Introduction
Left bundle branch pacing (LBBP) is a new method for treating patients with pacing indications. Studies have confirmed the feasibility and safety of LBBP, which can result in a narrow QRS duration and directly correct left bundle branch block (LBBB) with a low capture threshold.1 Following the first use of LBBP in 2017,2 LBBP quickly replaced other pacing strategies as a promising alternative for delivering physiological pacing to achieve electrical and mechanical synchrony of the left ventricle. Therefore, patients with LBBB and heart failure are likely to benefit from LBBP.
Left ventricular noncompaction (LVNC) is a rare congenital cardiomyopathy related to the arrest of myocardial development. It is characterized by prominent trabeculations on the ventricular luminal surface and regional wall motion abnormalities. There are currently no specific treatments. Treatment of LVNC mainly involves controlling symptoms, disease progression, and complications, the most common of which is heart failure. Therefore, cardiac resynchronization therapy (CRT) is one of these treatments.
Conventional CRT involves simultaneous biventricular pacing (BVP) achieved by inserting a lead electrode into the right ventricular myocardium and the cardiac vein through the coronary sinus (CS). Here, we present a case in which a patient who was diagnosed with LVNC and suffered from longstanding complete LBBB and heart failure underwent LBBP-optimized CRT, which significantly improved cardiac function.
Key Teaching Points.
-
•
Left ventricular noncompaction is a rare congenital cardiomyopathy related to the arrest of myocardial development. Specific treatments are currently not available. The effect of cardiac resynchronization therapy (CRT) on ventricular myocardium, such as left ventricular noncompaction, has been little reported.
-
•
Left bundle branch pacing–optimized CRT (LBBP-optimized CRT) is different from traditional CRT, which uses left bundle branch pacing (LBBP) instead of conventional right ventricular pacing.
-
•
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is used to evaluate the left ventricular mechanical synchrony after CRT. LBB+CS pacing turned out to be the optimal mode with the best left ventricular mechanical synchrony detected by SPECT MPI.
-
•
LBBP-optimized CRT may achieve both physiological pacing and left ventricular mechanical synchrony. Clinical trials are required to confirm the advantage of LBBP-optimized CRT over conventional CRT in improving cardiac function.
Case report
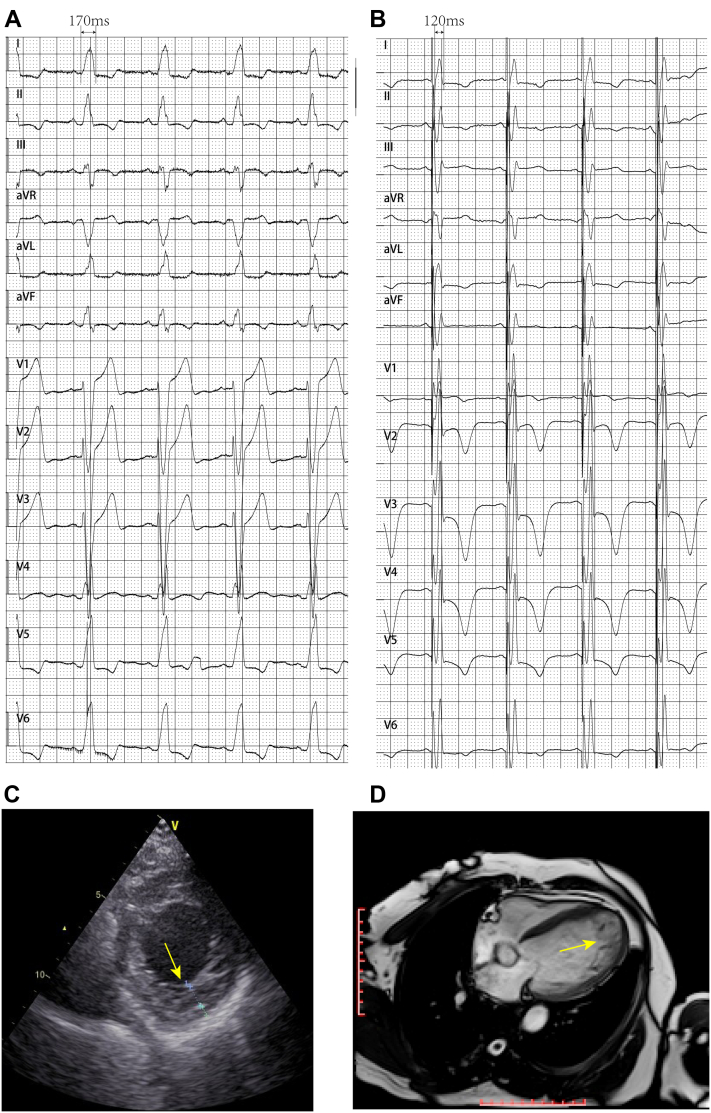
