Abstract
Actinobacillus actinomycetemcomitans, an oral pathogen, only occasionally causes nonoral infections. In this study 52 A. actinomycetemcomitans strains from 51 subjects with nonoral infections were serotyped and genotyped by arbitrarily primed PCR (AP-PCR) to determine whether a certain clone(s) is specifically associated with nonoral infections or particular in vitro antimicrobial susceptibility patterns. The promoter structure of leukotoxin genes was additionally investigated to find the deletion characteristic of highly leukotoxic A. actinomycetemcomitans strains. The nonoral A. actinomycetemcomitans strains included all five known serotypes and nonserotypeable strains, the most common serotypes being b (40%) and c (31%). AP-PCR distinguished 10 different genotypes. A. actinomycetemcomitans serotype b strains were more frequently found in blood samples of patients with bacteremia or endocarditis than in patients with focal infections. One AP-PCR genotype was significantly more frequently found among strains originating in focal infections than in blood samples. Resistance to benzylpenicillin was significantly more frequent among A. actinomycetemcomitans serotype b strains than among strains of other serotypes. No differences in the leukotoxin gene promoter region or benzylpenicillin resistance between nonoral and oral A. actinomycetemcomitans strains were observed. Nonoral A. actinomycetemcomitans strains showed great similarity to the oral strains, confirming that the oral cavity is the likely source of nonoral A. actinomycetemcomitans infections. The predominance of serotype b strains in endocarditis and bacteremia supports the hypothesis of a relationship between certain A. actinomycetemcomitans clones and some nonoral infections. The mechanisms behind the exceptionally high rate of occurrence of benzylpenicillin resistance among A. actinomycetemcomitans serotype b strains are to be elucidated in further studies.
Actinobacillus actinomycetemcomitans, a gram-negative facultatively anaerobic coccobacillus, is an important pathogen in periodontitis, a chronic tissue-destructive infection which may eventually lead to the loss of teeth (16, 44). Despite the rather common presence of the organism in the oral cavity, a literature review for nonoral A. actinomycetemcomitans infections revealed that less than 200 cases were reported during the last 30 years. These infections include endocarditis (9, 22, 23, 30, 40), pericarditis (20), pneumonia (43), septicemia (22, 39), and abscesses in various body sites (22). Approximately 0.6% of infective endocarditis cases are caused by A. actinomycetemcomitans (12). The rare recovery of A. actinomycetemcomitans from nonoral infections may be due to difficulties in growing and identifying the organism, and therefore, it may remain unrecognized or is misidentified (12, 25). It is also possible that only certain A. actinomycetemcomitans clones possess the capacity to cause invasive infections.
Of the five currently known A. actinomycetemcomitans serotypes (serotypes a through e) (32, 44), the most prevalent ones in the oral cavity are serotypes a, b, and c, making up more than 80% of strains at almost equal frequencies (32, 33). Serotype b is associated with periodontitis, and serotype c seems to be particularly frequent in periodontally healthy subjects (1, 44). The only study on the distribution of the three most common serotypes of A. actinomycetemcomitans in nonoral infections revealed a predominance of serotype c (45). However, no information on the presence of the two novel A. actinomycetemcomitans serotypes, serotypes d and e (15, 32), in nonoral infections is available.
Some oral A. actinomycetemcomitans clones may exert an elevated pathogenic potential to cause periodontal destruction, as suggested by several recent studies in which particular genotypes of the organism were associated with certain forms of periodontal diseases or gingival health (4, 8, 13, 19). One of the major virulence determinants of A. actinomycetemcomitans is leukotoxin, which is specifically cytotoxic to human polymorphonuclear leukocytes and monocytes (6, 37). All A. actinomycetemcomitans strains seem to have genes that code for leukotoxin (31). However, a deletion in the leukotoxin gene promoter region leads to expression of increased leukotoxic activity (7). Recently, colonization with A. actinomycetemcomitans strains with the particular deletion was reported to predict conversion from health to periodontal destruction in children (8).
The oral cavity is the ecological niche for A. actinomycetemcomitans. Therefore, it is likely that the source of nonoral A. actinomycetemcomitans infections is the oral cavity, especially in patients with periodontitis. A statement of the American Heart Association concerning prevention of infective endocarditis by prophylactic administration of amoxicillin (11) is complied with in dental care. There is a consensus among studies from different geographical locations that amoxicillin, among other ampicillin-group penicillins, generally exhibits good in vitro activity against oral strains of A. actinomycetemcomitans, although some amoxicillin-resistant A. actinomycetemcomitans strains have been detected (29, 34, 41).
The aim of the present study was to characterize serotypically and genotypically A. actinomycetemcomitans strains isolated from nonoral infections to find out if a certain serotype(s) or genotype(s) is specifically associated with nonoral A. actinomycetemcomitans infections. We also analyzed the promoter structure of A. actinomycetemcomitans leukotoxin genes in order to find signs of elevated pathogenicity among nonoral strains in comparison with the pathogenicities of oral strains. Additionally, to facilitate prediction of optimal candidates for antimicrobial therapy in these infections, we compared the antimicrobial susceptibilities of A. actinomycetemcomitans strains in relation to their recovery from nonoral or oral infections and between serotypes and genotypes.
