Highlights
-
•
Use of neoadjuvant chemotherapy is increasingly common among women with advanced stage endometrial cancer.
-
•
Compared to primary cytoreduction, neoadjuvant chemotherapy is associated with improved rates of optimal cytoreduction.
-
•
Neoadjuvant chemotherapy is associated with decreased perioperative morbidity and decreased operative times.
-
•
Neoadjuvant chemotherapy followed by surgery is associated with similar overall survival compared to primary surgery.
Keywords: Endometrial cancer, Uterine cancer, Hysterectomy, Neoadjuvant, Cytoreduction, Debulking
Abstract
Objective
While primary cytoreductive surgery (PCS) is considered the standard of care for women who present with stage IV endometrial cancer, neoadjuvant chemotherapy (NACT) followed by interval cytoreductive surgery (ICS) has emerged as an alternative treatment strategy. We summarized the literature and compared outcomes of PCS compared to NACT and ICS.
Methods
We conducted a systematic search on PubMed, Embase, Web of Science, and Scopus for articles published from January 1, 1990 to December 31, 2020. Key search terms included multiple descriptors of advanced disease status in combination with “endometrial cancer” and “neoadjuvant chemotherapy”. Our review included studies that examined survival and surgical outcomes of patients with stage III or IV endometrial cancer treated with neoadjuvant chemotherapy followed by interval cytoreductive surgery versus those who received primary cytoreductive surgery. We excluded studies examining only patients with leiomyosarcomas, carcinosarcomas, and stromal sarcomas due to the biologic heterogeneity of these malignancies.
Results
The nine included studies encompassed 5,844 patients, of which 1,317 (22.5%) received NACT and 4,527 received PCS (77.5%). With the exception of a single study, all were retrospective observational studies or case series. Use of NACT in patients with stage IV EC increased from 16.0% in 2010 to 23.9% in 2015. Five studies analyzed median overall survival and all but one reported no significant difference between NACT + ICS vs. PCS. Optimal cytoreduction (<1 cm of residual disease) rates were similar across both treatment groups in three separate analyses, however pooled data suggest improved rates of optimal cytoreduction for NACT + ICS vs. PCS patients (81.9% vs. 51.5% respectively). Patients receiving NACT experienced significantly shorter hospital admissions and lower operative times compared to PCS counterparts.
Conclusions
NACT followed by ICS reduces perioperative morbidity while offering similar overall survival.
1. Introduction
Endometrial cancer is the most common gynecologic cancer in developed nations and the fourth most common cancer in women (Lee et al., 2017, Rabinovich, 2016). Over 70% of endometrial cancer patients present with stage I disease which is associated with a greater than 90% five year survival rate (Galaal et al., 2016). Mortality markedly increases with advanced stages of disease; women with stages III and IV endometrial cancer experience five year survival rates of 56% and 17%, respectively (Siegel et al., 2019). Women with advanced endometrial cancer represent only 10–15% of all endometrial cancer cases, yet account for over half of all endometrial cancer deaths (Rabinovich, 2016, Palisoul and Mutch, 2016). Patients with inoperable endometrial cancer experience a median survival of only 2–8 months (Palisoul and Mutch, 2016).
Primary cytoreduction, followed by adjuvant chemotherapy and/or radiotherapy, represents the current mainstay of treatment for advanced stage endometrial cancer (Wright et al., 2012). Cytoreduction has emerged as the most important component of treatment (Burke et al., 2014). In multiple analyses, patients with stage III or IV endometrial cancer who underwent optimal cytoreduction to ≤1 cm of residual disease experienced an overall survival benefit compared to women left with bulky residual disease after surgery (Bristow et al., 2001, Shih et al., 2011, LAMBROU et al., 2004). Despite the survival benefit associated with optimal surgical cytoreduction, the procedure is associated with significant morbidity (Rabinovich, 2016). Over the last two decades the use of cytotoxic chemotherapy has progressively expanded (Cain et al., 2017, Deng et al., 2017, Wright et al., 2016, Blumenthal et al., 2018, Grossman et al., 2003, Dehal et al., 2018). However, to date, there have been few reports of the use of primary, or neoadjuvant chemotherapy (NACT), for women with stage IV endometrial cancer (Tobias et al., 2020).
Over the same time period, use of NACT followed by interval debulking surgery has become an accepted treatment modality for advanced ovarian cancer (Wright et al., 2016). Data from four randomized controlled trials demonstrated the non-inferiority of NACT followed by interval cytoreduction compared to primary cytoreduction for stage IIIC and IV ovarian cancer (Onda et al., 2008, Vergote et al., 2010, Kehoe et al., 2015, Fagotti et al., 2016).
In light of these findings and the similarities in presentation between advanced ovarian and endometrial cancer, a re-evaluation of NACT for endometrial cancer is warranted. We performed a systematic review to examine the use and outcomes associated with NACT for metastatic endometrial cancer. Specifically, we examined surgical outcomes, use of chemotherapy, and survival to inform management of endometrial cancer patients who are precluded from primary cytoreduction.
