Abstract
Purpose
Renal scintigraphy (RS) with either technetium-99 m diethylene-triamine-pentaacetate (Tc-99 m DTPA) or technetium-99 m mercaptoacetyltriglycine (Tc-99 m MAG3) has both been used to evaluate early allograft function after kidney transplantation (KT). This study was done to compare the predictive performance of RS using these two radiopharmaceuticals for prediction of outcomes during first 3 months of KT.
Methods
This retrospective study included patients who received KT then underwent both Tc-99 m DTPA and Tc-99 m MAG3 RS, successively. Receiver operating characteristic (ROC) curve analysis was used to determine the predictiveness of RS parameters on early clinical adverse outcomes of either (1) graft-related death, (2) need for graft resection, (3) delayed graft function requiring temporary dialysis, or (4) a serum creatinine level of ≥ 2.0 mg/dL at three months post-KT, as well as to predict biopsy-confirmed acute tubular necrosis and acute rejection.
Results
Of 187 patients included, 77 (41.2%) had at least one early adverse clinical outcome. Tc-99 m MAG3 RS was more predictive than Tc-99 m DTPA RS, in terms of AUCROC, in three parameters including time to peak (0.754 vs. 0.516, p-value 0.0001), 20-min to peak ratio (0.762 vs. 0.651, p-value 0.006), and 20-min to 3-min ratio (0.823 vs. 0.699, p-value 0.0005). Acute tubular necrosis was better predicted by Tc-99 m MAG3 RS while both were at best only modest in predicting acute rejection.
Conclusion
Three parameters which, when obtained from Tc-99 m MAG3 RS, had superior predictiveness compared with Tc-99 m DTPA RS, including time to peak, 20-min to peak ratio, and 20-min to 3-min ratio.
Keywords: Renal transplantation, Renal scintigraphy, Radionuclide imaging, Graft complication, Graft outcome
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem with prevalence of between 11 and 13% [1]. Patients with stage 5 CKD, also known as end-stage renal disease (ESRD), need either dialysis or kidney transplantation (KT) to prolong survival [2]. Although improvement of transplantation techniques and the use of immunosuppressive medication have improved post-transplantation outcomes, acute complications such as acute graft rejection, surgical complications, and delayed graft function still impact long-term outcome [3]. Renal scintigraphy (RS) is a nuclear medicine imaging study which has long been used for evaluation of patients after KT. The two most commonly used radiopharmaceuticals are technetium-99 m diethylene-triamine-pentaacetate (Tc-99 m DTPA) and technetium-99 m mercaptoacetyltriglycine (Tc-99 m MAG3). Tc-99 m DTPA is purely filtered by glomeruli and is therefore used to measure glomerular filtration rate (GFR) [4], whereas 89% to virtually 100% of Tc-99 m MAG3 is cleared by tubular secretion [5, 6] and has been used to estimate effective renal plasma flow rate (ERPF) in modern practice in place of I-131 ortho-iodohippurate. Since RS is a dynamic imaging study, many quantifiable parameters can be derived to represent the perfusion and function of kidneys. These parameters have been used to predict acute complications after KT, such as the Hilson’s perfusion index which has been reported to be associated with acute rejection [7, 8]. However, there have been only few studies that directly compared the predictive power between RS performed using Tc-99 m DTPA and Tc-99 m MAG3. This study aimed to determine whether Tc-99 m DTPA and Tc-99 m MAG3 are superior to one another for predicting early adverse clinical outcomes and two common renal pathologies, i.e., acute tubular necrosis and acute rejection, after KT.
Methods
Design and Setting
This retrospective cohort study was performed in Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand, a tertiary care academic hospital with an established multidisciplinary team that regularly performs KT.
Data Collection
The demographic and clinical data were collected from medical records; kidney biopsy results were obtained from pathology reporting system of our institute. The RS images were obtained from imaging database and archiving system and analyzed.
Population and Sampling
The population of interest were adults with ESRD who underwent KT. Consecutive sampling was used, i.e., all adult ESRD patients who underwent either living- or deceased-donor KT between 1 June 2013 and 31 March 2019, who also underwent Tc-99 m DTPA and Tc-99 m MAG3 RS within 14 days post-transplantation, were included. Patients who were lost to follow-up before the minimum follow-up time of 3 months after KT and those who had incomplete medical records were excluded. Some participants who developed adverse clinical outcomes underwent kidney biopsy after RS measurement to find out the causative renal pathologies. During the study period, a total of 298 KT patients underwent postoperative RS with both Tc-99 m DTPA and Tc-99 m MAG3. One case with missing medical records, one who underwent combined liver and kidney transplantation, and one who died from bacterial pneumonia unrelated to graft complications were excluded. A total of 108 patients underwent RS beyond 14 days of transplantation and were also excluded. Therefore, 187 patients remaining were included in the study. The average age of patients was 42.4 ± 10.3 years and most (61.0%) were males. The most common comorbidity associated with ESRD was hypertension found in 67.4% of patients, followed by glomerulonephritis which was the cause of ESRD in 15.0% of patients. Thirty-eight patients (20.3%) had no known concomitant diseases. The average duration of dialysis before receiving KT was 4.7 ± 2.8 years, and hemodialysis was the more common mode of dialysis (73.3%) before transplantation. Patient demographics are provided in Table 1.
