Abstract
Cognitive symptoms of schizophrenia are reported to be minimally responsive to treatment with antipsychotic medications, though variability exists and many prior studies have significant confounds. Here, we examined the response of cognitive symptoms to antipsychotic medications in 71 inpatients with schizophrenia on and off antipsychotic medications in a blinded, placebo-controlled, cross-over study design. Patients received either antipsychotic medication monotherapy or placebo for 4–6 weeks before switching conditions. Neuropsychological testing, including working memory, intelligence, episodic memory, and verbal fluency tests, was administered during each condition. Additionally, we assessed whether polygenic scores for cognitive ability (PGScog) related to variability in antipsychotic medication-induced changes in cognitive performance. Overall, significant changes in cognition were not observed in response to medications (p's > 0.05) except for in episodic memory (p = 0.01), which showed a medication-related improvement. Some antipsychotic medication-related cognitive changes were associated with genetic predisposition to cognitive ability: PGScog showed positive correlations with medication-induced improvements in verbal list learning (p = 0.02) and category fluency (p = 0.03). Our primary results reinforce the notion that in general, cognitive measures are minimally responsive to antipsychotic medication. However, PGScog results suggest that genetic variation may influence the ability of current treatments to affect cognitive change within this patient population. This study underscores the need for development of novel treatment options specifically targeting cognitive symptoms as well as the importance of genetic variability in treatment response for patients with schizophrenia.
Keywords: Psychosis, Cognition, Antipsychotic treatment, Genetics
1. Introduction
Cognitive dysfunction is well established as a core symptom of schizophrenia (Braff, 1993; Bowie and Harvey, 2005; Keefe and Harvey, 2012), present in the majority of patients with schizophrenia and linked to functional outcome (Green, 1996). Work in recent decades has shown that cognitive deficits are not primarily related to antipsychotic medication exposure or length of illness. Cognitive symptoms are evident in medication-naïve patients (Bilder et al., 2000; Bowie and Harvey, 2005; Fatouros-Bergman et al., 2014) and can predate first break psychotic episodes (Reichenberg et al., 2010; Seidman and Mirsky, 2017). Individuals with schizophrenia score on average one to two standard deviations below normal on many cognitive tests (Keefe et al., 2011; Keefe and Harvey, 2012; Schaefer et al., 2013). Deficits are widespread, and impairments in processing speed, working memory, and verbal memory among others are often noted (Fatouros-Bergman et al., 2014).
The mainstay of treatment for schizophrenia continues to be antipsychotic medications that act through dopamine receptor blockade. These medications have beneficial effects in mitigating positive symptoms of the disease state (e.g., hallucinations, delusions); however, their efficacy for cognitive dysfunction is in question, with only minimal improvement being previously reported (Keefe and Harvey, 2012; Nielsen et al., 2015). An abundance of data suggest that first-generation antipsychotic medications do not substantially improve cognitive deficits (Sharma, 2002; Bowie and Harvey, 2005). Numerous investigations tested whether second-generation antipsychotic medications might provide cognitive advantages relative to first-generation antipsychotic medications, and some modest cognitive improvements with quetiapine, risperidone, and olanzapine were initially reported (Keefe et al., 2007b). However, later reports highlighted important issues affecting early studies, such as not accounting for practice effects on retest, small sample sizes, short duration of treatment, and potential bias of industry sponsorship (Harvey et al., 2005; Keefe and Harvey, 2012).
Subsequent, large-scale, multisite studies failed to demonstrate a consistent advantage of second-generation over first-generation antipsychotic medications in regard to cognition in patients with schizophrenia. For example, the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study examined various second-generation antipsychotic medications against perphenazine in chronic patients and found small medication-related improvements in cognition across multiple medications but did not show significant advantage of second-generation antipsychotic medications over the first-generation (Harvey, 2007; Keefe et al., 2007a). The European Union First Episode Schizophrenia Trial (EUFEST), which compared open-label haloperidol to multiple second-generation antipsychotic medications in first episode patients, did find a modest improvement in cognitive symptoms in all treatment groups, but failed to demonstrate a significant benefit of second-generation antipsychotic medications over first-generation (Davidson et al., 2009). The modest cognitive improvements found with all antipsychotic medications in the EUFEST study, at least in part, may be confounded by practice effects, which can be difficult to directly address without a balanced cross-over design (Davidson et al., 2009; Keefe and Harvey, 2012). In addition, studies looking at specific second-generation antipsychotic medications, such as quetiapine, have found possible isolated domains of improvement (e.g., executive functions) but failed to demonstrate substantial overall efficacy for relieving cognitive dysfunction in patients with schizophrenia (Andersen et al., 2011). Overall, there is at best weak and variable support for positive effects for both first- and second-generation antipsychotic medications on cognition in schizophrenia. Some studies have attempted to clarify the confound of practice effects by using a longitudinally studied cohort of healthy comparators, but such an approach assumes comparable practice effects in patients and controls and this may not be the case in all domains (Goldberg et al., 2007; Szöke et al., 2008).
