Abstract
Weight gain during interpregnancy period is related to hypertensive disorders of pregnancy (HDP). However, in interpregnancy care/counseling, the unpredictability of the timing of the next conception and the difficulties in preventing age-related body weight gain must be considered while setting weight management goals. Therefore, we suggest considering the annual change in the body mass index (BMI). This study aimed to clarify the association between annual BMI changes during the interpregnancy period and HDP risk in subsequent pregnancies. A multicenter retrospective study of data from 2009 to 2019 examined the adjusted odds ratio (aOR) of HDP in subsequent pregnancies. The aORs in several annual BMI change categories were also calculated in the subgroups classified by HDP occurrence in the index pregnancy. This study included 1,746 pregnant women. A history of HDP (aOR, 16.76; 95% confidence interval [CI], 9.62 − 29.22), and annual BMI gain (aOR, 2.30; 95% CI, 1.76 − 3.01) were independent risk factors for HDP in subsequent pregnancies. An annual BMI increase of ≥ 1.0 kg/m2/year was related to HDP development in subsequent pregnancies for women without a history of HDP. This study provides data as a basis for interpregnancy care/counseling, but further research is necessary to validate our findings and confirm this relationship.
Subject terms: Health care, Medical research, Risk factors
Introduction
Hypertensive disorders of pregnancy (HDP) are a group of syndromes defined by the onset of hypertension during pregnancy with an incidence of 8–10%1. The recurrence rate of HDP is as high as 20–60% in a subsequent pregnancy2–5. Women with a history of HDP are at an increased risk of future cardiovascular disease (CVD) and mortality6–8, which is further increased in women with recurrent events compared to those with a single event9,10. Therefore, although HDP shows spontaneous postpartum remission, it affects the outcomes of subsequent pregnancies and women’s health later in life. Thus, there is an urgent need to establish a strategy to prevent HDP in subsequent pregnancies.
Interpregnancy care/counseling has recently been recognized for its beneficial role in maternal health and subsequent pregnancy outcomes11–13. In addition to a history of HDP and being overweight/obese (BMI ≥ 25.0 kg/m2), interpregnancy body mass index (BMI) gain, defined as the difference between the pre-pregnant BMI of the index pregnancy and that of the subsequent pregnancy, is reported to be associated with HDP3,14–17. The American College of Obstetricians and Gynecologists (ACOG) recommends body weight management during the interpregnancy period to reduce the recurrence risk of HDP; however, no clear goal has been stated to this effect11. The total BMI gain during the interpregnancy period is certainly a valuable indicator for detecting high-risk for HDP at the first visit for subsequent pregnancy; however, the metric has no role or relevance in the prevention of HDP in subsequent pregnancies at the interpregnancy care/counseling stage, which is provided just after childbirth (index pregnancy). Most women do not plan when they will have another baby, and it is difficult to plan a weight management goal. We should also consider the difficulty in preventing age-related weight gain, as reported previously18. Longitudinal studies have reported that approximately 0.5 kg is the mean spontaneous age-related annual weight gain (natural gain) in women younger than 50 years19,20. For Japanese women of average height (157.9 cm), approximately 0.2 kg/m2/year is implied as the spontaneous age-related annual BMI gain. Considering the unpredictability of the timing of next conception and the difficulty of compensation for age-related weight gain, goal setting based on the total change in BMI during the interpregnancy period can be ambiguous. This process of goal setting must include goals with a clear timeframe and allow for some weight gain in these women.
It is generally essential to consider the target population, attainability, and the timeframe when formulating these goals21. To address these issues, the concept of “annual BMI change” was applied in the present study to provide a standard for body weight management in interpregnancy care/counseling; annual BMI change would be preferable to total BMI change as the former would help set more realistic goals with an explicit timeframe. Moreover, “annual BMI change” has already been beneficial in various medical and healthcare fields, including those related to oncology22,23, CVD risks24,25, severe obstructive sleep apnea26, and diabetes mellitus27,28. However, no report has reported the association between the annual BMI change and HDP risk. Thus, it is necessary to evaluate whether an annual BMI gain of 0.2 (natural gain) or more kg/m2/year is related to HDP occurrence in the subsequent pregnancy.
This study aimed to examine the association between annual BMI changes during the interpregnancy period and HDP in a subsequent pregnancy. Furthermore, we also aimed to conduct several subgroup analyses considering the adequate annual BMI change, as we believe that it would be help in the interpregnancy care/counseling based on individual characteristics, including pre-pregnant BMI and the outcome of the index pregnancy.
Methods
Study population
This multicenter retrospective study used data obtained from an electronic medical records system. Data were collected for women aged 15 years or older who delivered at Nagoya University Hospital or TOYOTA Memorial Hospital, Aichi prefecture, or 12 private maternity facilities (Kishokai Medical Corporation located in Aichi and Gifu prefectures) in 2009–2019. We assessed the records directly and ascertained the data, including blood pressure if necessary. Women whose medical records for the index and the subsequent pregnancy could be obtained were included. The exclusion criteria were as follows: multiple pregnancy, stillbirth before 22 weeks of gestation, and data missing for maternal blood pressure and pre-pregnant BMI (Fig. 1). Women with chronic hypertension were also excluded. Since all patients with chronic hypertension are diagnosed with HDP at the point of conception1, including them in the study population was inappropriate for evaluating the risk of HDP development after conception. Women who developed HDP in a subsequent pregnancy were included in the HDP group, while those who did not were included in the non-HDP group. HDP was diagnosed according to the definitions proposed by the International Society for the Study of Hypertension in Pregnancy in 20181, but chronic hypertension was excluded for the abovementioned reasons. Thus, the HDP group included only women with gestational hypertension and preeclampsia.
