Abstract
Menisci are wedge‐shaped cartilage discs that are divided into two parts: the avascular and vascular regions. They are formed by fibrocartilage tissue, which contains round cartilage‐like cells and extracellular matrix. Meniscus injury in animals is a common orthopedic problem, but data on the natural healing process mainly deals with the vascular zone. The healing processes in the avascular zone of the meniscus are significantly limited. Thus, this study aimed to evaluate autologous growth plate chondrocytes' impact on the healing process of a damaged meniscus in the avascular zone based on a growing animal model. The study group consisted of 10 pigs at about three months of age. From each animal, chondrocytes from the iliac growth plate and from concentrated bone marrow were taken. Knee joints were divided into right (R) and left (L). The medial meniscus of the R knee joint was treated with a hyaluronic acid based scaffold incubated with bone marrow cells from marrow aspirates (nCHON). The medial meniscus of the L knee joint was treated with a hyaluronic acid based scaffold incubated with bone marrow cells from marrow aspirates supplemented with immature chondrocytes isolated from growth plates (wCHON). The meniscus was damaged in the avascular zone in both knee joints. Followingly, the damaged part of the meniscus was filled with a scaffold with cells from the concentrated bone marrow and from growth plate chondrocytes. In the control group, a scaffold with concentrated bone marrow cells was used. After three months the animals were euthanized and preparations (microscopic slides) were made from the meniscus' damaged part. A qualitative and quantitative analysis have been prepared. The wCHON group in comparison with the nCHON group showed a statistically significantly higher number of fusiform cells on the surface of the graft as well as better healing of the graft. In addition, the degree of vascularization was higher in specimens from the wCHON group than in the nCHON group. The results of our research on immature pig knees revealed that mesenchymal stem cell and growth plate chondrocytes could be treated as the cell source for meniscus reconstruction, and growth plate chondrocytes enhance healing processes in the avascular zone of the injured meniscus.
Keywords: growth plate chondrocytes, meniscus tear, mesenchymal stem cells, pigs, scaffold
The study aimed to evaluate autologous growth plate chondrocytes’ impact on the healing process of a damaged meniscus in the avascular zone based on a growing animal model. The study group consisted of 10 pigs at about three months of age. From each animal, chondrocytes from the iliac growth plate and from concentrated bone marrow were taken. The medial meniscus of the right knee joint was treated with a hyaluronic acid based scaffold incubated with bone marrow cells from marrow aspirates (nCHON). The medial meniscus of the left knee joint was treated with a hyaluronic acid‐based scaffold incubated with bone marrow cells from marrow aspirates supplemented with immature chondrocytes isolated from growth plates (wCHON). The meniscus was damaged in the avascular zone in both knee joints. The damaged part of the meniscus was filled with a scaffold with cells from the concentrated bone marrow and from growth plate chondrocytes. In the control group, a scaffold with concentrated bone marrow cells was used. After three months a qualitative and quantitative analysis have been prepared. The results showed that (1) MSCs and growth plate chondrocytes are a useful cell source for meniscus reconstruction, (2) growth plate chondrocytes enhancehealing processes in the avascular zone of the injured meniscus, (3) meniscus injury is becoming more common even in pediatric patients and can lead to several joint injuries of the knee, especially degenerative changes.

1. INTRODUCTION
Menisci play a crucial role in the knee joint, being involved in load distribution, joint stability, lubrication, proprioception and nutrition of the articular cartilage (Whitehouse et al., 2017). These wedge‐shaped cartilage discs are located between the condyle of the femur and tibia and are made of fibrocartilage tissue containing round, flat or irregularly‐shaped cartilage‐like cells and extracellular matrix that is rich in proteoglycans and collagen predominantly type I (McDevitt & Webber, 1990). However, there is a clear relationship between the shape of these cells and the type of collagen they produce (Von Der Mark et al., 1977). The meniscus is divided into two parts: the first avascular, in which mainly collagen II is found, while the second, vascular region is rich in collagen I (Chevrier et al., 2009; Zellner et al., 2017). A meniscus injury is a common clinical orthopedic problem and may be associated with osteoarthritis. Meniscal tears in the vascular zone may be treated successfully using various repair techniques, but the natural healing process in the avascular zone is limited (Heckmann et al., 2006; Rangger et al., 1995; Walker & Erkman, 1975; Weiss et al., 1989; Yu et al., 2015), while meniscal tears in the avascular zone are usually treated by arthroscopic excision. Meniscectomy results in abnormal stress concentrations on the articular cartilage which over time may cause degenerative changes (Liechti et al., 2019). However, in the recent past, the work has been increasingly focused on the therapeutic options to preserve the meniscus, even in the case of avascular zone damage (Beaufils & Pujol, 2018; Koch et al., 2017, 2018, 2019; Ruiz‐Ibán et al., 2011; Yu et al., 2015). Nevertheless, tissue engineering gives a potential chance for meniscus repair (Abdel‐Hamid et al., 2005; Takroni et al., 2016; Yamasaki et al., 2005). While these clinical problems mainly concern adults, they can also appear as rare processes in immature individuals. Thus, studies connected with this type of injury in juveniles are sporadic (Liechti et al., 2019).
The development of new treatments for meniscus injury in immature and adult organisms should be compared to obtain a broad understanding of the regenerative abilities of this tissue. Comparing changes in young and adult individuals will make it possible to describe the mechanisms occurring in the development or during differentiation of regenerative features. Based on our previous scientific research (Tomaszewski et al., 2019) we decided to check how the environment of cells with high proliferative potential could affect tissues' regenerative capabilities but in immature organism. Therefore, the aim of our preliminary study was to present an attempt to assess the influence of growth plate chondrocytes on the healing process of meniscus injury in the avascular zone using a hyaluronic acid scaffold seeded with mesenchymal stem cells (MSCs) in pigs.