One naturalistic study reported long-term outcomes (up to six to eight years) for recovered first-episode patients, many of whom were off medications at follow-up, and found improvements in cognition at later time points (Fu et al., 2019). These investigators found that patients who were off medication had improved processing speeds (compared to patients continuing medication), suggesting the possibility of adverse cognitive effects of antipsychotic medications for certain individuals. However, that study did not control for factors that led some patients to discontinue medications, such as severity of illness and medication side effects. Moreover, there is a dearth of studies that have documented the effects of antipsychotic treatment on cognition in chronic patients while controlling for practice effects and the passage of time in a systematic way.
As outlined above, there is considerable variability in reported findings about the effects of antipsychotic medications on cognition in schizophrenia. There is also considerable heterogeneity in the schizophrenia patient population itself (Liang and Greenwood, 2015), in both the range of cognitive abilities and in the timecourse with which cognitive deficits may emerge, likely due, in part, to genetic factors (Dickinson et al., 2020). Indeed, single-nucleotide polymorphism (SNP) heritability for general cognition was recently estimated at 21.5% (Trampush et al., 2017), and genome-wide association studies (GWAS) have demonstrated that many genetic loci are associated with cognition and intelligence (Sniekers et al., 2017; Davies et al., 2018; Savage et al., 2018). A number of these genetic loci are associated with risk for both schizophrenia and cognition, many demonstrating worse cognitive performance with higher schizophrenia risk (Smeland et al., 2017). Prior studies have not tested whether the responsiveness of cognition in schizophrenia to the effects of antipsychotic medications may relate to this genetic variation.
The current study extends the field's examination of the potential of antipsychotic medications to improve cognitive deficits observed in patients with schizophrenia in two ways. First, we use a placebo-controlled, cross-over design study, in an inpatient setting, to examine antipsychotic medication effects on cognitive performance while controlling for practice effects. Second, we examine the contribution of cognitive genetics to the prediction of changes in performance with antipsychotic treatment.
2. Methods
2.1. Participants
Seventy-one volunteers with diagnoses of schizophrenia (n = 64) or schizoaffective disorder (n = 7) were studied (aged 18–59 years, mean 29.8 ± 8.6 years old, 23 women, 33 current tobacco smokers Table 1), pursuant to a protocol approved by the National Institutes of Health (NIH) Institutional Review Board (IRB). All participants provided written informed consent prior to entering the study. Patients were screened for eligibility based on history and physical examination, routine laboratory testing including urine toxicology, and a radiologist-reviewed clinical brain magnetic resonance imaging (MRI) examination. Diagnosis was determined by a Structured Clinical Interview for DSM IV (SCID) (First et al., 1996) and confirmed by ongoing inpatient clinical evaluation. Individuals with confounding conditions such as major neurological disorders, major medical conditions, and active substance use disorders (except tobacco) were excluded from the study. Included participants were admitted as inpatients at the NIH Clinical Center and remained hospitalized during all study procedures. Mean duration of illness for participants was 8.1 (±7.6) years (with seven patients in the first year of illness, two patients who were antipsychotic medication naïve, and seven patients who had been free of antipsychotic medications for at least two weeks prior to admission to the inpatient unit). Fifteen patients had a history of non-tobacco substance use disorders in remission for at least six months (five with a history of alcohol use disorder, seven with a history of cannabinoid use disorder, and three with a history of more than one substance use disorder).
Table 1.
Demographics.
| Schizophrenia | n = 64 (90.1%) |
| Schizoaffective disorder | n = 7 (9.9%) |
| Male | n = 48 (67.6%) |
| Female | n = 23 (32.4%) |
| Mean age | 29.8 (±8.6) years |
| Mean years of education | 13.9 (±2.2) years |
| Mean duration of illness | 8.1 (±7.6) years |
| Mean age of first psychotic episode | 21.9 (±4.9) years |
| Treatment resistance | n = 34 |
| Family SES | 48.0 (±12.2) |
| Nonsmokers | n = 38 (53.5%) |
| Smokers | n = 33 (46.5%) |
| Mean chlorpromazine equivalents | 304.7 (±160.6) mg |
| Haloperidol | n = 4 (5.6%) |
| Aripiprazole | n = 10 (14.1%) |
| Olanzapine | n = 25 (35.2%) |
| Quetiapine | n = 6 (8.5%) |
| Risperidone | n = 21 (29.6%) |
| Ziprasidone | n = 3 (4.2%) |
| Other antipsychotic medication | n = 2 (2.8%) |
| Active arm first | n = 28 (39.4%) |
| Active arm second | n = 43 (60.6%) |
Demographic information for the 71 participants in the study including diagnosis, gender, age (±1 STD), race, years of education (±1 STD), duration of illness (±1 STD), age of first psychotic episode (±1 STD), treatment resistance (clozapine trial or at least 2 antipsychotic medication failures), family socioeconomic status (SES, ±1 STD) as calculated by using the Hollingshead four-factor index (Hollingshead, 1975), and current smoking (tobacco) status. Also included are chlorpromazine equivalents (using Woods's calculations except for molindone for which psychopharmacopeia.com was used; ±1STD) for medication used during the active arm (Woods, 2003), medication patients were on during the active arm, and which arm was active for patients. “Other antipsychotic medication” category includes molindone and trifluoperazine.
