Introduction
Arrhythmias can occur in patients with arrhythmogenic cardiomyopathy (ACM, also called arrhythmogenic right ventricular cardiomyopathy), despite the absence of structural abnormalities in the heart.1,2 The mechanism responsible for the onset of ventricular fibrillation (VF) in the early stage of ACM remains poorly understood. Here, we report a clinical case with a 24-year follow-up of a patient that did not have an identified genetic variation, and was resuscitated from VF. A significantly short QTc interval was the only abnormality at the time of the initial event, which argued that it arose from a purely electrical mechanism. Therefore, this study provides, for the first time, a well-documented case with a short JTc interval and early aborted sudden cardiac death in an individual with a concealed ACM.
Case report
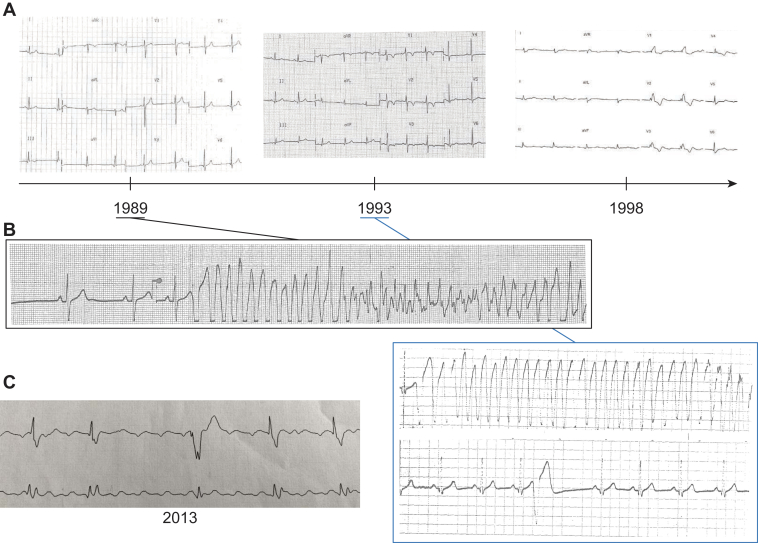
In 1989, a 34-year-old man was hospitalized to investigate the cause of repeated syncope, which occurred at rest. His mother had died of cancer, but she also experienced heart failure. His uncle had died suddenly at 57 years old. The physical examination was normal. The electrocardiogram (ECG) was in sinus rhythm, with a short QTc interval (338 ms; Bazett’s formula was used for the rate correction), but without any other abnormalities (Figure 1A). During monitoring in the hospital, the patient experienced VF (possibly caused by an arrhythmogenic premature ventricular contraction), which was treated with rapid external cardioversion (Figure 1B). This arrhythmia was triggered by a short coupling interval, premature ventricular beat, and left bundle branch block morphology (Figure 1B). In 2012, the patient had also presented with atrial arrhythmias (Figure 1C).
Figure 1.
Electrocardiograms and electrical characteristics. A: Twelve-lead electrocardiograms performed at the time of the initial event (left), 4 years later (middle), and 9 years later (right). B: Short premature ventricular beats that triggered ventricular fibrillation in 1989 and 1993. C: Plot shows how the durations of the corrected QT (ms, black, left axis) and QRS (ms, red, right axis) evolved in the index patient.