We chose animals at the age of 12 weeks, due to the presence of bone growth cartilage and the same size of the animal. It allows optimal performance of the surgery. Comparing results obtained using growth plate derived chondrocytes along with bone marrow cells seeded in scaffold and the construct implanted into the meniscal defect with the results derived from the control group (only bone marrow cells seeded in scaffold) would probably be helpful in elucidating proliferative capabilities of meniscus tissues during development in relation to the cell niche.
2. MATERIAL AND METHODS
The study group consisted of 10 pigs at the age of 12 weeks. Each animal was assigned a number. Knee joints were divided into right (R) and left (L). Surgical procedures were performed on both hind legs of all the animals—the medial meniscus was examined. However, the medial meniscus of the R knee joint was treated with a hyaluronic acid based scaffold incubated with bone marrow cells from marrow aspirates (nCHON—without growth plate chondrocytes). The medial meniscus of the L knee joint was treated with a hyaluronic acid‐based scaffold incubated with bone marrow cells from marrow aspirates supplemented with immature chondrocytes isolated from growth plates (wCHON—with growth plate chondrocytes). In the first stage of the experiment, in the operating room with the animal under analgosedation (atropine sulfate 0.05 mg/kg s.c., Polfarmex, Kutno; ketamine hydrochloride 3 mg/kg i.m., Biowet, Pulawy; and xylazine hydrochloride 1 mg/kg i.m., Riemser), the bone marrow aspiration was performed. The entire experiment described below has been presented in Figures 1 and 2.
FIGURE 1.

nCHON group—experiment scheme
FIGURE 2.
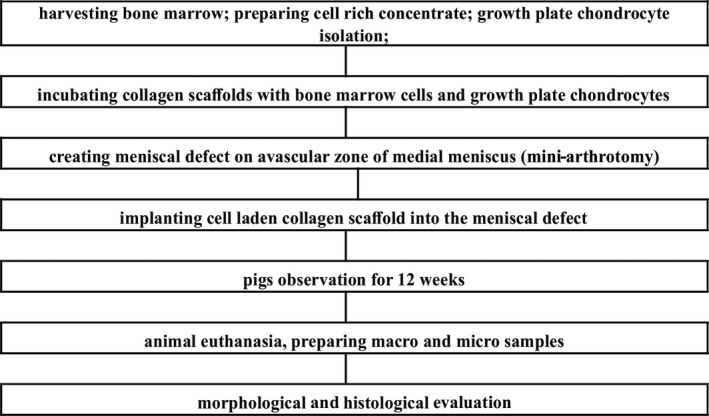
wCHON group—experiment scheme
2.1. Bone marrow harvesting
We used the Mini Marrowstim Concentration System (BIOMET). Each time, a bone marrow biopsy was performed from the posterior iliac spine to aspirate 30 ml of bone marrow (3 ml of heparin with 27 ml of bone marrow aspirate). The bone marrow were centrifuged for 15 min at 3200 rpm using relative centrifugal force (RCF) G‐force 1219 (room temperature—RT), yielding a 3‐layer distribution consisting of (1) a plasma rich layer, (2) a cell‐rich layer, and (3) a red blood cell layer according to the literature (Tomaszewski et al., 2019).
2.2. Cell‐rich concentrate preparation
The isolated cell‐rich concentrate obtained from a bone marrow biopsy was rinsed twice with Ham's F12 (PAN Biotech Cat. No. P‐04‐14500) and centrifuged at 200 g for 5 min (RT).
2.3. Growth plate chondrocyte isolation
Using the trepanning needle, which we used to aspirate the bone marrow, we retrieved a biopsy of the iliac growth cartilage. The cartilage samples were placed in a sterile tube containing cooled Ham's F12 (PAN Biotech Cat. No. P‐04‐14500). All samples were transported from the operating theatre to the Cell Culture Laboratory at the Center of Experimental Medicine. The growth plate biopsy was weighed, cut into small pieces (1 mm3 fragments), and rinsed in Ham's F12 (PAN Biotech, P‐04‐14500). The minced tissue was digested with Collagenase NB 4 (SERVA, 17454.02) at a concentration of 0.3 U/ml in Ham's F12 (PAN Biotech Cat. No. P‐04‐14500) at 37°C for 6 h. After digestion the growth plate cell suspension was centrifuged twice at 180 g for 10 min and rinsed with Ham's F12 (PAN Biotech Cat. No. P‐04‐14500). The obtained cells were suspended in 100 µl of Ham's F12 (PAN Biotech Cat. No. P‐04‐14500) with 20% serum (Gibco Cat. No. 10270). The chondrocyte digestion process from the growth plate was monitored by Zeiss inverted microscopy. Cell number and viability were difficult to assess due to the small amount of procured tissue. The procedure described above was conducted according to literature data (Brittberg et al., 1994; Visna et al., 2003).
2.4. Scaffold preparation
We cut the Hyalofast scaffold into 20 equal fragments similar to the predicted size of 6 mm in diameter of the meniscus defect. We chose Hyalofast because hyaluronic acid (HA) is released into the lesion in the degradation process, creating a microenvironment rich in HA. We divided the scaffolds into two groups: (1) the wCHON group (after rinsing with Ham's F12, the scaffolds were incubated with cell‐rich concentrate for 5 h and then with chondrocytes isolated from the iliac growth plate for another 7 h), and (2) the nCHON group (the scaffolds were incubated only with cell‐rich concentrate for 5 h). In the second stage of the experiment, the pigs were anaesthetized using 1% propofol 1.5–2.5 mg/kg i.v. (Fresenius Kabi, Austria GmbH) and fentanyl 2.5 mg/kg m.c. i.v. and inhaled 0.5% volume isoflurane.
2.5. Meniscus damage induction and scaffold implantation
While monitoring the vital parameters of the animals, we performed a mini‐arthrotomy separately on the right and left knee. Each time, a 5 mm diameter meniscal defect was made between the inner and middle third of the meniscus (which constituted about one‐third of the meniscus width). Caution was taken in order to remove the meniscus precisely without damaging the adjoining cartilage. In the nCHON group we implanted a scaffold incubated with bone marrow cells into the defect. In the second experimental group, wCHON, a scaffold incubated with bone marrow cells, and growth plate chondrocytes were implanted. Every graft was checked prior to implantation to confirm the graft and animal serial number. Every graft was stabilized with 3 vicryl 5–0 sutures (Johnson & Johnson). After confirming stable graft placement, the surgical wound was closed and the operated limb was secured with a sterile dressing. The animals were allowed to walk freely in their cages.