MATERIALS AND METHODS
A. actinomycetemcomitans strains.
The present collection of bacteria comprised 52 A. actinomycetemcomitans strains from 51 subjects diagnosed with various nonoral infections (Table 1) and 21 oral A. actinomycetemcomitans strains from 21 subjects. A. actinomycetemcomitans JP2, which expresses the deletion in the leukotoxin gene promoter region characteristic of the highly leukotoxic A. actinomycetemcomitans strains (7), was used as a reference strain in the PCR analysis of the leukotoxin gene promoter structure.
TABLE 1.
Origins of 52 nonoral A. actinomycetemcomitans strains from 51 subjects
| Infection | Sample source | No. of subjects with depicted infection |
|---|---|---|
| Endocarditis | Blood | 13 |
| Pneumonia or lung abscess | Aspirated pus, tissue biopsy specimen | 6 |
| Thoracic wall infection | Aspirated pus, tissue biopsy specimen | 3 |
| Elbow or finger abscesses | Aspirated pus | 2 |
| Infection of neck | Tissue biopsy specimen | 3 |
| Osteitisa | Excised bone | 2b |
| Bacteremia of unknown origin | Blood | 22 |
| Total | 51 |
Osteitis of the hand and an unknown site.
One of the two patients was infected with two strains of different serotypes, serotypes b and c.
The A. actinomycetemcomitans strains from nonoral infections were obtained from geographically distant locations: the Culture Collection at the University of Göteborg in Göteborg, Sweden (Sweden, n = 24; Austria, n = 1; Germany, n = 2; and United States, n = 1), from international research groups that possess published or unpublished data on nonoral A. actinomycetemcomitans infections (Iceland, n = 4; Belgium, n = 2; New Zealand, n = 6; and Taiwan, n = 4), and from patients with unpublished A. actinomycetemcomitans infections from hospitals in Finland (n = 8). A. actinomycetemcomitans strains were recovered from both mono- and polyinfections, although the possible existence of copathogens was not always known. After arrival at our laboratory the cultures of nonoral A. actinomycetemcomitans strains were plated on Trypticase soy-serum-bacitracin-vancomycin (TSBV) agar plates (35) and the plates were incubated in 5% CO2 in air at 37°C for 2 to 3 days. The species identification was confirmed as described previously (32). Briefly, colonies on TSBV agar plates were identified as A. actinomycetemcomitans if they presented a typical colony morphology, a positive catalase reaction, and a negative result for lactose fermentation. Subcultures starting from a single colony per sample were preserved in 20% skim milk at −70°C until they were used.
A total of 21 oral A. actinomycetemcomitans strains comprised the reference material in the analysis of the leukotoxin gene promoter structure and for antimicrobial susceptibility testing and were selected from our strain collection to comply with the serotype and genotype distributions of the A. actinomycetemcomitans strains from nonoral infections. The oral strains originated in subjects with periodontitis (n = 19), gingivitis (n = 1), or a healthy periodontium (n = 1) (age range, 14 to 71 years) and had been identified as described earlier (32).
Serotyping.
Serotyping of A. actinomycetemcomitans strains was performed by using autoclaved whole A. actinomycetemcomitans cell antigen extract and serotype-specific rabbit antisera in an immunodiffusion assay as described previously (32).
AP-PCR genotyping.
The random sequence oligonucleotide OPA-13 (5′-CAGCACCCAC-3′) (Operon Technologies, Inc., Alameda, Calif.) was used as a primer in the arbitrarily primed PCR (AP-PCR) analysis as previously reported in detail (2, 28).
Analysis of leukotoxin promoter structure by PCR amplification.
The primer pair 5′-ATA TTA AAT CTC CTT GT-3′ and 5′-ACC TGA TAA CAG TAT T-3′ (7) was used to amplify a DNA fragment from the leukotoxin promoter region of A. actinomycetemcomitans strains as described earlier (4).
Antimicrobial susceptibility testing.
The MICs of six antimicrobial agents for the 52 nonoral A. actinomycetemcomitans strains and 21 oral A. actinomycetemcomitans strains were determined by the agar dilution susceptibility testing method approved by the National Committee for Clinical Laboratory Standards (NCCLS) (26) with Haemophilus test medium. Haemophilus influenzae ATCC 49247 and ATCC 49766 and Haemophilus aphrophilus ATCC 13252 and NCTC 5906 were included as controls. Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 25922 were included as additional control strains. The six antimicrobial agents, supplied as standard powders by several manufacturers, included benzylpenicillin, amoxicillin, tetracycline, metronidazole, azithromycin, and trovafloxacin. The antimicrobial agent concentrations in the agar medium ranged from 0.06 to 32.0 mg/liter for all other agents except for metronidazole, which was used at concentrations ranging from 0.25 to 128.0 mg/liter. A. actinomycetemcomitans strains were grown on brucella blood agar plates in 5% CO2 in air at 37°C for 48 h. The bacterial masses from the plates were harvested, the masses were adjusted into suspensions with turbidities equal to the turbidity of a McFarland 0.5 standard, and the final inoculum (104 CFU per spot) was delivered onto the agar plates with a multipoint inoculator. After incubation in 37°C in 5% CO2 in air (metronidazole-containing plates, however, were incubated in anaerobic jars filled with mixed gas [85% N2, 10% H2, 5% CO2]) for 48 h, the MIC results were interpreted according to NCCLS guidelines (26, 27).
