Abstract
Purpose
In patients living with Anorexia Nervosa (AN), dehydration and haemoconcentration, may prevent a correct interpretation of laboratory nutritional parameters. Our study aims to evaluate if some indicators of disease severity, as body mass index (BMI), Phase Angle (PhA) and months of amenorrhea may be predictors of metabolic alterations (serum albumin, liver enzymes).
Methods
In 154 outpatients with AN, case history was collected, and anthropometric and laboratory parameters measured. Patients were divided according to the following tertiles (T) of BMI, duration of amenorrhea and PhA: (1) BMI (T1 < 15.6; T2 15.6–16.8; T3 > 16.8 kg/m2); (2) Amenorrhea duration (T1 < 7; T2 7–14; T3 > 14 months); (3) PhA value (T1 < 4.64; T2 4.64–5.35; T3: > 5.35°).
ROC curves were used to determine which of these three indicators (BMI, PhA and amenorrhea duration) might better identify patients belonging to Group A or B (less than 3 or more metabolic abnormalities).
Results
The most frequent registered metabolic alterations were for alkaline phosphatase (ALP), alanine aminotransferase, cholesterol and hemoglobin. Aspartate aminotransferase, ALP and gamma glutamyl transferase abnormalities were frequent in the first tertiles of all the three indicators. Albumin was low in the T1 of BMI and PhA. No differences in nutritional alterations emerged according to amenorrhea duration. PhA had the best performance (AUCs: 0.721) in identifying patients with 3 or more abnormalities, with the optimal cut-off value of 4.5°.
Conclusions
Our data confirmed PhA as the more reliable predictor of metabolic alterations, followed by BMI and amenorrhea duration, especially in the first tertile.
Evidence-based medicine
Level 2.
Keywords: Anorexia nervosa, Hepatic alterations, Nutrition alterations, Phase angle, Malnutrition
Introduction
Anorexia nervosa (AN) is a serious eating disorder that, in contrast to other mental health disorders, is often associated with serious clinical complications. Clinical complications account for more than half of the mortality in these patients [1]. and increase as a result of overall weight loss and duration of the illness [2, 3]. This condition of malnutrition progressively involves any organ, and medical complications can be serious or even life-threatening. Severe starvation induces protein and fat catabolism that leads to loss of cellular volume and function, resulting in functional impairment and structural changes in various organs, including the heart, brain, liver, intestines, kidneys, and muscles [4–6].
In the literature, hepatic complications and some nutritional alterations related to AN are increasingly being described. A previous study demonstrated that abnormal serum concentrations of liver enzymes were a frequent finding not only in hospitalized patients living with AN but also in outpatients [7]. AST (aspartate amino- transferase) and ALT (alanine amino- transferase) were inversely related to weight and BMI. Furthermore, discrepancies between changes in these two enzymes as well as decreased alkaline phosphatase (ALP) and increased GGT (gamma glutamyl transferase) were observed. Regarding some metabolic indicators of nutritional status, in addition to undernutrition, dehydration and haemoconcentration conditions might influence some parameters, such as serum albumin levels. Various studies showed that in patients living with AN, serum albumin and other parameter levels were not a reflection of nutritional status, as indicated by the lack of correlation with BMI values [8, 9].
Several studies have shown that BMI and duration of amenorrhoea play a major role in the severity of this disorder. Furthermore, phase angle (PhA), a parameter derived from bioimpedance analysis (BIA), can be considered an index of the integrity of cell membranes and can be used to evaluate the extracellular/intracellular water distribution: a low PhA (< 5°) is a common finding in severe malnutrition [10, 11]. A retrospective study evaluated the incidence of haematologic complications in a group of 318 AN female patients, suggesting that its incidence was strictly related to the malnutrition duration (i.e., duration of amenorrhea) and the severity of malnutrition defined either by low BMI or PhA [9]. Several studies have also demonstrated a close relationship between PhA, a bioimpedance variable, and nutritional status [12–14].
PhA discriminates between different forms of underweight and is an effective marker of qualitative changes in body composition. A low PhA has also been interpreted as a marker of clinically relevant malnutrition characterized by increased extracellular mass and concomitant reduced body cell mass and functional loss [15].
BMI, amenorrhea duration and PhA are criteria related to a common mechanism of chronic malnutrition state.