2.6. Morphological and histological evaluations
At 12 weeks after the performed surgical procedure, the animals were sacrificed (Morbitol 1.6 mg/kg i.v.), and the 20 treated knees were collected: 10 from the nCHON group and 10 from the wCHON group. The isolated organs were then fixed in 10% formaldehyde. After dehydration in a graded ethanol solutions, clearing in xylene, infiltrating twice in paraffin waxes (30 min, 24 h), the material was embedded in paraffin using standard methods (Slaoui & Fiette, 2011). After sample preparation and sectioning (5 µm thick) using a rotary microtome (Leica RM2125RT; Leica Biosystems), collecting on glass slides and deparaffinization, the histological sections were stained with three different staining methods: Mayer's hematoxylin and eosin, Safranin O, and Masson's trichrome. The histological results were independently evaluated by two pathologists using an Olympus BX51 microscope (Olympus America, Inc.) and afterwards averaged. Due to the lack of an ideal scale for the microscopic assessment of the meniscus, we selected parameters for histopathological evaluation based on observations of other authors; see Table 1 (Ishida et al., 2007; Pauli et al., 2011). Using Masson staining, HE staining, and Safranin O staining we managed to apply microscopic evaluation of preparations based on the following values: graft healing, fusiform cells, presence of fibroblasts in graft, presence of fibrochondrocytes in graft, presence of fibroblasts in meniscus, presence of fibrochondrocytes in meniscus, presence of residual scaffold, and presence of vessels. Eventually the following classification of the rate of variation of the meniscus parameters in accordance with the scale of lesions was applied: 0—extreme, 1—severe, 2—major, 3—moderate, 4—minor, 5—normal (Ruiz‐Ibán et al., 2011; Zellner et al., 2013). Histological staining (HE staining, Masson staining and Safranin O staining) was conducted according to protocols described in the literature (Hermyt et al., 2019; Kaczmarek et al., 2017; Kaczmarek et al., 2021; Kowalska et al., 2017; Sheehan & Hrapchak, 1980; Slaoui & Fiette, 2011).
TABLE 1.
The quantitative analysis based on classification of the rate of variation of the meniscus parameters in accordance with the scale of lesions in animals from wCHON (L) and nCHON (R) group: fusiform cells (FC), graft healing (GH), fibroblasts in graft (FBG), fibrochondrocytes in graft (FCG), fibroblasts in meniscus (FBM), fibrochondrocytes in meniscus (FCM), the presence of residual scaffold (RS), Masson staining (M), HE staining (HE), Safranin O staining (S), the presence of vessels (V)
| Animal number | FC | GH | FBG | FCG | FBM | FCM | RS | M | HE | S | V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 R | 0 | 1 | 3 | 0 | 0 | 4 | 4 | 1 | 1 | 1 | 1 |
| 1 L | 4 | 4 | 4 | 1 | 1 | 5 | 2 | 4 | 3 | 3 | 0 |
| 2 R | 1 | 0 | 5 | 0 | 5 | 4 | 3 | 2 | 2 | 1 | 2 |
| 2 L | 4 | 5 | 5 | 1 | 2 | 5 | 2 | 2 | 4 | 3 | 0 |
| 3 R | 2 | 3 | 4 | 2 | 3 | 2 | 2 | 2 | 2 | 1 | 1 |
| 3 L | 3 | 5 | 3 | 4 | 5 | 5 | 1 | 4 | 3 | 4 | 1 |
| 4 R | Death | ||||||||||
| 4 L | |||||||||||
| 5 R | 3 | 3 | 4 | 1 | 4 | 4 | 3 | 3 | 4 | 3 | 3 |
| 5 L | 4 | 3 | 4 | 1 | 4 | 2 | 4 | 3 | 3 | 3 | 3 |
| 6 R | 2 | 2 | 4 | 1 | 4 | 2 | 4 | 3 | 2 | 2 | 3 |
| 6 L | 5 | 5 | 4 | 4 | 4 | 2 | 3 | 4 | 4 | 4 | 2 |
| 7 R | 4 | 3 | 4 | 2 | 4 | 1 | 5 | 2 | 3 | 2 | 1 |
| 7 L | 5 | 5 | 4 | 2 | 4 | 3 | 2 | 4 | 4 | 5 | 1 |
| 8 R | 4 | 3 | 3 | 1 | 3 | 2 | 3 | 3 | 3 | 2 | 2 |
| 8 L | 4 | 4 | 4 | 2 | 3 | 2 | 3 | 4 | 3 | 4 | 0 |
| 9 R | 2 | 2 | 4 | 3 | 1 | 3 | 2 | 2 | 2 | 2 | 2 |
| 9 L | 3 | 3 | 4 | 2 | 4 | 1 | 1 | 3 | 3 | 3 | 0 |
| 10 R | 1 | 0 | 2 | 1 | 4 | 2 | 4 | 1 | 1 | 1 | 0 |
| 10 L | 3 | 4 | 4 | 4 | 4 | 2 | 2 | 3 | 3 | 3 | 0 |
2.7. Statistical analysis
To evaluate the difference between two groups, that is, treatment with scaffolds, concentrated bone marrow and growth plate chondrocytes (wCHON) and scaffold with concentrated bone marrow cells (nCHON), for each parameter (R version 3.5.1—2018‐07‐02), we used the Wilcoxon paired test and the bootstrap resampling method for additional verification of our findings, due to a relatively small size of the sample in the experiment. We calculated the average values re‐sampled 500 times using the R package boot.