2. Methods
2.1. Search strategy and information sources
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines (Liberati et al., 2009). A systematic search for articles published from January 1, 1990 to December 31, 2020 was performed in PubMed, Embase, Web of Science, and Scopus. Key search terms included descriptors of advanced disease status (“advanced stage”, “unresectable”, “metastatic”, “stage III”, “stage IV” and others) in combination with “endometrial cancer” and “neoadjuvant chemotherapy” (Supplemental Table 1). Where applicable, we searched using Medical Subject Heading (MeSH) terms “Neoadjuvant therapy”, “Endometrial neoplasms”, and “Uterine neoplasms” or other related exploding search terms. All literature searches were executed between March 3, 2020 and March 11, 2020.
2.2. Eligibility criteria
We sought to identify all reports that examined survival and surgical outcomes of patients with advanced endometrial cancer treated with neoadjuvant chemotherapy followed by interval cytoreductive surgery versus those who received primary cytoreductive surgery. Inclusion criteria were as follows: (1) patients with international FIGO stage III or IV endometrial cancer, (2) patients with endometrioid, clear cell, serous, and mixed histologic subtypes, (3) reporting of relevant survival outcomes, including overall survival, and (4) English language reports. We did not formulate selection criteria based on neoadjuvant chemotherapy regimens or cycle numbers as these parameters vary widely across practices. Reviews, case reports, and letters were excluded for systematic review. We excluded studies examining only patients with uterine leiomyosarcomas, carcinosarcomas, and stromal sarcomas due to the biological heterogeneity found in these malignancies.
2.3. Study selection and data extraction
All studies were screened for relevance by title and abstract by two independent reviewers (ABH and JW). Any disparities in opinion were resolved after both reviewers conferred and achieved consensus. The full texts of relevant studies were further screened by two independent reviewers (ABH and JW) according to the inclusion and exclusion criteria. Disparities in opinion were once again resolved after conference and consensus to produce a final group of included studies. The following data and descriptors were extracted from included studies: publication year, years of analysis, study type, endometrial cancer FIGO stages included, number of patients, histology, response to NACT, extent of debulking, progression to IDS or adjuvant chemotherapy, median overall survival (OS), and any other relevant additional findings.
2.4. Quality assessment
The NIH Study Quality Assessment Tools were used to evaluate each included study for the quality and risk of bias due to study design flaws or implementation (Study Quality Assessment Tools, 2020). Two independent reviewers (ABH and JW) scored each study according to NIH Study Quality Assessment Tool criteria and rated study quality as “good” (valid results with least risk of bias), “fair” (not enough bias to invalidate results), and “poor” (significant risk of bias). Disparities in ratings were resolved through discussion between the two reviewers.
2.5. Data synthesis
After literature search and review it was noted that there was significant heterogeneity among the identified studies. The decision was therefore made to review the studies descriptively rather than to perform a formal meta-analysis.
3. Results
3.1. Study selection
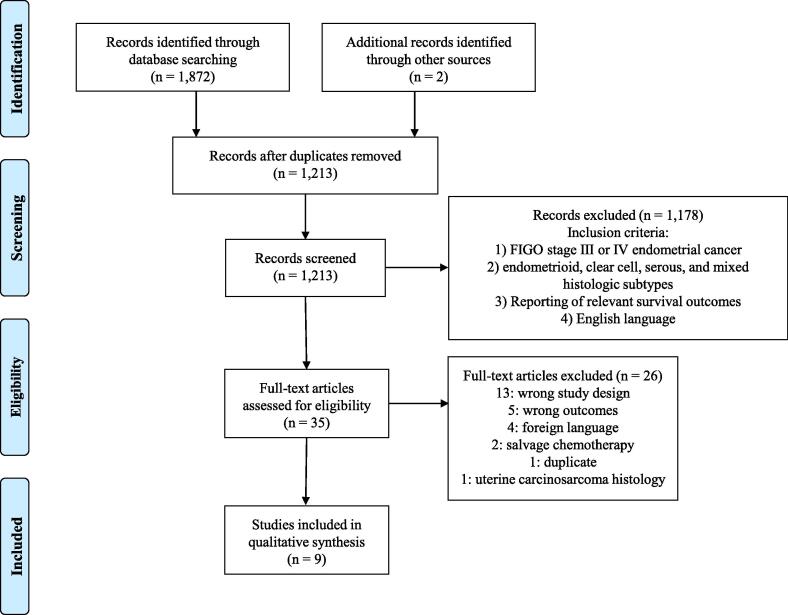
After systematic literature search, 1,213 unique articles were identified, of which 1,178 irrelevant articles were excluded during title and abstract screening. Of the thirty-five articles that underwent full-text screening, nine were selected for patient populations, study design, and outcomes appropriate to this systematic review. Twenty-six studies were excluded: thirteen for irrelevant study designs (review article, case report, or letters), five for lack of survival outcomes, four for non-English language formats, two for patients receiving salvage chemotherapy rather than NACT, one duplicate study, and one for including only patients with uterine carcinosarcomas (Fig. 1).
