Abstract
Background and Objective
Fabry disease, an X-linked lysosomal storage disorder characterized by absent or reduced alpha-galactosidase activity, is a lifelong disease that impairs patients’ quality of life. Patients with Fabry disease have a considerably shortened lifespan, with mortality being mainly due to renal failure, cardiovascular disease, or cerebrovascular disease. Enzyme replacement therapy with agalsidase alfa has been shown to attenuate the renal, cardiovascular, and neuropathic disease progression associated with Fabry disease. The objective of this study was to investigate the safety of a new animal component-free version of agalsidase alfa.
Methods
A phase III/IV, open-label, single-arm, multicenter safety study was conducted in Canadian patients with Fabry disease between August 2011 and September 2017 as a regulatory requirement to assess the safety of agalsidase alfa produced using an animal component-free bioreactor process. Eligible patients had a documented diagnosis of Fabry disease and satisfied current Canadian guidelines for receiving enzyme replacement therapy for Fabry disease. Following treatment with animal component-free bioreactor-processed agalsidase alfa, treatment-emergent adverse events were monitored, and post hoc analyses of infusion-related reactions by antidrug antibody and neutralizing antibody statuses were conducted. The data were analyzed using descriptive statistics.
Results
A total of 167 patients (mean [standard deviation] age, 48.9 [14.8] years), including six pediatric patients (< 18 years of age), received at least one full or partial infusion of agalsidase alfa animal component-free. Fewer than 5% of treatment-emergent adverse events (212/4446) observed in 40 patients were reported as infusion-related reactions. Antidrug antibody and neutralizing antibody status did not affect the proportion of patients with infusion-related reactions. No clinically significant changes in vital signs were observed in patients over the course of the study.
Conclusions
Long-term treatment with bioreactor-produced agalsidase alfa animal component-free did not reveal new safety signals in this population of Canadian patients with Fabry disease. The treatment-emergent adverse event profile was consistent with the clinical manifestations of the disease and the known safety profile of roller bottle-produced agalsidase alfa.
Clinical Trial Registration
ClinicalTrials.gov identifier NCT01298141.
Key Points
| Agalsidase alfa was initially produced using a roller bottle process, but a switch to an animal component-free bioreactor process was needed. |
| We studied the safety of agalsidase alfa animal component-free in Canadian patients with Fabry disease and found that long-term treatment with agalsidase alfa animal component-free had acceptable safety and tolerability. |
| Antidrug antibody and neutralizing antibody status did not affect the proportion of patients with infusion-related reactions. |
Introduction
Fabry disease is a rare, X-linked glycosphingolipid storage disorder caused by the deficiency of the enzyme alpha-galactosidase A (α-gal) due to mutations in a single gene, GLA, located on the X chromosome Xq22.1 (OMIM 300644) [1]. Reduced or absent α-gal activity leads to the accumulation of globotriaosylceramide and other glycosphingolipids, within the lysosomes of various cell types throughout the body, resulting in renal, cardiovascular, and cerebrovascular complications [1]. A severe reduction (under 5%) in α-gal function causes the classical phenotype of Fabry disease and triggers the early onset of symptoms including clustered angiokeratoma, cornea verticillata, acroparesthesia, and hypohidrosis or hyperhidrosis [1]. Additionally, patients with the classic variant may have cardiac involvement, e.g., left ventricular hypertrophy, cardiomyopathy, or arrhythmia, stroke or transient ischemic attack, and renal involvement, with albuminuria at a young age and progressive nephropathy in adulthood [2, 3]. Higher residual α-gal activity is typically seen in female heterozygotes; male patients with higher residual α-gal activity usually have the non-classical (late-onset) form of the disease with major involvement of a only single organ system (heart or kidney) and a more variable disease course [4]. Although there is no ethnic predisposition associated with the disease, there are geographic areas with higher prevalence. For example, in Canada, a large kindred of patients with Fabry disease has arisen in Nova Scotia dating to a common ancestor [5, 6].
Fabry disease is a lifelong disorder with a variable but progressive course [7] that impairs patients’ quality of life [8, 9] and may culminate in premature death [10] due to renal failure, cardiovascular disease, or stroke. Prior to developments in supportive care for renal function, such as the use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, deaths among patients with Fabry disease were mainly attributed to complications of renal failure (31% of cases) [11]. After such significant improvements were made in renal care, the leading cause of death in patients with Fabry disease became cardiac disease (38% of cases) [11]. Enzyme replacement therapy (ERT) with agalsidase alfa and others may attenuate renal, cardiovascular, and neuropathic disease progression characteristics of Fabry disease [12–15].
In Canada [16, 17], there are currently two approved forms of recombinant α-gal: agalsidase alfa (produced in human cell lines) [18] and agalsidase beta (produced in Chinese hamster ovary cells) [19]. The approved agalsidase alfa product was initially produced using a roller bottle (RB) process, which involved using bovine serum and animal-derived proteins [20]. Following a switch to an animal component-free (AF) bioreactor process aimed at improving operational efficiency and enhancing the robustness of the manufacturing approach [20], supplies of agalsidase alfa produced using the RB process became depleted. There were no changes to the agalsidase alfa drug product formulation, manufacturing site, or container closure; however, the new production protocol enabled a continuous supply of bioreactor-produced agalsidase alfa AF until its safety could be confirmed and it became commercially available. The primary objective of this study was to observe the safety of agalsidase alfa produced using an adapted cell line in suspension and an AF bioreactor process in Canadian patients with Fabry disease.
Patients and Methods
Study Design
This open-label, single-arm, multicenter safety study was performed between August 2011 and September 2017 in Canada as a regulatory requirement and was conducted according to the International Conference on Harmonization of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki (ClinicalTrials.gov identifier NCT01298141). All patients (or their legal guardians/parents) provided written informed content before enrolling in the study.