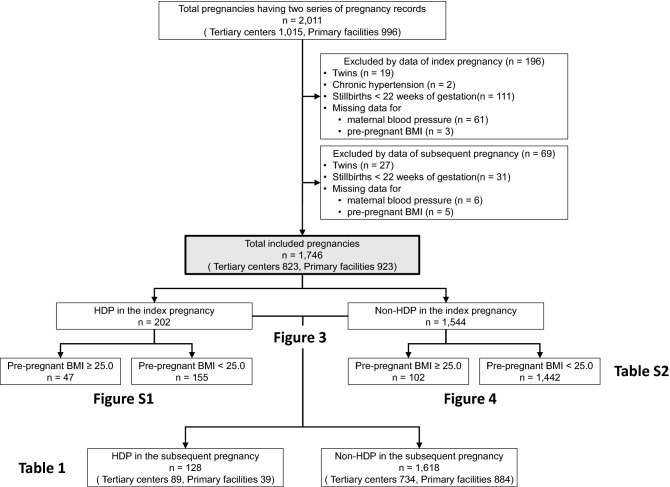
Figure 1.
Flow chart of the study subjects. Clinical data were obtained from 2011 women who delivered at two tertiary centers or 12 primary maternity care units for whom two serial pregnancy records were available were obtained. A total of 1746 women were eligible for this study after excluding 196 and 69 women based on the index and subsequent pregnancy data, respectively.
This study was approved by the Nagoya University Hospital ethics committee (approval number: 2015–0415) and all research was performed in accordance with relevant guidelines/regulations and the Declaration of Helsinki. The requirement for informed consent was waived by the Nagoya University Hospital ethics committee because of the retrospective nature of the study.
Definitions of variables
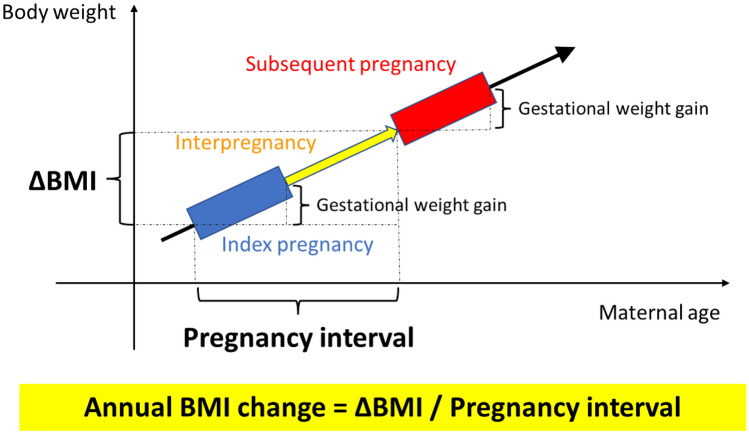
We used the self-reported maternal height and pre-pregnant body weight obtained during routine practice to calculate BMI (kg/m2) (weight in kg divided by squared height in m2). The subjects were categorized as underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥ 30.0 kg/m2) as reported by the World Health Organization consultation29. For the subgroup analyses, the participants were dichotomized on the basis of pre-pregnant BMI (< 25.0 or ≥ 25.0 kg/m2) in the index pregnancy30. Women with prepregnancy diabetes mellitus (DM) or hemoglobin A1C level ≥ 6.5% (48 mmol/mol) or fasting plasma glucose level ≥ 126 mg/dL during pregnancy were defined as having overt DM. Assisted reproductive technology (ART) was defined as conception after in vitro fertilization or intracytoplasmic sperm injection. Gestational weight gain was defined as the difference between pre-pregnant body weight and the weight of the woman before delivery. Gestational age (GA) was routinely assessed by the expected date of delivery (EDD) determined as based on the last menstruation period and the measurement of the crown-rump length by ultrasonography. In ART pregnancies, EDD was determined based on the age of the embryo and the date of transfer. Gestational DM (GDM) was diagnosed according to oral glucose tolerance test results of fasting plasma glucose level ≥ 92 mg/dL or 1-h and 2-h plasma glucose level after 75-g glucose loading of ≥ 180 mg/dL or ≥ 153 mg/dL, respectively. Light for date was diagnosed using the Japanese standards for birth weight according to pregnancy duration31. As shown in Fig. 2, we defined interpregnancy BMI change (ΔBMI) as a change in pre-pregnant BMI from the index pregnancy to the subsequent pregnancy, as previously reported14,30. Interpregnancy interval was defined as the interval from the EDD of the index pregnancy to that of the subsequent pregnancy, which is equivalent to the interval between the two conceptions. The annual BMI change was calculated as the ΔBMI/pregnancy interval. Annual BMI change during the interpregnancy period was categorized into five groups (< 0.0 [weight loss], ≥ 0.0– < 0.2 [reference, natural gain], ≥ 0.2– < 0.6, ≥ 0.6– < 1.0 and ≥ 1.0 kg/m2/year), since an annual gain of 0.2 kg/m2/year is considered normal and unavoidable19,20; 0.6 and 1.0 kg/m2/year gains are equivalent to approximately 1.5 and 2.5 kg/year increases in the weight of women of average height (157.9 cm), respectively. Comparisons were performed among these categories accordingly to establish body weight management goals keeping in mind the normal range of weight gain.
Figure 2.
Overview of the definitions of terms. We defined interpregnancy BMI change (ΔBMI) as a change in pre-pregnant BMI from the index pregnancy to the subsequent pregnancy. The interpregnancy interval was defined as the interval from the expected date of delivery of the index pregnancy to that of the subsequent pregnancy, which is equivalent to the interval between the two conceptions. The annual BMI change was calculated as the ΔBMI/pregnancy interval. Gestational weight gain was defined as the change between pre-pregnant body weight and that before delivery. BMI, body mass index.
Statistical analysis
Clinical characteristics and parameters (Table 1) of the HDP and non-HDP groups were compared using the χ2 test, Fisher’s exact test, Student’s t-test, Welch’s t-test, or the Mann–Whitney U test as appropriate. Crude and adjusted odds ratios (aORs) were calculated for HDP in the subsequent pregnancy using univariable and multivariable logistic regression analyses, respectively. Variables pertaining to the index pregnancy or interpregnancy period, which are known to be associated with HDP in subsequent pregnancies, were selected for analysis. In the multivariable logistic regression analysis, backward elimination methods were used to identify factors associated with HDP in the subsequent pregnancy, and variables with values of p < 0.25 in the univariable logistic regression analysis were entered in the multivariable logistic regression analysis. Multivariable logistic regression analysis of well-known risk factors for HDP3,14–17 was also performed for sensitivity analysis. We classified annual BMI gain into four or five categories based on their distributions as mentioned above and performed a multivariable analysis to determine how the aOR changed with a specific annual BMI change.