3. RESULTS
Results of the microscopic analysis (Figure 3) for the samples from nCHON and wCHON groups (Table 1) are summarized in Table 2 where, apart from the means, standard deviations, medians and percentiles, corresponding p‐values of the Wilcoxon test are presented. As the test's confidence level was 95%, there is a statistically significant effect provided that p < .05. Figure 4 presents selected parameters for which there were significant differences between the groups according to the Wilcoxon paired test.
FIGURE 3.

Histological assessment of meniscus using HE staining, Masson staining, and Safranin O staining. (a–c) meniscus nCHON—severe with no graft healing, (d–f) meniscus wCHON – normal healing with normal fusiform cells, but moderate residual scaffold, (g–i) meniscus wCHON with normal graft healing, normal fusiform cells, severe fibroblasts in graft, severe Masson staining, severe Safranin O staining, (j–l) meniscus nCHON – severe graft healing, major fibroblast graft, major residual scaffold, minor Masson staining, moderate Safranin O staining. (m) meniscus, (sc) scaffold. Light microscope, 200×
TABLE 2.
Mean (x), standard deviation (±SD), median (M) and quartile deviation (25th and 75th percentiles; Q25 and Q75) of the analysed parameters quantifying applied treatment: with nCHON and wCHON
| Parameter | nCHON | wCHON | p | ||
|---|---|---|---|---|---|
| x ± SD |
M Q25–Q75 |
x ± SD |
M Q25–Q75 |
||
| Fusiform cells | 2.11 ± 1.36 |
2.0 1.0–3.0 |
3.89 ± 0.78 |
4.0 3.0–4.0 |
.02 |
| Graft healing | 2.11 ± 1.62 |
2.0 1.0–3.0 |
4.22 ± 0.83 |
4.0 3.0–5.0 |
.01 |
| Fibrochondrocytes scafold | 1.22 ± 0.97 |
1.0 1.0–2.0 |
2.33 ± 1.32 |
2.0 1.0–4.0 |
.05 |
| Fibroblasts scaffold | 3.56 ± 0.88 |
4.0 3.0–4.0 |
4.11 ± 0.33 |
4.0 4.0–4.0 |
.27 |
| Fibrochondrocytes meniscus | 2.67 ± 1.12 |
2.0 2.0–4.0 |
3.0 ± 1.58 |
2.0 2.0–5.0 |
.60 |
| Fibroblasts meniscus | 3.11 ± 1.62 |
4.0 3.0–4.0 |
3.44 ± 1.24 |
4.0 3.0–4.0 |
.58 |
| Residual scaffold | 3.33 ± 1.00 |
3.00 3.0–4.0 |
2.22 ± 0.97 |
2.0 2.0–3.0 |
.03 |
| Masson staining | 2.11 ± 0.78 |
2.0 2.0–3.0 |
3.44 ± 0.73 |
4.00 3.0–4.0 |
.02 |
| HE staining | 2.22 ± 0.97 |
2.0 2.0–3.0 |
3.33 ± 0.50 |
3.0 3.0–4.0 |
.03 |
| Safranin O staining | 1.67 ± 0.71 |
2.00 1.0–2.0 |
3.55 ± 0.73 |
3.0 3.0–4.0 |
.01 |
| Vascularization | 1.67 ± 1.00 |
2.0 1.0–2.0 |
0.78 ± 1.09 |
0.0 0.0–1.0 |
.04 |
The p‐value of the Wilcoxon pair test in the last column indicate statistically significant difference of means provided that p < .05.
FIGURE 4.

Boxplots for selected quantifiers (according to Wilcoxon test) influenced by the applied treatment (no cells; nCHON and cells; wCHON; p < .05), corresponding to Table 2
Histological staining successfully compared the degree of variability of the analyzed meniscus parameters between the experimental groups. Safranin O staining, compared to Masson staining and HE staining, is more effective in the microscopic assessment of the rate of variation of damaged meniscus treated with growth plate chondrocytes in relation to a scaffold incubated only with bone marrow cells (respectively for the mentioned staining methods p = .01, p = .02, p = .03) (Table 2). The wCHON group in comparison with the nCHON group showed a statistically significantly higher number of fusiform cells on the surface of the graft (p = .02) as well as better healing of the graft (p = .01). Additionally in specimens in which a scaffold was incubated with bone marrow cells and growth plate chondrocytes were implanted, the presence of residual scaffolding was significantly lower than in the complementary group, in which the implanted scaffold was incubated only with bone marrow cells. The degree of vascularization was higher in specimens from the wCHON group than in the nCHON group (p = .04) (Figure 4, Table 2). Respectively to the scale of lesions in both experimental groups, no statistically significant differences were established in such parameters as the presence of fibroblasts or fibrochondrocytes in the graft, or presence of fibroblasts or fibrochondrocytes in the meniscus.
Since our knowledge about the properties of distribution that the sample analyzed previously comes from is strictly limited mainly due to the relatively small sample size in the experiment, we applied one of the most popular resampling methods, the bootstrap, for additional verification of our findings. Using the R package boot, average values for nCHON and wCHON cases resampled 500 times were calculated. The results, presented in Table 3, indicate that there is no apparent difference between bootstrap predictions and the estimates calculated for a sample originating from the experiment. It confirms the validity of the sample size applied in our analysis.
TABLE 3.
