Abstract
Objectives:
Frailty is a syndrome characterized by increased vulnerability and reduced ability to maintain homeostasis after stressful events that results in increased risk for poor outcomes. Frailty screening could potentially be valuable in cardiac surgery risk assessment. The purpose of this review is to evaluate the current literature linking multicomponent frailty assessment and invasive cardiac surgery outcomes.
Methods:
We searched PubMed, Embase, and CINAHL; 1887 articles met the search criteria, and each was independently reviewed by two reviewers.
Results:
The 19 eligible studies assessed 52,291 subjects using 17 different frailty measurements. The most commonly used tools were the Fried Frailty Phenotype and the Clinical Frailty Scale. Between 9% and 61% of participants were found to be frail in each study. All 19 studies included mortality as an outcome, 12 included surgical complications, 12 included hospital length of stay (LOS), 3 included quality of life, and 2 included functional status. Nine found statistically significant differences in survival between frail and non-frail patients, 6 of 12 found that frail patients had longer LOS, 4 of 12 found that frail patients were more likely to suffer major complications, and 2 of 2 found that frail patients were more likely to have a decrease in functional status.
Conclusion:
Though some studies lacked power, the majority confirmed that frail patients are more likely to experience poor outcomes. Further research is needed to determine which frailty measure provides the best predictive validity and to identify interventions to mitigate the risks that major cardiac surgery poses to frail patients.
Keywords: Frailty assessment, cardiac surgery, open-heart surgery, outcomes
INTRODUCTION
Frailty describes a syndrome characterized by increased vulnerability and a reduced ability to maintain homeostasis after physically and mentally stressful events.1,2 Frailty increases the risk for adverse outcomes including mortality, major morbidity, and decreased functional status and quality of life.1,3 Although the risk for frailty increases with age, not all older adults are frail, and frailty is not exclusive to the aged.2,4 Most importantly, frailty is not a static state and can progressively worsen or improve depending on intervention.2,5–7
Since the concept was first described in the scientific literature, two basic operationalizations of frailty have emerged, the frailty phenotype and the model of frailty as an accumulation of deficits.1,8 The frailty phenotype, first defined by Fried and colleagues, manifests as three or more objectively identifiable physical indicators including weakness (measured by grip strength), slowness (measured by gait speed), unintentional weight loss (10 or more pounds lost in the last year), exhaustion (self-report), and low physical activity (kilocalories expended in a week compared to age and sex norms).1 Many other frailty assessment tools, like the FRAIL Scale,9 the Comprehensive Assessment of Frailty,10 and the Short Physical Performance Battery11 measure some combination of the frailty phenotype dimensions, sometimes using alternative strategies like questionnaires or chair raises to assess weakness. The deficit index model pioneered by Rockwood and colleagues operationalizes frailty as the result of multiple impairments, like cognitive decline, self-care deficits, and comorbidity, making a person less resilient to stressors.8 While the operationalization is very different, both models conceptualize frailty as a multidimensional syndrome of reduced physical and psychological reserves that results in an increased risk for poor outcomes.
While attempts have been made to identify which of the over 70 frailty assessment tools is the most effective and efficient, further research is needed to determine which have the most utility in clinical practice. In 2019, the International Conference of Frailty and Sarcopenia Research acknowledged the large number of frailty screening and assessment tools and recommended five that have been found to be valid and reliable for a general population of older adults.2 Though the frailty construct has the potential to be a valuable aid in health care decision-making, this lack of consensus on which tools are the most appropriate can limit its measurement and use in the clinical setting.12,13 Despite this limitation, a growing body of literature suggests that the identification of frailty could be an important factor in refining strategies to improve overall health and quality of life in the aging population.2,13–16
Frailty screening has the potential to be valuable in the setting of cardiac surgery in particular. Cardiac surgery is especially stressful on the body and is more successful in patients who are relatively healthy at the time of surgery.17 Therefore, more research is needed to identify which cardiac surgery patients are at the greatest risk for poor outcomes and complications. Integrating frailty assessment into usual pre-operative care could allow time for cardiac prehabilitation to optimize patients for surgery18 or prepare high risk patients for longer recovery times. Because frail patients have decreased resilience to stressful events, they are at a higher risk to experience painful, costly, and potentially debilitating complications following cardiac intervention.3 Optimizing patients undergoing cardiac surgery and their post-surgery treatment plan has the potential to decrease mortality and post-operative complications, reduce hospital length of stay, and improve patient quality of life for years after surgery.12
Over the last decade, there has been an increased focus on frailty in influencing health outcomes. There is a need for an updated review of literature examining the association between frailty and major cardiac surgery outcomes. Two past systematic reviews have examined unvalidated or single-component frailty measurement tools, but these reviews have failed to illustrate frailty as a multi-faceted, measurable phenomenon.19,20 Additionally, one review focused on the minimally invasive cardiac procedure, transcatheter aortic valve replacement (TAVR), which does not require a full sternotomy and involves risks that are inherently different from those of more invasive surgical procedures like coronary artery bypass grafting (CABG) or surgical aortic valve replacement (AVR).21,22 Lastly, frailty research has increased dramatically in recent years, and there is a need to update past literature reviews like the one Sepheri and colleagues conducted in 2014 with the expanding body of research.23 Their review of just 6 studies no longer reflects the full body of literature on the subject. The purpose of this review is to fill these gaps and evaluate the current state of evidence linking multicomponent frailty assessment and invasive cardiac surgery outcomes.
METHODS
Data Sources and Search Strategy
A clinical informaticist assisted in developing a comprehensive literature search strategy using Embase, CINAHL, and PubMed from inception to February 2020. We searched each database using cardiac surgery or cardiovascular surgery, and frailty syndrome, frail elderly, frail*, or frailty assessment. We identified and reported relevant information in this paper according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PRISMA provides guidelines for authors on how to report the purpose, methods, and results of systematic reviews and meta-analyses.24
Inclusion and Exclusion Criteria
Studies were included in the analysis if they (1) designated a frailty assessment tool or score as the independent variable, (2) used a frailty measurement tool that is valid and multidimensional (rather than a single isolated measurement like 5-meter gait speed or BMI), (3) examined clinically relevant postoperative outcomes, (4) included a patient population undergoing an invasive cardiac surgery. We considered frailty assessment tools to be objective, multidimensional measures that operationalized frailty as a clinical syndrome manifested as either an observable phenotype or an accumulation of deficits. Frailty measurement tools were considered to be valid if the validity was reported in the article or if the authors referenced a validity study for the measure. We defined clinically relevant outcomes as mortality, hospital and ICU length of stay, hospital readmission, major adverse cardiac and cerebrovascular events (MACCEs), disability, functional status, quality of life, or some composite combination of these. Studies were excluded if they (1) examined a population undergoing minimally invasive cardiac interventions (like transcatheter aortic heart valve replacements or percutaneous coronary intervention), (2) investigated outcomes that were not clinically relevant (like cost), (3) were a review or meta-analysis of existing literature, (4) manuscripts were not written in English, or (5) if a full text publication could not be found (poster presentations, conference abstracts). Studies that surveyed both invasive and minimally invasive cardiac surgeries were included if they provided sub-group analysis for the invasive surgery group.