Patients
Eligible patients had a documented diagnosis of Fabry disease with evidence of cardiac, neurologic, and renal complications, or uncontrolled neuropathic pain or gastrointestinal symptoms from the disease thereby satisfying current Canadian guidelines for receiving ERT for Fabry disease [21]. The study population consisted of patients who had participated in the Canadian Fabry Disease Initiative (CFDI) study (ClinicalTrials.gov identifier NCT 00455104) and were receiving agalsidase alfa AF. Patients were maintained on the dose they were receiving at the time of enrollment in the CFDI study. The standard agalsidase alfa AF dose was defined as 0.2 mg/kg, administered by intravenous (IV) infusion over 40 (± 10) min every other week (± 5 days). As part of the regulatory approval conditions for agalsidase alfa in Canada, patients who had shown kidney or cardiac disease progression while receiving the standard every-other-week dose of agalsidase alfa were administered a higher once-weekly dose of 0.2 mg/kg, administered by IV infusion over ≥ 40 (± 10) min once weekly (± 2 days). Patients were enrolled over the course of the CFDI study, and there was wide variation in the duration of the follow-up period, which reflected the date of diagnosis of Fabry disease, the time taken to meet criteria for ERT, and the date at which consent was given for treatment. Patients who completed the present study continued to receive agalsidase alfa AF until it was approved for commercial use.
Safety Assessments
Safety was assessed by monitoring adverse events (AEs), serious AEs (SAEs), treatment-emergent AEs (TEAEs), vital signs, blood tests, and anti-agalsidase alfa antibodies. Adverse events were reported for the duration of the study period. Adverse events were coded using Medical Dictionary for Regulatory Activities (Version 13.1). The total number of TEAEs, defined as those occurring within 30 days of the last dose, were reported by system organ class. Adverse event monitoring and assessment of vital signs were assessed at each dosing visit.
Anti-drug antibodies (ADAs) to agalsidase alfa were detected by immunoglobulin G (IgG) enzyme-linked immunosorbent assay or electrochemiluminescence [22, 23], and in vitro neutralizing anti-drug antibodies (NAbs) were measured by an enzyme inhibition assay [22, 23]. Centralized laboratories were employed for antibody testing and laboratory tests at annual or biannual visits. Samples confirmed positive for ADAs were further characterized for NAbs. Patients with persisting NAbs were defined as those with a positive antibody titer at the time of testing and at a previous sampling. Patients with transient NAbs were defined as those with a negative antibody titer at the time of testing but a positive result at the previous sampling. Efficacy of ERT with agalsidase alfa was not assessed in this study.
Post hoc analyses of infusion-related reactions (IRRs) by antibody status were performed. A reaction was categorized as being related to an infusion if it began either during the infusion or within 12 h after the start of the infusion and was judged by the investigator as possibly or probably related to agalsidase alfa.
Statistical Analysis
Continuous data collected prior to the administration of agalsidase alfa AF and at subsequent visits were summarized using descriptive statistics. No formal statistical tests were conducted.
Results
Patient Characteristics at Baseline
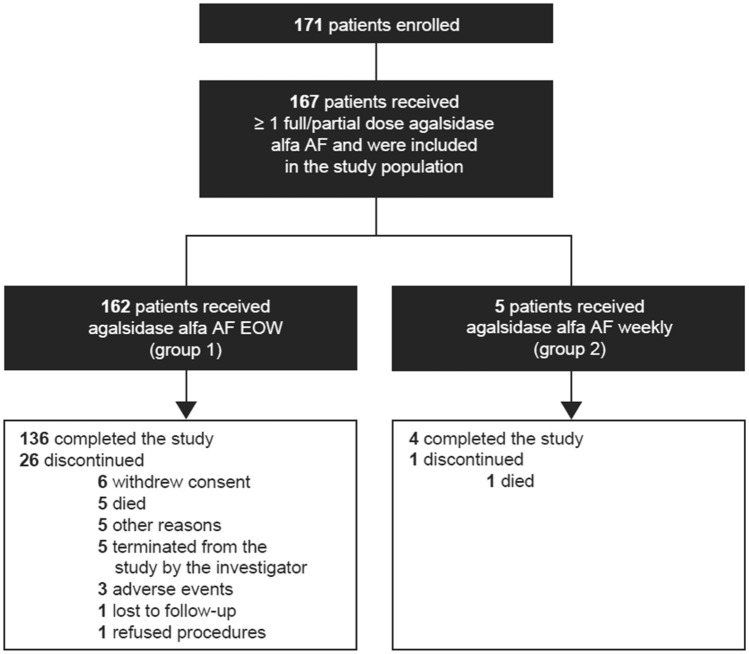
A total of 171 patients enrolled in the study, and 167 (162 taking agalsidase alfa AF every other week and five taking agalsidase alfa AF once weekly) received at least one full or partial infusion of agalsidase alfa AF and were included in the analyses as the study population (Fig. 1). Of the 167 patients, 140 (83.8%) completed the study and 27 (16.2%) discontinued prematurely. Reasons for discontinuation included death (n = 6; 3.6%), consent withdrawal (n = 6; 3.6%), termination of study by the investigator (n = 5; 3.0%), AEs (n = 3; 1.8%), refusal of study procedures (n = 1; 0.6%), lost to follow-up (n = 1; 0.6%), and other (n = 5; 3.0%). Because only five patients received once-weekly agalsidase alfa AF, there was insufficient power to detect differences between dose groups, and study findings are described for the overall study population rather than by enzyme dose group.
Fig. 1.
Study design. AF animal component-free, EOW every other week
Study patients, six of whom were pediatric patients (< 18 years of age), had a mean (standard deviation [SD]) age of 48.9 (14.8) years. The proportion of male patients was 54.5% (Table 1). At baseline, 45.5% of patients had received agalsidase alfa RB only, 1.2% had received agalsidase beta only, 27.5% had received a combination of previous treatments or unknown, and 25.7% were ERT naïve. In the overall study population, the mean (SD) duration of prior treatment with agalsidase alfa (RB and/or AF) and agalsidase beta was 49.1 (37.2) months and 65.7 (28.2) months, respectively. The mean (SD) duration of treatment with agalsidase alfa AF was 57.2 (21.7) months.
