Abstract
Background
Recent studies have shown an increased risk of acute kidney injury (AKI) induced by vancomycin + piperacillin-tazobactam (VPT) combination. In this study, the efficacy of intravenous magnesium sulfate in prevention of VPT induced AKI in critically ill patients admitted to the ICU has been evaluated.
Methods
In an open-label, placebo-controlled, randomized clinical trial, 72 adults (≥ 18 years old) who had indications to receive VPT as empiric therapy were assigned to the magnesium or control group in 1:1 ratio. Concomitant with VPT, intravenous infusion of magnesium sulfate was started for patients in the magnesium group. The target serum level of magnesium was defined 3 mg/dl. Patients in the control group received normal saline as placebo. The target serum level of magnesium was defined 1.9 mg/dl in this group. The study’s primary outcome was incidence of AKI during and up to 48 h after the treatment course. Escalation and de-escalation of VPT regimen, duration of hospitalization, length of ICU stay and 28-day mortality were secondary outcomes.
Results
Thirty patients in each group completed the examination. Five patients in the magnesium group and 11 patients in the control group experienced AKI (p = 0.072). De-escalation of VPT regimen was done approximately in 60% of patients. Duration of hospitalization and length of ICU stay were not statistically different between the groups. Finally, 28-day mortality was 23.33% in each group. Although the incidence of AKI was not statistically different between the groups in unadjusted logistic regression model, it became significant after adjusting for confounding factors [unadjusted model (OR = 0.34; 95% CI: 0.10–1.16, p = 0.084), adjusted model: (OR = 0.26; 95% CI: 0.07–0.96, p = 0.04)].
Conclusions
Administration of magnesium sulfate with the target serum levels around 3 mg/dL reduced the incidence of AKI in critically ill patients who were receiving VPT as empric therapy.
Graphical abstract
Keywords: Vancomycin, Piperacillin-tazobactam, Acute kidney injury, Magnesium
Introduction
Vancomycin + piperacillin-tazobactam (VPT) is widely used as an empiric regimen for treatment of infections in critically ill patients [1, 2]. Recent studies have shown an increased risk of acute kidney injury (AKI) induced by vancomycin in concomitant with piperacillin-tazobactam (PT) in both critically ill [3] and non-critically ill patients [4]. According to the Food and Drug Administration recommendation, VPT combination should be administered with caution. Monitoring of the kidney function is suggested during the treatment course [5].
The first meta-analysis in this regard was published in 2016 [6]. The next systematic review and meta-analysis in 2017, showed that the use of VPT is associated with threefold increase in the risk of AKI [7]. Also in 2018, two systematic reviews and meta-analyses were published in this topic [8, 9].
The probable mechanism of vancomycin induced nephrotoxicity is production of reactive oxygen species (ROS) and induction of oxidative stress [10]. On the other hand, a well-known type of nephrotoxicity induced by beta-lactam antibiotics is acute interstitial nephritis [11]. However, the exact mechanism of increased risk of AKI following administration of VPT is not clear. One hypothesis is an additive toxicity. Also, it is suggested that PT enhances the accumulation of vancomycin in the proximal tubule [11].
Nephrotoxicity associated with VPT can cause negative outcomes including increase in duration of hospital stay, medical costs, morbidity and mortality. Approximately 20% of the patients with drug-induced AKI required dialysis and mortality was about 25% in these patients. It should also be noted that multiple episodes of AKI can increase the risk of progression to chronic kidney disease [12].
One of the strategies to prevent AKI induced by VPT combination is the use of PT alternatives including cefepime and carbapenems. The combination of vancomycin and cefepime is rationale when the local data show microorganism susceptibility, otherwise the use of a carbapenem is preferred. The routine use of carbapenems is associated with the development of resistant pathogens [11]. So, different strategies should be considered to reduce the risk of AKI induced by VPT.
Various antioxidants were examined for prevention of AKI induced by nephrotoxins in preclinical models. For vancomycin, some compounds including erythropoietin, curcumin, vitamin C and E, cilastatin and melatonin were assessed [13]. Despite the primary promising effects, it is essential to be tested in the clinical settings.
Magnesium is the second intracellular and the fourth most common cation in the body [14]. As a cofactor, it has a key role in several enzymatic reactions. It also involved in regulation of muscles tone, ion transport across the membrane, release of neurotransmitters and integrity of the cellular membranes [14, 15]. Considering antioxidant and anti-inflammatory properties of magnesium and also hypomagnesemia as a common electrolyte imbalance in critically ill patients, magnesium supplementation can be a promising option in prevention of AKI induced by VPT [15, 16].
In this open-label, placebo-controlled, randomized clinical trial, the efficacy of intravenous (IV) magnesium sulfate in prevention of vancomycin + piperacillin-tazobactam induced acute kidney injury was evaluated.
Methods
Study design and participants
This study was designed as an open-label, placebo-controlled, randomized clinical trial to evaluate efficacy of intravenous magnesium sulfate in prevention of AKI induced by VPT in critically ill patients. It was performed from October 2019 to July 2020 in Imam Khomeini Hospital Center, Tehran, Iran. The ethics Committee of Tehran University of Medical Sciences approved the study (IR.TUMS.TIPS.REC.1398.080). Also, it was registered as a clinical trial (register ID: IRCT20100228003449N26). Informed consent was obtained from all participants or their first-degree relatives.
Critically ill patients (aged ≥ 18 years old) with creatinine clearance (CrCl) more than 30 ml/min [calculated according Cockcroft-Gault formula → CrCl= (× 0.85 for females)] who had indications to receive VPT as empiric therapy have been included. Patients with CrCl less than 30 ml/min, indications for renal replacement therapy prior to the enrolment and history of serious hypersensitivity reactions to vancomycin or beta-lactams were not eligible. Also patients were excluded during the study course if 1) received VPT treatment for less than 48 h, 2) died or discharged from ICU within 48 h of the inclusion and 3) with signs and symptoms of hypermagnesemia including neuromuscular (muscular paralysis, decreased deep tendon reflexes and apnea) or cardiovascular (systolic blood pressure less than 90 mm Hg, heart rate < 60 beats /minute and arrhythmia).
Considering the incidence rate of AKI in the control (35%) and magnesium (5%) group, it was suggested that at least 27 patients in each group should be included (α = 0.05, β = 0.2 and power = 0.80). The numbers of 35% and 5% were incidence of AKI in patients who were treated with VPT and vancomycin alone respectively [13, 17].