The aims of our retrospective cohort study are to evaluate if some indicators of disease severity, as body mass index (BMI), Phase Angle (PhA) and duration of amenorrhea may be predictors of metabolic alterations (serum albumin, liver enzymes) in a group of outpatients living with AN.
Subjects and methods
154 outpatients with AN who attended the clinic for Eating Disorders at the Clinical Nutrition Unit, Federico II University Hospital in Naples, from 1995 to 2005 were recruited. Our study is a retrospective cohort study; case history, anthropometric and laboratory parameters measured was collected. Patients with restricting subtype (AN-R) according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM V, 2013) were considered for this study [16].
Liver function tests were performed with routine enzymatic (AST, ALT, ALP, GGT, CHE) assays, and nutritional parameters, such as blood cholesterol (CHOL), glucose (GLU), albumin (ALB), hemoglobin (HB) and lymphocytes (LYM), were evaluated. Upon reviewing clinical records, we collected information about the amenorrhea’s months. Bioimpedance analysis (BIA) was performed at 50 kHz using Human Im Plus II (DS Medica-Milan) at room temperature (22–25 °C) after 20 min in the supine position; all subjects were in a fasted state (12 h) and voided prior to measurement. The measured BIA variables were resistance (R), reactance (Xc) and phase angle (PhA). Bioimpedance index (BI-index in cm2/ohm) was derived as the ratio height2/whole-body R. Using BIA, fat-free mass (FFM) and fat mass (FM) can be estimated by means of predictive equations, which include BIA variables and variables such as age, stature and weight, specific for AN female subjects [10].
Total sample was divided according to the following tertiles (T) of BMI, amenorrhea and PhA:
BMI (T1 < 15.6; T2 15.6–16.8; T3 > 16.8 kg/m2);
Amenorrhea duration (T1 < 7; T2 7–14; T3 > 14 months);
PhA value (T1 < 4.64; T2 4.64–5.35; T3: > 5.35°).
The study protocol was approved by the Ethical Committee of the Federico II University Hospital (Prot. N 37/17) and carried out according to the Declaration of Helsinki.
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA). The results are expressed as the mean ± standard deviation (SD). One-way analysis of variance was used for comparisons between subgroups, while Tukey’s test was used for pairwise comparisons. The χ2 test was used to assess differences in proportions. A p < 0.05 was considered statistically significant.
Receiver-operating characteristic (ROC) curves were used to determine the optimal cut-off value for continuous versus dichotomous variables, to determine which of these three indicators (BMI, PhA and amenorrhea duration) might better identify the highest number of metabolic abnormalities.
As a significant cut-off value was observed, the sensitivity and specificity were presented. While the area under the curve was evaluated, a 5% type-1 error level was called a statistically significant predictive value of the test variables.
Results
The general characteristics and body composition data obtained with BIA of 154 female AN-R patients (BMI range 13.1–18.5 kg/m2, amenorrhea duration range 3–72 months, PhA range 1.9–7.7°) are presented in Table 1.
Table 1.
General characteristics of 154 restrictive anorectic patients
| mean ± SD | min–max | |
|---|---|---|
| Age (years) | 21.1 ± 4.9 | 16–38 |
| Weight (kg) | 41.8 ± 4.6 | 29.2–53.5 |
| Stature (cm) | 161 ± 5.6 | 145–174 |
| BMI (kg/m2) | 16.1 ± 1.4 | 13.1–18.5 |
| Amenorrhea (months) | 13.7 ± 12.9 | 3–72 |
| FFM (kg) | 34.8 ± 4.6 | 25.4–50.7 |
| FM (kg) | 7.1 ± 3.4 | 0.5–15.7 |
| FM (%) | 16.6 ± 7.5 | 1.4–33 |
| Phase angle (°) | 4.93 ± 0.74 | 2.0–7.80 |
FFM fat-free mass, FM fat mass
Anthropometric data expressed for the three tertiles are illustrated in Table 2. Significant differences were observed for body weight and BMI (p < 0.05).
Table 2.
