Abstract
Abstract
To date, no meta-analytical study evaluating the benefits of resistance exercise intervention on muscular strength and power and functional capacity in acute hospitalized older adults was conducted. Then, to synthesize the emerging evidence on the effects of resistance exercise intervention on muscular strength and power and functional capacity in acute hospitalized older adults, two independent authors performed a systematic search (PubMed, Scopus, Web of Science, and SciELO) until January 2021. Randomized clinical trials were included regarding the effects of resistance exercise and hospital usual care. The Cochrane Collaboration assessment tool was used to analyze the risk of bias. The comparisons included muscular strength (isometric handgrip strength and one-repetition maximum test of leg press), muscular power (output during leg press exercise), and functional capacity (timed-up-and-go, and short physical performance battery). Resistance exercise intervention increased muscular strength (isometric handgrip strength: mean difference = 2.50 kg, 95% confidence interval (CI) = 1.33, 3.67; and one-repetition maximum test of leg press: mean difference = 19.28 kg, 95% confidence interval = 14.70, 23.86) and muscular power (mean difference = 29.52 W, 95% confidence interval = 28.84, 30.21), and functional capacity (timed-up-and-go: mean difference = 3.40 s, 95% confidence interval = 0.47, 6.36; and short physical performance battery: mean difference = 1.29 points, 95% confidence interval = 0.10, 2.48) at discharge compared with hospital usual care. This meta-analysis endorses the increase of muscular strength and power gains and improving the functional capacity in favor of resistance exercise intervention in acute hospitalized older adults.
Trial Registration
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020203658
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00446-7.
Keywords: Aging, Inflammation, Older adults, Physical performance, Strength training
Introduction
One in four adults will be aged over 65 years by 2050 [1]. Considering that cumulative effects during the aging process are associated with decreases in muscular strength and power, functional capacity, and muscle mass [2], older people become more vulnerable to the risk of injuries, falls, fractures, infections, and numerous complications related to chronic diseases [3, 24, 40], leading to an increase in hospitalization [4]. Although hospitalization may be a crucial life-saving strategy, the length of time a patient is bedridden can increase the risk of infection [3, 8] and muscle disuse-induced atrophy [6].
Many acute hospitalized older adults present lower physical activity levels [7, 8], rapid muscular strength, and power declines [9, 10], which are key elements to perform basic activities of daily living such as walking, balance, and standing from a seated position [6, 11, 12]. According to this approach, the development of strategies aiming to prevent muscular strength and power decreases, hospitalized-associated disability in older adults should be primary focus in the field of health care [6, 11]. Among strategies to provoke changes in the muscular strength and power, and functional capacity in acute hospitalized older adults, resistance exercise (RE) appears to be an attractive alternative when compared to hospital usual care [13–15].
In this regard, recent studies have supported the effectiveness of RE intervention as a safe alternative strategy to change functional capacity during hospitalization in older adults [13, 15–18, 37, 38]. For instance, individualized and multicomponent (structured) RE intervention performed during a short-time (5–7 days consecutively) promotes significant changes in functional capacity by mitigate the muscular strength and power decline over hospital usual care in older patients [13, 15–18]. Although qualitative synthesis has demonstrated benefits in favor of RE intervention compared with hospital usual care, to date, no meta-analytical study evaluating the changes of RE intervention over hospital usual care on muscular strength and power (stronger predictors of functional limitations), as well functional capacity (e.g., walking, balance, and standing from a seated position) in acute hospitalized older adults was conducted [14]. Therefore, this systematic review and meta-analysis aimed to evaluate the changes provoked by structured RE intervention (randomized clinical trials) on muscular strength and power and functional capacity (including direct measurements to assess balance, walking, and agility) when compared to usual care in acute hospitalized older adults.
Methods
Data sources and searches approach
This systematic review was performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19] registered on the International Prospective Register of Systematic Reviews (PROSPERO 2020 CRD42020203658). English language articles by title and abstract were retrieved from the earliest record up to January 2021 on PubMed/MEDLINE, Scopus, Web of Science, and SciELO by two independent authors (MASC and ALS). The search strategy combined the following terms: (“Aged” OR “Older people” OR “Older adults” OR “Older hospitalized”) AND (“Hospitalization” OR “Hospital-based” OR “Hospital admission”) AND (“Exercise therapy” OR “Physical exercise” OR “Exercise program” OR “Exercise” OR “Physical activity” OR “Training”) AND (“Muscle mass” OR “Muscular strength” OR “Muscle strength” OR “Muscle power” OR “Physical function” OR “Functional capacity” OR “Functional performance” OR “Physical performance” OR “Balance” OR “Mobility” OR “Gait speed”). However, we did not find randomized clinical trials involving muscle mass assessment using direct measurements (e.g., magnetic resonance imaging, ultrasound, dual-energy X-ray absorptiometry), RE intervention, and acute hospitalized older adults. In addition, gray literature (e.g., abstracts, conference papers, and editorials) was excluded. In case of disagreements, a third reviewer evaluated the article (CMCF).
