Abstract
International physical activity guidelines recommend that older adults accumulate 150 min/week of moderate-vigorous physical activity (MVPA). It is unclear whether meeting this recommendation is associated with better higher-order cognitive functions and if so, what are the neurophysiological mechanisms responsible for such a relationship. We tested the hypothesis that meeting MVPA guidelines is associated with better executive function in older adults, and explored if greater increases in prefrontal cortex oxygenation are implicated. Older adults who did (active, n = 19; 251 ± 79 min/week) or who did not (inactive, n = 16; 89 ± 33 min/week) achieve activity guidelines were compared. Executive function was determined via a computerized Stroop task while changes in left prefrontal cortex oxygenation (ΔO2Hb) were measured with functional near-infrared spectroscopy. Aerobic fitness ( 2peak) was determined using a graded, maximal cycle ergometry test. MVPA and sedentary time were objectively assessed over 5 days. Both groups had similar (both, P > 0.11) levels of aerobic fitness (24.9 ± 8.9 vs. 20.9 ± 5.6 ml/kg/min) and sedentary time (529 ± 60 vs. 571 ± 90 min/day). The active group had faster reaction times (1193 ± 230 vs. 1377 ± 239 ms, P < 0.001) and greater increases in prefrontal cortex ΔO2Hb (9.4 ± 5.6 a.u vs. 5.8 ± 3.4 a.u, P = 0.04) during the most executively demanding Stroop condition than the Inactive group. Weekly MVPA was negatively correlated to executive function reaction times (r = − 0.37, P = 0.03) but positively correlated to the ΔO2Hb responses (r = 0.39. P = 0.02) during the executive task. In older adults, meeting MVPA guidelines is associated with better executive function and larger increases in cerebral oxygenation among older adults.
Keywords: Cognitive aging, Habitual physical activity, Stroop task, Cerebral oxygenation, Objectively measured activity
Introduction
Advancing age is associated with a progressive decline in higher-order cognitive processes [1], known as executive functions that encompass decision-making, problem solving, planning and sequencing of responses, and multitasking. Adverse impairments in executive functions represent early warning signs implicated in the development of major neurocognitive disorders and diseases (e.g., dementia, Alzheimer’s disease) [2, 3]. However, these cognitive processes may be favorably modified through healthy lifestyle behaviors [4]. Specifically, engagement in sufficient physical activity may be a therapeutic strategy to preserving or improving executive function in older adults.
It is well established that greater aerobic fitness levels (i.e., V̇O2max) are associated with better executive function in older adults [5–7]. The neurophysiological mechanisms linking aerobic fitness and executive function are due, at least in part, to alterations in the oxygenation of the prefrontal cortex, the brain region responsible for the initiation of executive functions [8, 9]. Specifically, older females with higher aerobic fitness exhibited a faster reaction time and a greater increase in prefrontal oxygenation (ΔO2Hb) response than older females with lower aerobic fitness during the executive condition of the Stroop task [5]. Furthermore, Mekari et al. [6] observed that left prefrontal cortex ΔO2Hb mediated the relationship between aerobic fitness and Trail B response time (index of executive function), with more aerobically fit older adults exhibiting an inverse relationship between aerobic fitness and Trail B time that was not observed among less aerobically fit participants.
Physical activity recommendations suggest that older adults engage in 150 min of moderate-vigorous aerobic physical activity (MVPA) per week for health benefits [10, 11]. However, only ~ 12% of older adults achieve such guidelines [12]. Also, older adults typically spend the majority of their waking day (i.e., > 10 h) engaged in sedentary behaviors (e.g., sitting or lying down; [13]). Despite the evidence for the favorable influence of aerobic fitness on higher-order cognitive functions [5, 6], considerably less is known about the influence of objectively measured habitual activity on executive functions in older adults. Using wrist-worn accelerometry, Barnes et al. [14] observed faster executive function (via Trail Making Test) in older females who had more daily movement time, as determined via the mean number of accelerometry counts per day. Although interesting, this method of quantifying activity from accelerometer provides a poor resolution regarding the intensity of the activity (e.g., a high amount of light activity and low amount of vigorous activity would produce equivalent values). Furthermore, Kerr et al. [15] observed a positive relationship between time spent in waist-worn accelerometry-determined MVPA and executive function (via Trail Making Test) in older adults. A small sub-sample (10% of participants) achieved 30 min of MVPA per day, which was associated with a 20% faster speed in the executive function test [15]. Physical activity level and aerobic fitness are different constructs [16, 17] that are weakly-moderately related [18] with aerobic fitness impacted by independent factors (i.e., genetics, body composition, and sex) [19, 20]. The above studies [14, 15] did not fully consider the impact of aerobic fitness, which could confound the determination of physical activity level and executive function if their more active older adults were also more aerobically fit. Furthermore, the neurophysiological mechanisms equating objectively measured MVPA and executive function have not been fully explored in this population.
Given national and international recommendations are grounded in intensity-related physical activity [10, 11], we sought to determine the influence of engaging in MVPA and specifically meeting the recommended 150 min of MVPA per week on executive function. We tested the hypothesis that meeting physical activity guidelines (i.e., more MVPA) is associated with better (i.e., faster) executive function in older adults who do (active) versus do not (inactive) achieve these guidelines. Objective measures of aerobic fitness and habitual sedentary time were conducted as well. In addition, we determined prefrontal cortex oxygenation responses during the Stroop task to provide mechanistic insight as to whether more time spent engaging in MVPA was associated with larger oxygenation responses.
