Abstract
Background
There are limited real-life data on isavuconazole prophylaxis and treatment of invasive mold infections (IMI) in hematological patients and allogeneic hematopoietic cell transplant (HCT) recipients.
Objectives
Primary objective was to describe the indications of real-life isavuconazole administration at a university hospital. Secondary objectives included the description of liver function tests and QTc interval between baseline and end of treatment (EOT), clinical outcomes and breakthrough IMI by the EOT.
Patients/Methods
This was a 5-year single-center retrospective study of all adult patients with acute myeloid leukemia and/or allogeneic HCT recipients who received isavuconazole as prophylaxis and/or treatment between June 1, 2016, and July 31, 2020.
Results
Among 30 identified patients, the indications for isavuconazole administration were adverse events associated with prior antifungal treatment (N: 18, 60%: hepatotoxicity, renal insufficiency, long QTc interval, neurotoxicity, and potential drug–drug interactions in 6, 4, 3, 1 and 4 patients, respectively), clinical efficacy (N: 5, 16.6%), and other reasons (N: 10, 33.3%; 5/10 patients treated with isavuconazole to facilitate hospital discharge with orally administered appropriate treatment). Alanine aminotransferase significantly decreased from baseline (mean: 129 IU/L, range: 73, 202) to a mean of 48 IU/L (range: 20, 80) by day 14 (P-value: 0.02), 23.5 IU/L (range: 20, 27) by day 28 (P-value: 0.03) and 16.5 IU/L (range: 16, 17) by day 42 (P-value: 0.009). The QTc interval decreased from baseline (mean: 456.8 ms, range: 390, 533) to EOT (mean: 433.8 ms, range: 400, 472; P-value: 0.03). The mean isavuconazole plasma concentration was 2.9 mg/L (range: 0.9, 6.7). There was no breakthrough IMI observed.
Conclusion
Isavuconazole is a safe and reliable antifungal agent in complex hematological patients, with relatively low hepatotoxicity and QTc interval shortening properties.
Keywords: Isavuconazole, Prophylaxis, Treatment, Invasive mold infections, Acute myelogenous leukemia, Allogeneic hematopoietic cell transplant recipients
Background
Isavuconazole is a second-generation triazole with a broad spectrum of activity against Candida, Aspergillus and most Mucorales spp., excellent adverse event profile, limited need for drug-level monitoring and established clinical efficacy [1–3]. It is currently approved for the treatment of invasive aspergillosis and mucormycosis [4–6]. Today, there are limited real-life data on isavuconazole use as primary and secondary prophylaxis and treatment of invasive mold infections (IMI) in hematological patients and allogeneic hematopoietic cell transplant (HCT) recipients [7–14]. Although the existing data suggest a good tolerability and safety profile, a variable rate of breakthrough IMI, ranging from 3 to 18%, has been observed [7, 11, 12, 14].
In this study, we review our 5-year experience with the administration of isavuconazole in high-risk hematology patients with acute myelogenous leukemia and/or allogeneic HCT recipients. We focused on pertinent questions associated with the use of isavuconazole in clinical practice, including the reasons for isavuconazole selection and effect of isavuconazole on liver function tests and QTc interval.
Methods
This was a single-center retrospective observational cohort study to include all adult (≥ 18-year-old) patients with acute myeloid leukemia and/or allogeneic HCT recipients who received isavuconazole as prophylaxis and/or treatment between June 1, 2016 and July 31, 2020. In this real-life study, isavuconazole was used as primary treatment of invasive aspergillosis and other IMI, but also as primary antifungal prophylaxis and empirical antifungal treatment based on specific case requirements and as deemed appropriate by the clinical team. The study was approved by the local ethics committee (2020-01072). Patients with other than acute myeloid leukemia hematologic malignancies who did not receive an allogeneic HCT were not included.
Study Objectives
The primary objective of this study was to describe the indications of real-life isavuconazole administration at a tertiary care center. The following secondary objectives were studied: (1) description of reasons for isavuconazole discontinuation, (2) distribution of liver function tests before and during isavuconazole administration, (3) assessment of QTc interval between baseline and end of treatment (EOT), and (4) clinical outcomes and breakthrough IMI by the EOT.
Data Collection
Patients were retrospectively identified using the institutional HCT and pharmacy databases. The following data were collected using the institutional HCT database: (1) demographics, (2) underlying hematologic malignancy-related variables, including diagnosis, stage of disease, and administered treatments, and (3) pertinent HCT-associated variables, including conditioning regimen, HCT type and graft-versus-host disease (GvHD) grade ≥ 2. The following data were retrieved through chart review: (1) isavuconazole administration-related variables (indication for initiation and discontinuation, mode of administration, dose and duration), (2) laboratory values, including absolute neutrophil count (ANC), absolute lymphocyte count (ALC), alanine aminotransferase (ALT), gamma-glutamyl transferase (γ-GT), and total bilirubin 7 days prior to isavuconazole initiation (day 7), at baseline (day + 1) and on days + 7 (± 3 days), + 28 (± 3 days), + 42 (± 3 days), + 84 (± 3 days) and by EOT (± 7 days) of isavuconazole administration, (3) QTc interval at baseline and on days + 7 to + 14, + 28 to + 42 and by EOT (± 7 days) when available, and (4) administration of other than isavuconazole antifungal agents within 30 days prior and concomitantly with isavuconazole. Isavuconazole therapeutic drug monitoring (TDM) was performed as clinically indicated and relevant data were collected, when available.