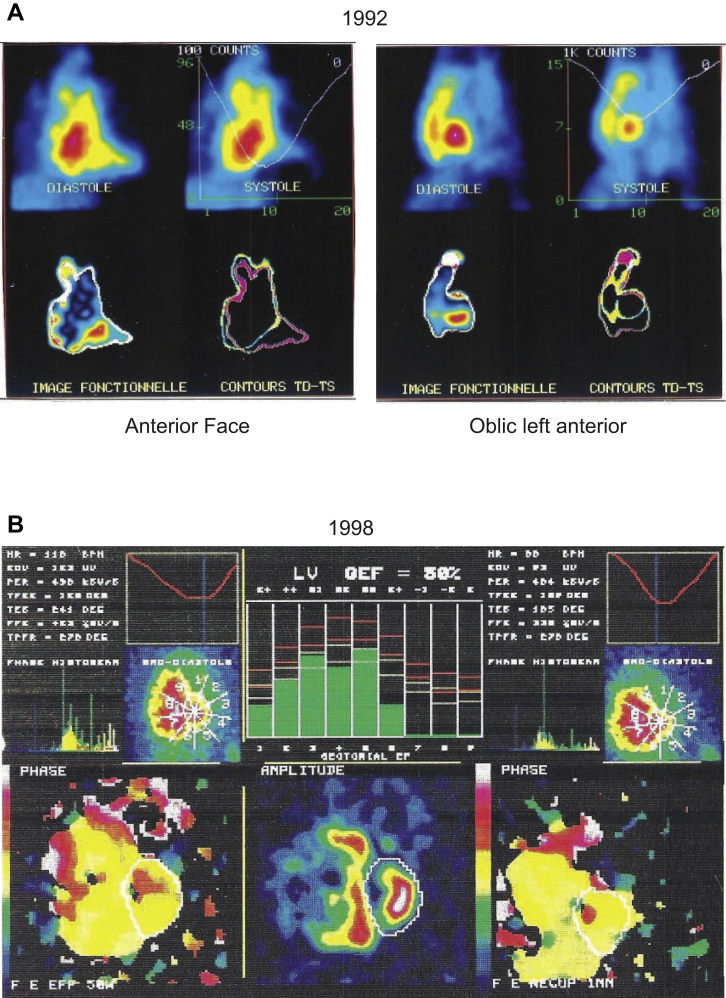
The patient had normal results on an echocardiogram, cardiac computed tomography scan, phase-analysis SPECT angio-scintigraphy (Figure 2), and an exercise stress test. The patient underwent automatic defibrillator implantation and was treated with nadolol. Two years later, he experienced palpitations and unsustained ventricular tachycardia. Sotalol (240 mg/day) reduced the symptoms. However, progressively, over 24 years of follow-up, the right ventricle (RV) became dilated (Figure 2) and the T waves became negative in leads V1 to V3 (Figure 1A). These signs led to a definitive ACM diagnosis. The QRS gradually widened, from 90 ms in 1989 to 200 ms in 1998, and the QTc fluctuated between 338 and 410 ms (Figure 1A–1C, Table 1).
Figure 2.
SPECT phase analysis imaging. A: Image acquired at the time of ventricular fibrillation occurrence, with no abnormalities. B: Nine years later, with frank right ventricular dilation.
Table 1.
Heart function parameters measured using electrocardiography, echocardiography, and scintigraphy
| Frequency (beats/min) | PR (ms) | QRS (ms) | QTc (ms) | JTc (ms) | LVEF (%) | RVEF (%)† | |
|---|---|---|---|---|---|---|---|
| 1989 | 54 | 140 | 90 | 338 | 248 | 65 | 50 |
| 1993 | 78 | 160 | 110 | 410 | 300 | - | - |
| 1998 | 62 | 350 | 200 | 350 | 150 | 60 | 20 |
| 2013 | Atrial arrhythmias | ||||||
LVEF = left ventricular ejection fraction; RVEF = right ventricular ejection fraction.
Measured by scintigraphy.
In 1998, SPECT imaging demonstrated a clear dilation of the RV (Figure 2). RV function, measured with scintigraphy, indicated a marked deterioration from 50% to 20% (Table 1). Despite medical treatment, including diuretics and angiotensin-converting enzyme inhibitors, his condition irreversibly deteriorated beyond repair. In the meantime, genetic testing revealed no class 3, 4, or 5 genetic variations, based on a gene panel that included ABCC9, ACTA1, ACTC1, ACTN2, AKAP9, ALPK3, ANK2, ANKRD1, APOA1, ATP2A2, BAG3, CACNA1C, CACNA1D, CACNA2D1, CACNB2, CALM1, CALM2, CALM3, CALR3, CASQ2, CAV3, CHRM2, CRYAB, CSRP3, CTF1, CTNNA3, DES, DMD, DOLK, DPP6, DSC2, DSG2, DSP, DTNA, EMD, GJA1, GJA5, GJC1, GLA, GPD1L, HCN4, HEY2, HFE, JPH2, JUP, KCNA5, KCNAB2, KCND3, KCNE1, KCNE1L, KCNE2, KCNE3, KCNH2, KCNJ2, KCNJ5, KCNJ8, KCNQ1, LAMA4, LAMP2, LDB3, LMNA, MOG1, MYBPC3, MYH6, MYH7, MYL2, MYL3, MYLK2, MYOM1, MYOZ2, MYPN, NEBL, NEXN, NKX2.5, NOS1AP, NPPA, NUP155, PDLIM3, PKP2, PLN, PRDM16, PRKAG2, PSEN1, PSEN2, PTPN11, RAF1, RBM20, RYR2, SCN10A, SCN1B, SCN2B, SCN3B, SCN4B, SCN5A, SCO2, SGCD, SLC8A1, SLMAP, SNTA1, STRN, SURF1, TAZ, TBX20, TBX5, TCAP, TGFB3, TMEM43, TMPO, TNNC1, TNNI3, TNNT2, TPM1, TRDN, TRPM4, TRPM7, TTN, TTR, and VCL. Ultimately, the patient received a heart transplant after a 24-year follow-up, mainly owing to a severe impairment in RV function.