Table 1.
Demographic and baseline characteristics
| Characteristic | Total (n = 167) |
|---|---|
| Age, mean (SD), years | 48.9 (14.8) |
| Patients <18 years of age, n (%) | 6 (3.6) |
| Male sex, n (%) | 91 (54.5) |
| Weight, mean (SD), kg | 74.9 (15.1) |
| Time since diagnosis, mean (SD), years | 11.0 (10.2) |
| Previous ERT status, n (%) | |
| Agalsidase alfa RB | 76 (45.5) |
| Othera | 46 (27.5) |
| Treatment naïve | 43 (25.7) |
| Agalsidase beta | 2 (1.2) |
| Exposure to agalsidase alfa AF, days | |
| Mean (SD) | 1601.2 (606.3) |
| Median (range) | 1896.0 (1.0–2167.0) |
| Medical history (body system), >50% patients, n (%) | |
| Heart | 162 (87.0) |
| Eyes, ears, nose, and throat | 157 (94.0) |
| Neurological | 149 (89.2) |
| Skin | 140 (83.8) |
| Genitourinary | 137 (82.0) |
| Abdominal | 131 (78.4) |
| Musculoskeletal | 131 (78.4) |
| Chest and lungs | 122 (73.1) |
| Head, neck, and thyroid | 103 (61.7) |
| Other organ systems | 147 (88.0) |
AF animal free, ERT enzyme replacement therapy, RB roller bottle, SD standard deviation
aThis group included patients who had previously received agalsidase alfa (RB or AF) and agalsidase beta, those who had received agalsidase alfa RB and agalsidase alfa AF, and those whose ERT status was unknown
Infusion Completion
Most infusions were complete rather than partial infusions. The mean (SD) number of infusions initiated and completed were identical at 116.0 (50.3), with the mean (SD) duration of the infusions being 47.4 (15.2) min. Eight partial infusions were recorded in seven patients (4.2% prevalence). In four of these patients, the partial infusions were attributed to the occurrence of AEs, which included non-radiating heaviness in the left upper chest, skin flushing, coughing, and frontal headache (all reported by one patient), shortness of breath (one patient), and other unspecified AEs (two patients). Additional reasons for receiving partial infusions included refusal of reinsertion of the IV infusion (one patient) and medication vials outside the acceptable temperature range (one patient). No reasons for receiving partial infusions were specified for one patient.
Analysis of AEs
TEAEs
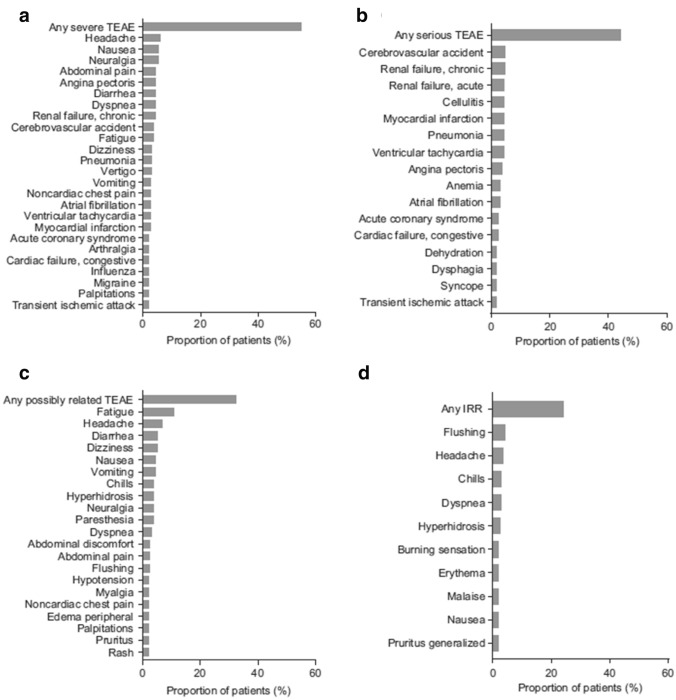
A total of 163 (97.6%) patients in the overall safety population experienced 4446 TEAEs, 11 patients (6.6%) reported TEAEs that were mild in severity, 60 patients (35.9%) reported TEAEs that were moderate in severity, and 92 patients (55.1%) reported severe TEAEs. The most common severe TEAEs that occurred in more than two patients were headache (n = 10; 6.0%); nausea and neuralgia (n = 9; 5.4% each); abdominal pain, angina pectoris, diarrhea, dyspnea, and chronic renal failure (n = 7; 4.2% each); and stroke and fatigue (n = 6; 3.6% each) (Fig. 2A). A total of 74 patients (44.3%) had 284 serious TEAEs (Table 2). The most common serious TEAEs that occurred in more than two patients were stroke and chronic renal failure (n = 8; 4.8% each); cellulitis, myocardial infarction, pneumonia, acute renal failure, and ventricular tachycardia (n = 7; 4.2% each); and angina pectoris (n = 6; 3.6%; Fig. 2B). Overall, 412 TEAEs in 79 patients were considered related to the study drug (Table 2). There were 25 study drug-related TEAEs classified as severe in 13 (7.8%) patients, the most frequent of which were related to pain (14 patients), gastrointestinal symptoms (seven patients), and cardiac symptoms (four patients: chest pain [two patients] and palpitations [two patients]). Three patients (1.8%) had 32 serious TEAEs that were considered related to agalsidase alfa AF, most of which were gastrointestinal disorders (11 events in two patients), nervous system disorders (six events in one patient), respiratory disorders (five events in one patient), or skin and subcutaneous disorders (five events in two patients). None of the ten life-threatening TEAEs that occurred in six patients was considered related to agalsidase alfa AF (Table 2).