Data Extraction
The titles and abstracts identified in the literature search were independently reviewed by two reviewers (AP and CM). Articles that obviously did not meet the study’s inclusion criteria were eliminated, and those that remained were evaluated in full text-review by two reviewers. Studies meeting the inclusion criteria were included in the study. Disputes for inclusion between reviewers were settled by a third author (PD).
Risk of Bias Assessment
Quality assessment of the included studies was performed independently by two reviewers using the Newcastle-Ottawa Scale (NOS) checklist. The NOS evaluates the risk of bias in nonrandomized studies based on the selection of participants, comparability of the experimental and control groups, and the assessment of the outcome. Conflicting results were settled after discussion and consensus was reached.
RESULTS
Study Selection
Based on the search terms, a total of 1,887 titles and abstracts were screened. The resulting 171 articles were read in full and were assessed for eligibility based on the established criteria. We identified 19 studies, seen in Table 1, that evaluated the ability of multicomponent frailty measurement tools to predict outcomes following invasive cardiac surgery. The study screening process is shown in Figure 1. In total, these studies assessed 52,291 subjects using 17 different frailty measurement tools.
Table 1:
Included Studies
| Population | Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author, Year and Country |
Study Design | Surgery | N | Age (mean ± SD) |
% Male | % Frail | Follow-up | Outcomes Measured | Association Between Frailty and Outcome |
| 1. Ad, 2016 USA |
Prospective observational cohort | Elective CABG or AVR | 166 | 74.1 ± 6.6 (limited to >=65) |
75 | 23 | 12 mo. |
|
Frailty was significantly associated with greater odds for discharge to intermediate care facilities after adjusting for pre-op characteristics. No other measured outcomes were significantly associated. |
| 2. Afilalo, 2012 USA and Canada |
Prospective observational cohort | CABG, AVR, or both | 152 | 75.9 ± 4.4 (limited to >=70) |
66 | 20–46 | Data not available |
|
Odds ratios for major morbidity or mortality in frail vs. non-frail patients were not statistically significant for any of the multicomponent frailty measurements but were for gait speed alone. |
| 3. Amabili, 2019 Belgium |
Prospective observational cohort | CABG, AVR, MVR, or other open-heart surgeries | 254 | 80 (limited to >=75) |
62 | 20.5 | 30 days |
|
Frailty scores significantly higher in patients who died than in those who did not. Using frailty in addition to EuroSCORE II had a better ability to predict 30-day mortality than EuroSCORE II alone. Frailty is associated with a higher probability of discharge to a health care facility rather than home. |
| 4. Back, 2019 Denmark |
Prospective observational cohort | Elective or subacute CABG, AVR, MVR, or combination | 604 | 73 (limited to >=65) |
79 | 25 | 30 days |
|
Frail patients had four times greater risk of 30-day mortality and MACCE compared to non-frail patients (though only MACCE were statistically significant). Frail patients had a statistically significant increased risk of re-operations, a-fib, renal failure, prolonged ventilation, GI complications, and delirium, increased total length of stay, and a higher probability of discharge to a health care facility rather than home. |
| 5. Dunlay, 2014 USA |
Prospective observational cohort | Destination LVAD implantation | 99 | 65.1 | 82 | 61.6 | Mean 1.9 years |
|
Higher deficit index scores were associated with higher one-year mortality. There was no difference initial hospital length of stay in frail vs. not frail. There was a trend toward discharge to inpatient rehab rather than home in frail patients, but it was not statistically significant. Frail patients had more readmissions than those who were not frail. |
| 6. Esses, 2018 USA |
Retrospective cohort - Secondary data from ACS NSQIP | Elective AVR | 3,088 | 69.24 ± 13 (limited to >=65) |
62 | 26–56 | 30 days |
|
Frailty was significantly associated with a higher risk for a major complication, 30-day mortality, and extended postoperative length of stay after aortic valve replacement. |
| 7. Henry, 2019 USA |
Prospective observational cohort | Elective CABG, AVR, MVR, or combination | 167 | 74.1 ± 6.5 (limited to >=65) |
70 | 9–28 | 12 mo. |
|
SOF frail patients were more likely to experience prolonged ventilation, pneumonia, longer ICU length of stay, and hospital readmission. Frail patients experienced no significantly different adverse clinical outcomes compared with non-frail patients other than discharge location. For both frailty assessment tools, pre-op physical QOL was lower in frail vs non-frail patients. |
| 8. Hosler, 2019 USA |
Prospective observational cohort | AVR and TAVR (only the AVR data is analyzed in this review) | 91 | 77.8 ± 5.3 (limited to >=70) |
66 | 41–62 | 12 mo. |
|
In the AVR cohort, patients deemed frail by the Clinical Frailty Scale or the FRAIL scale were more likely to have poor outcomes (die, have decreased functional status after surgery, or increase NYHA class) than those who were not, but the difference was not statistically significant. |
| 9. Jha, 2016 Australia |