Table 1.
Baseline characteristics and perinatal outcomes.
| HDP in the subsequent pregnancy | Non-HDP in the subsequent pregnancy | p Value | |
|---|---|---|---|
| n = 128 | n = 1,618 | ||
| Tertiary center | 89 (69.5) | 734 (45.4) | < 0.001* |
| Index pregnancy | |||
| Maternal age, years old | 31.3 ± 4.8 | 30.4 ± 4.8 | 0.054 |
| Pre-pregnant BMI | 24.3 ± 5.4 | 20.6 ± 2.9 | < 0.001* |
| Overweight/Obesity (BMI ≥ 25.0) | 47 (36.7) | 102 (6.3) | < 0.001* |
| Smokers | 0 (0.0) | 17 (1.1) | 1.000 |
| OvertDM | 4 (3.1) | 14 (0.9) | 0.055 |
| Hyperthyroidism | 1 (0.8) | 15 (0.9) | 1.000 |
| Hypothyroidism | 3 (2.3) | 32 (2.0) | 1.000 |
| Primiparity | 96 (75.0) | 1,111 (68.7) | 0.596 |
| ART | 20 (15.6) | 124 (7.7) | 0.001* |
| Gestational body weight gain, kg | 11.5 ± 4.8 | 10.9 ± 3.8 | 0.149 |
| HDP | 72 (56.3) | 130 (8.0) | < 0.001* |
| GA at the onset of HDP, weeks, median [p25, p75] | 32.9 [27.2, 37.3] | 36.7 [30.8, 38.7] | 0.005* |
| Early-onset HDP | 39/72 (54.2) | 41/130 (31.5) | 0.002* |
| PE | 17/72 (23.6) | 37/130 (28.5) | 0.456 |
| GDM | 11 (8.6) | 55 (3.4) | 0.005* |
| Stillbirth > 22 weeks of gestation | 1 (0.8) | 13 (0.8) | 1.000 |
| GA at delivery, weeks | 39.0 ± 2.1 | 39.1 ± 2.1 | 0.490 |
| Preterm birth (< 37 weeks of gestation) | 15 (11.7) | 118 (7.3) | 0.069 |
| Cesarean section | 39 (30.5) | 376 (23.2) | 0.066 |
| Neonatal sex, male | 59 (46.1) | 883 (54.6) | 0.064 |
| Neonatal height, cm | 49.2 ± 3.2 | 49.4 ± 2.9 | 0.428 |
| Birthweight, g | 2,970 ± 553 | 2,976 ± 494 | 0.891 |
| Light for date infant | 9 (7.0) | 124 (7.7) | 0.683 |
| Placental weight, g | 580.2 ± 125.6 | 571.1 ± 112.7 | 0.390 |
| Inter-pregnancy | |||
| Pregnancy interval, years, median [p25, p75] | 2.1 [1.6, 2.8] | 2.0 [1.7, 2.5] | 0.317 |
| ΔBMI | 0.99 ± 1.88 | 0.40 ± 1.37 | 0.001* |
| Anuual BMI change, kg/m2/year | 0.60 ± 1.42 | 0.19 ± 0.77 | 0.002* |
| Subsequent pregnancy | |||
| Maternal age, years old | 33.7 ± 5.0 | 32.7 ± 5.0 | 0.017* |
| Pre-pregnant BMI | 25.3 ± 5.7 | 21.0 ± 3.0 | < 0.001* |
| Overweight (BMI > 25.0) | 57 (44.5) | 137 (8.5) | < 0.001* |
| Smokers | 0 (0.0) | 17 (1.1) | 1.000 |
| OvertDM | 5 (3.9) | 16 (1.0) | 0.009* |
| Hyperthyroidism | 1 (0.8) | 15 (0.9) | 1.000 |
| Hypothyroidism | 3 (2.3) | 32 (2.0) | 1.000 |
| ART | 18 (14.1) | 122 (7.5) | 0.007* |
| Gestational body weight gain, kg | 10.3 ± 4.2 | 10.2 ± 3.6 | 0.688 |
| HDP | 128 (100) | 0 (0.0) | – |
| GA at the onset of HDP, weeks, median [p25, p75] | 36.5 [31.4, 38.0] | – | – |
| Early-onset HDP | 44 (34.4) | – | – |
| PE | 30 (23.4) | – | – |
| GDM | 20 (15.6) | 121 (7.5) | 0.003* |
| Stillbirth ≥ 22 weeks of gestation | 0 (0.0) | 1 (0.1) | 1.000 |
| GA at delivery, weeks | 38.8 ± 1.9 | 39.0 ± 1.5 | 0.141 |
| Preterm birth (< 37 weeks of gestation) | 11 (8.6) | 79 (4.9) | 0.068 |
| Cesarean section | 41 (32.0) | 371 (22.9) | 0.020* |
| Neonatal sex, male | 75 (58.6) | 818 (50.6) | 0.076 |
| Neonatal height, cm | 49.7 ± 3.4 | 49.8 ± 2.1 | 0.936 |
| Birthweight, g | 3,026 ± 601 | 3,054 ± 405 | 0.599 |
| Light for date infant | 12 (9.4) | 49 (3.0) | 0.002* |
| Placental weight, g | 600.9 ± 143.2 | 581.1 ± 108.9 | 0.127 |
HDP, hypertensive disorders of pregnancy; BMI, body mass index; DM, diabetes mellitus; ART, assisted reproductive technology; GA, gestational age; PE, preeclampsia; GDM, gestational diabetes mellitus.
Data are presented as means ± standard deviation or median [p25, p75] for continuous variables and n (%) for discrete variables.
*Statistically significant.
