Introduction
When faced with a patient surviving sudden cardiac death (SCD) following ventricular fibrillation (VF), the cardiac electrophysiologist is tasked with searching for an identifiable cause of the arrhythmia, whether it be structural, electrical, or genetic. Identifying a clear cause not only establishes a diagnosis but permits tailored patient counseling and management. Furthermore, the identification of modifiable or reversible elements may improve prognosis if properly addressed. In the most challenging cases, exhaustive and careful investigations offer no explanation and the patient is diagnosed with idiopathic VF.
With this case report, we aim to highlight the presence, in apparent idiopathic VF (IVF), of occult substrate that is detectable with high-density epicardial electroanatomic mapping that is amenable to catheter ablation. We also review the significance of early repolarization (ER) pattern in the presence of SCD and the electrophysiologic mechanisms underlying the J-wave anomaly.
Case report
We report the case of a 21-year-old male patient with a history of resuscitated SCD due to VF at age 19. Initial cardiac and metabolic work-up revealed no obvious cause for the arrhythmia, aside from an inferolateral ER pattern on resting electrocardiogram (ECG) (Figure 1A).
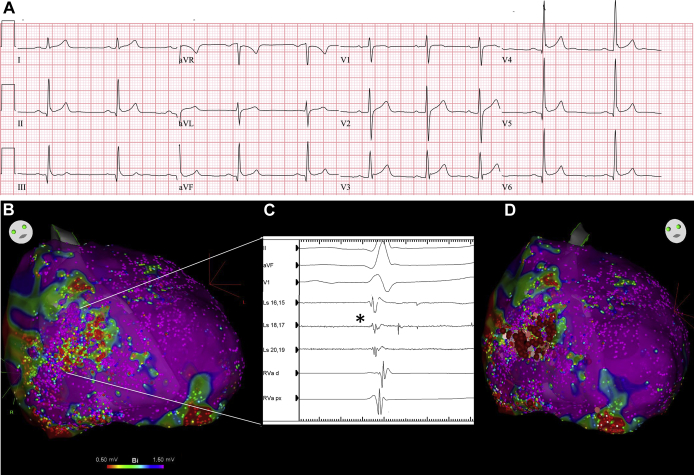
Figure 1.
A: Resting electrocardiogram of a 21-year-old male patient showing marked early repolarization in inferolateral leads. This patient displays the notching phenotype with enlarged QRS (106 ms) and concomitant upward ST-segment elevation. B: Epicardial voltage map of right ventricle (RV; anterior view). C: Corresponding epicardial electrograms registered in anterior aspect of RV outflow tract and RV free wall. Fragmented low-amplitude signals, as well as late potentials, were recorded with a PentaRay catheter (Biosense Webster, Inc, Diamond Bar, CA) (black asterisk). D: Ablation lesions targeting microstructural abnormalities.
The patient’s transthoracic echocardiogram revealed no abnormalities. Cardiac magnetic resonance imaging detected no structural abnormalities or late gadolinium enhancement. An exercise treadmill test failed to provoke arrhythmias or myocardial ischemia, and QTc interval was normal throughout rest, stress, and recovery. Procainamide pharmacological challenge did not provoke a Brugada pattern or any arrhythmias. The patient had African origins with no family history of SCD.
A subcutaneous intracardiac defibrillator was placed for secondary prevention of IVF and beta blockers were initiated.
During follow-up, the patient had 7 episodes of recurring VF requiring a total of 6 appropriate shocks, over the span of 10 months, despite medical therapy. Quinidine therapy was declined by the patient. Subcutaneous intracardiac defibrillator electrograms (EGMs) did not show a consistent triggering premature ventricular contraction morphology. Following the seventh event, the patient agreed to undergo electrophysiologic study and possible ablation.
Epicardial access was obtained and detailed high-density electroanatomic mapping was performed using the CARTO navigation system (Biosense Webster, Inc, Diamond Bar, CA) and a PentaRay mapping catheter (Biosense Webster, Inc). Abnormal signals, defined as bipolar EGMs of low voltage (<1 mV) and fractionated (>70 ms),1 were recorded in the anterior epicardial right ventricle (RV) outflow tract as well as the epicardial RV free wall. Figure 1B and 1C highlights the abnormal areas with the corresponding EGMs. Detailed endocardial voltage mapping was normal, and no spontaneous premature ventricular contractions were noted throughout the procedure with or without isoproterenol.
Abnormal signals were targeted for radiofrequency ablation (Figure 1D). A total of 37 minutes of radiofrequency ablation was performed using an irrigated contact force ablation catheter targeting >5 g contact at 40–50 W. Lesions were applied for 60 seconds or until the disappearance of mid-to-late components of fractionated potentials. For this particular case, given the lack of clear VF trigger, preprocedure electrophysiology study was not performed, and efforts were focused on the VF substrate. Therefore, complete elimination of all abnormal late fractionated EGMs was used as the ablation endpoint, as opposed to noninduciblity of sustained ventricular arrhythmias at study end. Subsequent remap following ablation was performed to confirm the complete abolition of late potentials. There were no immediate complications and the patient was discharged the next day; however, ECG remained unchanged.
Since the ablation, the patient remained free of VF recurrence over 5 months of follow-up.