Prospective observational cohort | Heart transplant or bridge to transplant VAD implantation | 120 | 53 ± 12 | 69 | 32.5 | Mean 337 days |
|
Survival was significantly worse in the frail group compared to the non-frail group in both the transplant and the VAD patients. Trends in the ICU and hospital lengths of stay showed longer times in the frail group vs. the non-frail group, but these differences were not significant. There was a significant association between NYHA class and frailty status but not between age and frailty status. |
| 10. Joseph, 2017 USA |
Prospective observational cohort | Bridge to transplant or destination LVAD implantation | 75 | 58 ± 11.9 | 75 | 58.7 | Median 314 days |
|
Frailty was not statistically significantly associated with 30-day mortality or extended hospital length of stay (> 30 days). Time to extubation was significantly longer in frail patients. |
| 11. Kovacs, 2017 Romania |
Prospective observational cohort | CABG, AVR, MVR, or combination | 57 | 70.2 ± 4.3 (limited to >=65) |
67 | Data not available | Data not available |
|
Using both frailty measurement tools, frail patients had statistically significantly longer mechanical ventilation times than patients who were not frail. All other outcomes were more likely in the frail population but were not statistically significant. |
| 12. Lal, 2019 New Zealand |
Prospective observational cohort | CABG, AVR, MVR, or combination | 96 | 74 (limited to >=65) |
68 | 10 | 12 mo. |
|
In both models unadjusted and adjusted for confounding variables, there was a significant association between frailty score and length of stay. For each point increase in EFS, there was a 17% lower chance of discharge on any particular day. High EFS scores were also significantly associated with a higher number of hospital readmission. |
| 13. Lytwyn, 2017 Canada |
Prospective observational cohort | CABG, AVR, MVR, or combination | 188 | 71 | 64 | 32–52 | 12 mo. |
|
Preop frailty was associated 2 to 3.5 times higher odds of poor functional survival, depending on the frailty tool used. All frailty tools used added significant prognostic value to the EuroSCORE II in determining functional survival after surgery. |
| 14. Miguelena-Hycka, 2018 Spain |
Prospective observational cohort | CABG, AVR, MVR, TVR, or combination | 137 | 78.4 ± 4.2 (limited to >=70) |
52 | 10–29 | 6 mo. |
|
6-month mortality was more likely with increasing frailty scores but not statistically significant. There was a statistically significant trend in the incidence of major in-hospital morbidity as frailty scores increased. Quality of life scores increased significantly after surgery for those who were frail and pre-frail. |
| 15. Reichart, 2018 Finland, France, Italy, Germany, Sweden, UK |
Prospective observational cohort | Isolated CABG | 6,156 | CFS 1–2: 65.3 CFS 3–4: 68.6 CFS 5–7: 69.9 |
83 | Pre-frail: 57.5 Frail: 3.2 |
Mean 1.2 years |
|
The CFS score, adjusted for covariates like age, comorbidities and EuroSCORE II, was a statistically significant independent predictor of 30-day mortality, prolonged inotropic support, acute kidney injury, severe bleeding and prolonged ICU length of stay. |
| 16. Rodrigues, 2017 Brazil |
Prospective observational cohort |
Elective CABG, AVR, MVR, or combination | 221 | No frailty: 70 Pre-frail: 72 (limited to >=65) |
66 | 65 | 30 days |
|
Pre-frail patients had significantly longer mechanical ventilation times and longer ICU and hospital lengths of stay. Additionally, pre-frail patients had a significantly higher risk of cardiovascular events, stroke, and in-hospital deaths than in the no-frailty group. |
| 17. Sohn, 2019 South Korea |
Prospective observational cohort | AVR with or without concomitant CABG or other open-heart surgery | 154 | 78.7 ± 3.6 (limited to >=75) |
51 | 26.6 | Median 40 mo. |
|
The higher frailty index scores were significantly associated with higher likelihood of overall mortality. Adding the frailty index with a cutoff of 0.25 and above being frail significantly improved the EuroSCORE II’s ability to predict all-cause mortality. |
| 18. Sunderman, 2014 Germany |
Prospective observational cohort | CABG, AVR, MVR, TVR, or combination (15% were TAVRs) | 450 | 79 ± 4 (limited to >=74) |
50 | Moderately frail: 42 Severely frail: 6.9 |
12 mo. |
|
Patients in the severely frail group were significantly more likely to die within 30 days of surgery than patients in the less frail groups. Both CAF scores and FORECAST are statistically significantly associated with 1-year mortality independent of age. Frail patients are more likely to die within one year of surgery than those who are not frail. |
| 19. Tran, 2018 Canada |
Secondary data from CorHealth Ontario Cardiac Registry | Isolated CABG | 40,083 | 65.84 ± 9.85 (limited to >=40) |
79 | 22 | Mean 4 years |
|
Controlling for sex and stratifying by age, frail patients were significantly more likely to die within 30 days than those who were not frail, except those older than 85. Frail patients also had statistically significantly higher rates of all-cause mortality than patients who were not. This difference was more obvious in those younger than 75. |
