Abstract
Objective
To evaluate the intraocular pressure (IOP)-lowering efficacy and safety of 10 and 15 µg bimatoprost implant in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).
Methods
This randomized, 20-month, multicenter, masked, parallel-group, phase 3 trial enrolled 528 patients with OAG or OHT and an open iridocorneal angle inferiorly in the study eye. Study eyes were administered 10 or 15 µg bimatoprost implant on day 1, week 16, and week 32, or twice-daily topical timolol maleate 0.5%. Primary endpoints were IOP and IOP change from baseline through week 12. Safety measures included treatment-emergent adverse events (TEAEs) and corneal endothelial cell density (CECD).
Results
Both 10 and 15 µg bimatoprost implant met the primary endpoint of noninferiority to timolol in IOP lowering through 12 weeks. Mean IOP reductions from baseline ranged from 6.2–7.4, 6.5–7.8, and 6.1–6.7 mmHg through week 12 in the 10 µg implant, 15 µg implant, and timolol groups, respectively. IOP lowering was similar after the second and third implant administrations. Probabilities of requiring no IOP-lowering treatment for 1 year after the third administration were 77.5% (10 µg implant) and 79.0% (15 µg implant). The most common TEAE was conjunctival hyperemia, typically temporally associated with the administration procedure. Corneal TEAEs of interest (primarily corneal endothelial cell loss, corneal edema, and corneal touch) were more frequent with the 15 than the 10 µg implant and generally were reported after repeated administrations. Loss in mean CECD from baseline to month 20 was ~ 5% in 10 µg implant-treated eyes and ~ 1% in topical timolol-treated eyes. Visual field progression (change in the mean deviation from baseline) was reduced in the 10 µg implant group compared with the timolol group.
Conclusions
The results corroborated the previous phase 3 study of the bimatoprost implant. The bimatoprost implant met the primary endpoint and effectively lowered IOP. The majority of patients required no additional treatment for 12 months after the third administration. The benefit-risk assessment favored the 10 over the 15 µg implant. Studies evaluating other administration regimens with reduced risk of corneal events are ongoing. The bimatoprost implant has the potential to improve adherence and reduce treatment burden in glaucoma.
Clinicaltrials.gov Identifier
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-021-01624-9.
Key Points
| The intracameral, sustained-release bimatoprost implant effectively lowered intraocular pressure in patients with open-angle glaucoma and ocular hypertension. |
| The effects of the implant on intraocular pressure were sustained beyond the expected duration of intraocular drug bioavailability. |
| The benefit/risk profile was most favorable for the 10 µg dose strength. |
Introduction
Glaucoma is a disease characterized by optic nerve damage and vision loss. It has been estimated to affect 76 million individuals aged 40–80 years [1] and is the leading cause of irreversible blindness [2]. The most common form of glaucoma is open-angle glaucoma (OAG), a chronic, progressive disease [3, 4]. The primary modifiable risk factor for glaucoma is intraocular pressure (IOP). There is abundant evidence that lowering IOP reduces the risk of development of glaucoma in individuals with ocular hypertension (OHT) as well as the risk of progression of glaucomatous optic nerve damage and vision loss [5–9]. Therefore, all approved treatment modalities in glaucoma, whether pharmacological or surgical, aim to lower IOP.
Initial therapy for OAG and OHT is typically pharmacologic. Eye drops containing topical ophthalmic solutions of IOP-lowering medications, such as prostaglandin analogs/prostamides (PGAs) and beta-blockers, are usually instilled once or twice daily. The PGAs (e.g., bimatoprost, latanoprost, tafluprost, and travoprost) are widely used in first-line therapy because they are most efficacious in lowering IOP and are well tolerated and systemically safe [10, 11]. Adherence to treatment is critical in glaucoma, as nonadherence is associated with worse visual outcomes [12, 13]. However, poor adherence to topical IOP-lowering therapy is endemic in glaucoma patients [14]. A study of adherence, as measured by the medication possession ratio, in 1234 newly diagnosed and treated patients with primary OAG estimated that only 20% of patients had persistently good adherence through 1 year of treatment [15]. Another study using pharmacy claims data similarly reported that patients who filled prescriptions for a topical PGA had medication available for dosing on only 37% of the days in the year [16]. Barriers to adherence to topical IOP-lowering therapy include forgetfulness, difficulty in instilling the eye drops, need for frequent administration, lack of understanding of the disease, the cost of medications, and side effects [14, 17–21]. Therefore, there is a need for alternative treatment modalities to deliver IOP-lowering medication without the need for daily eye drops.
Bimatoprost implant (Durysta; Allergan, an AbbVie company, Dublin, Ireland) was developed to address the problem of lack of adherence in glaucoma by lowering IOP without the need for patient or caregiver administration of daily topical eye drops [22]. This small, rod-shaped, biodegradable implant contains 10 µg bimatoprost in a drug delivery system consisting of polymers similar to those used in biodegradable sutures [23]. The implant is administered intracamerally with a single-use, prefilled 28-gauge applicator system [22] and provides slow, steady release of bimatoprost to reduce IOP as the polymer matrix is biodegraded through hydrolysis and metabolism to carbon dioxide and water [23, 24]. The implant was designed to release drug for 3–4 months. Drug release from the implant is complete within 90 days in vitro, and an in vivo study using beagle dogs administered a 15 µg implant similarly showed complete drug release and intraocular tissue drug levels below the limit of detection by 4.2 months after implant administration [23]. A drug-distribution study using beagle dogs further showed that drug concentrations achieved in the iris-ciliary body (a target tissue for IOP lowering) were 4400-fold higher after intracameral administration of a 15 µg bimatoprost implant than after 7 days of daily application of bimatoprost 0.03% eye drops [25]. In contrast, drug distribution to the bulbar conjunctiva, eyelid margins, and periorbital fat (tissues associated with topical PGA-related side effects) was below detectable levels or limited after bimatoprost implant administration compared with topical dosing [25]. These results suggest that targeted drug delivery with the intracameral bimatoprost implant has the potential to minimize periorbital and ocular surface adverse effects associated with topical PGA administration.
Two phase 3 studies to fulfill US Food and Drug Administration (FDA) registration requirements evaluated the efficacy and safety of bimatoprost implant 10 µg and 15 µg compared with twice-daily topical timolol in lowering IOP in patients with OAG or OHT after initial and repeated administrations. The ARTEMIS 1 study results were reported previously [23] and showed that both dose strengths of bimatoprost implant were noninferior to timolol drops in lowering IOP. IOP lowering was sustained in most patients beyond the expected duration of intraocular drug bioavailability predicted by results of the pharmacokinetics studies in vitro and in animals, as well as by findings that drug concentrations in aqueous humor samples taken from two subjects during the study were below the limit of quantitation at 3–4 months after their last implant administration [23]. The benefit/risk profile favored the 10 µg implant over the 15 µg implant [23].
We report here the results of the ARTEMIS 2 study, which was identical in design and clinical hypothesis to ARTEMIS 1 but involved different sites and different patients and, therefore, was independent of the ARTEMIS 1 study.
Methods
Study Design
This randomized, multicenter, subject- and efficacy evaluator-masked, parallel-group, active-controlled, 20-month phase 3 clinical trial (ARTEMIS 2, registered at ClinicalTrials.gov with the identifier NCT02250651) evaluated the efficacy and safety of bimatoprost implants in comparison with topical timolol for lowering IOP in subjects with OAG or OHT. The study was conducted at 114 sites in 15 countries (Argentina, Canada, Colombia, Czech Republic, Egypt, Germany, Italy, Malaysia, New Zealand, Singapore, South Africa, South Korea, Turkey, the UK, and the USA) in accordance with the International Conference on Harmonization E6 guideline for Good Clinical Practice. An institutional review board or ethics committee approved the study at each site, and all patients provided written informed consent.
Patients
The primary inclusion criteria included age ≥ 18 years with a diagnosis of OAG or OHT in each eye and both eyes requiring IOP-lowering treatment; study eye baseline IOP in the range of 22–32 mmHg at hour 0 (8 am ± 1 h) and 19–32 mmHg at hour 2 (2 h after hour 0); study eye inferior iridocorneal angle Shaffer grade of ≥ 3 on gonioscopy and peripheral anterior chamber depth of ≥ 1/2 corneal thickness by Van Herick estimation; and central corneal endothelial cell density (CECD) by specular microscopy of ≥ 1800 cells/mm2 by automated analysis at screening, with CECD in both eyes confirmed as qualified by the central reading center (CRC; Doheny Image Reading Center, Los Angeles, CA, USA) by baseline.
The primary exclusion criteria included history of closed-angle glaucoma or non-responsiveness to topical ophthalmic beta-blockers and/or PGAs; peripheral anterior synechiae in the inferior iridocorneal angle on gonioscopic examination at screening in either eye; history or evidence of complicated cataract surgery in the study eye; and any contraindication to beta-blocker therapy.
A complete listing of all patient eligibility criteria is provided in Online Supplementary Material (OSM), Resource 1.
Visit Schedule
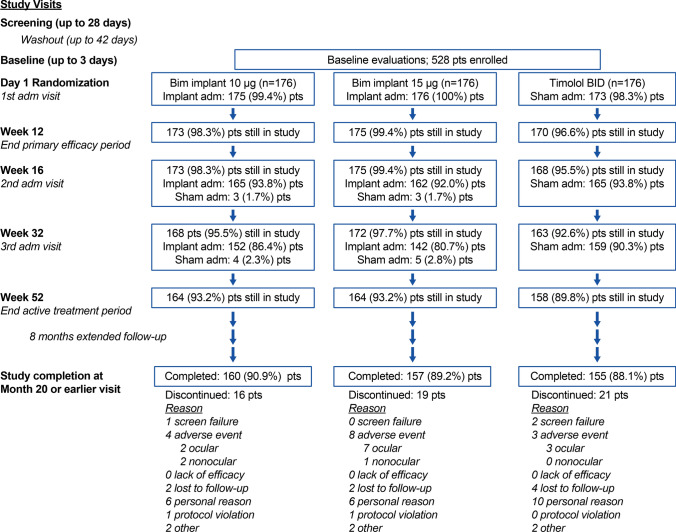
Study visits included screening and baseline visits; administration visits on day 1, week 16, and week 32; follow-up visits during the active treatment period at weeks 2, 6, 12, 15, 18, 22, 28, 31, 34, 38, 44, 48, and 52; and visits during an extended safety follow-up at months 14, 16, 18, and 20 (Fig. 1). If there were no safety concerns, patients who received fewer than three administrations of the bimatoprost implant or the sham procedure could complete and exit the study 12 months after the last administration received.