Definitions
Invasive fungal infections were defined based on established definitions by the European Organization for research and Treatment of Cancer/Mycoses Study Group [15]. Breakthrough IMI was defined as a probable/proven IMI diagnosed after a minimum of 7-day administration of appropriate antifungal treatment, as previously described [16, 17]. Clinical response was defined based on established guidelines [18]. Isavuconazole TDM was performed with liquid chromatography with tandem mass spectrometry (LC-MC/MC; RECIPE 200 Kit System, Germany). Empirical treatment was defined as administration of isavuconazole for the treatment of a suspicion of a possible IMI or in the setting of persistent neutropenic fever. Targeted antifungal treatment was defined as treatment of proven or probable IMI. Last follow-up day was considered the July 31, 2020, for those patients with ongoing isavuconazole administration. For these patients, EOT was considered to be the July 31, 2020, the day on which the database was sealed. Neutropenia was defined as an absolute neutrophil count < 500cells/mm3.
Statistical Analysis
Standard descriptive statistics were used to summarize the study population characteristics. The Fisher’s exact or Chi-square tests were used for categorical variables and t test for continuous variables. Continuous variables were presented as means, with standard deviation and range. Statistical analysis was performed using STATA 16.0 (StataCorp, College Station, TX).
Results
Baseline Patient Characteristics
We identified 30 patients treated with isavuconazole at our institution during the study period (Table 1). The median age was 59 years (range: 18, 76), and 14 (46.7%) patients were female. The most frequently identified underlying hematologic malignancy was acute myeloid leukemia/myelodysplastic syndrome (24, 80%), and 18 (60%) patients received an allogeneic HCT. A total of 26 (86.7%) patients had received a chemotherapy regimen within the last 30 days before isavuconazole administration. Fourteen (46.6%) patients were neutropenic before isavuconazole initiation with a mean duration of neutropenia of 23.2 days (range: 8, 35).
Table 1.
Baseline patient characteristics
| Variables | Patients N: 30 (%) |
|---|---|
| Demographics | |
| Age (years), mean (range) | 59 (18, 76) |
| Gender, female | 14 (46.7) |
| Underlying hematologic malignancy | |
| Acute myeloid leukemia/myelodysplastic syndrome | 24 (80) |
| Acute lymphoblastic leukemia | 2 (6.6) |
| Lymphoma | 2 (6.6) |
| Othera | 2 (6.6) |
| Chemotherapy within 30 days prior to isavuconazole | 26 (86.7) |
| Induction | 11 (36.7) |
| Consolidation | 9 (30) |
| Salvage | 2 (6.7) |
| Conditioning for allogeneic HCT | 4 (13.3) |
| Neutropenia prior to IVC administration, days Mean (range) | 23.2 (8, 35) |
| HCT-associated variablesb | 18 (60) |
| Conditioning regimen | |
| Myeloablative | 2 (11) |
| Reduced intensity | 16 (88) |
| HCT donor | |
| HLA-matched related | 5 (26.6) |
| HLA-matched unrelated | 6 (33.3) |
| Haploidentical | 7 (38.8) |
| HCT source | |
| Bone marrow | 4 (22) |
| Peripheral blood stem cells | 14 (78) |
| GvHD grade ≥ 2 before isavuconazole | 6 (33) |
| Indication for isavuconazole administration | |
| Treatment | 20 (77) |
| Proven IFIc | 9 (45) |
| Probable IFId | 5 (25) |
| Possible IFI | 6 (30) |
| Prophylaxis | 10 (33) |
IVC Isavuconazole, HCT hematopoietic cell transplant, HLA human leukocyte antigen, GvHD graft-versus host disease, IFI invasive fungal infection
aOther included: myeloid sarcoma (N: 1), multiple myeloma (N: 1)
bFor all HCT-related variables, denominator included the total number of HCT recipients (N: 18)
cProven IFI included 5 cases of invasive aspergillosis and 4 cases of mucormycosis
dProbable IFI included 5 cases of invasive aspergillosis
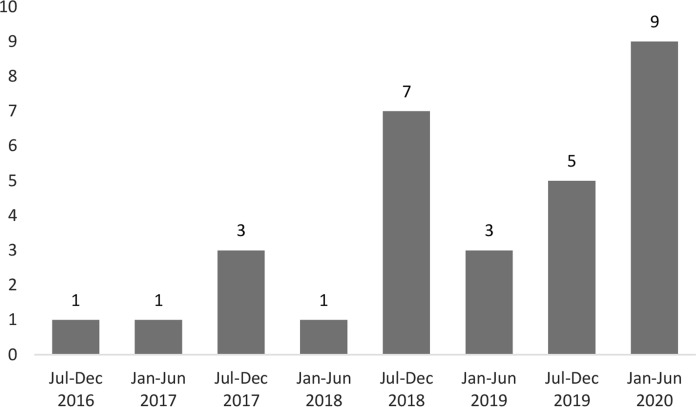
The distribution of isavuconazole administration during the study period is shown in Fig. 1. There was a significant increase in isavuconazole utilization during the second part of the study (July 2018 to July 2020) compared to the first part (June 2016–2018; P-value < 0.001). Isavuconazole was administered as primary prophylaxis in 10 (33.3%) patients and as treatment in 20 (66.6%) patients. Among 20 treated patients, 14 (70%) and 6 (30%) patients received isavuconazole as targeted and empirical therapy, respectively. Treatment was administered for a proven, probable and possible IMI in 9 (45%), 5 (25%) and 6 (30%) patients, respectively (Table 2).