Fig. 1.
PRISMA diagram of included studies.
3.2. Study characteristics
The nine included studies encompassed 5,844 patients, of whom 1,317 received NACT and 4,527 received PDS (Table 1) (Tobias et al., 2020, Bogani et al., 2019, de Lange et al., 2019, Eto et al., 2013, Holman et al., 2017, Khouri et al., 2019, Rajkumar et al., 2019, Vandenput et al., 2009, Wilkinson-Ryan et al., 2015). Tobias et al. study demonstrated that use of NACT in patients with stage IV EC increased from 16.0% in 2010 to 23.9% in 2015. This study accounted for the largest sample size, including 4,890 patients (Tobias et al., 2020). With the exception of the study by Vandenput et al., all included studies were retrospective observational studies or case series. No randomized controlled trials examining NACT in advanced endometrial cancer were found. In the only prospective study included, Vandenput et al. enrolled thirty patients to receive three to four cycles of NACT following laparoscopic confirmation of intraabdominal spread of disease (stage IV). (Vandenput et al., 2009)
Table 1.
Summary of studies examining neoadjuvant chemotherapy in advanced endometrial cancer.
| Reference | Study Design | Years Analyzed | Stages Included | NACT Regimen* | NACT | Histologies NACT | PDS | Histologies PDS |
|---|---|---|---|---|---|---|---|---|
| n | n (%) | n | n (%) | |||||
| Bogani et al., 2019 | Retrospective cohort, propensity-matched | 2005–2016 | IVb | 3-6 cycles paclitaxel/carboplatin or cisplatin ± doxorubicin | 15 | 15 (100) serous | 15 | 15 (100) serous |
| de Lange et al., 2019 | Retrospective case-series | 2005–2014 | III, IV | 1-6 cycles paclitaxel/carboplatin | 102 | 44 (43) endometrioid 44 (43) serous 4 (4) clear cell 10 (10) other or mixed |
– | – |
| Eto et al., 2013 | Retrospective cohort | 1996–2005 | IVb | 63% taxane + platinum 30% doxorubicin + platinum ± other 7% other Cycle numbers not reported |
125 | 68 (54) endometrioid 57 (46) non-endometrioid |
279 | 163 (58) endometrioid 116 (42) non-endometrioid |
| Holman et al., 2017 | Retrospective cohort | 1993–2012 | III, IV | NACT details not reported | 27 | 19 (70) serous 8 (30) mixed |
233 | 79 (34) serous 154 (66) mixed |
| Khouri et al., 2019 | Retrospective case-series | 2002–2016 | NR | 85% paclitaxel/carboplatin 10% carboplatin only 5% paclitaxel only Cycle numbers not reported |
39 | 11 (28) endometrioid 17 (44) serous 3 (8) clear cell 4 (10) carcinosarcoma 1 (3) neuroendocrine 3 (8) mixed |
– | – |
| Rajkumar et al., 2019 | Retrospective cohort | 2010–2016 | IIIC, IV | 3-6 cycles taxol/carboplatin or cisplatin/doxorubicin | 17 | NR | 28 | NR |
| Tobias et al., 2020 | Retrospective cohort | 2010–2015 | IV | NR | 952 | 223 (23) endometrioid 249 (26) serous 34 (4) clear cell 115 (12) carcinosarcoma 90 (9) sarcoma 188 (20) EM NOS 53 (6) other |
3938 | 1114 (28) endometrioid 828 (21) serous 117 (3) clear cell 612 (16) carcinosarcoma 554 (14) sarcoma 614 (16) EM NOS 99 (3) Other |
| Vandenput et al., 2009 | Prospective cohort | 1999–2007 | IV | 3-4 cycles paclitaxel/carboplatin or doxorubicin/cisplatin | 30 | 2 (7) endometrioid 27 (90) serous 1 (3) clear cell |
– | – |
| Wilkinson-Ryan et al., 2015 | Retrospective cohort | 2000–2013 | IV | 3-8 cycles taxane/platinum | 10 | 10 (100) serous or mixed histology with >50% serous component | 34 | 34 (100) serous or mixed histology with >50% serous component |
NACT = neoadjuvant chemotherapy, IDS = interval debulking surgery, PD = primary debulking surgery.
EM NOS = endometrioid not otherwise specified.
NR = not reported.
Summaries of the most common NACT regimens are listed, as studies reported highly variable NACT regimens.
