Abstract
The vertebrate transition to land is one of the most consequential, yet poorly understood periods in tetrapod evolution. Despite the importance of the water–land transition in establishing modern ecosystems, we still know very little about the life histories of the earliest tetrapods. Bone histology provides an exceptional opportunity to study the biology of early tetrapods and has the potential to reveal new insights into their life histories. Here, we examine the femoral bone histology from an ontogenetic series of Greererpeton, an early tetrapod from the Middle‐Late Mississippian (early Carboniferous) of North America. Thin‐sections and micro‐CT data show a moderately paced rate of bone deposition with significant cortical thickening through development. An interruption to regular bone deposition, as indicated by a zone of avascular tissue and growth marks, is notable at the same late juvenile stage of development throughout our sample. This suggests that an inherent aspect to the life history of juvenile Greererpeton resulted in a temporary reduction in bone deposition. We review several possible life history correlates for this bony signature including metamorphosis, an extended juvenile phase, environmental stress, and movement (migration/dispersal) between habitats. We argue that given the anatomy of Greererpeton, it is unlikely that events related to polymorphism (metamorphosis, extended juvenile phase) can explain the bony signature observed in our sample. Furthermore, the ubiquity of this signal in our sample indicates a taxon‐level rather than a population‐level trait, which is expected for an environmental stress. We conclude that movement via dispersal represents a likely correlate, as such events are a common life history strategy of aquatically bound vertebrates.
Keywords: bone histology, Carboniferous, dispersal, early tetrapod, femur
The bone histology from an ontogenetic series of the early tetrapod, Greererpeton, reveals a peculiar life history event highlighted by the yellow band in our schematic. It is possible that the period of stress endured by juvenile Greererpeton represents a dispersal event from natal to adult waterbodies.
1. INTRODUCTION
One of the most significant transitions in vertebrate evolution was the emergence of terrestrial tetrapods from previously water‐bound fish. Fossils that document tetrapod origins and their subsequent diversification onto land are largely regarded as amphibious with varying levels of terrestriality, as gleaned from gross morphological indicators (Clack, 2012; Dickson et al., 2021; Schoch, 2014). Regardless of any degree of terrestriality, these animals were likely reproductively tied to water, similar to modern amphibians, and spent at least a portion of their lives in an aquatic setting (Clack, 2012; Schoch, 2014). Despite the importance of the water–land transition in establishing modern ecosystems, enduring questions about the life histories of early tetrapods remain, such as how they grew or what proportion of their life cycles were spent in water versus on land. A major impediment to addressing these questions is the lack of ontogenetic data for the vast majority of early tetrapod fossils (Callier et al., 2009).
Bone histology provides an unparalleled opportunity to reveal life histories of extinct taxa and can serve as an important tool in understanding the water–land transition. Previous histological studies on early tetrapods have primarily focused on late Devonian or early Permian taxa. In Devonian taxa, such as the fin‐bearing tetrapodomorphs Eusthenopteron (Laurin et al., 2007; Meunier & Laurin, 2012; Sanchez et al., 2014) and Hyneria (Kamska et al., 2018) and the early tetrapod Acanthostega (Sanchez et al., 2016), histological studies have suggested particular life history traits including delayed limb ossification with an extended juvenile stage of development, low metabolic rate, and a largely aquatic lifestyle. With some exception (Laurin et al., 2007; Meunier & Laurin, 2012), these analyses have been conducted on the humerus, an element that is taxonomically informative for these groups yet is highly disparate from the morphology of modern tetrapod limb bones that have been used to validate paleohistological interpretations (Castanet et al., 2000; De Margerie et al., 2002; Margerie et al., 2004; Montes et al., 2007). Permian crown tetrapods, on the other hand, have been extensively sampled and have demonstrated a wide range of established ecologies and life history strategies (e.g., Bazzana et al., 2020; Boitsova et al., 2019; Estefa et al., 2020; Gee, Haridy & Reisz, 2020; Huttenlocker & Shelton, 2020; Konietzko‐Meier et al., 2016; Sanchez & Klembara, 2008).
Histological studies from the intervening Carboniferous Period are largely lacking, due to the rarity of tetrapod fossils preserved in Mississippian rock (Anderson et al., 2015; Clack, 2002; Coates & Clack, 1995; Otoo et al., 2019; Romer, 1956; Warren & Turner, 2004). Other than two studies examining pathological bone (Bishop et al., 2015; Herbst et al., 2019) and vertebral elements in a few Carboniferous taxa (Danto et al., 2016, 2017), there is a scarcity of osteohistological data for nearly 60 million years of tetrapod evolution (Figure 1). However, it is during this time period that the critical transition from fully aquatic to fully terrestrial tetrapods is inferred to have taken place (Dickson et al., 2021; Figure 1). Thus, improving histological and taxonomic sampling has the potential to reveal important insights into the growth, life histories, and potential ecologies of early tetrapods.
FIGURE 1.
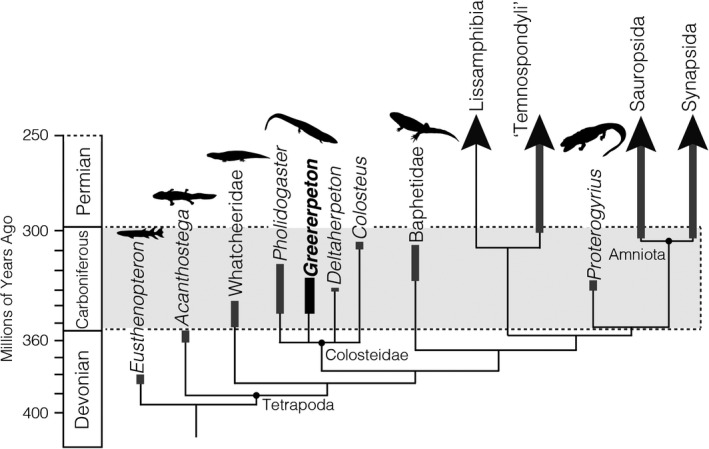
A simplified phylogeny on a geological timescale highlighting Carboniferous tetrapods and their temporal ranges (thickened bars). Greererpeton (bold) and other colosteids do not have a resolved phylogeny but are generally considered early diverging tetrapods. Further phylogenetic context is provided for modern tetrapod groups—lissamphibians, sauropsids (i.e., reptile line), and synapsids (i.e., mammal line)—as well as the stem amniote Proterogyrinus that commonly co‐occurs with Greererpeton. The phylogeny is adapted from Dickson et al. (2021) and silhouettes are sourced from PhyloPic
A major group of Carboniferous tetrapods is the Colosteidae (Cope, 1875), a clade of aquatically adapted predators with lateral systems, elongated trunks and tails, and comparatively short limbs (Clack, 2012; Clack & Milner, 2015; Godfrey, 1989a, 1989b; Smithson, 1982). Although the clade remains phylogenetically unstable (Clack et al., 2016; Ruta et al., 2003), it is generally considered an early diverging group of tetrapods (Figure 1) from the Middle Mississippian–Pennsylvanian (c. 345–315 mya) of North America and the United Kingdom (Clack, 2012; Clack & Milner, 2015). There are at least four described genera of colosteids, including Colosteus, Pholidogaster, and Deltaherpeton, and the largest and most well‐known, Greererpeton (Clack & Milner, 2015). Contrary to the trend of rare Carboniferous tetrapod fossils, Greererpeton is an abundant component of the fossiliferous sites of West Virginia (Godfrey, 1989a, 1989b; Smithson, 1982), Illinois (Bolt & Lombard, 2001; Schultze & Bolt, 1996), and possibly other localities in Kentucky (Greb et al., 2016). These Middle–Upper Mississippian (c. 345–325 mya) sites preserve freshwater alluvial systems with indications of brackish influences (Godfrey, 1989b; Greb et al., 2016) and existed in a warm, humid climate (Kahmann & Driese, 2008; Miller & Eriksson, 1999). The paleoenvironmental reconstructions of the Late Mississippian Appalachian Basin generally indicate an aquatic setting that varied with seasonality (i.e., flooding and drying; Kahmann & Driese, 2008; Miller & Eriksson, 1999), in terrain (i.e., upper oxbow systems) (Greb et al., 2016), and salinity (i.e., brackish incursions; Greb et al., 2016) indicating that aquatic inhabitants of these environments lived in flux.
The extraordinary abundance of Greererpeton fossils has provided an important window into the gross anatomy of a critical early tetrapod, including details on its ontogenetic growth (Godfrey, 1989a, 1989b). However, it has never been studied from a histological perspective. Here, we provide a new osteohistological dataset from an ontogenetic series of Greererpeton femora from the Middle–Upper Mississippian (early Carboniferous) deposits of West Virginia. We describe changes in growth patterns and bone deposition during Greererpeton development and reveal a previously unrecognized life history event that occurred during juvenile growth. We conclude by reviewing several possible explanations for this distinct and consistent histological signal in our sample.
2. METHODS
2.1. Specimen selection
Our sample consisted of four isolated femora and one humerus from the Museum of Comparative Zoology (MCZ). The femora MCZ VP 101550, MCZ VP 101552, and MCZ VP 101553 were collected from the Hinton Bonebed (Summers County, West Virginia), while MCZ VP 101557 and the humerus MCZ VPRA‐8952 were collected from the Greer Quarry (Mason Country, West Virginia). The Hinton Bonebed generally contains more fragmentary, disarticulated material, while the Greer Quarry preserves articulated material (Romer, 1941, 1969; Smithson, 1982). Although distinct, these two sites have previously been discussed with a reasonable geographic and geological overlap, though the Hinton Bonebed (Hinton Formation, Serpukhovian) is slightly younger than the Greer Quarry (Bluefield Formation, Late Viséan–Early Serpukhovian; Hotton, 1970; Panchen, 1970; Smithson, 1982). The four femoral specimens vary in size, comparable to the known size range described for Greererpeton (Godfrey, 1989b), with MCZ VP 101550 representing a small individual, MCZ VP 101552 and MCZ VP 101553 representing mid‐sized individuals, and MCZ VP 101557 representing a particularly large individual.
The three femora histologically sampled (MCZ VP 101550, VP 101552, and VP 101557) are fragmentary distal ends with intact metaphases (Figure S1). In addition, we scanned with micro‐computed tomography (μCT) a fourth, more complete femur (MCZ VP 101553; Figure S1). These femora are recovered from sites highly dominated by two tetrapod taxa: Greererpeton and Proterogyrinus. Greererpeton femora are distinct from Proterogyrinus in that they are gracile with a narrow condylar region where both the anterior and posterior condyles are comparatively sized and with an adductor crest that runs parallel rather than diagonal to the long axis of the bone (Figure S1; Godfrey, 1989b). Given the distinct anatomical divergence from Proterogyrinus, and the overwhelming abundance of Greererpeton from both the Hinton and Greer Quarry localities (Godfrey, 1989b), we classify our ontogenetic series as cf. Greererpeton, with the caveat that we lack apomorphies to classify our sample to the genus.
2.2. Thin sections
We made cross‐sectional thin sections of all three distal femora from as close to the mid‐diaphysis as possible, given the fragmentary nature of these specimens. Longitudinal sections were also made at the distal epiphyses of MCZ VP 101550 and VP 101552. All thin sections were made subsequent to standard specimen preservation and we followed protocols developed by Lamm (2013).