Anthropometric measurements (expressed as mean ± SD) according to BMI, amenorrhea duration and PhA in 154 restrictive anorectic patients
| Subgroups | Body mass index | Amenorrhea duration | Phase angle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1, n = 52 | T2, n = 49 | T3, n = 53 | T1, n = 52 | T2, n = 53 | T3, n = 49 | T1, n = 51 | T2, n = 50 | T3, n = 53 | |
| Age (years) | 21.2 ± 5.0 | 20.9 ± 5.2 | 21.1 ± 4.9 | 20.1 ± 4.8 | 21.4 ± 4.0 | 22.3 ± 5.4 | 21.2 ± 5.1 | 20.7 ± 4.7 | 21.4 ± 5.0 |
| Weight (kg) | 37.8 ± 3.6* | 41.8 ± 2.9 | 45.8 ± 3.3 | 42.4 ± 3.6 | 42.4 ± 5.0 | 40.7 ± 5.3** | 39.9 ± 4.6* | 43.0 ± 4.9 | 42.6 ± 3.9 |
| Height (cm) | 161 ± 6.3 | 161 ± 5.1 | 161 ± 5.3 | 161 ± 4.8 | 160 ± 5.9 | 161 ± 6.2 | 161 ± 5.8 | 162 ± 6.3 | 160 ± 4.4 |
| BMI (kg/m2) | 14.6 ± 0.8* | 16.2 ± 0.3 | 17.6 ± 0.4 | 16.4 ± 1.3 | 16.3 ± 1.3 | 15.7 ± 1.5** | 15.5 ± 1.4* | 16.3 ± 1.2 | 16.6 ± 1.3 |
T1: BMI < 15.6 kg/m2; amenorrhea duration < 7 months; PhA < 4.64°; T2: BMI 15.6–16.8 kg/m2; amenorrhea duration 7–14 months; PhA 4.64–5.35°, T3: BMI > 16.8 kg/m2; amenorrhea duration > 14 months; PhA > 5.35°
*p ≤ 0.05 versus T2 and T3
**p ≤ 0.05 versus T1 and T2
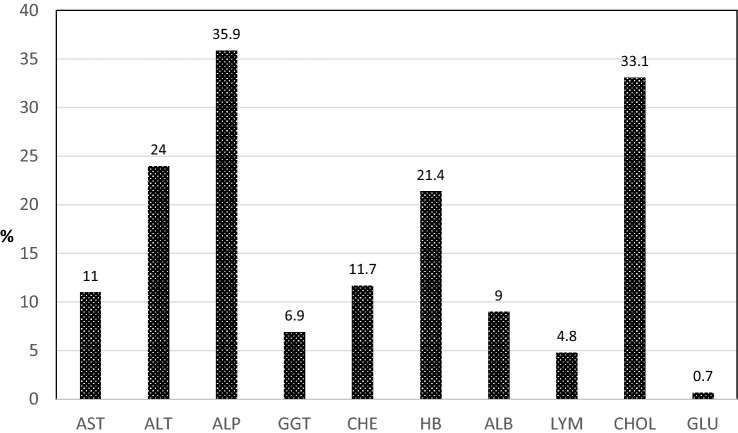
Distribution of abnormalities’ metabolic in the total sample is presented in Fig. 1. The most frequent ones were expressed for ALP, ALT, CHOL and HB.
Fig. 1.
Metabolic abnormalities (%) in 154 restrictive anorectic patients
BMI tertiles
A quite high prevalence of nutritional parameters’ abnormalities was observed in T1 patients. T1 had a lower ALB than T2 and T3. A low ALB was significantly higher in T1. GLU was mainly within the normal range, but also in T1, 13.5% of patients showed hypoglycaemia (Table 3).
Table 3.