Nearly all of the studies examined only patients with FIGO stage III or IV EC. The majority of NACT patients received three to six cycles (range: 1–8) of a taxane and platinum agent, although NACT regimens were highly variable between and within studies (Table 1). Three of the nine studies exclusively examined patients with serous carcinomas. Six of the studies included a comparator PDS arm, while the remaining three were case-series lacking a PCS arm. Bogani et al. and Tobias et al. selected a comparator arm via propensity score adjustment to account for allocation bias (Tobias et al., 2020, Bogani et al., 2019).
3.3. Quality assessment
Quality assessment and critical appraisal of included studies was conducted according to the NIH Study Quality Assessment Tools (Appendix 1). Separate assessment criteria were applied according to study design. Five studies were judged to be at low risk of bias, three studies at some risk of bias, and one study at high risk of bias. Major sources of bias include widely varying NACT regimens across studies (inconsistently implemented exposure measure) and lack of sample size or power description among studies.
3.4. Chemotherapeutic and surgical outcomes
Major chemotherapeutic and surgical outcomes of interest included response to NACT, performance of ICS, and extent of cytoreductive surgery are summarized in Table 2. Five studies reported a total of 257 patient responses to NACT, of which 178 (69%) experienced at least a partial response, 25 (10%) experienced stable disease, and 46 (18%) experienced progression of disease. Notably, Eto et al. reported NACT responses only for those patients that underwent ICS. Holman et al. found that patients receiving NACT experienced a significantly lower complete response rate than those receiving primary surgery with adjuvant chemotherapy and/or radiotherapy, which likely reflects selection bias inherent in nonrandomized, retrospective observational studies. (Holman et al., 2017) Wilkinson-Ryan et al. examined changes in CA-125 as proxies for responses to therapy, and observed no significant difference in treatment response between NACT and PDS cohorts at the conclusion of therapy (mean CA-125 decline of 80% and 92.5% respectively, P = 0.43). (Wilkinson-Ryan et al., 2015)
Table 2.
Chemotherapeutic and surgical outcomes for included studies.
| NACT | PDS | Response to NACT | NACT patients receiving IDS | Extent of Debulking Surgery |
Additional Findings | ||
|---|---|---|---|---|---|---|---|
| n | n | n (%) | n (%) | NACT+IDS, n(%) | PDS, n(%) | ||
| Bogani et al., 2019 | 15 | 15 | NR | 15 (100) | 14 (93) complete 1 (7) optimal |
13 (87) complete 2 (13) optimal |
p = 0.96 for debulking outcomes • NACT arm with significantly shorter hospital stays (4 vs. 6d, p = 0.011) • NACT arm trend towards shorter op. time (127 vs. 177.6, p = 0.072) and trend towards lower transfusion rates (6.6% vs. 33.3%, p = 0.067) |
| de Lange et al., 2019 | 102 | – | 4 (4) CR 73 (72) PR 9 (9) SD 11 (11) PD 2 (2) died during treatment 3 (3) no imaging available |
80 (78) | 48 (60) complete 23 (29) optimal 9 (11) suboptimal |
– | |
| Eto et al., 2013 | 125 | 279 | 40 (68) CR/PR 9 (15) SD 7 (12) PD 3 (5) not evaluable •responses to NACT reported only for patients who received IDS |
59 (47) | 19 (32) complete 15 (25) optimal 25 (43) suboptimal |
61 (22) complete 65 (23) optimal 153 (55) suboptimal |
p = 0.087 for optimal cytoreduction |
| Holman et al., 2017 | 27 | 233 | 8 (30) CR 9 (33) PR/SD 10 (37) PD |
17 (63) | NR | NR | |
| Khouri et al., 2019 | 39 | – | 22 (56) PR 1 (3) SD 16 (41) PD |
16 (41) | 13 (81) complete/optimal 3 (19) suboptimal |
– | |
| Rajkumar et al., 2019 | 17 | 28 | NR | 17 (100) | NR | NR | |
| Tobias et al., 2020 | 952 | 3938 | NR | 555 (58) | NR | NR | |
| Vandenput et al., 2009 | 30 | – | 2 (7) CR 20 (67) PR 6 (20) SD 2 (7) PD |
24 (80) | 22 (92) complete 2 (8) optimal |
– | |
| Wilkinson-Ryan et al., 2015 | 10 | 34 | Average decline in CA 125 post-NACT = 91 ± 11% | 10 (100) | 7 (70) complete 3 (30) optimal |
11 (32) complete 17 (50) optimal 6 (18) suboptimal |
p = 0.10 for debulking outcomes •NACT arm with significantly shorter mean operative time (136.9 vs. 202.6 min., p = 0.025) and length of hospital stay (3 vs. 5 d., p = 0.0021) •NACT arm with trend towards less blood loss (410ml vs. 781.8ml, p = 0.063) |
CR = complete response, PR = partial response, SD = stable disease, PD = progressive disease, NR = not reported.