We adopted a blinded, counter-balanced, cross-over design for the study (Eisenberg et al., 2017). After admission but prior to starting the blinded part of the study protocol, participants were transitioned to antipsychotic medication monotherapy with the exception of six subjects who were already medication free. Patients then received either antipsychotic medication monotherapy or placebo for approximately 4–6 weeks followed by a further 4–6 weeks of the alternate treatment condition (39.4% receiving active medication in the first arm, Table 1). Participants underwent a stepped crosstaper to transition between treatment conditions before the first arm, between arms, and after the second arm of the study. During the active arm, 91.5% of patients were treated with second-generation antipsychotic medication (see Table 1). Patients on first-generation antipsychotic medications in our study were permitted to take blinded anticholinergics to mitigate side effects to antipsychotic medication during the active medication arm. The six medication-free patients who entered the blinded part of the protocol without antipsychotic medications received antipsychotic medications in the second arm of the study (and therefore were not randomized to order of the active arm) and three of them were studied unblinded. For analytic purposes, antipsychotic medication classes were collapsed given the prior literature showing no substantially reproducible robust differential effects between first- and second-generation antipsychotic medications (Harvey, 2007; Keefe et al., 2007a; Davidson et al., 2009). The neuropsychological testing battery (see below) was performed only once per arm of the study (i.e., active medication and placebo conditions); after, on average, three weeks into the placebo arm. Concurrent symptomatology (less than one week prior to neuropsychological testing assessments) was measured with the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), which was administered in by trained, blinded clinician raters.
2.2. Neuropsychological testing
Patients completed a neuropsychological cognitive battery of testing which included working memory, IQ (intelligence quotient), episodic memory, and verbal fluency tests. The neuropsychological battery was expanded after the first 26 participants and therefore different numbers of participants were available for data analysis depending on the measure (see Table 2). The N-back task was used to assess working memory (Owen et al., 2005) and was administered with three levels of working memory load and a control task. Participants viewed a sequence of briefly presented, individual numbers (digits 1–4) on a video monitor and responded using a keypad. They were instructed to indicate: the currently presented number in the 0-back condition (sensorimotor control condition), the immediately previously presented number in the 1-back condition, the number two positions earlier in the sequence in the 2-back condition, and the number presented three positions earlier in the 3-back condition. The Wechsler Abbreviated Scale Intelligence (WASI) provided estimates of verbal, non-verbal, and overall IQ (Hays et al., 2002). For the Category Fluency test (a measure of processing speed and semantic fluency), participants were given 1 min in which to rapidly verbalize exemplars in response to a category prompt. The Story Memory task served as one index of verbal learning and episodic memory; participants listened to a paragraph-length story and were asked to repeat the story, immediately and after a 20–30 min delay. The Hopkins Verbal Learning Test (HVLT) Revised served as a second verbal memory index (Benedict et al., 1998). During three HVLT learning trials, participants heard and immediately recalled a list of words. They were also asked to recall the words after a 20–25 min delay and to recognize them from within a longer list of words (recognition trials).
Table 2.
Performance on neuropsychological testing.
| Task | N | Mean off (STD) | Mean on (STD) | F | Partial η2 | P |
|---|---|---|---|---|---|---|
| 0-back | 63 | 94.9% (12.0) | 96.8% (6.0) | 1.5 | 0.02 | 0.23 |
| 1-back | 62 | 75.7% (24.0) | 79.7% (20.9) | 1.4 | 0.02 | 0.25 |
| 2-back | 64 | 62.7% (24.8) | 64.0% (24.8) | 0.3 | 0.01 | 0.57 |
| 3-back | 65 | 54.1% (23.1) | 55.1% (21.6) | 0.2 | <0.01 | 0.69 |
| Verbal IQ | 43 | 105.3 (14.6) | 104.9 (14.5) | 0.2 | <0.01 | 0.68 |
| Non-verbal IQ | 44 | 109.0 (13.6) | 109.2 (14.1) | <0.1 | <0.01 | 0.89 |
| Overall IQ | 43 | 108.1 (14.3) | 108.0 (14.3) | <0.1 | <0.01 | 0.97 |
| Category fluency | 44 | 19.5 (8.8) | 20.1 (7.3) | 0.3 | 0.01 | 0.62 |
| Story memory immediate | 45 | 8.6 (4.9) | 9.4 (4.6) | 1.1 | 0.03 | 0.30 |
| Story memory delay | 45 | 7.0 (4.7) | 9.0 (5.7) | 7.0 | 0.14 | 0.01⁎ |
| HVLT immediate | 44 | 23.1 (7.2) | 23.2 (5.8) | <0.1 | <0.01 | 0.93 |
| HVLT delay | 44 | 7.6 (3.2) | 7.7 (2.6) | 0.1 | <0.01 | 0.82 |
| HVLT recognition | 37 | 10.5 (2.1) | 10.7 (1.8) | 0.1 | <0.01 | 0.73 |
Performance on N-back, WASI (Wechsler Abbreviated Scale Intelligence), category fluency, story, and HVLT (Hopkins Verbal Learning Test) off and on medication is depicted. N-back performance reflects accuracy. Intelligence estimates from the WASI are reflected in the verbal, non-verbal, and overall IQ average scores. Category fluency scores reflect average number of words listed. Story Memory scores reflect the average number of elements of the story remembered. HVLT scores reflect average number of words remembered and recognized.