Statistical methods.
The statistical significance of the differences between the frequency distributions were determined by chi-square statistics and Fisher's exact test.
RESULTS
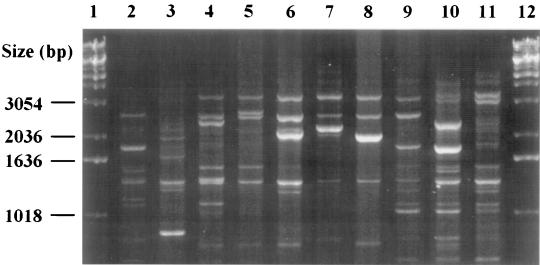
Strains of all five known serotypes and, additionally, a few nonserotypeable strains were recovered from among the 52 nonoral A. actinomycetemcomitans strains that originated from 51 subjects. Serotypes b (21 of 51; 41%) and c (16 of 51; 31%) were the most frequent serotypes in the subjects, whereas strains of serotypes a (8 of 51; 16%), d (4 of 51; 8%), and e (1 of 51; 2%) and nonserotypeable strains (2 of 51; 4%) occurred less commonly. One subject harbored two serotypes, serotypes b and c. Oligonucleotide OPA-13 distinguished a total of 10 AP-PCR genotypes (Fig. 1), with 1 to 3 genotypes within each serotype, among the 52 strains.
FIG. 1.
Ten different AP-PCR banding patterns (lanes 2 to 11) among 52 nonoral A. actinomycetemcomitans strains from 51 subjects. Lanes 1 and 12, DNA markers. The banding patterns in lanes 6, 8, 10, and 11 were not previously found in our studies with oral A. actinomycetemcomitans isolates (2, 3, 14, 28).
To determine possible differences in the serotype distributions in relation to the recovery site, the A. actinomycetemcomitans strains were divided into two groups according to their detection either from blood or from focal infections (Table 1). Table 2 shows the serotype and genotype distributions of the strains in blood and in focal infections when one A. actinomycetemcomitans strain per subject was examined; only strains from the subject colonized with strains of two different serotypes were excluded. Serotype b was (phi-square = 0.0714; P = 0.0553) more frequent in blood samples (17 of 35; 49%) than in focal infections (3 of 15; 20%). An association between the AP-PCR genotype and the origin of A. actinomycetemcomitans strains was noted when genotype 3 occurred statistically significantly (phi-square = 0.1330; P = 0.0197) more frequently in subjects with focal infections (5 of 15; 33%) than in those whose blood samples were tested (2 of 35; 6%) (Table 2). No statistically significant differences in the frequencies of other A. actinomycetemcomitans serotypes or genotypes in blood or in focal infections were observed.
TABLE 2.
Serotype and AP-PCR genotypea distributions among nonoral A. actinomycetemcomitans strains from 50 subjects, separately for samples from blood and focal infections, when one strain per subject was analyzed
| No. (%) of subjects infected with strains with depicted serotype
|
No. (%) of subjects infected with strains with depicted AP-PCR genotype within each serotype
|
||||||
|---|---|---|---|---|---|---|---|
| Serotype | Blood | Focal infection | Total | AP-PCR genotype | Blood | Focal infection | Total |
| a | 4 (11) | 4 (27) | 8 (16) | 1 | 3 (9) | 4 (27) | 7 (14) |
| 10 | 1 (3) | 0 | 1 (2) | ||||
| b | 17 (49)b | 3 (20) | 20 (40) | 2 | 7 (20) | 2 (13) | 9 (18) |
| 12 | 3 (9) | 0 | 3 (6) | ||||
| 23 | 7 (20) | 1 (7) | 8 (16) | ||||
| c | 9 (26) | 6 (40) | 15 (30) | 3 | 2 (6) | 5 (33)c | 7 (14) |
| 24 | 7 (20) | 1 (7) | 8 (16) | ||||
| d | 2 (6) | 2 (13) | 4 (8) | 22 | 1 (3) | 1 (7) | 2 (4) |
| 25 | 1 (3) | 1 (3) | 2 (4) | ||||
| e | 1 (3) | 0 | 1 (2) | 24 | 1 (3) | 0 | 1 (2) |
| xd | 2 (6) | 0 | 2 (4) | 26 | 2 (6) | 0 | 2 (4) |
| Total | 35 (100) | 15 (100) | 50 (100) | 35 (100) | 15 (100) | 50 (100) | |
AP-PCR genotype designations 1 to 17 are according to Asikainen et al. (3), designations 18 and 19 are according to Paju et al. (28), designations 20 to 22 are according to Dogan et al. (14), and designations 23 to 26 are according to this study.
For frequency of detection of serotype b versus that of the other serotypes in blood samples, P = 0.0553.
For frequency of detection of AP-PCR genotype 3 versus that of the other genotypes in focal infections, P = 0.0197.
x, nonserotypeable A. actinomycetemcomitans strains.