Fig. 2.
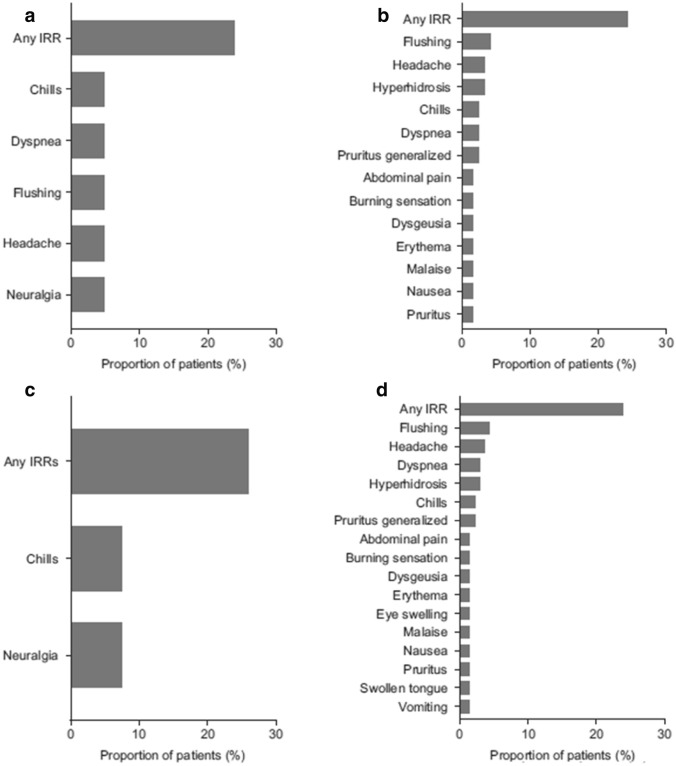
Most common [i.e., reported in more than two patients] (A) severe treatment-emergent adverse events (TEAEs), (B) serious TEAEs, (C) possibly related TEAEs, and (D) infusion-related reaction (IRRs) for the study population (n = 167). Medical Dictionary for Regulatory Activities Preferred Terms
Table 2.
Treatment-emergent adverse events (TEAEs)
| Parameter | Total (n = 167) |
|
|---|---|---|
| Patients, n (%) | TEAEs, n | |
| Any TEAE | 163 (97.6) | 4446 |
| Any severe TEAE | 92 (55.1) | 348 |
| Any serious TEAE | 74 (44.3) | 284 |
| Any serious life-threatening TEAE | 6 (3.6) | 10 |
| TEAE leading to treatment discontinuation | 7 (4.2) | 7 |
| TEAE leading to death | 3 (1.8) | 3 |
| Any study drug-related TEAE | 79 (47.3) | 412 |
| Any study drug-related severe TEAE | 13 (7.8) | 25 |
| Any study drug-related serious TEAE | 3 (1.8) | 32 |
| Any infusion-related TEAE | 40 (24.0) | 212 |
Percentages are based on the number of patients in the safety set for each treatment group
Seven (4.2%) patients discontinued the study after experiencing TEAEs of stroke (n = 2), congestive heart failure (n = 1), myocardial infarction (n = 1), abdominal pain (n = 1), adenoviral pneumonia (n = 1), and hip fracture (n = 1). The discontinuation of the study because of abdominal pain was assessed as being possibly related to agalsidase alfa AF. Following treatment, seven TEAE-related deaths occurred, including one death after study discontinuation due to stroke; none of these was considered related to treatment. Deaths in three patients were due to TEAEs of coronary artery disease, Fabry disease complications, and myocardial infarction, and occurred within 30 days of receiving the last dose of agalsidase alfa AF. Four patients died more than 30 days after the last dose of drug, owing to separate events of stroke, intracranial hemorrhage, septic shock, and sepsis.
Infusion-Related AEs
Among the 79 patients (47.3% of 167 patients) experiencing drug-related TEAEs in the study population, 54 (32.3% of 167 patients) reported TEAEs considered possibly related to the agalsidase alfa AF infusion (Fig. 2C). The most frequent TEAEs (occurring in more than two patients) in the study population reported as probably related to study drug were flushing (n = 5; 3.0%), cardiac disorders (n = 3; 1.8%), neuralgia (n = 3; 1.8%), burning sensation (n = 3; 1.8%), and hyperhidrosis (n = 3; 1.8%).
Of the total 4446 TEAEs, 212 observed in 40 patients (24.0%) were reported as IRRs, corresponding to an IRR prevalence of 4.8% (Table 2). Overall, the most frequently reported IRRs were flushing (n = 7; 4.2%), headache (n = 6; 3.6%), chills and dyspnea (n = 5; 3.0% each), hyperhidrosis (n = 4; 2.4%), and burning sensation, malaise, nausea, and generalized pruritus (n = 3; 1.8% each) (Fig. 2D). Most IRRs were mild (n = 21 patients; 12.6%) or moderate (n = 16 patients; 9.6%); three patients (1.8%) reported severe IRRs. Of the 32 drug-related treatment-emergent serious AEs, 19 were considered IRRs, and 15 of these were reported in a single patient. There were no clinically significant changes in vital signs in the overall safety population.