2.3. μ‐CT
The well‐preserved and complete femur MCZ VP 101553 and the humerus MCZ VPRA‐8952 were scanned using high‐resolution micro‐computed tomography (μ‐CT). The humerus was included as it is often used to describe bone histology in early tetrapods and it allowed us to make broad‐scale comparisons with the femoral sample. Both specimens were scanned using a Bruker Skyscan 1173 Micro‐CT system in the MCZ Digital Imaging Facility using 130 kV and 61 µm, with a resolution of 7.5 μm (MCZ VP 101553) and 20 μm (MCZ VPRA‐8952) and a 0.25 mm brass filter.
3. RESULTS
3.1. MCZ VP 101550
Cross sections through the shaft of the smallest specimen reveal a thick cortical wall and a large medullary cavity that makes up 35% of the total cross‐sectional area (Figure 2a). A poorly developed adductor crest is present on the ventral surface and the ventral cortex lacks abundant vascularity, although some radially oriented canals tend to be ventrally located (Figure 2a). Trabeculae are in low abundance throughout the medullary cavity forming only narrow spicules (Figure 2a). A slender band of endosteal deposition occurred as evidenced by the lamellar‐fibred bone that lines much of the endosteal surface with some development of trabecular spicules (Figure 2b). Besides the narrow layer of endosteally deposited tissue, the endosteal half of the cortex is composed of well‐vascularized woven‐ to parallel‐fibered tissue. These vascular canals are generally longitudinally oriented and there are large secondary osteons in addition to primary canals (Figure 2b). The periosteal half of the cortex is composed of poorly vascularized parallel‐fibered bone (Figure 2b). The few osteons that are present are primary canals and tend toward a more radial orientation (Figure 2b). At the periosteal surface, several layers of growth marks (approximately 10) are apparent in largely avascular parallel‐fibered matrix that measures around 600 µm (Figure 2b).
FIGURE 2.
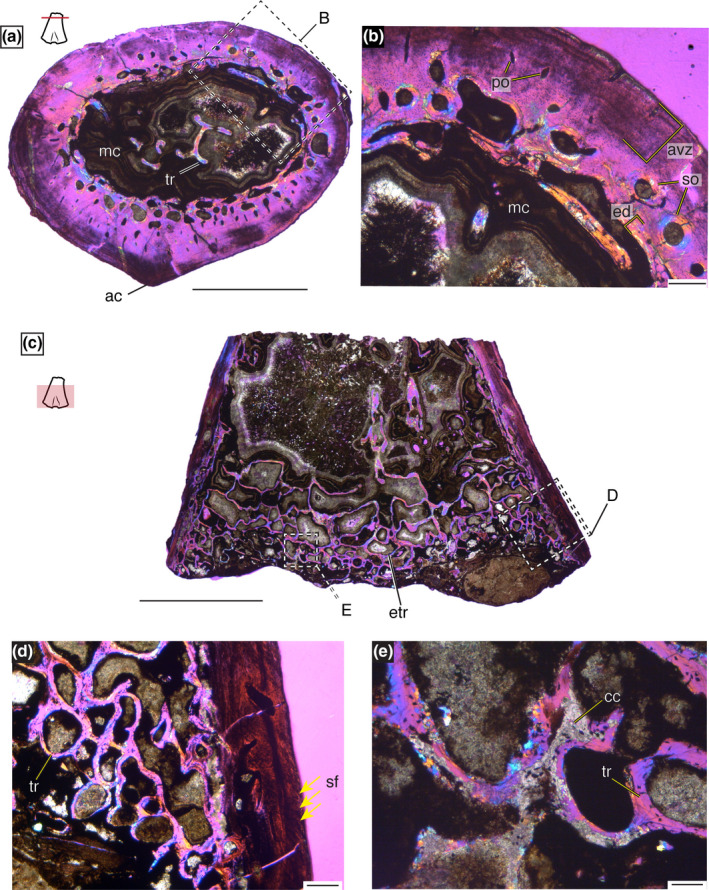
The histology of MCZ VP 101550 under cross‐polarized light with a lambda filter. (a) Entire cross‐sectional view of the metaphyseal section displays a robust cortex with little trabeculae in the medullary cavity and a poorly developed adductor crest. Scale bar = 2.5 mm. (b) High magnification of the cortex displaying three layers of tissue: endosteally deposited lamellar bone, fibrolamellar to parallel‐fibered vascularized tissue, and a periosteally deposited avascular zone with growth marks. Scale bar = 250 µm. (c) Longitudinal section through the distal portion of MCZ VP 101550 with abundant epiphyseal trabeculae that is greatly reduced toward the metaphysis. Scale bar = 5 mm. (d) High magnification of the cortex shows abundant Sharpey's fibers (arrows) and trabeculae in longitudinal view. Scale bar = 250 µm. (e) High magnification of active trabeculae building with both bone and calcified cartilage present. Scale bar = 100 µm. ac, adductor crest; avz, avascular zone; cc, calcified cartilage; ed, endosteal deposition; etr, epiphyseal trabeculae; mc, medullary cavity; po, primary osteon; sf, Sharpey's fibers; so, secondary osteon; tr, trabeculae
The distal longitudinal section of MCZ VP 101550 indicates that the epiphysis was largely unossified (Figure 2c). Trabeculae are rare in the metaphysis with increasing density toward the epiphysis (Figure 2c). The cortical bone preserves abundant, thick Sharpey's fibers arranged at nearly 45° angle to the long axis of the bone that likely represent sites of muscle attachment (Figure 2d). Calcified cartilage is present at points of longitudinal extension and at sites of trabeculae building indicating active longitudinal growth (Figure 2e).
3.2. MCZ VP 101552
MCZ VP 101552 preserves more of the diaphysis compared to the other two specimens histologically sectioned here. The specimen has been slightly taphonomically compressed, making the cross‐sectional circumference more dorsoventrally elongate. Thick cortical walls make up the majority of the cross section, and the medullary cavity (15% of total cross‐sectional area, although the specimen is laterally compressed) contains well‐developed trabeculae (Figure 3a). Some endosteal deposition is present giving rise not only to the endosteal‐most portion of the cortex but the trabeculae as well (Figure 3b). Moving periosteally, a region of vascularized woven‐ to parallel‐fibered bone is present with longitudinally oriented vasculature; this region has prevalent secondary remodeling that gives rise to large open vascular canals, as well as more constricted secondary osteons (Figure 3c). The adductor crest is well developed and, although the shape has likely been altered by taphonomic compression, there is evidence of increased cortical remodeling ventrally (Figure 3a).
FIGURE 3.
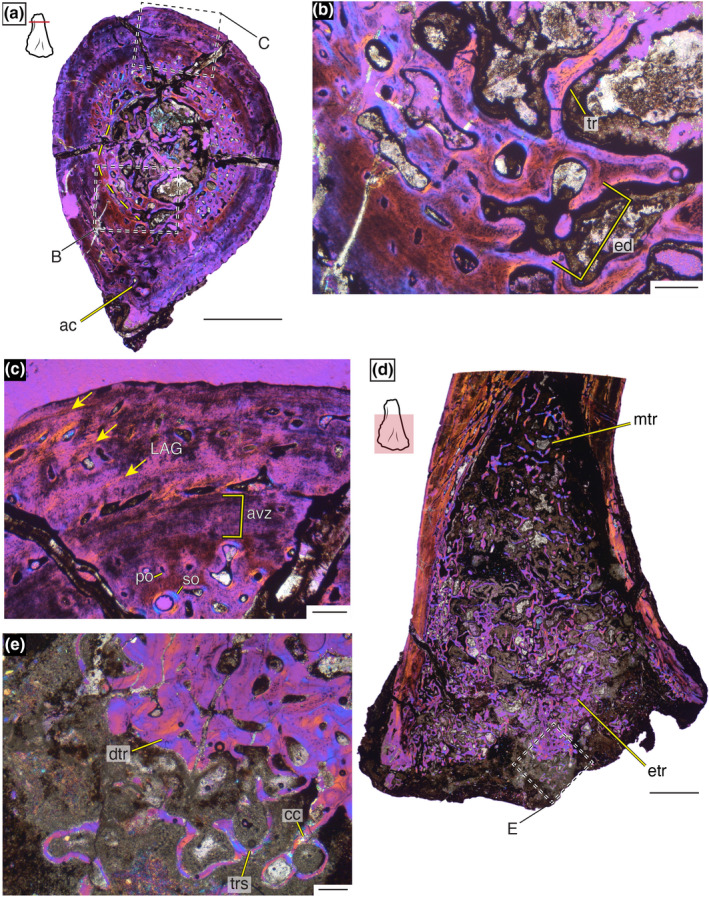
The histology of MCZ VP 101552 under cross‐polarized light with a lambda filter. (a) Cross‐sectional image of the transverse metaphyseal section reveals dense trabeculae in the medullary cavity and a developed, although taphonomically shifted, adductor crest. Yellow dashed line represents original endosteal surface. Scale bar = 2.5 mm. (b) High‐resolution image of endosteally deposited tissue and trabecular extensions into the medullary cavity. Scale bar = 250 µm. (c) Magnified image of the dorsal portion of the cortex highlighting three layers of tissue: vascularized endosteal portion of the cortex, an avascular zone with growth marks, and a layer that consists of vascularized tissue punctuated by LAGs (arrows). Scale bar = 250 µm. (d) Longitudinal section through the distal portion of MCZ VP 101552 highlights the variation in trabeculae at the epiphysis and metaphysis. Scale bar = 2.5 mm. (e) High magnification of a distal region of the epiphysis where both dense and loose trabeculae are found, and active trabeculae building is evident. Scale bar = 250 µm. ac, adductor crest; avz, avascular zone; dtr, dense trabeculae; ed, endosteal deposition; etr, epiphyseal trabeculae; LAG, line of arrested growth; mtr, metaphyseal trabeculae; po, primary osteon; so, secondary osteon; tr, trabeculae; trs, trabecular spicules
Periosteal to the well‐vascularized tissue layer is a distinct shift in tissue arrangement. This region is composed of generally avascular tissue with neatly organized layers of osteocytes arranged in parallel‐fibred tissue (Figure 3c). Although not uniform around the circumference of the bone, this region is approximately 400 µm thick and has about seven growth marks. Periosteal to this region, single layers of well‐organized longitudinal (although occasionally anastomosed) primary osteons in parallel‐fibred matrix are punctuated by single lines of arrested growth (LAGs; Figure 3c). The periosteal surface is scalloped with partial vascular canals and there is no evidence of an external fundamental system (EFS; Figure 3c).
A longitudinal section of the distal end of MCZ VP 101552 reveals an epiphysis that was not fully ossified and a medullary cavity with increasingly densely packed trabeculae toward the epiphysis (Figure 3d). The distal‐most portion of the femur is composed of both dense and loose trabeculae with evidence of calcified cartilage, suggesting at least some active trabeculae building, as well as calcified cartilage caps at the distal ends of the cortical bone, indicating active longitudinal extension (Figure 3e).