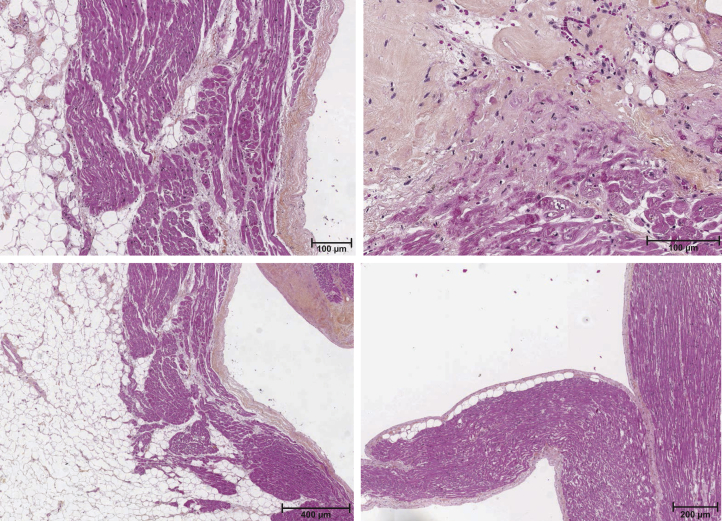
A heart examination revealed a weight of 450 g. Macroscopic observation revealed an important RV enlargement and left ventricular hypertrophy. The myocardial layers were replaced with fibrofatty tissue, mainly in the RV (Figure 3). There were lymphocytes in both the endocardium and epicardium. Extensive damaging fibrosis and myocardial necrosis were also present (Figure 3).
Figure 3.
Patient’s heart autopsy. Examples of a microscopic section of the patient’s explanted heart stained with hematoxylin, eosin, and saffron. Inflammation, adipose tissue, and myocardial necrosis are observed.
Discussion
This study described malignant ventricular arrhythmias (VA) in a patient with ACM without identified desmosomal mutation and without any initially detectable structural abnormality. To our knowledge, no previous study has collected information on genotype, cardiac tissue, and long-term follow-up data for a patient with ACM. It has been suggested that during the electrical phase, which usually starts after puberty, symptomatic VA occurs, and the primary manifestation can be VF.1,2 The exact cellular mechanisms underlying this electrical instability remain to be clarified. A murine model of arrhythmia (PKP2cKO mice) developed recently supported the myogenic origin of VA in patients with ACM.3 In the PKP2cKO mouse model, the QTc interval did not differ from that of control animals, but abnormal calcium handling was evidenced, and it could be corrected with flecainide treatment. Hence, one of the main challenges remains the clear ACM diagnosis at the very early stage of the disease.