Fig. 1.
Patient flow through the study. Adm administration, BID twice daily, Bim bimatoprost, pts patients
Randomization, Intervention, and Masking
After the screening visit to determine patient eligibility, patients using IOP-lowering medications began a washout period of up to 42 days before the baseline visit. The minimum washout period was 4 days for parasympathomimetics and carbonic anhydrase inhibitors, 14 days for sympathomimetics and alpha-adrenergic agonists, and 28 days for beta-adrenergic antagonists, prostaglandin analogs (PGAs), and fixed-combination medications. On day 1, subjects were randomized in a 1:1:1 ratio to one of three treatment groups: 10 µg bimatoprost implant, 15 µg bimatoprost implant, or timolol. The randomization was stratified by baseline study eye hour 0 IOP of ≤ 25 or > 25 mmHg. The sponsor provided a computer-generated randomization scheme, and an automated interactive voice response system/interactive web response system was used to manage the treatment assignments.
If both eyes were eligible to be the study eye, the eye with the higher IOP at baseline, or the right eye (if both eyes had the same IOP) was selected as the study eye. On administration day visits, an implant was administered to study eyes in the bimatoprost implant groups, and for masking, a sham procedure was administered to study eyes in the timolol treatment group and all fellow eyes (OSM, Resource 2). Eyes were prepared for intraocular injection using standard practice for an intraocular procedure, and a single-use, needled, prefilled applicator system was used to administer the bimatoprost implant intracamerally as described previously [23]. In the sham procedure, a needleless applicator was used to touch the cornea. The second and third administrations of the bimatoprost implant or the sham procedure could be withheld in the event of a safety concern.
Throughout the study, study eyes in the timolol group and all fellow eyes were treated with topical timolol maleate 0.5% (timolol) twice daily (BID); study eyes in the bimatoprost implant groups received vehicle eye drops BID for masking. Timolol and vehicle eye drops were provided in identically appearing masked bottles labeled for the eye of administration (“Left” or “Right”) and were administered by the patients at 8 am (± 1 h) and 8 pm (± 1 h) daily, beginning in the evening on the day 1 administration visit. On the morning of subsequent study visits, the drops were administered at the study site after the hour 0 IOP measurement.
For all patients, use of rescue (nonstudy) IOP-lowering medication in either eye was allowed during the first 52 weeks (after confirmation of IOP at a subsequent visit) if the investigator attested that it was required for safety reasons because of inadequate IOP control. After the week 52 visit, rescue was allowed in either eye if the investigator determined that the IOP was not adequately controlled at two consecutive visits. To maintain masking, patients in the bimatoprost implant groups who used rescue treatment only in the study eye received sham administrations in the study eye on any subsequent administration days. Any patient who used rescue treatment in both eyes discontinued use of all study-provided eye drops and received no further administrations of bimatoprost implant or sham procedure in either eye.
All patients, as well as the site personnel who collected efficacy data, were masked to the treatment and study eye assignment.
Outcome Measures
The main efficacy outcome measure was IOP evaluated at hours 0 and 2 with Goldmann applanation tonometry using a 2-person, masked reading method. The primary endpoints were the study eye IOP at hours 0 and 2 at weeks 2, 6, and 12, and the hour-matched IOP change from baseline in the study eye at hours 0 and 2 at week 12.
The main safety measures were treatment-emergent adverse events (TEAEs, defined as adverse events with onset or increased severity, or that became serious, on or after the first study treatment date); CECD on specular microscopy; central corneal thickness (CCT) evaluated with ultrasound (contact) pachymetry; biomicroscopy; gonioscopy with bimatoprost implant assessment; ophthalmoscopy; and best-corrected visual acuity (BCVA). Corneal TEAEs of interest (corneal touch, endothelial cell loss, edema, opacity, disorder, or thickening) and anterior segment inflammatory TEAEs of interest (iritis, anterior chamber cell, iris adhesions, anterior chamber flare, keratitis, uveitis, anterior chamber inflammation, iridocyclitis, and keratic precipitates) were also evaluated. Visual fields were evaluated at baseline, weeks 28 and 52, and month 20.
Statistical Analysis
The statistical analyses used the final database lock from the completed study and were performed with SAS version 9.4 software (SAS Institute Inc, Cary, NC, USA). Previous planned interim analyses used database locks at weeks 12 and 52. All statistical tests were two-sided with an alpha level of 0.05. The analyses of IOP used observed values in the intent-to-treat patient population. To avoid confounding of the efficacy data, IOP measurements taken after initiation of use of a rescue IOP-lowering medication or procedure in an eye were excluded from analysis.
Analysis of the IOP primary endpoint used a mixed-effect model for repeated measures (MMRM) with IOP as the response variable. The model used an unstructured covariance matrix for repeated measures and included fixed effects of treatment, timepoint (hours 0 and 2 at weeks 2, 6, and 12), treatment-by-timepoint interaction, and baseline IOP stratification (≤ 25 and > 25 mmHg); the hour-matched baseline IOP and the timepoint-by-baseline hour-matched IOP interaction were included as covariates. The difference between the 10 or 15 µg bimatoprost implant and timolol (bimatoprost implant minus timolol) and the corresponding two-sided 95% confidence interval (CI) for each timepoint was derived from the MMRM model. Noninferiority of the bimatoprost implant to timolol was established if the upper limit of the 95% CI was ≤ 1.5 mmHg for all six timepoints. If noninferiority to timolol was established, the bimatoprost implant was to be declared clinically noninferior to timolol if the upper limit of the 95% CI was ≤ 1.0 mmHg for three or more timepoints. Noninferiority of the 15 µg implant was tested first, followed by noninferiority testing of the 10 µg implant [23]. Superiority tests were performed after noninferiority was established. For patients who received repeat administration in the study eye, similar MMRM models were used to evaluate IOP after the second and third administration.
A similar MMRM approach was used to evaluate IOP change from baseline. The model included IOP hour-matched change from baseline as the response variable, with noninferiority of the bimatoprost implant to timolol established if the 95% CI of the between-group difference was within a 1.5 mmHg noninferiority margin at both hours 0 and 2 at week 12. Diurnal IOP and the number of patients who had used additional (rescue) IOP-lowering treatment in the study eye were summarized with descriptive statistics. Kaplan-Meier survival analyses evaluated the time to initial use of additional treatment in the study eye after the last administration, and for patients who received three administrations in the study eye, after the third administration. For these analyses, patients who did not use any additional treatment in the study eye were censored at their last visit.
Safety analyses by treatment group were based on the first study treatment actually received in the study eye. Results for fellow eyes were pooled across treatment groups. Rates of TEAEs were evaluated overall and by administration cycle (for patients who received the administration) and during the extended safety follow-up period (for patients who received three administrations). Analysis of visual fields used the mean deviation (MD) from Humphrey perimetry and excluded data collected after use of rescue IOP-lowering medication. When appropriate, post hoc statistical comparisons among groups and for 10 and 15 µg bimatoprost implant versus timolol were performed using chi-square or Fisher exact tests for categorical variables and analysis of covariance or MMRM models for continuous variables.
The planned sample size of approximately 600 patients used for the ARTEMIS 1 study [23] was modified for the ARTEMIS 2 study after discussion with the FDA, because evaluation of masked study data suggested lower IOP variability and a lower rate of patient rescue or discontinuation from the study during the primary efficacy period than had been assumed for the initial sample size calculation. Enrollment of approximately 510 patients (170 per treatment group) was planned to provide 95% power to show noninferiority of the 15 μg bimatoprost implant to timolol and 84% power to show noninferiority of the 10 μg bimatoprost implant to timolol using the updated estimate of IOP variability and estimates of between-group differences from a previous study [22], and assuming a study discontinuation or rescue rate of 5% within 12 weeks.
Results
Enrollment in the study began in December 2014, and the study was completed in May 2020. A total of 528 patients were enrolled in the study and randomly assigned to one of the three treatment groups. Baseline demographics and study eye characteristics were generally well balanced among the treatment groups (Table 1). However, by chance the proportion of patients who were Black or African American was higher in the timolol group than in the bimatoprost implant groups (Table 1).
Table 1.