Acronyms: AVR: aortic valve replacement or repair, CABG: coronary artery bypass graft, DVT: deep vein thrombosis, ICU: intensive care unit, MACCE: major cardiac or cerebrovascular event (includes all-cause mortality, stroke or transient ischemic attack, MI, acute coronary syndrome (including unstable angina), and left ventricular failure), MI: myocardial infarction, MVR: mitral valve replacement or repair, PE: pulmonary embolism, STS: Society of Thoracic Surgeons, TAVR: transcatheter aortic valve replacement, TVR: tricuspid valve replacement, UTI: urinary tract infection
Figure 1:
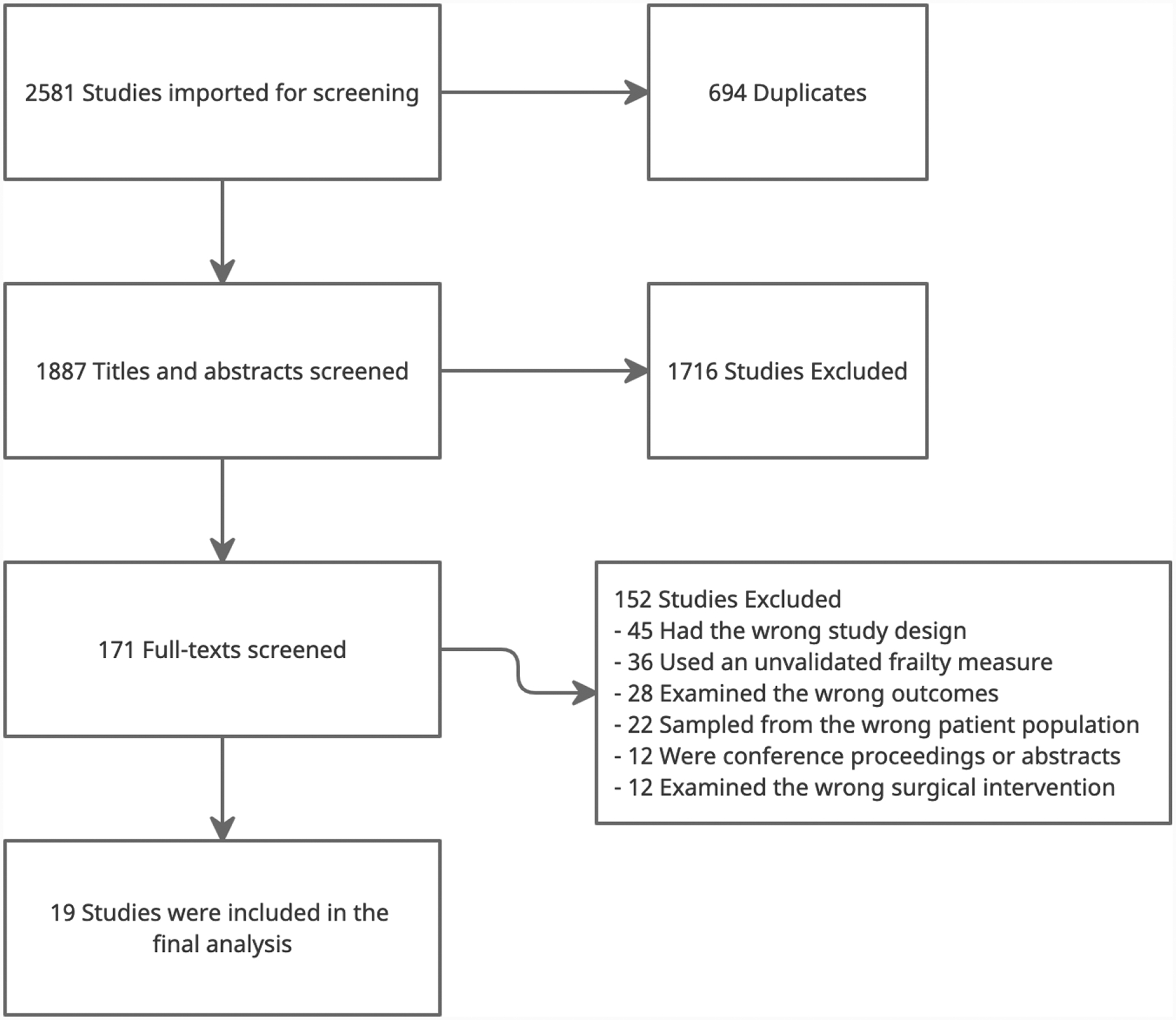
PRIMSA Diagram
Though all included studies analyzed patients receiving invasive cardiac surgery, the type of surgery varied. Fourteen studies involved CABG,4,14,25–37 fourteen involved AVR,14,25–33,35,36,38 nine involved mitral valve replacement (MVR),26–32,34,36 three involved left ventricular assist device (LVAD) implantation (destination only,39 bridge to transplant only,40 and both destination and bridge to transplant41), one involved heart transplants,40 and three involved other open-heart surgeries like aortic root replacements, tricuspid valve replacements, and arrhythmia surgeries.26,32,36
Frailty Measurement Tools
In many cases, authors referred to measurement tools by varying names, so we standardized the names in Table 2 alongside the authors’ names for the tool. The Fried Frailty Phenotype (with or without added psychological tests) and Clinical Frailty Scale were the most commonly used assessment tools in the included studies. Five studies used the Fried Frailty Phenotype,14,25,28,32,41 and three additional studies added a test of cognitive function and a depression scale, referred to as Fried+ in Table 2.14,31,40 Six studies used the Clinical Frailty Scale,29,31–34,38 three studies used the Edmonton Frailty Scale,26,29,30 and two studies used the Comprehensive Assessment of Frailty (CAF).27,36 In their 2014 study, Sunderman et al. condensed the CAF to create the FORECAST (Frailty predicts death One yeaR after Elective CArdiac Surgery Test) measure and validated it compared to the CAF data.36 Two studies used the FRAIL scale.32,38 Ten other validated frailty assessment tools were used, each by just one study. Eight studies compared multiple validated frailty assessment tools,4,14,28,29,31,32,36,38 while 11 just included one.25–27,30,33–35,37,39–41
Table 2:
Frailty Criteria
| Assessment Components | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author year | Multicomponent Frailty Measure | Scale First Author | Labs | Gait Speed | Strength | Weight Loss | Exhaustion | Physical Activity | Cognition | Disability | Comorbidities | Depression | Score |
| 1. Ad, 2016 | Frailty Phenotype (CHS frailty index) |
Fried | X 5m |
X grip |
X >10lbs |
X SR |
X kcal |
Range 0–5, 0–2 = not frail, 3 or greater = frail | |||||
| 2. Afilalo, 2012 | Frailty Phenotype (CHS frailty scale) |
Fried | X 5m |
X grip |
X >10lbs |
X SR |
X kcal |
Range 0–5, 0–2 = not frail, 3 or greater = frail | |||||
| Fried+ (expanded CHS frailty scale) | Fried | X 5m |
X grip |
X >10lbs |
X SR |
X kcal |
X MCA |
X GDS |
Range 0–5, 0–2 = not frail, 3 or greater = frail | ||||
| MacArthur Study of Successful Ageing (MSSA) frailty scale | Sarkisian | X 5m |
X grip |
X kcal |
X NR |
Range 0–4, higher scores = more frail | |||||||
| 3. Amabili, 2019 | Edmonton Frail Scale | Rolfson | X Chair rise |
X SR |
X clock |
X ADLs |
X SR |
X SR |
Range 0–17, 0–5 = not frail, 6–7 = vulnerable, 8–9 = mildly frail, 10–11 = moderately frail, 12–17 = severely frail | ||||
| 4. Back, 2019 | Comprehensive Assessment of Frailty | Sunderman | X | X 5m |
X Grip, chair rise |
X CES-D |
X kcal |
Range 1–35, 1–10 = not frail, 11–25 = moderately frail, 26 – 35 = severely frail | |||||
| 5. Dunlay, 2014 | Deficit Index (31 items) | Rockwood | X ADLs |
X EMR |
X EMR |
Range 0–1, > 0.32 = frail, 0.23–0.32 = intermediate frail, < 0.23 = not frail | |||||||
| 6. Esses, 2018 | Modified Frailty Index | Obeid | X ADLs |
X EMR |
X EMR |
Range 0–1, 0.2 or greater = frail | |||||||
| Risk Analysis Index | Melin | X | X ADLs |
X EMR |
15 or greater = frail | ||||||||
| Ganapathi Index | Ganapathi | X | X ADLs |
X EMR |
Range 0–6, 2 or greater = frail | ||||||||
| 7. Henry, 2019 | Frailty Phenotype (CHS frailty index) |
Fried | X 5m |
X grip |
X >10lbs |
X SR |
X kcal |
Range 0–5, 0–2 = not frail, 3 or greater = frail | |||||
| Study of Osteoporotic (SOF) Assessment | Ensrud | X Chair rise |
X >5% |
X SR |
Range 0–3, 2 or greater = frail | ||||||||
| 8. Hosler, 2019 |
FRAIL scale | Morley | X SR |
X >5% |
X SR |
X SR |
X SR |
Range 0–5, higher score = more frail | |||||
| Clinical Frailty Scale | Rockwood | X SR |
X MMSE |
X ADLs |
X EMR |
Range 1–9, higher score = more frail | |||||||
| Deficit Accumulation Frailty Index | Rockwood | X 5m |
X grip |
X SR |
X MMSE |
X ADLs |
X EMR |
Range 0–1, non-frail (< .10), pre-frail (.10 – .19), mildly frail (.20 – .29), moderately frail (.30 – .39), and severely frail (≥ .40) | |||||
| 9. Jha, 2016 | Fried+ (modified Fried phenotype) | Fried | X 5m |
X grip |
X >10lbs |
X SR |
X kcal |
X MCA |
X DMI-10 |
Range 0–5, 0–2 = not frail, 3 or greater = frail | |||
| 10. Joseph, 2017 | Frailty Phenotype (Fried criteria) |
Fried | X 5m |
X grip |
X >10lbs |
X SR |
X kcal |
Range 0–5, 0–2 = not frail, 3 or greater = frail | |||||
| 11. Kovacs, 2017 | Edmonton Frail Scale | Rolfson | X Chair rise |
X SR |
X clock |
X ADLs |
X SR |
X SR |
Range 0–17, 0–5 = not frail, 6–7 = vulnerable, 8–9 = mildly frail, 10–11 = moderately frail, 12–17 = severely frail | ||||