Mean bootstrap results for 500‐times resampling of original data
| Parameter | nCHON | wCHON |
|---|---|---|
| Fusiform cells | 2.12 | 3.88 |
| Graft healing | 1.88 | 4.22 |
| Fibrochondrocytes scafold | 1.21 | 2.29 |
| Fibroblasts scaffold | 3.67 | 3.99 |
| Fibrochondrocytes meniscus | 2.69 | 3.01 |
| Fibroblasts meniscus | 3.13 | 3.42 |
| Residual scaffold | 3.33 | 2.23 |
| Masson staining | 2.13 | 3.36 |
| HE staining | 2.24 | 3.37 |
| Safranin O staining | 1.66 | 3.50 |
| Vascularization | 1.51 | 0.86 |
4. DISCUSSION
A complete meniscus that forms a kind of cushion between the femur and tibia is essential for proper functioning of a knee joint. Any damage or injury affects its integrity, eventually disrupting its proper functioning and leading to osteoarthritic changes (Beaufils & Pujol, 2018; Petty & Lubowitz, 2011; Takroni et al., 2016). Regenerative‐medicine‐based meniscus therapy searches for new therapeutic approaches for meniscus lesions in both the vascular and avascular zones using cells, such as MSCs (Koch et al., 2017, 2018, 2019). Repairing meniscus damage is important to prevent late effects of meniscectomy (Beaufils & Pujol, 2018), especially as the natural healing process in the avascular meniscal zone is limited (Henning et al., 1987; Yu et al., 2015). The meniscus consists of two compartments, the inner avascular part where type II collagen is present and the outer vascular part where type I collagen is present (Zellner et al., 2017). The meniscal vascularity extends to 10%–25%, but there is no literature concerning that layer's size in the growing population (Whitehouse et al., 2017). In the case of severe meniscus damage in the avascular zone, arthroscopic removal of the damaged part is still considered a standard. If the damage constitutes a significant part of the meniscus, replacement of the damaged meniscus using allografts or a meniscal substitute (acellular scaffold) in some cases can be performed (Bulgheroni et al., 2010; LaPrade et al., 2010; Lubowitz et al., 2007; Ruiz‐Ibán et al., 2011; Spencer et al., 2012; Yu et al., 2015). Numerous studies have been conducted on adult organisms, while data on immature specimens are relatively scarce. Therefore, in our research, we focused on immature pigs, where we analyzed changes in the avascular zone of the meniscus. We paid special attention to the meniscus damage in the avascular zone, which was confirmed by histopathological examination. However, the finding of key significance is that we have also managed to show, based on this experiment, that autologous growth cartilage chondrocytes further influence the recovery. We found apparent changes in this zone when we used a hyaluronic acid‐based scaffold incubated with bone marrow cells from marrow aspirates supplemented with immature chondrocytes isolated from growth plates.
Many studies describe scaffolds used to treat meniscus damage, among them scaffolds built with type I collagen, type I and II collagen, glycosaminoglycan copolymers, and synthetic biodegradable polymers containing polyglycolic acid and polylactic acid (Ibarra et al., 2000; Sweigart & Athanasiou, 2001). Most of these studies were focused on finding a suitable substrate for the cells which would accelerate the regeneration process in the meniscal avascular zone. Nevertheless, the cell‐free scaffold reduced the regeneration quality compared to the scaffold with cells (Zellner et al., 2017). We chose the Hyalofast because in the degradation process, the hyaluronic acid is released into the lesion, creating a microenvironment rich in HA (Gobbi et al., 2017; Gobbi & Whyte, 2016). Hyaluronic acid participates in chondrogenesis of adipose derived mesenchymal stem cells as well as in chondrocytes co‐cultures (Amann et al., 2017). Moreover, MSCs combined with Hyalofast have been shown to differentiate into chondrocytes, which in our opinion is crucial in fibrocartilage tissue regeneration. The potential of restoring meniscus like fibrocartilage by BM‐MSCs has been described in many studies (Yamasaki et al., 2005; Yu et al., 2015; Zellner et al., 2017). The source of MSCs may be bone marrow, adipose tissue or synovium. However, there is a less therapeutic effect on meniscal repair using MSC synovium instead of bone marrow MSCs (Whitehouse et al., 2017; Yu et al., 2015). Due to this observation, we decided to use cells of bone marrow origin from the iliac crest (Angele et al., 2008; Gobbi et al., 2017; Gobbi & Whyte, 2016; Ibarra et al., 2000; Pabbruwe et al., 2010; Spencer et al., 2012; Sweigart & Athanasiou, 2001; Uchio et al., 2003; Wei et al., 2012; Yamasaki et al., 2005). However, after an injury involving damage to the meniscus in an avascular zone, the amount of MSCs from the synovial fluid increases. The MSCs have a high migrational potential toward the lesion, and this migration seems to be responsible for the healing processes of the damaged meniscus (Mandal et al., 2011; Matsukura et al., 2014; Nerurkar et al., 2011; Zellner et al., 2017).
MSCs can be delivered to the site of meniscus damage by direct application or intraarticular injection. Ruiz‐Ibán et al. reported better results of meniscal suture lesions in the avascular zone combined with the administration of MSCs derived from adipose tissue, instead of just meniscal suture (Pak et al., 2014; Ruiz‐Ibán et al., 2011). Yu et al. achieved the best results by soaking the scaffold with MSCs, and our studies confirmed this observation (Yu et al., 2015). The scaffold was prepared for clinical application by using the three‐dimensional shapes corresponding to the meniscal damage because the ability to adjust the shape of the scaffolds to the damage to the meniscus significantly improves the healing results (Zellner et al., 2013).
MSCs stimulate fibrocartilage tissue formation with an abundant extracellular matrix and improve the newly formed meniscal tissue integration. The experimental model was designed to minimize the spontaneous repair because in pediatric patients fewer complications and better healing of the damaged tissues compared to adult patients have been observed (Liechti et al., 2019). The defect in the avascular zone with a diameter of 5 mm constituted about 30% of the meniscus width. Two different tissue‐engineering strategies were used in this study: implantation of a HA scaffold seeded with MSCs (nCHON) and implantation of the HA scaffold which was seeded with bone marrow‐derived MSCs and autologous growth plate chondrocytes (wCHON). The repaired meniscus in wCHON had a higher number of fusiform cells on the graft surface (p = .02) and a better graft healing level (p = .01) compared to changes which appeared in the nCHON experimental group. Additionally, the presence of a residual scaffold was significantly lower and the degree of vascularization was also higher in wCHON. However, in both experimental groups, no statistically significant differences were found in the degree of variability of parameters such as the presence of fibroblasts or fibrochondrocytes in the graft and the presence of fibroblasts or fibrochondrocytes in the meniscus. The bone marrow‐derived cells have the potential to differentiate into the chondrogenic line in adult specimens. At the same time we suspect that immature chondrocytes isolated from growth plates accelerate the process of vascularization and healing in immature specimens, while their presence does not affect the accumulation of fibroblasts or fibrochondrocytes.