Table 3 shows the MICs at which 50% of isolates are inhibited (MIC50s) and the MIC90s of the six antimicrobial agents for all 73 A. actinomycetemcomitans strains tested. In all tests the MICs for the Haemophilus and the other control strains were in acceptable ranges. No differences in the MIC50s or the MIC90s were observed between the nonoral and oral A. actinomycetemcomitans strains. Amoxicillin, tetracycline, azithromycin, and trovafloxacin showed good activity against all A. actinomycetemcomitans strains, regardless of the infection site. According to the NCCLS breakpoints suggested for Haemophilus spp. susceptibility interpretation (26), 21 (29%) of 73 A. actinomycetemcomitans strains were resistant to benzylpenicillin, with all strains being of serotype a, b, or c (Table 4). Thus, none of the total of 11 serotype d, serotype e, or nonserotypeable A. actinomycetemcomitans strains (nonoral strains, n = 7; oral strains, n = 4) were resistant to benzylpenicillin.
TABLE 3.
MICs of six antimicrobial agents for nonoral and oral A. actinomycetemcomitans strains
| Antimicrobial agent | MIC (mg/liter)
|
||||||
|---|---|---|---|---|---|---|---|
| Nonoral A. actinomycetemcomitans strains (n = 52)
|
Oral A. actinomycetemcomitans strains (n = 21)
|
NCCLS breakpointsa (mg/liter [susceptible/ resistant]) | |||||
| Range | 50% | 90% | Range | 50% | 90% | ||
| Benzylpenicillinb | 0.5–8.0 | 2.0 | 4.0 | 1.0–8.0 | 2.0 | 4.0 | ≤1/≥4 |
| Amoxicillinb | 0.12–2.0 | 0.5 | 1.0 | 0.5–1.0 | 0.5 | 1.0 | ≤1/≥4 |
| Tetracycline | 0.25–4.0 | 0.5 | 1.0 | 0.25–1.0 | 1.0 | 1.0 | ≤2/≥8 |
| Azithromycin | 0.06–2.0 | 1.0 | 1.0 | 0.25–1.0 | 1.0 | 1.0 | ≤4/—c |
| Trovafloxacin | ≤0.06–0.12 | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | ≤1/— |
| Metronidazoled | 0.25–128.0 | 16.0 | 16.0 | 2.0–128.0 | 16.0 | 16.0 | ≤8/≥32 |
TABLE 4.
Benzylpenicillin resistance among nonoral and oral A. actinomycetemcomitans strains of various AP-PCR genotypesa among serotypes a, b, or c
| Serotype | Frequency of resistanceb
|
AP-PCR genotype | Frequency of resistanceb
|
||||
|---|---|---|---|---|---|---|---|
| Nonoral strains | Oral strains | Total | Nonoral strains | Oral strains | Total | ||
| a | 1/8 (13) | 1/3 (33) | 2/11 (18) | 1 | 1/7 (14) | 1/3 (33) | 2/10 (20) |
| 10 | 0/1 (0) | —c | 0/1 (0) | ||||
| b | 13/21 (62) | 5/8 (63) | 18/29 (62) | 2 | 7/9 (78) | 4/4 (100) | 11/13 (85) |
| 12 | 3/3 (100) | 1/2 (50) | 4/5 (80) | ||||
| 23 | 3/9 (33) | — | 3/9 (33) | ||||
| 8 | — | 0/1 (0) | 0/1 (0) | ||||
| 9 | — | 0/1 (0) | 0/1 (0) | ||||
| c | 1/16 (6) | 0/6 (0) | 1/22 (5) | 3 | 0/8 (0) | 0/4 (0) | 0/12 (0) |
| 24 | 1/8 (13) | — | 1/8 (13) | ||||
| 4 | — | 0/1 (0) | 0/1 (0) | ||||
| 14 | — | 0/1 (0) | 0/1 (0) | ||||
For AP-PCR genotype designations, see footnote a of Table 2.
Number of resistant strain(s)/number of strains with the depicted sample origin and serotype or AP-PCR genotype definition (percent).
—, no strains of the depicted AP-PCR genotype were detected.
Table 4 shows that resistance to benzylpenicillin occurred among A. actinomycetemcomitans serotype b strains (18 of 29; 62%) statistically significantly (phi-square = 0.3567; P = 0.0000) more frequently than among strains of the other serotypes (3 of 44; 7%), including 11 serotype d, serotype e, or nonserotypeable strains. The same phenomenon was seen for both nonoral (phi-square = 0.3607; P = 0.0000) and oral (phi-square = 0.3471; P = 0.0139) A. actinomycetemcomitans strains. Within serotype b, strains of the AP-PCR genotypes 2 and 12 combined together (15 of 18; 83%) exhibited resistance to benzylpenicillin statistically significantly (phi-square = 0.3143; P = 0.0041) more often than strains of the three other serotype b genotypes (3 of 11; 27%). Similarly, strains of the AP-PCR genotypes 2 and 12 combined exhibited resistance to benzylpenicillin statistically significantly (phi-square = 0.4755; P = 0.0000) more frequently than strains of all the other A. actinomycetemcomitans genotypes (6 of 55; 11%).