Indicates effects with p < 0.05.
2.3. Genetic testing
Procedures for genotyping, phasing, and imputation have been previously reported (Gregory et al., 2019). Briefly, genotyping was performed on blood lymphocytes using Illumina BeadChips (550 K-2.5 M SNP chips), was phased using Shapeit, and imputed using Impute2 software using the 1000 Genomes Phase 3 data as a reference panel. After QC measures, this resulted in an imputed genome of 5.7 million SNPs. Ancestry-related covariates were calculated using the ‘pca’ function in Plink Version 1.9 (https://www.cog-genomics.org/plink2/). Polygenic scores for cognition (PGScog, where higher scores indicate greater propensity for cognitive ability) were calculated using the beta values from the summary statistics of a genome-wide association meta-analysis of general cognition in 78,308 individuals of European descent (Sniekers et al., 2017) as weights for the “score” function in Plink. A set of 10 differently thresholded PGScog were reduced to a single score through principal components analysis using previously reported methods. This approach allows examination of the bulk of common cognitive genetic variance while minimizing multiple comparisons (Bergen et al., 2019; Dickinson et al., 2020). PGScog were available only for a subset of our patients (n = 38, unrelated individuals of European descent) in order to best match allele frequency variation in the population that was tested in the reference GWAS.
2.4. Statistical analyses
Statistical analyses used the General Linear Model (GLM) function as implemented in SPSS (IBM Corp, Build 1.0.0. 1327, IBM SPSS Statistical Subscription, Armonk, NY, https://www.ibm.com/analytics/spss-statistics-software). Repeated measures were conducted to analyze the effect of medication state (i.e., placebo versus active) on performance for the various measures. For chlorpromazine equivalents (CPZE; all antipsychotic medications and dosages were converted to chlorpromazine equivalents as a standard measure of antipsychotic medication exposure) (Woods, 2003) and genetic analyses, separately across cognitive variables, we used percent change in cognitive performance between medications arms as the dependent variable in separate univariate GLMs (percent change was calculated as the performance difference in the on versus off conditions divided by the mean of on and off performance). In the CPZE analyses, CPZE was the between-subjects covariate. In the genetic analyses, GLMs were performed such that PGScog was included as a covariate of interest, along with the first three ancestry-related components as covariates of no-interest to account for population stratification. Finally, in an additional set of GLM analyses, we tested the association of PGScog with cognitive performance under each treatment condition (on- or off-medication) with the same covariates as identified above for genetic analyses. If there were insufficient data for a participant (missing on and/or off medication data for a measure), that participant was excluded from the analysis of that measure only (n's per analysis are reflected in Table 2 and the subset with genetic data available for PGScog analysis is reported in Supplemental Table 1). To facilitate full assessment and potential follow-up by other investigators, we highlight in the Results section those findings that met an intendedly liberal statistical threshold of p < 0.05, uncorrected. Statistical results for all analyses are detailed in Table 2 and in Supplemental Table 1, again to enable full assessment by other investigators. PANSS data during the active and placebo conditions were compared using the Wilcoxon signed-rank test with 17 patients being excluded due to insufficient PANSS data. Pearson correlations were conducted comparing percent change in performance between arms for each of the cognitive measures to both illness duration and age of first psychotic episode. Student's t-tests were used to compare percent change in cognitive performance and treatment resistance (defined as clozapine trial history or at least 2 failed antipsychotic medication trials).
3. Results
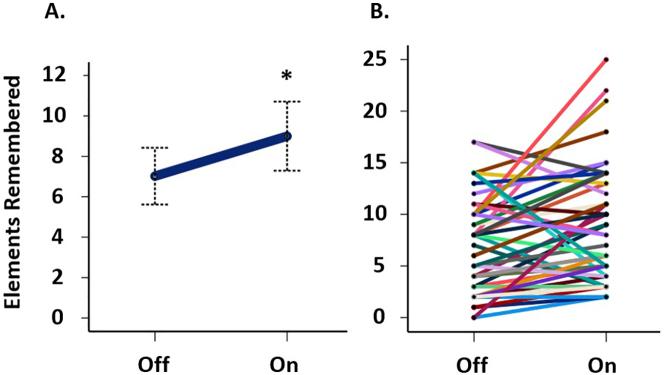
Patients were prescribed an average antipsychotic medication chlorpromazine equivalent dose of 304.7 mg (±160.6 mg) during the active arm of the study (Table 1). Symptomatology, as reflected by PANSS, indicated mild/moderate symptoms in the study sample. The average PANSS total score on-medication (56.5 (±12.5)) was less than off-medication (62.5 (±16.1), p < 0.001, Supplemental Table 2). There were no notable changes in cognitive performance across the battery of neuropsychological tests when comparing study arms except for the story memory delay condition (Table 2), in which patients recalled fewer story elements after a delay (31.7% reduction) during the off-medication compared to the on-medication condition (n = 45, F = 7.0, p = 0.01, Fig. 1). A post hoc analysis limited to patients who received active medication during the first arm of the study yielded a similar result (n = 25, F = 7.3, p = 0.01). In addition, chlorpromazine equivalents did not impact performance on any of the tests in the cognitive battery between study conditions. Illness duration, age of first psychotic episode, and treatment resistance were not associated (p > 0.05) with percent change in performance across the cognitive measures between arms.