Patients were randomly assigned (in 1:1 ratio) to the magnesium or control group. The method of randomization was permuted block randomization that has been done by a biostatistician (not involved in the trial). The biostatistician prepared a list of blocks with a size of 6 patients in each block and provided to the clinical investigators. According to the allocation ratio, three patients in each block were assigned to the magnesium and control groups.
Procedures
Vancomycin was initiated with a loading dose of 25 mg/kg and then 15 mg/kg every 8–12 h as maintenance regimen. The vancomycin dose was adjusted based on the serum trough level (target = 15–20 mcg/mL). Assessment of serum vancomycin level was done according chemiluminescent immunoassay method. Each gram of vancomycin was infused during at least 1 h. PT was administered as 4.5 g every 6 h. The first dose was infused over 30 min while the infusion time of next doses was 4 h.
Concomitant with VPT, infusion of magnesium sulfate was started in the magnesium group. The target serum level of magnesium was defined 3 mg/dL in this group. The required dose of magnesium sulfate was calculated according to the rule of thumb [18]. Based on this rule, each gram of magnesium sulfate is expected to increase the magnesium serum level by 0.18 mg/dL. Each gram of magnesium sulfate was diluted in 50 ml of normal saline and was infused over at least one hour. The cardiovascular and hemodynamic conditions of the patients were monitored during the infusion time. The serum level of magnesium was assessed twice to three times daily according Colorimetric (Xylidyl blue) method.
Patients in the control group received normal saline as placebo. The target serum level of magnesium was defined 1.9 mg/dL in this group. These patients received magnesium sulfate if the magnesium serum levels dropped to less than 1.9 mg/dL during the treatment course. For each patient, demographic data, underlying diseases, history of medications, acute physiology and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA) scores, baseline vital signs and laboratory data were recorded. The patients were daily followed in terms of the vital sings, laboratory parameters, microbial cultures, medications and intake/output throughout the treatment.
Outcomes
The primary outcome of study was incidence of AKI during and up to 48 h after the treatment course. The definition of AKI was based on the kidney disease improving global outcomes (KDIGO) criteria [19].
Escalation and de-escalation of VPT regimen, duration of hospitalization, length of ICU stay and 28-day mortality were considered as secondary outcomes.
Definitions
At least 0.3 mg/dl increase in serum creatinine within 48 h or at least 50% increase in serum creatinine relative to baseline within the previous 7 days was considered AKI.
Staging of AKI was done according to:
-
Stage 1:
1.5 to 1.9 times increase in serum creatinine relative to baseline
-
Stage 2:
2 to 2.9 times increase in serum creatinine relative to baseline
-
Stage 3:
3 times increase in serum creatinine relative to baseline or creatinine ≥ 4 mg/dl or need for renal replacement therapy.
The change of vancomycin to linezolid or piperacillin-tazobactam to a carbapenem was considered as the antibiotic regimen escalation. The change was considered according to the patient’s clinical conditions (worsening of signs and symptoms, hypotension, vasopressor requirement, increase in the dose of vasopressor and organ dysfunction) or microbial culture results.
The change of VPT to a narrow spectrum antibiotic regimen such as clindamycin + a fluoroquinolone was considered as de-escalation. Similar to the escalation, it was done according to the microbial culture results and clinical conditions of the patients (substantial improvement in the clinical status and organs' function and vasopressor weaning).
Non-susceptible pathogens to at least one antibiotic from at least three classes of antibiotics were defined as Multi-Drug Resistant (MDR) pathogens [20].
Susceptible pathogens to only two categories of antibiotics were considered as Extensively Drug Resistant (XDR) [20].
Statistical analysis
The continuous and categorical variables were shown as mean ± standard deviation (SD) and frequency (percentage) respectively. Two independent sample t-test and Fisher’s exact test were applied for comparison of continuous and categorical variables respectively.
The Odds Ratio (OR) and 95% confidence interval (CI) for the incidence of AKI was calculated by logistic regression. Due to the difference between the groups regarding sex and history of diabetes mellitus, the primary outcome was adjusted accordingly.
For comparison of the incidence of AKI between the groups, Kaplan–Meier curve was plotted and compared with the Log-Rank test.
The p-value < 0.05 was considered as statistical significance. All statistical analyses were performed by statistical package for social sciences (SPSS) version 21.0.
The analysis has been done as per-protocol and patients who received VPT for less than 48 h were not included.
Results
Patients
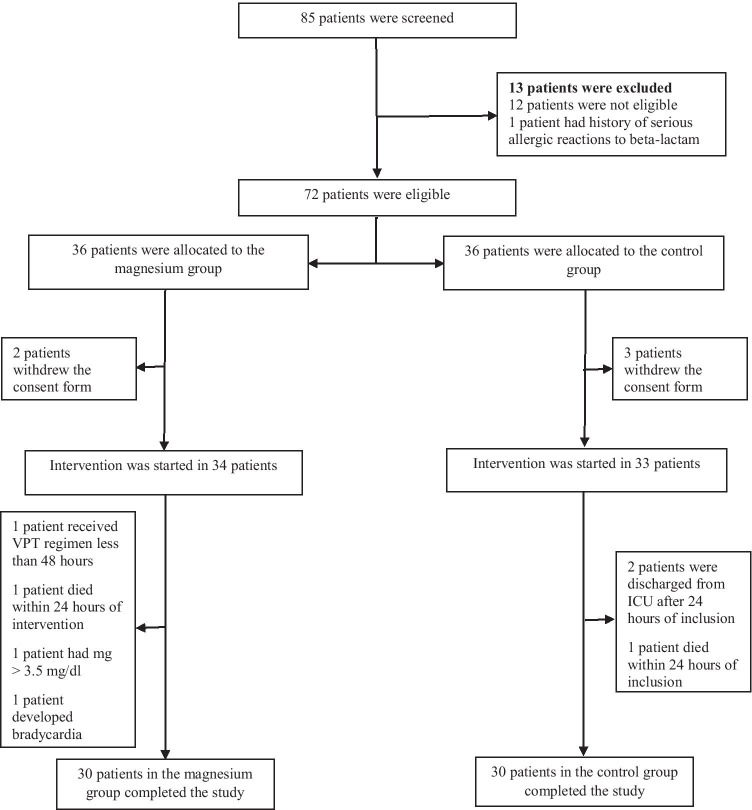
Twelve out of 85 patients had CrCl < 30 ml/min and one patient had history of serious hypersensitivity reactions to beta-lactam. The study was initiated with 72 patients (36 patients in each group). During the study, a number of patients were excluded. Finally, 30 patients in each group completed the treatment (Fig. 1).