According to the NCCLS breakpoints for anaerobic bacteria (27), 4 (5%) of 73 A. actinomycetemcomitans strains were resistant to metronidazole; 2 were of serotype b and two were of serotype c. All four strains had different AP-PCR genotypes.
All 73 nonoral and oral A. actinomycetemcomitans strains included in the study displayed the 1,000-bp leukotoxin gene promoter amplicon, whereas reference strain JP2 generated the expected 470-bp amplicon.
DISCUSSION
The study material comprised 52 nonoral A. actinomycetemcomitans strains from 51 subjects with various nonoral infections and 21 oral A. actinomycetemcomitans strains from 21 subjects. The nonoral strains were collected from distinct geographic locations worldwide, whereas the oral strains originated in our culture collection. Our hypothesis was that the A. actinomycetemcomitans strains involved in nonoral infections would represent especially virulent A. actinomycetemcomitans clones. Therefore, we compared the frequencies of detection and antimicrobial susceptibilities of A. actinomycetemcomitans strains of various serotypes and genotypes obtained from nonoral and oral sampling sites. We additionally analyzed the leukotoxin gene promoter structure in order to find differences between nonoral and oral A. actinomycetemcomitans strains.
The serotype and AP-PCR genotype characterizations of the present nonoral A. actinomycetemcomitans strains showed wide heterogeneity, with the major serotypes being serotypes a, b, and c (15, 40, and 31% of strains, respectively) and with smaller proportions of serotype d, serotype e, and nonserotypeable strains (8, 2, and 4%, respectively). The AP-PCR technique, a rapid and useful method for the clonal analysis of A. actinomycetemcomitans (2, 36), distinguished 10 different genotypes among the present nonoral A. actinomycetemcomitans strains. This result shows that a variety of clones may cause nonoral A. actinomycetemcomitans infections. The serotype distribution of the present nonoral A. actinomycetemcomitans strains resembles that of oral A. actinomycetemcomitans strains when all five serotypes are determined (32, 33, 42), supporting the concept that the oral cavity is the ecological niche of A. actinomycetemcomitans. Unfortunately, no information on the oral carriage of A. actinomycetemcomitans or the periodontal status of the patients who contributed the present nonoral strains was available, and, thus, there is no direct evidence of the plausible sources of the bacterium in these nonoral infections. Nevertheless, the clonal identities of the A. actinomycetemcomitans strains recovered from blood and the oral cavity have been confirmed in patients with endarteritis, endocarditis, or bacteremia (24, 30, 39), which suggests that the origin of the present nonoral A. actinomycetemcomitans strains was also the human oral cavity.
Four of the AP-PCR genotypes comprising as many as 22 (42%) of all 52 strains were not found in our earlier studies (2, 3, 14, 28). However, since our studies have mainly included oral A. actinomycetemcomitans strains from Finnish subjects, the present finding of previously undetectable AP-PCR genotypes hardly suggests specific A. actinomycetemcomitans clones in nonoral infections but more likely is due to the widespread geographic origins of the present strains.
Our findings differ from the previous results on the serotype distribution of nonoral A. actinomycetemcomitans (45). The data of Zambon and coworkers (45) suggested that serotype c predominates in nonoral infections; 22 (73%) of the 30 A. actinomycetemcomitans strains were of serotype c. The numbers of A. actinomycetemcomitans strains included in the present study and in that of Zambon and coworkers (45) are still limited, which, together with the different serotyping methods, may account for the different results between the two studies.
A. actinomycetemcomitans serotype b is strongly associated with periodontal disease (1, 44). In the present study serotype b was the predominant (41% of subjects) serotype in nonoral infections and was more prevalent (49 versus 20%; P = 0.0553) (Table 2) in blood samples of endocarditis and bacteremia patients than in focal infections, which are likely less severe than blood infections. This suggests that due to serotype-dependent factors some A. actinomycetemcomitans strains may exhibit tropism for certain tissues, such as the endocardium, and may contribute to the course of nonoral infections. The importance of certain A. actinomycetemcomitans clones in nonoral infections is further supported by the finding that one A. actinomycetemcomitans AP-PCR genotype was significantly (P = 0.0197) more frequently found in focal infections than in blood samples. Previously, among a total of 15 distinguishable A. actinomycetemcomitans AP-PCR genotypes, strains of this particular AP-PCR genotype were the most frequently detected (32%) in the oral cavities of periodontally healthy subjects (2). Thus, further studies are needed to determine whether certain characteristics enable strains of this genotype to colonize healthy oral cavities and preferentially cause localized nonoral infections.
None of the present oral or nonoral A. actinomycetemcomitans strains produced the amplicon characteristic of the deletion of the leukotoxin gene promoter of a highly toxic oral A. actinomycetemcomitans strain that is mostly detected among juvenile periodontitis patients of African origin (8, 18). The nonoral A. actinomycetemcomitans strains originated from Taiwan, New Zealand, and the United States, but a majority (69%) was received from northern Europe, where the virulent clonal type characterized by high-level production of leukotoxin has not been detected (4, 17). Although the ethnic origin or race of the patients who contributed the nonoral A. actinomycetemcomitans strains in the present study were not known, the result supports the current assumption that the deletion of the leukotoxin promoter structure is rare (7).