Distribution (%) of hemato-biochemical parameters’ abnormalities according to BMI, amenorrhea duration and PhA tertiles in 154 restrictive anorectic patients
| Subgroups | Body mass index | Amenorrhea duration | Phase angle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1, n = 52 | T2, n = 49 | T3, n = 53 | T1, n = 52 | T2, n = 53 | T3, n = 49 | T1, n = 51 | T2, n = 50 | T3, n = 53 | |
| Parameter (limits) | |||||||||
| ALB < 3.5 g/dL | 21.2* | 8.2 | 11.3 | 12.7 | 14.3 | 14.3 | 19.6* | 12.0 | 9.4 |
| CHOL > 190 mgdL | 42.3 | 36.7 | 34.0 | 38.4 | 31.0 | 42.9 | 37.3 | 26.0 | 49.1 |
| HDL < 45 mg/dL | 8.6 | 9.8 | 10.9 | 9.6 | 9.1 | 10.8 | 14.8* | 6.8 | 9.8 |
| GLU < 60 mg/dl | 13.5* | 4.1 | 7.6 | 4.8 | 9.5 | 10.2 | 5.9 | 6.0 | 11.3 |
| HB < 12.0 g/dL | 23.1 | 20.8 | 18.9 | 20 | 31.0 | 18.8 | 29.4*** | 20.4 | 13.2 |
| LYM < 1.200 × 103/μL | 6.4 | 2.2 | 4.2 | 3.5 | 5.0 | 4.7 | 4.3 | 4.0 | 4.3 |
| AST > 35 U/L | 9.6 | 16.3** | 5.7 | 9.5 | 4.8 | 16.3* | 13.7* | 1.0* | 7.5 |
| ALT > 35 U/L | 23.1 | 26.5 | 18.9 | 28.6*** | 21.4 | 15.2 | 17.6 | 24.0 | 26.4*** |
| ALP > 104 U/L | 32.6 | 53.0** | 43.4 | 44.4 | 38.1 | 44.9 | 58.8*** | 40 | 30.2 |
| GGT > 36n U/L | 13.5*** | 12.3 | 1.9 | 3.2 | 11.9 | 14.3*** | 11.8*** | 8.0 | 7.6 |
| CHE < 6.000 U/L | 10 | 16.4 | 8 | 14 | 11.4 | 9.1 | 14.6 | 8.3 | 12.2 |
ALB albumin, CHOL cholesterol, GLU fasting blood glucose, HB hemoglobin, LYM lymphocytes, ALT alanine aminotransferase, AST aspartate amino transaminase, ALP alkaline phosphatase, GGT gamma glutamyl transferase, T1: BMI < 15.6 kg/m2; amenorrhea duration < 7 months; PhA < 4.64°, T2: BMI 15.6–16.8 kg/m2; amenorrhea duration 7–14 months; PhA 4.64–5.35°, T3: BMI > 16.8 kg/m2; amenorrhea duration > 14 months; PhA > 5.35°
*p ≤ 0.05 between T1 and T2
**p ≤ 0.05 between T2 and T3
***p ≤ 0.05 between T3 and T1
No significant difference between the BMI subgroups was found for the other parameters (Table 3).
Regarding liver enzymes, Table 3 illustrates statistically significant differences in distribution observed for AST and GGT.
Amenorrhea duration tertiles
We observed no difference in nutritional alterations, we found a higher hypercholesterolemia distribution (42.9%) 42.9% in T3 (n.s.). Higher values of ALP and GGT was evident in T3 (p ≤ 0.005) (Table 3).
Phase angle tertiles
We observed an increased distribution of nutritional abnormalities in the T1 PhA. Low serum HB was more prevalent in T1 than in T3 (p ≤ 0.005); ALB and low HDL cholesterol were significantly higher in T1 than in the other groups (Table 3).
Hepatic alterations were frequent in T1 for AST, ALP and GGT as opposed only to a higher value of ALT in T3 (p ≤ 0.005).
ROC analysis
For this analysis, only six metabolic parameters belonging to the routine haemato-biochemistry evaluation (AST, ALT, ALP, CHE, HB), were selected because they occur in total sample as abnormalities in more than 10%. Patients were divided into two groups based on the number of biochemical abnormalities (Group A ≤ 3; Group B ≥ 3). The hemato-biochemical parameters and body composition data between the groups A and B are presented in Table 4.
Table 4.