A 63-year-old female patient presented symptomatic NYHA class III systolic heart failure and complete LBBB with a 170-ms QRS duration on electrocardiogram (ECG) (Figure 1A). She suffered from shortness of breath, orthopnea, and lower extremity edema for 2 months. Echocardiography revealed LVNC (Figure 1C), enlargement of the left ventricular end-diastolic diameter (LVEDD, 57 mm) and left ventricular end-systolic diameter (LVESD, 49 mm), and a low left ventricular ejection fraction (LVEF, 28%). Cardiac magnetic resonance imaging revealed a large left ventricle (67 mm), LVEF of 25%, prominent ventricular apical trabeculae (24 mm, compacted ratio >2.3), and ventricular motor dissonance associated with the LBBB (Figure 1D). Her oral medications included furosemide, spironolactone, sacubitril/valsartan, and metoprolol succinate tablets.
Figure 1.
A: Baseline electrocardiogram showing complete left bundle branch block. B: Biventricular pacing ECG showing a shortened QRS duration. C: Echocardiography revealing left ventricular noncompaction. D: Cardiac magnetic resonance image showing prominent ventricular apical trabeculae.
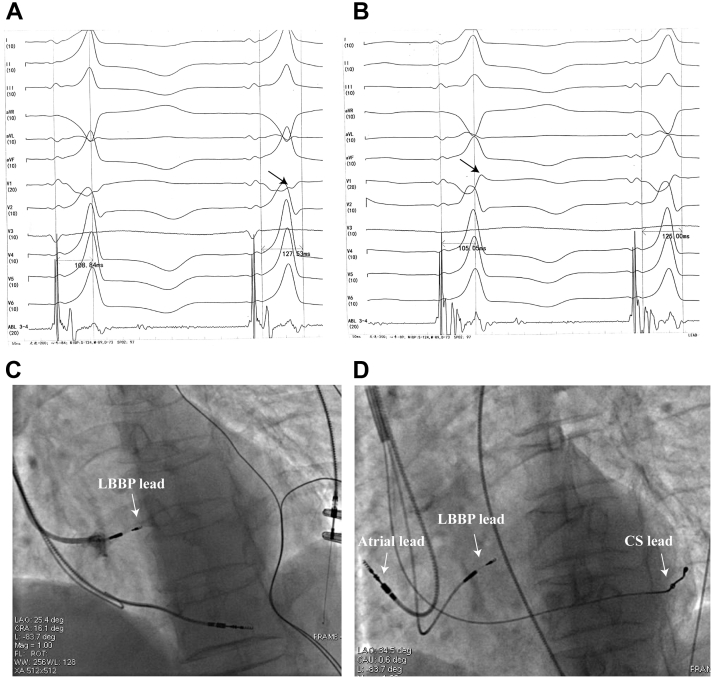
The patient had indications for CRT-D. However, she did not consent to CRT-D for economic reasons. Therefore she received an LBBP-optimized CRT pacemaker with a SelectSecure pacing lead (model 3830, 69 cm; Medtronic, Minneapolis, MN) in the left bundle branch (LBB) area, a screw-in pacing lead in the right atrium, and a left ventricular pacing lead in the epicardial lateral cardiac vein. All pacing leads were introduced transvenously into the right ventricle via the left subclavian vein. First, a screw-in pacing lead was implanted into the apical right ventricular septum in order to provide backup pacing in case third-degree block occurred owing to right bundle branch block injury during subsequent lead placement. Then, the SelectSecure pacing lead delivered through a fixed-curve sheath (C315 His) was implanted into the LBB area using a 9-partition method3 to achieve LBBP and correct the LBBB. When the paced QRS complex showed a “W” pattern in lead V1 (Figure 2A), the pacing lead was screwed into the right interventricular septum until the left ventricular septum was reached, without protruding into the left ventricular cavity (Figure 2C). The notch at the nadir of the “W” in lead V1 gradually increased to form an R wave, and the ECG QRS complex morphology changed to a right bundle branch block pattern (Figure 2B). The peak left ventricular activation time was 105 ms, the capture threshold was 0.5 V (0.4 ms), and the QRS duration was 120 ms (Figure 1B and Figure 2B). Next, the lead previously placed in the right ventricular septum was unscrewed and implanted into the right atrium. Finally, the left ventricular pacing lead was inserted into the lateral cardiac vein through the CS (Figure 2D). By shortening the paced atrioventricular interval (110 ms), continuous BVP (LBB+CS pacing) was guaranteed.