Fig. 1.
Consort flowchart of the study
The demographic characteristics, baseline diseases (except diabetes mellitus) and past drug history of patients were not statistically different between the groups. The mean ± SD age of patients were 58 ± 14 and 60 ± 15 years in the magnesium and control groups respectively (p = 0.544). In the magnesium group, 56.67% of the patients were male while this value was 36.67% in the control group (p = 0.098). Baseline diseases were detected in 76.67% and 86.67% of patients in the magnesium and control groups respectively (p = 0.253). Eight patients in the control group had diabetes mellitus while only two patients had such comorbidity in the magnesium group (p = 0.040). Other baseline characteristics of the patients are demonstrated in Table 1.
Table 1.
Baseline characteristics of patients
| Characteristic | Magnesium group (N = 30) | Control group (N = 30) | p-value |
|---|---|---|---|
| Age(year), mean ± SD | 58 ± 14 | 60 ± 15 | 0.544 |
| Male sex, n (%) | 17(56.67) | 11(36.67) | 0.098 |
| Female sex, n (%) | 13(43.33) | 19(63.33) | |
| Comorbidities, n (%) | |||
| Any | 23(76.67) | 26(86.67) | 0.253 |
| Hypertension | 13(43.33) | 13(43.33) | 0.603 |
| Ischemic heart disease | 10(33.33) | 13(43.33) | 0.298 |
| Immunocompromising condition | 10(33.33) | 6(20.0) | 0.191 |
| Diabetes mellitus | 2(6.67) | 8(26.67) | 0.04 |
| Chronic lung disease | 1(3.33) | 2(6.67) | 0.5 |
| Rheumatologic disease | 1(3.33) | 3(10.0) | 0.306 |
| Hypothyroidism | 0 | 3(10.0) | 0.119 |
| Drug history, n (%) | |||
| Aspirin | 10(33.33) | 11(36.67) | 0.5 |
| Statin | 10(33.33) | 7(23.33) | 0.284 |
| ARB | 8(26.67) | 11(36.67) | 0.290 |
| Beta-blockers | 8(26.67) | 6(20.0) | 0.381 |
| Furosemide | 4(13.33) | 4(13.33) | 0.647 |
| CCB | 3(10.0) | 3(10.0) | 0.665 |
| Spironolactone | 3(10) | 2(6.67) | 0.5 |
| PPI | 3(10) | 2(6.67) | 0.5 |
| ACEI | 2(6.67) | 2(6.67) | 0.694 |
| Insulin | 2(6.67) | 2(6.67) | 0.694 |
| Metformin | 1(3.33) | 6(20.0) | 0.051 |
| Immunosuppresants | 1(3.33) | 1(3.33) | 0.754 |
| Corticosteroids | 0 | 1(3.33) | 0.5 |
| Levothyroxine | 0 | 3(10) | 0.119 |
ARB: angiotensin receptor blocker, CCB: calcium channel blocker, PPI: proton pomp inhibitor, ACEI: angiotensin converting enzyme inhibitor
The baseline laboratory data of the patients are shown in Table 2. There is no significant difference in this regard between the groups (except for sodium level). The mean ± SD serum level of magnesium at baseline were 2.01 ± 0.08 and 1.99 ± 0.11 mg/dL in the magnesium and control groups respectively (p = 0.607). Following infusion of magnesium sulfate, the mean ± SD daily serum magnesium level was 2.81 ± 0.18 mg/dL in the magnesium group. The mean ± SD daily serum magnesium level was 1.85 ± 0.06 mg/dL in the control group.
Table 2.
Baseline laboratory data of patients
| Laboratory data, mean ± SD | Magnesium group | Control group | p-value |
|---|---|---|---|
| White Blood Cell (cells /μl) | 13,813 ± 6253 | 12,171 ± 6375 | 0.322 |
| PMN (%) | 83.72 ± 8.05 | 81.05 ± 11.45 | 0.317 |
| Hemoglobin(g/dl) | 10.08 ± 2.25 | 10.27 ± 1.89 | 0.734 |
| Platelet count (cells × 103/ μl) | 232 ± 142 | 242 ± 145 | 0.793 |
| Sodium(meq/l) | 140 ± 5 | 135 ± 6 | 0.005 |
| Potassium(meq/l) | 3.75 ± 0.46 | 3.87 ± 0.67 | 0.438 |
| Magnesium(mg/dl) | 2.01 ± 0.08 | 1.99 ± 0.11 | 0.607 |
| Calcium(mg/dl) | 8.01 ± 0.72 | 8.19 ± 0.61 | 0.317 |
| Urea (mg/dl) | 42 ± 25 | 37 ± 17 | 0.306 |
| Serum creatinine(mg/dl) | 0.95 ± 0.21 | 0.98 ± 0.24 | 0.577 |
| Creatinine clearance(ml/min) | 83 ± 37 | 72 ± 23 | 0.181 |
| Aspartate aminotransferase(u/l) | 56 ± 47 | 46 ± 56 | 0.520 |
| Alanine aminotransferase(u/l) | 37 ± 42 | 30 ± 29 | 0.570 |
| Alkaline phosphatase(u/l) | 249 ± 148 | 381 ± 373 | 0.161 |
| Total bilirubin(mg/dl) | 1.32 ± 1.24 | 1.03 ± 1.03 | 0.425 |
| C-reactive protein(mg/dl) | 116 ± 80 | 102 ± 67 | 0.475 |
| Erythrocyte sedimentation rate(mm/h) | 52 ± 42 | 68 ± 40 | 0.209 |
| APACHE score | 13 ± 4 | 13 ± 3 | 0.802 |
| SOFA score | 3 ± 2 | 2 ± 2 | 0.450 |
As are shown in Table 3, the mean daily dose and serum trough level of vancomycin were comparable between the groups. The mean ± SD duration of the treatment were 4.4 ± 2.75 and 4.8 ± 1.64 days in the magnesium and control groups respectively (p = 0.534). In the magnesium and control groups, 19 and 18 patients respectively received at least one nephrotoxic agent concomitant with VPT (p = 0.500). Although, more patients in the magnesium group received furosemide, vasopressors or angiotensin receptor blockers (ARBs) but the difference was not statistically significant. Contrast media was applied in only one patient in the magnesium group. A non-steroidal anti-inflammatory drug (NSAID) was administrated in only one patient in the control group. Only two patients in the control group received acyclovir.