3.3. MCZ VP 101553
μ‐CT scans of the complete femur, MCZ VP 101553, contain lower‐resolution details, but vasculature and trabecular organization is discernable. Generally, the micro‐anatomy of the comparably sized MCZ VP 101552 is similar to what is observable in MCZ VP 101553. In longitudinal section (Figure 4a), there are apparent changes in trabecular density and cortical thickness at the diaphysis, the metaphyses, and the epiphyses. At the diaphysis, there is a narrow medullary space (14% of cross‐sectional area) encapsulated by the formation of circular trabeculae (hereinafter referred to as an endosteal ring) and large secondary and primary osteons are visible, although it is likely that many smaller osteons are present but are not visible at this resolution (Figure 4a,c). The proximal (Figure 4b) and distal (Figure 4d) metaphyses show an expansion of the medullary space and reduced compacta. This proximal and distal expansion of the medullary space takes place abruptly from the mid‐shaft within the diaphysis and continues until there are only narrow cortical borders at the epiphyses (Figure 4a). Metaphyseal trabecular networks are composed of more loosely packed large boney struts (Figure 4a,b), while epiphyseal trabeculae are abundant, narrow boney struts (Figure 4a,d).
FIGURE 4.
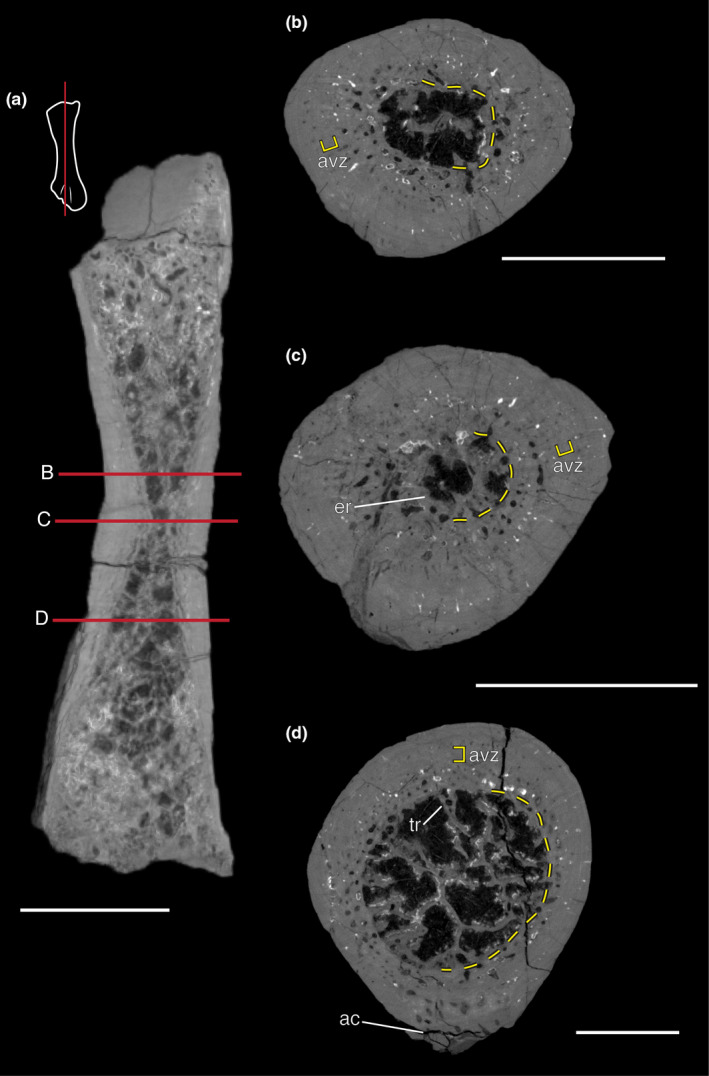
Micro‐CT imagery of the more complete femur MCZ VP 101553. (a) Longitudinal section with indicators (red lines) of where representative cross‐sections were taken. Notice a thickening of the cortex toward the mid‐shaft. (b) The proximal section that was taken at the interface of the diaphysis and metaphysis represent an intermedial cortical thickness and medullary cavity size. (c) At the diaphysis, the medullary cavity is dramatically reduced and encircled by tissue that has been endosteally deposited. (d) A distal section at the metaphysis highlights a trabecular network that extends from the endosteal surface of the cortex. The adductor crest on this specimen is significantly worn but still visible in this portion of the femur. In all specimens, a faint avascular zone is discernable and the purported original endosteal surface is indicated by a yellow dashed lined. Cross sections are not to scale and all scale bars = 5 mm. ac, adductor crest; avz, hypothesized avascular zone; er, endosteal ring; po, primary osteon; so, secondary osteon; tr, trabeculae
3.4. MCZ VP 101557
The cross section of the largest femur, MCZ VP 101557, shows a very thick cortex with a narrow (2% of cross‐sectional area) medullary cavity (Figure 5a). Trabecular development and endosteal deposition appear to have given rise to the narrow medullary cavity. A few bony spicules occupy the center of the medullary cavity and are extensions of an endosteal ring formed by lamellar bone (Figure 5b). This endosteal ring is separated from the cortex by bony struts that appear to be extensions of endosteally deposited tissue (Figure 5b). This portion of the cortex is composed of radially oriented primary vascular canals in a woven matrix with plump and abundant osteocyte lacunae. There is a distinct delineation in tissue organization that occurs in portions of the cortex that have not been remodeled (Figure 5c). There is also increased secondary remodeling compared to more periosteal tissue, indicating more recently deposited tissue (Figure 5c,d). Both the tissue organization and secondary remodeling suggest that this woven tissue was endosteally deposited.
FIGURE 5.
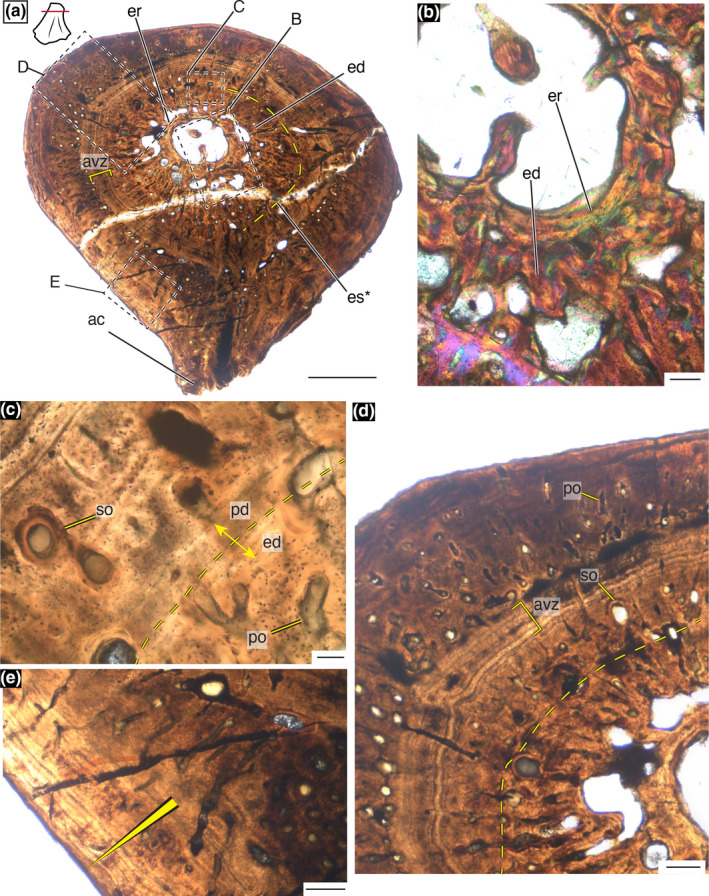
The cross‐sectional histology of MCZ VP 101557. (a) Entire cross‐section with a robust adductor crest and significant endosteal deposition from the original endosteal surface. Scale bar = 2.5mm. (b) High magnification under cross‐polarized light with a lambda plate demonstrates the unorganized tissues of the endosteally deposited bone and the more lamellar organization to the endosteal ring that defines a narrow medullary cavity. Scale bar = 250 µm. (c) Details of the delineation between periosteally deposited bone and endosteally deposited bone. Scale bar = 100 µm. (d) The avascular zone is sandwiched between layers of vascularized tissue. Scale bar = 1000 µm. (e) At the periosteal surface (yellow tag), vascularity becomes less abundant and growth marks become more abundant indicating at least the onset of skeletal maturity. Scale bar = 250 µm. ac, adductor crest; avz, avascular zone; ed, endosteal deposition; er, endosteal ring; es*; original endosteal surface; po, primary osteon; so, secondary osteon
Periosteally, canals become more longitudinally oriented and secondary remodeling becomes more prevalent toward the mid‐cortex after which point a distinctive shift in tissue occurs (Figure 5d). A region of avascular lamellar bone is present in the mid‐cortex containing 7–10 growth marks and is approximately 450 µm thick (Figure 5d). Periosteal to this region, longitudinal and occasionally, radially oriented canals in parallel‐fibred bone make up nearly half of the cortex (Figure 5d). Gradually, this bone transitions periosteally to a poorly vascularized tissue composed of parallel‐ to lamellar‐fibred matrix with loosely packed growth marks (Figure 5e).
3.5. MCZ VPRA‐8952
High‐density mineral inclusions of this specimen render internal microstructure analysis of the humerus challenging. However, there are evident anatomical distinctions between the humerus and femur of Greererpeton that influence life history interpretations. As in other early tetrapod humeri (Ruta et al., 2003; Dickson et al., 2021), the L‐shape morphology of MCZ VPRA‐9852 is formed by a narrow cortex and abundant trabecular network (Figure 6a). The cortex is thickest at the “mid‐diaphysis” and particularly thin at the distal epiphysis (Figure 6b). From the available resolution, it appears that the cortex is largely avascular.
FIGURE 6.
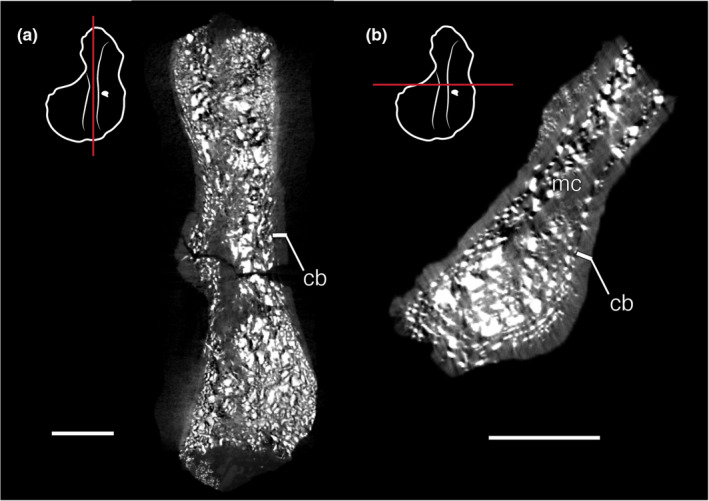
Micro‐CT imagery of Greererpeton humerus MCZ VPRA‐8952. In both longitudinal (a) and cross‐section (b), the cortex is remarkably narrow compared to the femur, and a trabecular network constitutes the vast majority of the internal structure. Scale bars = 5 mm. cb, cortical bone; mc, medullary cavity
4. DISCUSSION
4.1. Growth patterns through ontogeny
From the specimens examined here, we propose a growth series for Greererpeton bone histology (Figure 7). Although interspecific variation likely occurs to a certain extent, we describe commonalities within samples that reflect a generalized growth pattern for Greererpeton. As in the most modern tetrapods, the earliest stages of bone deposition record a relatively rapid growth rate as indicated by the more densely vascularized endosteal tissue. However, following this initial period, growth was dramatically slowed for an extended period of time, as evident by a portion of the cortex (400–600 µm) that is composed of avascular, lamellar‐ to parallel‐fibred tissue with 7–10 growth marks (Figure 7e–g). These growth marks do not appear as hyper‐mineralized distinct lines of arrested growth (LAGs) nor do they appear as a distinct layers of parallel‐fibered bone as in annuli (Huttenlocker et al., 2013). As such, we define this large band of tissue present throughout our histological sample simply as an avascular zone. Growth resumes following this period of slowed deposition with an abrupt shift to tissues that contain primarily, but not exclusively, radially oriented canals in a largely parallel‐fibred matrix, and with occasional single LAGs (Figures 3c and 5d). This stage of ontogeny and bone deposition makes up a considerable portion of the cortex in the largest specimen. However, there is a gradual slowing toward the periosteal surface (1000 µm) that is characterized by more frequent growth marks and reduced vascularity, suggesting at least the approach of skeletal maturity (Figure 5e). Concurrently, there is increased endosteal deposition and trabeculae building that dramatically narrows the medullary cavity (from 35% to 2% of the cross‐sectional area). This narrowing in subadult specimens (e.g., MCZ VP 101552, and VP 101553) is constrained to the diaphysis where an endosteal ring initially forms. However, with increasing size, endosteal deposition is initiated in the metaphysis resulting in an endosteal ring throughout the shaft of the bone as observed in the metaphysis of the very large adult included in our sample (MCZ VP 101557).