Fig. 1.
Story memory delay: Performance on story memory delay task off and on medication (A) with participants (n = 45) collapsed (*p < 0.05) and (B) by participant (each line representing a patient). Error bars (A) represent 95% confidence intervals.
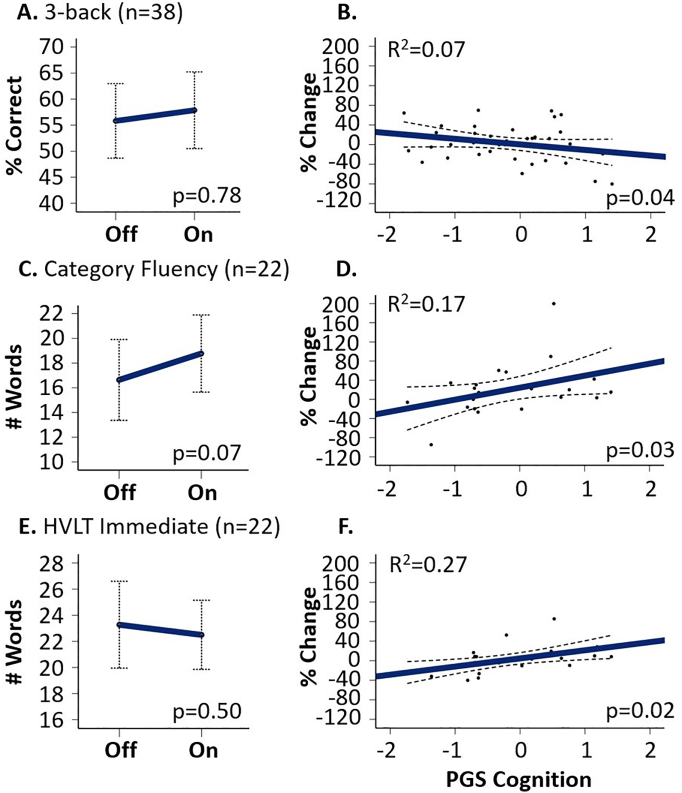
In the subset of patients for which genetic information was also available, the percent change in performance on certain cognitive variables from the off- to the on-medication conditions varied as a function of the genetic propensity for cognition (PGScog) (Supplemental Table 1), although the variables did not show treatment responses in main analyses. As shown in Fig. 2, PGScog was associated with performance change for the 3-back, Category Fluency, and HVLT Immediate conditions. The main effect of condition on story memory delay persisted in this subsample (n = 23, F = 6.3, p = 0.02); however, we did not find an association between PGScog and change in story memory performance (p = 0.20) (Supplemental Table 1).
Fig. 2.
Cognitive performance on and off medication and as related to cognitive PGS covariate: A and B depict 3-back performance off and on medication (A) as well as percent change in performance as a function of PGS for cognition (B). C and D depict Category Fluency performance off and on medication (C) as well as percent change in performance as a function of PGScog (D). E and F depict HVLT Immediate performance off and on medication (E) as well as performance as a function of PGScog (F). Error bars (A, C, E) indication 95% confidence intervals. Dots (B, D, F) represent individual patient's percent change in performance, with more positive values indicating better performance on medication than off. Solid lines (B, D, F) are the fit lines and thin dashed lines are the mean 95% confidence intervals.
For category fluency (Fig. 2C and D), there was a trend toward greater performance on medications in main analyses (F = 3.9, p = 0.07). This improvement was related to PGScog such that more advantageous cognitive genetics (i.e., higher PGScog) predicted greater improvement in performance on medication (n = 22, F = 5.6, R2 = 0.17, p = 0.03). When on and off medication performance measures were analyzed separately, there was no relationship of performance with PGScog during either treatment condition (p = 0.67 for the on-medication condition and p = 0.11 for the off condition; Supplemental Fig. 1C and D).
Similarly, for the HVLT immediate test (Fig. 2E and F), there was an association between PGScog and HVLT immediate percent change in performance (n = 22, F = 6.3, R2 = 0.27, p = 0.02), such that more advantageous cognitive genetics predicted improvement in performance on medications. Considering the on and off medication performance separately, there was no relationship between performance and PGScog during either study arm (p = 0.85 for the on-medication condition and p = 0.19 for the off condition; Supplemental Fig. 1E and F).