Baseline demographics and study eye characteristics (ITT population)
| Parameter | Bimatoprost implant 10 µg (n = 176) |
Bimatoprost implant 15 µg (n = 176) |
Timolol BID (n = 176) |
P Valuea Bim 10 µg vs. timolol |
P Valuea Bim 15 µg vs. timolol |
|---|---|---|---|---|---|
| Age, mean (SD), years | 62.5 (12.7) | 63.8 (10.7) | 61.4 (12.4) | 0.446 | 0.055 |
| Range | 23–88 | 24–85 | 19–90 | ||
| Gender, n (%) | 0.831 | 0.749 | |||
| Male | 86 (48.9) | 85 (48.3) | 88 (50.0) | ||
| Female | 90 (51.1) | 91 (51.7) | 88 (50.0) | ||
| Race, n (%) | 0.078 | 0.036 | |||
| White | 115 (65.3) | 116 (65.9) | 104 (59.1) | ||
| Hispanic | 22 (12.5) | 27 (15.3) | 21 (11.9) | ||
| Black or AA | 20 (11.4) | 19 (10.8) | 36 (20.5) | ||
| Asian | 11 (6.3) | 6 (3.4) | 13 (7.4) | ||
| Other | 8 (4.5) | 8 (4.5) | 2 (1.1) | ||
| Iris color, n (%) | 0.968 | 0.136 | |||
| Brown | 82 (46.6) | 81 (46.0) | 82 (46.6) | ||
| Dark brown | 30 (17.0) | 16 (9.1) | 31 (17.6) | ||
| Blue | 24 (13.6) | 20 (11.4) | 20 (11.4) | ||
| Hazel | 6 (3.4) | 7 (4.0) | 9 (5.1) | ||
| Green | 5 (2.8) | 2 (1.1) | 2 (1.1) | ||
| Gray | 0 | 4 (2.3) | 1 (0.6) | ||
| Green/brown | 10 (5.7) | 18 (10.2) | 12 (6.8) | ||
| Blue/brown | 14 (8.0) | 20 (11.4) | 14 (8.0) | ||
| Other | 3 (1.7) | 7 (4.0) | 3 (1.7) | ||
| Not reported | 2 (1.1) | 1 (0.6) | 2 (1.1) | ||
| Diagnosis, n (%) | 0.575 | 0.557 | |||
| OAG | |||||
| Primary | 131 (74.4) | 118 (67.0) | 125 (71.0) | ||
| Pigmentary | 3 (1.7) | 7 (4.0) | 4 (2.3) | ||
| Pseudoexfoliation | 1 (0.6) | 2 (1.1) | 2 (1.1) | ||
| OHT | 41 (23.3) | 49 (27.8) | 45 (25.6) | ||
| Lens status | > 0.999 | 0.295 | |||
| Phakic | 135 (76.7) | 143 (81.3) | 135 (76.7) | ||
| Pseudophakic | 41 (23.3) | 33 (18.8) | 41 (23.3) | ||
| CECD, mean (SD), cells/mm2 | 2434.2 (310.8) | 2487.7 (301.9) | 2469.2 (352.4) | 0.324 | 0.598 |
| Range | 1824–3215 | 1811–3719 | 1698–3643 | ||
| IOP, mean (SD), mmHg | |||||
| Hour 0 | 24.3 (2.4) | 24.4 (2.5) | 24.5 (2.5) | 0.500 | 0.790 |
| Hour 2 | 23.2 (2.8) | 23.4 (2.8) | 23.4 (3.1) | 0.533 | 0.943 |
| Diurnal | 23.7 (2.5) | 23.9 (2.4) | 23.9 (2.5) | 0.446 | 0.857 |
AA African American, BID twice daily, Bim bimatoprost implant, CECD central corneal endothelial cell density, IOP intraocular pressure, ITT intent-to-treat, OAG open-angle glaucoma, OHT ocular hypertension, SD standard deviation
aP value based on two-sample t test for continuous variables and chi-square test for categorical values
The mean age of the study population was 62.6 years; 49.1% of the patients were male, 63.4% were White, and 70.8% were diagnosed with primary OAG in the study eye. Most of the patients had phakic eyes and required washout of previous topical IOP-lowering medication before study enrollment. The baseline mean diurnal IOP was 23.7, 23.9, and 23.9 mmHg in the 10 µg implant, 15 µg implant, and timolol groups, respectively. CECD was required to be at least 1800 cells/mm2 at study entry, and at baseline the mean CECD ranged from 2434 to 2488 cells/mm2 among the treatment groups.
Figure 1 shows the flow of patients through the study. Completion rates for the 20-month study were high and comparable among the treatment groups. The study was completed by 90.9%, 89.2%, and 88.1% of patients in the 10 µg implant, 15 µg implant, and timolol groups, respectively.
Study drug exposure was evaluated in the safety population of all treated patients. In the 10 µg implant group, 152 patients (86.9%) received three implant administrations, 13 patients (7.4%) received two implant administrations, and ten patients (5.7%) received one implant administration. In the 15 µg implant group, 142 patients (80.7%) received three implant administrations, 20 patients (11.4%) received two implant administrations, and 14 patients (8.0%) received one implant administration.
On gonioscopy, the implants were typically observed in the inferior iridocorneal angle. Figure 2 shows the appearance of implants on gonioscopy in a representative patient. The implants frequently swelled after administration as they became hydrated and degraded. After the first administration on day 1, investigators reported visible implant on gonioscopy at week 12 in 96.5% (164/170) of study eyes in the 10 µg implant group, and for implants with size assessments, 46.4% (58/125) were reported to be 51–100% of initial size, whereas 26.4% (33/125) were reported to be 101–150% of initial size. In the 15 µg implant group, visible implant was reported in 98.8% (168/170) of study eyes at week 12, and for implants with size assessments, 38.1% (48/126) were reported to be 51–100% of initial size, whereas 47.6% (60/126) were reported to be 101–150% of initial size. At week 52, the implant administered on day 1 in both the 10 and 15 µg treatment groups was typically reported to be no longer visible or was estimated to be less than 25% of its initial size. At the end of the extended safety follow-up (month 20), one or more visible implants were reported in 88.4% (130/147) of study eyes in the 10 µg implant group and 89.1% (122/137) of study eyes in the 15 µg implant group, and implants from each administration typically were reported to be no longer visible or were estimated to be ≤ 25% of their initial size.
Fig. 2.
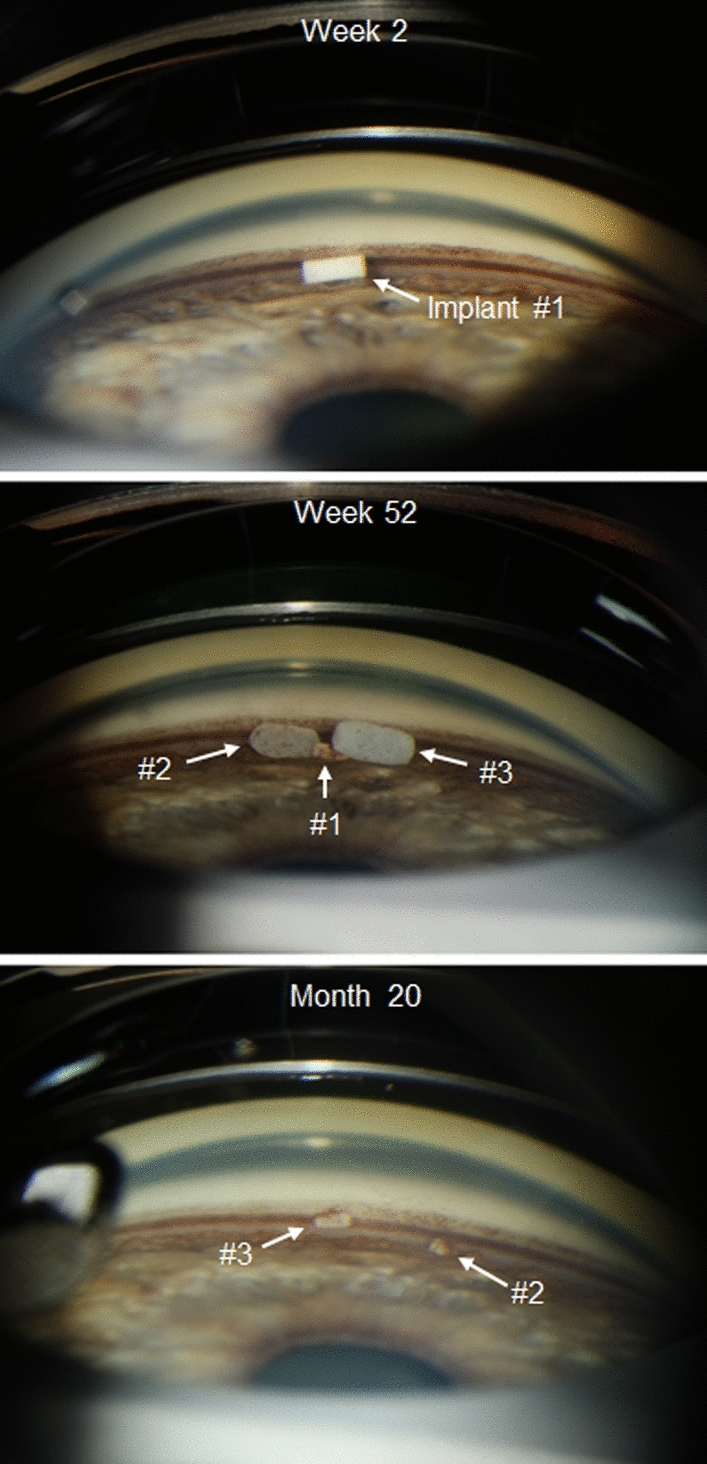
Gonioscopic photographs of the study eye iridocorneal angle in a representative patient in the 10 μg bimatoprost implant treatment group. Photographs were taken at week 2, week 52, and month 20. Implants #1, #2, and #3 were administered at day 1, week 16, and week 32, respectively
Primary Endpoints: Intraocular Pressure (IOP) Lowering Through Week 12
The mean IOP in study eyes was consistently lower with the 10 and 15 µg bimatoprost implants compared with timolol BID at hours 0 and 2 at weeks 2, 6, and 12 (Fig. 3a). Moreover, the mean change from baseline IOP in study eyes was consistently larger with the 10 and 15 µg bimatoprost implants compared with timolol BID at hours 0 and 2 at weeks 2, 6, and 12 (Fig. 4a). Both the 10 and 15 µg bimatoprost implants met the a priori criteria for statistical and clinical noninferiority to timolol BID; the upper limit of the 95% CI of the difference from timolol in mean IOP (Fig. 3b) and mean change from baseline IOP (Fig. 4b) was < 1 mmHg for each implant dose strength at both hours 0 and 2 at weeks 2, 6, and 12. The upper limit of the 95% CI of the difference from timolol was < 0 mmHg, indicating superiority of the bimatoprost implant to timolol in mean IOP and mean change from baseline IOP, at one of the six time points for the 10 µg implant and four of the six time points for the 15 µg implant.
Fig. 3.