Methods
Participants
Thirty-five older adults were recruited from the Acadia University Active Aging program and dichotomized into those who achieved ≥ 150 min of objectively measured MVPA per week (active, n = 19; 9♀) versus those who did not (inactive, n = 16; 12♀). These two groups were compared cross-sectionally. Based on an estimated moderate effect size (r = 0.50) between MVPA and our outcome measures (reaction time, ΔO2Hb), a sample size calculation estimated that 26 participants were needed assuming a two-tailed, α = 0.05 and β = 80% power (G*Power, v3.1 [21]). Participants had no physical limitations to exercise and a resting blood pressure < 140/90 mmHg. In the present study, an older adult was defined as > 55 years of age [22]; however, the proportion of participants > 65 years are presented for completeness. All participants were healthy and had normal-to-corrected vision. None of the participants had a history of neurological or psychiatric disorders, color blindness, surgery with general anesthesia during the previous 6 months, involuntary tremors, epilepsy, or drug/alcohol problems. Participants were excluded if they scored < 25 out of 30 on the Mini-Mental State Examination. All criteria were assessed during a telephone screening and the first meeting at the research center with a certified exercise physiologist. Four participants were taking levothyroxine for hypothyroidism (inactive: n = 3; active: n = 1). To treat high-blood pressure, two of these same participants (inactive: n = 1; active: n = 1) were prescribed nifedipine (calcium channel blocker) and eprosartan (angiotensin-receptor blocker). Participants continued taking all prescribed medications throughout the duration of the study. Research Ethics Board approval was attained from Dalhousie University and Acadia University. Participants were informed of the methods and study design verbally and in writing before providing written informed consent. Participants’ aerobic fitness and habitual activity data have been presented in determining the influence of habitual activity with peripheral vascular function [23] or baroreflex function [24]. One inactive participant in [24] did not participate in the cognitive testing aspect of the study and therefore is not included (inactive: n = 16). Most participants’ (n = 32) reaction time (not cerebral oxygenation or habitual activity) have been presented in determining the impact of difference exercise interventions on cognitive function [25]. The analyses and purpose in the present study are unique and used to answer an independent research question.
Experimental design
Participants reported for two separate laboratory experimental sessions in a thermoneutral environment (21 °C). During the first session, participants signed the consent form and completed questionnaires on their health and mental status followed by a clinical neuropsychological test (Stroop task; described below). Participants concluded the session with a continuous, graded, maximal cycling exercise test (see details below). The second session consisted of being equipped with the activity monitors for a 5-day measurement period. Visits 1 and 2 were separated by a minimum of 48 h. Participants were asked to refrain from consuming caffeine, smoking, and drinking alcohol within 6 h of any testing. Assessments were performed 6-h post-prandial and after participants avoided vigorous physical activity for a minimum of 24 h.
Experimental outcomes
Anthropometrics and peak aerobic fitness
Height and weight were measured using a calibrated stadiometer (Health-O-Meter, McCook Il, USA) to the nearest 0.5 cm and 0.1 kg, respectively. An incremental and maximal exercise test on a cycle ergometer (Lode Excalibur Sport, Groningen, The Netherlands) was administered to determine peak oxygen consumption (V̇O2peak) via a mixing chamber-based commercial metabolic system (TrueOne 2400®, Parvomedics Inc., Sandy, UT) in a laboratory setting. The determination of maximal aerobic fitness is described in detail elsewhere [25, 26]. Following a 5-min warm-up period of light-intensity cycling (30–50 W), the workload was set at 1 W/kg body mass and gradually increased by 15 W/min until voluntary exhaustion. Strong verbal encouragement was provided throughout the test. Upon completion of the test, the workload was immediately reduced to the warm-up level for a 5-min cool-down period. The primary criterion for the attainment of V̇O2max was a plateau in relative V̇O2 (change < 2.1 ml/min/kg) despite an increase in workload. In the absence of a plateau, attainment of V̇O2peak was based upon a respiratory exchange ratio of ≥ 1.10 and the inability to maintain a pedaling cadence of 60 revolutions/min. Furthermore, V̇O2max was considered to be the highest V̇O2 value attained during the test if the following criteria were observed: (1) a respiratory exchange ratio ≥ 1.10 and (2) a peak heart rate ≥ 95% age-predicted maximum (i.e., 220–age). Most participants (n = 19) attained both criteria and therefore the term V̇O2max is used throughout.
Activity monitors
Participants wore a PiezoRx (StepsCount, ON, Canada) during all waking hours and an activPAL inclinometer (Pal Technologies Ltd. Glasgow, UK) 24 h/day concurrently for 5 full days (including at least 1 weekend day), consistent with recommendations for activity monitoring in older adults [27] and considerations for habitual activity monitoring [28]. The PiezoRx is a valid measure of step count [29] and physical activity intensity [29, 30]. The PiezoRx is a medical grade physical activity monitor that uses step rate thresholds to determine time spent in light- (LPA), moderate- (MPA), and vigorous-intensity physical activity (VPA). Based on the observations of our lab [29, 31, 32], the step rate thresholds for moderate-intensity (i.e., 3 metabolic equivalents) and vigorous-intensity (i.e., 6 metabolic equivalents) physical activity in absolute terms were set at 110 and 130 steps per minute, respectively. Placement of the PiezoRx was standardized by securing it on their waistband or belt in line with their right mid-thigh as per manufacturer’s recommendations. The activPAL inclinometer was used to determine the amount of waking time spent standing versus sitting/lying. The activPAL was waterproofed and secured using Tegaderm™ transparent medical dressing to the midline of their right thigh, one-third of the way between the hip and the knee. ActivPAL protocols were based on previous research outlining important considerations for field-based research [28].
Cognitive tests
Participants initially completed the Mini-Mental State Exam, with scores ranging from 27 to 30 across all participants. As previously described [25], participants completed a computerized, modified Stroop task consisting of 3 conditions using their right hand in the same order (naming, interference, switching). The answers were mapped to the letters “u,” “i,” “o,” and “p” on a keyboard, with the specific finger designations as “index finger (u) – red,” “middle finger (i) – green,” “ring finger (o) – blue,” and “little finger (p) – yellow.” Before each condition, participants completed 12 practice trials for condition 1 (naming; congruent naming) and condition 2 (interference) but 20 practice trials for condition 3 (switching). Each condition consisted of 60 trials composed of a fixation cross appearing in the center of the screen for 500 ms, followed by presentation of the word for 3000 ms. Participants rested in the seated position between conditions (5 min).