Study selection
Two independent authors (MASC and ALS) performed the systematic search and completed the study selection. The eligibility criteria were determined according to PICOS (population, intervention, comparators, outcome, and study design). Randomized clinical trials (RCT) in hospitalized older people (defined as age ≥ 65 years) [42] were examined, comparing RE intervention with hospital usual care and reporting muscular strength and/or power and functional capacity (balance, mobility, or gait speed). Hospital usual care was characterized by daily medical assessment, standard medical and pharmacological care therapy (including antibiotic, systemic steroids, inhaled bronchodilators, and oxygen), and full-time nursing assistance [37, 38]. Noteworthy, the geriatricians may orientated to the patient to perform standard physical rehabilitation (mainly focused on walking exercises) for mitigate functional capacity declines [13, 15–18]. However, hospitalized older adults with usual care spend most time in bed, even the individual can walk without the help of a nurse (walk approximately 600 steps per day (only 12 min daily walking)] [34, 35]. Initially, the publications were first retrieved and preliminary screened by title and abstract. After exclusion of duplicate publications, the identified articles were included in the review if they matched the following criteria: (a) RCT study; (b) hospitalized older adults undergoing acute medical illness; (c) structured RE intervention (exercises performed against resistance) performed in the hospital compared to hospital usual care; (d) measurements of isometric handgrip strength (HGS) and/or one-repetition maximum test (1RM test), timed-up-and-go test (TUG), Short Physical Performance Battery (SPPB), including sit-to-stand test (muscular power), balance and gait speed. Then, studies were excluded following exclusion criteria: (1) patients with chronic respiratory, circulatory, infectious, renal, urological, neurological, gastrointestinal, and musculoskeletal disorders—also cancer and HIV patients undergoing treatment; (2) intervention using the vibrating platform, Tai Chi Chuan, dance, exergames, and physical activities as exercise; (3) use of nutritional supplementation during hospitalization; (4) absence of information on the evaluations of the studied outcomes. The agreement between MASC and ALS was kappa = 0.89, P < 0.001. Eventual disagreements were discussed with a third author (CMCF).
Data extraction and quality assessments
The quality of included studies was performed using the “risk of bias” assessment tool of the Cochrane Collaboration. The quality of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias were classified as high (“ + ”), low (“- “), or unclear (“?”) risk of bias [20]. Quality assessments of both reviewers were compared, and disagreements in the scores were resolved by discussion. Two authors (MASC and ALS) independently extracted the following data from each study for analysis: author/year, number of participants within each group, baseline participants’ characteristics, intervention details, pre- and post-data from all outcomes. In circumstances when standard deviations were not available, these values were calculated using traditional statistical methods, assuming a correlation of 0.50 between the baseline and post-intervention scores within each subject [21]. Similarly, when studies reported standard error, the values were converted to standard deviation (SD). Hozo’s equations [22] were used to estimate mean and SD in the investigations with non-parametric data reporting median and range.
Data syntheses and analyses
Meta-analysis was conducted using Review Manager Software (RevMan software package version 5.4). RevMan was used to calculate the effect size of RE intervention on isometric HGS, 1RM leg press, and SPPB in hospitalized older people. The variation (pre-minus post-intervention) from all included studies was used to calculate the mean difference and 95% confidence interval (CI) and these were conducted using the DerSimonian-Laird random-effects inverse variance model all outcomes [39, 40]. Weighted percentages were based on the sample sizes of respective studies. Statistical significance was assumed as P < 0.05 in a Z test analysis to examine whether effect size was significantly different from zero. Study heterogeneity was evaluated using the I2 statistic, and Cochrane’s Q. Values of I2 higher than 50 and 75% were considered moderate and high heterogeneity. For Cochrane’s Q, significant heterogeneity exists when the Q value exceeds the degrees of freedom (df) of the estimate. Moreover, publication bias was tested visually using a funnel plot. Effect sizes were calculated, and values of 0.00–0.19 were considered trivial, 0.20–0.49 as small, 0.50–0.79 as moderate, and > 0.80 as large. Sensitivity analyses were performed by excluding one trial at a time according to the risk of bias to test the robustness of the pooled results. Forest plots were generated to illustrate the between study-level effect sizes along with a 95% CI [41].