Table 1.
Patient demographics presented as mean ± standard deviation unless indicated otherwise
| Characteristic of participants (n = 187) | |
|---|---|
| Age (years) | 42.4 ± 10.3 |
| Sex, n (%) | |
| Male | 114 (61.0%) |
| Female | 73 (39.0%) |
| Weight (kg) | 59.6 ± 11.7 |
| Height (cm) | 161.5 ± 8.0 |
| BMI (kg/m2) | 22.8 ± 3.9 |
| Underlying diseases, n (%) | |
| Hypertension | 126 (67.4%) |
| Diabetes mellitus | 19 (10.2%) |
| SLE | 4 (2.1%) |
| Renal stone | 11 (5.9%) |
| Glomerulonephritis | 28 (15.0%) |
| IgA nephropathy | 17 (9.1%) |
| Gout | 20 (10.7%) |
| ADPKD | 6 (3.2%) |
| Dialysis duration before KT (years) | 4.7 ± 2.8 |
| Dialysis mode before KT, n (%) | |
| Peritoneal dialysis | 50 (26.7%) |
| Hemodialysis | 137 (73.3%) |
| Preoperative serum creatinine (mg/dL) | 10.7 ± 3.5 |
| Preoperative BUN (mg/dL) | 51.0 ± 18.5 |
Abbreviations: SD, standard deviation; SLE, systemic lupus erythematosus; ADPKD, autosomal dominant polycystic kidney disease; BUN, blood urea nitrogen
Outcomes of Interest
For the purpose of this study, patients were classified as having early adverse clinical outcomes if the patient had at least one of the following events during the first 3 months of follow-up: (1) died of graft-related complications, (2) needed graft resection, (3) had delayed graft function defined as the need for temporary dialysis within the first week after transplantation, or (4) had a serum creatinine level of ≥ 2.0 mg/dL at 3 months after transplantation. Secondary outcomes including the pathological diagnoses of acute tubular necrosis and any acute graft rejection occurring within the first 3 months of KT were also analyzed.
Renal Scintigraphy
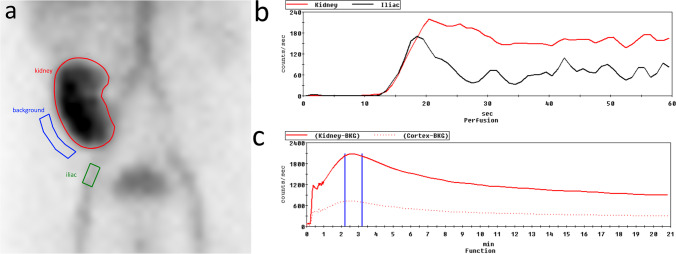
Renal scintigraphy was performed at the Division of Nuclear Medicine, Department of Radiology, Faculty of Medicine. In all patients, RS with Tc-99 m DTPA and Tc-99 m MAG3 were done in succession. First, an indwelling IV catheter attached to a three-way stopcock was inserted into the cubital vein before the beginning of the examination. The first RS was done with 37 MBq of Tc-99 m DTPA. The second RS was done with 185 MBq of Tc-99 m MAG3 which followed immediately after completion of the first RS. Image acquisition was done with the patient in supine position under a Discovery NM/CT 670 gamma camera and SPECT/CT system (GE Healthcare, IL, USA) equipped with a low-energy all-purpose collimator. Dynamic imaging using list mode acquisition was started simultaneously with bolus injection of the radiotracer with imaging matrix of 1,024 × 1,024 × 16, photopeak of 140 keV, and energy window of ± 15%. Images were acquired in two phases: the perfusion phase at 1 s/frame during the first minute and the function phase at 20 s/frame for the remaining 20 min. Radioactivity in the syringe was measured before and after injection by placing the syringe under the gamma camera at a distance of 30 cm for a duration of 60 s. Analysis of the images was done using Xeleris 3.0 software provided by the manufacturer. Whole kidney region of interest (ROI) was drawn around the renal graft and used to calculate the parameters unless there was significant retention in the renal pelvis, in which case a cortical ROI was instead used. Semilunar soft tissue background ROI was drawn at the inferolateral region of the graft. Iliac artery ROI was drawn at the iliac artery just distal to the renal graft. Several key renal scintigraphic parameters were examined. This included quantified renal function as represented by glomerular filtration rate (GFR) obtained from Tc-99 m DTPA RS and effective renal plasma flow rate (ERPF) obtained from Tc-99 m MAG3 RS. Hilson’s perfusion index, time to peak, 20-min to peak ratio, and 20-min to 3-min ratio from RS using both Tc-99 m DTPA and Tc-99 m MAG3 were also examined. Image analysis was done by an experienced nuclear medicine physician with 10 years of experience in analyzing and interpreting RS in renal transplant recipients. An example of ROI placement is demonstrated in Fig. 1.