In the first condition (naming), the participant read 1 of 4 words (RED, BLUE, YELLOW, or GREEN) and pressed the key that corresponded to the same colour as their meaning (e.g., pressed “u” when RED is written in red font). This condition is the simplest condition and provides a measure of processing speed. The second condition consisted of an interference task, which required identifying the color of a color-word, the meaning of the word being incongruent with the colour itself (e.g. press the “i” key when the word BLUE is written in green font). This condition is more difficult, as it requires participants to inhibit their reading of the color word and only respond to the color of the font. The third condition consisted of a switching task, which was identical to the interference task, except that for 25% of the trials (15 of 60 trials), a square appeared instead of the fixation cross, and participants were asked to identify the color-word, instead of naming its colour (e.g., press the “o” key when the word BLUE is written in green font). The reading trials appeared randomly throughout the block, which makes this condition one of cognitive flexibility as the participant had to remember the rules (square vs fixation cross) and respond based on these rules. During practice and experimental trials, a visual feedback (“Error”) was given for incorrect responses only. Reaction times (ms) and total errors were recorded. There were no accuracy-speed trade-off observed and all participants demonstrated a high accuracy (condition 1 and condition 2: > 98% accuracy; condition 3: > 90% accuracy), as expected [5]. Therefore, only reaction times are presented.
Cerebral oxygenation
The concentration changes of HbO2 (ΔO2Hb) and HHb (ΔHHB) were acquired with the PortaLite fNIRS system (Artinirs Medical Systems, Elst, Netherlands). Neither a Stroop nor group effect was observed for ΔHHb (all, P > 0.11); therefore, only ΔO2Hb values are presented. Cerebral oxygenation was recorded on the left side of the prefrontal cortex in accordance with existing literature showing sensitivity of tests of executive function to frontal regions in the left hemisphere [33]. Specifically, the functional near infra-red spectroscopy probe was placed on the participant’s left forehead on the Fp1 prefrontal cortex region, using the 10/20 positioning system, which refers to the distances between adjacent electrodes covering 10–20% of the front of the skull. The probe was tightly secured with a black bandage wrapped around the participant’s forehead to reduce the amount of interference from background light. The Portalite system uses Bluetooth technology, which allows participants to walk and move without the restriction of the wires. A 2-wavelength continuous measurement system was used in accordance with the absorption characteristics of light, with standard wavelengths of 760 nm and 850 nm. Optode distances between the receiver and transmitters were 30, 35, and 40 mm. The absorption of which was measured, and concentration changes in O2Hb were calculated using the difference in absorbance based on the Beer-Lambert law. Because continuous-wave technology does not measure optical path lengths [34], only changes in the concentration of O2Hb relative to baseline (see below) could be inferred assuming both a path length factor and partial volume.
Continuous NIRS measurements were recorded during the 3 conditions of the Stroop task. “Events” were manually inserted into the test’s time frame to indicate a change of condition (i.e., “event 1- begin Stroop 1”), to ensure that separation between the Stroop conditions was specified for data analysis. Data were averaged over every task component (baseline, Stroop 1, Stroop 2, Stroop 3) and normalized to express the magnitude of change from the baseline period. The baseline period occurred immediately before the Stroop task in which participants sat quietly and were asked to close their eyes to eliminate external visual stimuli and establish a 60-s baseline of O2Hb data (last 60 s of a 5-min rest period). Continuous O2Hb data throughout the Stroop task were sampled at 10 Hz and filtered with a Savitzky-Golay smoothing algorithm before analysis. All data analyses were completed in the Oxysoft analysis software (Artinis Medical Systems, Netherlands).
Data analysis
Due to equipment malfunctions, one participants’ activPAL data (active group) and one participants’ O2Hb data (inactive group) were removed. Therefore, sedentary/standing time and cerebral oxygenation are presented for the remaining participants (n = 34). All activity monitor data were analyzed by an investigator blind to the cognitive data, and vice versa.
Relative V̇O2max data were averaged over 15-s intervals for the duration of the graded exercise protocol. Peak V̇O2 was considered the greatest 30-s averaged V̇O2max. All participants wore the PiezoRx for 5 days with a minimum 10 h of wear time per day. The PiezoRx data were then multiplied to estimate one full week of activity. Participants self-reported their daily PiezoRx wear time and waking hours to accommodate activPAL analysis. ActivPAL data were analyzed using a customized LabVIEW program (LabVIEW 2013; National Instruments, Austin, TX) that confirmed waking hours and summarized daily averages of awake time spent standing, sitting, and lying down.
Reaction times for each trial (60 trials per condition) were averaged for each of the 3 Stroop test conditions. Oxyhemoglobin measures were averaged during each condition and presented as an absolute increase (ΔO2Hb) from the baseline period.
Statistical analysis
All data were assessed for normality using a Shapiro–Wilk test, and non-normalized data were log-transformed prior to statistical analysis. Baseline descriptive characteristics, pedometer wear time, V̇O2max, habitual physical activity time, sedentary time, and standing time were compared between active and inactive groups via independent samples t-tests. The proportion of males/females or those younger/older than 65 years between groups was compared via chi-square analysis. The effects of meeting physical activity guidelines on Stroop test reaction times and ΔO2Hb were compared using a between-subjects (group (active, inactive) × Stroop condition (naming, interference, switching)) repeated measures analysis of variance (ANOVA). The variance of differences was assessed using Mauchly’s test of sphericity and when violated, the Greenhouse–Geisser correction to the degrees of freedom was applied. Bonferroni post hoc testing was conducted on statistically significant ANOVAs. The repeated measure ANOVAs for reaction time and ΔO2Hb were also conducted with the inclusion of aerobic fitness or sedentary time as covariates to determine the independent effects of each variable. Some multicollinearity was observed with MVPA being weakly-moderately related to aerobic fitness (r = 0.45; P = 0.01) but not sedentary time (P = 0.19).
