The incidence of progressive neovascular retinopathies (NRs) that result in progressive vision loss is expected to increase by ~33% in adults of developed nations by 2030, including the United States and United Kingdom [1–5]. Antiangiogenic treatments, such as Lucentis® (ranibizumab), Avastin® (bevacizumab) and Eylea® (aflibercept), have demonstrated their efficacy in improving the visual acuity (VA) of patients suffering of NRs while undergoing treatment [6, 7]. However, declining VA in patients and increased prevalence of retinal scarring, post-treatment, have raised concerns [8, 9]. Although contemporary antiangiogenic treatments have aimed to decelerate the progression of NRs, retinal scarring developed as a consequence of aberrant angiogenesis remains a primary, and understudied, factor in irreversible vision loss.
Müller glia (MG) respond to retinal insult via reactive gliosis, a group of processes that regulate changes in their cellular and molecular behaviour. Although initial MG gliosis is neuroprotective, the prolonged dysregulation of retinal homoeostasis as a result of NRs leads to disruptive behaviours and subsequent glial scar formation. Emerging therapies that address the progression of gliosis from aberrant angiogenesis will thereby target scarring as a separate, contributing factor to vision loss from NRs.
The collaboration of bioengineering and medicine is paramount to further the understanding of MG gliosis, and advance the development of therapeutics against vision loss. However, the complex pathology of the retina is affected by a myriad of regulatory components that make it nearly impossible to identify specific, transient biochemical changes that lead to the onset of gliosis. The application of microscale, in vitro assays can help to assess the individual contribution of MG to retinal scarring within controlled cellular systems. Microfluidics (μFs) are powerful platforms able to recreate geometrical and/or physiological conditions of complex organs and tissues with high accuracy. For instance, μFs can be designed to mimic the spatial constriction of the retina, develop concentration gradients of molecules modelled by regulation of pressure as well as impose desired flow rates, and diffusion to the micro/nano level [10]. The ability of μFs to regulate fluids and chemical reactions at the microscale increases their sensitivity to biomolecular changes in cells, reduces time for analysis and cost of reagents. Likewise, μFs can be readily designed using computer-aided design (CAD) software and inexpensively manufactured via 3D-printing, computer numerical controlled machines and lithography [11]. Moreover, these devices can be tailored to be compatible with conventional imaging techniques, such as brightfield and fluorescence microscopy, which can help identify behavioural changes of cells in real time.
The complexity of μFs to study the retina vary depending on their application to recreate specific phenomena. For instance, retina-on-a-chip models have modelled the retinal environment for the growth of organoids and 3D structures consisting of several cell types within a perfused platform that mimics the retinal vasculature [12]. These platforms provide unique ways to quantify cellular behaviours with high precision as they change in response to pharmacological treatments, becoming ideal drug testing platforms to study and model retinopathies. On the other hand, less complex μFs offer a customizable environment to characterise specific behaviours of a single cell type in response to treatment. For instance, a bench-top μF device, the gLL, short for ‘glia lane’ has served as a pivotal platform to study MG behaviours related to gliosis under different conditions in our laboratory, as seen in Fig. 1. Particularly, we have demonstrated the chemotactic ability of MG in response to concentration gradients of the vascular endothelial growth factor (VEGF) and its ability to upregulate the epidermal growth factor receptor, both behaviours characteristic of late stage gliosis [13, 14]. Nonetheless, additional studies are needed to effectively characterise changes in MG behaviour, such as proliferation and hypertrophy in response to anti-VEGF drugs. These studies will greatly aid researchers to quantitatively assess the response of MG to these treatments and the individual contribution of MG to gliosis via changes in their behaviour. Newfound knowledge will promote the development of therapies that discourage angiogenesis, while preventing the onset of gliosis. Recently, research groups have evaluated the survival of MG when exposed to Lucentis® and Eylea® in vitro, demonstrating that these anti-VEGF drugs do not affect these cells in stress conditions, such as a high glucose environment. However, further studies are required to gain insight in any behavioural changes that MG may undergo upon anti-VEGF treatment [15]. The study of MG behaviours upon anti-VEGF drug treatment is imperative to characterise changes that may contribute to gliosis, and μFs are a precise, tuneable and inexpensive platform to meet this challenge.
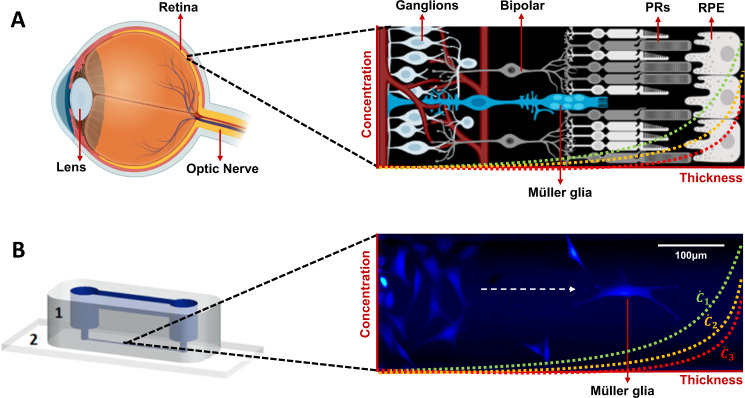
Fig. 1. Müller glia in vivo and in vitro.