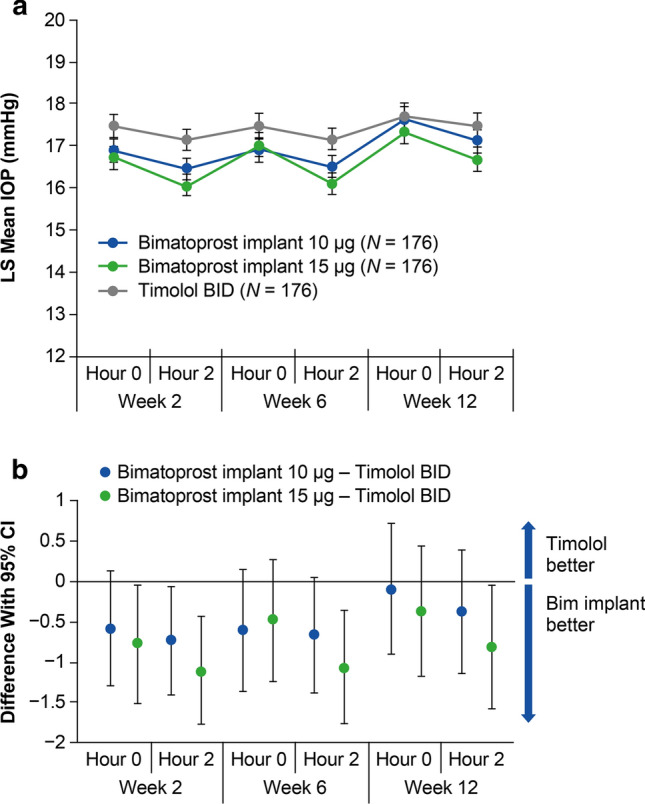
Primary endpoint of mean IOP through week 12. a LS mean IOP in study eyes at hours 0 and 2 at weeks 2, 6, and 12. b The 95% CIs of the between-group differences show that both the 10 and 15 µg bimatoprost implants met the prespecified criteria for statistical and clinical noninferiority to timolol BID. BID twice daily, Bim bimatoprost, CI confidence interval, IOP intraocular pressure, LS least-squares
Fig. 4.
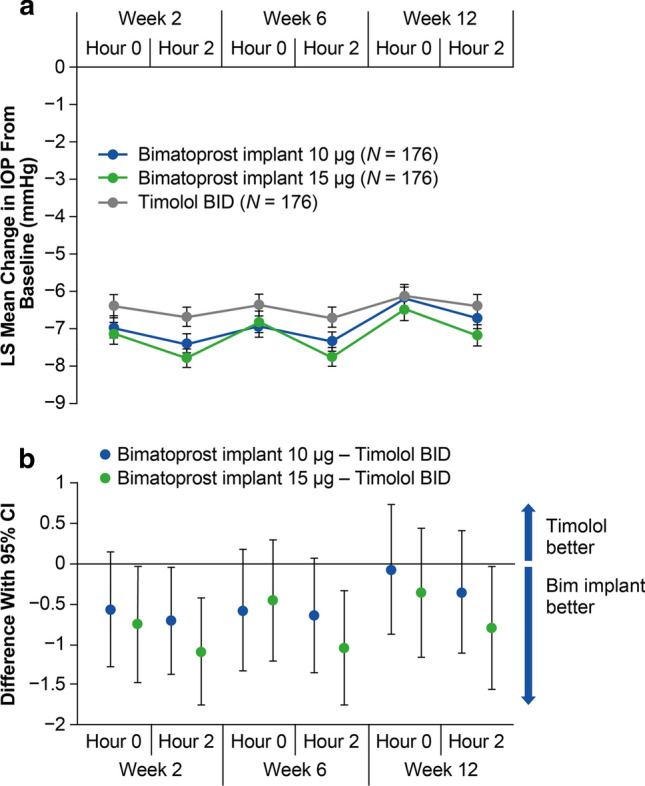
Primary endpoint of mean change in IOP from baseline through week 12. a LS mean change in IOP from baseline in study eyes at hours 0 and 2 at weeks 2, 6, and 12. b The 95% CIs of the between-group differences show that both the 10 and 15 µg bimatoprost implants met the prespecified criteria for statistical and clinical noninferiority to timolol BID. BID twice daily, Bim bimatoprost, CI confidence interval, IOP intraocular pressure, LS least-squares
Efficacy After Repeated Administration
The bimatoprost implant provided similar IOP lowering after repeated administrations (OSM, Resource 3).
Efficacy Throughout the Study
The mean IOP in study eyes was reduced from baseline in each treatment group at all follow-up visits during the active treatment period of the study (OSM, Resource 4). At week 52 (the end of the active treatment period), the percentage of patients who remained in the study and had not received rescue treatment in the study eye was 81.8% (144/176) in the 10 µg implant group, 77.8% (137/176) in the 15 µg implant group, and 84.1% (148/176) in the timolol BID group. The week 52 mean (standard deviation (SD)) diurnal IOP in the study eye of these patients was 17.8 (3.8) mmHg in the 10 µg implant group, 17.1 (3.6) mmHg in the 15 µg implant group, and 17.2 (3.3) mmHg in the timolol BID group.
The mean IOP in study eyes remained controlled in each treatment group throughout the extended safety follow-up (OSM, Resource 4). In the bimatoprost implant groups, patients received their last administration no later than 8 months into the study, whereas patients in the timolol group continued their daily treatment through month 20. The percentage of patients who reached month 20 without receiving rescue treatment in the study eye was 64.8% (114/176) in the 10 µg implant group, 64.2% (113/176) in the 15 µg implant group, and 78.4% (138/176) in the timolol BID group. The mean (SD) diurnal IOP in the study eye of these patients at month 20 was 18.2 (4.0) mmHg in the 10 µg implant group, 17.6 (3.5) mmHg in the 15 µg implant group, and 17.2 (3.4) mmHg in the timolol BID group.
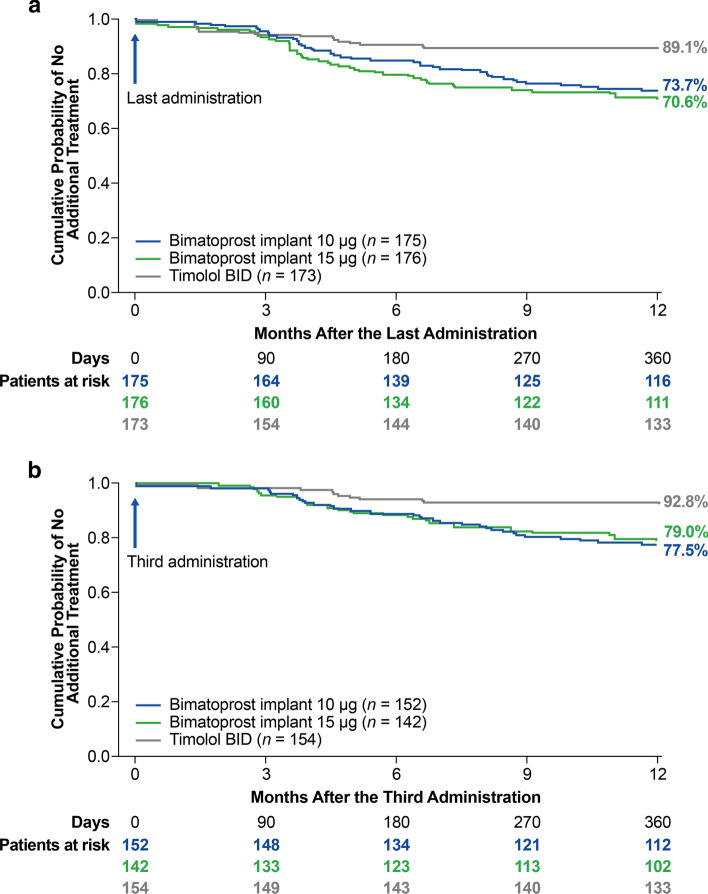
Kaplan-Meier survival analysis evaluated time to use of rescue IOP-lowering treatment in the study eye (Fig. 5). The estimated probability of not requiring rescue treatment in the study eye for 1 year after the last administration was 73.7% in the 10 µg implant-treated eyes and 70.6% in the 15 µg implant-treated eyes (Fig. 5a). For patients who received three administrations, the estimated probability of not requiring rescue treatment in the study eye for 1 year after the third administration was 77.5% in the 10 µg implant-treated eyes and 79.0% in the 15 µg implant-treated eyes (Fig. 5b).
Fig. 5.
Kaplan-Meier survival analysis of time to initial use of additional IOP-lowering treatment in the study eye a after the last bimatoprost implant or sham administration and b after the third administration in patients who received three administrations. BID twice daily, IOP intraocular pressure
Safety Outcomes
Overall rates of TEAEs in each treatment group are summarized in Table 2. One or more TEAEs were reported in 74.9%, 88.1%, and 70.5% of patients in the 10 µg implant, 15 µg implant, and timolol groups, respectively. Treatment-related TEAEs, almost all ocular, were reported in 48.0%, 61.9%, and 20.8% of patients in the 10 µg implant, 15 µg implant, and timolol groups, respectively.
Table 2.
Summary of treatment-emergent adverse events (safety population)
| TEAE | Overall Incidence, n (%) |
P Valuea Bim 10 µg vs. timolol |
P Valuea Bim 15 µg vs. timolol |
||
|---|---|---|---|---|---|
| Bimatoprost implant 10 µg (n = 175) |
Bimatoprost implant 15 µg (n = 176) |
Timolol BID (n = 173) |
|||
| Any TEAE | 131 (74.9) | 155 (88.1) | 122 (70.5) | 0.364 | < 0.001 |
| Any treatment-related TEAE | 84 (48.0) | 109 (61.9) | 36 (20.8) | < 0.001 | < 0.001 |
| Ocular | 84 (48.0) | 109 (61.9) | 35 (20.2) | < 0.001 | < 0.001 |
| Nonocular | 3 (1.7) | 5 (2.8) | 2 (1.2) | > 0.999 | 0.448 |
| Any serious TEAE | 22 (12.6) | 36 (20.5) | 16 (9.2) | 0.320 | 0.003 |
| Ocular | 6 (3.4) | 13 (7.4) | 0 | 0.030 | < 0.001 |
| Nonocular | 17 (9.7) | 25 (14.2) | 16 (9.2) | 0.882 | 0.151 |
| Deathb | 0 | 1 (0.6) | 1 (0.6) | 0.497 | > 0.999 |
BID twice daily, Bim bimatoprost implant, TEAE treatment-emergent adverse event
aP value based on chi-square test or Fisher exact test (when any frequency was < 5)
bDeath from tumor metastases (15 µg implant group) and complications of hip fracture (timolol group) considered to be unrelated to treatment
Ocular TEAEs in the study eye, listed in OSM, Resource 5, were mostly mild or moderate in severity and were reported in 62.3%, 80.7%, and 48.0% of patients in the 10 µg implant, 15 µg implant, and timolol groups, respectively. The most common of these TEAEs were conjunctival hyperemia, foreign body sensation, and conjunctival hemorrhage, which typically were reported within 2 days after the study treatment in association with the administration procedure (Table 3). The occurrence of conjunctival hyperemia and foreign body sensation within 2 days after the administration procedure was likely related to the use of povidone-iodine solution in the sterile preparation for the procedure. The median duration of TEAEs in study eyes after the first administration was 12.5, 14, and 15 days for conjunctival hyperemia; 5, 2, and 3 days for foreign body sensation; and 12, 15, and 15 days for conjunctival hemorrhage in the 10 µg implant, 15 µg implant, and timolol groups, respectively. Iris hyperpigmentation in the study eye was reported as a TEAE in five patients in both the 10 and the 15 µg bimatoprost implant groups. There were no TEAE reports of eyelash growth or periorbital fat atrophy in any treatment group.