Table 3.
Characteristics of VPT regimen and microbiologic data
| Variable | Magnesium group (N = 30) | Control group (N = 30) | p-value |
|---|---|---|---|
| Vancomycin dose(mg/24 h), mean ± SD | 1933 ± 639 | 1766 ± 552 | 0.285 |
| Vancomycin through level(mcg/mL), mean ± SD | 17.15 ± 3.06 | 17.14 ± 3.03 | 0.996 |
| Piperacillin-tazobactam dose(g/24 h), mean ± SD | 14.55 ± 1.93 | 14.25 ± 1.70 | 0.527 |
| Duration of treatment (days), mean ± SD | 4.43 ± 2.75 | 4.80 ± 1.64 | 0.534 |
| Concomitant nephrotoxic agents, n (%) | |||
| any | 19 (63.33) | 18 (60.0) | 0.5 |
| Furosemide | 11(36.67) | 7(23.33) | 0.199 |
| vasopressor | 9 (30) | 3(10.0) | 0.052 |
| ARB | 7(23.33) | 6(20.0) | 0.5 |
| ACEI | 1(3.33) | 3(10.0) | 0.306 |
| CNI | 1(3.33) | 1(3.33) | 0.754 |
| Contrast media | 1(3.33) | 0 | 0.5 |
| NSAID | 0 | 1(3.33) | 0.5 |
| Acyclovir | 0 | 2(6.67) | 0.246 |
| Culture results, n (%) | |||
| Positive culture | 10(33.33) | 12(40) | 0.395 |
| Gram positive organism | 2(20.0) | 6(50.0) | 0.156 |
| Gram negative organism | 8(80) | 6(50.0) | |
| MDR pathogen | 3 (37.50) | 2 (33.33) | 0.657 |
| XDR pathogen | 5(62.50) | 4 (66.67) | |
ARB: angiotensin receptor blocker, ACEI: angiotensin converting enzyme inhibitor, CNI: calcineurin inhibitor, NSAID: non-steroidal anti-inflammatory drug, MDR: multi-drug resistant, XDR: extensively drug resistant
Microbial cultures were positive in 37% of the patients. Bacteremia, pneumonia and intra-abdominal complications were common sources of infection. Other microbiology data are shown in Table 3.
Clinical outcomes
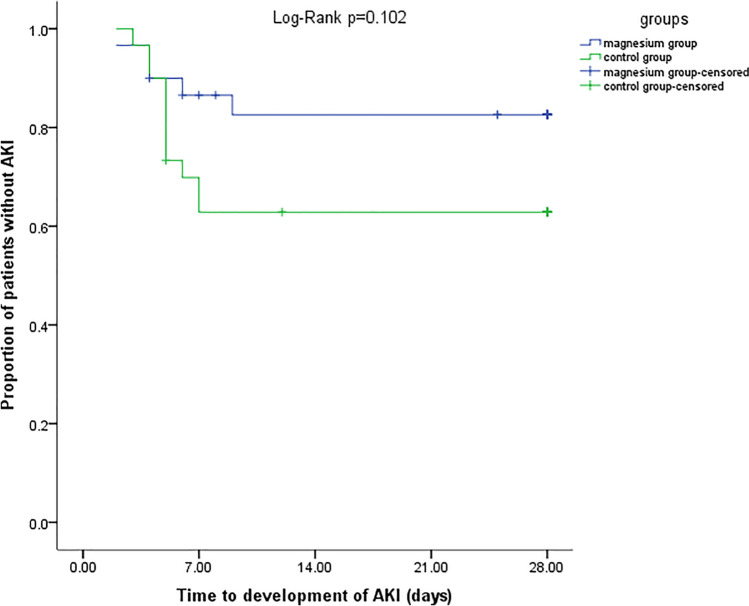
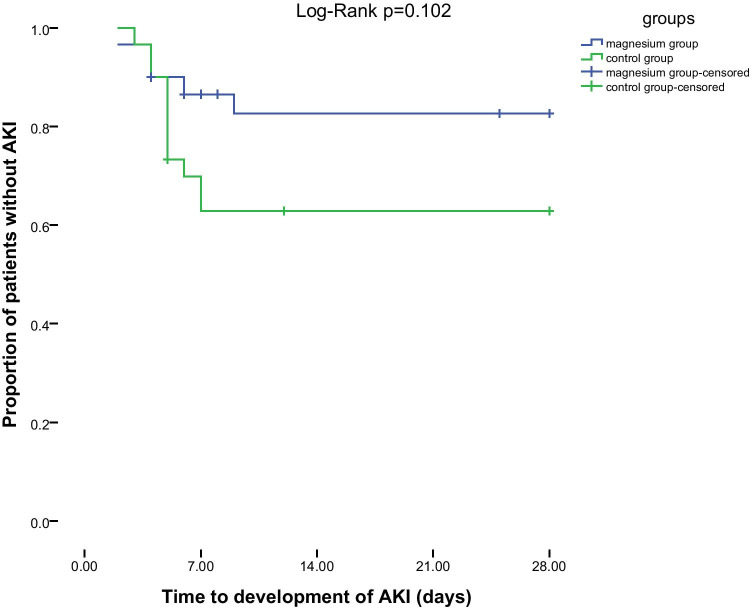
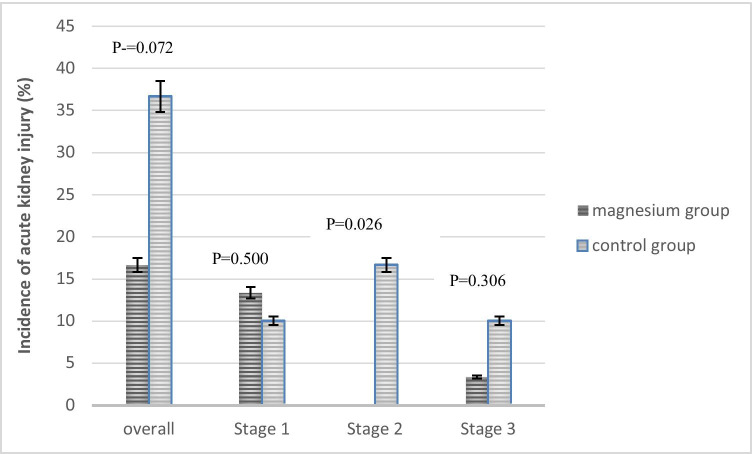
Five patients in the magnesium group and 11 patients in the control group developed AKI (p = 0.072) (Fig. 2). In the magnesium group, 4 and 1 patients experienced stages 1 and 3 of AKI respectively. In the control group 3, 5, 3 patients experienced stages 1, 2 and 3 of AKI respectively (Fig. 3). Three patients in the control group but no patient in the magnesium group needed renal replacement therapy. Recovery of AKI occurred in 3 and 5 patients in the magnesium and control groups respectively during the hospitalization course. De-escalation of VPT was done in 60% of patients in the magnesium group and 63.33% of patients in the control group. The mean ± SD of length of ICU stay in the magnesium and control groups were 11 ± 6 and 13 ± 8 days respectively (p = 0.241). Also length of hospital stay was not statistically different between the groups (17 ± 6 vs. 16 ± 8 days in the magnesium and control groups respectively, p = 0.557). Finally, 28-day mortality rate was 23.33% in each group (Table 4).