Hemato-biochemical parameters and body composition (expressed as mean ± SD) between the groups A and B
| Group A < 3 abnormalities | Group B ≥ 3 abnormalities | |
|---|---|---|
| Age (years) | 21.2 ± 4.8 | 21.5 ± 5.9 |
| Weight (kg) | 42.1 ± 4.5 | 41.8 ± 4.9 |
| Stature (cm) | 160.6 ± 5.5 | 162.8 ± 4.3 |
| BMI (kg/m2) | 16.3 ± 1.3 | 15.7 ± 1.3 |
| Amenorrhea (months) | 13.7 ± 14.0 | 14.9 ± 13.0 |
| FFM (kg) | 37.7 ± 3.6 | 38.6 ± 4.1 |
| FM (kg) | 4.4 ± 1.5 | 3.2 ± 1.9* |
| FM % | 10.3 ± 2.9 | 7.4 ± 4.3* |
| Phase angle (°) | 5.0 ± 0.66 | 4.3 ± 1.0* |
| AST (U/L) | 23.4 ± 9.9 | 40.3 ± 23.3* |
| ALT (U/L) | 26.7 ± 24.1 | 67.5 ± 61.1* |
| ALP (U/L) | 86.7 ± 48.9 | 132.8 ± 103.5* |
| CHE (U/L) | 6469 ± 2067 | 6806 ± 2303 |
| HB (g/dL) | 12.9 ± 1.1 | 12.2 ± 0.9* |
| CHOL (mgdL) | 179 ± 36 | 183 ± 42 |
*p ≤ 0.05
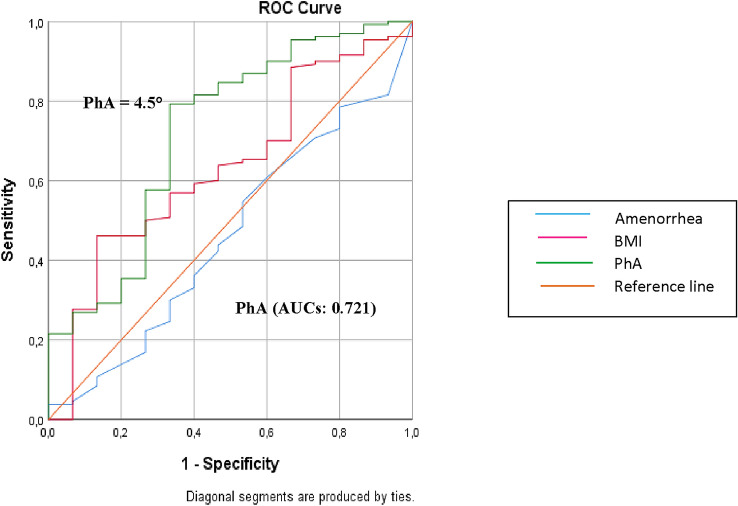
ROC curves were used to determine which of these three indicators (BMI, PhA and amenorrhea duration) might better identify patients belonging to Group A (less than 3 metabolic abnormalities) or Group B (at least 3 metabolic abnormalities). PhA had the best performance (AUCs: 0.721) in identifying patients with three or more abnormalities, with the optimal cut-off value of 4.5°, whereas BMI and amenorrhea duration proved to be inadequate indicators (AUCs: 0.632 and 0.460, respectively) (Fig. 2).
Fig. 2.
Receiver-operating characteristic (ROC) curve indicator of three or more number of metabolic abnormalities
Discussion
We evaluated which of these nutritional indicators among BMI, PhA and amenorrhea duration, played a central role in metabolic alterations, especially for some nutritional and liver parameters, in a large cohort of AN-R outpatients.
Our data show that in these patients with chronic malnutrition, albumin is not a good mirror of nutritional status. Analysis of the data indicates that the prevalence of low serum albumin is very similar in different of amenorrhea duration, but a higher prevalence is only related to lower BMI. Our results are in accordance with a systematic review that considered albumin to be an unsuitable nutritional marker in patients with severe caloric restriction, including AN [16, 17].
Although the pathophysiological mechanisms are not completely known, they are considered to be related to the atrophy of fat cells and the gelatinous transformation in bone marrow as a consequence of starvation due to the marked restriction of carbohydrates and fats, which are the source of fat in bone marrow [18]. It remains controversial whether there is a direct correlation between hematological changes and the severity of disease. In a previous study, we reported that these abnormalities might be related to body composition parameters (i.e., PhA and BMI) [9].
As presented by our data, the amenorrhea duration does not have an effect on nutritional alterations, and blood albumin and glycose concentration are the only parameters on which BMI showed an effect. Instead, PhA appears to be a more sensitive issue and seems to play a major role in these alterations unless cholesterolemia is not influenced by a lower PhA.
Our study also showed that AST alterations were changed in all subgroups according to worsening clinical status. PhA had a significant influence on all hepatic enzymes, which were more apparent in the tertiles with the lowest PhA.