Results
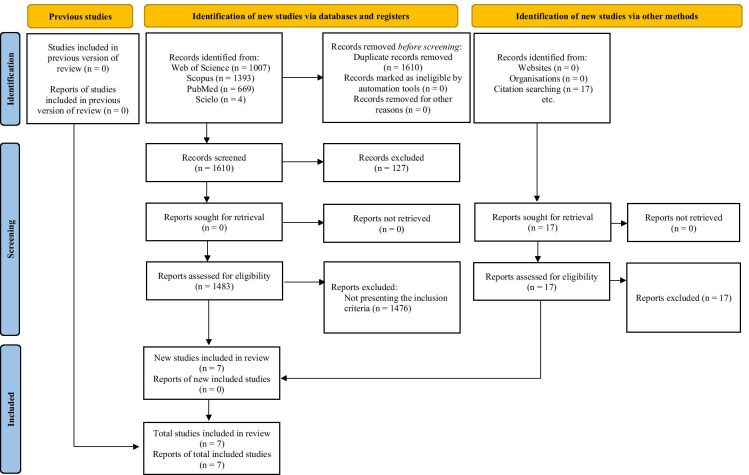
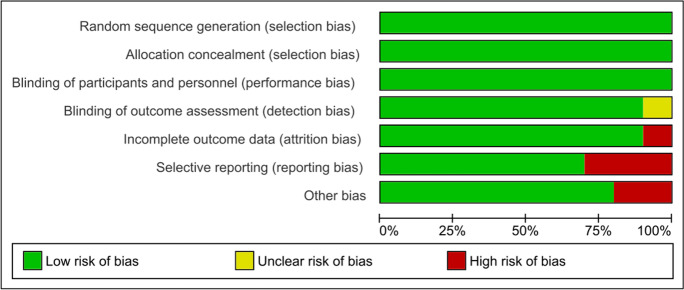
The selection processes retrieved 3090 full-text as documented in the PRISMA flow diagram (Fig. 1). After excluding abstracts, conference papers, editorials, and duplicated reviews and meta-analysis studies, 1483 studies were then assessed according to PICOS eligibility criteria. Afterward, 1476 studies were excluded for not presenting the inclusion criteria (e.g., no structured RE intervention, use of protein supplementation during hospitalization, absence of dependent variables of interest). Therefore, a total of 7 RCT’s were included in the qualitative synthesis for meta-analysis [i.e., muscular strength measure by isometric HGS and (or) 1RM leg press, muscular power measure by leg-peak power, and functional capacity measure by TUG], whereas a total of 4 studies were included in the quantitative synthesis (i.e., functional capacity measure by SPPB scale). Moreover, all studies included in both qualitative and quantitative syntheses present a low risk of bias (Fig. 2).
Fig. 1.
PRISMA flow diagram of the study selection process
Fig. 2.
Risk of bias summary
Participants’ characteristics
The participants’ characteristics included in the systematic review and meta-analysis are presented in Table 1. Two thousand four hundred ninety-eight patients with a range between 65 to 102 years (male and female) were hospitalized for acute medical illness. Most studies included acute hospitalized older adults for different medical conditions (e.g., fall, respiratory, circulatory, infectious, renal, urological, neurological, gastrointestinal, and musculoskeletal conditions). Moreover, all participants presented low functional capacity [according to score obtained by SPPB scale and (or) Barthel Index scores, and another physical performance tests]. However, there were no adverse effects (i.e., signs or symptoms) reported by participants during the trials, and no patient had to interrupt the intervention. Finally, the average length of stay was between 5 and 12 days (median = 8).
Table 1.