Table 3.
Treatment-emergent ocular adverse events in study eyes by time of onset after bimatoprost implant or sham procedure administration
| TEAE | Onset within 2 days, n (%) | Onset after 2 days, n (%) | ||||
|---|---|---|---|---|---|---|
| Bimatoprost implant 10 µg (n = 175) |
Bimatoprost implant 15 µg (n = 176) |
Timolol BID (n = 173) |
Bimatoprost implant 10 µg (n = 175) |
Bimatoprost implant 15 µg (n = 176) |
Timolol BID (n = 173) |
|
| Conjunctival hyperemia | 36 (20.6) | 56 (31.8) | 16 (9.2) | 23 (13.1) | 39 (22.2) | 5 (2.9) |
| Conjunctival hemorrhage | 16 (9.1) | 12 (6.8) | 11 (6.4) | 0 | 2 (1.1) | 3 (1.7) |
| Foreign body sensation in eye | 13 (7.4) | 14 (8.0) | 2 (1.2) | 7 (4.0) | 5 (2.8) | 0 |
| Eye pain | 11 (6.3) | 15 (8.5) | 5 (2.9) | 0 | 11 (6.3) | 3 (1.7) |
| Photophobia | 11 (6.3) | 12 (6.8) | 0 | 2 (1.1) | 3 (1.7) | 0 |
| Eye irritation | 7 (4.0) | 7 (4.0) | 3 (1.7) | 3 (1.7) | 5 (2.8) | 4 (2.3) |
| Aqueous humor leakage | 5 (2.9) | 1 (0.6) | 0 | 0 | 0 | 0 |
| Punctate keratitis | 5 (2.9) | 8 (4.5) | 2 (1.2) | 3 (1.7) | 6 (3.4) | 5 (2.9) |
| Anterior chamber cell | 4 (2.3) | 6 (3.4) | 1 (0.6) | 4 (2.3) | 6 (3.4) | 0 |
| Dry eye | 4 (2.3) | 9 (5.1) | 4 (2.3) | 8 (4.6) | 9 (5.1) | 3 (1.7) |
| Lacrimation increased | 4 (2.3) | 5 (2.8) | 1 (0.6) | 2 (1.1) | 5 (2.8) | 0 |
| Vision blurred | 4 (2.3) | 4 (2.3) | 0 | 5 (2.9) | 3 (1.7) | 1 (0.6) |
| Ocular discomfort | 3 (1.7) | 7 (4.0) | 1 (0.6) | 0 | 5 (2.8) | 0 |
| Iritis | 2 (1.1) | 3 (1.7) | 0 | 7 (4.0) | 6 (3.4) | 0 |
| Blepharitis | 1 (0.6) | 2 (1.1) | 0 | 8 (4.6) | 5 (2.8) | 5 (2.9) |
| Vitreous detachment | 1 (0.6) | 0 | 0 | 2 (1.1) | 4 (2.3) | 1 (0.6) |
| Conjunctivitis allergic | 0 | 1 (0.6) | 0 | 5 (2.9) | 2 (1.1) | 2 (1.2) |
| Corneal edema | 0 | 2 (1.1) | 0 | 6 (3.4) | 21 (11.9) | 0 |
| Corneal endothelial cell loss | 0 | 0 | 0 | 14 (8.0) | 45 (25.6) | 1 (0.6) |
| Corneal touch | 0 | 0 | 0 | 2 (1.1) | 4 (2.3) | 0 |
| Erythema of eyelid | 0 | 1 (0.6) | 1 (0.6) | 4 (2.3) | 2 (1.1) | 0 |
| Intraocular pressure increased | 0 | 2 (1.1) | 1 (0.6) | 14 (8.0) | 15 (8.5) | 5 (2.9) |
| Iris adhesions | 0 | 0 | 0 | 4 (2.3) | 3 (1.7) | 0 |
| Iris hyperpigmentation | 0 | 0 | 0 | 5 (2.9) | 5 (2.8) | 0 |
| Vitreous floaters | 0 | 0 | 0 | 5 (2.9) | 1 (0.6) | 2 (1.2) |
| Overalla | 77 (44.0) | 95 (54.0) | 42 (24.3) | 84 (48.0) | 119 (67.6) | 60 (34.7) |
| P valueb | < 0.001 | < 0.001 | – | 0.012 | < 0.001 | – |
BID twice daily, TEAE treatment-emergent adverse event. All ocular TEAEs in study eyes that were reported in ≥ 2% of subjects in any treatment group within 2 days or after 2 days following administration are listed
aAny ocular TEAE in the study eye
bP value for comparison of overall TEAE vs. timolol (chi-square test)
Serious ocular TEAEs (all in the study eye) were reported more frequently in the 10 and 15 µg implant groups (3.4% and 7.4% of patients, respectively) than in the timolol group (no patients) (P = 0.030 and P < 0.001 vs. timolol, respectively). In both bimatoprost implant groups, the most common study eye serious ocular TEAE was corneal endothelial cell loss (CECL). The incidence rates for corneal TEAEs of interest (mainly CECL, corneal edema, and corneal touch) and inflammatory TEAEs of interest (mainly anterior chamber cell and iritis) in study eyes were higher in the bimatoprost implant groups than in the timolol group and were higher in the 15 µg implant group than in the 10 µg implant group. Corneal TEAEs of interest were most commonly reported after repeated administrations. In the 10 µg implant group, occurrence or worsening of CECL was reported in no patients after the first and second administrations, 3.8% (6/156) of patients after the third administration (through the end of the week 52 visit window), and 5.8% (9/155) of patients during the extended safety follow-up through month 20. In the 15 µg implant group, occurrence or worsening of CECL was reported in 0.6% (1/176) of patients after the first administration, 4.2% (7/165) of patients after the second administration, 11.6% (17/147) of patients after the third administration, and 14.6% (21/144) of patients during the extended safety follow-up through month 20.
Implants were removed because of a TEAE, most commonly corneal edema or CECL, in five patients (2.9%) in the 10 µg implant group and 19 patients (10.8%) in the 15 µg implant group. The removal of implants was after the first, second, and third administrations, respectively, in one, one, and three patients in the 10 µg group and none, four, and 15 patients in the 15 µg group. The TEAE leading to the single implant removal after the first administration (in the 10 µg implant group) was “product administered at an inappropriate site” (the implant was accidentally injected into the corneal stroma). An additional patient in the 10 µg implant group underwent implant removal after the second administration because the implant resided in the injection track at the cornea.
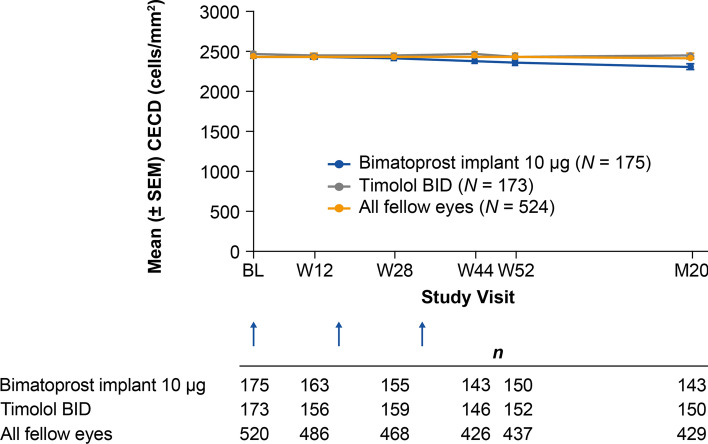
Evaluation of CECD on specular microscopy showed a time-dependent loss of CECD in study eyes in the bimatoprost implant groups, with greater loss in the 15 µg implant group (Table 4). At month 20, the mean CECD was approximately 5% lower than the baseline value for study eyes in the 10 µg implant group compared with approximately 1% lower than the baseline value for study eyes in the timolol BID group and for all fellow eyes treated with timolol BID (Fig. 6). The proportion of study eyes with a ≥ 20% decrease in CECD from baseline in the 10 µg implant, 15 µg implant, and timolol BID groups, respectively, was 0% (0/163), 1.2% (2/166), and 0% (0/156) at 12 weeks after the first administration (week 12), 0.6% (1/155), 3.9% (6/154), and 0% (0/159) at 12 weeks after the second administration (week 28), 3.5% (5/143), 10.3% (14/136), and 0% (0/146) at 12 weeks after the third administration (week 44), 5.3% (8/150), 15.4% (21/136), and 1.3% (2/152) at the end of the active treatment period (week 52), and 8.1% (14/173), 24.4% (43/176), and 0.6% (1/173) at month 20 or the last study visit before exit.
Table 4.