Fig. 1.
Analysis of renal scintigraphy. a Placement of regions of interest around the kidney (red), soft tissue background (blue), and iliac artery (green). b Perfusion time-activity curve of activity during the first 1–60 s. c Renogram of radiotracer activity in the kidney during the 20 min of image acquisition
Statistical Analysis
Descriptive statistics were used to summarize the demographic characteristics and renal scintigraphic parameters. For assessing the predictiveness of RS parameters in forecasting the outcomes of interest, receiver operating characteristic (ROC) curve analysis and the area under the ROC curve (AUCROC) were determined for each RS parameter, using the logistic regression framework. DeLong’s test was used to compare the AUCROC between parameters derived from Tc-99 m DTPA RS and Tc-99 m MAG3 RS to determine whether there were differences in predictive performance between these two radiopharmaceuticals. Analysis was conducted in R version 4.03 [9]. The “pROC” package was used for the ROC curve analysis and comparison [10]. Figures were produced using the “ggplot2” package [11].
Results
Kidney Transplantation and Postoperative Course
Of the 187 patients, 177 (94.7%) underwent deceased-donor KT and 153 (81.8%) received kidney graft from a standard criteria donor. The average donor age was 38.8 ± 12.7 years, the average cold ischemic time was 16.6 ± 5.7 h, and the average operation time was 1.8 ± 0.5 h. Table 2 summarizes adverse clinical outcomes during the first 3 months of follow-up. A total of 51 patients (27.3%) required temporary dialysis due to delayed graft function. There were three cases (1.6%) of severe graft complication requiring graft resection. Two patients (1.1%) died during the first 3 months of follow-up associated with graft failure and severe cytomegalovirus and cryptococcal infections. At 3 months of follow-up, 32 subjects (17.1%) had serum creatinine ≥ 2.0 mg/dL. Overall, there were 77 patients (41.2%) who had at least one adverse clinical outcome, and among these 77 patients, 66 cases of them underwent kidney biopsies which revealed acute rejection in 26 (39.4%) and acute tubular necrosis in 34 patients (51.5%). Several cases of mechanical complications during the early postoperative period were also of note. Two patients had vascular anastomotic bleeding which required immediate repair after the initial operation. Two had renal transplant artery stenosis and one had renal artery kinking, which were successfully corrected by angioplasty stenting during the same admission. There was one case with urine leak from the ureteric anastomotic site which resolved after conservative management. There were 8 cases of perinephric hematoma detected on postoperative ultrasound, but none interfered with renal scintigraphy image processing.
Table 2.