Pearson’s correlations evaluated the relationship between V̇O2max, step counts, LPA, MVPA time, standing time, and sedentary time versus Stroop test reaction times for each condition and ΔO2Hb data for each condition in the pooled sample. Partial correlations controlling for aerobic fitness or sedentary time were conducted between MVPA with reaction time and ΔO2Hb for the Switching condition. All statistics were completed in SPSS Version 26.0 (IBM, NY). Statistical significance was accepted as p < 0.05. All data are presented as means ± standard deviations.
Results
There were no differences in age, body mass index, V̇O2max, or sedentary time between the groups (Table 1). The active group accumulated more steps per day and spent more time per week engaged in light, moderate, and vigorous physical activity (Table 1). The active (h/day) and inactive reported similar (both, P > 0.51) PiezoRx wear times (14.1 ± 1.4 vs. 14.1 ± 1.4 h/day) and waking hours (15.4 ± 1.3 vs. 15.6 ± 1.0 h/day).
Table 1.
Participant descriptive characteristics and activity-related data in those who met the MVPA guidelines (active) and those who did not (inactive)
| Active (n = 19) | Inactive (n = 16) | P-value | |
|---|---|---|---|
| Participant characteristics | |||
| Age (years) | 66 ± 5 [57–73] | 68 ± 7 [56–83] | 0.24 |
| Age (> 65 years; %) | 13 (68%) | 10 (65%) | 0.61 |
| Sex (males, females) | 10♂, 9♀ | 4♂, 12♀ | 0.10 |
| Body mass index (kg/m2) | 25 ± 3 [21–33] | 28 ± 5 [19–37] | 0.13 |
| V̇O2max (mlO2/kg/min) | 24.9 ± 8.9 [13–45] | 20.9 ± 5.6 [13–33] | 0.13 |
| Habitual activity | |||
| Step count (steps/day) | 11,402 ± 3305 [5318–17535] | 6734 ± 1601 [4747–9727] | < 0.001 |
| LPA (mins/week) | 521 ± 192 [158–985] | 378 ± 127 [118–647] | 0.01 |
| MVPA (mins/week) | 251 ± 79 [151–424] | 89 ± 33 [44–137] | < 0.001 |
| MPA (mins/week) | 233 ± 72 [141–401] | 79 ± 17 [35–134] | < 0.001 |
| VPA (mins/week) | 18 ± 13 [1–57] | 10 ± 8 [2–26] | 0.04 |
| Standing time (mins/day) | 393 ± 71 [257–486] | 365 ± 71 [276–550] | 0.25 |
| Sedentary time (mins/day) | 529 ± 60 [405–648] | 571 ± 90 [379–704] | 0.11 |
Data are presented as means ± SD. LPA, light physical activity; MVPA, moderate-vigorous physical activity; MPA, moderate physical activity; VPA, vigorous physical activity; V̇O2max, maximal oxygen uptake. Data were analyzed using independent samples t-tests, with the exception of the proportion of each sex and adults > 65 years (chi-square)
Values presented in bold are considered statistically significant (p < 0.05)
Reaction times
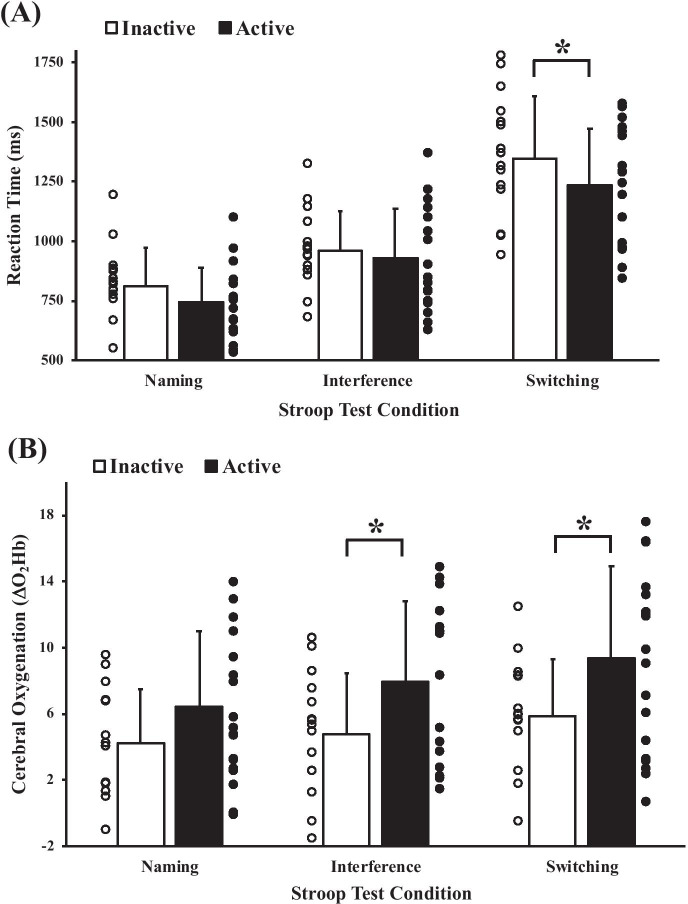
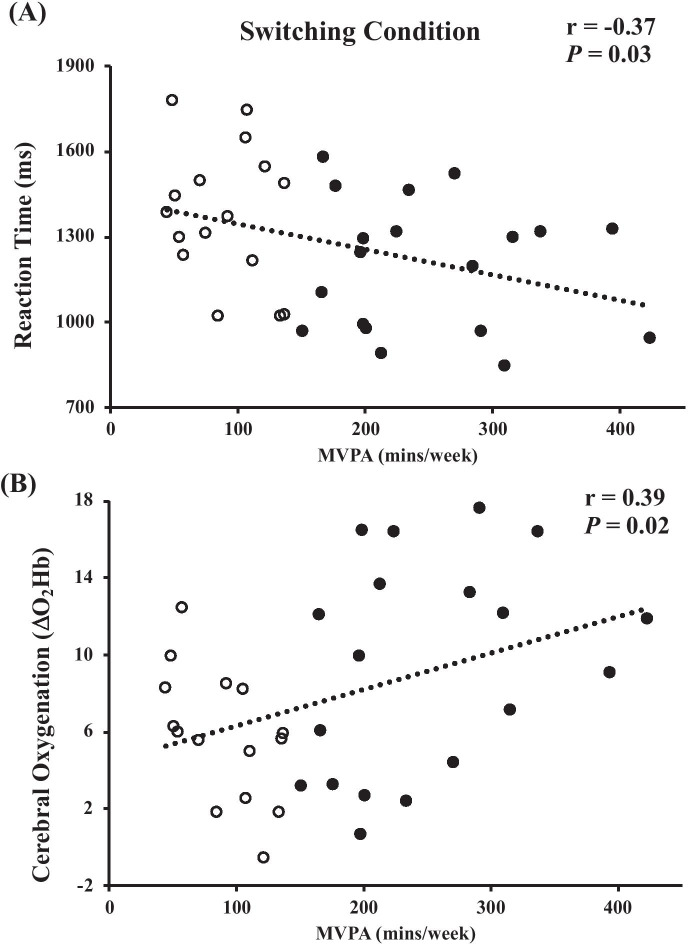
As expected, reaction times were faster during the interference condition (active: 927 ± 209 ms; inactive: 958 ± 167 ms) in comparison to the switching condition (active: 1193 ± 230 ms; inactive: 1377 ± 239 ms; P < 0.001), which were both slower versus the naming condition (active: 744 ± 146 ms; inactive: 814 ± 159 ms; both, P < 0.001). As presented in Fig. 1A, The active and inactive groups produced similar reaction times for the naming and interference conditions (both, P > 0.19), but the active group was faster during the switching condition (P = 0.03). These results were unchanged (switching: P = 0.03) after controlling for sedentary time but accounting for aerobic fitness resulted in no between-group differences (switching: P = 0.09). Weekly MVPA was negatively correlated to reaction time during the switching condition (Fig. 2A; r = − 0.37, P = 0.03), but not within the naming (r = − 0.03, P = 0.86) or interference (r = 0.02, P = 0.91) conditions. Similarly, V̇O2max was negatively associated with the switching condition reaction times (r = − 0.43, P = 0.01). Controlling for V̇O2max, but not sedentary time (P = 0.05), eliminated the relationship between MVPA and switching condition reaction time (r = − 0.21, P = 0.24). No other relationships were observed between reaction times of any condition with step count, LPA, V̇O2max (naming and interference conditions only), sedentary time, or standing time (r-range: − 0.30–0.30; all, P > 0.09).