Fig. 1.
Administration of isavuconazole during the study period. The y-axis depicts the number of patients treated with isavuconazole per year
Table 2.
Detailed description of 20 patients, who were treated with isavuconazole for a proven, probable or possible invasive mold infection
| Age | Gender | Heme Malignancy |
Chemotherapy 30 days before IVC |
Antifungal 30 days before IVC |
Allo- HCT | GvHD |
|---|---|---|---|---|---|---|
| 76 | Male | AML | Induction | PCZ, ECH | ||
| 68 | Male | MDS | Consolidation | FCZ | Yes | No |
| 51 | Female | AML | Induction | L-AMB | ||
| 41 | Female | MDS | Consolidation | PCZ | Yes | No |
| 42 | Male | AML | Induction | L-AMB | ||
| 62 | Female | AML | Induction | L-AMB | ||
| 61 | Male | ALL | Consolidation | L-AMB + ECH + VCZ | Yes | Yes |
| 65 | Male | AML | Consolidation | L-AMB | Yes | Yes |
| 50 | Female | MDS | Consolidation | PCZ | Yes | No |
| 60 | Male | AML | Salvage | PCZ | Yes | No |
| 67 | Male | AML | Induction | VCZ | ||
| 70 | Female | AML | Induction | VCZ | ||
| 74 | Male | AML | Induction | PCZ | Yes | No |
| 17 | Male | ALL | Consolidation | ECH | Yes | No |
| 59 | Female | Lymphoma | None | L-AMB | Yes | No |
| 58 | Male | MDS | None | PCZ, ECH | Yes | Yes |
| 74 | Female | MDS | Salvage | L-AMB + ECH | ||
| 69 | Male | AML | Induction | FCZ | Yes | No |
| 43 | Male | AML | Conditioning | L-AMB | Yes | Yes |
| 53 | Male | Lymphoma | Conditioning | L-AMB | Yes | No |
| Age | Gender | Neutropenia days before IVC |
Neutropenia days after IVC |
Treatment Type |
IFI | Reason for IVC start | Reason of IVC stop | IVC days |
|---|---|---|---|---|---|---|---|---|
| 76 | Male | 26 | 0 | Targeted | Proven IA | Hepatotoxicity | Death | 124 |
| 68 | Male | 21 | 4 | Empirical | Possible IA | Renal insufficiency | No IFI | 7 |
| 51 | Female | 19 | 30 | Empirical | Possible IA | Simplification | AE (rash) | 2 |
| 41 | Female | 0 | 0 | Targeted | Proven IA | Neurotoxicity | Cost | 9 |
| 42 | Male | 13 | 10 | Targeted | Probable IA | Hepatotoxicity | Simplification | 6 |
| 62 | Female | 8 | 15 | Targeted | Probable IA | Simplification | Hematological disease progression | 11 |
| 61 | Male | 0 | 0 | Targeted | Proven mucormycosis | Better CNS penetration | AE (rash) | 1 |
| 65 | Male | 0 | 0 | Empirical | Possible IA | Hepatotoxicity, low PCZ level | Simplification | 81 |
| 50 | Female | 0 | 0 | Targeted | Proven IA | Long QTc | Death | 4 |
| 60 | Male | 0 | 15 | Targeted | Probable IA | Empirical | Hematological disease progression | 20 |
| 67 | Male | 0 | 0 | Targeted | Proven mucormycosis | Combination treatment | Ongoing | 568 |
| 70 | Female | 0 | 0 | Targeted | Probable IA | Hepatotoxicity | End of treatment | 257 |
| 74 | Male | 30 | 10 | Empirical | Possible IA | Renal insufficiency | Simplification | 69 |
| 17 | Male | 0 | 0 | Targeted | Proven IA | Simplification | End of treatment | 270 |
| 59 | Female | 0 | 0 | Empirical | Possible IA | Renal insufficiency | Cost | 36 |
| 58 | Male | 0 | 0 | Targeted | Proven cryptococcosis | Hepatotoxicity | Cost | 127 |
| 74 | Female | 10 | 7 | Targeted | Proven Mucormycosis | Combination treatment | Ongoing | 151 |
| 69 | Male | 0 | 0 | Targeted | Probable IA | Empirical | Hematological disease progression | 165 |
| 43 | Male | 0 | 0 | Targeted | Proven IA | Simplification | Ongoing | 170 |
| 53 | Male | 30 | 0 | Empirical | Possible IA | Simplification | No IFI | 10 |
IVC Isavuconazole, HCT hematopoietic cell transplant, GvHD graft versus host disease, IFI invasive fungal infection, AML acute myelogenous leukemia, POS posaconazole, ECH echinocandin, IA invasive aspergillosis, MDS myelodysplastic syndrome, FCZ: fluconazole, L-AMB liposomal amphotericin-B, AE adverse event, VCZ voriconazole, ALL acute lymphoblastic leukemia, CNS central nervous system
Isavuconazole Administration Data
All patients received a loading dose of isavuconazole and 200 mg once daily as regular dose (Table 3). Mean duration of isavuconazole administration was 87.6 days (range: 1, 568), 12.2 days (range: 1, 127) and 117.3 days (range: 1, 568) for intravenous and oral administration, respectively. The vast majority of patients (N: 27, 90%) had received another antifungal agent within 30 days prior to isavuconazole, including posaconazole (N: 7, either intravenously or as an extended release oral formulation), liposomal amphotericin-B (N: 6), fluconazole (N: 6), voriconazole (N: 2), and an echinocandin (N: 5).