FIGURE 7.
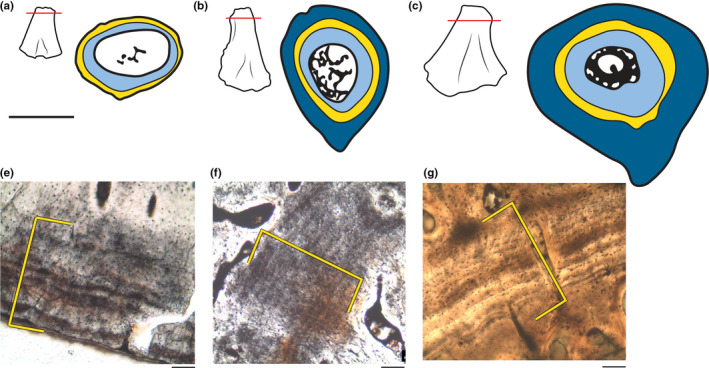
Summary growth series of Greererpeton as established by the femoral bone histology presented in this study. Early bone growth is characterized by vascularized, primarily longitudinally oriented canals in a woven‐ to parallel‐fibered tissue (light blue). This relatively elevated rate of bone deposition is interrupted by an avascular zone that contains 7–10 growth marks (yellow band in a–c and the brackets e–g). In the subadult (b,f) and adult specimens (c,g), bone deposition resumes with periosteal deposition of vascularized tissue in a parallel‐fibered matrix (dark blue) and endosteal development of trabeculae that continues until only a narrow medullary cavity is present (black)
Histological data confirm that relative size reflects distinct ontogenetic stages in our sample. The smallest specimen, MCZ VP 101550, has a thick avascular zone at its periosteal surface and lacks calcified cartilage in the medullary cavity (Figure 2); two features that would typically indicate later stages of ontogeny (Calderón et al., 2019; Cormack, 1979; Estefa et al., 2020, 2021; Horner et al., 1999; Jordana et al., 2016; Nacarino‐Meneses et al., 2016; Sanchez et al., 2014; Sanchez et al., 2016). Based on these criteria alone, we may interpret MCZ VP 101550 as an adult of a different, smaller‐bodied species, regardless of the morphological similarities, suggesting similar taxonomic assignment. However, there is evidence of calcified cartilage in the distal epiphysis indicating active longitudinal extension and bone growth (Figure 2d,e). Furthermore, the avascular zone at the periosteal surface of MCZ VP 101550 is captured in the mid‐cortex of the larger specimens (Figures 3a, 4b–d and 5a). Therefore, the interpretation of the histology of MCZ VP 101550 requires an adjustment when placed in an ontogenetic context. Rather than an adult of a smaller species, it is evident that MCZ VP 101550 represents a juvenile Greererpeton. This reiterates the importance of collecting an ontogenetic sample when applying bone histology to describe growth strategies in the fossil record.
In addition to fluctuations in depositional rate through ontogeny, there is an increased development of the adductor crest in Greererpeton. The adductor crest forms on the distal ventral surface of the femoral shaft and is presumably the insertion site of the m. adductor femoralis (Holmes, 2003; Molnar et al., 2020; Smithson, 1985). Early in Greererpeton ontogeny, the cross section of the adductor crest extends only slightly ventrally forming a small protrusion of the cortex (Figure 2a). This diminutive adductor crest is a feature noted in juvenile early tetrapods (Clack & Finney, 2005). By subadulthood, the adductor crest extends much more ventrally and is formed by the coupling of endosteal secondary remodeling and rapid periosteal deposition (Figure 3a). Finally, by near skeletal maturity, the adductor crest on the distal metaphysis has a significant ventral extension with abundant Sharpey's fibers indicating a ligamentous attachment for the m. adductor femoralis (Figure 5a). Bone is highly susceptible to mechanical inputs and will remodel and deposit according to loading stressors (Carter et al., 1996; Enlow, 1963; Goldman et al., 2009; Hoyte & Enlow, 1966; Mcfarlin et al., 2008; Parfitt, 2010; Rabey et al., 2015) that will increase through ontogeny and with body size (Goldman et al., 2009; Montoya‐Sanhueza & Chinsamy, 2017; Sumner & Andriacchi, 1996) even in aquatic and semi‐aquatic tetrapods (Leclair & Castanet, 1987; Witten & Huysseune, 2007).
It is crucial that the growth series presented here is from data captured in the thick femoral cortices, as it provides a robust record of bone deposition through the animals’ life span. This is in stark contrast with the narrow cortex preserved in the humerus (Figure 6), an element that has been a significant source of data in other early tetrapod life history studies (e.g., Sanchez et al., 2014; Sanchez et al., 2016). For early tetrapods, whose humeri are morphologically distinct from modern tetrapods, this study raises caution when selecting appendicular elements for histological analysis, as different bones may preserve varying amounts of the animal's growth and life history.
4.2. Periods of slowed bone deposition
Our ontogenetic series documents two substantial periods of slowed bone deposition; one early in ontogeny that is recorded throughout the femoral sample as an avascular zone (Figure 7e–g) and another one later in ontogeny at the periosteal surface (Figure 5e). These two zones, while both representing slowed bone deposition, are histologically distinct. The avascular zone early in ontogeny is defined by an abrupt shift in tissue type at both the endosteal and periosteal surfaces, whereas the late‐stage slowed growth shows a gradual shift in bone tissue that is more consistent with an animal approaching skeletal maturity. Although the trend toward skeletal maturity is supported by the size of MCZ VP 101557, which is among the largest specimens known from Greererpeton (Godfrey, 1989b), the periosteal surface lacks the tightly packed LAGs commonly associated with a true EFS, a feature associated with skeletal and/or sexual maturity in many amniotes (e.g., Jordana et al., 2016; Nacarino‐Meneses et al., 2016; Woodward et al., 2013).
In addition to these substantial zones of reduced depositional rate, there is variable evidence of seasonal signals in our Greererpeton sample. Seasonally stressed environments commonly result in the complete, or nearly complete, cessation of bone deposition in a variety of vertebrates, often resulting in LAGs or other growth marks at regular, periodic intervals (Castanet, 1994; Castanet & Smirina, 1990; Köhler & Moyà‐Solà, 2009). To endure seasonally unfavorable conditions, vertebrates undertake a variety of energy‐saving strategies (i.e., torpor) including hibernation, daily torpor, brumation, and aestivation (Geiser, 2013). Many modern amphibians and aquatic reptiles, who often serve as analogues for early tetrapod ecology and physiology, undergo seasonal aestivation or brumation to conserve energy. This results in complete cessation of bone deposition leaving single or double LAGs in the bone histology that represents a periodic shutdown of metabolic activity (Castanet, 1994; Sanchez, Steyer, et al., 2010).
In our sample, there are at least three single LAGs alternating with periods of active growth and, thus, likely represent a seasonal signal (Figure 3c). However, these seasonal signals are not universally present in the subadult and adult specimens. While the subadult specimen, MCZ VP 101552, displays exemplar LAGs interrupting regular growth of its parallel‐fibred tissue consistent with seasonal adaptations, in the large adult specimen, MCZ VP 101557, seasonal markers are less apparent. Fossil and modern amphibious vertebrates are known to be particularly susceptible to small differences and fluctuations in habitat (Alcobendas & Castanet, 2000; Esteban et al., 1996; Gee et al., 2020; Sanchez & Schoch, 2013; Sanchez, Steyer, et al., 2010; Sinsch, 2015); therefore, this dissimilarity may result from minor geographical and/or environmental differences between the Hinton Bonebed and Greer Quarry populations. This suggests that although these two localities share a generalized environmental context (see Introduction), there were seasonal events specific to these localities.
4.3. Possible interpretations for the avascular zone
The most notable feature of Greererpeton femoral bone histology is the avascular zone that contains 7–10 growth marks (Figure 7e–g). This zone occurs irrespective of specimen size or locality, and at the same general point during ontogeny, suggesting it represents a consistent life history event inherent to the growth and development of Greererpeton. Growth marks are most commonly associated with seasonality, as mentioned above. However, the typical seasonal LAGs as exemplified in MCZ VP 101552 are histologically distinct from the unique avascular zone in two ways. First, in ectotherms, seasonal single or double LAGs represent near complete cessation of bone deposition (Alcobendas & Castanet, 2000; Castanet, 1994; Castanet & Smirina, 1990; Esteban et al., 1996). The avascular zone found in Greererpeton is composed of multiple growth marks interspersed with bone matrix, indicating a significant slowdown but not a complete quiescence of metabolic activity and represents a greater amount of bone deposition than what is typically represented by an annulus. Second, this zone occurs only once during the growth of Greererpeton, even in the largest and presumably oldest specimen (MCZ VP 101557) that, based on its large body size, likely lived for several years and, thus, several seasons after the event. Therefore, we conclude seasonality alone is an unlikely explanation for the avascular zone present in our Greererpeton sample (Table 1).
TABLE 1.