Study characteristics included in the systematic review and meta-analysis
| Study (country) | Sample | Protocol intervention | Endpoints | Main outcomes |
|---|---|---|---|---|
| Martínez-Velilla et al. 2020 (Spain) |
UC: n = 49 patients (28 female) RE: n = 54 patients (25 female) Mean age: ~ 86 years (≥ 75 years) Diseases: type II diabetic patients, respiratory, cardiovascular, neurological, musculoskeletal, urinary, and other Patients with low physical function |
UC: Occasionally, the patients performed a standard physiotherapy focused on walking exercises as recommended by the geriatricians RE: Twice-day supervised sessions performed whole-body exercises (at 30–60% of 1RM) during 20 min per session |
Functional capacity assessment: SPPB scale Muscular strength assessment: isometric HGS |
UC: ↔ SPPB scale, ↔ isometric HGS; RE: ↑ SPPB scale, ↑ isometric HGS |
|
McCullagh et al. 2019 (Ireland) |
UC: n = 95 patients (39 female) RE: n = 95 patients (41 female) Mean age: ~ 80 years (≥ 65 years) Diseases: lower impairment in several systems, according to Cumulative Illness Rating Scale-Geriatrics Patients with low physical function |
UC: Composed by breathing and stretching exercises RE: Once-day session performed whole-body exercises using body weight as external resistance during 20–40 min per session |
Functional capacity assessment: SPPB scale |
UC: ↑ SPPB scale, RE: ↑↑ SPPB scale |
|
Morton et al. 2007 (Australia) |
UC: 126 patients (68 female) RE: 110 patients (61 female) Mean age: ~ 79 years (≥ 65 years) Diseases: respiratory, circulatory, digestive, genitourinary, and other Patients with low physical function |
UC: Daily medical assessment, 24–h nursing assistance, and allied health service on referral from medical, nursing or other allied health staff RE: Twice-day supervised sessions performed whole-body exercises using body weight as external resistance during 20–30 min |
Functional capacity assessment: TUG |
UC: ↔ TUG RE: ↔ TUG |
|
Ortiz-Alonso et al. 2019 (Spain) continue |
UC: 125 patients (67 female) RE: 143 patients (86 female) Mean age: ~ 88 years (75–102 years) Diseases: respiratory, circulatory, renal, neurological, digestive, and falls Patients with low physical function |
UC: Occasionally, the patients performed a standard physiotherapy focused on walking exercises as recommended by the geriatricians RE: Twice-day sessions performed whole-body exercises using body weight as external resistance during ~ 20 min |
Functional capacity assessment: SPPB scale |
UC: ↔ SPPB scale, RE: ↑ SPPB scale |
|
Raymond et al. 2017 (Australia) |
UC: 232 patients (134 female) RE: 236 patients (149 female) Mean age: ~ 84 years (≥ 65 years) Diseases: respiratory, renal, circulatory, neurological, dementia, musculoskeletal, and other (e.g., fall) Patients with low physical function |
UC: Comprised individual physiotherapy sessions (gait retraining, aerobic, balance and strength exercises, range of movement, transfers and stairs practice) RE: Once-day supervised sessions performed whole-body exercises using body weight as external resistance (two sets of 8–12 repetition maximum) |
Functional capacity assessment: TUG |
UC: ↔ TUG; RE: ↔ TUG |
|
Sáez de Asteasu et al. 2019 (Spain) |
UC: 65 patients (32 female) RE: 65 patients (32 female) Mean age: ~ 87 years (≥ 75 years) Diseases: respiratory, circulatory, infectious, gastrointestinal, neurologic, and other Patients with moderate dependence |
UC: Occasionally, the patients performed a standard physiotherapy focused on walking exercises as recommended by the geriatricians RE: Twice-day supervised sessions performed whole-body exercises using specific equipment (at 30–60% of 1RM) during 20 min per session |
Functional capacity assessment: SPPB scale Muscular strength assessment: 1RM leg press exercise Muscular power assessments: Muscle power leg press exercise at 50% of 1RM |
UC: ↔ SPPB scale, ↔ sit-to-stand test, ↔ 1RM leg press exercise, ↔ muscle power; RE: ↑ SPPB scale, ↑ sit-to-stand test, ↑ 1RM leg press exercise, ↑ muscle power leg press exercise at 50% of 1RM |
|
Sáez de Asteasu et al. 2020 (Spain) |
UC: 185 patients (109 female) RE: 185 patients (100 female) Mean age: ~ 87 years (75–101 years) Diseases: respiratory, circulatory, infectious, gastrointestinal, neurologic, and other Patients with low physical function |
UC: Occasionally, the patients performed a standard physiotherapy focused on walking exercises as recommended by the geriatricians RE: Twice-day supervised sessions performed whole-body exercises using specific equipment (at 30–60% of 1RM) during 20 min per session |
Muscular strength assessment: 1RM leg press exercise Muscular power assessment: Muscle power leg press exercise at 45% of 1RM |
UC: ↔ 1RM leg press exercise, ↔ muscle power leg press exercise at 45% of 1RM; RE: ↑ 1RM leg press exercise, ↑ muscle power leg press exercise at 45% of 1RM |
UC hospital usual care, RE resistance exercise, SPPB short physical performance battery, HGS handgrip strength, TUG timed-up-and-go test, 1RM one-repetition maximum test.
Intervention characteristics
Three RCTs were performed with RE progressive intervention using specific equipment (i.e., machine, external load, or cycle ergometer) and four RCTs were composed by body weight RE intervention (Table 1). On average, RE intervention were performed during 20–40 min per session and 5–7 days consecutively per week. Additionally, in five RCTs, the RE intervention were performed more than once per day (up to two times per day).