All nine studies reported the proportion of NACT patients receiving ICS (Table 2). In the studies by Bogani et al., Rajkumar et al., and Wilkinson-Ryan et al., receipt of IDS was an inclusion criterion for patients in the NACT arm. Excluding patients from the aforementioned studies, 58.9% of NACT patients overall received ICS, driven largely by the sample size of Tobias et al. The most commonly reported contraindications to ICS included disease progression and/or surgically unresectable tumor burden, although reasons for exclusion from IDS were infrequently reported. (de Lange et al., 2019, Khouri et al., 2019, Vandenput et al., 2009)
The extent of cytoreductive surgery was classified as complete (no gross residual disease), optimal (≤1cm residual disease), and suboptimal (greater than1cm residual disease). Six studies reported surgical outcomes for patients receiving NACT followed by ICS, encompassing 204 patients in total. Of these patients, 167 (81.9%) received complete/optimal cytoreduction and 37 (18.1%) received suboptimal cytoreduction. Only three studies compared surgical outcomes between NACT with ICS and PCS arms, encompassing 328 PCS patients in total. Of these PCS patients, 169 (51.5%) received complete/optimal cytoreduction and 159 (48.5%) received suboptimal cytoreduction.
Vandenput et al. reported the highest rate of complete cytoreduction (92%) among patients receiving NACT with ICS. Cytoreduction rates were similar between NACT + ICS and PCS patients in the analysis by Bogani et al. (P = 0.96). Eto et al. compared rates of optimal/complete cytoreduction between NACT with ICS and PCS arms and found no significant difference (57% vs. 45%, P = 0.087). Wilkinson-Ryan et al. similarly concluded that rates of complete (70% NACT + ICS vs. 32% PCS) and optimal (30% NACT with ICS vs. 50% PCS) cytoreduction were similar between treatment groups (P = 0.10).
Patients who received NACT experienced significantly shorter hospital admissions compared to PCS counterparts in the studies of Bogani et al. (4 vs. 6 days, P = 0.011) and Wilkinson-Ryan et al. (3 vs. 5 days, P = 0.0021). Wilkinson-Ryan et al. additionally reported significantly lower operative times in patients who received NACT with ICS compared to those who underwent PCS (136.9 vs. 202.6 min, P = 0.025), Bogani et al. noted similar trends (127 vs. 177.6 min, P = 0.072).
3.5. Survival outcomes
Median overall survival (OS) among patients receiving NACT ranged from 12 months (Eto et al.) to 27 months (de Lange et al.) (Table 3) (de Lange et al., 2019, Eto et al., 2013). Four studies analyzed differences in median OS between patients who received NACT + ICS vs. PCS and all reported no significant difference in survival across treatment groups. Eto et al. reported equivalent median OS in patients receiving NACT with ICS vs. PCS (21 months for both, P = 0.84). Bogani et al., Rajkumar et al., and Wilkinson-Ryan et al. reported similar findings in their comparisons of NACT + ICS and PCS patients.
Table 3.
Survival outcomes and additional findings of included studies.
| Median OS (months) |
|||||
|---|---|---|---|---|---|
| Reference | NACT Overall | NACT + IDS subgroup | PDS | Additional findings | |
| Bogani et al., 2019 | – | 16.7 | 18 | p = 0.35 | |
| de Lange et al., 2019 | 27 | NR | – | •Subgroup analysis of median OS (months) by extent of debulking Complete/optimal = 41 Suboptimal = 16 No surgery = 13 |
|
| Eto et al., 2013 | 12 | 21 | 21 | p = 0.84 for NACT+IDS vs. PDS | • Median OS for NACT patients who did not receive IDS = 7 months |
| Holman et al., 2017 | 20.4 | NR | – | • Subgroup analysis of median OS (months) by treatment modalities PDS + adj. CT = 30.8 PDS + adj. RT = 38.3 PDS + adj. CT + adj. RT = 48.8 |
|
| Khouri et al., 2019 | NR | 16 | – | • Improved median OS associated with IDS vs. no IDS (16 months vs. 6 months, p = 0.037) • Improved median OS associated with partial response to NACT vs. no response (15 months vs. 5 months, p = 0.015) |
|
| Rajkumar et al., 2019 | – | 29 | 22.5 | p = 0.57, HR=1.26, 95%CI (0.56-2.85) | • Subgroup analysis of median OS (months) by extent of debulking Complete/optimal = 29 Suboptimal = 17.5 |
| Tobias et al., 2020 | 19 (95% CI: 17-21) |
NR | 25 (95% CI: 24-27) |
• Survival curves of overall NACT and overall PDS arms cross after 3 months • Survival curves of NACT+IDS and PDS+adj. CT arms cross after 8 months |
|
| Vandenput et al., 2009 | 23 | NR | – | • Median OS for NACT patients who did not undergo IDS (n=6) = 12 months | |
| Wilkinson-Ryan et al., 2015 | – | 17.3 | 20.7 | p = 0.23 | |
NACT = neoadjuvant chemotherapy, PDS = primary debulking surgery, adj. CT = adjuvant chemotherapy, adj. RT = adjuvant radiotherapy, NR = not reported.