Moreover, it is worth emphasizing that the effect of meniscus treatment may depend on the MSC differentiation stage at the implantation phase. Pabbruwe et al. presented results indicating that chondrogenically differentiated MSCs (transforming growth factor beta 1; TGF‐ß1) were more poorly integrated than undifferentiated cells in animals (Pabbruwe et al., 2010). Culturing MSCs in chondrogenic media gives significantly better meniscal regeneration compared with MSC cultured in basal media in animal models (Al Faqeh et al., 2012). However, the improvement in the healing of meniscus damage in the avascular zone is also possible due to the use of MSCs as well as other cells. Zellner et al. observed the influence of MSCs and the scaffold on the healing of meniscal damage and additionally by adding cells from the meniscus, which significantly improved the regeneration process by adding cells from the meniscus. It significantly improved the regeneration process (Zellner et al., 2017). But there is a lack of data concerning the use of autologous growth plate chondrocytes in a growing animal model for meniscal repair. Our study has shown that the implantation of the scaffold with MSCs and autologous growth plate chondrocytes into the meniscal defect provides better repair than seen in scaffolds without chondrocytes. In the presence of fusiform cells, the scaffolds were also less residual. Our reports in this field are the same as other authors (Murphy et al., 2003; Port et al., 1996; Yu et al., 2015). The use of scaffold incubated with MSCs and chondrocytes also resulted in better macroscopic meniscal regeneration and defect filling compared with the repair after implantation of chondrocyte‐free scaffolds.
Our study limitation is that it is an animal study, but we used pigs, which seem to have a knee‐like structure and similar healing processes. There is no follow‐up concerning the possible osteoarthritis in the knee joint after the meniscus was damaged and repaired. Further tests are required to obtain additional information on regenerative processes, especially those related to the accumulation of fibroblasts or fibrochondrocytes in immature animals. They should involve the impact of synovial MSC on meniscal healing and the mechanical stress on scaffolds after the repair of the meniscal region. It should be mentioned that the lack of an assessment of cell density/viability was a limitation for these studies. Therefore, it is necessary to conduct further research taking into account these parameters.
5. CONCLUSIONS
The results of our research on immature pig knees show that (1) MSCs and growth plate chondrocytes are a useful cell source for meniscus reconstruction, (2) growth plate chondrocytes enhance healing processes in the avascular zone of the injured meniscus, (3) meniscus injury is becoming more common even in pediatric patients and can lead to several joint injuries of the knee, especially degenerative changes.
CONFLICT OF INTEREST
Author declare that they do not have any conflict of interests.
ACKNOWLEDGEMENTS
We are very thankful to Dr. Aleksandra Wysocka‐Wycisk for her technical assistance and Richard Ashcroft for the language correction. The study was wholly funded by a grant from the Medical University of Silesia. We received permission for the implementation of our study from the Local Bioethics Committee of the Medical University of Silesia in Katowice (resolution number 29/2016 from 12/04/2016).
Tomaszewski, R. , Rost‐Roszkowska, M. , Wilczek, G. , Gap, A. & Wiktor, Ł. (2021) Changes in the avascular area of the meniscus using mesenchymal stem cells and growth plate chondrocytes in a pig model. Journal of Anatomy, 239, 1409–1418. 10.1111/joa.13508
Data Availability Statement
Data will be shared on request.
REFERENCES
- Abdel‐Hamid, M. , Hussein, M.R. , Ahmad, A.F. & Elgezawi, E.M. (2005) Enhancement of the repair of meniscal wounds in the red‐white zone (middle third) by the injection of bone marrow cells in canine animal model. International Journal of Experimental Pathology, 86, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Faqeh, H. , Nor Hamdan, B.M. , Chen, H.C. , Aminuddin, B.S. & Ruszymah, B.H. (2012) The potential of intra‐articular injection of chondrogenic‐induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Experimental Gerontology, 47, 658–664. [DOI] [PubMed] [Google Scholar]
- Amann, E. , Wolff, P. , Breel, E. , van Griensven, M. & Balmayor, E.R. (2017) Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co‐cultures. Acta Biomaterialia, 52, 130–144. [DOI] [PubMed] [Google Scholar]
- Angele, P. , Johnstone, B. , Kujat, R. , Zellner, J. , Nerlich, M. , Goldberg, V. et al. (2008) Stem cell based tissue engineering for meniscus repair. Journal of Biomedical Materials Research Part A, 85, 445–455. [DOI] [PubMed] [Google Scholar]
- Beaufils, P. & Pujol, N. (2018) Meniscal repair: technique. Orthopaedics and Traumatology: Surgery and Research, 104, 137–145. [DOI] [PubMed] [Google Scholar]
- Brittberg, M. , Lindahl, A. , Nilsson, A. , Ohlsson, C. , Isaksson, O. & Peterson, L. (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. The New England Journal of Medicine, 331(14), 889–895. [DOI] [PubMed] [Google Scholar]
- Bulgheroni, P. , Murena, L. , Ratti, C. , Bulgheroni, E. , Ronga, M. & Cherubino, P. (2010) Follow‐up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. The Knee, 17, 224–229. [DOI] [PubMed] [Google Scholar]
- Chevrier, A. , Iliescu Nelea, M. , Hurtig, M.B. , Hoemann, C. & Buschmann, M.D. (2009) Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. Journal of Orthopaedic Research, 27(9), 1197–1203. [DOI] [PubMed] [Google Scholar]