A Schematic of the retina featuring Müller glia among other relevant cells of the retina, such as photoreceptors (PRs) and retinal pigmented epithelium (RPE) cells. This schematic features a representation of the vascular network within the retina. Schematics were created with BioRender.com. B rMC-1 model of Müller glia cells within a microchannel of the gLL microfluidic device (1: polymer, 2: glass slide) [13]. Cells were labelled with Calcein AM (2 μM). Concentration gradients are depicted in both the retina schematic and the microchannel of the gLL as a juxtaposition of the ability of both (in vitro and in vivo) platforms to develop concentration gradients ().
Developed nations face an increasing incidence of vision loss among their population, despite access to optimal healthcare. This challenge requires the synergistic collaboration between medicine and engineering to develop new strategies to restore vision. The advancement of anti-VEGF therapies against retinopathies has demonstrated pharmacological efficacy to improve visual outcome in patients. Yet, there is still a significant gap on the understanding of how these agents play a role in retinal gliosis. The use of μFs will help examine this phenomenon by recreating geometric and physiological parameters of the retina, while isolating the individual response of MG to stimulus. Understanding the mechanisms that may influence MG response under anti-VEGF treatment will help identify the best pharmacological treatments, as well as their dosage for early, mild and late stages of NR, moving forward in the fight against blindness [16].
Acknowledgments
Funding
U.S. Department of Health & Human Services |NIH| National Eye Institute (NEI)—R21 EY031439-01. U.S. Department of Health & Human Services |NIH| National Institute of General Medical Sciences (NIGMS)—1 T32 GM 135141.
Author contributions
Both authors contributed equally to the idea, preparation and writing of the manuscript (JSP and MV).
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Eye Institute. Diabetic retinopathy data and statistics. United States; 2020. Available from: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/diabetic-retinopathy-data-and-statistics.
- 2.National Eye Institute. Age-related macular degeneration (AMD) data and statistics. United States; 2019. Available from: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics.
- 3.National Eye Institute. Glaucoma data and statistics. United States; 2019. Available from: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-andstatistics/glaucoma-data-and-statistics.
- 4.Royal National Institute of Blind People. Key information and statistics on sight loss in the UK > Sight Loss Data Tool. United Kingdom; 2020. Available from: https://www.rnib.org.uk/professionals/knowledge-and-research-hub/key-information-and-statistics/sight-loss-data-tool.
- 5.Mathur R, Douglas I, Bhaskaran K, Smeeth L. Diabetic eye disease: a UK Incidence and Prevalence Study. Royal United Kingdom: National Institute of Blind People; 2017. Available from: https://www.rnib.org.uk/sites/default/files/Diabetic%20eye%20disease.%20A%20UK%20Incidence%20and%20Prevalence%20Study%20-%20Full%20report.pdf.
- 6.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Group S-US seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–9. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Cheema MR, DaCosta J, Talks J. Ten-year real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration. Clin Ophthalmol. 2021;15:279–87. doi: 10.2147/OPTH.S269162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:656–66. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel E, Pan W, Ying GS, Kim BJ, Grunwald JE, Ferris FL, 3rd, et al. Development and course of scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2018;125:1037–46. doi: 10.1016/j.ophtha.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vazquez M. Microfluidic and microscale assays to examine regenerative strategies in the neuro retina. Micromachines. 2020;11:1089. doi: 10.3390/mi11121089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiwari SK, Bhat S, Mahato KK. Design and fabrication of low-cost microfluidic channel for biomedical application. Sci Rep. 2020;10:9215. doi: 10.1038/s41598-020-65995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Achberger K, Probst C, Haderspeck J, Bolz J, Rogal J, Chuchuy J, et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife. 2019;8:e46188. doi: 10.7554/eLife.46188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pena JS, Vazquez M. VEGF upregulates EGFR expression to stimulate chemotactic behaviors in the rMC-1 model of Muller glia. Brain Sci. 2020;10:330. doi: 10.3390/brainsci10060330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pena JS, Robles D, Zhang S, Vazquez M. A milled microdevice to advance glia-mediated therapies in the adult nervous system. Micromachines. 2019;10:513. doi: 10.3390/mi10080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen W, Yau B, Lee SR, Zhu L, Yam M, Gillies MC. Effects of ranibizumab and aflibercept on human muller cells and photoreceptors under stress conditions. Int J Mol Sc. 2017;18:533. doi: 10.3390/ijms18030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna S, Komati R, Eichenbaum DA, Hariprasad I, Ciulla TA, Hariprasad SM. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: a comparative review. BMJ Open Ophthalmol. 2019;4:e000398. doi: 10.1136/bmjophth-2019-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]