Table 3.
Data on isavuconazole administration
| Variables | Patients N: 30 (%) |
|---|---|
| Dose | |
| Loading dose 200 mg 3 times daily for 2 days | 30 (100) |
| Maintenance dose 200 mg once daily | 30 (100) |
| Formulation | |
| Intravenous only | 10 (33) |
| Orally only | 10 (33) |
| Intravenous followed by orally | 10 (33) |
| Duration | |
| Overall duration, days mean (range) | 87.6 (1, 568) |
| Intravenous administration duration, days mean (range) | 12.2 (1, 127) |
| Oral administration duration, days mean (range) | 117.3 (1, 568) |
| Prior administration of other antifungal agentsa | 27 (90) |
| Posaconazole | 7 |
| Liposomal amphotericin-B | 6 |
| Fluconazole | 6 |
| Voriconazole | 2 |
| Echinocandin | 5 |
| No antifungal | 3 |
| Concomitant administration with other antifungal agents | 1 (3.3) |
| Neutropenia during IVC administration, days mean (range) | 18.8 (4, 30) |
| Indication for IVC administrationb | |
| Adverse events of prior treatment | 14(46.6) |
| Hepatotoxicity | 6 |
| Renal insufficiency | 4 |
| Long QTc interval | 3 |
| Central nervous system | 1 |
| Potential drug–drug interactions | 4 (13.3%) |
| Clinical efficacy | 5 (16.6) |
| Sub-therapeutic posaconazole level | 1 |
| Combination treatment | 4 |
| Otherc | 10 (33.3) |
| Indication for IVC discontinuationb | |
| Treatment completion | 5 (16.7) |
| Treatment de-escalation | 5 (16.7) |
| Adverse events | 5 (16.7) |
| Rash | 2 |
| Hepatotoxicity | 2 |
| Drug–drug interactions | 1 |
| Insurance coverage | 3 (10) |
| Death | 3 (10) |
| Disease progression | 3 (10) |
| Ongoingd | 3 (10) |
IVC Isavuconazole, IFI invasive fungal infection
aAdministration of other antifungal agents during the 30 days prior to isavuconazole administration. A patient could have received more than one antifungal agent prior to isavuconazole administration
bA patient could have more than one indication for isavuconazole administration and/or discontinuation
cOther reasons for initiation of treatment with isavuconazole included the following: (1) transition from intravenous to orally administered treatment in 5 patients, (2) treating team choice for two cases of probable pulmonary invasive aspergillosis and a patient with proven pulmonary and cerebral Rhizomucor pusillus mucormycosis, (3) no specific indication in two patients
dThree patients were still on isavuconazole at the end of the study period
Reasons for Isavuconazole Initiation
Patients received isavuconazole for: (1) adverse events and potential drug–drug interactions associated with the administration of prior antifungal treatment (N: 18, 60%), (2) clinical efficacy (N: 5, 16.6%), and (3) other reasons (N: 10, 33.3%). Among patients with adverse events associated with prior antifungal agents, isavuconazole was started due to elevated liver tests in 6 patients, underlying renal insufficiency in 4 patients, long QTc interval in 3 patients, underlying central nervous system toxicity in 1 patient, and potential interactions between voriconazole or posaconazole and chemotherapy in 4 patients. In 5 patients requiring administration of isavuconazole for clinical efficacy reasons, the selection of isavuconazole was based on sub-therapeutic drug levels of posaconazole in 1 patient and in severe IMI requiring combination treatment in 4 patients: (1) isavuconazole with an echinocandin for the treatment of a probable IMI due to Aspergillus terreus, (2) isavuconazole with an echinocandin in a patient with severe neutropenic enterocolitis for broader anti-Candida coverage, (3) isavuconazole with liposomal amphotericin-B for the treatment of a proven pulmonary IMI due to Rhizomucor and (4) isavuconazole with liposomal amphotericin-B and an echinocandin for the treatment of a disseminated Rhizomucor infection. In the rest of cases, isavuconazole was used for treatment simplification in 5 patients, replacing liposomal amphotericin-B (N: 3) and an echinocandin (N: 2), to facilitate hospital discharge with an orally administered appropriate treatment. Finally, isavuconazole was used as primary antifungal treatment in two cases of probable pulmonary aspergillosis and one patient with proven pulmonary and cerebral mucormycosis, respectively. In two patients, the reason for isavuconazole selection was not documented in the patient charts.
Reasons for Isavuconazole Discontinuation
Isavuconazole treatment was discontinued prematurely in 5 (16%) patients due to: (1) type-I drug hypersensitivity reaction (N: 2), (2) elevated liver enzymes (N: 2) and (3) a potential drug–drug interaction with a chemotherapy treatment (N: 1). Type-I drug hypersensitivity reaction was suspected in a patient who developed a maculopapular rash, nausea and vomiting after 24 h of isavuconazole treatment initiation and in one patient with a maculopapular rash and severe cardiothoracic pain with elevated cardiac enzymes just after the first infusion. The latter was subsequently attributed to administration of isavuconazole intravenously without using the specific filter required for administration. Although an isavuconazole-related allergic reaction was not confirmed, isavuconazole treatment was not re-initiated in any of these two patients. Among the two patients whose isavuconazole was discontinued due to moderate liver function abnormalities, one had received chemotherapy within 48 h prior and in the second patient isavuconazole was co-administered with several other potentially hepatotoxic agents.