Only two studies conducted “intention to treat” analyses comparing the survival of all patients who received NACT, regardless of receipt of ICS, to all patients who received PCS. Eto et al. reported a median OS of 12 and 21 months for the overall NACT and NACT with ICS cohorts, respectively. Tobias et al. reported a median survival of 19 months (95% CI: 17–21 months) for the overall NACT group and 25 months (95% CI: 24–27 months) for the PCS group. Tobias et al. additionally performed dual analyses, with an intention to treat (overall NACT vs. overall PCS) and a “per protocol” analysis (NACT with ICS vs. PCS with postoperative chemotherapy). Both analyses revealed a time-varying relationship between NACT and survival whereby NACT patients experienced superior survival in the first months of follow-up, after which time the survival curves of NACT and PCS patients crossed after 3 months and 8 months in the intention to treat and per protocol analyses, respectively, and after which time survival was superior for PCS.
Several studies conducted novel subgroup analyses, highlighted in Table 3. Both de Lange et al. and Rajkumar et al. demonstrated increasing survival in women who received more extensive cytoreductive surgeries (de Lange et al., 2019, Rajkumar et al., 2019). De Lange et al. reported median OS of 41, 16 and 13 months for women who received complete/optimal, suboptimal, and no cytoreductive surgery respectively. Receipt of ICS improved median OS from 7 months for women receiving NACT without ICS to 21 months for those receiving NACT with ICS in the analysis by Eto et al.
4. Discussion
These data suggest that use of a strategy of neoadjuvant chemotherapy with interval cytoreductive surgery is increasingly common among women with advanced stage endometrial cancer. Compared to primary cytoreduction, NACT with ICS appears to be associated with improved rates of optimal cytoreduction, decreased perioperative morbidity, and decreased operative times. While the relationships between NACT and PCS are complex, patients undergoing NACT followed by ICS experience similar overall survival compared to those receiving PCS.
Neoadjuvant therapy has been employed successfully in a spectrum of solid tumors, often as a technique for reducing surgical morbidity and improving the extent of cytoreduction, or as a method of downstaging advanced disease. In a large retrospective study of patients in the National Cancer Database (NCDB), Dehal et al. demonstrated improved 3-year survival for patients with T4b colon cancer (invasion of extra-colonic organs), but no benefit for patients with T3 or T4a disease (Dehal et al., 2018). A RCT comparing NACT vs. PCS in 317 patients with muscle-invasive bladder cancer found a 33% mortality reduction in the NACT arm, attributed largely to the ability of NACT to downstage disease (Grossman et al., 2003). In a meta-analysis of seven RCTs by Fu et al., patients with esophageal adenocarcinoma experienced improved overall survival (HR 0.74; 95% CI, 0.63–0.88) with neoadjuvant chemoradiotherapy (Fu et al., 2015). In the setting of breast cancer, NACT is most commonly used in early, rather than advanced, disease, allowing for the possibility of breast-conserving surgery (Cain et al., 2017). Collectively, these studies highlight the importance of careful patient selection in optimizing survival benefits from NACT. Despite limited data, it appears that use of NACT is increasing for women with stage IV endometrial cancer (Tobias et al., 2020).
Gynecologic oncologists have increasingly employed NACT followed by ICS in advanced ovarian cancer. Pooled analysis of the EORTC 55,971 and CHORUS trials demonstrated similar overall survival in women with stages IIIA-IV ovarian cancer treated with NACT vs. PCS. (27.6 vs. 26.9 months, respectively) (Vergote et al., 2010, Vergote et al., 2018). The SCORPION and JCOG0602 trials further demonstrated decreased perioperative morbidity, operative times, and length of hospital stay with NACT + ICS (Fagotti et al., 2016, Onda et al., 2016). Given these findings, the Society of Gynecologic Oncology and the American Society of Clinical Oncology endorse NACT for patients with advanced ovarian cancer who are deemed at high risk of perioperative morbidity or have low probability of cytoreduction to <1 cm of residual disease with PCS (Wright et al., 2016). Endometrial cancer resembles ovarian cancer in its propensity for peritoneal spread, and has therefore drawn focused interest as a disease site candidate for NACT (Bogani et al., 2019, Holman et al., 2017, Wilkinson-Ryan et al., 2015). Given the poor overall outcomes of women with metastatic endometrial it is unclear why NACT has not received more attention in this setting. Further, many women with endometrial cancer are elderly with significant comorbidities, a population that may derive the most benefit from decreased perioperative morbidity.
Our findings suggest that NACT followed by ICS reduces perioperative morbidity while offering similar overall survival. The findings of the large analysis by Tobias et al. are intriguing as survival was initially superior for patients who received NACT but among patients who survived longer, overall survival was more favorable for those who underwent PCS. These findings indicate that PCS may be associated with significant perioperative morbidity and mortality or delay in receipt of chemotherapy. However, for those patients who can undergo primary cytoreduction without undue morbidity and go on to receive chemotherapy, long term outcomes may be more favorable. NACT may also be useful in identifying chemotherapy resistant disease, allowing early optimization of end-of-life care. Developing algorithms or clinical trials to differentiate patients who will benefit most from NACT vs. PCS will be critical in supporting clinical decision-making for advanced EC.