Figure 2.
A: QRS complex at the screw site showing a “W” pattern in lead V1. B: QRS complex morphology altered to a right branch block pattern. C: Sheath angiography in left anterior oblique (LAO) view showing the depth of the lead. D: Final fluoroscopic image with a SelectSecure pacing lead (Medtronic, Minneapolis, MN) in the left bundle branch pacing area, a pacing lead in the right atrium, and a left ventricular pacing lead in the epicardial lateral cardiac vein.
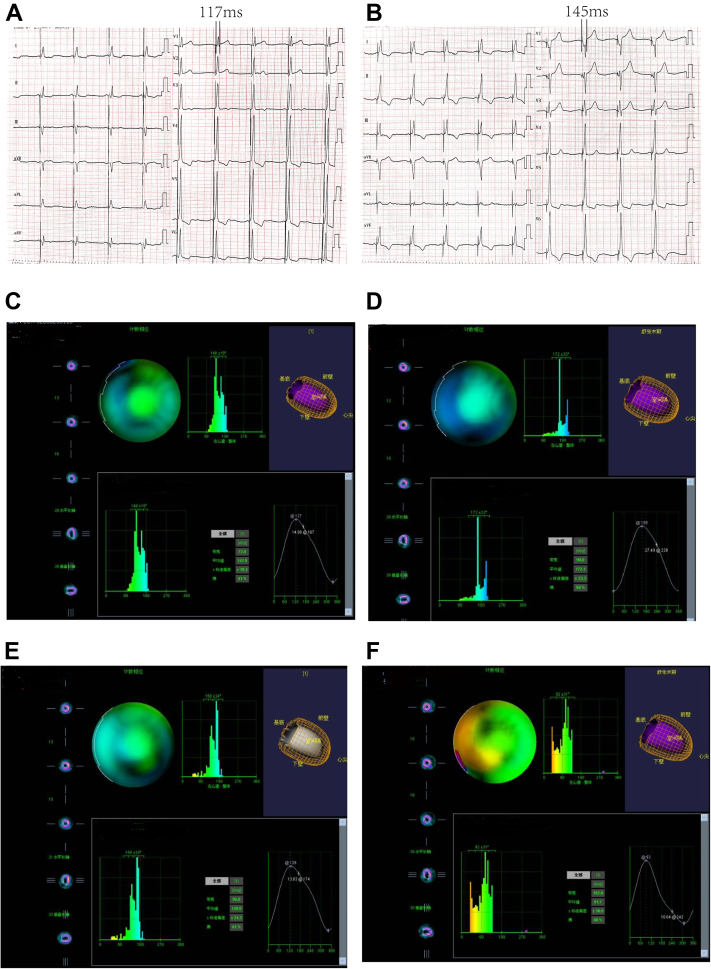
To maximize the chance of response to CRT, we tried to find the best pacing mode. Both LBBP and BVP were considered. After the procedure, ECG at different pacing mode indicated that BVP resulted in a narrower QRS duration (117 ms; Figure 3A) than LBBP only (145 ms; Figure 3B). Three days after implantation, echocardiography was performed again by the same echocardiographic specialist. The LVEF had increased remarkably (38%). Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) was then performed. The phase standard deviation (PSD) and phase histogram bandwidth (PHB) at different pacing modes were used to evaluate left ventricular mechanical synchrony. SPECT MPI revealed better left ventricular mechanical synchrony with BVP (PHB = 72°; PSD = 19.3; Figure 3C) than LBBP (PHB = 90°; PSD = 23.3; Figure 3D) or left ventricular pacing (PHB = 96°; PSD = 24.3; Figure 3E) alone. The 3 above-mentioned pacing modes were better than the no-pacing state in which the pacemaker was turned off (PHB = 102°; PSD = 91.7; Figure 3F). Therefore, the patient was discharged with continuous BVP.