Fig. 2.
Kaplan–Meier plot for comparison of occurrence of AKI in two groups
Fig. 3.
The incidence of acute kidney injury in the magnesium and control group
Table 4.
Clinical outcomes of patients
| Variable | Magnesium group (N = 30) | Control group (N = 30) | p value |
|---|---|---|---|
| AKI, n (%) | |||
| Any stage | 5(16.67) | 11(36.67) | 0.072 |
| Stage 1 | 4 (13.33) | 3 (10.0) | - |
| Stage 2 | 0 | 5(16.67) | |
| Stage 3 | 1(3.33) | 3(10.0) | |
| RRT | 0 | 3 (10.0) | 0.727 |
| AKI recovery in hospitalization course | 3 (10.0) | 5(16.67) | 0.5 |
| Change of VPT regimen, n (%) | |||
| Escalation | 12(40) | 11(36.67) | 0.5 |
| De-escalation or discontinuation | 18(60) | 19(63.33) | |
| Intubation rate, n (%) | 12(40) | 7(23.33) | 0.153 |
| Length of stay in hospital (days), mean ± SD | 17 ± 6 | 16 ± 8 | 0.557 |
| Length of stay in ICU (days), mean ± SD | 11 ± 6 | 13 ± 8 | 0.241 |
| 28-day mortality, n (%) | 7(23.33) | 7(23.33) | 0.619 |
AKI: acute kidney injury, RRT: renal replacement therapy
Although incidence of AKI was not statistically different between the groups in unadjusted logistic regression model, it became significant after adjusting for confounders [unadjusted model (OR = 0.34; 95% CI: 0.10–1.16, p = 0.084), adjusted model: (OR = 0.26; 95% CI: 0.07–0.96, p = 0.04)]. According to the adjusted model, magnesium sulfate reduced the odds of AKI by 74% (Table 5).
Table 5.
Logistic regression analysis of the effect of intravenous magnesium sulfate on odds of AKI
| Model | OR (95% CI) | P-value |
|---|---|---|
| Unadjusted | 0.34 (0.10–1.16) | 0.086 |
| Adjusted for sex and diabetes mellitus | 0.26 (0.07–0.96) | 0.043 |
Infusion of magnesium sulfate tolerated well in most of the patients. Only in two patients due to bradycardia and serum magnesium levels > 3.5 mg/dl, the infusion of magnesium sulfate was stopped.
Discussion
This study was first randomized clinical trial in which the efficacy of intravenous magnesium sulfate in prevention of acute kidney injury induced by vancomycin + pipracillin-tazobactam was investigated. The incidence of AKI of VPT was reduced by concomitant administration of magnesium sulfate critically ill patients. Of patients who developed nephrotoxicity in the magnesium group, most (80%) were in stage 1 of AKI, no patient required renal replacement therapy and recovery of the renal function occurred in 60% of the individuals during the hospitalization course. Treatment with VPT regimen was successful in about 60% of the patients and the regimen was de-escalated. Although the sample size of study was small and two groups had some differences in the baseline characteristics (sex and history of diabetes mellitus), it is suggested that magnesium sulfate can be a promising option for prevention of AKI induced by VPT. Magnesium sulfate is an available, safe and inexpensive supplement and if its efficacy proved in future large clinical trials, management of patients who needed broad spectrum antibiotics will change significantly particularly in critical care settings. Indeed, in the road of antibiotic stewardship programs, VPT regimen can be reconsidered as a safe empiric regimen and carbapenem-based regimens will be reserved.
Despite the use of permuted block randomization, two groups were not matched in terms of sex and diabetes mellitus that small sample size can be one of the reasons. However, the primary logistic regression model was adjusted accordingly.
Duration of treatment was about 4 days in both groups. One of the issues about studies that considered incidence of AKI induced by VPT is short duration of exposure. Short course VPT therapy (≥ 24 but ≤ 72 h) did not increase the risk of AKI in comparison with other regimens (vancomycin + cefepime or vancomycin + meropenem) in critically ill patients [21]. In the study of Blevins et al., that median duration of VPT therapy was 4 days and the incidence rates of AKI in VPT, vancomycin + cefepime and vancomycin + meropenem combinations were 39.3%, 24.2% and 23.5% respectively. Accordingly VPT increased the risk of AKI by 2.16 as an independent risk factor [22]. Also in O'Callaghan et al. study that the risk proportion of AKI in patients who treated with VPT were 1.9 to 2.2 times more than patients who treated with other antibiotic combinations, the median course of treatment was 4 days [3]. In our study, magnesium sulfate reduced the incidence of AKI in patients who received VPT therapy for at least 4 days. However, this effect should be confirmed in future studies with large sample size and in patients who longer treatment is indicated.
One of the most important risk factors for development of AKI in critically ill patients is the use of nephrotoxic agents such as loop diuretics, vancomycin, angiotensin converting enzyme inhibitors (ACEIs), ARBs, contrast media and vasopressors [23]. Although more patients in the magnesium group received furosemide or vasopressors than patients in the control group, the incidence of AKI was lower in the magnesium group. A small number of patients in the control group received a NSAID or acyclovir. However, none of those patients developed AKI.