Analysis of Agalsidase Alfa AF Immunogenicity
ADA and NAb Assessment
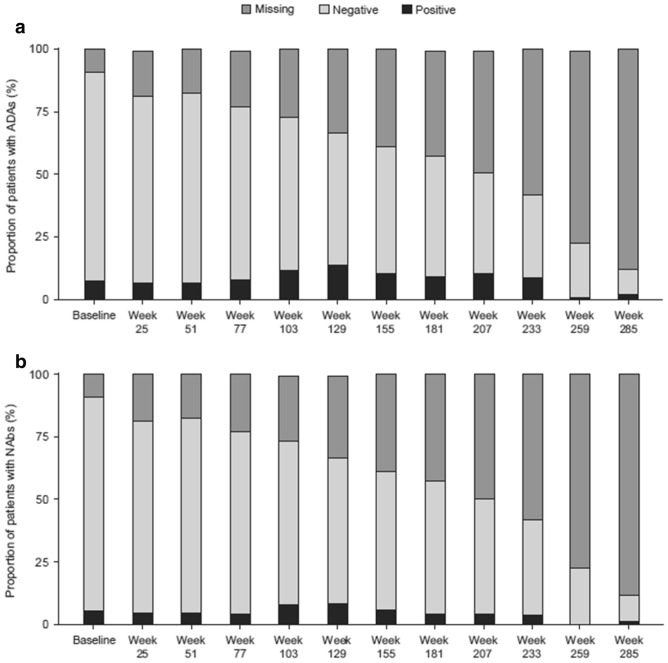
Among the 167 patients in the study population, at least one positive ADA result was reported for 42 (25.1%) patients at any time during the study period; of these, 12 (7.2%) patients tested positive at baseline, including four patients who had previously received agalsidase alfa RB. There were no apparent trends in ADA results with exposure over the treatment period, and no difference in the occurrence of IRRs as TEAEs between patients with or without ADAs. Samples positive for the presence of ADAs were evaluated further for the presence of NAbs: 27 (16.2%) patients who were ADA positive during the study also had at least one NAb-positive result. Only two of the 27 patients positive for NAbs were female: one was positive at baseline, whereas the other, a known Fabry disease homozygote, was positive at her last study visit (at week 233). Of the 12 patients who were positive for ADAs at baseline, nine also tested positive for NAbs at baseline. Of 23 patients who were positive for ADAs at week 129, 14 tested positive for NAbs; of three patients who tested positive for ADAs at week 285, two tested positive for NAbs. Interpreting trends of changing ADA or NAb titers over time was not possible owing to the large amount of missing data (Fig. 3).
Fig. 3.
Proportion of patients with positive or negative antidrug antibody (ADA) status (A) and neutralizing antibody (Nab) status (B) over time. Baseline was defined as the time at which data were collected prior to the first administration of the study drug
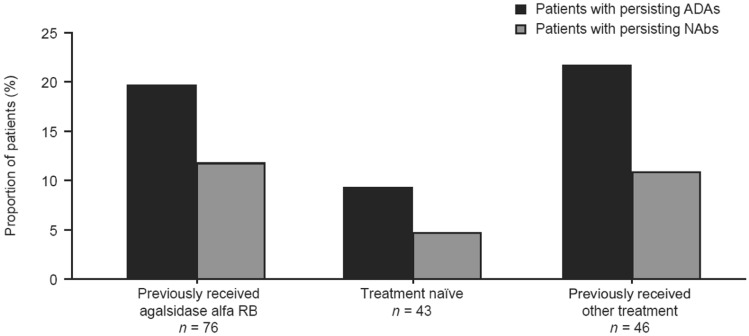
Of the 42 patients with at least one positive ADA result, 29 (69.0%) had persisting ADAs, 16 (38.1%) had persisting NAbs, and five (11.9%) had transient NAbs. Nine (21.4%) patients were negative for NAbs at all times of testing. Eleven (26.2%) patients were tested for NAbs only at a single time point. Of the 29 patients with persisting ADAs, ten had received other ERT treatment (both agalsidase alfa [RB or AF] and agalsidase beta; both agalsidase alfa RB and agalsidase alfa AF; or unknown ERT status) prior to the study, 15 had been previously treated with agalsidase alfa RB, and four were naïve to ERT. Of 16 patients with persisting NAbs, nine had been previously treated with agalsidase alfa RB, five had received other ERT treatment, and two were naïve to treatment. The proportion of patients with persisting ADAs and NAbs by ERT status are shown in Fig. 4. One patient who was naïve to treatment before the start of the study had a transient positive immunoglobulin E (IgE) result at week 103, although no ADAs or NAbs were detected during the same visit. This patient did not report any IRRs and experienced one treatment-emergent serious AE of osteonecrosis.
Fig. 4.
Persisting antidrug antibody [ADAs] (n = 29) and neutralizing antibodies [Nabs] (n = 16) by previous enzyme replacement therapy status. For patients with more than one positive or negative result for ADA/NAb, patients with persisting ADAs/NAbs were defined as those with a positive antibody titer at the time of testing and at the previous sampling. Other treatment included agalsidase alfa (roller bottle [RB] or animal component-free) and agalsidase beta, those who had received agalsidase alfa RB and agalsidase alfa animal component-free, and those whose enzyme replacement therapy status was unknown
IRRs by ADA/NAb Status
Antidrug antibody and NAb status did not affect the proportion of patients with drug-related AEs or IRRs. Of 79 patients with drug-related AEs, 18 (22.8%) had ADAs and nine (11.4%) had NAbs. Ten of 42 (23.8%) patients with ADAs and 30 of 123 (24.4%) patients without ADAs had IRRs (Fig. 5A, B). Similarly, seven of 27 (25.9%) patients with NAbs and 33 of 138 (23.9%) patients without NAbs had IRRs (Fig. 5C, D). Of seven patients receiving agalsidase alfa AF who died, only one (14.3%) was positive for both ADAs and NAbs at baseline and at all study visits; this patient’s death was reported as being due to a TEAE of coronary artery disease occurring within 30 days of the last agalsidase alfa AF dose.
Fig. 5.
Infusion-related reactions (IRRs) occurring in two or more patients who were antidrug antibody positive (A), antidrug antibody negative (B), neutralizing antibody positive (C), or neutralizing antibody negative (D) in the study population (n = 167). Medical Dictionary for Regulatory Activities Preferred Terms
Discussion
To ensure an uninterrupted supply of agalsidase alfa for Canadian patients with Fabry disease, the upstream production process for agalsidase alfa was switched from an RB process to an AF bioreactor process. The safety of agalsidase alfa RB has been demonstrated in several clinical trials [24–26] and with over 18 years of post-marketing experience [2, 27–29]. This study aimed to confirm the safety profile of agalsidase alfa AF in the context of the well-characterized historical safety profile of agalsidase alfa RB.