Amoxicillin, tetracycline, azithromycin, and trovafloxacin exhibited good activities against both nonoral and oral A. actinomycetemcomitans strains. However, when applying the NCCLS guidelines (26, 27) in the interpretation of the MIC results, it was seen that approximately 30% of all A. actinomycetemcomitans strains, strains of both nonoral and oral origins, were resistant to benzylpenicillin or metronidazole. Our present results largely corroborate those of previous studies from our laboratory and elsewhere on the antimicrobial susceptibilities of oral A. actinomycetemcomitans strains (5, 29, 34, 41). A. actinomycetemcomitans strains do not produce penicillinase (34); thus, the resistance to benzylpenicillin is probably not beta-lactamase mediated but, instead, may be related to changes in penicillin-binding proteins, as observed among strains of H. influenzae (10), a close phylogenetic relative of A. actinomycetemcomitans. Interestingly, in the present study serotype b strains were statistically significantly most frequently (P = 0.0000; Table 4) resistant to benzylpenicillin, whereas only a few strains of the other serotypes were resistant to benzylpenicillin. Additionally, two AP-PCR genotypes among serotype b strains exhibited benzylpenicillin resistance significantly more often (P = 0.0041; Table 4) than the other serotype b genotypes. It is not known whether serotype b strains or, particularly, whether some clones within serotype b have specific properties, such as alterations in penicillin-binding proteins, that would allow them to exhibit increased resistance to benzylpenicillins.
According to the present guidelines of NCCLS (27), two nonoral and two oral A. actinomycetemcomitans strains, comprising 5% of all strains tested in the present study, were resistant to metronidazole. As has been shown in earlier studies, resistance to metronidazole occurs among oral A. actinomycetemcomitans strains (5, 21, 29, 34, 41), which can be expected due to its oxygen tolerance. Amoxicillin, the currently recommended antimicrobial agent for use as endocarditis prophylaxis in dental procedures (11), showed good activity against all of the present A. actinomycetemcomitans strains, regardless of the origin of the infection site, and therefore can be anticipated to be effective as endocarditis prophylaxis for periodontitis patients harboring oral A. actinomycetemcomitans. Our results for amoxicillin corroborate previous results for oral A. actinomycetemcomitans strains (41). Also, trovafloxacin, a new quinolone, which has excellent activity against several microaerophilic bacterial species (38) but whose activity has not previously been tested against A. actinomycetemcomitans, showed high levels of activity against the present strains. However, to date no information on the in vivo efficacy of trovafloxacin against A. actinomycetemcomitans infections is available.
In conclusion, the serotype and genotype characteristics of nonoral A. actinomycetemcomitans strains highly resembled those of the oral strains and suggest that the origin of the strains was the human oral cavity. The predominance of serotype b strains in nonoral A. actinomycetemcomitans infections and the relationship between serotype b strains and bacteremia or endocarditis as well as between certain AP-PCR genotypes and focal infections support the hypothesis that certain A. actinomycetemcomitans clones are important contributors to nonoral infections. However, the relatively small sample sizes in the present comparisons provoke the need for additional studies with larger sample sizes to prove the relationship between an A. actinomycetemcomitans strain and a specific infection. Additionally, further studies on the exceptional resistance of A. actinomycetemcomitans serotype b strains to benzylpenicillin are warranted.
ACKNOWLEDGMENTS
We thank Geert Claeys, Laboratory for Bacteriology and Virology, Ghent University Hospital, Ghent, Belgium; Peter Holbrook, Faculty of Odontology, University of Iceland, Reykjavik, Iceland; Kwen-Tay Luh, Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan, Republic of China; Patricia Short, Institute of Environmental Science and Research Limited, Kenepuru Science Center, Porirua, New Zealand; and Georges Wauters, Université Catholique de Louvain, Brussels, Belgium, for kind help in supplying the nonoral A. actinomycetemcomitans strains. We acknowledge Jørgen Slots, University of Southern California, Los Angeles, for the kind gift of A. actinomycetemcomitans JP2. We thank Marja Piekkola for excellent technical assistance.
This study was supported by grants from the Academy of Finland (grant 1012374), the University of Helsinki (Scholarship for Young Researchers), and the Finnish Dental Society.