Potential correlates for the avascular zone (AVZ) in the femoral bone histology of Greererpeton
| Life history event | Cessation of growth? | Post‐ossification juvenile event? | Single event? | Supporting evidence | Opposing evidence |
|---|---|---|---|---|---|
| Seasonal stress | Yes | Yes | No | Known to be recorded in bone microstructure 1 | AVZ is histologically distinct from a repetitive seasonal LAG or annuli |
| Metamorphosis | Yes | Yes | Yes | Causes metabolic stress and occurs early in ontogeny 2 | No anatomical evidence of metamorphosis in Greererpeton 3 |
| Extended juvenile phase | Yes | Yes | Yes | Common in modern amphibians that are also tied to aquatic settings 4 | Greererpeton was not polymorphic 3 and this event occurred well before skeletal maturity 4 |
| Juvenile environmental stress | Yes | Yes | Yes | Resource availability can pause juvenile growth 5 | Occurs on a population level 6 ; the AVZ is found at a taxon level |
| Juveniles may not be able to escape poor living conditions | Late‐stage juveniles typically leave environmentally stressed conditions 7 | ||||
| Migration | Yes | Yes | No | Common in aquatically bound modern tetrapods 8 ; can be recorded by bone histology 9 | Occurs multiple times during the lifetime of an animal 8 |
| Dispersal | Yes | Yes | Yes | Common in aquatically bound modern tetrapods 8 ; occurs once in late‐stage juveniles 8 | Tetrapod dispersal commonly involves terrestrial excursions 8 ; Greererpeton was primarily aquatic in habit 3 |
While the avascular zone may not represent typical seasonality, it does signify that all young Greererpeton endured a temporary period of reduced bone deposition. Curiously, this interval is somewhat counter to the stereotyped growth patterns of modern tetrapods where elevated juvenile growth rates are followed by reduced or halted growth in adulthood. As such, we explore several potential life history correlates found in modern taxa with similar, primarily, aquatically bound ecologies (Table 1). These potential explanations for the life history event recorded in our sample are difficult to test due to the lack of modern histological data of such an event. Modern analogues are critical in grounding paleohistological interpretations and this is particularly challenging in fossil groups that have no modern descendants and are separated from modern taxa by more than 300 million years of divergent evolution. While early tetrapods are often compared to modern amphibians (e.g., Clack, 2012; Molnar et al., 2020; Sanchez, de Ricqlès, et al., 2010; Sanchez & Klembara, 2008), there are discrepancies in body size and the extremely derived life histories of modern amphibians likely confounds many morphological and/or physiological comparisons of growth through ontogeny. Thus, the applicability of this comparison remains questionable (Bazzana et al., 2020), and we broaden our modern vertebrate group comparison in deciphering likely explanations for the avascular zone, all while acknowledging the limitation of drawing comparisons between Greererpeton and modern taxa. Given these constraints, in our consideration of possible life history events, we establish the following criteria determined by the bone histology observed in our sample: (1) the event must incur reduced bone deposition and/or metabolic stress, (2) it must happen after limb ossification is initiated but well before skeletal maturity, and (3) it must occur as a singular event that is not repeated during the lifetime of the individual.
-
Metamorphosis. Perhaps the most tempting explanation for the juvenile life history event observed in Greererpeton is metamorphosis. Metamorphosis is typically defined as an abrupt morphological shift during post‐embryonic development that is often associated with a change in ecological niche and triggered by environmental cues (Fritzsch, 1990; Laudet, 2011; Schoch, 2007, 2009, 2014). While metamorphosis occurs in many teleost fishes, as well as lampreys, the origins of tetrapod metamorphosis is generally regarded as a derived condition within amphibians (Fritzsch, 1990; Schoch, 2001, 2009). Gradual morphological and ecological changes through ontogeny have been noted in some Paleozoic tetrapods, and especially temnospondyli amphibians such as Eryops (Sanchez & Klembara, 2008; Schoch, 2009; Sinsch, 2015); yet it is unclear whether these gradual changes create metabolic stress or interrupt juvenile bone deposition and growth.
Metamorphosis has the potential to be recorded in bone histology as it results in significant metabolic stress (Kirschman et al., 2017). Furthermore, in modern metamorphic amphibians, ossification of the femur begins with metamorphosis (Gao et al., 2018) and previous histological analyses of amphibian bone commonly ascribe the endosteal‐most LAG as a metamorphic line (Alcobendas & Castanet, 2000; Esteban et al., 1996; Olgun et al., 2005; Rozenblut & Ogielska, 2005; Sinsch, 2015). However, there is a scarcity of experimental data supporting this assertion and it is unclear whether metamorphosis is directly correlated with a single LAG, multiple LAGs, or any bony signature at all. More notably, no fossil or modern amphibian has been described with an avascular zone comparable to what we have described here in Greererpeton.
Extended Juvenile Phase. The period of slowed juvenile bone deposition in Greererpeton could be the result of the temporary retention of a juvenile body size as a natural stage of development. By in large, the most common extension of a juvenile phase occurs in amphibians that frequently employ facultative paedomorphism, or the retention of juvenile characteristics into sexual maturity. Facultative paedomorphism is controlled by a genetic–environmental interaction where the cost–benefit analysis of this polymorphism rewards the occupation of a niche space unfilled by adult morphology (Denoël et al., 2005). Given the seasonal pond and oxbow paleoenvironmental settings ascribed to Greererpeton localities (Greb et al., 2016; Retallack, 2011; Turner & Eriksson, 1999), it is conceivable that decelerating the rate of bone deposition and maintaining a smaller body size during a drying season with reduced habitat availability allowed juveniles to occupy small waterbodies that could not sustain much larger adults. However, both the Hinton Bonebed and Greer Quarry preserve individuals of various ontogenetic size, from juvenile to adult, and there is no indication of polymorphism in the population sampled (Godfrey, 1989a, 1989b); a feature that has been documented in other fossil ontogenetic series (Sanchez & Schoch, 2013; Schoch & Fröbisch, 2006; Teschner et al., 2018).
Juvenile Environmental Stress. Klein et al. (2009) describe an example of interrupted juvenile growth recorded in the femoral bone histology of a captive alligator that superficially resembles the avascular zone described here as it documents a more extensive period of bone deposition compared to that preserved in annuli. The authors specifically illustrate a substantial “inner ring of slow growth” at a juvenile stage of development, which they correlate with a period of poor living conditions in both environment and food resources. Once the alligator was removed from its poor living conditions, active growth and bone deposition resumed until the animal reached maturity. Given the similarity of this histological signature to the avascular zone in Greererpeton, another possible explanation is that the zone represents a period of limited resource availability that uniquely affects juvenile growth. The event described in the juvenile alligator, however, took place in a captive setting in a single individual, and thus, it is unclear whether similar juvenile stress events could be inherent to the life histories of wild populations. Furthermore, in modern systems, environmentally induced stress events are normally experienced at the population‐level rather than taxon‐level (Auer et al., 2018; Moore et al., 2016; Mueller & Diamond, 2001). The avascular zone of Greererpeton is ubiquitous in our sample irrespective of locality, indicating that this represents a taxon‐level trait. A taxon‐level juvenile mark has been described in sea turtle species, where the first cyclical growth mark deposited is an annulus (Snover & Hohn, 2004). In this instance, juvenile growth is interrupted by a seasonal signal that presents as an annulus rather than a LAG likely because of elevated background growth rates. As previously discussed, the avascular zone is distinct from a true annulus and, furthermore, Greererpeton lacks the consistent skeletochronological markers akin to those described in sea turtle species that gives rise to these juvenile annuli.
-
Movement Between Habitats. For modern tetrapod taxa that are either reproductively tied to water (i.e., amphibians) or are semi‐aquatic (e.g., crocodilians), movement to/from bodies of water is an essential component of their life histories, although it often comes at a metabolic and survivability cost (Cayuela et al., 2020; Rothermel & Semlitsch, 2002). Movement between natal and adult habitats is important to maintain healthy populations as it helps to combat intraspecific competition, resource limitations, and can increase genetic drift (Cayuela et al., 2020). It also typically occurs in older juveniles that are 1–3 years of age (Hutton, 1989; Lance et al., 2011; Semlitsch, 2008), well after the onset of bone ossification and prior to skeletal maturity.
Movement behavior can be separated into two categories: migration and dispersal (Semlitsch, 2008). Migration is defined by seasonal and iterative movements between habitats that occur throughout the lifetime of the animal. As such, we would expect migration to present as a cyclical bony signature rather than a singular ontogenetic event. Dispersal, on the other hand, is a unidirectional movement—from a natal to an adult habitat—that occurs once during development. It is a fundamental mechanism in ecology and is critical to populations that are spatially structured, such as tetrapods tied to waterbodies where abiotic factors, like hydroperiod or temperature, and biotic factors, like competition and predation, often promote emigration (Cayuela et al., 2020; Miller et al., 2015). In modern amphibians, dispersal is an essential component of their life histories because of their reproductive dependence on waterbodies (Cayuela et al., 2020; Grant et al., 2010; Miller et al., 2015). These dispersal events occur in post‐metamorphic juveniles but prior to reaching sexual maturity (Cayuela et al., 2020). In crocodylians, and especially the highly aquatic gharials, dispersal also tends to occur in late‐stage juveniles in response to density‐dependent factors (Hutton, 1989; Whitaker & Basu, 1981).
4.4. Evaluation of possible life history correlates for the avascular zone
The above proposed explanations for the avascular zone in Greererpeton do not currently have well‐established osteohistological correlates; however, we can make determinations on their likelihoods based on the anatomy of Greererpeton, as well as the prevalence of such events in modern ecosystems.
We consider metamorphosis as an unlikely correlate for the avascular zone, as the gross anatomy of Greererpeton through ontogeny shows little proportional change except for the orbits (Godfrey, 1989a, 1989b). Furthermore, within tetrapods, metamorphosis is usually considered a derived amphibian life history trait (Fritzsch, 1990; Schoch, 2007). Based on this, the retention of a juvenile size is also unlikely as such developmental and ecological strategies have only been documented in modern polymorphic vertebrates (i.e., fish and amphibians) and are usually associated with the initiation of sexual maturity and reduced growth (Denoël et al., 2005). In addition to not being polymorphic, our Greererpeton sample shows substantial bone deposition subsequent to the avascular zone and the overall size of the femur significantly increases into adulthood (Figure S1). These characteristics of Greererpeton growth run counter to the expectations established in modern vertebrates that employ facultative paedomorphism.
Periods of metabolic stress uniquely experienced by juveniles can occur in response to unfavorable environmental conditions and can manifest in bone histology (Guarino et al., 1995; Hemelaar, 1985; Klein et al., 2009). However, environmentally induced fluctuations in metabolic activity typically occur at a population‐level rather than being inherent to the life history of a species. Our sample of Greererpeton consists of individuals from two localities that vary in space and time, yet the avascular zone persists at the same stage of development, indicating a taxon‐level trait. Furthermore, in comparable modern ecosystems where the interface between water and land is in flux, juveniles are those that most frequently move away from resource limitations and environmental hardship (Hutton, 1989; Semlitsch, 2008). For these reasons, we find an environmental stress event solely experienced by juveniles to be a poorly supported explanation for the avascular zone.
In contrast, a common life history feature of aquatically bound tetrapods is movement between natal and adult habitats. Of the two types of movement behaviors—migration and dispersal—dispersal is the more likely candidate for the avascular zone in Greererpeton because it is a singular event. Dispersal is also a critical component of the development and ecologies of spatially constrained tetrapods and is characterized by the following: (1) it is a natural component of many species’ life histories; (2) it occurs at a late juvenile stage, typically within the first three years; and (3) it causes significant metabolic and physiological stress, especially when it occurs over land. The avascular zone in Greererpeton meets these three criteria more favorably than the alternative hypotheses described here.