Mean (standard deviation) central corneal endothelial cell density in study eyes by specular microscopy, cells/mm2 (safety population)
| Visit | Bimatoprost implant 10 µg (n = 175) |
Bimatoprost implant 15 µg (n = 176) |
Timolol BID (n = 173) |
All fellow eyes (n = 524) |
P Valuea Bim 10 µg vs. Timolol |
P Valuea Bim 15 µg vs. Timolol |
|---|---|---|---|---|---|---|
| Baseline | 2435.6 (311.2) | 2487.7 (301.9) | 2469.2 (355.1) | 2437.1 (346.2) | – | – |
| Week 12 | 2425.4 (319.4) | 2462.0 (310.8) | 2448.8 (343.5) | 2428.7 (359.4) | 0.027 | 0.791 |
| Week 28 | 2411.2 (327.3) | 2411.5 (354.5) | 2453.9 (355.3) | 2427.4 (361.5) | 0.280 | < 0.001 |
| Week 44 | 2382.9 (366.9) | 2309.6 (504.0) | 2461.2 (361.6) | 2431.0 (372.3) | 0.188 | < 0.001 |
| Week 52 | 2355.0 (404.0) | 2237.9 (557.5) | 2437.1 (372.6) | 2420.1 (375.5) | 0.059 | < 0.001 |
| Month 20 | 2303.6 (454.1) | 2091.6 (638.9) | 2451.5 (375.5) | 2418.1 (369.8) | 0.001 | < 0.001 |
BID twice daily, Bim bimatoprost implant
aP values are based on a mixed-effect model for repeated measures including treatment, visit, treatment-by-visit interaction, baseline central corneal endothelial cell density, and visit-by-baseline interaction
Fig. 6.
Mean CECD in study eyes in the 10 µg bimatoprost implant and timolol treatment groups. The timing of implant or sham administration is shown with arrows. Statistical comparisons are reported in Table 4. BID twice daily, Bim bimatoprost, BL baseline, CECD corneal endothelial cell density, M month, SEM standard error of the mean, W week
The mean BCVA for all study eyes and for study eyes with a corneal TEAE of interest was stable from baseline to the last available visit in each treatment group (Table 5). Among eyes with a corneal TEAE of interest, none in the 10 µg implant group and two in the 15 µg implant group had a greater than two-line loss in BCVA from baseline at their last study visit (Table 5). Also, in each treatment group, mean changes from baseline in the study eye CCT at each visit were small and not considered to be clinically significant. Among patients with a report of a corneal TEAE of interest, the study eye mean change in CCT from baseline at the last available visit was + 1.6 µm (n = 19), + 4.5 µm (n = 55), and + 28 µm (n = 1) in the 10 µg implant, 15 µg implant, and timolol groups, respectively.
Table 5.
Best-corrected visual acuity in study eyes
| Safety parameter | All patients | Patients with corneal TEAE of interesta | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bimatoprost implant 10 µg (n = 175) | Bimatoprost implant 15 µg (n = 176) | Timolol BID (n = 173) |
P Value Bim 10 µg vs. Timolol |
P Value Bim 15 µg vs. Timolol |
Bimatoprost implant 10 µg (n = 19) |
Bimatoprost implant 15 µg (n = 55) | Timolol BID (n = 1) |
||
| Mean BCVA (SD), letters | |||||||||
| Baseline | 82.7 (5.6) | 81.7 (6.4) | 82.2 (6.4) | 84.5 (5.0) | 82.5 (5.8) | 80 | |||
| Month 20 or last visit | 83.3 (5.9) | 81.6 (6.9) | 82.7 (7.0) | 0.662b | 0.193b | 84.7 (6.3) | 81.6 (6.5) | 80 | |
| Patients with > two-line (ten-letter) loss in BCVA from baseline at month 20 or last visit, n (%) | 2 (1.1) | 5 (2.8) | 3 (1.7) | 0.684c | 0.723c | 0 | 2 (3.6) | 0 | |
BCVA best-corrected visual acuity, BID twice daily, Bim bimatoprost implant, SD standard deviation, TEAE treatment-emergent adverse event
aAll patients with a TEAE report of corneal endothelial cell loss, corneal edema, corneal opacity, corneal touch, corneal disorder, or corneal thickening who had a baseline BCVA assessment
bP value based on an analysis of covariance model including treatment with baseline BCVA as the covariate
cP value based on Fisher exact test
At baseline, the mean (SD) MD in study eyes was − 2.21 (4.01), − 1.56 (3.19), and − 1.78 (5.80) dB in the 10 µg implant, 15 µg implant, and timolol groups, respectively. Analysis of the change in the MD from baseline during the study showed progression of visual field loss in the timolol group; the progression of visual field loss was similar or reduced (P ≤ 0.037 vs. timolol at week 52) in the bimatoprost implant groups (Fig. 7). The mean change in MD from baseline was − 0.08, − 0.26, and − 0.96 dB at week 52 and − 0.37, − 0.60, and − 1.18 dB at month 20 in the 10 µg implant, 15 µg implant, and timolol groups, respectively (Fig. 7).
Fig. 7.
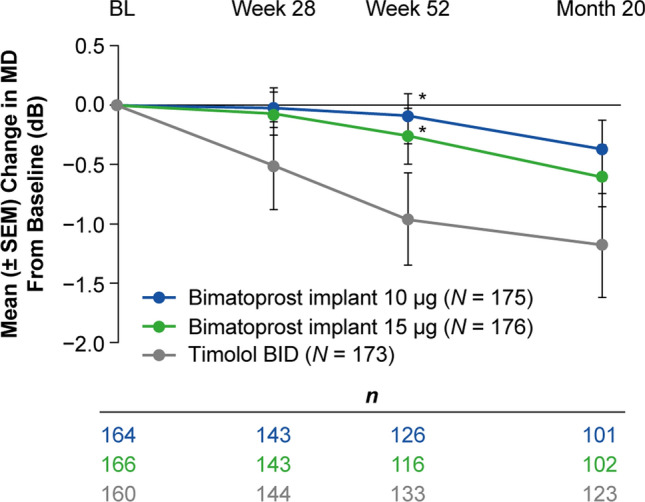
Mean change in the visual field MD from baseline by Humphrey perimetry in study eyes. Test results from eyes that had received rescue IOP-lowering treatment were excluded from the analysis. The number of eyes included in the analysis of change in MD from baseline at visits from week 28 to month 20 ranged from 101 to 143 in the 10 µg bimatoprost implant group, 102 to 143 in the 15 µg bimatoprost implant group, and 123 to 144 in the timolol BID group. *P ≤ 0.037 vs. timolol based on a mixed-effect model for repeated measures including treatment, visit, treatment-by-visit interaction, baseline MD, and visit-by-baseline interaction. BID twice daily, BL baseline, IOP intraocular pressure, MD mean deviation, SEM standard error of the mean
Discussion
In this study, both the 10 and 15 µg bimatoprost implants were noninferior to topical timolol in lowering IOP through 12 weeks. The study design included three administrations of implant at a fixed 4-month dosing interval, and efficacy after the second and third administrations was similar. Both dose strengths of implant provided IOP control in most patients for 1 year after the last implant administration without any additional IOP-lowering treatment. With the 4-month fixed-dosing interval used, the 10 µg implant demonstrated a better corneal safety profile than the 15 µg implant, presumably related to its smaller size. Both dose strengths of implant are cylindrical in shape with the same diameter, but the 15 µg implant is 50% longer than the 10 µg implant.
The results of the ARTEMIS 2 study essentially replicated those of the ARTEMIS 1 study. This is a positive finding for this novel treatment, as it provides an additional dataset with consistent results that clinicians can use to understand the efficacy and safety of the implant. In addition, the replication of the results in a second study, at different sites, reduces any potential bias.
One notable difference between the two ARTEMIS studies was that the ARTEMIS 2 study was planned to enroll fewer patients than ARTEMIS 1. However, the ARTEMIS 2 study still was adequately powered, and both dose strengths of implant met the primary endpoint of noninferiority to topical timolol in lowering IOP. A difference in patient disposition was also evident between the two studies: in the ARTEMIS 1 study, the study completion rate was comparable in the 10 µg implant and timolol groups and lower in the 15 µg implant group, whereas in the ARTEMIS 2 study, study completion rates were similarly high (approximately 90%) across all treatment groups. The reason for this difference in results between the two studies is unknown.
The most common TEAEs (conjunctival hyperemia, conjunctival hemorrhage, and foreign body sensation) typically occurred within 2 days after administration and were related to the administration procedure. Some of these events were likely caused by the procedure preparation, which included povidone-iodine irrigation. There were no reports of eyelash growth or periorbital fat atrophy, consistent with the findings of the ARTEMIS 1 study and a drug distribution study in dogs [25]. These results suggest that targeted drug delivery with the bimatoprost implant may be successful in reducing periocular adverse effects associated with topical PGAs. Anterior segment TEAEs of interest (most commonly iritis and anterior chamber cells) occurred in some patients in the bimatoprost implant groups. These TEAEs were typically mild in severity and transient. The TEAEs of most clinical concern were the corneal AEs reported in the bimatoprost implant groups. The frequency of these TEAEs was higher with the larger (15 µg) implant and after repeated administration, consistent with the premise that the corneal TEAEs result from a physical interaction between the cornea and implants [23]. As many as three implants were present in the angle at the same time because of the slow rate of implant biodegradation and the 16-week fixed-interval administration schedule used in the study. There were no TEAE reports of CECL in patients after the first or second administration of the 10 µg implant. However, when multiple implants were administered at 4-month intervals, the CECD was decreased compared with timolol, and the difference between the 10 µg implant and timolol became statistically significant at 20 months.
The analysis of time to rescue demonstrated an extended duration of efficacy of the bimatoprost implant, with high probability (> 70%) of patients requiring no rescue for 1 year after the third or last administration. These results are consistent with those of the ARTEMIS 1 study [23] and the previous 2-year, phase 1/2 dose-ranging study of the bimatoprost implant in patients with OAG (APOLLO) [26]. In the phase 1/2 study, 68%, 40%, and 28% of study eyes that received bimatoprost implant 6, 10, 15, or 20 µg (two 10 µg implants) on day 1 were controlled without any additional treatment up to months 6, 12, and 24, respectively [26].