Findings from this study revealed that the prevalence and type of TEAEs reported with agalsidase alfa AF were consistent with those reported with agalsidase alfa RB. In this study, the most commonly reported serious TEAEs were stroke, chronic renal failure, cellulitis, myocardial infarction, pneumonia, acute renal failure, ventricular tachycardia, and angina pectoris, in line with findings from previous studies using agalsidase alfa RB [18, 25, 30–32]. The majority of occurrences of these TEAEs were considered to be unrelated to agalsidase alfa AF. In previous studies [25, 31, 33], 0.0–6.6% of serious TEAEs were considered related to agalsidase alfa RB, compared with 6.4% of serious TEAEs related to agalsidase alfa AF in the present study. Similarly, 24.0% of patients reported IRRs in this study, at the higher end of the range reported in prior clinical trials of agalsidase alfa RB (12.6–25.0% of adult patients) and were mostly mild or moderate in severity [18, 25, 33, 34].
Overall, 25.1% of patients in the present study tested positive for IgG ADAs, and IgE was transiently detected in a single female patient, who experienced one treatment-emergent serious AE of osteonecrosis and did not report any IRRs. This is higher than reported in previous studies, where anti-agalsidase alfa IgG antibodies were detected in 6.6–20.0% of patients [25, 29, 31, 34] and no anti-agalsidase alfa IgE antibodies were reported [29, 31]. However, the mean duration of exposure in this long-term study, at 4.4 years, was longer than in most studies (two 0.5-year studies, one 1-year study, and one long-term phase IV study with a mean follow-up of 3.5 years) [25, 29, 31]. Furthermore, not all ADAs possess neutralizing activity [35], and no clear pattern between ADAs and IRRs could be established, therefore conclusions relating to potential clinical impact cannot be drawn.
Although this study population consisted mainly of adults, six pediatric patients were also included. Canadian guidelines for the initiation of ERT for patients with Fabry disease are applicable to all patients with the condition, regardless of age [21]. The safety profile for pediatric patients who received agalsidase alfa has been shown previously to be similar to that observed for adults [36]. Moreover, an earlier 55-week phase II study of agalsidase alfa AF in 14 children aged ≥ 7 years with Fabry disease showed that this treatment was well tolerated [37]. In the present study, definitive conclusions relating to the induction of ERT in children could not be made owing to the small number of patients.
There were a number of limitations to this study, for example, the inclusion of patients receiving agalsidase alfa AF either once weekly or every other week. Patients receiving the once-weekly regimen were included in the study to reflect real-world variations in dose as well as continued evaluation of patients included in earlier studies with agalsidase alfa; however, the low number of patients receiving once-weekly agalsidase alfa AF precluded separate analysis. Although this study confirms that agalsidase alfa AF administered every other week is well tolerated in patients with Fabry disease, it was not possible to confirm if the weekly 0.2-mg/kg regimen would change the safety profile of the drug relative to the approved 0.2-mg/kg every-other-week regimen. Previous studies have indicated comparable efficacy with once-weekly and every-other-week treatment regimens [25], clinical outcomes data were not collected during this study thus no correlation could be explored between clinical outcomes and ERT dosing, or likewise, between clinical outcomes and ADAs, NAbs, or TEAEs. Furthermore, owing to a large amount of missing ADA and NAb data over time in the study population, trends over time in these two parameters could not be sufficiently interpreted. Last, as only two patients in the study switched from agalsidase beta to agalsidase alfa AF, it was not possible to compare patients receiving different forms of ERT.
Conclusions
Long-term treatment with bioreactor-produced agalsidase alfa AF was generally well tolerated and did not reveal any new safety signals in this population of Canadian adults and children with Fabry disease above the already-known safety profile of agalsidase alfa RB. The TEAE profile was consistent with the clinical manifestations of the disease, with few patients discontinuing treatment because of TEAEs. Antidrug antibody and neutralizing antibody status did not affect the proportion of patients with infusion-related reactions. Although IgE ADAs were detected in one patient, there were no associated IRRs. Overall, this study confirms that the safety profile of agalsidase alfa AF is similar to that previously reported with agalsidase alfa RB in patients with Fabry disease.
Acknowledgements
The authors thank the investigators and staff from the centers participating in the study, including Cheryl Rockman-Greenberg, MD (University of Manitoba, Winnipeg, MB, Canada); Sarah Dyack, MD (Izaak Walton Killam Health Centre, Halifax, NS, Canada); Bruno Maranda, MD (Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, QC, Canada); Jennifer MacKenzie, MD (Kingston General Hospital, Kingston, ON, Canada); Chitra Prasad, MD (London Health Sciences Centre, Victoria Hospital, London, ON, Canada); and Alicia Chan, MD (University of Alberta Hospital, Edmonton, AB, Canada). Under the direction of the authors, Vanessa Ducas, PhD, and Latoya M. Mitchell, PhD, CMPP, employees of Excel Medical Affairs, provided writing assistance for this manuscript. Editorial assistance in formatting, proofreading, copy editing, and fact checking also was provided by Excel Medical Affairs. Shire International GmbH, a Takeda company, provided funding to Excel Medical Affairs for support in writing and editing this manuscript. The sponsor was involved in the study design, data collection, analysis, and the decision to submit the manuscript for publication, but had no involvement in the interpretation of the data.