Data are presented as mean ± standard deviation or median [p25, p75] for continuous variables and number (percentage) for categorical variables. Statistical significance was set at p < 0.05. The statistical analyses were conducted using SPSS version 27.0 for Windows software (SPSS, Inc., Chicago, IL, USA).
Results
Participants
A total of 2,011 pregnant women (tertiary centers, n = 1,015; primary maternity care units, n = 996) were included. Among them, 206 women were excluded due to multiple pregnancies (n = 19 and 27), chronic hypertension (n = 2 and 0), stillbirth before 22 weeks of gestation (n = 111 and 31), and missing data for maternal blood pressure (n = 61 and 6) or pre-pregnant BMI (n = 3 and 5) in the index and subsequent pregnancy, respectively (Fig. 1). The remaining 1,746 pregnant women (tertiary centers, n = 823; primary maternity care units, n = 923) were finally included.
Comparison of clinical parameters between the HDP and non-HDP groups
HDP occurred in a subsequent pregnancy in 128/1,746 (7.3%) participants, 69.5% of whom were treated at tertiary centers. Table 1 presents data of women who developed HDP in the subsequent pregnancy (HDP group) and those who did not develop HDP in the subsequent pregnancy (non-HDP group).
With regard to the variables of the index pregnancy (HDP group vs. non-HDP group), there were no significant differences in maternal age (31.3 ± 4.8 vs. 30.4 ± 4.8 years, p = 0.054), the incidence of smoking between the two groups (0.0 vs. 1.1%, p = 1.000). Pre-pregnant BMI (24.3 ± 5.4 vs. 20.6 ± 2.9 kg/m2, p < 0.001) and the incidence of HDP (56.3 vs. 8.0%; p < 0.001) were significantly higher in HDP group. The median GA at the onset of HDP was lower in the HDP group than in the non-HDP group (median [p25-p75]; 32.9 [27.2–37.3] vs. 36.7 [30.3–38.7] weeks, p = 0.005); therefore, the HDP group included more patients with early-onset HDP than those in the non-HDP group (54.2 vs. 31.5%, p = 0.002). The incidence of conception by ART and GDM was also higher in the HDP group than in the non-HDP group (15.6 vs. 7.7%, p = 0.001 and 8.6 vs. 3.4%, p = 0.005, respectively).
Regarding the variables pertaining to the interpregnancy period (HDP group vs. non-HDP group), the pregnancy interval did not differ significantly between the two groups (median [p25-p75]; 2.1 [1.6–2.8] vs. 2.0 [1.7–2.5] years, p = 0.317), whereas the ΔBMI and annual BMI change were significantly higher in the HDP group than in the non-HDP group (0.99 ± 1.88 vs. 0.40 ± 1.37 kg/m2, p = 0.001 and 0.60 ± 1.42 vs. 0.19 ± 0.77 kg/m2/year, p = 0.002, respectively).
With regard to the variables pertaining to the subsequent pregnancy (HDP group vs. non-HDP group), the incidence of conception by ART and GDM was significantly higher in the HDP group than in the non-HDP group (14.1% vs. 7.5%, p = 0.007 and 15.6% vs. 7.5%, p = 0.003, respectively). The mean pre-pregnant BMI was also higher in the HDP group than in the non-HDP group (25.3 ± 5.7 vs. 21.0 ± 3.0 kg/m2, p < 0.001). Regarding the perinatal outcomes of the subsequent pregnancy, the Cesarean section and light for date rates were significantly higher in the HDP group than in the non-HDP group (32.0% vs. 22.9%, p = 0.020 and 9.4% vs. 3.0%, p = 0.002, respectively).
Risk factors for HDP in the subsequent pregnancy
Based on the results of univariable and multivariable analyses performed using the backward elimination method (Table 2), five variables remained in the final set, and the history of HDP (HDP development in the index pregnancy) showed the highest aOR for HDP in the subsequent pregnancy (aOR, 16.76; 95% confidence interval [CI], 9.62–29.22), followed by annual BMI gain in the interpregnancy period (aOR, 2.30; 95% CI, 1.76–3.01), pre-pregnant BMI in the index pregnancy (aOR, 1.25; 95% CI, 1.17–1.33), and maternal age in the index pregnancy (aOR, 1.07; 95% CI, 1.01–1.13). Those results were also similar after controlling simultaneously for potential confounding variables: maternal age, pre-pregnant BMI, gestational body weight gain, overt DM, ART, HDP, GDM in the index pregnancy; interpregnancy period, and annual BMI change during the interpregnancy period (Table S1, Model 1). Furthermore, the results were similar even after adjusting for the tertiary centers (Table S1, Model 2).
Table 2.
Univariable and multivariable logistic regression analysis of factors potentially associated with HDP in a subsequent pregnancy.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p Value | aOR | 95%CI | p Value | |
| Index pregnancy | ||||||
| Maternal age†, years old | 1.04 | (1.00–1.08) | 0.054 | 1.07 | (1.01–1.13) | 0.021* |
| Pre-pregnant BMI†, kg/m2 | 1.25 | (1.20–1.30) | < 0.001 | 1.25 | (1.17–1.33) | < 0.001* |
| Primiparity† | 1.14 | (0.70–1.84) | 0.596 | |||
| Gestational body weight gain†, kg | 1.04 | (1.00–1.09) | 0.077 | – | – | – |
| Overt DM† | 3.24 | (1.05–10.01) | 0.041 | – | – | – |
| ART† | 2.28 | (1.37–3.82) | 0.002 | – | – | – |
| HDP† | 14.72 | (9.94–21.79) | < 0.001 | 16.76 | (9.62–29.22) | < 0.001* |
| GDM† | 2.53 | (1.29–4.97) | 0.007 | – | – | – |
| Stillbirth ≥ 22 weeks of gestation† | 0.91 | (0.12–7.02) | 0.928 | |||
| GA at delivery†, weeks | 0.97 | (0.90–1.05) | 0.490 | |||
| Preterm birth (< 37 weeks of gestation)† | 1.69 | (0.95–2.99) | 0.072 | – | – | – |
| Cesarean section† | 1.45 | (0.98–2.14) | 0.067 | – | – | – |
| Neonatal sex†, male | 0.71 | (0.50–1.02) | 0.065 | – | – | – |
| Neonatal height†, cm | 0.98 | (0.93–1.03) | 0.428 | |||
| Birthweight†, g | 1.00 | (1.00–1.00) | 0.891 | |||
| Light for date infant† | 0.86 | (0.43–1.75) | 0.683 | |||
| Placental weight†, g | 1.00 | (1.00–1.00) | 0.390 | |||
| Inter-pregnancy | ||||||
| Pregnancy interval, years | 1.26 | (1.08–1.48) | 0.004 | 1.57 | (1.25–1.97) | < 0.001* |
| Anuual BMI change, kg/m2/year | 1.57 | (1.31–1.87) | < 0.001 | 2.30 | (1.76–3.01) | < 0.001* |
HDP, hypertensive disorders of pregnancy; OR, odds ratio; CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; ART, assisted reproductive technology; GA, gestational age; PE, preeclampsia; GDM, gestational diabetes mellitus.