| Clinical Frailty Scale | Rockwood | X SR |
X MMSE |
X ADLs |
X EMR |
Range 1–7, higher score = more frail | |||||||
| 12. Lal, 2019 | Edmonton Frail Scale | Rolfson | X Chair rise |
X SR |
X clock |
X ADLs |
X SR |
X SR |
Range 0–17, 0–5 = not frail, 6–7 = vulnerable, 8–18 = frail | ||||
| 13. Lywtyn, 2017 | Fried+ (modified Fried criteria) | Fried | X 5m |
X grip |
X >10lbs |
X SR |
X kcal |
X MCA |
X GDS |
Range 0–5, 0–2 = not frail, 3 or greater = frail | |||
| Short Performance Physical Battery | Da Camara | X | X Chair rise |
Range 0–12, 9 or greater = frail | |||||||||
| Clinical Frailty Scale | Rockwood | X SR |
X MMSE |
X ADLs |
X EMR |
Range 1–7, higher score = more frail | |||||||
| 14. Miguelena-Hycka, 2018 | Frailty Phenotype (Fried frailty scale) |
Fried | X 5m |
X grip |
X >10lbs |
X SR |
X kcal |
Range 0–5, 0–2 = not frail, 3 or greater = frail | |||||
| Clinical Frailty Scale | Rockwood | X SR |
X MMSE |
X ADLs |
X EMR |
Range 1–7, higher score = more frail | |||||||
| FRAIL scale | Morley | X SR |
X >5% |
X SR |
X SR |
X SR |
Range 0–5, higher score = more frail | ||||||
| 15.Reichart, 2018 | Clinical Frailty Scale | Rockwood | X SR |
X MMSE |
X ADLs |
X EMR |
Range 1–7, 1–2 = fit, 3–4 = pre-frail, 5–7 = frail | ||||||
| 16. Rodrigues, 2017 |
Clinical Frailty Scale | Rockwood | X SR |
X MMSE |
X ADLs |
X EMR |
Range 1–7, 1–3 = no frailty, 4 or more = pre-frail | ||||||
| 17. Sohn, 2019 | Frailty Index with laboratory data | Blodgett | X | X EMR |
Range 0–1, 0.25 or greater = frail | ||||||||
| 18. Sunderman, 2014 | Comprehensive Assessment of Frailty | Sunderman | X | X 5m |
X Grip, chair rise |
X CES-D |
X kcal |
Range 1–35, 1–10 = not frail, 11–25 = moderately frail, 26 – 35 = severely frail | |||||
| FORECAST (shortened CAF) | Sunderman | X | X Grip, chair rise |
X kcal |
Range 0–14, 1–4 = not frail, 4–6 = moderately frail, 6–14 = severely frail | ||||||||
| 19. Tran, 2018 | Adjusted Clinical Groups frailty indicator | Abrams | X NR |
X NR |
X NR |
X NR |
X NR |
Range 0–10, 1 or greater = frail | |||||
Acronyms and abbreviations: 5m: 5-meter gait speed, grip: grip strength using a dynamometer, SR: self-report, kcal: kilocalories expended in one week, MMSE: Mini-Mental Status Exam, CES-D: Center for Epidemiologic Studies Depression score, NR: not reported, ADLs: Activities of Daily Living (eating, bathing, dressing, toileting, mobility, and grooming), EMR: Electronic Medical Record chart audit, >5%: 5% or more decrease in total body weight in the last year, MCA: Montreal Cognitive Assessment test, GDS: Geriatric Depression Scale, DMI-10: Depression in Medical Illness-10
Domains of Frailty
Among the seventeen assessment tools used by the studies in this review, many different domains were measured in order to diagnose frailty. Physical activity and weight loss were most commonly measured. Sixteen studies used at least one frailty assessment tool that included a measure of physical activity,14,25–29,31–34,36–38,40,41 and fourteen studies used a tool that measured recent weight loss.14,25–32,36–38,40,41 Thirteen studies used at least one tool that measured cognition,4,14,26,29–34,37–40 twelve included disability,4,26,29–34,36–39 and thirteen included comorbidities.4,26,29–39 Ten studies measured gait speed,14,25,27,28,31,32,36,38,40,41 ten measured strength,14,25,27,28,31,32,36,38,40,41 and nine asked the participants questions about their levels of exhaustion.14,25,27,28,31,32,36,38,40,41 Seven took depressive symptoms into consideration,14,26,29–31,39,40 and five analyzed lab data as part of the frailty assessment.4,27,35,36,39
Depending on the measurement tool used, the percentage of frail patients varied widely between studies. In the 16 studies that used a dichotomous frailty determination, anywhere between 9% and 61% of participants were found to be frail. Three studies analyzed frailty as an ordinal variable.30,32,36
Study Characteristics
Sample sizes varied between studies from 57 to over 40,000. 17 of the 19 studies prospectively collected primary observational data, while two involved secondary data analysis.4,37 With the exception of Reichart and colleagues’ study that used primary data from dozens of European hospitals, studies that examined secondary data used larger samples than those that collected primary data.34
All but two studies’ participants had a mean age greater than 65 years. Jha and Joseph’s study populations were slightly younger at 53 ± 12 years and 58 ± 11.9 years, respectively.40,41 Fourteen studies used age as an exclusion criterion, most often limiting selection to participants who were 65 years or older.4,14,25–30,32,33,35,37,38 Additionally, all but one study had mostly male participants.36 Six of those studies had at least 75% male participants.25,27,34,37,39,41
Outcome Measures
Study authors chose to measure many different outcomes. The most common outcome measured was mortality, with all nineteen studies including this variable. Twelve studies collected data on complications following surgery,4,14,25,27–35 twelve on overall hospital length of stay,4,25–30,33,34,39–41 eight on ICU length of stay,25–27,29,30,33,34,40 six on discharge location,25–28,33,39 five on hospital readmission,25,27,28,30,39 and three each on quality of life28,31,32 and ventilation time.29,33,41 Two studies collected data on functional status post-surgery.31,38