As pharmacokinetics data for the bimatoprost implant (from drug distribution studies in dogs and aqueous samples after implant administration in humans) show that drug release is complete within 3–4 months [23], continued drug presence is unlikely to account for the sustained IOP lowering observed for 1 year or longer after implant administration. Matrix metalloproteinase (MMP)-induced durable tissue remodeling of aqueous outflow pathways has been proposed as a more likely explanation for the extended duration of IOP lowering after bimatoprost implant administration [23, 26–28]. Topical PGAs reduce IOP by inducing a concentration-dependent upregulation of MMP expression and activity in the ciliary body and trabecular meshwork, which leads to increased extracellular matrix turnover and tissue remodeling that decreases the resistance to aqueous outflow through the unconventional (uveoscleral) and conventional (trabecular meshwork) pathways [29–36]. The drug concentrations achieved in target tissues with the bimatoprost implant are orders of magnitude higher than those achieved with topical dosing [25], and these higher drug concentrations have been shown to produce much greater upregulation of MMPs in human ciliary body cell cultures [36]. The higher drug concentrations achieved in outflow tissues by the implant are proposed to produce a greater upregulation of MMPs, which causes a more durable tissue remodeling, leading to sustained IOP lowering [23, 26–28].
The larger 15 µg bimatoprost implant was removed from subsequent development following the CECL observed in the ARTEMIS studies, but bimatoprost implant 10 µg has been approved by the FDA for single intracameral administration for the reduction of IOP in patients with OAG or OHT. The duration of effect demonstrated in the ARTEMIS and APOLLO studies suggests the potential for the bimatoprost implant to effectively control IOP when used in treatment regimens with long intervals between administrations. Ongoing studies (NCT03850782, NCT03891446) are evaluating the safety and efficacy of as-needed administration of the implant.
By chance, there was an imbalance in race/ethnicity among treatment groups at randomization. We do not believe this imbalance in race/ethnicity was a confounding factor in the analyses, because a preplanned subgroup analysis of the pooled ARTEMIS 1 and ARTEMIS 2 study data (with larger sample size), which was performed for FDA drug approval, showed that overall IOP lowering was similar among racial/ethnicity subgroups (FDA summary basis for approval section 7.1.2). Another study limitation may have been the requirement for implant administrations at a fixed 4-month interval, because many patients may have had adequate IOP control and not needed repeated administrations. This issue is being addressed by the ongoing studies that are evaluating an as-needed dosing regimen. In addition, the Kaplan-Meier analysis was an indirect measure of the maintenance of the IOP-lowering effect over time. The decision of when to rescue was up to the investigator, and there were no defined IOP criteria for rescue.
A strength of this study was the collection and analysis of visual field MD data. Large population-based studies in treated patients with glaucoma have demonstrated worsening of the visual field over time, as assessed by the mean change in the visual field MD, with reported progression rates generally ranging from − 0.32 dB/year [37] to − 0.8 dB/year [38]. Given these expected visual field progression rates, even with the current medical and surgical methods to treat glaucoma, the cumulative incidence of blindness in at least one eye among patients diagnosed with glaucoma and visual field loss was 26.5% after 10 years [39], which is unacceptable when life expectancies are increasing and patients are living longer with glaucoma [40]. The timolol group in our studies progressed − 0.97 dB in the first 12 months, in the range of what is expected from other studies [41]. In contrast, the mean change in visual field MD from baseline over 1 year was − 0.08 dB for patients using the commercialized dose strength (10 µg) of the bimatoprost implant in the treatment regimen used in this study, demonstrating stability of the visual field over time compared with timolol (Fig. 7).
Small changes in the visual field MD in patients with early glaucoma correspond to a significant loss of neural tissue, and patients with a mean MD of − 2 dB, as seen at baseline in this study, have already lost ~ 30% of their retinal ganglion cells (RGCs) [42]. Based on the relationship between the MD and number of RGCs reported by Medeiros et al. [42], the difference of ~ 0.8 dB in change from baseline MD at month 20 between the 10 µg implant-treated and timolol-treated eyes, which may appear small, corresponds to the preservation of an estimated 50,000 RGCs (~ 7%) with bimatoprost implant treatment compared with topical timolol treatment. These results suggest that with the treatment regimen used in this study, the bimatoprost implant has the potential to preserve more RGCs and improve visual field preservation over time in patients with early glaucoma.
The reformulation of bimatoprost into a sustained-release implant has provided a novel, drop-free, drug-delivery option for glaucoma therapy. The implant addresses many barriers to adherence to topical therapy, and the continuous drug release from the implant may potentially provide more stable 24/7 IOP control, leading to better preservation of the visual field. In many patients, intracameral delivery of bimatoprost with the implant may reduce the burden of treatment by providing long-term, sustained IOP control.
In summary, the efficacy and safety profiles of bimatoprost implant that were demonstrated in this study replicated those in the ARTEMIS 1 study, demonstrating that the results are robust. The implant effectively lowered IOP, and in many patients the IOP control provided by the implant persisted beyond the expected duration of intraocular drug bioavailability. The incidence of corneal TEAEs of interest was higher in the bimatoprost implant groups than in the timolol group. The study used a fixed dosing regimen of three administrations at 16-week intervals, and with this dosing regimen, the smaller 10 µg implant had a better safety profile and benefit-risk ratio than the 15 µg implant. The results suggest a potential for bimatoprost implant treatment to reduce the progression of visual field loss. Ongoing studies are evaluating 24-h IOP control with the bimatoprost implant and the effects of the implant on the visual field. Studies are also in progress to understand ideal treatment intervals, given the unexpectedly long duration of action of the implant.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Kevin Wang, PhD, an independent contractor funded by AbbVie, contributed to the statistical analyses for the article under the direction of the authors. Writing and editorial assistance was provided to the authors by Evidence Scientific Solutions, Inc (Philadelphia, PA) and funded by Allergan, an AbbVie company. All authors met ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
ARTEMIS 2 Study Group Principal Investigators: Argentina: Arturo Alezzandrini; Gabriel Bercovich; Pablo Deromedis; Federico Furno Sola; Carolina Gentile; Simon Lerner; Anahi Lupinacci; Carlos Zeolite; Canada: Catherine Birt; Andrew Crichton; Sebastien Gagne; Michael Giunta; Paul Harasymowycz; Delan Jinapriya; Marcelo Nicolela; Donald Nixon; Patrick Saurel; David Yan; Darana Yuen; Colombia: Santiago Arango; Sandra Belalcázar; Alexander Martinez; Juan Camilo Parra Restrepo; Czech Republic: Vladimir Korda; Jana Kadlecova; Jitka Svacinova; Egypt: Hany Khairy; Hani El Ibiary; Zeinab El Sanabary; Germany: Katharina Bell; Roman Greslechner; Jöerg Koch; Katrin Lorenz; Isabel Oberacher-Velten; Stefanie Schmickler; Claudie Schuart; Hagen Thieme; Italy: Francesco Bandello; Carlos Cagini; Michele Figus; Leonardo Mastropasqua; Luca Rossetti; Maurizio Giacinto Uva; Malaysia: Sandragasu Thayanithi; New Zealand: Anthony Wells; Singapore: Rahat Husain; Victor Koh; Dawn Lim; Aung Tin; South Africa: Petrus Gous; Lynette Venter; South Korea: Changwon Kee; Michael Kook; Ki-Ho Park; Turkey: Muhsin Eraslan; Ozcan Kayikcioglu; Nilgun Yildirim; United Kingdom: Rupert Bourne; Anshoo Choudhary; Francesca Cordeiro; Vincent Dubois; James Kirwan; Sheng Lim; Keith Martin; Antony Nithy; Avinash Prabhu; Andrew Tatham; United States: Ahmad Amir; Jason Bacharach; Howard Barnebey; Allen Beck; Lance Bergstrom; Navaneet Borisuth; James D. Branch; Jonathan Briggs; Stephen Bylsma; Peter Chang; William Christie; Frank Cotter; Michael Depenbusch; Damien F. Goldberg; Jack Greiner; Shailesh Gupta; Ron Gutmark; Ying Han; Sebastian Heersink; Malik Kahook; Albert Khouri; Joshua Kim; Howard Kushnick; Christopher Lin; Jodi Luchs; Arindel Maharaj, Steven L. Mansberger; Frank Mares; Eydie Miller-Ellis; Satish Modi; Matthew Paul; Ian Pitha; Robert Saltzmann; Michelle Sato; Michael Savestsky; Bruce Segal; Zachary Segal; Janet Serle; Mark Sherwood; Inder Singh; Stephen E. Smith; Julia Song; Robert Sorenson; Lawrence Tenkman; Navin Tekwani; Carl Tubbs; Farrell Tyson; Gianmarco Vizzeri; Steven Vold; Qui Vu; Kimberly S. Warren; David Wirta
Declarations
Funding
This study was sponsored by Allergan prior to its acquisition by AbbVie Inc. The study sponsor provided funding for manuscript development and the open access fee.
Conflict of interest
Jason Bacharach is a consultant, and is on the speaker’s bureau, for Allergan (an AbbVie company). Andrew Tatham is a consultant for Allergan (an AbbVie company), has received research support from Alcon and Allergan (an AbbVie company), and is a speaker for Alcon, Allergan (an AbbVie company), Glaukos, Heidelberg Engineering, and Santen. Gloria Ferguson and Hagen Thieme have no financial interests to disclose. Sandra Belalcázar has been a speaker for Allergan (an AbbVie company). Margot L. Goodkin, Qiang Guo, Michael R. Robinson, and Marina Bejanian are employees of AbbVie Inc, and may hold AbbVie stock. Michelle Y. Chen and Jeen Liu were Allergan employees at the time of this work. David L. Wirta is a consultant for Allergan (an AbbVie company) and Eyenovia, and has received research support from Aerpio, Allergan (an AbbVie company), Annexon, Dompe, Eyenovia, Mallinckrodt, Nicox, Novaliq, Novartis, Santen, and SilkTech.
Availability of data and material
Allergan will share de-identified patient level data and study-level data, including protocols and clinical study reports, for phase II, III, or IV trials completed after 2008 that are registered to ClinicalTrials.gov or EudraCT, have received regulatory approval in the USA and/or the EU in a given indication and the primary manuscript from the trial has been published. To request access to the data, the researcher must sign a data use agreement, and any shared data are to be used for non-commercial purposes. More information can be found on http://www.allerganclinicaltrials.com/.
Ethics approval
An institutional review board or ethics committee approved the study at each site.
Consent to participate
All patients in this study provided written informed consent before undergoing any study-related procedure.