Distribution of Liver Function Tests During Isavuconazole Administration
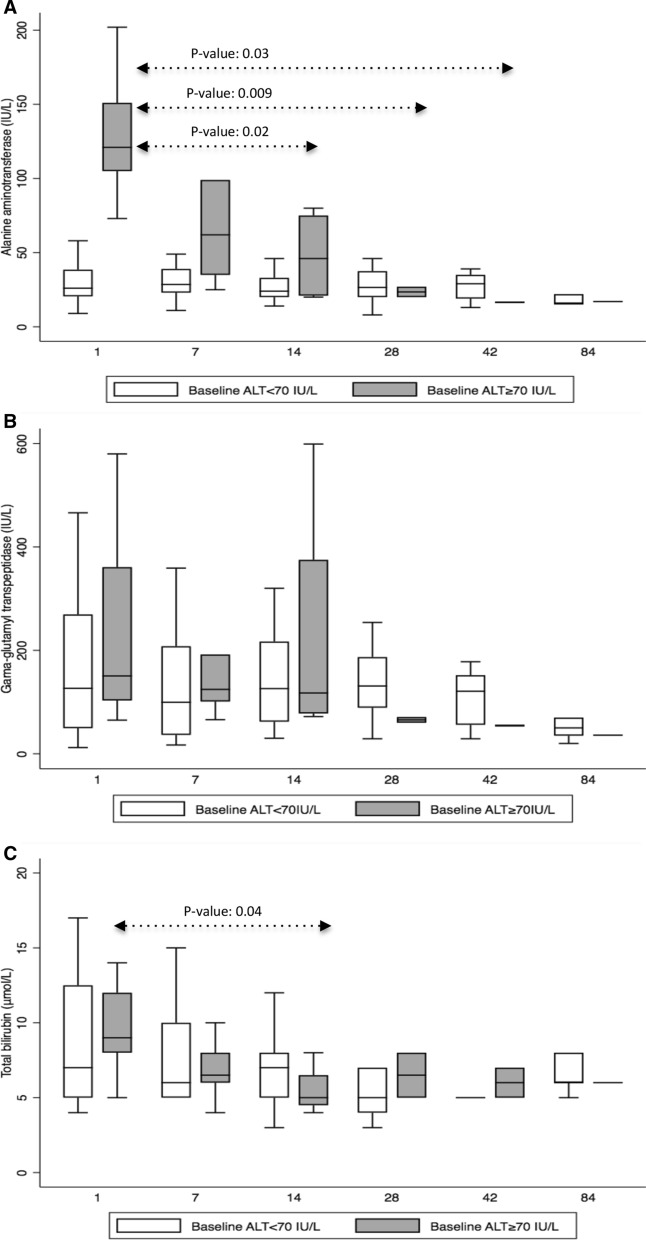
The mean ALT value at baseline was 49.3 IU/L (range: 9, 202). As isavuconazole was administered in 6 (20%) patients due to liver function test abnormalities associated with prior antifungal treatments, we divided patients in two groups: those 6 (20%) patients with prior hepatotoxicity (baseline ALT ≥ 70 IU/L) and 24 (80%) patients without baseline liver function abnormalities (baseline ALT < 70 IU/L). We analyzed the distribution of ALT, γ-GT and total bilirubin during the first 84 days of isavuconazole administration between the two groups (Fig. 2). In the baseline-hepatotoxicity group, ALT decreased from a mean of 129 IU/L (range: 73, 202) at baseline to 106.5 IU/L (range: 25, 356) by day + 7 (P-value: 0.58), 48 IU/L (range: 20, 80) by day + 14 (P-value: 0.02), 23.5 IU/L (range: 20, 27) by day + 28 (P-value: 0.03), 16.5 IU/L (range: 16, 17) by day + 42 (P-value: 0.009) and 17 IU/L (range: 17, 17) by day + 84 (P-value: not available, only one patient; Fig. 2a). There were no significant differences in γ-GT mean values between baseline and day + 84 of isavuconazole administration in the hepatotoxicity group (Fig. 2b). Total bilirubin decreased from a mean of 9.5 μmol/L (range: 5, 14) at baseline to 6.8 μmol/L (range: 4, 10) by day 7 (P-value: 0.08), 5.5 μmol/L (range: 4, 8) by day 14 (P-value: 0.04), 6.5 μmol/L (range: 5, 8) by day + 28 (P-value: 0.23), 6 μmol/L (range: 5, 7) by day + 42 (P-value: 0.18) and 6 μmol/L (range 6, 6) by day + 84 (P-value: not available, only one patient; Fig. 2c). Among 24 patients with baseline ALT < 70 IU/L, ALT, γ-GT and total bilirubin remained stable from baseline to day + 84 of isavuconazole administration without significant differences (data not shown).
Fig. 2.