Muscular strength and power
Three RCTs assessed endpoint-related muscular strength using isometric HGS or 1-RM leg press (Table 2). Regarding muscular strength, RE intervention increased HGS [mean difference = 2.50 kg, 95% CI (1.33, 3.67), heterogeneity: not applicable, I2 = 78%, P = 0.029] and 1RM leg press [mean difference = 19.28 kg (14.70, 23.86), heterogeneity: P = 0.005, I2 = 87%, P < 0.001)]. Two RCTs assessed endpoint-related muscular power by output during leg press exercise at lower-load intensity (Table 2). Hence, RE intervention increased muscular power [mean difference = 29.52 W (28.84, 30.21), heterogeneity: P = 0.54, I2 = 0%, P < 0.001)].
Table 2.
Meta-analysis performed on the effects of resistance exercise intervention compared to hospital usual care on muscular strength and power, and functional capacity in acute hospitalized older adults
| Outcomes | k | Mean difference (95% CI) | I2 | P |
|---|---|---|---|---|
| Isometric HGS (kg) | 1 | 2.50 (1.33, 3.67) | 78% | 0.029* |
| 1RM leg press (kg) | 2 | 19.28 (14.70, 23.86) | 87% | < 0.0001* |
| Leg-peak of power (W) | 2 | 29.52 (28.84, 30.21) | 0% | < 0.0001* |
| Time-up-and-go (s) | 2 | 3.40 (0.47, 6.36) | 93% | 0.020* |
| Test for overall effect | 7 | 14.31 (6.44, 22.18) | 99% | 0.0008* |
Calculation based on random-effects model. Results are expressed as mean difference and 95% confidence intervals (95% CI). k number of studies included in effect, I2 heterogeneity, kg kilogram, W watts, s second, isometric HGS handgrip strength, 1RM one-repetition maximum test.
*P < 0.05 in favor RE intervention vs. favor hospital usual care treatment.
Functional capacity
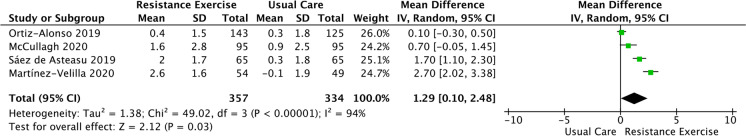
Six RCTs assessed endpoint-related functional capacity by TUG (Table 2) and SPPB scale (Fig. 3). Overall, the RE intervention improves TUG [mean difference = 3.40 s, 95%CI (0.47, 6.36), heterogeneity: P < 0.001, I2 = 93%, P = 0.0200] (Table 2) and SPPB scale [mean difference = 1.29 points, 95%CI (0.10, 2.48), heterogeneity: P < 0.0001, I2 = 94%, P < 0.001] (Fig. 3).
Fig. 3.
Meta-analysis performed on the effects of resistance exercise compared to hospital usual care on short physical performance battery in acute hospitalized older adults. Notes: Calculation based on a random-effects model. Results are expressed as mean difference and 95% confidence intervals (95% CI). k, kappa coefficient; I2, heterogeneity
Discussion
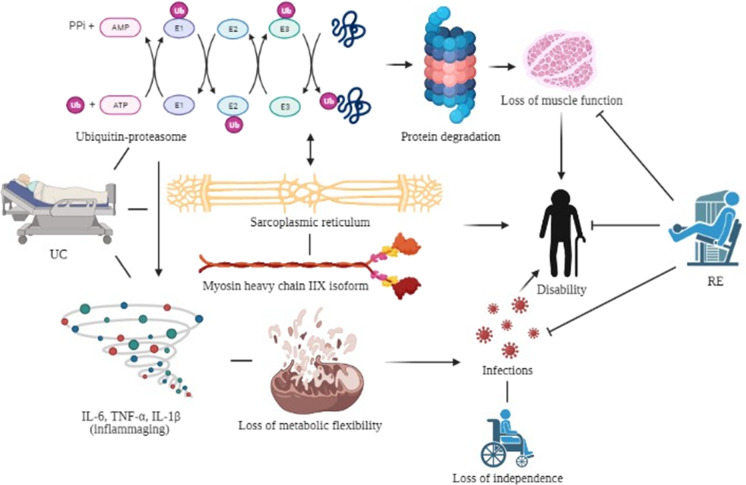
To date, and the best of our knowledge, this study is the first summarized meta-analytical evidence supporting the effectiveness of RE intervention on muscular strength and power, and functional capacity in acute hospitalized older adults. Overall, these findings may be explained, at least in part, by the daily frequency of RE intervention. Most studies (5/7 RCTs) reported that hospitalized patients had exercised twice a day (morning and evening) since the RE frequency of the stimulus appears to provide cumulative benefits in acute hospitalized older people. On the other hand, none participants have related signs or symptoms of adverse effects during the intervention. In addition, no patient dropout during the intervention, indicating high compliance. Therefore, this meta-analysis highlights the importance of including RE intervention for acute hospitalized older adults as primary focus in the field of health care to improve muscular strength and power and functional capacity (Fig. 4).