Details of adverse outcomes occurring within the first 3 months after transplantation
| Patient outcomes | Count (%) |
|---|---|
| Number of cases | 187 (100.0) |
| Underwent graft resection | 3 (1.6) |
| Delay graft function (need for dialysis) | 51 (27.3) |
| Death | 2 (1.1) |
| Serum creatinine at 3 months of ≥ 2.0 mg/dL | 32 (17.1) |
| Three-month composite adverse outcomes | 77 (41.2) |
Renal Scintigraphic Parameters in Predicting Outcomes
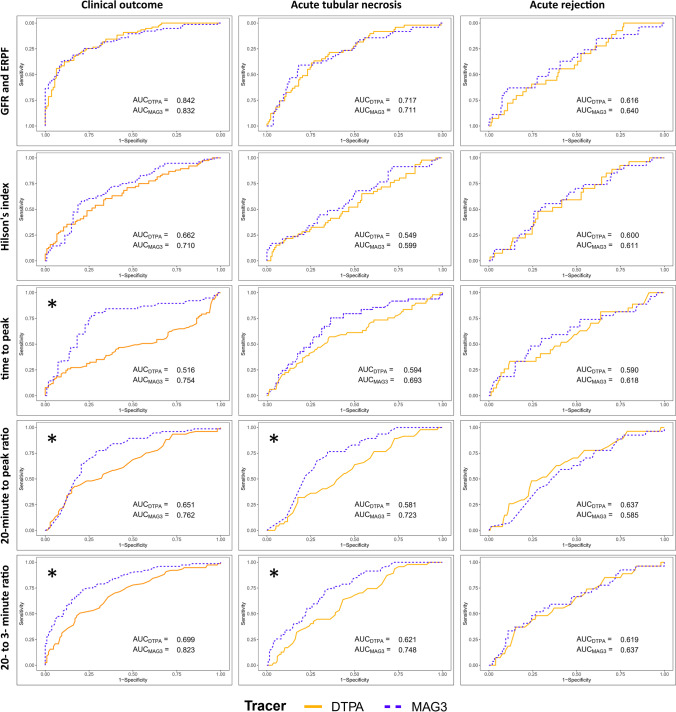
Results of ROC analyses to determine the predictive performance of scintigraphic parameters for prediction of adverse clinical outcomes within the first 3 months after transplantation are presented in Table 3 and ROC curves are presented in Fig. 2. For predicting early adverse clinical outcome, quantified renal functions in terms of GFR (from Tc-99 m DTPA RS) and ERPF (from Tc-99 m MAG3 RS) were the strongest parameters with AUCROC of 0.842 and 0.832, respectively, but there was no difference between GFR and ERPF as determined by DeLong’s test with a p-value of 0.69. Hilson’s perfusion index was only moderately predictive of outcome and there was no difference between Hilson’s perfusion index from Tc-99 m DTPA and Tc-99 m MAG3, although there was a trend toward Tc-99 m MAG3 being more predictive (AUCROC 0.710 vs. 0.662, p-value 0.27). There were three parameters where Tc-99 m MAG3 was found to be superior to Tc-99 m DTPA including time to peak (AUCROC 0.754 vs. 0.516, p-value 0.0001), 20-min to peak ratio (AUCROC 0.762 vs. 0.651, p-value 0.006), and 20-min to 3-min ratio (AUCROC 0.823 vs. 0.699, p-value 0.0005), as determined by DeLong’s test. For acute tubular necrosis, there were two parameters which Tc-99 m MAG3 was found to be superior to Tc-99 m DTPA including 20-min to peak ratio (AUCROC 0.723 vs. 0.581, p-value 0.002) and 20-min to 3-min ratio (AUCROC 0.748 vs. 0.621, p-value 0.004), as shown in Table 4. For acute graft rejection within the first 3 months after KT, parameters derived from either Tc-99 m DTPA or Tc-99 m MAG3 had only modest predictiveness with AUCROC ranging from 0.585 to 0.640 and no significant difference between these two radiopharmaceuticals in any parameter was found, as shown in Table 5.
Table 3.
Comparison of quantitative renal scintigraphy parameters between Tc-99 m DTPA and Tc-99 m MAG3 for prediction of adverse clinical outcomes within 3 months after transplantation
| Parameter | AUCROC (95% CI) | p-value* | Cutoff** | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Quantified function (mL/min) | 0.69 | ||||
| Tc-99 m DTPA (GFR) | 0.842 (0.786, 0.897) | 26.6 | 72.7 | 79.1 | |
| Tc-99 m MAG3 (ERPF) | 0.832 (0.772, 0.893) | 161.9 | 75.3 | 78.2 | |
| Hilson’s perfusion index | 0.27 | ||||
| Tc-99 m DTPA | 0.662 (0.582, 0.743) | 107.1 | 60.1 | 65.7 | |
| Tc-99 m MAG3 | 0.710 (0.634, 0.786) | 114.8 | 57.9 | 79.4 | |
| Time to peak (minutes) | 0.0001 | ||||
| Tc-99 m DTPA | 0.516 (0.426, 0.606) | 1.3 | 29.9 | 86.4 | |
| Tc-99 m MAG3 | 0.754 (0.680, 0.829) | 4.8 | 80.1 | 71.8 | |
| 20-min to peak ratio (%) | 0.006 | ||||
| Tc-99 m DTPA | 0.651 (0.570, 0.731) | 65.5 | 42.9 | 83.0 | |
| Tc-99 m MAG3 | 0.762 (0.692, 0.832) | 86.5 | 76.3 | 71.0 | |
| 20-min to 3-min ratio (%) | 0.0005 | ||||
| Tc-99 m DTPA | 0.699 (0.622, 0.776) | 71.5 | 50.6 | 80.2 | |
| Tc-99 m MAG3 | 0.823 (0.762, 0.885) | 106.5 | 73.7 | 78.5 |
Note: *p-value obtained by using DeLong’s test for comparison of area under receiver operator characteristic curve. **Cutoff values which yield the maximum Youden’s index value
Fig. 2.