We acknowledge several important limitations in this systematic review. The majority of studies included were retrospective studies with relatively small cohorts. No randomized controlled trials have been performed examining NACT in EC, thus limiting the strength of the clinical recommendations made on the basis of existing evidence. Second, conclusions about survival drawn from this review are skewed by the large sample size of Tobias et al., which accounts for 72% of NACT patients and 87% of PCS patients included in our pooled cohort. However, surgical outcomes, perioperative morbidity, and chemotherapeutic outcomes were unaffected by Tobias et al., which focused only on time-varying survival outcomes. Third, NACT regimens varied widely between studies in terms of agents used and number of cycles administered, which prevents our study from commenting on the efficacy of different regimens. Similarly, included studies examined outcomes across many histologic subtypes of EC. There are undoubtedly genetic differences that carry implications for treatment across EC subtypes, thus this review can only offer insight into NACT for EC patients as a whole (Chang et al., 2019).
Our review suggests that NACT followed by ICS represents a viable alternative to PCS in select patients with advanced EC. Those patients who receive ICS experience improved cytoreduction and decreased perioperative and postoperative complications. The current evidence rests solely on small retrospective cohort studies and a single, large observational analysis. Based on the findings of our paired meta-analysis, every attempt should be made to perform complete cytoreduction in women with metastatic endometrial cancer chosen form PCS. Further characterization of surgical, survival, and chemotherapeutic outcomes, ideally through a multi-institutional RCT, will be necessary to refine an algorithm for NACT candidate selection. Further, as therapy for endometrial cancer evolves and incorporates targeted therapeutics, further work to characterize outcomes of women treated with NACT will be essential.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dr. Wright has served as a consultant for and Clovis Oncology, received royalties from UpToDate, and received research funding from Merck. Dr. Previs has served as a consult from Myriad and has received research funding from Myriad and is supported by grants from the NIH 1K12HD103083-01, AAOGF-GOG Foundation and the Emerson Collective.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2021.100887.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Blumenthal G.M., Bunn P.A., Chaft J.E., et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J. Thorac. Oncol. 2018;13(12):1818–1831. doi: 10.1016/j.jtho.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Bogani G., Ditto A., Leone Roberti Maggiore U., et al. Neoadjuvant chemotherapy followed by interval debulking surgery for unresectable stage IVB Serous endometrial cancer. Tumori. 2019;105(1):92–97. doi: 10.1177/0300891618784785. [DOI] [PubMed] [Google Scholar]
- Bristow R.E., Duska L.R., Montz F.J. The role of cytoreductive surgery in the management of stage IV uterine papillary serous carcinoma. Gynecol. Oncol. 2001;81(1):92–99. doi: 10.1006/gyno.2000.6110. [DOI] [PubMed] [Google Scholar]
- Burke W.M., Orr J., Leitao M., et al. Endometrial cancer: A review and current management strategies: Part i. Gynecol. Oncol. 2014;134(2):385–392. doi: 10.1016/j.ygyno.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Cain H., Macpherson I.R., Beresford M., Pinder S.E., Pong J., Dixon J.M. Neoadjuvant Therapy in Early Breast Cancer: Treatment Considerations and Common Debates in Practice. Clin. Oncol. 2017;29(10):642–652. doi: 10.1016/j.clon.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Chang Z., Talukdar S., Mullany S.A., Winterhoff B. Molecular characterization of endometrial cancer and therapeutic implications. Curr. Opin. Obstet. Gynecol. 2019;31(1):24–30. doi: 10.1097/GCO.0000000000000508. [DOI] [PubMed] [Google Scholar]
- de Lange N.M., Ezendam N.P.M., Kwon J.S., et al. Neoadjuvant chemotherapy followed by surgery for advanced-stage endometrial cancer. Curr. Oncol. 2019;26(2):226–232. doi: 10.3747/co.26.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehal A., Graff-Baker A.N., Vuong B., et al. Neoadjuvant Chemotherapy Improves Survival in Patients with Clinical T4b Colon Cancer. J. Gastrointest. Surg. 2018;22(2):242–249. doi: 10.1007/s11605-017-3566-z. [DOI] [PubMed] [Google Scholar]
- Deng H.Y., Wang W.P., Wang Y.C., et al. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur. J. Cardio-thoracic Surg. 2017;51(3):421–431. doi: 10.1093/ejcts/ezw315. [DOI] [PubMed] [Google Scholar]