In contrast to those findings, for the 3-back measure (Fig. 2A and B), individuals with advantageous cognitive genetics showed less improvement in task performance with antipsychotic treatment (n = 38, F = 4.4, R2 = 0.07, p = 0.04). When examining on and off medication performance separately, there was no relationship with PGScog for either condition (active medication condition p = 0.15 and placebo condition p = 0.75; Supplemental Fig. 1A and B).
We also considered whether anticholinergics could have confounded our results for the few (n = 3) patients that were on them during the blinded part of the study. The story memory delay, category fluency, and HVLT immediate effects outlined above were not impacted as there were no patients on anticholinergics included in these analyses. The 3-back by PGScog relationship trended in the same direction (n = 36, F = 3.9, R2 = 0.09, p = 0.06) as outlined above when patients taking anticholinergic medication were excluded from the analysis.
4. Discussion
In considering the effects of antipsychotic medications across a battery of cognitive measures in patients with schizophrenia, we found improved performance with antipsychotic treatment for story memory delay. We did not observe meaningful changes for any other measures despite modest symptom improvement on medication. Some interesting findings emerged from examination of variability in cognitive genetics in this sample. Medication-related performance changes in category fluency and verbal list learning were positively associated with PGScog, while the reverse was true for the most challenging working memory condition tested (3-back). These results may suggest a complex interaction between genetic variation, cognitive task difficulty, and antipsychotic medication-induced cognitive performance changes in patients with schizophrenia.
The failure to find statistically robust differences in cognitive performance between antipsychotic treatment conditions – apart from delayed story memory – was not entirely surprising given the mixed literature on the effects of antipsychotic medications on cognition in patients with schizophrenia (Keefe and Harvey, 2012). In a study by Goldberg et al. (2007), comparing first episode schizophrenia patients to healthy volunteers on a broad cognitive battery, investigators found that only two of sixteen cognitive measures showed medication-related improvements in patients that exceeded the practice effects observed in healthy volunteers (Goldberg et al., 2007). The counterbalanced, placebo-controlled cross-over design employed here helped to address practice effects and avoid false positive findings not due to antipsychotic treatment. For example, post hoc analysis of the story memory delay data confirmed that the story delay result was not solely due to performance improvement in individuals who were first off and then on medication (active arm second condition), suggesting that practice effects were not a major factor. Of interest, one of the two effects that survived correction for practice effects in the prior study (Goldberg et al., 2007) was a verbal episodic memory variable similar to the story memory test found to improve with treatment in the current study. However, it is also true that a different episodic verbal memory measure in the present study (HVLT delay) showed no effect of treatment.
The results of the current study also highlighted possible differential relationships of PGScog with antipsychotic medication response across cognitive measures. Higher scores on our index of cognitive genetics was positively correlated with better verbal performance with antipsychotic treatment on the category fluency and HVLT (immediate) tasks – with relatively strong effect sizes (R2 = 0.17 and 0.27, respectively) – although this influence was not consistent for other measures of verbal performance in the battery. Verbal performance deficits have been well-documented in patients with schizophrenia (Bowie and Harvey, 2005) and show amelioration with antipsychotic treatment for some measures in prior studies (Nielsen et al., 2015; Veselinović et al., 2019). The findings add a layer to this complexity.
In contrast to the verbal performance results, higher PGScog was associated with worse performance for the 3-back task during the on-medication condition, although the effect size of this result was more modest (R2 = 0.07). It is not clear whether these different associations with PGScog reflect cognitive domain differences in cognitive operations and neural circuitry, other cognitive task differences (e.g., in load or difficulty), or a combination of these and other factors. However, the findings do highlights that the influence of genetic factors on the cognitive effects of antipsychotic treatment should be explored more deeply in future work.
Concerning study limitations, the sample size was limited by the high clinical demands of the current study, even within the well-controlled setting of an inpatient research ward, and did not allow for comparisons between specific antipsychotic medications or classes of antipsychotic medications. Although there were too few patients on first-generation antipsychotic medications to directly compare to second-generation antipsychotic medications in the current study, second-generation antipsychotic medications have not reliably shown superiority to first-generation antipsychotic medications with respect to cognition (Harvey, 2007; Keefe et al., 2007a; Davidson et al., 2009; Keefe and Harvey, 2012). Furthermore, there was no effect when CPZE was considered as a covariate in the analyses, suggesting that small variations in the estimated degree of dopaminergic blockade by antipsychotic medications alone are unlikely to be a strong determinant of cognitive performance differences. Nonetheless, future work would be needed to specifically test effects of different antipsychotic medications/classes.