Hypomagnesemia is common in hospitalized patients. Approximately 7–11% and 65% of medical and critically ill patients suffered from hypomagnesemia [14]. Magnesium has several physiological and regulatory functions in the body. Recently, hypomagnesemia has been suggested as a risk factor for development of chronic kidney and end stage renal diseases. It seems that there is a correlation between hypomagnesemia and the rate of kidney function decline [24]. Magnesium deficiency is associated with inflammation, thrombosis and endothelial dysfunction [25]. Increased release of substance P and pro-inflammatory cytokines and activation of nuclear factor-kappa β (NFĸB) were detected following hypomagnesemia. Increased intracellular calcium content following hypomagnesemia, caused TNF-α release as one of the main pro-inflammatory cytokines [26]. Magnesium supplementation can suppress release of the pro-inflammatory cytokines and attenuates inflammatory responses. Oral magnesium supplementation significantly reduced serum C- reactive protein level [27]. Also magnesium sulfate inhibited the inflammatory responses via NFĸB pathway [28].
Magnesium is a potent anti-oxidant cation. Through blocking of NMDA receptors and attenuating the intracellular calcium accumulation, magnesium suppressed the release of reactive oxygen species (ROS) and other free radicals [29].
Magnesium deficiency is known as a predisposing factor for ischemia–reperfusion (I-R) injury. In the presence of magnesium deficiency, higher amounts of ROS and lipid peroxidation byproducts were detected following reperfusion [30]. Treatment with magnesium decreased I-R injury [31]. Also, it reduced the neuron apoptosis induced by I-R injury [32]. In a clinical study, protective role of magnesium supplementation against I-R injury was detected in patients following liver transplantation [33]. It seems that via blocking NMDA and calcium channels and induction of vasodilation, magnesium prevents I-R injury [31].
Magnesium is an essential element for the activity of different enzymes such as Na–K-ATPase, enolase, pyrophosphatase and pyruvate kinase and subsequently regulates energy production and transfer through glycolysis and Krebs cycle [14, 34].
Direct cytotoxic effect on the proximal tubule and loop of Henle is the main mechanism of cisplatin- induced nephrotoxicity. Following impairment of the reabsorption capacity of the renal tubules, hypomagnesemia is common in the patients who are receiving cisplatin. In such condition, not only patients are predisposed to the serious adverse effects of hypomagnesemia but also it can aggravate nephrotoxicity of cisplatin [35]. On the other hand, the nephroprotective role of magnesium supplementation against cisplatin-induced nephrotoxicity has been shown in the clinical practice. Yoshida et al., assessed the nephroprotective effect of magnesium sulfate in 496 patients with thoracic cancers who had indications of cisplatin therapy. Patients received 2 g magnesium sulfate (16 mEq magnesium) in 500 ml of normal saline before cisplatin administration. Pretreatment with magnesium substantially decreased the incidence of cisplatin-induced nephrotoxicity [36]. In another cohort study, protective role of magnesium against cisplatin-induced AKI were evaluated in 28 patients with cervical cancer. Renal function was reserved in more numbers of patients who received 15 mEq magnesium compared with the control group. [37]. Renoprotective effect of magnesium was investigated in 58 patients with head and neck cancers who had indications of cisplatin therapy. None of the patients who received magnesium sulfate developed nephrotoxicity [38]. In an ongoing multi-center randomized clinical trial the efficacy of magnesium supplementation in prevention of cisplatin-induced nephrotoxicity is under investigation [39].
At present, magnesium supplementation is a component of the standard of care in patients who have indications of chemotherapy with regimens containing cisplatin. These patients should be hydrated with magnesium-containing normal saline before cisplatin administration [40].
Similar to cisplatin, hypomagnesemia is a result and also a risk factor of nephrotoxicity induced by cyclosporine. Cyclosporine causes both acute and chronic nephrotoxicity. The main mechanism of acute form is renal arterial vasoconstriction. However, inflammation and fibrosis following infiltration of mononuclear cells are the main culprits in the chronic nephrotoxicity. Interestingly, magnesium supplementation decreased both forms of the nephrotoxicity. Vasodilatory effect and decrease in chemo-attraction and infiltration of monocytes or macrophages have been suggested as mechanisms for nephroprotective effects of magnesium against cyclosporine induced acute and chronic nephrotoxicity respectively [41, 42].
In contrast with cisplatin and cyclosporine, there is limited evidence about association between PT and hypomagnesemia. Piperacillin induced hypomagnesemia and hypokalemia was reported in critically ill patients. The serum levels of magnesium, potassium and calcium were measured before and after treatment with piperacillin. Serum levels of all electrolytes were significantly decreased following the treatment. Although renal function of patients remained stable during the treatment course, the authors attributed the changes to subclinical tubular dysfunction induced by piperacillin [43]. Also, PT induced changes in the serum electrolytes levels particularly hypokalemia have been described in some case reports [44, 45]. Finally, it seems that hypomagnesemia is a consequence of treatment with PT but it is not clear whether it causes or aggravates PT induced tubular dysfunction.
This randomized clinical trial was the first study that has been designed to evaluate the efficacy of magnesium for prevention of AKI induced by VPT. Our study has several limitations including small sample size, open label design, and difference in some baseline characteristics of the patients.
Conclusion
Magnesium sulfate with the target serum level of about 3 mg/dL reduced the incidence of acute kidney injury induced by vancomycin + piperacillin-tazobactam without serious adverse effects in critically ill patients. Our data support the concomitant administration of magnesium sulfate in patients who had indications of vancomycin + piperacillin-tazobactam as empiric therapy. However, close monitoring of the hemodynamic parameters is recommended. Well-designed randomized clinical trials with large sample size are needed to confirm the nephroprotective role of magnesium against vancomycin + piperacillin-tazobactam induced nephrotoxicity.
Acknowledgements
Office of Vice- Chancellor for Research of Tehran University of Medical Sciences, Tehran, Iran supported this work. With thanks of nursing staffs of general ICU of Imam Khomeini Hospital especially Hayedeh Bahrampour, Sayedeh Sedigheh Safavi and Sara Goodarzi for their kind support.
Author contribution
Hossein Khalili: Conceptualization, project manager, review and final editing of the manuscript.
Hamid Rahmani: Data gathering, statistical analysis, primary drafting of the manuscript.