Abbreviations
- α-gal
Alpha-galactosidase
- ADA
Antidrug antibody
- AE
Adverse event
- AF
Animal component–free
- CFDI
Canadian Fabry Disease Initiative
- ERT
Enzyme replacement therapy
- IgE
Immunoglobulin E
- IgG
Immunoglobulin G
- IRR
Infusion-related reaction
- IV
Intravenous
- NAb
Neutralizing antibody
- RB
Roller bottle
- TEAE
Treatment-emergent adverse event
Declarations
Funding
This study was funded by Shire Human Genetic Therapies, a Takeda company.
Conflict of interest
Dr. Khan reports grants and personal fees from Takeda, outside of the submitted work. Dr. Sirrs reports grants from and advisory board participation for Sanofi Genzyme, grants and travel support from Takeda, and grants and travel support from Amicus, outside of the submitted work. Dr. Bichet reports grants and travel support from and speaker bureau participation for Amicus Therapeutics and Sanofi Genzyme, outside of the submitted work. Dr. Morel reports personal fees from Takeda, outside of the submitted work. Dr. Tocoian and Dr. Lan were employees of Takeda at the time of the study. Dr. West reports grants, personal fees, and travel support from Amicus Therapeutics, Idorsia, Protalix, Sanofi Genzyme, and Takeda, outside of the submitted work.
Ethics approval
This study was conducted according to the International Conference on Harmonization of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki (ClinicalTrials.gov identifier NCT01298141). The study was approved by the relevant institutional review board.
Consent to participate
All patients (or their legal guardians/parents) provided written informed content before enrolling in the study.
Consent for publication
Not applicable.
Availability of data and material
The datasets, including redacted study protocol, redacted statistical analysis plan, and individual participants’ data (after de-identification), behind the results reported in this article, will be available 3 months after the submission of a request through https://clinicaltrials.takeda.com/takedas-commitment?commitment=5 by researchers who provide a methodologically sound proposal in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Code availability
Not applicable.
Author contributions
AK, SMS, DGB, CFM, AT, LL, and MLW each provided substantial contributions to the interpretation of data for the work, drafted and revised the manuscript critically for important intellectual content, and approved the final version to be published. LL provided the statistical analysis of the data.
References
- 1.Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffmann R, Ries M, Timmons M, Flaherty JT, Brady RO. Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant. 2006;21:345–354. doi: 10.1093/ndt/gfi152. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz A, Germain DP, Desnick RJ, Politei J, Mauer M, Burlina A, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123:416–427. doi: 10.1016/j.ymgme.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Arends M, Wanner C, Hughes D, Mehta A, Oder D, Watkinson OT, et al. Characterization of classical and nonclassical Fabry disease: a multicenter study. J Am Soc Nephrol. 2017;28:1631–1641. doi: 10.1681/ASN.2016090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkilionis AJ, Riddell DC, Spence MW, Fenwick RG. Fabry disease in a large Nova Scotia kindred: carrier detection using leucocyte alpha-galactosidase activity and an Ncol polymorphism detected by an alpha-galactosidase cDNA clone. J Med Genet. 1991;28:232–240. doi: 10.1136/jmg.28.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. 2008;372:1427–1435. doi: 10.1016/S0140-6736(08)61589-5. [DOI] [PubMed] [Google Scholar]
- 7.MacDermot J, MacDermot KD. Neuropathic pain in Anderson-Fabry disease: pathology and therapeutic options. Eur J Pharmacol. 2001;429:121–125. doi: 10.1016/S0014-2999(01)01312-7. [DOI] [PubMed] [Google Scholar]
- 8.Gold KF, Pastores GM, Botteman MF, Yeh JM, Sweeney S, Aliski W, et al. Quality of life of patients with Fabry disease. Qual Life Res. 2002;11:317–327. doi: 10.1023/a:1015511908710. [DOI] [PubMed] [Google Scholar]
- 9.Miners AH, Holmes A, Sherr L, Jenkinson C, MacDermot KD. Assessment of health-related quality-of-life in males with Anderson Fabry disease before therapeutic intervention. Qual Life Res. 2002;11:127–133. doi: 10.1023/A:1015009210639. [DOI] [PubMed] [Google Scholar]
- 10.Barbey F, Hayoz D, Widmer U, Burnier M. Efficacy of enzyme replacement therapy in Fabry disease. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:277–286. doi: 10.2174/1568016043356192. [DOI] [PubMed] [Google Scholar]
- 11.Mehta A, Clarke JTR, Giugliani R, Elliott P, Linhart A, Beck M, FOS Investigators et al. Natural course of Fabry disease: changing pattern of causes of death in FOS—Fabry Outcome Survey. J Med Genet. 2009;46:548–552. doi: 10.1136/jmg.2008.065904. [DOI] [PubMed] [Google Scholar]
- 12.Beck M, Hughes D, Kampmann C, Larroque S, Mehta A, Pintos-Morell G, Fabry Outcome Survey Study Group et al. Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: a Fabry Outcome Survey analysis. Mol Genet Metab Rep. 2015;3:21–27. doi: 10.1016/j.ymgmr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feriozzi S, Torras J, Cybulla M, Nicholls K, Sunder-Plassmann G, West M, FOS Investigators The effectiveness of long-term agalsidase alfa therapy in the treatment of Fabry nephropathy. Clin J Am Soc Nephrol. 2012;7:60–69. doi: 10.2215/CJN.03130411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kampmann C, Perrin A, Beck M. Effectiveness of agalsidase alfa enzyme replacement in Fabry disease: cardiac outcomes after 10 years’ treatment. Orphanet J Rare Dis. 2015;10:125. doi: 10.1186/s13023-015-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Dib R, Gomaa H, Carvalho RP, Camargo SE, Bazan R, Barretti P, et al. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst Rev. 2016;7:CD006663. doi: 10.1002/14651858.CD006663.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shire Canada. Replagal part III: patient medication information. https://www.takeda.com/4ab39a/siteassets/en-ca/home/what-we-do/our-medicines/product-monographs/replagal/replagal-pm-en.pdf. Accessed 9 Dec 2020.