Discussion
An ER pattern on ECG, described as a slurring or notching at the end of the QRS complex with or without ST-segment elevation, was initially described as a common and benign finding for many years. However, its association with VF in patients with structurally normal hearts is now well established, with recognition of an ER syndrome.1,2 Inferior or multiple lead involvement, taller and dynamic J waves (with augmentation of the J wave after sudden pauses or at lower heart rates), and limited ST-segment elevation with horizontal or descending pattern are some of the electrocardiographic features attributed to this rarer malignant form of ER.3
In recent years, others have used advanced electroanatomic mapping techniques as well as guided biopsies to better characterize the mechanisms underlying ER pattern on ECG in patients surviving IVF.4,5
Both depolarization and repolarization abnormalities have been described in the pathogenesis of the ER pattern.5 Nademanee and colleagues1,5 report 2 distinct groups of patients with ER syndrome: those in whom detailed electroanatomic mapping detects depolarization abnormalities, most often located in the RV epicardium; and those in whom no depolarization abnormalities are detected and where Purkinje ectopy is most often found to be the driver of VF. Abnormal EGMs can also be found in the latter group, but they are identified as ER potentials because they either are not in continuity with adjacent depolarization wave (electrical gap > 100 ms) or lack the distinctive sharpness of depolarization potentials (“hump” pattern).5
In the first group, J waves appear to be the reflection of delayed activation in the altered myocardium; in the latter, it is a transmural voltage gradient during repolarization, from epicardium to endocardium, that translates into J-wave or ST-segment elevation.3,5
The abnormal EGMs noted in the abnormal-depolarization group have been termed microstructural abnormalities, given the absence of structural cardiac disease using multiple imaging modalities, and may correspond to focal areas of fibrofatty replacement separating sparse myocardial fibers.4 They also resemble the substrate described in patients with Brugada syndrome, as well as in IVF survivors without ER or Brugada.6 The microstructurally abnormal areas demonstrated in our patient, epicardial RV outflow tract and anterior RV, in fact correspond to the most common sites of abnormal substrate in Brugada syndrome. Catheter ablation of such substrate has shown good to excellent results in terms of preventing VF recurrence.1,4,6 Although the substrate is most often located in epicardial RV, it has also been reported in epicardial inferolateral left ventricle and may coincide with the origin of ventricular ectopy.7 Patients with the abnormal-depolarization phenotype usually do not respond to quinidine therapy and are more likely to have an SCN5A mutation.1
Our patient demonstrated clear depolarization abnormalities along the RV epicardium. Interestingly, electroanatomic mapping failed to link these abnormal potentials to the inferior J wave itself. Moreover, abolition of the fractionated signals did not result in normalization of ER pattern, though it thus far appears to have successfully prevented VF. This suggests ER might have been a bystander phenomenon (given the frequency of this pattern in young male adults), with the microstructural abnormalities being the main substrate of IVF. Etiology of this localized altered myocardium remains unclear. If genetic testing detects no pathogenic variant of usual culprit genes, especially in the absence of family history, the hypothesis of prior occult myocarditis might be evoked.
In conclusion, this case is an example of IVF in which microstructural RV epicardial abnormalities were present. This ventricular substrate was targeted for catheter ablation with a favorable outcome.
When presented with a case of recurring IVF, even in the absence of a clear singular trigger, microstructurally abnormal myocardium should be considered as possible substrate and target for ablation.
Key Teaching Points.
-
•
Early repolarization syndrome patients may demonstrate depolarization abnormalities along the right ventricular epicardium.
-
•
These abnormalities, defined as microstructural, may not be detected by standard cardiac imaging.
-
•
This substrate may be targeted for ablation and prevent recurrence of ventricular fibrillation.
Footnotes
Disclosures: Dr Hadjis discloses consultant fees from Biosense Webster, Abbott Medical, and Medtronic. Funding Sources: The authors have no funding sources to disclose.
References
- 1.Nademanee K., Haissaguerre M., Hocini M., et al. Mapping and ablation of ventricular fibrillation associated with early repolarization syndrome. Circulation. 2019;140:1477–1490. doi: 10.1161/CIRCULATIONAHA.118.039022. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M., Derval N., Sacher F., et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 3.Patton K.K., Ellinor P.T., Ezekowitz M., et al. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation. 2016;133:1520–1529. doi: 10.1161/CIR.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 4.Boukens B.J., Benchacholamas V., Amersfoort S.V., et al. Structurally abnormal myocardium underlies ventricular fibrillation storms in a patient diagnosed with the early repolarization pattern. JACC Clin Electrophysiol. 2020;6:1395–1404. doi: 10.1016/j.jacep.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Haissaguerre M., Nademanee K., Hocini M., et al. Depolarization versus repolarization abnormality underlying inferolateral J-wave syndromes: new concepts in sudden cardiac death with apparently normal hearts. Heart Rhythm. 2019;16:781–790. doi: 10.1016/j.hrthm.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haissaguerre M., Duchateau J., Dubois R., et al. Idiopathic ventricular fibrillation: role of Purkinje system and microstructural myocardial abnormalities. JACC Clin Electrophysiol. 2020;6:591–608. doi: 10.1016/j.jacep.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voskoboinik A., Hsia H., Moss J., et al. The many faces of early repolarization syndrome: a single-center case series. Heart Rhythm. 2020;17:273–281. doi: 10.1016/j.hrthm.2019.09.013. [DOI] [PubMed] [Google Scholar]