Mostafa Mohammadi: Patients’ selection and monitoring during the study course.
Mohamadreza Salehi: Patients’ selection and clinical evaluation.
Zahra Mostafavi: Data gathering and patients’ nursing.
Data availability
Data are available per request and contact with the corresponding author.
Declarations
Conflict of interest
There is no conflict of interest for all authors to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hossein Khalili, Email: khalilih@tums.ac.ir.
Hamid Rahmani, Email: hmdrahmani89@gmail.com.
Mostafa Mohammadi, Email: mohammady_mm@tums.ac.ir.
Mohamadreza Salehi, Email: salehi.mohamad3@gmail.com.
Zahra Mostafavi, Email: za.mostafavi@gmail.com.
References
- 1.Ciarambino T, Giannico OV, Campanile A, et al. Acute kidney injury and vancomycin/piperacillin/tazobactam in adult patients: a systematic review. Intern Emerg Med. 2020;15(2):327–331. doi: 10.1007/s11739-020-02287-2. [DOI] [PubMed] [Google Scholar]
- 2.Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The Risk of Acute Kidney Injury in Critically Ill Patients Receiving Concomitant Vancomycin with Piperacillin-Tazobactam or Cefepime. J Intensive Care Med. 2019 doi: 10.1177/0885066619828290. [DOI] [PubMed] [Google Scholar]
- 3.O'Callaghan K, Hay K, Lavana J, McNamara JF. Acute kidney injury with combination vancomycin and piperacillin-tazobactam therapy in the ICU: A retrospective cohort study. Int J Antimicrob Agents. 2020;56(1):106010. doi: 10.1016/j.ijantimicag.2020.106010. [DOI] [PubMed] [Google Scholar]
- 4.Balcı C, Uzun Ö, Arıcı M, Hayran SA, Yüce D, Ünal S. Nephrotoxicity of piperacillin/tazobactam combined with vancomycin: should it be a concern? Int J Antimicrob Agents. 2018;52(2):180–184. doi: 10.1016/j.ijantimicag.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Blair K, Covington EW. Incidence and Risk Factors of Acute Kidney Injury in Patients Receiving Concomitant Vancomycin and Continuous-Infusion Piperacillin/Tazobactam: A Retrospective Cohort Study. Ann Pharmacother. 2020;54(11):1096–1101. doi: 10.1177/1060028020921170. [DOI] [PubMed] [Google Scholar]
- 6.Giuliano CA, Patel CR, Kale-Pradhan PB. Is the Combination of Piperacillin-Tazobactam and Vancomycin Associated with Development of Acute Kidney Injury? A Meta-analysis. Pharmacotherapy. 2016;36(12):1217–1228. doi: 10.1002/phar.1851. [DOI] [PubMed] [Google Scholar]
- 7.Hammond DA, Smith MN, Li C, Hayes SM, Lusardi K, Bookstaver PB. Systematic Review and Meta-Analysis of Acute Kidney Injury Associated with Concomitant Vancomycin and Piperacillin/tazobactam. Clin Infect Dis. 2017;64(5):666–674. doi: 10.1093/cid/ciw811. [DOI] [PubMed] [Google Scholar]
- 8.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin plus Piperacillin-Tazobactam and Acute Kidney Injury in Adults: A Systematic Review and Meta-Analysis. Crit Care Med. 2018;46(1):12–20. doi: 10.1097/CCM.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 9.Chen XY, Xu RX, Zhou X, Liu Y, Hu CY, Xie XF. Acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam administration: a systematic review and meta-analysis. Int Urol Nephrol. 2018;50(11):2019–2026. doi: 10.1007/s11255-018-1870-5. [DOI] [PubMed] [Google Scholar]
- 10.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–55. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 11.Watkins RR, Deresinski S. Increasing Evidence of the Nephrotoxicity of Piperacillin/Tazobactam and Vancomycin Combination Therapy-What Is the Clinician to Do? Clin Infect Dis. 2017;65(12):2137–2143. doi: 10.1093/cid/cix675. [DOI] [PubMed] [Google Scholar]
- 12.Kane-Gill SL, Goldstein SL. Drug-Induced Acute Kidney Injury: A Focus on Risk Assessment for Prevention. Crit Care Clin. 2015;31(4):675–684. doi: 10.1016/j.ccc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Elyasi S, Khalili H, Hatamkhani S, Dashti-Khavidaki S. Prevention of vancomycin induced nephrotoxicity: a review of preclinical data. Eur J Clin Pharmacol. 2013;69(4):747–754. doi: 10.1007/s00228-012-1406-3. [DOI] [PubMed] [Google Scholar]
- 14.Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology. Br J Anaesth. 1999;83(2):302–320. doi: 10.1093/bja/83.2.302. [DOI] [PubMed] [Google Scholar]
- 15.van de Wal-Visscher ER, Kooman JP, van der Sande FM. Magnesium in Chronic Kidney Disease: Should We Care? Blood Purif. 2018;45(1–3):173–178. doi: 10.1159/000485212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen BA, Bruserud Ø. Hypomagnesemia in critically ill patients. J Intensive Care. 2018;6:21. doi: 10.1186/s40560-018-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes DM, Smotherman C, Birch A, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34(7):662–669. doi: 10.1002/phar.1428. [DOI] [PubMed] [Google Scholar]
- 18.Hammond DA, Stojakovic J, Kathe N, et al. Effectiveness and Safety of Magnesium Replacement in Critically Ill Patients Admitted to the Medical Intensive Care Unit in an Academic Medical Center: A Retrospective Cohort Study. J Intensive Care Med. 2019;34(11–12):967–972. doi: 10.1177/0885066617720631. [DOI] [PubMed] [Google Scholar]
- 19.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 20.Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother. 2018;73(1):22–32. doi: 10.1093/jac/dkx368. [DOI] [PubMed] [Google Scholar]