†Parameteres of the index pregnancy.
*Statistically significant.
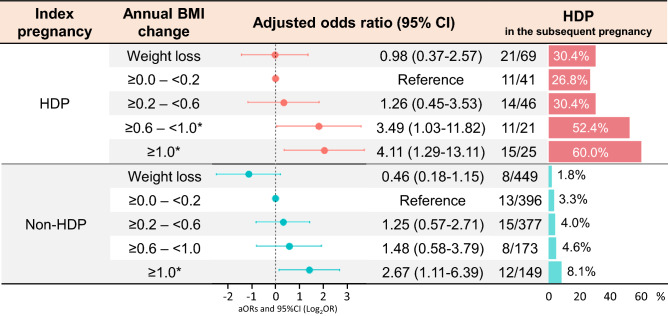
The aORs of HDP in the subsequent pregnancy were also evaluated by classifying annual BMI changes into five categories distribution (Fig. 3). In the subgroup of HDP in the index pregnancy (Fig. 3, upper panel), the aORs in the 0.6–1.0 kg/m2/year gain and ≥ 1.0 kg/m2/year gain groups were approximately 3.49 (95% CI, 1.03–11.82) and 4.11 (95% CI, 1.29–13.11), respectively. However, in the subgroup of non-HDP in the index pregnancy (Fig. 3, lower panel), the aOR was significantly increased only in the ≥ 1.0 kg/m2/year gain (aOR, 2.67; 95% CI, 1.11–6.39).
Figure 3.
Adjusted odds ratios of classified annual BMI change for HDP in the subsequent pregnancy. The multivariable models were adjusted for maternal age in the index pregnancy, pre-pregnant BMI in the index pregnancy, classified annual BMI change, and interpregnancy interval. The values on the left side of the graph are expressed as log2OR. The right side of the graph shows the incidence of HDP in subsequent pregnancies according to the degree of annual BMI change. The number of HDP events in the subsequent pregnancy/total number is also shown according to the degree of annual BMI change. BMI, body mass index; CI, confidence interval; HDP, hypertensive disorders of pregnancy *Statistically significant.
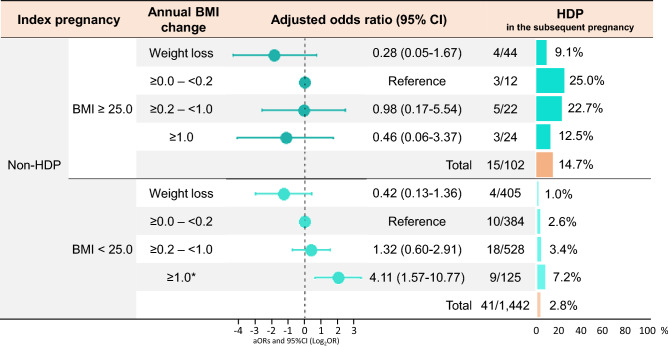
We further analyzed for women without a history of HDP, who were divided according to pre-pregnant BMI (≥ or < 25.0 kg/m2) in the index pregnancy (Fig. 4) since the recurrent risk was high in women with a history of HDP. The annual BMI change during the interpregnancy period was categorized into four groups (< 0.0 [weight loss], ≥ 0.0– < 0.2 [reference], ≥ 0.2– < 1.0, and ≥ 1.0 kg/m2/year) since the aORs of HDP in the subsequent pregnancy were increased in ≥ 1.0 kg/m2/year, respectively, in women without a history of HDP (Fig. 3, lower panel). Among patients without a history of HDP, the prevalence of HDP in the subsequent pregnancy was higher in women with pre-pregnant BMI of ≥ 25.0 kg/m2 in the index pregnancy (15/102, 14.7%) than in those with pre-pregnant BMI of < 25.0 kg/m2 in the index pregnancy (41/1,442, 2.8%). In women with pre-pregnant BMI ≥ 25.0 kg/m2, the prevalence of HDP was lower in the weight loss group than in the weight gain groups (4/44, 9.1% vs. 11/58, 19.0%), although the difference was not significant (aOR 0.28, 95%CI, 0.05–1.67) (Fig. 4, upper panel). In women with pre-pregnant BMI of < 25.0 kg/m2, the aOR for HDP in the subsequent pregnancy was significant only when they had an annual weight gain of ≥ 1.0 kg/m2/year (aOR, 4.11; 95% CI, 1.57–10.77) (Fig. 4, lower panel). Further, among the women with pre-pregnant BMI of < 25.0 kg/m2 and who did not develop HDP in the index pregnancy, those women who developed HDP in the subsequent pregnancy had significantly higher gestational weight gain in the index pregnancy and greater annual BMI change than those without HDP in the subsequent pregnancy, although there were no significant differences in maternal age and pregnancy interval (Table S2).
Figure 4.
Adjusted odds ratios for HDP in the subsequent pregnancy among those without the history of HDP in the index pregnancy. The multivariable models were adjusted for maternal age in the index pregnancy, pregnancy interval, and classified annual BMI change. Values on the left side of the graph are expressed as log2OR. The right side of the graph shows the incidence of HDP in the subsequent pregnancy according to the degree of annual BMI change. The number of HDP in the subsequent pregnancy/total number is also shown according to the degree of annual BMI change. BMI, body mass index; CI, confidence interval; HDP, hypertensive disorders in pregnancy.