Another consideration in interpreting the current study is the timing of cognitive measurements. The washout period used in the current study is thought to be sufficient to minimize acute effects of antipsychotic medication while ensuring a feasible and well-tolerated duration of participation (Gilbert et al., 1995; Wyatt et al., 1999). However, more enduring medication effects on the brain may still be present even after several weeks of washout, and the timecourse of possible cognitive changes in response to medication treatment or withdrawal is unknown. In addition, the length of controlled antipsychotic treatment is a limitation of the study. We are only able to report findings for the time period we studied and therefore shorter- or longer-term effects of medication on cognition cannot be extrapolated from this study. Finally, in light of substantial illness heterogeneity in schizophrenia, it is possible that the present results, obtained from people in the community with largely mild to moderate symptomatology who were willing and able to consent for and complete an extensive voluntary inpatient study, do not generalize to all individuals with this illness. Future work is needed to understand cognitive antipsychotic medication effects more broadly in this complex condition.
Despite these limitations, the current study moves forward our understanding of the effects of antipsychotic medications on cognition in patients with schizophrenia. The results suggest again that antipsychotic treatments do not greatly impact the cognitive deficits observed in the patient population overall, even when studied in a cross-over design that mitigates practice effects and excepting circumscribed narrative memory results which may offer some limited avenues for further investigation. However, variability across individuals in cognitive responses to medication is substantial and may be genetically mediated, as evidenced by varying relationships with PGScog. Given the differential cognitive changes observed, our study supports future research into genetic influences on treatment efficacy and highlights the need for further development of treatments targeted toward improving cognition in the schizophrenia patient population, as current antipsychotic treatment options continue to fall short.
5. Conclusions
For the cohort of patients with schizophrenia in our study, antipsychotic treatment effects on cognitive performance were inconsistent. However, taking into account genetic variability highlighted more robust effects on a number of measures. Thus, our study stresses the importance of considering subsets of patients with schizophrenia when evaluating treatment effects of cognition.
Funding
This work was supported by the Division of Intramural Research Programs, National Institute of Mental Health (NIMH), United States, to the NIMH Clinical and Translational Neuroscience Branch (ZIAMH002652; NCT00001247, NCT00001486).
CRediT authorship contribution statement
R.K.B: Conceptualization, Formal analysis, Writing – Original Draft, Writing – Review & Editing; D.D.: Conceptualization, Formal Analysis, Investigation, Data Curation, Writing – Review & Editing, Project administration; D.P.E.: Conceptualization, Formal Analysis, Investigation, Writing – Review & Editing, Project administration; M.D.G.: Formal Analysis, Data Curation, Writing – Review & Editing; J.A.A.: Investigation, Writing – Review & Editing; K.F.B: Conceptualization, Writing – Review & Editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors have nothing to declare.
Acknowledgments
We would like to thank the research participants and their families who made this work possible as well as the multidisciplinary staff of the Clinical & Translational Neuroscience Branch (CTNB) Inpatient Clinical Research Unit who supported our volunteers clinically throughout their participation. We are grateful as well to the CTNB research staff who assisted with the data collection. Some of this work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scog.2021.100223.
Appendix A. Supplementary data
References
- Andersen R., Fagerlund B., Rasmussen H., et al. Cognitive effects of six months of treatment with quetiapine in antipsychotic-naive first-episode schizophrenia. Psychiatry Res. 2011;187(1–2):49–54. doi: 10.1016/j.psychres.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Benedict R.H.B., Schretlen D., Groninger L., Brandt J. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- Bergen S.E., Ploner A., Howrigan D., et al. Joint contributions of rare copy number variants and common SNPs to risk for schizophrenia. Am. J. Psychiatry. 2019;176(1):29–35. doi: 10.1176/appi.ajp.2018.17040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder R.M., Goldman R.S., Robinson D., et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am. J. Psychiatry. 2000;157(4):549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bowie C.R., Harvey P.D. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr. Clin. N. Am. 2005;28(3):613–633. doi: 10.1016/j.psc.2005.05.004. 626. [DOI] [PubMed] [Google Scholar]
- Braff D.L. Information processing and attention dysfunctions in schizophrenia. Schizophr. Bull. 1993;19(2):233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Davidson M., Galderisi S., Weiser M., et al. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST) Am. J. Psychiatry. 2009;166(6):675–682. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- Davies G., Lam M., Harris S.E., et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 2018;9(1):2098. doi: 10.1038/s41467-018-04362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D., Zaidman S.R., Giangrande E.J., Eisenberg D.P., Gregory M.D., Berman K.F. Distinct polygenic score profiles in schizophrenia subgroups with different trajectories of cognitive development. Am. J. Psychiatry. 2020;177(4):298–307. doi: 10.1176/appi.ajp.2019.19050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D.P., Yankowitz L., Ianni A.M., et al. Presynaptic dopamine synthesis capacity in schizophrenia and striatal blood flow change during antipsychotic treatment and medication-free conditions. Neuropsychopharmacology. 2017;42(11):2232–2241. doi: 10.1038/npp.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros-Bergman H., Cervenka S., Flyckt L., Edman G., Farde L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr. Res. 2014;158(1–3):156–162. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.I., Williams J.B.W. Biometrics Research; New York: 1996. User's Guide for the SCID-I for DSM IV Axis I Disorders - Research Version. [Google Scholar]