Distribution of (a) alanine aminotransferase (ALT; IU/L), (b) gamma-glutamyl transferase (γ-GT; IU/L), and (c) total bilirubin (µmol/L) at baseline (day + 1) and on days + 7(± 3 days), + 14(± 3 days), + 28(± 3 days), + 42(± 3 days), and + 84(± 3 days) of isavuconazole administration presented in box plots, for patients with and without baseline hepatotoxicity, defined as ALT ≥ 70 IU/L. Boxes represent the median and 25th and 75th percentiles, whiskers represent the range of maximum and minimum values within the interquartile range. Outliers are not shown. P-values are presented only for statistically significant differences. The X-axis represents days of isavuconazole administration: baseline (day + 1) and days +7(± 3 days), + 14(± 3 days), + 28(± 3 days), + 42(± 3 days), and + 84(± 3 days)
Description of QTc Interval During Isavuconazole Administration
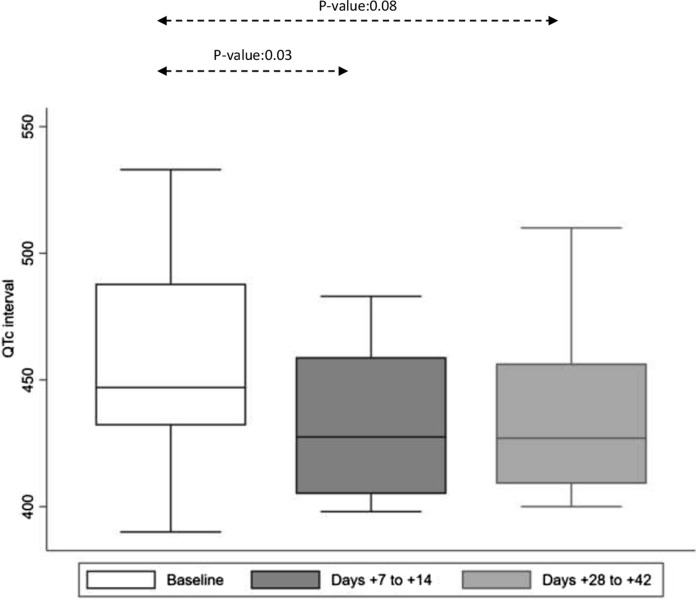
A total of 29 patients had available QTc data at baseline, with a mean QTc interval at 456.8 ms (range: 390, 533; Fig. 3). Among the 12 patients with available QTc interval by the EOT, the mean QTc was at 433.8 ms (range: 400, 472), significantly shorter than at baseline (P-value: 0.03). Fourteen patients had available QTc interval data between days 7–14 of isavuconazole administration, with a mean of 432.2 ms (range: 398, 483), significantly shorter from baseline (P-value: 0.03). There was a trend for shorter QTc interval between baseline and days 28–42 (mean: 435.4, range: 400, 510; P-value: 0.08).
Fig. 3.
Distribution of QTc interval in msec at baseline and during isavuconazole administration presented as box plots. QTc interval measurement was available for 29 patients at baseline. Due to the fact that QTc interval measurements were not available at all time points during the study period, we included QTc between 7 and 14 (14 patients) and 28–42 days (12 patients) of isavuconazole administration. There was a significant decrease in QTc interval from baseline to days 7–14 and a trend for shorter QTc interval between days 28–42 of isavuconazole administration. Boxes represent the median and 25th and 75th percentiles; whiskers represent the range of maximum and minimum values within the interquartile range
Isavuconazole TDM
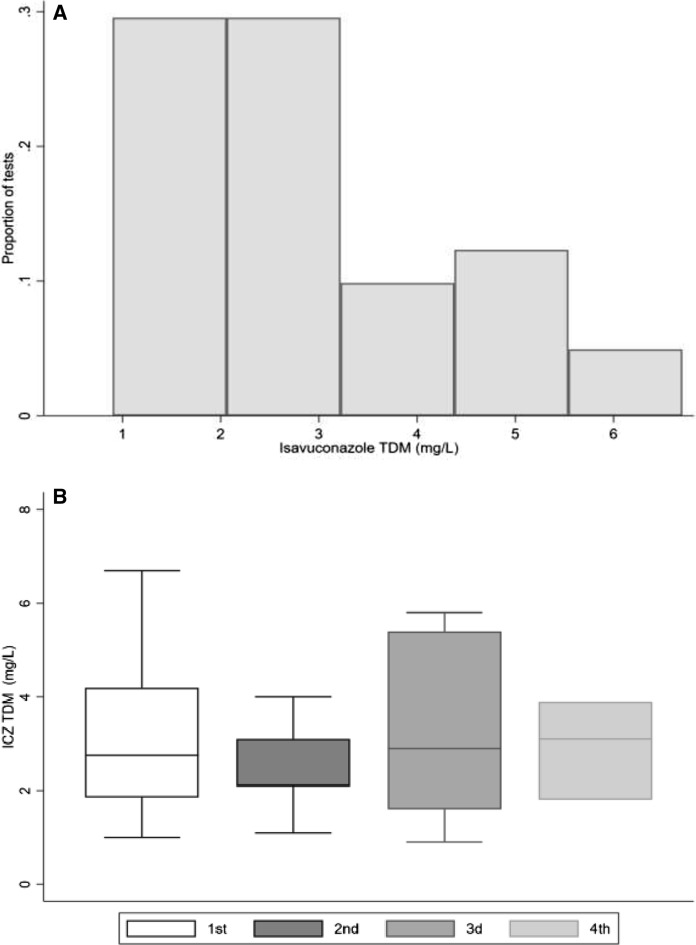
A total of 35 TDM tests were performed in 16 (43.3%) patients (median: 1 test/patient, range: 0–4). The mean isavuconazole concentration was 2.9 mg/L (range: 0.9, 6.7; Fig. 4a). Seven patients had only one isavuconazole TDM, while 2, 4 and 3 patients had 2, 3 and 4 TDM tests performed, respectively. The first TDM was performed at a median of 9 days (range: 3, 168) after isavuconazole initiation. The mean concentration of isavuconazole was 3.0 mg/L (range: 1, 6.7), 2.5 mg/L (range: 1.1, 4.0), 3.3 mg/L (range: 0.9, 5.8) and 2.9 mg/L (range: 1.8, 3.9) on the 1st, 2nd, 3rd and 4th measurements, respectively (Fig. 4b). There was no statistically significant difference between the four different measurements of isavuconazole concentrations.