- 17.Sanofi Canada. Fabrazyme product monograph. Sanofi Genzyme, Canada. 2017. http://products.sanofi.ca/en/fabrazyme-en.pdf. Accessed 9 Dec 2020.
- 18.European Medicines Agency. European public assessment report for Replagal: product information. 2008. https://www.ema.europa.eu/en/medicines/human/EPAR/replagal. Accessed 9 Dec 2020.
- 19.European Medicines Agency. European public assessment report for Fabrazyme: product information. 2009. https://www.ema.europa.eu/en/medicines/human/EPAR/fabrazyme. Accessed 9 Dec 2020.
- 20.Australian Government, Department of Health and Ageing Therapeutic Goods Administration. Australian public assessment report for agalsidase alfa ghu (submission number: PM-2009-01140-3-3). 2019. https://www.tga.gov.au/sites/default/files/auspar-replagal.pdf. Accessed 9 Dec 2020.
- 21.Sirrs S, Bichet BG, Iwanochko M, Khan A, Moore D, Oudit G, West ML. Canadian Fabry disease treatment guidelines. 2019. https://garrod.ca/wp-content/uploads/2020/02/Canadian-Fabry-Treatment-Guidelines-2019-final.pdf. Accessed 9 Dec 2020.
- 22.US Food and Drug Administration. Immunogenicity testing of therapeutic protein products: developing and validating assays for anti-drug antibody detection. Guidance for industry. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-testing-therapeutic-protein-products-developing-and-validating-assays-anti-drug. Accessed 9 Dec 2020.
- 23.Shibata H, Nishimura K, Miyama C, Tada M, Suzuki T, Saito Y, et al. Comparison of different immunoassay methods to detect human anti-drug antibody using the WHO erythropoietin antibody reference panel for analytes. J Immunol Methods. 2018;452:73–77. doi: 10.1016/j.jim.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Hughes DA, Deegan PB, Milligan A, Wright N, Butler LH, Jacobs A, et al. A randomised, double-blind, placebo-controlled, crossover study to assess the efficacy and safety of three dosing schedules of agalsidase alfa enzyme replacement therapy for Fabry disease. Mol Genet Metab. 2013;109:269–275. doi: 10.1016/j.ymgme.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Goláň L, Goker-Alpan O, Holida M, Kantola I, Klopotowski M, Kuusisto J, et al. Evaluation of the efficacy and safety of three dosing regimens of agalsidase alfa enzyme replacement therapy in adults with Fabry disease. Drug Des Dev Ther. 2015;9:3435–3444. doi: 10.2147/DDDT.S80928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuboi K, Yamamoto H. Efficacy and safety of enzyme-replacement-therapy with agalsidase alfa in 36 treatment-naïve Fabry disease patients. BMC Pharmacol Toxicol. 2017;18:43. doi: 10.1186/s40360-017-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramaswami U, Parini R, Pintos-Morell G, Kalkum G, Kampmann C, Beck M, FOS Investigators Fabry disease in children and response to enzyme replacement therapy: results from the Fabry Outcome Survey. Clin Genet. 2012;81:485–490. doi: 10.1111/j.1399-0004.2011.01671.x. [DOI] [PubMed] [Google Scholar]
- 28.Linhart A, Kampmann C, Zamorano JL, Sunder-Plassmann G, Beck M, Mehta A, et al. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry Outcome Survey. Eur Heart J. 2007;28:1228–1235. doi: 10.1093/eurheartj/ehm153. [DOI] [PubMed] [Google Scholar]
- 29.Pastores GM, Boyd E, Crandall K, Whelan A, Piersall L, Barnett N. Safety and pharmacokinetics of agalsidase alfa in patients with Fabry disease and end-stage renal disease. Nephrol Dial Transplant. 2007;22:1920–1925. doi: 10.1093/ndt/gfm096. [DOI] [PubMed] [Google Scholar]
- 30.Schiffmann R. Enzyme replacement in Fabry disease: the essence is in the kidney. Ann Intern Med. 2007;146:142–144. doi: 10.7326/0003-4819-146-2-200701160-00147. [DOI] [PubMed] [Google Scholar]
- 31.Hughes DA, Elliott PM, Shah J, Zuckerman J, Coghlan G, Brookes J, et al. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008;94:153–158. doi: 10.1136/hrt.2006.104026. [DOI] [PubMed] [Google Scholar]
- 32.Schiffmann R, Kopp JB, Austin HA, 3rd, Sabnis S, Moore DF, Weibel T, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 33.Goker-Alpan O, Nedd K, Shankar SP, Lien YH, Weinreb N, Wijatyk A, et al. Effect and tolerability of agalsidase alfa in patients with Fabry disease who were treatment naive or formerly treated with agalsidase beta or agalsidase alfa. JIMD Rep. 2015;23:7–15. doi: 10.1007/8904_2015_422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasa H, Nagao M, Kino K. Safety and effectiveness of enzyme replacement therapy with agalsidase alfa in patients with Fabry disease: post-marketing surveillance in Japan. Mol Genet Metab. 2019;126:448–459. doi: 10.1016/j.ymgme.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Lenders M, Schmitz B, Brand SM, Brand E. Neutralizing anti-drug antibodies in Fabry disease have no obvious clinical impact? Orphanet J Rare Dis. 2018;13:171. doi: 10.1186/s13023-018-0916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbey F, Livio F. Safety of enzyme replacement therapy. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry disease: perspectives from 5 years of FOS. Oxford: Oxford PharmaGenesis; 2006. [Google Scholar]
- 37.Goker-Alpan O, Longo N, McDonald M, Shankar SP, Schiffmann R, Chang P, et al. An open-label clinical trial of agalsidase alfa enzyme replacement therapy in children with Fabry disease who are naïve to enzyme replacement therapy. Drug Des Dev Ther. 2016;10:1771–1781. doi: 10.2147/DDDT.S102761. [DOI] [PMC free article] [PubMed] [Google Scholar]