Receiver operating characteristic curves of quantified renal scintigraphy parameters from Tc-99 m DTPA and Tc-99 m MAG3 RS for prediction of clinical outcomes, acute tubular necrosis, and acute rejection. *Denotes parameters which Tc-99 m MAG3 has significantly higher predictiveness as determined by DeLong’s test for comparison of areas under ROC curves
Table 4.
Comparison of quantitative renal scintigraphy parameters between Tc-99 m DTPA and Tc-99 m MAG3 for prediction of acute tubular necrosis
| Parameter | AUCROC (95% CI) | p-value* | Cutoff** | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Quantified function (mL/min) | 0.82 | ||||
| Tc-99 m DTPA (GFR) | 0.717 (0.636, 0.798) | 23.5 | 63.3 | 73.2 | |
| Tc-99 m MAG3 (ERPF) | 0.711 (0.624, 0.798) | 127.2 | 59.2 | 81.9 | |
| Hilson’s perfusion index | 0.34 | ||||
| Tc-99 m DTPA | 0.549 (0.451, 0.646) | 178.3 | 21.7 | 89.6 | |
| Tc-99 m MAG3 | 0.599 (0.504, 0.693) | 73.5 | 91.5 | 28.7 | |
| Time to peak (minutes) | 0.15 | ||||
| Tc-99 m DTPA | 0.594 (0.497, 0.691) | 1.6 | 57.1 | 64.5 | |
| Tc-99 m MAG3 | 0.693 (0.606, 0.780) | 6.6 | 63.8 | 75.5 | |
| 20-min to peak ratio (%) | 0.002 | ||||
| Tc-99 m DTPA | 0.581 (0.491, 0.672) | 46.5 | 89.4 | 27.2 | |
| Tc-99 m MAG3 | 0.723 (0.648, 0.800) | 90.5 | 76.6 | 65.4 | |
| 20-min to 3-min ratio (%) | 0.004 | ||||
| Tc-99 m DTPA | 0.621 (0.533, 0.709) | 53.5 | 93.6 | 27.2 | |
| Tc-99 m MAG3 | 0.748 (0.673, 0.823) | 106.5 | 72.3 | 66.9 |
Note: *p-value obtained by using DeLong’s test for comparison of area under receiver operator characteristic curve. **Cutoff values which yield the maximum Youden’s index value
Table 5.
Comparison of quantitative renal scintigraphy parameters between Tc-99 m DTPA and Tc-99 m MAG3 for prediction of acute rejection
| Parameter | AUCROC (95% CI) | p-value* | Cutoff** | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Quantified function (mL/min) | 0.50 | ||||
| Tc-99 m DTPA (GFR) | 0.616 (0.509, 0.772) | 54.4 | 100 | 23.1 | |
| Tc-99 m MAG3 (ERPF) | 0.640 (0.522, 0.759) | 98.5 | 37.0 | 89.4 | |
| Hilson’s perfusion index | 0.89 | ||||
| Tc-99 m DTPA | 0.600 (0.492, 0.708) | 128.8 | 48.1 | 72.1 | |
| Tc-99 m MAG3 | 0.611 (0.499, 0.722) | 115.4 | 67.9 | 55.6 | |
| Time to peak (minutes) | 0.73 | ||||
| Tc-99 m DTPA | 0.590 (0.469, 0.711) | 3.1 | 33.3 | 88.1 | |
| Tc-99 m MAG3 | 0.618 (0.494, 0.742) | 19.2 | 55.6 | 70.0 | |
| 20-min to peak ratio (%) | 0.32 | ||||
| Tc-99 m DTPA | 0.637 (0.528, 0.747) | 59.5 | 62.9 | 61.5 | |
| Tc-99 m MAG3 | 0.585 (0.475, 0.695) | 92.5 | 59.3 | 59.0 | |
| 20-min to 3-min ratio (%) | 0.77 | ||||
| Tc-99 m DTPA | 0.619 (0.505, 0.734) | 79.5 | 37.0 | 84.6 | |
| Tc-99 m MAG3 | 0.637 (0.520, 0.754) | 134.5 | 51.9 | 73.1 |
Note: *p-value obtained by using DeLong’s test for comparison of area under receiver operator characteristic curve. **Cutoff values which yield the maximum Youden’s index value
Discussion
In this retrospective cohort study, we determined that renal scintigraphy using Tc-99 m MAG3 was superior to Tc-99 m DTPA in terms of predicting early adverse clinical outcomes comprised of important early clinical adverse graft-related complications, during the first 3 months after KT. Parameters in which Tc-99 m MAG3 RS were found to be significantly superior to Tc-99 m DTPA were those related to the excretion phase of the renogram, including time to peak, 20-min to peak ratio, and 20-min to 3-min ratio. This is likely explained by the fact that Tc-99 m MAG3 has a higher extraction fraction than Tc-99 m DTPA [4] which results in better quality RS images and renograms. The renogram obtained from Tc-99 m DTPA had less variation between those with and without adverse outcomes, whereas Tc-99 m MAG3 showed more distinct differences. A good example is the 20-min to 3-min ratio, which in the Tc-99 m DTPA RS had a narrower range of 23–130% compared with a corresponding range of 26–271% from Tc-99 m MAG3 RS, with higher values associated with adverse outcomes. The 20-min to peak and 20-min to 3-min ratios were also superior for predicting a pathological finding of acute tubular necrosis. This is likely because cortical retention of Tc-99 m MAG3 is