Many of the articles mentioned trends and non-statistically significant results. For the purpose of this review, we will only discuss the results that are statistically significant (based on p-values of <0.05). Of the nineteen articles that measured all-cause mortality as an outcome, nine found statistically significant differences in survival between frail and non-frail patients.4,26,33–36,39,40 Six of twelve found that frail patients had longer lengths of stay,4,27,28,30,33,34 four of twelve found that frail patients were more likely to suffer a major complication as a result of cardiac surgery,4,27,32,33 four of six found that frail patients were more likely to be discharged to a rehabilitation facility than those who were not frail,25–28 and three of five found that frail patients were more likely to be readmitted to the hospital.28,30,39 All three studies that measured time-to-extubation found that frail patients had longer mechanical ventilation times than non-frail patients.29,33,41 Lastly, both studies that measured functional status pre and post-surgery found that frail patients were statistically more likely to have a decrease in functional status.31,38
Risk of Bias Assessment
The results of the risk of bias assessment are shown in Table 3. Using the NOS checklist, the studies as a whole presented low to medium risk of bias. Five studies had medium risk of bias based on their sample selection,29,30,33,38,39 four on the comparability of the experimental and control groups,25,29,35,41 and two in the measurement of the outcomes.29,33 Overall, all but two studies had low risk of bias. Two had medium risk.29,33
Table 3:
Risk of Bias Assessment
| Selection | Comparability | Outcome | Overall Risk of Bias | |
|---|---|---|---|---|
| 1. Ad, 2016 | Low | Medium | Low | Low |
| 2. Afilalo, 2012 | Low | Low | Low | Low |
| 3. Amabili, 2019 | Low | Low | Low | Low |
| 4. Back, 2019 | Low | Low | Low | Low |
| 5. Dunlay, 2014 | Medium | Low | Low | Low |
| 6. Esses, 2018 | Low | Low | Low | Low |
| 7. Henry, 2019 | Low | Low | Low | Low |
| 8. Hosler, 2019 | Medium | Low | Low | Low |
| 9. Jha, 2016 | Low | Low | Low | Low |
| 10. Joseph, 2017 | Low | Medium | Low | Low |
| 11. Kovacs, 2017 | Medium | Medium | Medium | Medium |
| 12. Lal, 2019 | Medium | Low | Low | Low |
| 13. Lywtyn, 2017 | Low | Low | Low | Low |
| 14. Miguelena-Hycka, 2018 | Low | Low | Low | Low |
| 15.Reichart, 2018 | Low | Low | Low | Low |
| 16. Rodrigues, 2017 | Medium | Low | Medium | Medium |
| 17. Sohn, 2019 | Low | Medium | Low | Low |
| 18. Sunderman, 2014 | Low | Low | Low | Low |
| 19. Tran, 2018 | Low | Low | Low | Low |
DISCUSSION
The goal of this review was to systematically summarize the evidence describing the link between invasive cardiac surgery, frailty, and postoperative outcomes. Despite the heterogeneity of the frailty measurement tools, we found strong evidence that frailty is associated with a higher risk for mortality, major morbidity, increased hospital and ICU length of stay, and decreases in quality of life and functional status after invasive cardiac surgery. Though some studies lacked the power to prove statistical significance and could only demonstrate trends, the majority of these studies confirmed that frail and pre-frail patients are more likely to experience poor outcomes. Furthermore, of the ten studies that did not find a statistically significant difference in mortality between frail and non-frail patients, six cited a small sample size as a major limitation.14,25,29,30,38,41 This supports the adoption of frailty screening as part of the routine risk assessment before major cardiac surgery.
One noteworthy observation gathered from this review is the heterogeneity of the frailty assessment tools employed. Even in this relatively specific patient population, 17 different assessment tools were used. The studies that used assessment tools that operationalize frailty as an accumulation of deficits, like the Clinical Frailty Scale, tended to show a higher prevalence of frailty than studies who used tools that relied more heavily on physical indicators, like the Fried Frailty Phenotype. No single scale stood out as being the most valid in predicting outcomes. Based on the study and the tool used, the prevalence of frailty varied from 9 to 61%. This speaks to the need for a consensus on which frailty measurement tool is the most accurate and efficient in predicting outcomes in this population.
Another interesting observation from this review was the preponderance of male patients in the included studies. All but one of the studies36 had a majority of male participants, and 6 studies had 75% or more male participants.25,27,34,37,39,41 The prevalence of frailty in the general population is higher in women than in men.2 While a higher percentage of men undergo cardiac surgery, women undergoing cardiac surgery are more likely to be older, frailer and have more comorbidities than their male counterparts.42 While many of the studies controlled for sex in their statistical analysis, none discussed the potential implications of sex-based differences. Further research would be useful to examine the potential moderating effect of sex on the relationship between frailty and cardiac surgery outcomes.