Figure 3.
A: Left bundle branch (LBB)-only-paced electrocardiogram (ECG). B: LBB+CS-paced ECG. C: Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) under biventricular pacing. D: SPECT MPI under LBB pacing. E: SPECT MPI under left ventricular pacing. F: SPECT MPI in the no-pacing state with the pacemaker turned off.
At the 1-month follow-up, the patient demonstrated a substantial improvement in heart failure signs: the LVEDD, LVESD, left ventricular end-diastolic volume, and left ventricular end-systolic volume had decreased to 51 mm, 39 mm, 126 mL, and 65 mL, respectively. Six months later, the LVEDD, LVESD, left ventricular end-diastolic volume, and left ventricular end-systolic volume had reduced from a baseline of 57 mm, 49 mm, 156 mL, and 112 mL to 47 mm, 36 mm, 104 mL, and 53 mL, respectively, and the LVEF had increased from a baseline of 28% to 49%. Correspondingly, orthopnea and lower extremity edema had resolved, and shortness of breath was significantly relieved. She improved from NYHA class III to class I.
Discussion
Our case is worth describing because LBBP-optimized CRT was used to treat LVNC with LBBB, which has not yet been reported. CRT has been demonstrated to improve heart failure symptoms, left ventricular function, and even survival in approximately 70% of patients with heart failure with a reduced ejection fraction, especially in patients with LBBB and dilated cardiomyopathy.4 However, there have been few reports on the effect of CRT on the ventricular myocardium. Approximately 30% of heart failure patients are nonresponders, who may suffer high-risk heart failure with <50% survival at 5 years. Therefore, it is important to choose the appropriate pacing strategy. To find the best pacing modes, we performed SPECT MPI to evaluate left ventricular mechanical synchrony and ultimately chose BVP.
The patient described in our report was a super-responder to BVP, showing normalization of left ventricular function along with significant structural remodeling, although she had LVNC as a comorbidity. Her LVEDD was 58 mm prior to BVP and decreased to 47 mm following treatment. Her ejection fraction had increased from 28% to 49% 6 months after LBBP-optimized CRT. We speculate that LBBP corrected the LBBB and that CS lead pacing ameliorated interventricular mechanical dyssynchrony caused by LVNC.
The importance of this case study lies in the fact that the LBBB observed in our patient was combined with LVNC. The observed cardiac dysfunction was the result of a combination of these 2 diseases; LBBP alone may not completely normalize conduction abnormalities resulting from LBBB and intraventricular conduction defects. Consequently, it was not sufficient to correct only the LBBB. Therefore, we implemented CRT following the guidelines. After LBBP-optimized CRT, SPECT MPI verified that we had chosen the correct treatment. The left ventricular mechanical synchrony was optimized under the LBB+CS pacing state with PHB = 72° and PSD = 19.3. LBBP-optimized CRT successfully achieved both physiological pacing and left ventricular mechanical synchrony. However, clinical trials are required to confirm the advantage of LBBP-optimized CRT over conventional CRT in improving cardiac function.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding sources: The work described in this article was supported by the National Natural Science Foundation of China (81770472 and 81760051).
Contributor Information
Changxi Hu, Email: 1030725300@qq.com.
Qingwei Ji, Email: jqw124@163.com.
References
- 1.Hou X., Qian Z., Wang Y., et al. Feasibility and cardiac synchrony of permanent left bundle branch pacing through the interventricular septum. Europace. 2019;21:1694–1702. doi: 10.1093/europace/euz188. [DOI] [PubMed] [Google Scholar]
- 2.Huang W., Su L., Wu S., et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1731–1736. doi: 10.1016/j.cjca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Jiang H., Hou X., Qian Z., et al. A novel 9-partition method using fluoroscopic images for guiding left bundle branch pacing. Heart Rhythm. 2020;17:1759–1767. doi: 10.1016/j.hrthm.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Proclemer A., Muser D., Facchin D. What we can learn from "super-responders.". Heart Fail Clin. 2017;13:225–232. doi: 10.1016/j.hfc.2016.07.018. [DOI] [PubMed] [Google Scholar]