Fig. 4.
Potential mechanisms involved in mitigating deleterious effect induced by resistance exercise intervention over usual care treatment during the acute hospitalization in older adults
The decline in muscular strength with advancing age is widely recognized as an essential factor contributing to a longer length of stay in hospital and earlier death [23, 24]. Thus, intervention strategies to improve the muscular strength of acute hospitalized older people are required [14]. In the current study, RE intervention shows isometric HGS and 1RM leg press increase over hospital usual care in acute hospitalized older adults. In this regard, our result is important because hospitalization condition in older adults is associated with functional decline [10, 11]. Also, hospitalization-associated disability is related to impairments on general health in older adults [6, 8]. Therefore, our findings indicate that RE might be an effective intervention strategy to obtain improvements in muscular strength (at upper and lower limbs), and consequently attenuating and (or) preventing the loss of muscular strength in acute hospitalized older adults (Fig. 4) [25–27].
The muscular power has been demonstrated to be a fundamental element to perform basic activities of daily living such as walking, balance, and standing from a seated position [6, 11, 12]. Hence, a structured exercise program applied during acute hospitalization might prevent muscular power declines in older adults. Our study demonstrated that RE intervention appears to be effective and safe compared to hospital usual care in improving muscular power by output during leg press exercise at the lower-load intensity in acute hospitalized older adults. Our findings have important clinical implications because muscular power declines at an earlier and faster rate during aging than muscular strength [23, 28]. Indeed, muscular power has been more strongly associated with a decline in functional capacity than muscular strength in older adults [29, 30]. Moreover, the muscular power output plays an important mediator role on functional capacity endpoints in acute hospitalized older adults [31]. Thus, the RE intervention presents potential therapeutic and functionally effects on improvement muscular power in acute hospitalized older adults (Fig. 4).
Effective strategies which improve functional capacity or delay further declines in acute hospitalized older adults by healthy lifestyle practices featuring regular exercise are needed [32]. In the current meta-analysis, only RE improved SPPB scale and TUG (strong predictors of functional capacity and fragility) in acute hospitalization older adults. According to this approach, RE intervention is the most robust overall evidence regarding functional capacity-preserving effects with aging, especially in acute hospitalization in older adults [14]. Our findings support that decline in functional capacity may be mitigated by RE in this population. Therefore, evidence endorses the RE prescription to promote health span extension and should be considered a frontline intervention to prevent harmful effects on hospitalization (e.g., infection and mortality) in older adults [43] (Fig. 4).
Limitations and strengths
While we are confident that RE intervention is a promising strategy to improve muscular strength and power and functional capacity during acute hospitalization, some limitations of this meta-analysis must be presented. The aspect that should not be overlooked is the small number of studies included in the analysis (7 RCTs). Although RE intervention has emerged as a strategy to improve muscle function outcomes, fewer studies investigated the effect of RE intervention in acute hospitalized older adults. In contrast, most studies included in this meta-analysis presented a low risk of bias due to a robust methodological approach and individualized orientation by health and fitness professionals. However, there is a need to be cautious with general interpretation due to studies heterogeneity (moderate to high). The strong point in this meta-analysis is pioneering on evaluating the effects of RE intervention which most patients completed the intervention with high compliance. In summary, our findings suggest that RE intervention is an effective and safe intervention in acute hospitalized older adults [14, 33].
Future perspectives
Muscular strength is considered a global measure of overall health status. Furthermore, a causal relationship between muscular strength and functional decline can be argued, even when the mechanisms involved are unclear. Noteworthy, as vital signs, when sure functional signs display abnormalities, clinicians should be encouraged to search for subjacent mechanisms related to the cross-talk axis muscle-adipose-brain. Future studies should consider investigating the role of RE intervention on muscle mass (pleiotropic effects), adiposity (potent regulator of inflammatory response), and fast walking speed (a strong predictor of death risk-related) in acute hospitalized older adults.