a prominent feature in this condition due to the tubular dysfunction which is characteristic of this complication [12, 13], thus causing these parameters to be higher in patients with acute tubular necrosis. For predicting acute graft rejection, since Tc-99 m MAG3 is a tubular secreting radiopharmaceutical and the most common rejection type, acute antibody-mediated rejection has been linked to peritubular capillaritis which affects peritubular blood flow and renal tubular function [14], cortical retention of Tc-99 m MAG3 should be theoretically prominent in graft rejection, and result in the 20-min to peak and 20-min to 3-min ratio from Tc-99 m MAG3 RS is more predictive than Tc-99 m DTPA RS. However, this was not the case in our study since there were no significant differences between these parameters from the two radiopharmaceuticals. This could be due to the small number of rejections in the cohort which was not enough to demonstrate a significant difference. Moreover, the overall AUCROC of all parameters regardless of radiopharmaceutical were at best only modest in predicting the graft rejection and kidney biopsy is still needed for diagnosis in suspected cases.
Quantified functions in terms of GFR from Tc-99 m DTPA RS and ERPF from Tc-99 m MAG3 RS were highly predictive of early adverse outcome with rather high AUCROC values of 0.842 and 0.832, respectively. Previous studies have determined that early renal function after transplantation in terms of serum creatinine level is related to longer term function and graft survival [15, 16]. Both GFR and ERPF obtained from RS are also measures of renal function; therefore, they are expected to be good predictors of allograft function and outcome. However, there was no difference in the predictive performance between GFR and ERPF. This is not beyond expectation, since estimations of GFR and ERPF are both based on the degree of accumulation of the radiopharmaceutical in the early phase of the renogram before the beginning of the excretory phase [17, 18]. Perfusion was also moderately predictive of early adverse outcomes as represented by Hilson’s perfusion index. Impaired perfusion as determined by either Tc-99 m DTPA or Tc-99 m MAG3 has traditionally been associated with acute rejection [7, 19] and poorer perfusion has also been linked to poorer graft function and graft loss [20, 21]. We found no difference in predictive power of Hilson’s perfusion index obtained from Tc-99 m DTPA RS and Tc-99 m MAG3 RS to forecast early adverse outcomes. This is expected since Hilson’s perfusion index is typically assessed within the first seconds after radiotracer injection and is therefore less likely to be significantly impacted by functional radiopharmaceutical kinetics.
Few studies have compared the predictiveness of Tc-99 m DTPA RS and Tc-99 m MAG3 RS. One study compared Tc-99 m DTPA RS and Tc-99 m MAG3 RS for diagnosis of chronic allograft nephropathy and acute rejection in 48 patients. The study found that the patterns of perfusion time-activity curves were different among normal grafts, chronic allograft nephropathy, and acute rejection. Hilson’s perfusion index and peak-to-plateau ratio were also examined. However, function parameters were not examined [22]. Our study differs from the aforementioned study in that we examined both function and perfusion parameters, those commonly reported in routine RS. Moreover, the outcome measures in our study were also different, i.e., clinical outcome, acute rejection, and acute tubular necrosis. All patients received RS with both radiopharmaceuticals so the variation between patients affecting the adverse outcomes was not an issue. The patient cohort had typical characteristics, i.e., middle aged, with typical underlying diseases, most had undergone deceased-donor kidney transplantations from standard criteria donors. Adverse outcome was found in 41.2% of patients, most of which was due to delayed graft function and failure of serum creatinine to decline. This figure is similar to that found in previous studies [23, 24]. These typical patient and adverse outcome profiles should make the study findings generalizable.