Fig. 1.
Comparison of Stroop task reaction times (A) and prefrontal cortex oxygenation responses (B) between older adults who meet physical activity guidelines (active) and those who do not (inactive). Group data are presented as means ± SD. Individual data are presented as the circles (active: black circles; inactive: white circles). Data were analyzed via a mixed group (active, inactive) by Stroop task condition (naming, interference, switching) repeated measures ANOVA. Bonferroni post hoc testing was conducted on statistically significant ANOVAs. *P < 0.05. A main effect of time was observed (i.e., Stroop effect) for reaction times and ΔO2Hb with naming < interference < switching (symbols not presented in figure)
Fig. 2.
Pooled sample correlations between MVPA with switching condition reaction times (A) and switching condition ΔO2Hb (B). Inactive and active participants are denoted as white and black circles, respectively
Cerebral oxygenation
ΔO2Hb was greatest during the Switching task (active: 9.4 ± 5.6 a.u.; inactive: 5.8 ± 3.4 a.u.) in comparison to the interference (active: 7.9 ± 4.8 a.u.; inactive: 4.7 ± 3.7 a.u.; P = 0.04), which were both greater than the naming condition (active: 6.4 ± 4.5 a.u.; inactive: 4.3 ± 3.2 a.u.; both, P < 0.04). As presented in Fig. 1B, the active group produced greater ΔO2Hb responses in the interference (P < 0.046) and switching conditions (P = 0.04), but not in the naming condition (P = 0.13) in comparison to the inactive group. These results were unchanged (interference: P = 0.03; switching: P = 0.05) after controlling for sedentary time. However, accounting for aerobic fitness resulted in no between-group differences in ΔO2Hb within either of these conditions (both, P = 0.09). Habitual MVPA was positively correlated to ΔO2Hb responses during the switching condition (Fig. 2B; r = 0.39, P = 0.02), but not in the interference (P = 0.065) or naming conditions (P = 0.08). V̇O2max was also positively associated with ΔO2Hb responses during the naming (r = 0.40, P = 0.02) and switching conditions (r = 0.34, P = 0.045). Controlling for V̇O2max, but not sedentary time (P = 0.04), eliminated the relationship between MVPA and switching condition ΔO2Hb (r = 0.28, P = 0.12). No other relationships were observed between ΔO2Hb responses within any condition versus step count, LPA, V̇O2max (interference condition only), sedentary time, or standing time (r-range: − 0.22–0.29; all, P > 0.10).
Discussion
Our results demonstrate that older adults who achieved MVPA recommendations had superior cognitive flexibility than their insufficiently active peers, independent of time spent engaging in habitual sedentary time. The better executive functions may have been due, in part, to the greater prefrontal cortex oxygenation responses in the active versus inactive older adults. Interestingly, neither step count nor sedentary time was associated with executive function or the intra-Stroop condition ΔO2Hb responses. Similar to aerobic fitness, we demonstrate that habitual MVPA is an important contributor to executive function in cognitively healthy older adults, with such cognitive benefits possibly mediated by neurophysiological changes in the oxygenation of the prefrontal cortex.