Conclusion
This meta-analysis suggests that RE intervention can increase muscular strength and power and improving functional capacity in acute hospitalized older adults. Moreover, acute hospitalized older patients undergoing RE intervention have strong protection against harmful effects.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
MASC and CSP contributed in planning. MASC, CMCF, ALS, PCS, and GK conducted data analysis, synthesis, and reporting of the work. MASC, CMCF, ALS, PCS, GK, and CSP contributed in data synthesis and revising of the work. MASC, CMCF, and CSP contributed in data extraction. MI, ESC, and CSP contributed in revising the work.
Funding
This study was supported by National Council of Technological and Scientific Development (CNPq/Brazil) and São Paulo Research Foundation (FAPESP/Brazil). MASC received a doctorate scholarship from the CNPq (Process 140473/2020–3), and CSP received a Post Doctorate scholarship from the FAPESP (Process 2018/23402–0). M.I. is funded in part by a research grant PI17/01814 of the Ministerio de Economía, Industria y Competitividad of Spain (ISCIII, FEDER).
Data availability
The data and materials analyzed during the present study are available from corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Open access
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shaw AC, Joshi S, Greenwood H, et al. Aging of the innate immune system. Curr Opin Immunol. 2010;22(4):507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guadalupe-Grau A, Carnicero JA, Gómez-Cabello A, et al. Association of regional muscle strength with mortality and hospitalisation in older people. Age Ageing. 2015;44(5):790–795. doi: 10.1093/ageing/afv080. [DOI] [PubMed] [Google Scholar]
- 4.Gill TM, Gahbauer EA, Han L, et al. The role of intervening hospital admissions on trajectories of disability in the last year of life: prospective cohort study of older people. BMJ. 2015;20(5):350–361. doi: 10.1136/bmj.h2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulop T, Dupuis G, Baehl S, et al. From inflamm-aging to immune-paralysis: a slippery slope during aging for immune-adaptation. Biogerontology. 2016;17(1):147–157. doi: 10.1007/s10522-015-9615-7. [DOI] [PubMed] [Google Scholar]
- 6.Covinsky KE, Pierluissi E, Johnston CB, et al. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 7.Pavon JM, Sloane RJ, Pieper CF, et al. Accelerometer-measured hospital physical activity and hospital-acquired disability in older adults. J Am Geriatr Soc. 2020;68(2):261–265. doi: 10.1111/jgs.16231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-Velilla N, Urbistondo-Lasa G, Veintemilla-Erice E, et al. Determining the hours hospitalised patients are bedridden due to their medical condition and functional impairment and secondary mortality. Rev Esp Geriatr Gerontol. 2013;48(2):96. doi: 10.1016/j.regg.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Mudge AM, O’Rourke P, Denaro CP. Timing and risk factors for functional changes associated with medical hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2010;65(8):866–872. doi: 10.1093/gerona/glq069. [DOI] [PubMed] [Google Scholar]
- 10.Fortinsky RH, Covinsky KE, Palmer RM, et al. Effects of functional status changes before and during hospitalization on nursing home admission of older adults. J Gerontol A Biol Sci Med Sci. 1999;54(10):521–526. doi: 10.1093/gerona/54.10.m521. [DOI] [PubMed] [Google Scholar]
- 11.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 12.Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455–461. doi: 10.1016/j.jamda.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortiz-Alonso J, Bustamante-Ara N, Valenzuela PL, et al. Effect of a simple exercise program on hospitalization-associated disability in older patients: a randomized controlled trial. J Am Med Dir Assoc. 2020;21(4):531–537. doi: 10.1016/j.jamda.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Valenzuela PL, Morales JS, Castillo-García A, et al. Effects of exercise interventions on the functional status of acutely hospitalised older adults: a systematic review and meta-analysis. Ageing Res Rev. 2020;61(8):101076. doi: 10.1016/j.arr.2020.101076. [DOI] [PubMed] [Google Scholar]
- 15.Saez de Asteasu ML, Martínez-Velilla N, Zambom-Ferraresi F, et al. Physical exercise improves function in acutely hospitalized older patients: secondary analysis of a randomized clinical trial. J Am Med Dir Assoc. 2019;20(7):866–873. 10.1016/j.jamda.2019.04.001. [DOI] [PubMed]
- 16.Martínez-Velilla N, Valenzuela PL, Sáez de Asteasu ML, et al. Effects of a tailored exercise intervention in acutely hospitalized oldest old diabetic adults: an ancillary analysis. J Clin Endocrinol Metab. 2021;106(2):e899-e906. 10.1210/clinem/dgaa809. [DOI] [PubMed]