The HDP recurrence rate was as high as 93.3% in women who had a pre-pregnant BMI of ≥ 25.0 kg/m2 in the index pregnancy and who had an annual BMI gain ≥ 0.6 kg/m2/year in the interpregnancy period (Fig. S1, upper panel). In women with HDP occurrence in the index pregnancy, the results were similar among the two subgroups with pregnant BMI of ≥ and < 25.0 kg/m2 in the index pregnancy; aOR and the prevalence of HDP was the highest in women with annual BMI gain ≥ 0.6 kg/m2/year during the interpregnancy period.
Discussion
This is the first study to evaluate the association between HDP in a subsequent pregnancy and annual BMI change during the interpregnancy period. The annual BMI gain during the interpregnancy period was significantly related to HDP in subsequent pregnancies. Maternal age, pre-pregnant BMI, HDP in the index pregnancy, and interpregnancy interval were also variables significantly associated with HDP in the subsequent pregnancy. Among these variables, a history of HDP in the index pregnancy was most strongly related to HDP in the subsequent pregnancy. Thus, annual BMI change were investigated in two subgroups: women with or without a history of HDP in the index pregnancy. For women without a history of HDP in the index pregnancy, an annual BMI gain of ≥ 1.0 kg/m2/year was associated with HDP in the subsequent pregnancy. Further, in the subgroup with pre-pregnant BMI < 25.0 in the index pregnancy, an annual BMI gain of ≥ 1.0 kg/m2/year was associated with HDP in the subsequent pregnancy, although it was not related to HDP in the subgroup with pre-pregnant BMI ≥ 25.0 in the index pregnancy. However, for women with a history of HDP in the index pregnancy, an annual BMI gain of ≥ 0.6 kg/m2/year was related to HDP in the subsequent pregnancy. These results suggest that in interpregnancy care/counseling, appropriate annual BMI goals is better to be provided to the women based on their characteristics, including the presence or absence of HDP in the index pregnancy.
Several previous studies suggested that women with a history of HDP are at higher risk for HDP in the subsequent pregnancy2,3,5. The present study showed an approximate recurrence rate of 35.6%, which is consistent with previous reports2,3,5. The recurrence rate is also affected by the other factors including maternal age, pre-pregnant BMI, and interpregnancy interval3,5,14,15,32,33. The present data also showed similar trends. Overall interpregnancy BMI gain was also reported to be related to HDP in the subsequent pregnancy16,17, which is consistent with the present data. The mean annual BMI change was approximately 0.2 kg/m2/year in this study population. An annual BMI gain of < 0.6 kg/m2/year was not associated with HDP recurrence in the subsequent pregnancy, although exceeding annual BMI gain ≥ 0.6 kg/m2/year was significant.
Interpregnancy care/counseling is now recommended to improve subsequent pregnancy outcomes and long-term health11–13,34,35. In the present study, BMI gain during the interpregnancy period was significantly associated with HDP in the subsequent pregnancy, whereas no significant difference was observed in gestational weight gain. Gestational weight gain has been reported to be associated with the development of HDP in that pregnancy36; however, it was not significantly associated with HDP in the subsequent pregnancy as determined in this study. Thus, the BMI gain during the interpregnancy period might be more critical.
Although there are currently no clear recommendations for women with a history of HDP, those with a history of preterm births are recommended to avoid short interpregnancy intervals34. Thus, we must gather additional evidence for interpregnancy care protocols to prevent HDP. A previous systematic review and meta-analysis reported that a significant interpregnancy weight gain increases the risk of GDM, preeclampsia, and large for gestational age births16. Another systematic review and meta-analysis reported that weight gain increases a risk of HDP17. The results of the present study support those of these systematic reviews and meta-analyses. But the present study is the first to demonstrate the association between annual BMI gain and the development of HDP in subsequent pregnancy in women with or without a history of HDP in the index pregnancy. These results would provide a standard for body weight management in interpregnancy care/counseling.
HDP is a complex syndrome involving several factors, including genetic variants37,38, a history of HDP, and a pre-pregnant BMI ≥ 25.0. In interpregnancy care/counseling, women who have HDP in the index pregnancy should also be recommended to use aspirin during the subsequent pregnancy to prevent HDP11. Additionally, those with a pre-pregnant BMI of ≥ 25.0 might be informed that approximately 90% of them might develop HDP in the subsequent pregnancy if their annual BMI increase by ≥ 0.6 kg/m2/year during the interpregnancy period (Fig. S1). Aspirin would be unsuitable for women without a history of HDP in the index pregnancy to prevent HDP. Thus, the annual BMI change might be significant for these patients, and this was the focus of this study. Among those with pre-pregnant BMI < 25.0, an annual BMI gain of ≥ 1.0 kg/m2/year was associated with an approximately 4.1-fold increased risk of HDP in the subsequent pregnancy. Those with a pre-pregnant BMI of < 25.0, and no history of HDP in the index pregnancy are more likely to develop HDP in the subsequent pregnancy if they had high gestational weight gain in the index pregnancy and high annual BMI gain during the interpregnancy period, which would lead to overweight/obesity (BMI of ≥ 25.0) in the subsequent pregnancy (Table S2). This suggests that this population might be susceptible to weight gain. We are also going to explore whether weight gain would cause HDP in these low-risk women directly or indirectly.
However, among those with pre-pregnant BMI ≥ 25.0, annual BMI change was not associated with the risk of HDP in the subsequent pregnancy, but the prevalence of HDP in the subsequent pregnancy was not very low. Furthermore, women with weight loss during the interpregnancy period had a lower incidence of HDP in the subsequent pregnancy than women with annual BMI gain, although no significant difference was detected in this study. These results suggest that the appropriate standard of annual BMI change during the interpregnancy period might vary depending on the pre-pregnant BMI status and history of HDP in the index pregnancy. These findings would be helpful in interpregnancy care/counseling based on individual characteristics. However, further studies are needed to understand whether weight management using annual BMI scale is possible to prevent HDP in subsequent pregnancies.