In modern pond and alluvial ecosystems, similar to those reconstructed for Greererpeton (Godfrey, 1989a, 1989b; Greb et al., 2016; Smithson, 1982), dispersal may occur in a completely aquatic setting, but frequently transpires across the terrestrial landscape (Cayuela et al., 2020; Davenport & Abdul Matin, 1990; Grant et al., 2010; Hutton, 1989; Miller et al., 2015; Rothermel & Semlitsch, 2002; Semlitsch, 2008; Smith & Green, 2005; Whitaker & Basu, 1981). Under a dispersal scenario, it may be anticipated that juvenile Greererpeton would exclusively navigate waterways to reach adult habitats due to their aquatic lifestyle. In particular, it is often assumed that a persistent cranial lateral line system in early tetrapods precludes extended exposure to air, thus restricting terrestrial excursions; this is primarily based on the fact that most modern amphibians lose the lateral line system during metamorphosis (Fritzsch, 1989; Schoch, 2001). However, unlike modern amphibians, Greererpeton did not metamorphose and maintained its lateral line system into adulthood. This retention of a lateral line system does not necessarily preclude temporary terrestrial excursions as experimental and developmental evidence suggests extensive flexibility in lateral line system development and function (Gayer, 2018; Rizzato et al., 2020) and that aquatic vertebrates with lateral line systems can survive days to years in aerial settings (Davenport & Abdul Matin, 1990; Healy, 1975; Schoch, 2001; Standen, Du & Larsson, 2014). Moreover, recent functional analyses have revealed that although primarily aquatic in habit, Greererpeton limb morphology had adaptations supporting at least limited terrestrial capabilities (Dickson et al., 2021). Due to the significant metabolic stress experienced by juvenile Greererpeton, dispersal—either through water or particularly over land—is a potential explanation for the avascular zone.
5. CONCLUSIONS
Here, we demonstrate the utility of bone histology in providing novel insights into the growth, ecology, and life strategies of early tetrapods. Furthermore, we demonstrate the importance of obtaining an ontogenetic series of appendicular elements with thick cortices that can provide a robust record of growth. Greererpeton growth patterns are characterized by moderately paced but active bone deposition, including a significant amount of periosteal and endosteal deposition into skeletal maturity. We find evidence of a major life history event early in the development of Greererpeton that resulted in an extended, yet temporary period of reduced bone deposition. This event is recorded in the bone histology as a distinct avascular zone that interrupts juvenile growth. Due to its unique histological features, the avascular zone does not appear to be driven by annual seasonality as it occurs only once within the cortex and does not represent complete cessation of metabolic activity and, in general, the bone histology of Greererpeton does not contain consistent skeletochronological markers. Metamorphosis is also an unlikely explanation, as Greererpeton was primarily aquatic in habit and its external anatomy shows no significant proportional change during growth. The retention of a juvenile body size in response to intraspecific niche partitioning is, thus, also unlikely as this life strategy only occurs in polymorphic species. Furthermore, the ubiquity of the avascular zone in our sample suggests that this is not a juvenile‐specific population response to environmental cues but instead a taxon‐level trait. Alternatively, in modern tetrapods that are spatially constrained (i.e., tied to water), movement between waterbodies is a common and critical component of their ecologies and such movements could be expected in early tetrapods such as Greererpeton. We rule out migration as a possible correlate for the avascular zone due to its repetitive nature; however, unidirectional dispersal from a natal to adult waterbody cannot be excluded as a possible explanation for the life history event, as captured in bone histology. Under this scenario, juvenile Greererpeton may have dispersed, either through water or over land, resulting in a period of metabolic stress and the temporarily reduction in bone deposition and growth. Given the fundamental importance of dispersal in structuring modern ecosystems, it is intriguing to consider dispersal as one potential mechanism for increased terrestrial diversification and expansion during the initial stages of tetrapod evolution.
AUTHOR CONTRIBUTIONS
MRW and SEP conceived and designed the project; MRW collected and analyzed the data; and MRW and SEP wrote and edited the manuscript.
Supporting information
Fig S1
ACKNOWLEDGMENTS
We thank C. Byrd, C. Green, E. Biedron, and C. Capobianco for specimen preparation and curation. T. Smithson, P. Bishop, and T. Simões provided helpful comments and feedback on this project and manuscript. We thank the editor, B. Gee, and an anonymous reviewer for helpful feedback on the manuscript.
Whitney, M.R. & Pierce, S.E. (2021) Osteohistology of Greererpeton provides insight into the life history of an early Carboniferous tetrapod. Journal of Anatomy, 239, 1256–1272. 10.1111/joa.13520
DATA AVAILABILITY STATEMENT
All original histological images are available via MorphoSource through the MCZ Vertebrate Paleontology database. Images are organized under the project: Greererpeton Osteohistology.
REFERENCES
- Alcobendas, M. & Castanet, J. (2000) Bone growth plasticity among populations of Salamandra salamandra: interactions between internal and external factors. Herpetologica, 56(1), 14–26. https://www.jstor.org/stable/3893123 [Google Scholar]
- Alex Smith, M. & Green, D.M. (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography, 28(1), 110–128. 10.1111/j.0906-7590.2005.04042.x [DOI] [Google Scholar]
- Anderson, J.S. , Smithson, T. , Mansky, C.F. , Meyer, T. & Clack, J. (2015) A diverse tetrapod fauna at the base of “Romer’s Gap”. PLoS ONE, 10(4), e0125446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer, S.K. , Dick, C.A. , Metcalfe, N.B. & Reznick, D.N. (2018) Metabolic rate evolves rapidly and in parallel with the pace of life history. Nature Communications, 9, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzana, K.D. , Gee, B.M. , Bevitt, J.J. & Reisz, R.R. (2020) Postcranial anatomy and histology of Seymouria, and the terrestriality of seymouriamorphs. PeerJ, 8, e8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, P.J. , Walmsley, C.W. , Phillips, M.J. , Quayle, M.R. , Boisvert, C.A. & McHenry, C.R. (2015) Oldest pathology in a tetrapod bone illuminates the origin of terrestrial vertebrates. PLoS ONE, 10(5), e0125723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitsova, E.A. , Skutschas, P.P. , Sennikov, A.G. , Golubev, V.K. , Masuytin, V.V. & Masuytina, O.A. (2019) Bone histology of two pareiasaurs from Russia (Deltavjatia rossica and Scutosaurus karpinskii) with implications for pareiasaurian paleobiology. Biological Journal of the Linnean Society, 128, 289–310. [Google Scholar]
- Bolt, J.R. & Lombard, R.E. (2001) The mandible of the primitive tetrapod Greererpeton, and the early evolution of the tetrapod lower jaw. Journal of Paleontology, 75(5), 1016–1042. [DOI] [Google Scholar]
- Calderón, T. , DeMiguel, D. , Arnold, W. , Stalder, G. & Köhler, M. (2019) Calibration of life history traits with epiphyseal closure, dental eruption and bone histology in captive and wild red deer. Journal of Anatomy, 235, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callier, V. , Clack, J.A. & Ahlberg, P.E. (2009) Contrasting developmental trajectories in the earliest known tetrapod forelimbs. Science, 324, 364–367. [DOI] [PubMed] [Google Scholar]
- Carter, D.R. , Van Der Meulen, M.C.H. & Beaupré, G.S. (1996) Mechanical factors in bone growth and development. Bone, 18(1), 5S–S10. [DOI] [PubMed] [Google Scholar]
- Castanet, J. (1994) Age estimation and longevity in reptiles. Gerontology, 40(2–4), 174–192. [DOI] [PubMed] [Google Scholar]
- Castanet, J. , Curry Rogers, K. , Cubo, J. & Jacques‐Boisard, J. (2000) Periosteal bone growth rates in extant ratites (ostriche and emu). Implications for assessing growth in dinosaurs. Comptes Rendus de l'Académie des Sciences ‐ Series III ‐ Sciences de la Vie, 323, 543–550. [DOI] [PubMed] [Google Scholar]
- Castanet, J. & Smirina, E. (1990) Introduction to the skeletochronological method in amphibians and reptiles. Annales des Sciences Naturelles, 11(4), 191–196. [Google Scholar]
- Cayuela, H. , Valenzuela‐Sánchez, A. , Teulier, L. , Martínez‐Solano, Í. , Léna, J.‐P. , Merilä, J. et al. (2020) Determinants and consequences of dispersal in vertebrates with complex life cycles: a review of pond‐breeding amphibians. Quarterly Review of Biology, 85(2), 133–158. [Google Scholar]
- Clack, J.A. (2002) An early tetrapod from ‘Romer’s Gap’. Nature, 418, 72–76. [DOI] [PubMed] [Google Scholar]
- Clack, J.A. (2012) Gaining ground: the origin and evolution of tetrapods. 2nd ed., Bloomington: Indiana University Press. [Google Scholar]
- Clack, J.A. , Bennett, C.E. , Carpenter, D.K. , Davies, S.J. , Fraser, N.C. , Kearsey, T.I. et al. (2016) Phylogenetic and environmental context of a Tournaisian tetrapod fauna. Nature Ecology & Evolution, 1(1). [DOI] [PubMed] [Google Scholar]
- Clack, J.A. & Finney, S.M. (2005) Pederpes finneyae, an articulated tetrapod from the tournaisian of Western Scotland. Journal of Systematic Palaeontology, 2(4), 311–346. [Google Scholar]
- Clack, J.A. & Milner, A.R. (2015) Basal tetrapoda. Part 31A. Sues, H.D. (Ed). München: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- Coates, M.I. & Clack, J.A. (1995) Romer’s gap: tetrapod origins and terrestriality. Bulletin du Muséum national d’historie naturelle, 17, 373–388. [Google Scholar]
- Conover, D.O. & Schultz, E.T. (1995) Phenotypic similarity and the evolutionary significance of countergradient variation. Trends in Ecology & Evolution, 10(6), 248–252. [DOI] [PubMed] [Google Scholar]
- Cope, E.D. (1875) Check‐list of North American Batrachia and Reptilia. Bulletin of the United States National Museum, 1, 1–104. [Google Scholar]
- Cormack, D.H. (1979) Ham’s histology. Philadelphia: J. B. Lippincott Company. [Google Scholar]
- Danto, M. , Witzmann, F. & Fröbisch, N.B. (2016) Vertebral development in paleozoic and mesozoic tetrapods revealed by paleohistological data. PLoS ONE, 11(4), e0152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danto, M. , Witzmann, F. , Pierce, S.E. & Fröbisch, N.B. (2017) Intercentrum versus pleurocentrum growth in early tetrapods: a paleohistological approach. Journal of Morphology, 278(9), 1262–1283. [DOI] [PubMed] [Google Scholar]