- 17.McCullagh R, O’Connell E, O’Meara S, et al. Augmented exercise in hospital improves physical performance and reduces negative post hospitalization events: a randomized controlled trial. BMC Geriatr. 2020;7(2):20–46. doi: 10.1186/s12877-020-1436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saez de Asteasu ML, Martínez‐Velilla N, Zambom‐Ferraresi F, et al. Changes in muscle power after usual care or early structured exercise intervention in acutely hospitalized older adults. J Cachexia Sarcopenia Muscle. 2020;11(4):997–1006. 10.1002/jcsm.12564. [DOI] [PMC free article] [PubMed]
- 19.Page MJ, McKenzie JE, Bossuyt PM, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;18(10):343. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 22.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;20(4):5–13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31(2):119–125. doi: 10.1093/ageing/31.2.119. [DOI] [PubMed] [Google Scholar]
- 25.Attaix D, Ventadour S, Codran A, et al. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–186. doi: 10.1042/EB0410173. [DOI] [PubMed] [Google Scholar]
- 26.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307(6):e469–e484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmassani ZS, Reidy PT, McKenzie AI, et al. Disuse-induced insulin resistance susceptibility coincides with a dysregulated skeletal muscle metabolic transcriptome. J Appl Physiol (1985). 2019;126(5):1419–1429. 10.1152/japplphysiol.01093.2018. [DOI] [PMC free article] [PubMed]
- 28.Reid KF, Pasha E, Doros G, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014;114(1):29–39. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Md JFB, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50(3):461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 30.Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55(4):192–199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 31.Saez de Asteasu ML, Martínez-Velilla N, Zambom-Ferraresi F, et al. Role of muscle power output as a mediator between gait variability and gait velocity in hospitalized older adults. Exp Gerontol. 2019;124:110631. 10.1016/j.exger.2019.110631 [DOI] [PubMed]
- 32.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594(8):2001–2024. doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramírez-Vélez R, García-Hermoso A, Martínez-Velilla N, et al. Effects of exercise interventions on inflammatory parameters in acutely hospitalized older patients: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2021;10(2):290. doi: 10.3390/jcm10020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullagh R, Dillon C, Dahly D, et al. Walking in hospital is associated with a shorter length of stay in older medical inpatients. Physiol Meas. 2016;37(10):1872. doi: 10.1088/0967-3334/37/10/1872. [DOI] [PubMed] [Google Scholar]
- 35.Fisher SR, Goodwin JS, Protas EJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59(1):91–95. doi: 10.1111/j.1532-5415.2010.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metter EJ, Talbot LA, Schrager M, et al. Arm-cranking muscle power and arm isometric muscle strength are independent predictors of all-cause mortality in men. J ApplPhysiol. 2004;96(2):814–821. doi: 10.1152/japplphysiol.00370.2003. [DOI] [PubMed] [Google Scholar]
- 37.Morton NA, Keating JL, Berlowitz DJ, et al. Additional exercise does not change hospital or patient outcomes in older medical patients: a controlled clinical trial. Aust J Physiother. 2007;53(2):105–111. doi: 10.1016/s0004-9514(07)70043-0. [DOI] [PubMed] [Google Scholar]
- 38.Raymond MJM, Jeffs KJ, Winter A, et al. The effects of a high-intensity functional exercise group on clinical outcomes in hospitalised older adults: an assessor-blinded, randomised-controlled trial. Age Ageing. 2017;46(2):208–213. doi: 10.1093/ageing/afw215. [DOI] [PubMed] [Google Scholar]
- 39.Hedges LV, Olkin I. Statistical methods for meta-analysis Academic press. 2014 doi: 10.2307/1164953. [DOI] [Google Scholar]
- 40.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane, updated February 2021. Available from www.training.cochrane.org/handbook.
- 41.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Integrated care for older people: guidelines on community-level interventions to manage declines in intrinsic capacity. 2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK488250/. [PubMed]
- 43.Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, Aubertin-Leheudre M, Bernabei R, Cadore EL, Cesari M, Chen LK, de Souto Barreto P, Duque G, Ferrucci L, Fielding RA, García-Hermoso A, Gutiérrez-Robledo LM, Harridge SDR, Kirk B, Kritchevsky S, Landi F, Lazarus N, Martin FC, Marzetti E, Pahor M, Ramírez-Vélez R, Rodriguez-Mañas L, Rolland Y, Ruiz JG, Theou O, Villareal DT, Waters DL, Won Won C, Woo J, Vellas B, Fiatarone Singh M. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. 2021;25(7):824–853. doi: 10.1007/s12603-021-1665-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and materials analyzed during the present study are available from corresponding author on reasonable request.
Not applicable.