Fig. 4.
a Therapeutic drug monitoring isavuconazole concentrations (mg/L) presented as a histogram. All isavuconazole measurements are included. b Isavuconazole plasma concentrations (mg/L) presented as box plots for 16, 9, 7 and 3 patients who had 1, 2, 3 and 4 different isavuconazole concentration measurements, respectively. Boxes represent the median and 25th and 75th percentiles; whiskers represent the range of maximum and minimum values within the interquartile range. Outliers are not shown
Clinical Outcomes
None of the 30 patients receiving isavuconazole developed a breakthrough IMI during the study period. Three (10%) patients died due to uncontrolled underlying malignancy. None of the patients included died due to an IMI.
Discussion
This 5-year single-center retrospective cohort study further expands our insight of the major clinical considerations associated with the use of isavuconazole in a real-life setting. We detail the reasons leading to the selection of this agent, its effect on liver function and cardiac rhythm, and a stable and reliable plasma concentrations over time.
Selecting the appropriate molecule or orally administered treatment sequence for the prophylaxis or treatment of IMI in complex poly-medicated and fragile hematological patients and/or allogeneic HCT recipients remains a major challenge. A large number of variables need to be considered, including, but not limited to, possible adverse events, drug–drug interactions, tolerability, bioavailability, absorption and availability of orally administered treatment. Nevertheless, antifungal agent administration is frequently interrupted, for instance voriconazole prophylaxis is discontinued in almost 50% of allogeneic HCT recipients receiving this agent [19, 20]. The need for safer, better tolerated antifungal treatment was the driving force for isavuconazole selection in the vast majority of cases in our study, due to adverse events or drug–drug interactions associated with a previously administered antifungal treatment. A large number of treatments administered in hematologic malignancy patients, including chemotherapy agents, are metabolized through the cytochrome P-450 (CYP) pathway, with potential important interactions with agents, such as voriconazole and posaconazole, both strong inhibitors of CYP3A4. Isavuconazole being a modest CYP3A4 inhibitor has fewer significant interactions allowing easier co-administration with other agents [1]. Well-known azole-related toxicities, including hepatotoxicity and prolongation of QTc interval, were the driving reasons leading to the replacement of another azole by isavuconazole in this study.
Indeed, isavuconazole appears to be less hepatotoxic when compared to other azoles. In the pivotal validation clinical trial, isavuconazole was associated with significantly less frequent liver test abnormalities, when compared to voriconazole [2]. Similarly, in a cohort of high-risk patients with hematologic malignancy from MD Anderson treated with posaconazole, liver tests rapidly dropped after transition from posaconazole to isavuconazole [9]. Consistent with prior reports and despite the small number of patients, our data suggest that isavuconazole may be safely used, even in patients with abnormal baseline liver function with rapid decline of transaminases within the first two weeks after isavuconazole initiation. Notably, the degree of baseline hepatotoxicity was relatively low, with a mean ALT in the “baseline hepatotoxicity” group of 129 IU/L. Furthermore, other interventions, such as discontinuation of other potentially hepatotoxic agents, which could have happened concomitantly with the introduction of isavuconazole, were not considered. More data are required to further study the safety and effect of isavuconazole on liver function in high-risk patients.
Hematology malignancy patients and allogeneic HCT recipients frequently require co-administration of more than one agents with potential QTc-prolonging effect (e.g., broad-spectrum azoles, macrolides, fluoroquinolones, chemotherapy agents, etc.). The unique characteristic of isavuconazole of shortening the QTc interval may allow clinicians to use this agent in patients with prolonged QTc interval, in whom an azole would have been previously avoided. Currently, there are few data on the beneficial effect of isavuconazole on the QTc interval [21]. Our data demonstrate a clear and robust decrease in the QTc interval under treatment with isavuconazole, which can be of use in specific cases.
Treating severe IMI, particularly those due to Mucorales spp., frequently requires administration of intravenously administered amphotericin-B as a first-line therapy [6, 22]. With more patients infected by angioinvasive molds surviving and eventually being discharged from the hospital, transition to an orally administered, effective and well-tolerated treatment is frequently required in clinical practice. Isavuconazole was used in almost one-third of patients in this series as a transition to an orally administered treatment to facilitate hospital discharge. Considering the excellent bioavailability, reliable pharmacokinetics and plasma concentrations and broad-spectrum activity of this agent, isavuconazole appears to be a desirable option for a step-down orally administered treatment of IMI high-risk patients [6].
As previously demonstrated, the rate of breakthrough IMI in patients treated with isavuconazole is variable, ranging from 3 to 18% [8, 11, 12, 14]. Breakthrough IMI has been mostly due to non-Aspergillus molds infections or non-albicans Candida invasive candidiasis, predominately reported in patients who had received prior antifungal treatments, with relapsed/refractory leukemia and prolonged neutropenia at the time of isavuconazole introduction. None of our patients receiving isavuconazole developed a b-IMI during the study period. This may be, in part, due to the small numbers of high-risk patients with relapsed/refractory leukemia and the relatively short follow-up period of our study.