Here, the only ECG abnormality was the short QTc (and JTc) interval found at the first clinical event. We recently published another study that highlighted abnormal QT dynamicity, with a short QT or JT interval, in a patient with ACM. We demonstrated that the duration of the cellular action potential had shortened, and that the pacing rate had lost some of its ability to adapt.4 Most patients with ACM exhibit a right ventricular parietal block, a reduced QRS amplitude, an epsilon wave, and an inversion in the T wave in V1–V3. However, the dynamicity of the QT or JT interval has not been investigated.5 Blom and colleagues6 described 2 patients with VF at an early stage of ACM. In both cases, the surface ECG displayed a wide QRS (>140 ms), particularly in leads V1–V3. Therefore, the short QT interval may have been neglected or masked by the widened QRS interval; moreover, this abnormality could mainly require a slow heart rate for detection. In addition, ACM is a progressive disease, and short myocardial repolarization may be evident only for a short period. The hypothesis that a short repolarization time might accompany ACM was supported by the high efficacy of sotalol in some patients with ACM.7 It should be noted that a high dose of the drug (eg, 320–480 mg/dL vs the normal dose of 160–320 mg/dL) was shown to have a greater antiarrhythmic efficacy.7, 8, 9 The reverse-use dependence of the effect of sotalol may underlie its efficacy and safety in a context of short repolarization time. In the present case, with right-dominant ACM, the short coupling interval, where the VF triggered ventricular premature beats, also suggested that the refractory period was shortened, which supported the use of drugs that prolong ventricular repolarization in patients with ACM. With the onset of structural disease, this type of ventricular premature beats gives way to additional, long-coupling interval beats, owing to the presence of scarring. It is not known whether a specific antiarrhythmic drug therapy has the potential to slow ACM progression. In our patient, sotalol might have had this effect, based on the long time delay (24 years) between the acute event and the heart transplant.
In conclusion, a short QTc or JTc interval should be investigated in young patients with syncope or presumed idiopathic VF. We also suggest that Holter monitoring should be performed to facilitate the quantification of the dynamicity of ventricular repolarization, with a QTe/RR slope analysis. This information could potentially assist in identifying patients with ACM that are prone to major arrhythmic events in the early stages of the disease. Our findings also suggested that the 2010 task force criteria could be revised to increase diagnostic sensitivity, particularly in family members that are affected but asymptomatic. Finally, this new ECG signature has suggested that it might be worthwhile to conduct a randomized controlled trial for evaluating sotalol or a pure, class 3 antiarrhythmic drug in patients with ACM.
Key Teaching Points.
-
•
The present case report suggests a myogenic origin of arrhythmogenic cardiomyopathy (ACM) electrical instability.
-
•
A short QTc interval at the early stage of ACM suggests that class 3 antiarrhythmic drug should be the medication of first choice in this setting.
-
•
Short myocardial repolarization may be obvious only during the early stage of ACM and thus worth looking for in patients with ventricular arrhythmias and apparently normal heart.
Footnotes
Conflict of interest: None declared (all authors). Funding: AM received postdoctoral funding from AFM telethon and Fond Marion Elisabeth Brancher.
References
- 1.Basso C., Corrado D., Marcus F.I., Nava A., Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D., Link M.S., Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. doi: 10.1056/NEJMra1509267. [DOI] [PubMed] [Google Scholar]
- 3.Cerrone M., Montnach J., Lin X., et al. Plakophilin-2 is required for transcription of genes that control calcium cycling and cardiac rhythm. Nat Commun. 2017;8 doi: 10.1038/s41467-017-00127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreau A., Reisqs J., Delanoe-Ayari H., et al. Deciphering DSC2 arrhythmogenic cardiomyopathy electrical instability: from ion channels to ECG and tailored drug therapy. Clin Transl Med. 2021;11:e319. doi: 10.1002/ctm2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L., Liu L., Kowey P., Fontaine G. The electrocardiographic manifestations of arrhythmogenic right ventricular dysplasia. Curr Cardiol Rev. 2014;10:237–245. doi: 10.2174/1573403X10666140514102928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom L.J., Te Riele A.S.J.M., Vink A., Hauer R.N.W., Hassink R.J. Late evolution of arrhythmogenic cardiomyopathy in patients with initial presentation as idiopathic ventricular fibrillation. HeartRhythm Case Rep. 2019;5:25–30. doi: 10.1016/j.hrcr.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wichter T., Borggrefe M., Haverkamp W., Chen X., Breithardt G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation. 1992;86:29–37. doi: 10.1161/01.cir.86.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Marcus G.M., Glidden D.V., Polonsky B., et al. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy. A report from the North American ARVC Registry. J Am Coll Cardiol. 2009;54:609–615. doi: 10.1016/j.jacc.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ermakov S., Scheinman M. Arrhythmogenic right ventricular cardiomyopathy – antiarrhythmic therapy. Arrhythm Electrophysiol Rev. 2015;4:86–89. doi: 10.15420/aer.2015.04.02.86. [DOI] [PMC free article] [PubMed] [Google Scholar]