REFERENCES
- 1.Asikainen S, Lai C-H, Alaluusua S, Slots J. Distribution of Actinobacillus actinomycetemcomitans serotypes in periodontal health and disease. Oral Microbiol Immunol. 1991;6:115–118. doi: 10.1111/j.1399-302x.1991.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 2.Asikainen S, Chen C, Slots J. Actinobacillus actinomycetemcomitans genotypes in relation to serotypes and periodontal status. Oral Microbiol Immunol. 1995;10:65–68. doi: 10.1111/j.1399-302x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 3.Asikainen S, Chen C, Slots J. Likelihood of transmitting Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in families with periodontitis. Oral Microbiol Immunol. 1996;11:387–394. doi: 10.1111/j.1399-302x.1996.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 4.Asikainen S, Chen C, Saarela M, Saxén L, Slots J. Clonal specificity of Actinobacillus actinomycetemcomitans in destructive periodontal disease. Clin Infect Dis. 1997;25(Suppl. 2):S227–S229. doi: 10.1086/516211. [DOI] [PubMed] [Google Scholar]
- 5.Avila-Campos M J, Carvalho M A R, Zelante F. Distribution of biotypes and antimicrobial susceptibility of Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 1995;10:382–384. doi: 10.1111/j.1399-302x.1995.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 6.Baehni P, Tsai C-C, McArthur W P, Hammond B F, Taichman N S. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979;24:233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brogan J M, Lally E T, Poulsen K, Kilian M, Demuth D R. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect Immun. 1994;62:501–508. doi: 10.1128/iai.62.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bueno L C, Mayer M P A, DiRienzo J M. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J Periodontol. 1998;69:998–1007. doi: 10.1902/jop.1998.69.9.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y-C, Chang S-C, Luh K-T, Hsieh W-C. Actinobacillus actinomycetemcomitans endocarditis: a report of four cases and review of the literature. Q J Med. 1991;81:871–878. [PubMed] [Google Scholar]
- 10.Clairoux N, Picard M, Brochu A, Rousseau N, Gourde P, Beauchamp D, Parr T R, Jr, Bergeron M G, Malouin F. Molecular basis of the non-beta-lactamase-mediated resistance to beta-lactam antibiotics in strains of Haemophilus influenzae isolated in Canada. Antimicrob Agents Chemother. 1992;36:1504–1513. doi: 10.1128/aac.36.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dajani A S, Taubert K A, Wilson W, Bolger A F, Bayer A, Ferrieri P, Gewitz M H, Shulman S T, Nouri S, Newburger J W, Hutto C, Pallasch T J, Gage T W, Levison M E, Peter G, Zuccaro G., Jr Prevention of bacterial endocarditis. Recommendations by the American Heart Association. Circulation. 1997;96:358–366. doi: 10.1161/01.cir.96.1.358. [DOI] [PubMed] [Google Scholar]
- 12.Das M, Badley A D, Cockerill F R, Steckelberg J M, Wilson W R. Infective endocarditis caused by HACEK microorganisms. Annu Rev Med. 1997;48:25–33. doi: 10.1146/annurev.med.48.1.25. [DOI] [PubMed] [Google Scholar]
- 13.DiRienzo J M, Slots J, Sixou M, Sol M-A, Harmon R, McKay T L. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect Immun. 1994;62:3058–3065. doi: 10.1128/iai.62.8.3058-3065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dogan, B., M. Saarela, and S. Asikainen. Oral Microbiol. Immunol., in press. [DOI] [PubMed]
- 15.Gmür R, McNabb H, van Steenbergen T J M, Baehni P, Mombelli A, van Winkelhoff A J, Guggenheim B. Seroclassification of hitherto nontypeable Actinobacillus actinomycetemcomitans strains: evidence for a new serotype e. Oral Microbiol Immunol. 1993;8:116–120. doi: 10.1111/j.1399-302x.1993.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 16.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 17.Haubek D, Poulsen K, Asikainen S, Kilian M. Evidence for absence in northern Europe of especially virulent clonal types of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1995;33:395–401. doi: 10.1128/jcm.33.2.395-401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haubek D, Poulsen K, Westergaard J, Dahlèn G, Kilian M. Highly toxic clone of Actinobacillus actinomycetemcomitans in geographically widespread cases of juvenile periodontitis in adolescents of African origin. J Clin Microbiol. 1996;34:1576–1578. doi: 10.1128/jcm.34.6.1576-1578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He T, Hayashi J, Yamamoto M, Ishikawa I. Genotypic characterization of Actinobacillus actinomycetemcomitans isolated from periodontitis patients by arbitrarily primed polymerase chain reaction. J Periodontol. 1998;69:69–75. doi: 10.1902/jop.1998.69.1.69. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz E A, Pugsley M P, Turbes P G, Clark R B. Pericarditis caused by Actinobacillus actinomycetemcomitans. J Infect Dis. 1987;155:152–153. doi: 10.1093/infdis/155.1.152. [DOI] [PubMed] [Google Scholar]
- 21.Jousimies-Somer H, Asikainen S, Suomala P, Summanen P. Activity of metronidazole and its hydroxy metabolite against clinical isolates of Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 1988;3:32–34. doi: 10.1111/j.1399-302x.1988.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan A H, Weber D J, Odone E Z, Perfect J R. Infection due to Actinobacillus actinomycetemcomitans: 15 cases and review. Rev Infect Dis. 1989;11:46–63. doi: 10.1093/clinids/11.1.46. [DOI] [PubMed] [Google Scholar]