Although the key strength of this study is a head-to-head comparison of Tc-99 m DTPA RS and Tc-99 m MAG3 RS, there are some limitations. Because of the retrospective nature, covariates could not be controlled; however, all RS were done using the same standard protocol of our center which should minimize variation. The residual activity at the end of the Tc-99 m DTPA RS may have interfered with the Tc-99 m MAG3 RS, which was done immediately after. However, the interference should not have significantly impacted the results since the dose of Tc-99 m MAG3 was 5 times higher than that of Tc-99 m DTPA which should negate the small amount of shine-through from the end of the Tc-99 m DTPA study. Another issue that might be of concern is the low dose (37 MBq) of Tc-99 m DTPA used in this study which is lower than what is recommended in most guidelines. However, this should have no substantial impact on the study findings. In most cases, the Tc-99 m DTPA RS had adequate image quality for image processing, and when in doubt, the images were compared with the Tc-99 m MAG3 RS to ensure comparable ROI placement. Moreover, the five parameters reported in this study should be reliably compared despite the lower dose of Tc-99 m DTPA. The GFR (Tc-99 m DTPA) and ERPF (Tc-99 m MAG3) as well as Hilson’s perfusion index obtained from both radiopharmaceuticals should be comparable since they are obtained in the early phases of the renogram before tracer kinetics take effect which explains the lack of difference in predictiveness. As for time to peak, 20-min to peak ratio, and 20-min to 3-min ratio, there were discrepancies between Tc-99 m DTPA and Tc-99 m MAG3, which is likely not due to the lower dose of the former, but due to the different mechanism of excretion. For example, in cases with acute tubular necrosis, there was significant cortical retention of Tc-99 m MAG3 resulting in the higher values of these three parameters compared with Tc-99 m DTPA which showed only poor uptake and not progressive cortical retention; even if the dose of DTPA were to be increased, the findings would not likely change.
Conclusion
Tc-99 m MAG3 RS is likely superior to Tc-99 m DTPA RS for prediction of early adverse outcomes during the first 3 months post-kidney transplantation. Tc-99 m MAG3 RS parameters which had superior predictiveness were time to peak, 20-min to peak ratio, and 20-min to 3-min ratio. GFR derived from Tc-99 m DTPA RS and ERPF measured by Tc-99 m MAG3 RS were comparable in their high predictiveness of early adverse clinical outcomes.
Acknowledgements
We would like to acknowledge Professor John F Smith, for english editing the MS via Publication Clinic KKU, Thailand.
Author Contribution
The study was designed by Daris, Bandit, and Sirirat. Material preparation and data collection were performed by Daris. The data analysis was performed by Daris and Sirirat. The first draft of the manuscript was written by Daris, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
Please contact the corresponding author for data requests.
Declarations
Conflict of Interest
Daris Theerakulpisut, Bandit Thinkhamrop, and Sirirat Anutrakulchai declare no conflict of interest.
Ethics Approval
The study was reviewed and approved by the Khon Kaen University Ethics Committee for Human Research (reference number HE631182). Since all data were obtained from available medical records and imaging databases, the informed consent requirement was waived. All procedures performed in studies involving human participants were in accordance with the Helsinki declaration as revised in 2013 and its later amendments.
Consent for Publication
Not applicable
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daris Theerakulpisut, Email: daris.th@gmail.com.
Bandit Thinkhamrop, Email: karawa@gmail.com.
Sirirat Anutrakulchai, Email: sirirt_a@kku.ac.th.
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia GG, Harden P, Chapman J, World Kidney Day Steering Committee The global role of kidney transplantation. Curr Opin Organ Transplant. 2012;17:362–7. doi: 10.1097/MOT.0b013e328354c277. [DOI] [PubMed] [Google Scholar]
- 3.Thiruchelvam PTR, Willicombe M, Hakim N, Taube D, Papalois V. Renal transplantation. BMJ. 2011;343:d7300. doi: 10.1136/bmj.d7300. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AT. Radionuclides in nephrourology, part 1: radiopharmaceuticals, quality control, and quantitative indices. J Nucl Med. 2014;55:608–615. doi: 10.2967/jnumed.113.133447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bubeck B, Brandau W, Weber E, Kälble T, Parekh N, Georgi P. Pharmacokinetics of technetium-99m-MAG3 in humans. J Nucl Med. 1990;31:1285–1293. [PubMed] [Google Scholar]
- 6.Eshima D, Taylor A. Technetium-99m (99mTc) mercaptoacetyltriglycine: update on the new 99mTc renal tubular function agent. Semin Nucl Med. 1992;22:61–73. doi: 10.1016/S0001-2998(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 7.Hilson AJ, Maisey MN, Brown CB, Ogg CS, Bewick MS. Dynamic renal transplant imaging with Tc-99m DTPA (Sn) supplemented by a transplant perfusion index in the management of renal transplants. J Nucl Med. 1978;19:994–1000. [PubMed] [Google Scholar]
- 8.Hilson AJ. The renal transplant perfusion index: where are we now? Eur J Nucl Med. 1991;18:227–228. doi: 10.1007/BF00186644. [DOI] [PubMed] [Google Scholar]
- 9.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2020. https://www.R-project.org. Accessed 12 Jul 2020.