- Davenport, J. & Matin, A.K.M.A. (1990) Terrestrial locomotion in the climbing perch, Anabas testudineus (Bloch) (Anabantidea, Pisces). Journal of Fish Biology, 37(1), 175–184. 10.1111/j.1095-8649.1990.tb05938.x [DOI] [Google Scholar]
- de Margerie, E. , Cubo, J. & Castanet, J. (2002) Bone typology and growth rate: testing and quantifying ‘Amprino’s rule’ in the mallard (Anas platyrhynchos). Comptes Rendus Biologies, 325(3), 221–230. 10.1016/s1631-0691(02)01429-4 [DOI] [PubMed] [Google Scholar]
- Denoël, M. , Joly, P. & Whiteman, H.H. (2005) Evolutionary ecology of facultative paedomorphosis in newts and salamanders. Biological Reviews of the Cambridge Philosophical Society, 80(4), 663–671. [DOI] [PubMed] [Google Scholar]
- Dickson, B.V. , Clack, J.A. , Smithson, T.R. & Pierce, S.E. (2021) Functional adaptive landscapes predict terrestrial capacity at the origin of limbs. Nature, 589, 242–245. [DOI] [PubMed] [Google Scholar]
- Enlow, D.H. (1963) Principles of bone remodeling. Springfield: Charles C. Thomas Company. [Google Scholar]
- Esteban, M. , García‐París, M. & Castanet, J. (1996) Use of bone histology in estimating the age of frogs (Rana perezi) from a warm temperate climate area. Canadian Journal of Zoology, 74(10), 1914–1921. 10.1139/z96-216 [DOI] [Google Scholar]
- Estefa, J. , Klembara, J. , Tafforeau, P. & Sanchez, S. (2020) Limb‐bone development of seymouriamorphs: implications for the evolution of growth strategy in stem amniotes. Frontiers in Earth Science, 8. 10.3389/feart.2020.00097 [DOI] [Google Scholar]
- Estefa, J. , Tafforeau, P. , Clement, A.M. , Klembara, J. , Niedźwiedzki, G. , Berruyer, C. et al. (2021) New light shed on the early evolution of limb‐bone growth plate and bone marrow. eLife, 10, e51581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch, B. (1989) Diversity and regression in the amphibian lateral line and electrosensory system. In: Coombs, S. , Görner, P. & Münz, H. (Eds.) The mechanosensory lateral line. New York, NY: Springer, pp. 99–114. [Google Scholar]
- Fritzsch, B. (1990) The evolution of metamorphosis in amphibians. Journal of Neurobiology, 21(7), 1011–1021. [DOI] [PubMed] [Google Scholar]
- Gao, J. , Li, X. , Zhang, Y. & Wang, H. (2018) Endochondral ossification in hindlimbs during Bufo gargarizans metamorphosis: A model of studying skeletal development in vertebrates. Developmental Dynamics, 247(10), 1121–1134. 10.1002/dvdy.24669 [DOI] [PubMed] [Google Scholar]
- Gayer, W.A. (2018) Water transport in the lateral line canal of the intertidal fish Xiphister mucosus (Girard 1858) and its significance to evaporative water with preliminary observations of the metabolic consequences of water loss. Portland State University. [Google Scholar]
- Gee, B.M. , Haridy, Y. & Reisz, R.R. (2020) Histological skeletochronology indicates developmental plasticity in the early Permian stem lissamphibian Doleserpeton annectens. Ecology and Evolution, 10(4), 2153–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser, F. (2013) Hibernation. Current Biology, 23(5), 188–193. [DOI] [PubMed] [Google Scholar]
- Godfrey, S.J. (1989a) Ontogenetic changes in the skull of the Carboniferous tetrapod Greererpeton burkemorani Romer, 1969. Philosophical Transaction of the Royal Society of London. B, Biological Sciences, 323(1213), 135–153. 10.1098/rstb.1989.0003 [DOI] [Google Scholar]
- Godfrey, S.J. (1989b) The postcranial skeletal anatomy of the carboniferous tetrapod Greererpeton burkemorani . Philosophical Transaction of the Royal Society of London. B, Biological Sciences, 323(1213), 75–133. 10.1098/rstb.1989.0002 [DOI] [Google Scholar]
- Goldman, H.M. , Mcfarlin, S.C. , Cooper, D. , Thomas, C. & Clement, J.G. (2009) Ontogenetic patterning of cortical bone microstructure and geometry at the human mid‐shaft femur. Anatomical Record, 292, 48–64. [DOI] [PubMed] [Google Scholar]
- Grant, E.H.C. , Nichols, J.D. , Lowe, W.H. & Fagan, W.F. (2010) Use of multiple dispersal pathways facilitates amphibian persistence in stream networks. Proceedings of the National Academy of Sciences of the United States of America, 107(15), 6936–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb, S.F. , Storrs, G.W. , Garcia, W.J. & Eble, C.F. (2016) Late Mississippian vertebrate palaeoecology and taphonomy, Buffalo Wallow Formation, western Kentucky, USA. Lethaia, 49(2), 199–218. 10.1111/let.12138 [DOI] [Google Scholar]
- Guarino, F.M. , Angelini, F. , Giacoma, C. & Cavallotto, L. (1995) Age determination by skeletochronology in low‐ and high‐elevation populations of Rana italica . Scientia Herpetologica, 1995, 187–191. [Google Scholar]
- Healy, W.R. (1975) Terrestrial activity and home range in efts of Notophthalmus viridescens . American Midland Naturalist, 93(1), 131. [Google Scholar]
- Hemelaar, A. (1985) An improved method to estimate the number of year rings resorbed in phalanges of Bufo bufo (L.) and its application to populations from different latitudes and altitudes. Amphibia‐Reptilia, 6(4), 323–341. [Google Scholar]
- Herbst, E.C. , Doube, M. , Smithson, T.R. , Clack, J.A. & Hutchinson, J.R. (2019) Bony lesions in early tetrapods and the evolution of mineralized tissue repair. Paleobiology, 45(4), 676–697. 10.1017/pab.2019.31 [DOI] [Google Scholar]
- Holmes, R.B. (2003) The hind limb of Captorhinus aguti and the step cycle of basal amniotes. Canadian Journal of Earth Sciences, 40(4), 515–526. 10.1139/e02-039 [DOI] [Google Scholar]
- Horner, J.R. , de Ricqlès, A. & Padian, K. (1999) Variation in dinosaur skeletochronology indicators: implications for age assessment and physiology. Paleobiology, 25(3), 295–304. 10.1017/s0094837300021308 [DOI] [Google Scholar]
- Hotton, N. (1970) Manchchunkia bassa gen. et. sp. nov. an anthracosaur (Amphibia: Labrinthodontia) from the upper Mississippian. Kirtlandia, 1, 12, 1–38. [Google Scholar]
- Hoyte, D.A.N. & Enlow, D.H. (1966) Wolff's law and the problem of muscle attachment on resorptive surfaces of bone. American Journal of Physical Anthropology, 24(2), 205–213. 10.1002/ajpa.1330240209 [DOI] [PubMed] [Google Scholar]
- Huttenlocker, A.K. & Shelton, C.D. (2020) Bone histology of varanopids (Synapsida) from Richards Spur, Oklahoma, sheds light on growth patterns and lifestyle in early terrestrial colonizers. Philosophical Transactions of the Royal Society B: Biological Sciences, 375, 20190142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocker, A.K. , Woodward, H.N. & Hall, B.K. (2013) The biology of bone. In: Padian, K. & Lamm, E.‐T. (Eds.) Bone histology of fossil tetrapods. Berkley: University of California Press, pp. 13–34. [Google Scholar]
- Hutton, J. (1989) Movements, home range, dispersal and the separation of size classes in Nile crocodiles. American Zoologist, 29(3), 1033–1049. 10.1093/icb/29.3.1033 [DOI] [Google Scholar]
- Jordana, X. , Marín‐Moratalla, N. , Moncunill‐Solè, B. , Nacarino‐Meneses, C. & Köhler, M. (2016) Ontogenetic changes in the histological features of zonal bone tissue of ruminants: a quantitative approach. Comptes Rendus Palevol, 15, 255–266. [Google Scholar]
- Kahmann, J.A. & Driese, S.G. (2008) Paleopedology and geochemistry of Late Mississippian (Chesterian) Pennington Formation paleosols at Pound Gap, Kentucky, USA: Implications for high‐frequency climate variations. Palaeogeography, Palaeoclimatology, Palaeoecology, 259(4), 357–381. 10.1016/j.palaeo.2007.09.022 [DOI] [Google Scholar]
- Kamska, V. , Daeschler, E.B. , Downs, J.P. , Ahlberg, P.E. , Tafforeau, P. & Sanchez, S. (2018) Long‐bone development and life‐history traits of the Devonian tristichopterid Hyneria lindae . Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 109(1–2), 75–86. 10.1017/s175569101800083x [DOI] [Google Scholar]
- Kirschman, L.J. , McCue, M.D. , Boyles, J.G. & Warne, R.W. (2017) Exogenous stress hormones alter energetic and nutrient costs of development and metamorphosis. Journal of Experimental Biology, 220(18), 3391–3397. [DOI] [PubMed] [Google Scholar]
- Klein, N. , Scheyer, T. & Tütken, T. (2009) Skeletochronology and isotopic analysis of a captive individual of Alligator mississippiensis Daudin, 1802. Fossil Record, 12(2), 121–131. 10.1002/mmng.200900002 [DOI] [Google Scholar]
- Köhler, M. , Marín‐Moratalla, N. , Jordana, X. & Aanes, R. (2012) Seasonal bone growth and physiology in endotherms shed light on dinosaur physiology. Nature, 487, 358–361. [DOI] [PubMed] [Google Scholar]
- Köhler, M. & Moyà‐Solà, S. (2009) Physiological and life history strategies of a fossil large mammal in a resource‐limited environment. Proceedings of the National Academy of Sciences of the United States of America, 106(48), 20354–20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konietzko‐Meier, D. , Shelton, C.D. & Martin Sander, P. (2016) The discrepancy between morphological and microanatomical patterns of anamniotic stegocephalian postcrania from the Early Permian Briar Creek Bonebed (Texas). Comptes Rendus Palevol, 15(1–2), 103–114. 10.1016/j.crpv.2015.06.005 [DOI] [Google Scholar]
- Lamm, E.‐T. (2013) Preparation and sectioning of specimens. In: Padian, K. & Lamm, E.‐T. (Eds.) Bone histology of fossil tetrapods. Berkley: University of California Press, pp. 55–160. [Google Scholar]
- Lance, V.A. , Elsey, R.M. , Trosclair, P.L. & Nunez, L.A. (2011) Long‐distance movement by American alligators in southwest Louisiana. Southeastern Naturalist, 10(3), 389–398. 10.1656/058.010.0301 [DOI] [Google Scholar]
- Laudet, V. (2011) The origins and evolution of vertebrate metamorphosis. Current Biology, 21(18), R726–R737. 10.1016/j.cub.2011.07.030 [DOI] [PubMed] [Google Scholar]
- Laurin, M. , Meunier, F. J. , Germain, D. & Lemoine, M. (2007) A microanatomical and histological study of the paired fin skeleton of the Devonian sarcopterygian Eusthenopteron foordi . Journal of Paleontology, 81(1), 143–153. https://doi.org/10.1666/0022‐3360(2007)81[143:amahso]2.0.co;2 [Google Scholar]
- Leclair, R. & Castanet, J. (1987) A skeletochronological assessment of age and growth in the frog Rana pipiens schreber (Amphibia, Anura) from southwestern Quebec. Copeia, 1987(2), 361. 10.2307/1445771 [DOI] [Google Scholar]
- Margerie, E.D. , Robin, J.‐P. , Verrier, D. , Cubo, J. , Groscolas, R. & Castanet, J. (2004) Assessing a relationship between bone microstructure and growth rate: a fluorescent labelling study in the king penguin chick (Aptenodytes patagonicus). Journal of Experimental Biology, 207, 869–879. [DOI] [PubMed] [Google Scholar]