- Eto T., Saito T., Shimokawa M., et al. Status of treatment for the overall population of patients with stage IVb endometrial cancer, and evaluation of the role of preoperative chemotherapy: a retrospective multi-institutional study of 426 patients in Japan. Gynecol. Oncol. 2013;131(3):574–580. doi: 10.1016/j.ygyno.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Fagotti A., Ferrandina G., Vizzielli G., et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Fu T., Bu Z.-D., Li Z.-Y., et al. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: A meta-analysis of randomized clinical trials. BMC Cancer. 2015;15(1) doi: 10.1186/s12885-015-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaal, K., M, A.M., Bryant, A., Ad, L., Ta, L., 2016. Adjuvant chemotherapy for advanced endometrial cancer (Review). 5. doi: 10.1002/14651858.CD010681.pub2.www.cochranelibrary.com. [DOI] [PMC free article] [PubMed]
- Grossman H.B., Natale R.B., Tangen C.M., et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- Holman L.L., Pal N., Iglesias D.A., et al. Factors prognostic of survival in advanced-stage uterine serous carcinoma. Gynecol. Oncol. 2017;146(1):27–33. doi: 10.1016/j.ygyno.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe S., Hook J., Nankivell M., et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- Khouri O.R., Frey M.K., Musa F., et al. Neoadjuvant chemotherapy in patients with advanced endometrial cancer. Cancer Chemother. Pharmacol. 2019;84(2):281–285. doi: 10.1007/s00280-019-03838-x. [DOI] [PubMed] [Google Scholar]
- Lambrou N., Gomezmarin O., Mirhashemi R., et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival1. Gynecol. Oncol. 2004;93(3):653–658. doi: 10.1016/j.ygyno.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Lee Y.C., Lheureux S., Oza A.M. Treatment strategies for endometrial cancer: Current practice and perspective. Curr. Opin. Obstet. Gynecol. 2017;29(1):47–58. doi: 10.1097/GCO.0000000000000338. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T., Matsumoto K., Shibata T., et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan clinical oncology group study JCOG0602. Jpn. J. Clin. Oncol. 2008;38(1):74–77. doi: 10.1093/jjco/hym145. [DOI] [PubMed] [Google Scholar]
- Onda T., Satoh T., Saito T., et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Gr. Eur. J. Cancer. 2016;64(June):22–31. doi: 10.1016/j.ejca.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Palisoul M., Mutch D.G. The clinical management of inoperable endometrial carcinoma. Expert Rev. Anticancer Ther. 2016;16(5):515–521. doi: 10.1586/14737140.2016.1168699. [DOI] [PubMed] [Google Scholar]
- Rabinovich A. Neo-adjuvant chemotherapy for advanced stage endometrial carcinoma: a glimmer of hope in select patients. Arch. Gynecol. Obstet. 2016;293(1):47–53. doi: 10.1007/s00404-015-3841-8. [DOI] [PubMed] [Google Scholar]
- Rajkumar S., Nath R., Lane G., Mehra G., Begum S., Sayasneh A. Advanced stage (IIIC/IV) endometrial cancer: Role of cytoreduction and determinants of survival. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;234(December 2016):26–31. doi: 10.1016/j.ejogrb.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Shih K.K., Yun E., Gardner G.J., Barakat R.R., Chi D.S., Leitao M.M. Surgical cytoreduction in stage IV endometrioid endometrial carcinoma. Gynecol. Oncol. 2011;122(3):608–611. doi: 10.1016/j.ygyno.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Study Quality Assessment Tools, 2020. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed August 15, 2020.
- Tobias C.J., Chen L., Melamed A., et al. Association of Neoadjuvant Chemotherapy with Overall Survival in Women with Metastatic Endometrial Cancer. JAMA Netw. Open. 2020;3(12):e2028612. doi: 10.1001/jamanetworkopen.2020.28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenput I., Van Calster B., Capoen A., et al. Neoadjuvant chemotherapy followed by interval debulking surgery in patients with serous endometrial cancer with transperitoneal spread (stage IV): a new preferred treatment? Br. J. Cancer. 2009;101(2):244–249. doi: 10.1038/sj.bjc.6605157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergote I., Tropé C.G., Amant F., et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- Vergote I., Coens C., Nankivell M., et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018;19(12):1680–1687. doi: 10.1016/S1470-2045(18)30566-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Ryan I., Frolova A.I., Liu J., et al. Neoadjuvant chemotherapy versus primary cytoreductive surgery for stage IV uterine serous carcinoma. Int. J. Gynecol. Cancer. 2015;25(1):63–68. doi: 10.1097/IGC.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A.A., Bohlke K., Armstrong D.K., et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of gynecologic oncology and American society of clinical oncology clinical practice guideline. Obstet. Gynecol. Surv. 2016;71(12):717–718. doi: 10.1097/01.ogx.0000508241.20157.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.D., Medel N.I.B., Sehouli J., Fujiwara K., Herzog T.J. Contemporary management of endometrial cancer. Lancet. 2012;379(9823):1352–1360. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.