- Gobbi, A. , Scotti, C. , Karnatzikos, G. , Mudhigere, A. , Castro, M. & Peretti, G.M. (2017) Onestep surgery with multipotent stem cells and Hyaluronan‐based scaffold for the treatment of full‐thickness chondral defects of the knee in patients older than 45 years. Knee Surgery, Sports Traumatology, Arthroscopy, 25, 2494–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi, A. & Whyte, G.P. (2016) One‐stage cartilage repair using a hyaluronic acid– based scaffold with activated bone marrow–derived mesenchymal stem cells compared with microfracture five‐year follow‐up. The American Journal of Sports Medicine, 44, 2846–2854. [DOI] [PubMed] [Google Scholar]
- Heckmann, T.P. , Barber‐Westin, S.D. & Noyes, F.R. (2006) Meniscal repair and transplantation: indications, techniques, rehabilitation, and clinical outcome. Journal of Orthopaedic and Sports Physical Therapy, 36, 795–814. [DOI] [PubMed] [Google Scholar]
- Henning, C.E. , Lynch, M.A. & Clark, J.R. (1987) Vascularity for healing of meniscus repairs. Arthroscopy: The Journal of Arthroscopic and Related Surgery, 3, 13–18. [DOI] [PubMed] [Google Scholar]
- Hermyt, M. , Janiszewska, K. & Rupik, W. (2019) Squamate egg tooth development revisited using three‐dimensional reconstructions of brown anole (Anolis sagrei, Squamata, Dactyloidae) dentition. Journal of Anatomy, 236, 1004–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra, C. , Koski, J.A. & Warren, R.F. (2000) Tissue engineering meniscus. Orthopedic Clinics of North America, 31, 411–418. [DOI] [PubMed] [Google Scholar]
- Ishida, K. , Kuroda, R. , Miwa, M. , Tabata, Y. , Hokugo, A. , Kawamoto, T. et al. (2007) The regenerative effects of platelet‐rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Engineering, 3, 1103–1112. [DOI] [PubMed] [Google Scholar]
- Kaczmarek, P. , Hermyt, M. & Rupik, W. (2017) Embryology of the VNO and associated structures in the grass snake Natrix natrix (Squamata: Natricinae): a 3D perspective. Frontiers in Zoology, 14, 1. 10.1186/s12983-017-0188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek, P. , Metscher, B. & Rupik, W. (2021) Embryology of the naso‐palatal complex in Gekkota based on detailed 3D analysis in Lepidodactylus lugubris and Eublepharis macularius . Journal of Anatomy, 238, 249–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, M. , Achatz, F.P. , Lang, S. , Pfeifer, C.G. , Pattappa, G. , Kujat, R. et al. (2018) Tissue engineering of large full‐size meniscus defects by a polyurethane scaffold: accelerated regeneration by mesenchymal stromal cells. Stem Cells International, 2018, 8207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, M. , Ehrenreich, T. , Koehl, G. , Pattappa, G. , Pfeifer, C. , Loibl, M. et al. (2017) Do cell based tissue engineering products for meniscus regeneration influence vascularization? Clinical Hemorheology and Microcirculation, 67(2), 125–140. [DOI] [PubMed] [Google Scholar]
- Koch, M. , Hammer, S. , Fuellerer, J. , Lang, S. , Pfeifer, ChG , Pattappa, G. et al. (2019) Bone marrow aspirate concentrate for the treatment of avascular meniscus tears in a one‐step procedure — evaluation of an in vivo model. International Journal of Molecular Science, 20, 1120. 10.3390/ijms20051120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska, M. , Hermyt, M. & Rupik, W. (2017) Three‐dimensional reconstruction of the embryonic pancreas in the grass snake Natrix natrix L. (Lepidosauria, Serpentes) based on histological studies. Zoology, 121, 91–110. [DOI] [PubMed] [Google Scholar]
- LaPrade, R.F. , Wills, N.J. , Spiridonov, S.I. & Perkinson, S. (2010) A prospective outcomes study of meniscal allograft transplantation. The American Journal of Sports Medicine, 38, 1804–1812. [DOI] [PubMed] [Google Scholar]
- Liechti, D.J. , Constantinescu, D.S. , Ridley, T.J. , Chahla, J. , Mitchell, J.J. & Vap, A.R. (2019) Meniscal repair in pediatric populations: a systematic review of outcomes. Orthopaedic Journal of Sports Medicine, 7(5), 2325967119843355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubowitz, J.H. , Verdonk, P.C.M. , Reid, J.B. & Verdonk, R. (2007) Meniscus allograft transplantation: a current concepts review. Knee Surgery, Sports Traumatology, Arthroscopy, 15, 476–492. [DOI] [PubMed] [Google Scholar]
- Mandal, B.B. , Park, S.H. , Gil, E.S. & Kaplan, D.L. (2011) Stem cell‐based meniscus tissue engineering. Tissue Engineering Part A., 17, 2749–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura, Y. , Muneta, T. , Tsuji, K. , Koga, H. & Sekiya, I. (2014) Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clinical Orthopaedics and Related Research, 472(5), 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt, C.A. & Webber, R.J. (1990) The ultrastructure and biochemistry of meniscal cartilage. Clinical Orthopaedics, 252, 8–18. [PubMed] [Google Scholar]
- Murphy, J.M. , Fink, D.J. , Hunziker, E.B. & Barry, F.P. (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis and Rheumatology, 48, 3464–3474. [DOI] [PubMed] [Google Scholar]
- Nerurkar, N.L. , Han, W. , Mauck, R.L. & Elliott, D.M. (2011) Homologous structure‐function relationships between native fibrocartilage and tissue engineered from MSC‐seeded nanofibrous scaffolds. Biomaterials, 32, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabbruwe, M.B. , Kafienah, W. , Tarlton, J.F. , Mistry, S. , Fox, D.J. & Hollander, A.P. (2010) Repair of meniscal cartilage white zone tears using a stem cell/collagen‐scaffold implant. Biomaterials, 31, 2583–2591. [DOI] [PubMed] [Google Scholar]