In addition to its retrospective monocentric nature, the small number of patients included with a relatively short follow-up further limit our study. Furthermore, only a few patients received isavuconazole as primary antifungal prophylaxis, and the majority of patients had isavuconazole administered as replacement and not as a first-line treatment. Nevertheless, our data suggest that isavuconazole is a safe antifungal agent in complex and poly-medicated hematological patients, given the relatively low risk of drug interactions and hepatotoxicity and a shortening effect on the QTc interval. With its use increasing overtime, more data on the long-term efficacy and safety profile of this compound are required.
Acknowledgements
We thank all our patients and all doctors and nurses who participate in their care.
Author Contributions
IK and DN contributed to the study conception and design. Material preparation, data collection and analysis were performed by IK, EG and DN. The first draft of the manuscript was written by IK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding provided by Université de Genève. This study was, in part, supported by a research grant from Pfizer (59276319—WP2485056).
Data Availability
Data are available upon request.
Declarations
Conflict of interest
IK, SML, EG, NV, CD have no conflicts of interest. YC has received consulting fees from MSD. DN has received research support from MSD and Pfizer and consulting fees from Roche Diagnostics, MSD, Pfizer, Basilea, and Gilead.
Ethical Approval
The study was approved by the local ethics committee. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to Participate
All patients had signed an informed consent form for utilization of their medical data.
Consent for Publication
All patients had signed an informed consent form for utilization and publication of their medical data.
Footnotes
This study was presented –in part- as Poster Presentation 6727, at the Swiss Society of Infectious Disease SSI Joint Annual Meeting 2021, Montreux, Switzerland
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miceli MH, Kauffman CA. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis. 2015;61(10):1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 2.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 3.Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR, 3rd, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 4.Patterson TF, Thompson GR, 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rausch CR, DiPippo AJ, Bose P, Kontoyiannis DP. Breakthrough fungal infections in patients with leukemia receiving isavuconazole. Clin Infect Dis. 2018;67(10):1610–1613. doi: 10.1093/cid/ciy406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen CD, Tallman GB, Hakki M, Lewis JS. Isavuconazole to prevent invasive fungal infection in immunocompromised adults: initial experience at an academic medical centre. Mycoses. 2019;62(8):665–672. doi: 10.1111/myc.12924. [DOI] [PubMed] [Google Scholar]
- 9.DiPippo AJ, Rausch CR, Kontoyiannis DP. Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients. Mycoses. 2019;62(1):81–86. doi: 10.1111/myc.12851. [DOI] [PubMed] [Google Scholar]
- 10.Hassouna H, Athans V, Brizendine KD. Real-world use-Isavuconazole at a large academic medical center. Mycoses. 2019;62(6):534–541. doi: 10.1111/myc.12910. [DOI] [PubMed] [Google Scholar]
- 11.Bose P, McCue D, Wurster S, Wiederhold NP, Konopleva M, Kadia TM, et al. Isavuconazole as primary anti-fungal prophylaxis in patients with acute myeloid leukemia or myelodysplastic syndrome: an open-label, prospective, phase II study. Clin Infect Dis. 2020;72:1755. doi: 10.1093/cid/ciaa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, Perlin DS, Zhao Y, Noble BN, Lewis JS, Strasfeld L, et al. Isavuconazole prophylaxis in patients with hematologic malignancies and hematopoietic cell transplant recipients. Clin Infect Dis. 2020;70(5):723–730. doi: 10.1093/cid/ciz282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz S, Cornely OA, Hamed K, Marty FM, Maertens J, Rahav G, et al. Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med Mycol. 2020;58(4):417–424. doi: 10.1093/mmy/myz103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern A, Su Y, Lee YJ, Seo S, Shaffer B, Tamari R, et al. A single-center, open-label trial of isavuconazole prophylaxis against invasive fungal infection in patients undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2020;26(6):1195–1202. doi: 10.1016/j.bbmt.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornely OA, Hoenigl M, Lass-Florl C, Chen SC, Kontoyiannis DP, Morrissey CO, et al. Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European confederation of medical mycology. Mycoses. 2019;62(9):716–729. doi: 10.1111/myc.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenks JD, Gangneux JP, Schwartz IS, Alastruey-Izquierdo A, Lagrou K, Thompson Iii GR, et al. Diagnosis of breakthrough fungal infections in the clinical mycology laboratory: an ecmm consensus statement. J Fungi. 2020;6(4):216. doi: 10.3390/jof6040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: mycoses study group and european organization for research and treatment of cancer consensus criteria. Clin Infect Dis. 2008;47(5):674–683. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan SY, Hughes RM, Woo K, Perales MA, Neofytos D, Papanicolaou G. Reasons for voriconazole prophylaxis discontinuation in allogeneic hematopoietic cell transplant recipients: a real-life paradigm. Med Mycol. 2020 doi: 10.1093/mmy/myaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amigues I, Cohen N, Chung D, Seo SK, Plescia C, Jakubowski A, et al. Hepatic safety of voriconazole after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2010;16(1):46–52. doi: 10.1016/j.bbmt.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keirns J, Desai A, Kowalski D, Lademacher C, Mujais S, Parker B, et al. QT Interval Shortening With Isavuconazole: In Vitro and In Vivo effects on cardiac repolarization. Clin Pharmacol Ther. 2017;101(6):782–790. doi: 10.1002/cpt.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood. 2011;118(5):1216–1224. doi: 10.1182/blood-2011-03-316430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.