- 21.Schreier DJ, Kashani KB, Sakhuja A, et al. Incidence of Acute Kidney Injury Among Critically Ill Patients With Brief Empiric Use of Antipseudomonal β-Lactams With Vancomycin. Clin Infect Dis. 2019;68(9):1456–1462. doi: 10.1093/cid/ciy724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blevins AM, Lashinsky JN, McCammon C, Kollef M, Micek S, Juang P. Incidence of Acute Kidney Injury in Critically Ill Patients Receiving Vancomycin with Concomitant Piperacillin-Tazobactam, Cefepime, or Meropenem. Antimicrob Agents Chemother. 2019;63(5):e02658–e2718. doi: 10.1128/AAC.02658-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cartin-Ceba R, Kashiouris M, Plataki M, Kor DJ, Gajic O, Casey ET. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract. 2012;2012:691013. doi: 10.1155/2012/691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebholz CM, Tin A, Liu Y, et al. Dietary Magnesium and Kidney Function Decline: The Healthy Aging in Neighborhoods of Diversity across the Life Span Study. Am J Nephrol. 2016;44(5):381–387. doi: 10.1159/000450861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier JA, Malpuech-Brugère C, Zimowska W, Rayssiguier Y, Mazur A. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 2004;1689(1):13–21. doi: 10.1016/j.bbadis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res. 2018;18(11):25–34. doi: 10.2147/JIR.S136742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almoznino-Sarafian D, Berman S, Mor A, et al. Magnesium and C-reactive protein in heart failure: an anti-inflammatory effect of magnesium administration? Eur J Nutr. 2007;46(4):230–237. doi: 10.1007/s00394-007-0655-x. [DOI] [PubMed] [Google Scholar]
- 28.Rochelson B, Dowling O, Schwartz N, Metz CN. Magnesium sulfate suppresses inflammatory responses by human umbilical vein endothelial cells (HuVECs) through the NFkappaB pathway. J Reprod Immunol. 2007;73(2):101–107. doi: 10.1016/j.jri.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Shcharbina N, Nechipurenko N, Matusevich L, Anatskaia L. The antioxidant effect of magnesium and its protective role for blood-brain barrier in acute stroke - model and clinical studies. 2014. 10.13140/2.1.4839.5206. https://www.researchgate.net/publication/263215418_The_Antioxidant_Effect_of_Magnesium_and_Its_Protective_Role_for_Blood-Brain_Barrier_in_Acute_Stroke_-_Model_and_Clinical_Studies. Accessed 5 May 2021.
- 30.Weglicki WB, Phillips TM, Mak IT, et al. Cytokines, neuropeptides, and reperfusion injury during magnesium deficiency. Ann N Y Acad Sci. 1994;17(723):246–257. doi: 10.1111/j.1749-6632.1994.tb36731.x. [DOI] [PubMed] [Google Scholar]
- 31.Akan M, Ozbilgin S, Boztas N, et al. Effect of magnesium sulfate on renal ischemia-reperfusion injury in streptozotocin-induced diabetic rats. Eur Rev Med Pharmacol Sci. 2016;20(8):1642–1655. [PubMed] [Google Scholar]
- 32.Zhou H, Ma Y, Zhou Y, et al. Effects of magnesium sulfate on neuron apoptosis and expression of caspase-3, bax and bcl-2 after cerebral ischemia-reperfusion injury. Chin Med J (Engl) 2003;116(10):1532–1534. [PubMed] [Google Scholar]
- 33.Kim JE, Jeon JP, No HC, et al. The effects of magnesium pretreatment on reperfusion injury during living donor liver transplantation. Korean J Anesthesiol. 2011;60(6):408–415. doi: 10.4097/kjae.2011.60.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan MF. The role of magnesium in clinical biochemistry: an overview. Ann Clin Biochem. 1991;28(Pt 1):19–26. doi: 10.1177/000456329102800103. [DOI] [PubMed] [Google Scholar]
- 35.Lajer H, Kristensen M, Hansen HH, et al. Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemother Pharmacol. 2005;56(5):535–542. doi: 10.1007/s00280-005-1010-7. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida T, Niho S, Toda M, et al. Protective effect of magnesium preloading on cisplatin-induced nephrotoxicity: a retrospective study. Jpn J Clin Oncol. 2014;44(4):346–354. doi: 10.1093/jjco/hyu004. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Watanabe K, Tsukiyama I, et al. Nephroprotective effects of hydration with magnesium in patients with cervical cancer receiving cisplatin. Anticancer Res. 2015;35(4):2199–2204. [PubMed] [Google Scholar]
- 38.Saito Y, Kobayashi M, Yamada T, et al. Premedication with intravenous magnesium has a protective effect against cisplatin-induced nephrotoxicity. Support Care Cancer. 2017;25(2):481–487. doi: 10.1007/s00520-016-3426-5. [DOI] [PubMed] [Google Scholar]
- 39.Makimoto A, Matsui M, Chin M, et al. Magnesium supplementation therapy to prevent cisplatin-induced acute nephrotoxicity in pediatric cancer: A protocol for a randomized phase 2 trial. Contemp Clin Trials Commun. 2019;22(16):100440. doi: 10.1016/j.conctc.2019.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seung AH. Adverse effects of chemotherapy and targeted agents. In: Alldredge BK, Corelli RL, Ernst ME, Guglielmo BJ, Jacobson PA, Kradjan WA, Williams BR, editors. Applied therapeutics: the clinical use of drugs. 10rd ed. Wolters Kluwer: Lippincott Williams & Wilkins; 2013. pp. 2109–2142.
- 41.Asai T, Nakatani T, Yamanaka S, et al. Magnesium supplementation prevents experimental chronic cyclosporine a nephrotoxicity via renin-angiotensin system independent mechanism. Transplantation. 2002;74(6):784–791. doi: 10.1097/00007890-200209270-00009. [DOI] [PubMed] [Google Scholar]
- 42.Miura K, Nakatani T, Asai T, et al. Role of hypomagnesemia in chronic cyclosporine nephropathy. Transplantation. 2002;73(3):340–347. doi: 10.1097/00007890-200202150-00005. [DOI] [PubMed] [Google Scholar]
- 43.Polderman KH, Girbes AR. Piperacillin-induced magnesium and potassium loss in intensive care unit patients. Intensive Care Med. 2002;28(4):520–522. doi: 10.1007/s00134-002-1244-3. [DOI] [PubMed] [Google Scholar]
- 44.Hussain S, Syed S, Baloch K. Electrolytes imbalance: a rare side effect of piperacillin/ tazobactam therapy. J Coll Physicians Surg Pak. 2010;20(6):419–420. [PubMed] [Google Scholar]
- 45.Zaki SA, Lad V. Piperacillin-tazobactam-induced hypokalemia and metabolic alkalosis. Indian J Pharmacol. 2011;43(5):609–610. doi: 10.4103/0253-7613.84986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available per request and contact with the corresponding author.