FUTURE RESEARCH NEEDS
Foremost, a standard frailty assessment that is feasible to administer in the preoperative setting and establishes a definite correlation with important postoperative outcomes would not only advance the science, but also shift the discussion of frailty from a scientific construct to implementation. While it might be impossible to gain consensus on a single frailty measurement tool for all patient populations, it would be beneficial to standardize within the cardiac surgery risk assessment sphere to make comparisons and meta-analyses more feasible. It is clear that further research is needed to identify which measure provides the best predictive validity and which components of frailty assessment correlate best with postoperative outcomes.
Additionally, with the amount of data supporting the integration of frailty assessment into surgical risk calculation, large national datasets like the Society for Thoracic Surgeons database or the UK Adult Cardiac Surgical Database should include additional frailty and geriatric-focused data. While nearly one third of patients undergoing cardiac surgery are 75 years old or older,19 the Society for Thoracic Surgeons database includes only one frailty assessment measure: five-meter gait speed.19 National data sets are created to track trends in health and ultimately enable surgeons to improve the quality of their practice, and they should evolve to mirror the aging population.
Another takeaway from this review that most studies focused on outcomes that are more relevant to clinicians than to patients. While all at least included mortality as an outcome, there was an obvious lack of consideration for patient-centered and patient-reported outcomes. Only four studies measured patients’ post-surgical functional status or quality of life. The passing of the Affordable Care Act and the establishment of the Patient Centered Outcomes Research Institute (PCORI) have encouraged health care providers to consider patient preferences more highly and to include their voice in clinical decision making.43 This focus should translate into the research that is being conducted as well. While postoperative acute kidney injury and prolonged ventilation time are important clinical outcomes, they may not be as meaningful to patients as health-related quality of life or functional status. Designing studies that employ community-based participatory research strategies will not only aid researchers in defining outcomes that have significance to this patient population but also increase awareness of frailty and strategies to prevent and reverse it.
While there is an ever-growing body of literature supporting the integration of frailty assessment into surgical risk calculation, there has been less consideration for testing and implementing new strategies to prevent and reverse frailty. Preliminary research suggests that cardiac prehabilitation is one possible solution to optimizing frail patients before their surgery.18 Additionally, nutritional support before and after cardiac surgery shows promise. One recent study by Goldfarb and colleagues found that 96% of frail cardiac surgery patients were not meeting their nutritional needs in the weeks following their surgery.44 They also found that higher weight loss following the surgery was associated with a higher risk of being re-hospitalized and falling.44 Further research into interventions to mitigate the risks that major cardiac surgery poses to frail patients is needed to improve clinical and institutional outcomes.
Limitations
There are several limitations that must be recognized in this literature review. Firstly, because it is neither feasible nor reasonable to assess frailty in someone who needs emergency surgery, every one of the studies in this review excluded patients undergoing emergent cardiac surgery. Excluding this population means that this review only represents non-emergent cardiac surgery. Secondly, we chose to exclude studies that utilized a single component frailty measurement. While five-meter gait speed is commonly used as a proxy measurement for frailty because it is simple, quick, and requires no special equipment, it fails to represent frailty as a multifaceted, dynamic construct. For this reason, we chose to exclude studies that used only five-meter gait speed as a measurement for frailty. Lastly, our results have some bias because we limited our search to studies that were published and written in English. Despite these limitations, this review highlights important areas for future research and identifies several multicomponent frailty assessment tools that show promise in predicting cardiac surgery outcomes.
Conclusions
Measuring frailty as an indicator of postoperative outcomes is a recent but critically important effort. The variety of measurement tools to assess frailty in the invasive cardiac surgical population demonstrates a lack of consensus on an appropriate tool. There is great opportunity to improve outcomes for this patient population by converging on a common set of appropriate measurements. The evidence in this review suggest the Fried Frailty phenotype and Clinical Frailty Scale are most commonly used in the setting of cardiac surgery, and that many different frailty tools show promising utility and should be considered as part of an overall strategy to reduce frailty-associated complications. Findings also make apparent the importance of future studies that incorporate patient-centered outcomes like quality-of-life and functional status.
What’s New:
This review provides an updated, more complete picture of the body of literature linking multicomponent frailty assessment and invasive cardiac surgery outcomes.
Unlike previous systematic reviews, this review utilizes the now accepted definition of frailty as a multi-faceted, measurable phenomenon. It highlights the heterogeneity of the current frailty measurement tools being used, describes and compares them in detail, and defines the domains most commonly being measured.
This review highlights the need for frailty research driven by patient-centered outcomes.
Conflict of Interest Statement
Authors have nothing to disclose regarding commercial support
Footnotes
Conflicts of interest: None
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M146–M157. [DOI] [PubMed] [Google Scholar]
- 2.Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J Nutr Health Aging. 2019;23(9):771–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe R, Iqbal J, Murali-Krishnan R, et al. Role of frailty assessment in patients undergoing cardiac interventions. Open Heart. 2014;1(1):e000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esses G, Andreopoulos E, Lin HM, Arya S, Deiner S. A Comparison of Three Frailty Indices in Predicting Morbidity and Mortality After On-Pump Aortic Valve Replacement. Anesth Analg. 2018;126(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajabali N, Rolfson D, Bagshaw SM. Assessment and Utility of Frailty Measures in Critical Illness, Cardiology, and Cardiac Surgery. Can J Cardiol. 2016;32(9):1157–1165. [DOI] [PubMed] [Google Scholar]