- McFarlin, S.C. , Terranova, C.J. , Zihlman, A.L. , Enlow, D.H. & Bromage, T.G. (2008) Regional variability in secondary remodeling within long bone cortices of catarrhine primates: the influence of bone growth history. Journal of Anatomy, 213, 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier, F.J. & Laurin, M. (2012) A microanatomical and histological study of the fin long bones of the Devonian sarcopterygian Eusthenopteron foordi . Acta Zoologica, 93(1), 88–97. 10.1111/j.1463-6395.2010.00489.x [DOI] [Google Scholar]
- Miller, D.J. & Eriksson, K.A. (1999) Linked sequence development and global climate change: the Upper Mississippian record in the Appalachian basin. Geology, 27(1), 35. [DOI] [Google Scholar]
- Miller, W.L. , Snodgrass, J.W. & Gasparich, G.E. (2015) The importance of terrestrial dispersal for connectivity among headwater salamander populations. Ecosphere, 6(10), 1–9. [Google Scholar]
- Molnar, J.L. , Diogo, R. , Hutchinson, J.R. & Pierce, S.E. (2020) Evolution of hindlimb muscle anatomy across the tetrapod water‐to‐land transition, including comparisons with forelimb anatomy. The Anatomical Record, 303(2), 218–234. 10.1002/ar.23997 [DOI] [PubMed] [Google Scholar]
- Montes, L. , Le Roy, N. , Perret, M. , De Buffrenil, V. , Castanet, J. & Cubo, J. (2007) Relationships between bone growth rate, body mass and resting metabolic rate in growing amniotes: a phylogenetic approach. Biological Journal of the Linnean Society, 92(1), 63–76. 10.1111/j.1095-8312.2007.00881.x [DOI] [Google Scholar]
- Montoya‐Sanhueza, G. & Chinsamy, A. (2017) Long bone histology of the subterranean rodent Bathyergus suillus (Bathyergidae): ontogenetic pattern of cortical bone thickening. Journal of Anatomy, 230(2), 203–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M.P. , Riesch, R. & Martin, R.A. (2016) The predictability and magnitude of life‐history divergence to ecological agents of selection: a meta‐analysis in livebearing fishes. Ecology Letters, 19(4), 435–442. [DOI] [PubMed] [Google Scholar]
- Mueller, P. & Diamond, J. (2001) Metabolic rate and environmental productivity: well‐provisioned animals evolved to run and idle fast. Proceedings of the National Academy of Sciences of the United States of America, 98(22), 12550–12554. 10.1073/pnas.221456698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarino‐Meneses, C. , Jordana, X. & Köhler, M. (2016) Histological variability in the limb bones of the Asiatic wild ass and its significance for life history inferences. PeerJ, 4, e2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgun, K. , Uzum, N. , Avci, A. & Miaud, C. (2005) Age, size and growth of the southern Triturus karelinii in a population from Turkey. Amphibia‐Reptilia, 26, 223–230. [Google Scholar]
- Otoo, B.K.A. , Clack, J.A. , Smithson, T.R. , Bennett, C.E. , Kearsey, T.I. & Coates, M.I. (2019) A fish and tetrapod fauna from Romer’s Gap preserved in Scottish Tournaisian floodplain deposits. Palaentology, 8, 225–253. [Google Scholar]
- Panchen, A.L. (1970) Hanbuch der Paläoherpetologie. Stuttgart: Fischer. [Google Scholar]
- Parfitt, A.M. (2010) Skeletal heterogeneity and the purposes of bone remodelling: implications for the understanding of osteoporosis. In: Marcus, R. , Feldman, D. , Nelson, D. & Rosen, C. (Eds.) Fundamentals of osteoporosis. Cambridge, MA: Academic Press, Elsevier, p. 537. [Google Scholar]
- Rabey, K.N. , Green, D.J. , Taylor, A.B. , Begun, D.R. , Richmond, B.G. & McFarlin, S.C. (2015) Locomotor activity influences muscle architecture and bone growth but not muscle attachment site morphology. Journal of Human Evolution, 78, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack, G.J. (2011) Woodland hypothesis for Devonian tetrapod evolution. The Journal of Geology, 119(3), 235–258. 10.1086/659144 [DOI] [Google Scholar]
- Rizzato, P.P. , Pospisilova, A. , Hilton, E.J. & Bockmann, F.A. (2020) Ontogeny and homology of cranial bones associated with lateral‐line canals of the Senegal Bichir, Polypterus senegalus (Actinopterygii: Cladistii: Polypteriformes), with a discussion on the formation of lateral‐line canal bones in fishes. Journal of Anatomy, 237(3), 439–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer, A.S. (1941) Earliest land vertebrates of this continent. Science, 94(2438), 279. [DOI] [PubMed] [Google Scholar]
- Romer, A.S. (1956) The early evolution of land vertebrates. Proceedings of the American Philosophical Society, 100, 157–167. [Google Scholar]
- Romer, A.S. (1969) A temnospondylous labyrinthodont from the Lower Carboniferous. Kirtlandia, 6, 1–20. [Google Scholar]
- Rothermel, B.B. & Semlitsch, R.D. (2002) An experimental investigation of landscape resistance of forest versus old‐field habitats to emigrating juvenile amphibians. Conservation Biology, 16(5), 1324–1332. 10.1046/j.1523-1739.2002.01085.x [DOI] [Google Scholar]
- Rozenblut, B. & Ogielska, M. (2005) Development and growth of long bones in European water frogs (Amphibia: Anura: Ranidae), with remarks on age determination. Journal of Morphology, 265(3), 304–317. [DOI] [PubMed] [Google Scholar]
- Ruta, M. , Coates, M.I. & Quicke, D.L.J. (2003) Early tetrapod relationships revisited. Biological Reviews of the Cambridge Philosophical Society, 78(2), 251–345. 10.1017/s1464793102006103 [DOI] [PubMed] [Google Scholar]
- Sanchez, S. , De Ricqlès, A. , Schoch, R. & Steyer, J.S. (2010) Developmental plasticity of limb bone microstructural organization in Apateon: Histological evidence of paedomorphic conditions in branchiosaurs. Evolution and Development, 12(3), 315–328. [DOI] [PubMed] [Google Scholar]
- Sanchez, S. , Klembara, J. , Castanet, J. & Steyer, J.S. (2008) Salamander‐like development in a seymouriamorph revealed by palaeohistology. Biology Letters, 4, 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, S. & Schoch, R.R. (2013) Bone histology reveals a high environmental and metabolic plasticity as a successful evolutionary strategy in a long‐lived homeostatic Triassic temnospondyl. Evolutionary Biology, 40(4), 627–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, S. , Steyer, J.S. , Schoch, R.R. & De Ricqlès, A. (2010) Palaeoecological and palaeoenvironmental influences revealed by long‐bone palaeohistology: the example of the Permian branchiosaurid Apateon . Geological Society, London, Special Publications, 339(1), 139–149. 10.1144/sp339.12 [DOI] [Google Scholar]
- Sanchez, S. , Tafforeau, P. & Ahlberg, P.E. (2014) The humerus of Eusthenopteron: a puzzling organization presaging the establishment of tetrapod limb bone marrow. Proceedings of the Royal Society B: Biological Sciences, 281(1782), 20140299. 10.1098/rspb.2014.0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, S. , Tafforeau, P. , Clack, J.A. & Ahlberg, P.E. (2016) Life history of the stem tetrapod Acanthostega revealed by synchrotron microtomography. Nature, 537(7620), 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch, R.R. (2001) Can metamorphosis be recognised in Palaeozoic amphibians? Neues Jahrbuch für Geologie und Paläontologie ‐ Abhandlungen, 220(3), 335–367. 10.1127/njgpa/220/2001/335 [DOI] [Google Scholar]
- Schoch, R.R. (2007) The evolution of metamorphosis in temnospondyls. Lethaia, 35(4), 309–327. 10.1111/j.1502-3931.2002.tb00091.x [DOI] [Google Scholar]
- Schoch, R.R. (2009) Evolution of life cycles in early amphibians. Annual Review of Earth and Planetary Sciences, 37(1), 135–162. 10.1146/annurev.earth.031208.100113 [DOI] [Google Scholar]
- Schoch, R.R. (2014) Amphibian evolution: the life of early land vertebrates. Oxford: John Wiley & Sons. [Google Scholar]
- Schoch, R.R. & Fröbisch, N.B. (2006) Metamorphosis and neoteny: alternative pathways in an extinct amphibian clade. Evolution, 60(7), 1467. [PubMed] [Google Scholar]
- Schultze, H.‐P. & Bolt, J.R. (1996) The lungfish Tranodis and the tetrapod fauna from the Upper Mississippian of North America. Special Papers in Palaeontology, 52, 31–54. [Google Scholar]
- Semlitsch, R.D. (2008) Differentiating migration and dispersal processes for pond‐breeding amphibians. Journal of Wildlife Management, 72(1), 260–267. 10.2193/2007-082 [DOI] [Google Scholar]
- Sinsch, U. (2015) Review: skeletochronological assessment of demographic life‐history traits in amphibians. Herpetological Journal, 25, 5–13. [Google Scholar]
- Smithson, T.R. (1982) The cranial morphology of Greererpeton burkemorani Romer (Amphibia: Temnospondyli). Zoological Journal of the Linnean Society, 76(1), 29–90. 10.1111/j.1096-3642.1982.tb01955.x [DOI] [Google Scholar]
- Smithson, T.R. (1985) The morphology and relationships of the Carboniferous amphibian Eoherpeton watsoni Panchen. Zoological Journal of the Linnean Society, 85(4), 317–410. 10.1111/j.1096-3642.1985.tb01517.x [DOI] [Google Scholar]
- Snover, M.L. & Hohn, A.A. (2004) Validation and interpretation of annual skeletal marks in loggerhead (Caretta caretta) and Kemp’s ridley (Lepidochelys kempii) sea turtles. Fishery Bulletin, 102(4), 682–692. [Google Scholar]
- Standen, E.M. , Du, T.Y. & Larsson, H.C.E. (2014) Developmental plasticity and the origin of tetrapods. Nature, 513(7516), 54–58. 10.1038/nature13708 [DOI] [PubMed] [Google Scholar]
- Sumner, D.R. & Andriacchi, T.P. (1996) Adaptation to differential loading: comparison of growth‐related changes in cross‐sectional properties of the human femur and humerus. Bone, 19(2), 121–126. [DOI] [PubMed] [Google Scholar]
- Teschner, E.M. , Sander, P.M. & Konietzko‐Meier, D. (2018) Variability of growth pattern observed in Metoposaurus krasiejowensis humeri and its biological meaning. Journal of Iberian Geology, 44(1), 99–111. 10.1007/s41513-017-0038-y [DOI] [Google Scholar]
- Turner, B.R. & Eriksson, K.A. (1999) Meander bend reconstruction from an Upper Mississipian muddy point bar at Possum Hollow, West Virginia, USA. Fluvial Sedimentology VI, 28, 363–379. [Google Scholar]
- Warren, A. & Turner, S. (2004) The first stem tetrapod from the Lower Carboniferous of Gondwana. Palaeontology, 47(1), 151–184. [Google Scholar]
- Whitaker, R. & Basu, D. (1981) The Gharial Gavialis gangeticus: a review. Journal of the Bombay Natural History Society, 79, 531–548. [Google Scholar]
- Witten, P.E. & Huysseune, A. (2007) Mechanisms of chondrogenesis and osteogenesis in fins. In: Hall, B.K. (Ed.) Fins into limbs: evolution, development, and transformation. Chicago: The University of Chicago Press, pp. 79–92. [Google Scholar]
- Woodward, H.N. , Padian, K. & Lee, A.H. (2013) Skeletochronology. In: Padian, K. & Lamm, E.‐T. (Eds.) Bone histology of fossil tetrapods. Berkeley: University of California Press, pp. 195–216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
All original histological images are available via MorphoSource through the MCZ Vertebrate Paleontology database. Images are organized under the project: Greererpeton Osteohistology.