ALP changed in the tertiles of both amenorrhea duration and PhA. ALP is often considered to be a hepatic marker, but human serum contains a mixture of ALP isoenzymes from bones, liver and intestines. We can hypothesize that the increased ALP levels observed in AN woman with the worst PhA were probably associated with increased bone turnover due to osteomalacia or secondary osteoporosis, which are frequently observed in these patients [19]. In malnourished patients with AN, marked hypertransaminasemia is recognized as a factor of severity, so it is an indicator of a worse prognosis [4, 7, 20]. These alterations seem to reflect different aspects of impaired liver function. Our study also illustrated that GGT changed in all three subgroups according to the worsening of the illness, but it was not specifically related to the changes in PhA.
In our study, we observed decreased AST and ALT levels, probably linked to the young age of our sample. We also hypothesized that the subjects with more severe disease, as reflected by the lower PhA and a longer duration of illness, would have reached a state of biochemical adaptation. When we verified the AST/ALT ratio, a diagnostic index for alcoholic liver disease, it was greater than 2.0 in only 2% of the total sample, it was less than 1.0 in 50% of the sample, and for the remaining 48%, the ratio ranged from 1.0 to 2.0. This normal ratio confirmed absolutely negligible alcohol intake in these patients.
Even if there have been several studies regarding changes in aminotransferase levels in AN patients, they have shown no consistent patterns in the results. Some studies have revealed abnormal values, but most results lie within normal limits. Other studies have reported that blood liver enzymes increase following refeeding syndrome [21].
According to our previous studies, PhA could be a sensible marker of nutritional status in patients with AN, enabling the extracellular/intracellular water distribution to be identified. Furthermore, recent studies, also conducted by our group, identified PhA as a prognostic predictor of malnutrition risk; those studies demonstrate the potential to use this parameter to monitor treatment because it is a more reliable parameter than BMI for monitoring nutritional state improvement in patients with AN [11, 13]. Our study confirmed the role of PhA as a screening parameter for diagnostic investigations that are not always used in routine clinical practice; a low PhA is as the more reliable predictor of metabolic alterations followed by BMI and amenorrhea duration,
There are some study limitations due to the retrospective design of this study. On the other hand, the major strengths of the study were the well characterized and homogenous study group and body composition data that aptly represent these patients with anorexia nervosa.
What is already known on this subject?
Dehydration and haemoconcentration in anorexia nervosa may prevent a correct interpretation of laboratory nutritional parameters. In these patients with chronic malnutrition, nutritional and hepatic parameters is not a good mirror of nutritional status, so our study could be identified as some indicators, as body mass index, phase angle and duration of amenorrhea may be predictors of metabolic alterations.
What does this study add?
Our results enable us to consider the importance of a bioimpedance analysis (BIA) to determine a PhA value to evaluate a state of a chronic malnutrition status in AN-r patients enabling the extracellular/intracellular water distribution to be identified. It confirmed the role of PhA as a screening parameter for diagnostic investigations that are not always used in routine clinical practice; a low PhA is as the more reliable predictor of metabolic alterations followed by BMI and amenorrhea duration.
Author contributions
ES. FP. and FC designed the study; ES and RS and DM collected the data; MM analyzed the data; EDF and CDC interpreted the data; and FP and ES wrote the manuscript. All read and approved the final manuscript.
Funding
Open Access funding provided by Università degli Studi di Napoli Federico II.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study protocol was approved by the Ethical Committee of the Federico II University Hospital (Prot. N 37/17) and carried out according to the Declaration of Helsinki.