Older adults who self-report more intensity-related physical activity generally perform better on tests of executive function than those who report less activity [35–37]; however, subjective measures are prone to large degrees of error [22] and make comparisons with activity recommendations challenging. Studies incorporating objective measures of activity have observed a positive relationship between daily physical activity and Trail B time in older females [14], and between MVPA and Trail B–Trail A time in older adults living in a continuing care retirement community [15]. Trail tasks provide a simple measure of executive function [38] but are less cognitively challenging than the switching condition of the Stroop task. Importantly, these studies did not measure their participants’ aerobic fitness levels, which could contribute to the greater executive function in their more active groups [5, 6]. The present analysis supports that more time spent engaging in MVPA is associated with better performance on the most executively demanding challenging condition of the Stroop task. Importantly, the reaction times in the present study are reflective of another study of a similar nature in an older population [5].
Our results demonstrate that active older adults are able to perform better on tests of executive function by means of increasing prefrontal cortex oxygenation (Fig. 1B). Unlike the active group, the inactive group did not exhibit a large increase in ΔO2Hb between the naming, interference, and switching conditions, which coincided with their slower reaction times in the switching condition (Fig. 2B, D). This finding is consistent with other studies linking higher aerobic fitness with better executive function and increased prefrontal cortex oxygenation in older adults [5, 6]. Specifically, both Mekari et al. [6] and Dupuy et al. [5] observed faster reaction times and greater prefrontal cortex oxygenation responses in their more aerobically fit versus less aerobically fit older adults. Furthermore, Colcombe et al. [9] observed higher activation in prefrontal regions (via magnetic resonance imaging) during a cognitive flanker task (attention and executive function) in higher fit older adults as compared to lower fit older adults. Our findings confirm that engaging in recommended amounts of intensity-related physical activity is related to the greater cerebral oxygenation responses observed during executive tasks in more active older adults. This novel observation may provide mechanistic insight into the cognitive benefits of active aging. The results of our study provide strong rationale for future interventional studies that aim to determine the impact of assisting older adults meet physical activity recommendation on cognitive function and the associated mechanisms (i.e., cerebral oxygenation). Other potential mechanisms that may contribute to our observed differences between the reaction times and ΔO2Hb responses of the active and inactive groups may involve cerebrovascular function [39], brain-derived neurotrophic factors, insulin-like growth factor 1 [40], or cerebral lactate metabolism [41]. Given the positive impact of structured aerobic exercise training on these mechanisms, it is plausible that habitual MVPA also promotes favorable alterations in cerebrovascular function, neurotrophins, and/or cerebral lactate metabolism that contributes to the greater cognitive function among older adults who achieve activity guidelines.
The similar aerobic fitness levels despite such large differences in habitual MVPA (251 vs 89 min/week) are an interesting observation of the present study. This may be explained by genetic factors, which have been shown to largely influence aerobic fitness [19, 20]. In addition, the group differences were driven primarily by MPA and not VPA (Table 1). It is likely that time spent in VPA is a greater determinant of aerobic fitness and that time spent engaging in MPA may not be sufficient to translate to improvements in aerobic fitness. We verified that higher aerobic fitness was associated with faster reaction times and larger O2Hb responses in the switching condition. Certainly, the findings of our study demonstrate that higher aerobic fitness not only elicits cognitive benefits in older adults but also suggests that MVPA time is an important contributor and may be an easier lifestyle behavior to modify than V̇O2max. It is challenging to determine whether habitual activity intensity or aerobic fitness is more important for cognitive health or if they mediate one another. Both measures are often highly related but inherently different constructs (physical behaviour versus cardiorespiratory fitness). Nevertheless, the observation in that our active and inactive groups were similar in aerobic fitness levels permitted the unique opportunity to evaluate the impact of MVPA when aerobic fitness was partially controlled for. Regardless of which physical factor is more important, strategies to increase aerobic fitness inherently rely on engaging in more MVPA. Interventional studies that concurrently measure changes in these physical factors, cognition, and cerebral oxygenation are needed to better characterize which factors mediate or drive the relationship between movement and brain health in older adults. We did not observe a relationship between sedentary time with any cognitive measure. To our knowledge, this is the first study to investigate the influence of true habitual sedentary time (i.e., via thigh-worn inclinometry) on executive function and demonstrates that intensity-related physical activity and aerobic fitness may have a greater impact than time spent sitting/lying on such outcomes. Corroborating the importance of MVPA is the lack of relationship observed between executive function with either step counts or LPA. Herein, our results suggest that the cognitive benefits may be grounded in the intensity by which the activity is conducted, rather than simply accumulating more steps at a lower intensity. Therefore, healthy older adults who are at an age-related risk of developing cognitive diseases [42, 43] may benefit most from increasing MVPA versus step counts, and that these cognitive benefits may be conferred when performing activity at a moderate intensity (e.g., ~ 110 steps/min).