- 6.Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. 2018;59(5):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray NW, Smart RR, Jakobi JM, Jones GR. Exercise prescription to reverse frailty. Appl Physiol Nutr Metab. 2016;41(10):1112–1116. [DOI] [PubMed] [Google Scholar]
- 8.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal. 2005;173(5):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morley JE, Malmstrom TK, Miller DK. A SIMPLE FRAILTY QUESTIONNAIRE (FRAIL) PREDICTS OUTCOMES IN MIDDLE AGED AFRICAN AMERICANS. Journal of Nutrition, Health, and Aging. 2012;16(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39(1):33–37. [DOI] [PubMed] [Google Scholar]
- 11.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afilalo J, Lauck S, Kim DH, et al. FRAILTY ASSESSMENT IN OLDER ADULTS UNDERGOING TRANSCATHETER OR SURGICAL AORTIC VALVE REPLACEMENT: THE FRAILTY-AVR STUDY. JACC (Journal of the American College of Cardiology). 2016;67(13):8. [Google Scholar]
- 13.Walston J, Buta B, Xue QL. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin Geriatr Med. 2018;34(1):25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5(2):222–228. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIsaac DI, MacDonald DB, Aucoin SD. Frailty for Perioperative Clinicians: A Narrative Review. Anesth Analg. 2020;130(6):1450–1460. [DOI] [PubMed] [Google Scholar]
- 17.Montrief T, Koyfman A, Long B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am J Emerg Med. 2018;36(12):2289–2297. [DOI] [PubMed] [Google Scholar]
- 18.Yau DKW, Wong MKH, Wong W-T, et al. PREhabilitation for improving QUality of recovery after ELective cardiac surgery (PREQUEL) study: protocol of a randomised controlled trial. BMJ Open. 2019;9(5):e027974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Ding X. The incremental predictive value of frailty measures in elderly patients undergoing cardiac surgery: A systematic review. Clin Cardiol. 2018;41(8):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furukawa H, Tanemoto K. Frailty in cardiothoracic surgery: systematic review of the literature. General Thoracic and Cardiovascular Surgery. 2015;63(8):425–433. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative Frailty Assessment and Outcomes at 6 Months or Later in Older Adults Undergoing Cardiac Surgical Procedures: A Systematic Review. Annals of internal medicine. 2016;165(9):650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolte D, Vlahakes GJ, Palacios IF, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J Am Coll Cardiol. 2019;74(12):1532–1540. [DOI] [PubMed] [Google Scholar]
- 23.Sepehri AB, Beggs T, Hassan AMDPF, et al. The impact of frailty on outcomes after cardiac surgery: A systematic review. Journal of Thoracic and Cardiovascular Surgery, The. 2014;148(6):3110–3117. [DOI] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ad N, Holmes SD, Halpin L, Shuman DJ, Miller CE, Lamont D. The Effects of Frailty in Patients Undergoing Elective Cardiac Surgery. J Card Surg. 2016;31(4):187–194. [DOI] [PubMed] [Google Scholar]
- 26.Amabili P, Wozolek A, Noirot I, et al. The Edmonton Frail Scale Improves the Prediction of 30-Day Mortality in Elderly Patients Undergoing Cardiac Surgery: A Prospective Observational Study. J Cardiothorac Vasc Anesth. 2019;33(4):945–952. [DOI] [PubMed] [Google Scholar]
- 27.Back C, Hornum M, Olsen PS, Moller CH. 30-day mortality in frail patients undergoing cardiac surgery: the results of the frailty in cardiac surgery (FICS) copenhagen study. Scand Cardiovasc J. 2019;53(6):348–354. [DOI] [PubMed] [Google Scholar]
- 28.Henry L, Halpin L, Barnett SD, Pritchard G, Sarin E, Speir AM. Frailty in the Cardiac Surgical Patient: Comparison of Frailty Tools and Associated Outcomes. Ann Thorac Surg. 2019;108(1):16–22. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs J, Moraru L, Antal K, Cioc A, Voidazan S, Szabo A. Are frailty scales better than anesthesia or surgical scales to determine risk in cardiac surgery? Korean J Anesthesiol. 2017;70(2):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lal S, Gray A, Kim E, et al. Frailty in Elderly Patients Undergoing Cardiac Surgery Increases Hospital Stay and 12-Month Readmission Rate. Heart Lung Circ. 2019;29(8):1187–1194. [DOI] [PubMed] [Google Scholar]
- 31.Lytwyn J, Stammers AN, Kehler DS, et al. The impact of frailty on functional survival in patients 1 year after cardiac surgery. J Thorac Cardiovasc Surg. 2017;154(6):1990–1999. [DOI] [PubMed] [Google Scholar]
- 32.Miguelena-Hycka J, Lopez-Menendez J, Prada PC, et al. Influence of Preoperative Frailty on Health-Related Quality of Life After Cardiac Surgery. Ann Thorac Surg. 2019;108(1):23–29. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues MK, Marques A, Lobo DML, Umeda IIK, Oliveira MF. Pre-Frailty Increases the Risk of Adverse Events in Older Patients Undergoing Cardiovascular Surgery. Arq Bras Cardiol. 2017;109(4):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reichart D, Rosato S, Nammas W, et al. Clinical frailty scale and outcome after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2018;54(6):1102–1109. [DOI] [PubMed] [Google Scholar]
- 35.Sohn B, Choi JW, Hwang HY, Jang MJ, Kim KH, Kim KB. Frailty Index is Associated with Adverse Outcomes after Aortic Valve Replacement in Elderly Patients. J Korean Med Sci. 2019;34(31):e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundermann SH, Dademasch A, Seifert B, et al. Frailty is a predictor of short- and mid-term mortality after elective cardiac surgery independently of age. Interact Cardiovasc Thorac Surg. 2014;18(5):580–585. [DOI] [PubMed] [Google Scholar]
- 37.Tran DTT, Tu JV, Dupuis JY, Bader Eddeen A, Sun LY. Association of Frailty and Long-Term Survival in Patients Undergoing Coronary Artery Bypass Grafting. J Am Heart Assoc. 2018;7(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosler QP, Maltagliati AJ, Shi SM, et al. A Practical Two-Stage Frailty Assessment for Older Adults Undergoing Aortic Valve Replacement. J Am Geriatr Soc. 2019;67(10):2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunlay SM, Park SJ, Joyce LD, et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant. 2014;33(4):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jha SR, Hannu MK, Chang S, et al. The Prevalence and Prognostic Significance of Frailty in Patients With Advanced Heart Failure Referred for Heart Transplantation. Transplantation. 2016;100(2):429–436. [DOI] [PubMed] [Google Scholar]
- 41.Joseph SM, Manghelli JL, Vader JM, et al. Prospective Assessment of Frailty Using the Fried Criteria in Patients Undergoing Left Ventricular Assist Device Therapy. Am J Cardiol. 2017;120(8):1349–1354. [DOI] [PubMed] [Google Scholar]
- 42.Johnston A, Mesana TG, Lee DS, Eddeen AB, Sun LY. Sex Differences in Long-Term Survival After Major Cardiac Surgery: A Population-Based Cohort Study. Journal of the American Heart Association. 2019;8(17):e013260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barksdale DJ, Newhouse R, Miller JA. The Patient-Centered Outcomes Research Institute (PCORI): information for academic nursing. Nurs Outlook. 2014;62(3):192–200. [DOI] [PubMed] [Google Scholar]
- 44.Goldfarb M, Marcano Y, Schafer D, et al. Dietary protein intake in older adults undergoing cardiac surgery. Nutrition, Metabolism and Cardiovascular Diseases. 2019;29(10):1095–1100. [DOI] [PubMed] [Google Scholar]