Other studies reported that an interpregnancy interval of more than five years increased a risk of developing HDP in a subsequent pregnancy33. The present study also showed that a longer interpregnancy interval was an independent risk factor for HDP in the subsequent pregnancy; however, the interpregnancy interval exceeded five years in only 2.1% (36/1,746) of patients in this study.
Strengths and limitations
This study has the following strengths. First, this is the first to assess the association between HDP in the subsequent pregnancy and the annual BMI changes during the interpregnancy period. Second, the association was reanalyzed by stratifying by other factors including a history of HDP and pre-pregnant BMI in the index pregnancy. Third, since this was a multicenter study, both tertiary care centers and primary maternity care units participated in this study. The study population included women with various risk levels, which could minimize selection bias. Previous studies of the recurrent risk of HDP included only women who gave birth at tertiary centers14,15,39. Furthermore, the data in this study were detailed and reliable as required by national registry studies.
This study has several limitations. First, only women for whom both index and subsequent pregnancy records were available were included. The following populations were not included: women who did not have a subsequent baby; and those who delivered a subsequent baby at a non-participating institute. Women with life-threatening complications, including brain hemorrhage, would not have a subsequent pregnancy and were also excluded. This impact is expected to be minimal because such severe cases are rare. However, we also could not include women who had an abortion in a subsequent pregnancy or those who developed infertility after the index pregnancy. These populations may have higher BMI gains than the study population, but this was not the focus of this study. The exclusion of individuals for missing data might have caused some bias. Those individuals were older and had a longer interpregnancy period, but other variables, including pre-pregnant BMI in the index pregnancy, ΔBMI, and annual BMI change, were not significantly different from those in this study population (data not shown). Therefore, the bias was minimal. Second, we did not follow up postpartum weight. Annual BMI gain was not measured as an annual check, but it was calculated according to ΔBMI and interpregnancy interval. However, these values would be correlated with each other because the mean interpregnancy interval was 2.3 years. Weight gain from prepregnancy to 18 months postpartum was recently reported as related to the subsequent risk of CVD in Danish women40. Interpregnancy health checkups for women who hope to have subsequent pregnancies have not yet been implemented in the clinical setting in Japan, but we plan to do so based on the present study's findings. Additionally, self-reported weight was used to calculate BMI in this study. However, most participants measured their weights at the prenatal visit in the first trimester, so the difference between the self-reported and actual values is likely to be minimal. Third, the analysis did not include maternal medication as a variable. Although aspirin use is one of the strategies to reduce the risk of HDP recurrence11, aspirin has not been used to prevent recurrent HDP in Japan. Thus, although we did not collect information on medications, we presume that only a few participants of this study would have used aspirin.
Conclusion
In this study, a history of HDP was most strongly associated with HDP in a subsequent pregnancy. Furthermore, an annual weight gain during the interpregnancy period of ≥ 0.6 kg/m2/year was related to HDP recurrence in women with a history of HDP. For women without a history of HDP in the index pregnancy, a value of ≥ 1.0 kg/m2/year was associated with HDP in the subsequent pregnancy. However, it remains unclear whether HDP in the subsequent pregnancy can be prevented by controlling the annual BMI gain under these values.
Further research is needed to determine whether managing the annual BMI gain can prevent HDP in a subsequent pregnancy, which could reduce the risk of future CVD. Our study findings can be the basis for such research. Despite the challenge it presents, preventing HDP recurrence will improve the future health outcomes of women.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author contributions
S.T., T.K., and M.Yo. conceived the study. S.T., T.K., and F.K. performed the statistical analyses. S.T., T.K., T.U., K.I., T.N.-K., Y.M., Y.I., S.Y., M.Ya., Y.K., and H.O. collected and interpreted the clinical data. S.T. and T.K. drafted the manuscript. All authors contributed to the interpretation of the results and the approval of the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01976-y.
References
- 1.Brown MA, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 2.CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group CLASP: A randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343:619–629. doi: 10.1016/S0140-6736(94)92633-6. [DOI] [PubMed] [Google Scholar]
- 3.Lykke JA, Paidas MJ, Langhoff-Roos J. Recurring complications in second pregnancy. Obstet. Gynecol. 2009;113:1217–1224. doi: 10.1097/AOG.0b013e3181a66f2d. [DOI] [PubMed] [Google Scholar]
- 4.van Oostwaard MF, et al. Prediction of recurrence of hypertensive disorders of pregnancy between 34 and 37 weeks of gestation: A retrospective cohort study. BJOG. 2012;119:840–847. doi: 10.1111/j.1471-0528.2012.03312.x. [DOI] [PubMed] [Google Scholar]
- 5.Ebbing C, Rasmussen S, Skjaerven R, Irgens LM. Risk factors for recurrence of hypertensive disorders of pregnancy, a population-based cohort study. Acta Obstet. Gynecol. Scand. 2017;96:243–250. doi: 10.1111/aogs.13066. [DOI] [PubMed] [Google Scholar]
- 6.Stuart JJ, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: An observational cohort study. Ann. Intern. Med. 2018;169:224–232. doi: 10.7326/m17-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haug EB, et al. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: Analysis of the nord-trondelag health study. JAMA Cardiol. 2019;4:628–635. doi: 10.1001/jamacardio.2019.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu R, et al. Hypertensive disorders of pregnancy and risk of cardiovascular disease-related morbidity and mortality: A systematic review and meta-analysis. Cardiology. 2020;145:633–647. doi: 10.1159/000508036. [DOI] [PubMed] [Google Scholar]