Although this study is strengthened by observing group differences in objectively measured physical activity and consideration of directly measured aerobic fitness and sedentary time, as well as the inclusion of mechanistic insights from our functional near-infrared spectroscopy measures, the study is not without limitations. The study implements a cross-sectional design and therefore is unable to establish causality between MVPA and cognitive functions. As well, the findings of the present study are specific to cognitively healthy older adults (MMSE ≥ 25) and may not be extrapolated to older adults with cognitive diseases or even older adults who are more susceptible to cognitive declines (85 + years) [42, 43]. However, given that most major cognitive diseases and disorders are initially characterized by impairments in executive function [2, 3], our findings are important in identifying moderate physical activity as a therapeutic strategy that may prevent the development of these adverse conditions. Although not statistically different, there were a disproportionate number of males versus females between the active (10♂, 9♀) and inactive groups (4♂, 12♀). However, this is a minor limitation given that females typically perform better on executive function tasks and exhibit less of an age-related decline in executive function than males [44]. Accordingly, it is possible that our study may even be underestimating the importance of MVPA on executive function among older persons in that an even greater group difference would be expected if groups were perfectly matched for sex. Clearly, future interventional studies should examine whether increases or reductions in habitual MVPA influence executive functions and the associated neurophysiological mechanisms among older adults with and without cognitive impairments. We defined older adults as those > 55 years [22], but the World Health Organization has unique activity guidelines for adults > 65 years. While we are insufficiently powered to analyze each of these age groups independently (see Table 1 for age distribution), the intensity-related aerobic physical activity portion of these guidelines is identical between younger and older adults (> 150 min of weekly MVPA), suggesting this to be a minor limitation. Functional near-infrared spectroscopy-derived measures have been well adopted by researchers due to their ability to non-invasively determine cerebral oxygenation during multiple conditions (rest, intra-exercise, etc.) with good temporal resolution [5, 6, 45, 46]. However, these measures do not provide good spatial resolution, poor depth penetrations, and permit changes in O2Hb only and not absolute values. Given that our cerebral oxygenation data were recorded from the left prefrontal cortex, we cannot rule out the possibility that the inactive group relied more so on bi-lateral increases in O2Hb than the active group, or that participants in the inactive group had a greater baseline O2Hb, which would limit the magnitude of the ΔO2Hb. Greater bi-lateral increases in the inactive versus active group are unlikely, with previous research demonstrating that higher fit older females exhibit greater right side prefrontal cortex (inferior frontal gyrus) oxygenation during the Stroop task compared to their less fit counterparts [5]. A greater baseline O2Hb is also unlikely given that individuals who self-report more physical activity tend to have greater cerebral blood flow at rest [47]. Nevertheless, the results of the present study are in accordance with results of the literature and they lead to a further understanding of the neurophysiological mechanisms that underpin the protective nature of habitual intensity-related physical activity on higher-order cognitive functions in older adults.
Independent of sedentary time, older adults who meet MVPA guidelines were faster on the executive portion of the Stroop task than their peers who did not achieve intensity-related recommendations. Our results support that this observation may be mediated by larger oxygenation responses in the prefrontal cortex among the active versus inactive groups. Future interventional studies are needed to investigate the cognitive effects and associated mechanisms on assisting the large proportion of insufficiently active older adults progressively work towards meeting activity recommendations.
Funding
Support for the work was provided by a Dalhousie University, Faculty of Health Research Development grant (to D.S. Kimmerly), a Nova Scotia Health Research Foundation (NSHRF) Development/Innovation grant (to D.S. Kimmerly and S. Mekary), as well as the Acadia University McCain Foundation Fund (to S. Mekary). M.W. O’Brien was supported by a Killam PreDoctoral Scholarship, Heart & Stroke Foundation of Canada BrightRed Scholarship, a Nova Scotia Graduate Scholarship, a Research Nova Scotia-Scotia Scholars Award, and Fredrick Banting and Charles Best CIHR Doctoral Award.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fjell AM, Sneve MH, Grydeland H, et al. The disconnected brain and executive function decline in aging. Cereb Cortex. 2017;27:2303–2317. doi: 10.1093/cercor/bhw082. [DOI] [PubMed] [Google Scholar]
- 2.Clark LR, Schiehser DM, Weissberger GH, et al. Specific measures of executive function predict cognitive decline in older adults. J Int Neuropsychol Soc. 2012;18:118–127. doi: 10.1017/S1355617711001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy G, Jacobs DM, Tang M-X, et al. Memory and executive function impairment predict dementia in Parkinson’s disease. Mov Disord. 2002;17:1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 4.Shea TB, Remington R. Cognitive improvement in healthy older adults can parallel that of younger adults following lifestyle modification: support for cognitive reserve during aging. J Alzheimers Dis Rep. 2018;2:201–205. doi: 10.3233/adr-180056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupuy O, Gauthier CJ, Fraser SA, et al. Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front Hum Neurosci. 2015; 9.10.3389/fnhum.2015.00066. [DOI] [PMC free article] [PubMed]
- 6.Mekari S, Dupuy O, Martins R, et al. The effects of cardiorespiratory fitness on executive function and prefrontal oxygenation in older adults. GeroScience. 2019;41:681–690. doi: 10.1007/s11357-019-00128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freudenberger P, Petrovic K, Sen A, et al. Fitness and cognition in the elderly. Neurology. 2016;86:418–424. doi: 10.1212/WNL.0000000000002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuter-Lorenz PA, Festini SB, Jantz TK. Executive functions and neurocognitive aging. In: Handbook of the Psychology of Aging. 8th ed. Elsevier; 2015, pp 245–262.
- 9.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tremblay MS, Warburton DE, Janssen I, et al. New Canadian physical activity guidelines. Appl Physiol Nutr Metab. 2011;36:36–46. doi: 10.1139/H11-009. [DOI] [PubMed] [Google Scholar]
- 11.Physical Activity Guidelines Advisory Committee . 2018 Physical activity guidelines advisory committee scientific report. Washington: US Department of Health and Human; 2018. [Google Scholar]
- 12.Statistics-Canada. Directly measured physical activity of adults, 2012 and 2013. Health Fact Sheet. 2015.