Authors' contributions
Study design: MRR, MB, JL; data collection: JB, AT, GF, SB, HT, DLW; statistical analysis: QG, JL; data interpretation: JB, AT, GF, SB, HT, MLG, MYC, QG, JL, MRR, MB, DLW; revisions and approval of the manuscript: JB, AT, GF, SB, HT, MLG, MYC, QG, JL, MRR, MB, DLW.
Code availability
Not applicable.
Footnotes
The members of the ARTEMIS 2 Study Group are presented in the Acknowledgements section.
Contributor Information
Jason Bacharach, Email: jbacharach@northbayeye.com.
the ARTEMIS 2 Study Group:
Arturo Alezzandrini, Gabriel Bercovich, Pablo Deromedis, Federico Furno Sola, Carolina Gentile, Simon Lerner, Anahi Lupinacci, Carlos Zeolite, Catherine Birt, Andrew Crichton, Sebastien Gagne, Michael Giunta, Paul Harasymowycz, Delan Jinapriya, Marcelo Nicolela, Donald Nixon, Patrick Saurel, David Yan, Darana Yuen, Santiago Arango, Sandra Belalcázar, Alexander Martinez, Juan Camilo Parra Restrepo, Vladimir Korda, Jana Kadlecova, Jitka Svacinova, Hany Khairy, Hani El Ibiary, Zeinab El Sanabary, Katharina Bell, Roman Greslechner, Jöerg Koch, Katrin Lorenz, Isabel Oberacher-Velten, Stefanie Schmickler, Claudie Schuart, Hagen Thieme, Francesco Bandello, Carlos Cagini, Michele Figus, Leonardo Mastropasqua, Luca Rossetti, Maurizio Giacinto Uva, Sandragasu Thayanithi, Anthony Wells, Rahat Husain, Victor Koh, Dawn Lim, Aung Tin, Petrus Gous, Lynette Venter, Changwon Kee, Michael Kook, Ki-Ho Park, Muhsin Eraslan, Ozcan Kayikcioglu, Nilgun Yildirim, Rupert Bourne, Anshoo Choudhary, Francesca Cordeiro, Vincent Dubois, James Kirwan, Sheng Lim, Keith Martin, Antony Nithy, Avinash Prabhu, Andrew Tatham, Ahmad Amir, Jason Bacharach, Howard Barnebey, Allen Beck, Lance Bergstrom, Navaneet Borisuth, James D. Branch, Jonathan Briggs, Stephen Bylsma, Peter Chang, William Christie, Frank Cotter, Michael Depenbusch, Damien F. Goldberg, Jack Greiner, Shailesh Gupta, Ron Gutmark, Ying Han, Sebastian Heersink, Malik Kahook, Albert Khouri, Joshua Kim, Howard Kushnick, Christopher Lin, Jodi Luchs, Arindel Maharaj, Steven L. Mansberger, Frank Mares, Eydie Miller-Ellis, Satish Modi, Matthew Paul, Ian Pitha, Robert Saltzmann, Michelle Sato, Michael Savestsky, Bruce Segal, Zachary Segal, Janet Serle, Mark Sherwood, Inder Singh, Stephen E. Smith, Julia Song, Robert Sorenson, Lawrence Tenkman, Navin Tekwani, Carl Tubbs, Farrell Tyson, Gianmarco Vizzeri, Steven Vold, Qui Vu, Kimberly S. Warren, and David Wirta
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. doi: 10.1016/s2214-109x(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183–2193. doi: 10.1016/s0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 5.The AGIS Investigators The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 6.Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 7.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 8.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 9.De Moraes CG, Demirel S, Gardiner SK, Liebmann JM, Cioffi GA, Ritch R, et al. Effect of treatment on the rate of visual field change in the ocular hypertension treatment study observation group. Invest Ophthalmol Vis Sci. 2012;53:1704–1709. doi: 10.1167/iovs.11-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T, Lindsley K, Rouse B, Hong H, Shi Q, Friedman DS, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 2016;123:129–140. doi: 10.1016/j.ophtha.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008;53(Suppl 1):S93–105. doi: 10.1016/j.survophthal.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Sleath B, Blalock S, Covert D, Stone JL, Skinner AC, Muir K, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118:2398–2402. doi: 10.1016/j.ophtha.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman-Casey PA, Niziol LM, Gillespie BW, Janz NK, Lichter PR, Musch DC. The association between medication adherence and visual field progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2020;127:477–483. doi: 10.1016/j.ophtha.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112:953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Newman-Casey PA, Robin AL, Blachley T, Farris K, Heisler M, Resnicow K, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122:1308–1316. doi: 10.1016/j.ophtha.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15:728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreer LE, Girkin CA, Campbell L, Wood A, Gao L, Owsley C. Glaucoma medication adherence among African Americans: program development. Optom Vis Sci. 2013;90:883–897. doi: 10.1097/opx.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansouri K, Iliev ME, Rohrer K, Shaarawy T. Compliance and knowledge about glaucoma in patients at tertiary glaucoma units. Int Ophthalmol. 2011;31:369–376. doi: 10.1007/s10792-011-9468-2. [DOI] [PubMed] [Google Scholar]
- 19.Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122:2010–2021. doi: 10.1016/j.ophtha.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GF, Hollander DA, Williams JM. Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2013;29:1515–1522. doi: 10.1185/03007995.2013.833898. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman TJ, Hahn SR, Gelb L, Tan H, Kim EE. The impact of ocular adverse effects in patients treated with topical prostaglandin analogs: changes in prescription patterns and patient persistence. J Ocul Pharmacol Ther. 2009;25:145–152. doi: 10.1089/jop.2008.0072. [DOI] [PubMed] [Google Scholar]
- 22.Lewis RA, Christie WC, Day DG, Craven ER, Walters T, Bejanian M, et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am J Ophthalmol. 2017;175:137–147. doi: 10.1016/j.ajo.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros FA, Walters TR, Kolko M, Coote M, Bejanian M, Goodkin ML, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1) Ophthalmology. 2020;127:1627–1641. doi: 10.1016/j.ophtha.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Lee SS, Hughes P, Ross AD, Robinson MR. Biodegradable implants for sustained drug release in the eye. Pharm Res. 2010;27:2043–2053. doi: 10.1007/s11095-010-0159-x. [DOI] [PubMed] [Google Scholar]
- 25.Seal JR, Robinson MR, Burke J, Bejanian M, Coote M, Attar M. Intracameral sustained-release bimatoprost implant delivers bimatoprost to target tissues with reduced drug exposure to off-target tissues. J Ocul Pharmacol Ther. 2019;35:50–57. doi: 10.1089/jop.2018.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craven ER, Walters T, Christie WC, Day DG, Lewis RA, Goodkin ML, et al. 24-Month phase I/II clinical trial of bimatoprost sustained-release implant (Bimatoprost SR) in glaucoma patients. Drugs. 2020;80:167–179. doi: 10.1007/s40265-019-01248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SS, Dibas M, Almazan A, Robinson MR. Dose-response of intracameral bimatoprost sustained-release implant and topical bimatoprost in lowering intraocular pressure. J Ocul Pharmacol Ther. 2019;35:138–144. doi: 10.1089/jop.2018.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinreb RN, Robinson MR, Dibas M, Stamer WD. Matrix metalloproteinases and glaucoma treatment. J Ocul Pharmacol Ther. 2020;36:208–228. doi: 10.1089/jop.2019.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lütjen-Drecoll E, Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2 alpha. Exp Eye Res. 1988;47:761–769. doi: 10.1016/0014-4835(88)90043-7. [DOI] [PubMed] [Google Scholar]
- 30.Kim JW, Lindsey JD, Wang N, Weinreb RN. Increased human scleral permeability with prostaglandin exposure. Invest Ophthalmol Vis Sci. 2001;42:1514–1521. [PubMed] [Google Scholar]
- 31.Weinreb RN, Lindsey JD. Metalloproteinase gene transcription in human ciliary muscle cells with latanoprost. Invest Ophthalmol Vis Sci. 2002;43:716–722. [PubMed] [Google Scholar]
- 32.Richter M, Krauss AH, Woodward DF, Lutjen-Drecoll E. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest Ophthalmol Vis Sci. 2003;44:4419–4426. doi: 10.1167/iovs.02-1281. [DOI] [PubMed] [Google Scholar]
- 33.Oh DJ, Martin JL, Williams AJ, Russell P, Birk DE, Rhee DJ. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:3887–3895. doi: 10.1167/iovs.06-0036. [DOI] [PubMed] [Google Scholar]
- 34.Wan Z, Woodward DF, Cornell CL, Fliri HG, Martos JL, Pettit SN, et al. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007;48:4107–4115. doi: 10.1167/iovs.07-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ooi YH, Oh DJ, Rhee DJ. Effect of bimatoprost, latanoprost, and unoprostone on matrix metalloproteinases and their inhibitors in human ciliary body smooth muscle cells. Invest Ophthalmol Vis Sci. 2009;50:5259–5265. doi: 10.1167/iovs.08-3356. [DOI] [PubMed] [Google Scholar]
- 36.Yamada H, Yoneda M, Gosho M, Kato T, Zako M. Bimatoprost, latanoprost, and tafluprost induce differential expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases. BMC Ophthalmol. 2016;16:26. doi: 10.1186/s12886-016-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aptel F, Aryal-Charles N, Giraud JM, El Chehab H, Delbarre M, Chiquet C, et al. Progression of visual field in patients with primary open-angle glaucoma—ProgF study 1. Acta Ophthalmol. 2015;93:e615–e620. doi: 10.1111/aos.12788. [DOI] [PubMed] [Google Scholar]
- 38.Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol. 2013;91:406–412. doi: 10.1111/j.1755-3768.2012.02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. Am J Ophthalmol. 2013;156:724–730. doi: 10.1016/j.ajo.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 40.Susanna R, Jr, De Moraes CG, Cioffi GA, Ritch R. Why do people (still) go blind from glaucoma? Transl Vis Sci Technol. 2015;4:1. doi: 10.1167/tvst.4.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoyama Y, Kawasaki R, Takahashi H, Maekawa S, Tsuda S, Omodaka K, et al. Effects of brimonidine and timolol on the progression of visual field defects in open-angle glaucoma: a single-center randomized trial. J Glaucoma. 2019;28:575–583. doi: 10.1097/ijg.0000000000001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53:6939–6946. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.