- 23.Kristinsson K G, Thorgeirsson G, Holbrook W P. Actinobacillus actinomycetemcomitans and endocarditis. J Infect Dis. 1988;157:599. doi: 10.1093/infdis/157.3.599. [DOI] [PubMed] [Google Scholar]
- 24.Martín M C, Andrés M T, Fierro J F, Méndez F J. Endarteritis and mycotic aortic aneurysm caused by an oral strain of Actinobacillus actinomycetemcomitans. Eur J Clin Microbiol Infect Dis. 1998;17:104–107. doi: 10.1007/BF01682165. [DOI] [PubMed] [Google Scholar]
- 25.Morello J A. Microbial agents in endocarditis. Clin Microbiol Newsl. 1980;2:1–3. [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. Approved standard M7-A4. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Approved standard M11-A4. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 28.Paju S, Saarela M, Alaluusua S, Fives-Taylor P, Asikainen S. Characterization of serologically nontypeable Actinobacillus actinomycetemcomitans isolates. J Clin Microbiol. 1998;36:2019–2022. doi: 10.1128/jcm.36.7.2019-2022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajukanta R, Asikainen S, Saarela M, Alaluusua S, Jousimies-Somer H. In vitro antimicrobial susceptibility of different serotypes of Actinobacillus actinomycetemcomitans. Scand J Dent Res. 1993;101:299–303. doi: 10.1111/j.1600-0722.1993.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 30.Pierce C S, Bartholomew W R, Amsterdam D, Neter E, Zambon J J. Endocarditis due to Actinobacillus actinomycetemcomitans serotype c and patient immune response. J Infect Dis. 1984;149:479. doi: 10.1093/infdis/149.3.479. [DOI] [PubMed] [Google Scholar]
- 31.Poulsen K, Theilade E, Lally E T, Demuth D R, Kilian M. Population structure of Actinobacillus actinomycetemcomitans: a framework for studies of disease-associated properties. Microbiology. 1994;140:2049–2060. doi: 10.1099/13500872-140-8-2049. [DOI] [PubMed] [Google Scholar]
- 32.Saarela M, Asikainen S, Alaluusua S, Pyhälä L, Lai C-H, Jousimies-Somer H. Frequency and stability of mono- or poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d or e. Oral Microbiol Immunol. 1992;7:277–279. doi: 10.1111/j.1399-302x.1992.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 33.Saarela M, Dogan B, Alaluusua S, Asikainen S. Persistence of oral colonization by the same Actinobacillus actinomycetemcomitans strain(s) J Periodontol. 1999;70:504–509. doi: 10.1902/jop.1999.70.5.504. [DOI] [PubMed] [Google Scholar]
- 34.Slots J, Evans R T, Lobbins P M, Genco R J. In vitro antimicrobial susceptibility of Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1980;18:9–12. doi: 10.1128/aac.18.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slots J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1982;15:606–609. doi: 10.1128/jcm.15.4.606-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slots J, Liu B, DiRienzo M, Chen C. Evaluating two methods for fingerprinting genomes of Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 1993;8:337–343. doi: 10.1111/j.1399-302x.1993.tb00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai C C, McArthur W P, Baehni P C, Hammond B F, Taichman N S. Extraction and partial characterization of a leukotoxin from a plaque-derived gram-negative microorganism. Infect Immun. 1979;25:427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Väisänen M-L, Mättö J, Salminen K, Jousimies-Somer H. In vitro activity of trovafloxacin against anaerobic bacteria. Rev Med Microbiol. 1997;8(Suppl. 1):S81–S83. [Google Scholar]
- 39.van Winkelhoff A J, Overbeek B P, Pavicic M J A M P, van den Bergh J P A, Ernst J P M G, de Graaff J. Long-standing bacteremia caused by oral Actinobacillus actinomycetemcomitans in a patient with a pacemaker. Clin Infect Dis. 1993;16:216–218. doi: 10.1093/clind/16.2.216. [DOI] [PubMed] [Google Scholar]
- 40.Verhaaren H, Claeys G, Verschraegen G, de Niel C, Leroy J, Clement D. Endocarditis from a dental focus. Importance of oral hygiene in valvar heart disease. Int J Cardiol. 1989;23:343–347. doi: 10.1016/0167-5273(89)90194-0. [DOI] [PubMed] [Google Scholar]
- 41.Walker C B, Pappas J D, Tyler K Z, Cohen S, Gordon J M. Antibiotic susceptibilities of periodontal bacteria. In vitro susceptibilities to eight antimicrobial agents. J Periodontol. 1985;56(11 Suppl.):67–74. doi: 10.1902/jop.1985.56.11s.67. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto M, Nishihara T, Koseki T, He T, Yamato K, Zhang Y-J, Nakashima K, Oda S, Ishikawa I. Prevalence of Actinobacillus actinomycetemcomitans serotypes in Japanese patients with periodontitis. J Periodont Res. 1997;32:676–681. doi: 10.1111/j.1600-0765.1997.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 43.Yuan A, Yang P-C, Lee L-N, Chang D-B, Kuo S-H, Luh K-T. Actinobacillus actinomycetemcomitans pneumonia with chest wall involvement and rib destruction. Chest. 1992;101:1450–1452. doi: 10.1378/chest.101.5.1450. [DOI] [PubMed] [Google Scholar]
- 44.Zambon J J, Slots J, Genco R J. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983;41:19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zambon J J, Umemoto T, De Nardin E, Nakazawa F, Christersson L A, Genco R J. Actinobacillus actinomycetemcomitans in the pathogenesis of human periodontal disease. Adv Dent Res. 1988;2:269–274. doi: 10.1177/08959374880020021101. [DOI] [PubMed] [Google Scholar]