google Scholar]
- Pinheiro J.C., Bates D.M. Mixed-Effects Models in S and S-Plus; 2000. Linear mixed-effects models: basic concepts and examples; pp. 3–56. [Google Scholar]
- Quinn G.P., Keough M.J. Cambridge University Press; 2002. Experimental Design and Data Analysis for Biologists. [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Ramsey G., Bastian M.L., Van Schaik C. Animal innovation defined and operationalized. Behav. Brain Sci. 2007;30:393–407. doi: 10.1017/S0140525X07002373. [DOI] [PubMed] [Google Scholar]
- Reader S.M., Laland K.N. Oxford University Press; 2003. Animal Innovation. [DOI] [Google Scholar]
- Rijksen H.D. H. Veenman; 1978. A fieldstudy on Sumatran orang utans (Pongo pygmaeus abelii, Lesson 1827): ecology, behaviour and conservation. [Google Scholar]
- Salmi R., Muñoz M. The context of non-vocal sounds in wild western gorillas (Gorilla gorilla gorilla): chest beating and hand clapping. Primates. 2020;82:1–11. doi: 10.1007/s10329-019-00782-5. [DOI] [PubMed] [Google Scholar]
- Schielzeth H., Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 2009;20:416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton I., Knott C., Morrogh-Bernard H., Wich S., van Schaik C. In: Orangutans: Geographic Variation in Behavioral Ecology and Conservation. Wich S.A., Utami-Atmoko S.S., Mitra Setia T., van Schaik C.P., editors. Oxford University Press; 2009. Ranging behavior of orangutan females and social organization; pp. 205–212. [Google Scholar]
- Snowdon C.T., Hausberger M., editors. Social Influences on Vocal Development. Cambridge University Press; 1997. [Google Scholar]
- Taglialatela J.P., Reamer L., Schapiro S.J., Hopkins W.D. Social learning of a communicative signal in captive chimpanzees. Biol. Lett. 2012;8:498–501. doi: 10.1098/rsbl.2012.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennie C., Bandini E., Van Schaik C.P., Hopper L.M. The zone of latent solutions and its relevance to understanding ape cultures. Biol. Philos. 2020;35:1–42. doi: 10.1007/s10539-020-09769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M., Call J., Nagell K., Olguin R., Carpenter M. The learning and use of gestural signals by young chimpanzees: a trans-generational study. Primates. 1994;35:137–154. doi: 10.1007/bf02382050. [DOI] [Google Scholar]
- Tomasello M., Call J., Warren J., Frost G.T., Carpenter M., Nagell K. The ontogeny of chimpanzee gestural signals: a comparison across groups and generations. Evol. Commun. 1997;1:223–259. doi: 10.1075/eoc.1.2.04tom. [DOI] [Google Scholar]
- van Schaik C.P. The socioecology of fission-fusion sociality in orangutans. Primates. 1999;40:69–86. doi: 10.1007/BF02557703. [DOI] [PubMed] [Google Scholar]
- van Schaik C.P. John Wiley & Sons; 2016. The Primate Origins of Human Nature. [Google Scholar]
- van Schaik C.P., van Noordwijk M.A., Wich S.A. Innovation in wild Bornean orangutans (Pongo pygmaeus wurmbii) Behaviour. 2006;143:839–876. [Google Scholar]
- Wegdell F., Hammerschmidt K., Fischer J. Conserved alarm calls but rapid auditory learning in monkey responses to novel flying objects. Nat. Ecol. Evol. 2019;3:1039–1042. doi: 10.1038/s41559-019-0903-5. [DOI] [PubMed] [Google Scholar]
- Wheeler B.C., Fischer J. Functionally referential signals: a promising paradigm whose time has passed. Evol. Anthropol. 2012;21:195–205. doi: 10.1002/evan.21319. [DOI] [PubMed] [Google Scholar]
- Wich S.A., Krützen M., Lameira A.R., Nater A., Arora N., Bastian M.L., Meulman E., Morrogh-Bernard H.C., Utami Atmoko S.S., Pamungkas J. Call cultures in orang-utans? PLoS One. 2012;7:e36180. doi: 10.1371/journal.pone.0036180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wich S.A., Swartz K.B., Hardus M.E., Lameira A.R., Stromberg E., Shumaker R.W. A case of spontaneous acquisition of a human sound by an orangutan. Primates. 2009;50:56–64. doi: 10.1007/s10329-008-0117-y. [DOI] [PubMed] [Google Scholar]
- Wilson S.F. Environmental influences on the activity of captive apes. Zoo Biol. 1982;1:201–209. doi: 10.1002/zoo.1430010304. [DOI] [Google Scholar]
- Zucker E.L., Mitchell G., Maple T. Adult male-offspring play interactions within a captive group of orang-utans (Pongo pygmaeus) Primates. 1978;19:379–384. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
9
Data Availability Statement
-
•
Data have been deposited at Zenodo and is publicly available as of the date of publication. DOI is listed in the key resources table.
-
•
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOI is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request