- Fu S., Czajkowski N., Torgalsboen A.K. Cognitive, work and social outcomes in fully recovered first-episode schizophrenia: on and off antipsychotic medication. Psychiatry. 2019;82(1):42–56. doi: 10.1080/00332747.2018.1550735. [DOI] [PubMed] [Google Scholar]
- Gilbert P.L., Harris M.J., McAdams L.A., Jeste D.V. Neuroleptic withdrawal in schizophrenic patients. A review of the literature. Arch. Gen. Psychiatry. 1995;52(3):173–188. doi: 10.1001/archpsyc.1995.03950150005001. [DOI] [PubMed] [Google Scholar]
- Goldberg T.E., Goldman R.S., Burdick K.E., et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch. Gen. Psychiatry. 2007;64(10):1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Gregory M.D., Kippenhan J.S., Callicott J.H., et al. Sequence variation associated with SLC12A5 gene expression is linked to brain structure and function in healthy adults. Cereb. Cortex. 2019;29(11):4654–4661. doi: 10.1093/cercor/bhy344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.D. Cognitive outcomes in the CATIE schizophrenia trial: why do they seem different from previous results? Psychiatry (Edgmont) 2007;4(9):20–23. [PMC free article] [PubMed] [Google Scholar]
- Harvey P.D., Rabinowitz J., Eerdekens M., Davidson M. Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am. J. Psychiatry. 2005;162(10):1888–1895. doi: 10.1176/appi.ajp.162.10.1888. [DOI] [PubMed] [Google Scholar]
- Hays J.R., Reas D.L., Shaw J.B. Concurrent validity of the wechsler abbreviated scale of intelligence and the Kaufman brief intelligence test among psychiatric inpatients. Psychol. Rep. 2002;90(2):355–359. doi: 10.2466/pr0.2002.90.2.355. [DOI] [PubMed] [Google Scholar]
- Hollingshead A.B. 1975. Four Factor Index of Social Status. unpublished. [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Harvey P.D. Cognitive impairment in schizophrenia. Handb. Exp. Pharmacol. 2012;213:11–37. doi: 10.1007/978-3-642-25758-2_2. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Bilder R.M., Davis S.M., et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Sweeney J.A., Gu H., et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am. J. Psychiatry. 2007;164(7):1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Fox K.H., Harvey P.D., Cucchiaro J., Siu C., Loebel A. Characteristics of the MATRICS consensus cognitive battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr. Res. 2011;125(2–3):161–168. doi: 10.1016/j.schres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Liang S.G., Greenwood T.A. The impact of clinical heterogeneity in schizophrenia on genomic analyses. Schizophr. Res. 2015;161(2–3):490–495. doi: 10.1016/j.schres.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R.E., Levander S., Kjaersdam Telleus G., Jensen S.O., Ostergaard Christensen T., Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia–a meta-analysis of randomized clinical trials. Acta Psychiatr. Scand. 2015;131(3):185–196. doi: 10.1111/acps.12374. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A., Caspi A., Harrington H., et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am. J. Psychiatry. 2010;167(2):160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J.E., Jansen P.R., Stringer S., et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 2018;50(7):912–919. doi: 10.1038/s41588-018-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J., Giangrande E., Weinberger D.R., Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr. Res. 2013;150(1):42–50. doi: 10.1016/j.schres.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman L.J., Mirsky A.F. Evolving notions of schizophrenia as a developmental neurocognitive disorder. J. Int. Neuropsychol. Soc. 2017;23(9–10):881–892. doi: 10.1017/S1355617717001114. [DOI] [PubMed] [Google Scholar]
- Sharma T. Impact on cognition of the use of antipsychotics. Curr. Med. Res. Opin. 2002;18(Suppl. 3):s13–s17. doi: 10.1185/030079902125001074. [DOI] [PubMed] [Google Scholar]
- Smeland O.B., Frei O., Kauppi K., et al. Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal-numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry. 2017;74(10):1065–1075. doi: 10.1001/jamapsychiatry.2017.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniekers S., Stringer S., Watanabe K., et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat. Genet. 2017;49(7):1107–1112. doi: 10.1038/ng.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöke A., Trandafir A., Dupont M.E., Méary A., Schürhoff F., Leboyer M. Longitudinal studies of cognition in schizophrenia: meta-analysis. Br. J. Psychiatry. 2008;192(4):248–257. doi: 10.1192/bjp.bp.106.029009. [DOI] [PubMed] [Google Scholar]
- Trampush J.W., Yang M.L.Z., Yu J., et al. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: a report from the COGENT consortium. Mol. Psychiatry. 2017;22(11):1651–1652. doi: 10.1038/mp.2017.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselinović T., Scharpenberg M., Heinze M., et al. Disparate effects of first and second generation antipsychotics on cognition in schizophrenia - findings from the randomized NeSSy trial. Eur. Neuropsychopharmacol. 2019;29(6):720–739. doi: 10.1016/j.euroneuro.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Wyatt R.J., Henter I.D., Bartko J.J. The long-term effects of placebo in patients with chronic schizophrenia. Biol. Psychiatry. 1999;46(8):1092–1105. doi: 10.1016/s0006-3223(99)00227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.