Infomed consent
No informed consent was necessary for the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mehler PS, Brown C. Anorexia nervosa-medical complications. J Eat Disord. 2015;3:11. doi: 10.1186/s40337-015-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowden DJ, Kilburn-Toppin F, Scoffings DJ. Radiology of eating disorders: a pictorial review. Radiographics. 2013;4:1171–1193. doi: 10.1148/rg.334125160. [DOI] [PubMed] [Google Scholar]
- 3.Westmoreland P, Krantz MJ, Mehler PS. Medical complications of anorexia nervosa and bulimia. Am J Med. 2016;1:30–37. doi: 10.1016/j.amjmed.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 4.De Caprio C, Pasanisi F, Contaldo F. Gastrointestinal complications in a patient with eating disorders. Eat Weight Disord. 2000;4:228–230. doi: 10.1186/2050-2974-1-39. [DOI] [PubMed] [Google Scholar]
- 5.Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165:561–566. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 6.Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord. 2003;34:383–396. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- 7.Montagnese C, Scalfi L, Signorini A, Contaldo F. Cholinesterase and other serum liver enzymes in underweight outpatients with eating disorders. Int J Eat Disord. 2007;8:746–750. doi: 10.1002/eat.20432. [DOI] [PubMed] [Google Scholar]
- 8.De HJ, Schepper J, Vanbesien YV. Albumin and pre-albumin levels do not reflect the nutritional status of female adolescents with restrictive eating disorders. Acta Paediatr. 2016;4:167–169. doi: 10.1111/apa.13312. [DOI] [PubMed] [Google Scholar]
- 9.De Filippo E, Marra M, Alfinito F, Di Guglielmo ML, Majorano M, et al. Hematological complications in anorexia nervosa. Eur J Clin Nutr. 2016;11:1305–1308. doi: 10.1038/ejcn.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra M, Sammarco R, De Filippo E, Caldara A, Speranza E, Scalfi L, et al. Prediction of body composition in anorexia nervosa: results from a retrospective study. Clin Nutr. 2018;5:1670–1674. doi: 10.1016/j.clnu.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Marra M, Sammarco R, De Filippo E, De Caprio C, Speranza E, Contaldo F, et al. Resting energy expenditure, body composition and phase angle in anorectic, ballet dancers and constitutionally lean males. Nutrients. 2019;3:502. doi: 10.3390/nu11030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra M, De Filippo E, Signorini A, Silvestri E, Pasanisi F, Contaldo F, et al. Phase angle is a predictor of basal metabolic rate in female patients with anorexia nervosa. Physiol Meas. 2004;2:145–152. doi: 10.1088/0967-3334/26/2/014. [DOI] [PubMed] [Google Scholar]
- 13.Marra M, Caldara A, Montagnese C, De Filippo E, Pasanisi F, Contaldo F, et al. Bioelectrical impedance phase angle in constitutionally lean females, ballet dancers and patients with anorexia nervosa. Eur J Clin Nutr. 2008;7:905–908. doi: 10.1038/ejcn.2008.54. [DOI] [PubMed] [Google Scholar]
- 14.Małecka-Massalska T, Popiołek J, Teter M, Homa-Mlak I, Dec M, et al. Application of phase angle for evaluation of the nutrition status of patients with anorexia nervosa. Psychiatr Pol. 2017;6:1121–1131. doi: 10.12740/PP/67500. [DOI] [PubMed] [Google Scholar]
- 15.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;6:509–516. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 16.Diagnostic and statistical manual of mental disorders, 5th edn (2013). American Psychiatric Association. Philadelphia
- 17.Narayanan V, Gaudiani JL, Mehler PS. Serum albumin levels may not correlate with weight status in severe anorexia nervosa. Eat Disord. 2008;17:322–326. doi: 10.1080/10640260902991202. [DOI] [PubMed] [Google Scholar]
- 18.Hutter G, Ganepola S, Hofmann WK. The hematology of anorexia nervosa. J Eat Disord. 2009;42:293–300. doi: 10.1002/eat.20610. [DOI] [PubMed] [Google Scholar]
- 19.Rosen E, Bakshi N, Watters A, Rosen HR, Mehler FS. Hepatic complications of anorexia nervosa. Dig Digest Dis Sci. 2017;11:977–2981. doi: 10.1007/s10620-017-4766-9. [DOI] [PubMed] [Google Scholar]
- 20.Tomita K, Haga H, Ishii G, Katsumi T, Sato C, et al. Clinical manifestations of liver injury in patients with anorexia nervosa. Hepatol Res. 2014;10:26–31. doi: 10.1111/hepr.12202. [DOI] [PubMed] [Google Scholar]
- 21.Gaudiani JL, Sabel AL, Mascolo M, Mehler PS. Severe anorexia nervosa: outcomes from a medical stabilization unit. Int J Eat Disord. 2012;1:85–92. doi: 10.1002/eat.20889. [DOI] [PubMed] [Google Scholar]