- Pak, J. , Lee, J.H. & Lee, S.H. (2014) Regenerative repair of damaged meniscus with autologous adipose tissue‐derived stem cells. BioMed Research International, 2014, 436029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli, C. , Grogan, S.P. , Patil, S. , Otsuki, S. , Hasegawa, A. , Koziol, J. et al. (2011) Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage, 19, 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty, C.A. & Lubowitz, J.H. (2011) Does arthroscopic partial meniscectomy result in knee osteoarthritis? Arthroscopy: The Journal of Arthroscopic and Related Surgery, 27, 419–424. [DOI] [PubMed] [Google Scholar]
- Port, J. , Jackson, D.W. , Lee, T.Q. & Simon, T.M. (1996) Meniscal repair supplemented with exogenous fibrin clot and autogenous cultured marrow cells in the goat model. The American Journal of Sports Medicine, 24, 547–555. [DOI] [PubMed] [Google Scholar]
- Rangger, C. , Klestil, T. , Gloetzer, W. , Kemmler, G. & Benedetto, K.P. (1995) Osteoarthritis after arthroscopic partial meniscectomy. The American Journal of Sports Medicine, 23, 240–244. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Ibán, M.Á. , Díaz‐Heredia, J. , García‐Gómez, I. , Gonzalez‐Lizán, F. , Elías‐Martín, E. & Abraira, V. (2011) The effect of the addition of adipose ‐derived mesenchymal stem cells to a meniscal repair in the avascular zone; an experimental study in rabbits. Arthroscopy: The Journal of Arthroscopic and Related Surgery, 27(12), 1688–1696. [DOI] [PubMed] [Google Scholar]
- Sheehan, D.C. & Hrapchak, B.B. (1980) Theory and practice of histotechnology, 2nd edition. St. Louis, MO: Mosby. [Google Scholar]
- Slaoui, M. & Fiette, L. (2011) Histopathology procedures: from tissue sampling to histopathological evaluation. In: Clifton, N.J. (Ed.) Drug Safety Evaluation: Methods and Protocols, Methods in Molecular Biology. Totowa, NJ: Humana Press, Springer Science+Business Media, LLC, Vol. 691, pp. 69–82. [DOI] [PubMed] [Google Scholar]
- Spencer, S.J. , Saithna, A. , Carmont, M.R. , Dhillon, M.S. , Thompson, P. & Spalding, T. (2012) Meniscal scaffolds: early experience and review of the literature. The Knee, 19, 760–765. [DOI] [PubMed] [Google Scholar]
- Sweigart, M.A. & Athanasiou, K.A. (2001) Toward tissue engineering of the knee meniscus. Tissue Engineering, 7, 111–129. [DOI] [PubMed] [Google Scholar]
- Takroni, T. , Laouar, L. , Adesida, A. , Elliott, J.A.W. & Jomha, N.M. (2016) Anatomical study: comparing the human, sheep and pig knee meniscus. Journal of Experimental Orthopaedics, 3, 35. 10.1186/s40634-016-0071-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski, R. , Wiktor, Ł. & Gap, A. (2019) Enhancement of cartilage repair through the addition of growth plate chondrocytes in an immature skeleton animal model. Journal of Orthopaedic Surgery and Research, 14, 260. 10.1186/s13018-019-1302-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchio, Y. , Ochi, M. , Adachi, N. , Kawasaki, K. & Iwasa, J. (2003) Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second‐look arthroscopy. Arthroscopy: The Journal of Arthroscopic and Related Surgery, 19, 463–469. [DOI] [PubMed] [Google Scholar]
- Visna, P. , Adler, J. , Pasa, L. , Kocis, J. , Cizmar, I. & Horky, D. (2003) Autologous chondrocyte transplantation for the treatment of articular defects of the knee. Scripta Medica (Brno), 76(3), 241–250. [Google Scholar]
- Von Der Mark, K. , Gauss, V. , Von Der Mark, H. & Müller, P. (1977) Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature, 267, 531–532. [DOI] [PubMed] [Google Scholar]
- Walker, P.S. & Erkman, M.J. (1975) The role of the meniscus in force transmission across the knee. Clinical Orthopedics, 109, 184–192. [DOI] [PubMed] [Google Scholar]
- Wei, L.C. , Gao, S.G. , Xu, M. , Jiang, W. , Tian, J. & Lei, G.H. (2012) A novel hypothesis: the application of platelet‐rich plasma can promote the clinical healing of white–white meniscal tears. Medical Science Monitor, 18, 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C.A. , Lundberg, M. , Hamberg, P. , DeHaven, K.E. & Gillquist, J. (1989) Non‐operative treatment of meniscal tears. The Journal of Bone and Joint Surgery, 71‐A, 811–822. [PubMed] [Google Scholar]
- Whitehouse, M.R. , Howells, N.R. , Parry, M.C. , Austin, E. , Kafienah, W. , Brady, K. et al. (2017) Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first‐in‐human study. Stem Cells Translational Medicine, 6(4), 1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, T. , Deie, M. , Shinomiya, R. , Izuta, Y. , Yasunaga, Y. , Yanada, S. et al. (2005) Meniscal regeneration using tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. Journal of Biomedical Materials Research Part A, 75, 23–30. [DOI] [PubMed] [Google Scholar]
- Yu, H. , Adesida, A.B. & Jomha, N.M. (2015) Meniscus repair using mesenchymal stem cells ‐ a comprehensive review. Stem Cell Research and Therapy, 6(1), 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner, J. , Hierl, K. , Mueller, M. , Pfeifer, C. , Berner, A. , Dienstknecht, T. et al. (2013) Stem cell‐based tissue‐engineering for treatment of meniscal tears in the avascular zone. Journal of Biomedical Materials Research Part B, 101(7), 1133–1142. [DOI] [PubMed] [Google Scholar]
- Zellner, J. , Pattappa, G. , Koch, M. , Lang, S. , Weber, J. , Pfeifer, C.G. et al. (2017) Autologous mesenchymal stem cells or meniscal cells: what is the best cell source for regenerative meniscus treatment in an early osteoarthritis situation? Stem Cell Research and Therapy, 8(1), 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared on request.