- 10.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wickham H. ggplot2: elegant graphics for data analysis. Springer-Verlag. 2016. https://ggplot2.tidyverse.org. Accessed 12 Jul 2020.
- 12.Dubovsky EV, Russell CD, Erbas B. Radionuclide evaluation of renal transplants. Semin Nucl Med. 1995;25:49–59. doi: 10.1016/S0001-2998(05)80006-6. [DOI] [PubMed] [Google Scholar]
- 13.Khor YM, Lam WWC, Wong WY, Whatt Goh AS. Role of nuclear medicine imaging in evaluation of complications following renal transplant. Proc Singap Healthc. 2015;24:233–242. doi: 10.1177/2010105815611813. [DOI] [Google Scholar]
- 14.Racusen LC, Bagnasco SM. Peritubular capillaritis in the renal allograft takes center stage. Kidney Int. 2015;88:218–220. doi: 10.1038/ki.2015.99. [DOI] [PubMed] [Google Scholar]
- 15.Johnston O, O’kelly P, Spencer S, Donohoe J, Walshe JJ, Little DM, et al. Reduced graft function (with or without dialysis) vs immediate graft function–a comparison of long-term renal allograft survival. Nephrol Dial Transpl. 2006;21:2270–4. doi: 10.1093/ndt/gfl103. [DOI] [PubMed] [Google Scholar]
- 16.Aitken E, Cooper C, Dempster N, McDermott M, Ceresa C, Kingsmore D. Delayed graft function is a syndrome rather than a diagnosis. Exp Clin Transpl. 2015;13:19–25. [PubMed] [Google Scholar]
- 17.Gates GF. Glomerular filtration rate: estimation from fractional renal accumulation of 99mTc-DTPA (stannous) Am J Roentgenol. 1982;138:565–570. doi: 10.2214/ajr.138.3.565. [DOI] [PubMed] [Google Scholar]
- 18.Fine EJ, Axelrod M, Gorkin J, Saleemi K, Blaufox MD. Measurement of effective renal plasma flow: a comparison of methods. J Nucl Med. 1987;28:1393–1400. [PubMed] [Google Scholar]
- 19.Carmody E, Greene A, Brennan P, Donohue J, Carmody M, Keeling F. Sequential Tc 99m mercaptoacetyl-triglycine (MAG3) renography as an evaluator of early renal transplant function. Clin Transplant. 1993;7:245–249. [PubMed] [Google Scholar]
- 20.Aktas A, Demirag A, Moray G, Karakayali H, Haberal M. Comparison of the scintigraphically determined function of the transplanted kidney before and after transplantation. Transplant Proc. 1999;31:3324–3325. doi: 10.1016/S0041-1345(99)00811-8. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SK, Lewis G, Rogers K, Attia J. Quantitative Tc-99m DTPA renal transplant scintigraphy predicts graft survival in the very early postoperative period. Nucl Med Commun. 2012;33:1292–1299. doi: 10.1097/MNM.0b013e328359db96. [DOI] [PubMed] [Google Scholar]
- 22.Aktaş A, Aras M, Colak T, Gençoğlu A, Karakayali H. Comparison of Tc-99m DTPA and Tc-99m MAG3 perfusion time-activity curves in patients with renal allograft dysfunction. Transplant Proc. 2006;38:449–453. doi: 10.1016/j.transproceed.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Wu WK, Famure O, Li Y, Kim SJ. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 2015;88:851–858. doi: 10.1038/ki.2015.190. [DOI] [PubMed] [Google Scholar]
- 24.Melih KV, Boynuegri B, Mustafa C, Nilgun A. Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation. Transplant Proc. 2019;51:1096–1100. doi: 10.1016/j.transproceed.2019.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the corresponding author for data requests.