- 13.Harvey JA, Chastin SFM, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471–487. doi: 10.1123/japa.2014-0164. [DOI] [PubMed] [Google Scholar]
- 14.Barnes DE, Blackwell T, Stone KL, et al. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. 2008;56:1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr J, Marshall SJ, Patterson RE, et al. Objectively measured physical activity is related to cognitive function in older adults. J Am Geriatr Soc. 2013;61:1927–1931. doi: 10.1111/jgs.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thivel D, Tremblay A, Genin PM, et al. Physical activity, inactivity, and sedentary behaviors: definitions and implications in cccupational health. Front Public Heal. 2018; 6.10.3389/fpubh.2018.00288. [DOI] [PMC free article] [PubMed]
- 17.Tremblay MS, Aubert S, Barnes JD, et al. Sedentary behavior research network (SBRN) – terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nokes N. Relationship between physical activity and aerobic fitness. J Sports Med Phys Fitness. 2009;49:136–141. [PubMed] [Google Scholar]
- 19.Costa AM, Breitenfeld L, Silva AJ, et al. Genetic inheritance effects on endurance and muscle strength. Sport Med. 2012;42:449–458. doi: 10.2165/11650560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Zeiher J, Ombrellaro KJ, Perumal N, et al. Correlates and determinants of cardiorespiratory fitness in adults: a systematic review. Sport Med - Open. 2019;5:39. doi: 10.1186/s40798-019-0211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 22.Sattler MC, Jaunig J, Tösch C, et al. Current evidence of measurement properties of physical activity questionnaires for older adults: an updated systematic review. Sport Med. 2020;50:1271–1315. doi: 10.1007/s40279-020-01268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien MW, Robinson SA, Frayne RJ, et al. Achieving Canadian physical activity guidelines is associated with better vascular function independent of aerobic fitness and sedentary time in older adults. Appl Physiol Nutr Metab. 2018;43:1003–1009. doi: 10.1139/apnm-2018-0033. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien MW, Johns JA, Dorey TW, et al. Meeting international aerobic physical activity guidelines is associated with enhanced cardiovagal baroreflex sensitivity in healthy older adults. Clin Auton Res. 2020;30:139–148. doi: 10.1007/s10286-019-00641-9. [DOI] [PubMed] [Google Scholar]
- 25.Mekari S, Neyedli HF, Fraser S, et al. High-intensity interval training improves cognitive flexibility in older adults. Brain Sci. 2020;10:1–12. doi: 10.3390/brainsci10110796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien MW, Johns JA, Robinson SA, et al. Impact of high-intensity interval training, moderate-intensity continuous training, and resistance training on endothelial function in older adults. Med Sci Sport Exerc. 2020;52:1057–1067. doi: 10.1249/MSS.0000000000002226. [DOI] [PubMed] [Google Scholar]
- 27.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. doi: 10.1186/1479-5868-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwardson C, Winkler E, Bodicoat D, et al. Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Heal Sci. 2017;6:162–178. doi: 10.1016/j.jshs.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien M, Wojcik WR, D’Entremont L, Fowles JR. Validation of the PiezoRx® step count and moderate to vigorous physical activity times in free living conditions in adults: a pilot study. Int J Exerc Sci. 2018;11:541–551. [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien M, Wojcik W, Fowles J. Medical-grade physical activity monitoring for measuring step count and moderate-to-vigorous physical activity: validity and reliability study. JMIR mHealth uHealth. 2018;6:e10706. doi: 10.2196/10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Brien M, Kivell MJ, Wojcik WR, et al. Influence of anthropometrics on step-rate thresholds for moderate and vigorous physical activity in older adults: scientific modeling study. JMIR Aging. 2018;1:e12363. doi: 10.2196/12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien MW, Wojcik WR, Fowles JR. Validity and interinstrument reliability of a medical grade physical activity monitor in older adults. J Meas Phys Behav. 2021;4:31–38. doi: 10.1123/jmpb.2019-0074. [DOI] [Google Scholar]
- 33.Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43:1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Ekkekakis P. Illuminating the black box: Investigating prefrontal cortical hemodynamics during exercise with near-infrared spectroscopy. J Sport Exerc Psychol. 2009;31:505–553. doi: 10.1123/jsep.31.4.505. [DOI] [PubMed] [Google Scholar]
- 35.Bixby WR, Spalding TW, Haufler AJ, et al. The unique relation of physical activity to executive function in older men and women. Med Sci Sports Exerc. 2007;39:1408–1416. doi: 10.1249/mss.0b013e31806ad708. [DOI] [PubMed] [Google Scholar]
- 36.Busse AL, Gil G, Santarém JM, Jacob Filho W. Physical activity and cognition in the elderly: a review. Dement Neuropsychol. 2009;3:204–208. doi: 10.1590/S1980-57642009DN30300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gajewski PD, Falkenstein M. Long-term habitual physical activity is associated with lower distractibility in a Stroop interference task in aging: Behavioral and ERP evidence. Brain Cogn. 2015;98:87–101. doi: 10.1016/j.bandc.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39:222–232. doi: 10.1016/j.intell.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guadagni V, Drogos LL, Tyndall AV, et al. Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology. 2020;94:e2245–e2257. doi: 10.1212/WNL.0000000000009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Churchill JD, Galvez R, Colcombe S, et al. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23:941–955. doi: 10.1016/S0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 41.Tsukamoto H, Suga T, Takenaka S, et al. Greater impact of acute high-intensity interval exercise on post-exercise executive function compared to moderate-intensity continuous exercise. Physiol Behav. 2016;155:224–230. doi: 10.1016/j.physbeh.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Murman DL. The impact of age on cognition. Semin Hear. 2015;36:111–121. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons: Higher than previously reported. JAMA J Am Med Assoc. 1989;262:2551–2556. doi: 10.1001/jama.1989.03430180093036. [DOI] [PubMed] [Google Scholar]
- 44.McCarrey AC, An Y, Kitner-Triolo MH, et al. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31:166–175. doi: 10.1037/pag0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herold F, Wiegel P, Scholkmann F, Müller N. Applications of functional near-infrared spectroscopy (fNIRS) neuroimaging in exercise–cognition science: a systematic, methodology-focused review. J Clin Med. 2018;7:466. doi: 10.3390/jcm7120466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mekari S, Fraser S, Bosquet L, et al. The relationship between exercise intensity, cerebral oxygenation and cognitive performance in young adults. Eur J Appl Physiol. 2015;115:2189–2197. doi: 10.1007/s00421-015-3199-4. [DOI] [PubMed] [Google Scholar]
- 47.Bailey DM, Marley CJ, Brugniaux JV, et al. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke. 2013;44:3235–3238. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]