- 9.Brouwers L, et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: A systematic review and meta-analysis. BJOG. 2018;125:1642–1654. doi: 10.1111/1471-0528.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theilen LH, et al. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am. J. Obstet. Gynecol. 2018;219:107e101–107e106. doi: 10.1016/j.ajog.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of, O., Gynecologists & Society for Maternal-Fetal, M Obstetric care consensus no. 8: Interpregnancy care. Obstet. Gynecol. 2019;133:e51–e72. doi: 10.1097/AOG.0000000000003025. [DOI] [PubMed] [Google Scholar]
- 12.Randel A. Interpregnancy care: Guidelines from ACOG and SMFM. Am. Fam. Physician. 2019;100:121–123. [PubMed] [Google Scholar]
- 13.Erondu C, Dunlop A. Interpregnancy care: An opportunity to improve women's health and reduce the risk of maternal morbidity and mortality. J. Public Health Manag. Pract. 2021;27:S155–S158. doi: 10.1097/PHH.0000000000001319. [DOI] [PubMed] [Google Scholar]
- 14.Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am. J. Obstet. Gynecol. 2006;194:916–920. doi: 10.1016/j.ajog.2005.10.819. [DOI] [PubMed] [Google Scholar]
- 15.Brown MA, et al. Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG. 2007;114:984–993. doi: 10.1111/j.1471-0528.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- 16.Teulings N, Masconi KL, Ozanne SE, Aiken CE, Wood AM. Effect of interpregnancy weight change on perinatal outcomes: Systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19:386. doi: 10.1186/s12884-019-2566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Hortelano JA, et al. Interpregnancy weight change and hypertension during pregnancy: A systematic review and meta-analysis. Obstet. Gynecol. 2020;135:68–79. doi: 10.1097/AOG.0000000000003573. [DOI] [PubMed] [Google Scholar]
- 18.Williams PT, Wood PD. The effects of changing exercise levels on weight and age-related weight gain. Int. J. Obes. (Lond.) 2006;30:543–551. doi: 10.1038/sj.ijo.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosell M, Appleby P, Spencer E, Key T. Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int. J. Obes. (Lond.) 2006;30:1389–1396. doi: 10.1038/sj.ijo.0803305. [DOI] [PubMed] [Google Scholar]
- 20.Tanamas SK, Shaw JE, Backholer K, Magliano DJ, Peeters A. Twelve-year weight change, waist circumference change and incident obesity: The Australian diabetes, obesity and lifestyle study. Obesity (Silver Spring) 2014;22:1538–1545. doi: 10.1002/oby.20704. [DOI] [PubMed] [Google Scholar]
- 21.Bovend'Eerdt TJ, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clin. Rehabil. 2009;23:352–361. doi: 10.1177/0269215508101741. [DOI] [PubMed] [Google Scholar]
- 22.Park SL, et al. Body size, adult BMI gain and endometrial cancer risk: The multiethnic cohort. Int. J. Cancer. 2010;126:490–499. doi: 10.1002/ijc.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muskens IS, et al. Body mass index, comorbidities, and hormonal factors in relation to meningioma in an ethnically diverse population: The Multiethnic Cohort. Neuro Oncol. 2019;21:498–507. doi: 10.1093/neuonc/noz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong W, et al. Effects of longitudinal body mass index variability on microvasculature over 5 years in adult Chinese. Obesity (Silver Spring) 2016;24:743–749. doi: 10.1002/oby.21398. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, et al. Sleep duration and cardiovascular risk factors in children and adolescents: A systematic review. Sleep Med. Rev. 2020;53:101338. doi: 10.1016/j.smrv.2020.101338. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RF, et al. Weight gain velocity as a predictor of severe obstructive sleep apnea among obese adolescents. Laryngoscope. 2020;130:1339–1342. doi: 10.1002/lary.28296. [DOI] [PubMed] [Google Scholar]
- 27.Alderisio A, et al. Long-term body weight trajectories and metabolic control in type 1 diabetes patients on insulin pump or multiple daily injections: A 10-year retrospective controlled study. Nutr. Metab. Cardiovasc. Dis. 2019;29:1110–1117. doi: 10.1016/j.numecd.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Polemiti E, et al. BMI and BMI change following incident type 2 diabetes and risk of microvascular and macrovascular complications: The EPIC-Potsdam study. Diabetologia. 2021;64:814–825. doi: 10.1007/s00125-020-05362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Consultation WE Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/s0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 30.Sorbye LM, et al. Interpregnancy weight change and recurrence of gestational diabetes mellitus: A population-based cohort study. BJOG. 2020;127:1608–1616. doi: 10.1111/1471-0528.16364. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi A, et al. Neurodevelopment in full-term small for gestational age infants: A nationwide Japanese population-based study. Brain Dev. 2016;38:529–537. doi: 10.1016/j.braindev.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Cormick G, et al. Inter-pregnancy interval and risk of recurrent pre-eclampsia: Systematic review and meta-analysis. Reprod. Health. 2016;13:83. doi: 10.1186/s12978-016-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebremedhin AT, et al. Interpregnancy interval and hypertensive disorders of pregnancy: A population-based cohort study. Paediatr. Perinat. Epidemiol. 2021;35:404–414. doi: 10.1111/ppe.12668. [DOI] [PubMed] [Google Scholar]
- 34.American College of, N.-M. et al. Interpregnancy care. Am. J. Obstet. Gynecol. 2019;220:22–18. doi: 10.1016/j.ajog.2018.11.1098. [DOI] [Google Scholar]
- 35.Hartman S, Brown E, Holub D, Horst M, Loomis E. Optimizing interconception care: Rationale for the IMPLICIT model. Semin. Perinatol. 2020;44:151247. doi: 10.1016/j.semperi.2020.151247. [DOI] [PubMed] [Google Scholar]
- 36.Dude AM, et al. Weight gain in early, mid, and late pregnancy and hypertensive disorders of pregnancy. Pregnancy Hypertens. 2020;20:50–55. doi: 10.1016/j.preghy.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohta K, et al. Association between a variant of the glutathione S-transferase P1 gene (GSTP1) and hypertension in pregnancy in Japanese: Interaction with parity, age, and genetic factors. Semin. Thromb. Hemost. 2003;29:653–659. doi: 10.1055/s-2004-815636. [DOI] [PubMed] [Google Scholar]
- 38.Kobashi G, et al. A1166C variant of angiotensin II type 1 receptor gene is associated with severe hypertension in pregnancy independently of T235 variant of angiotensinogen gene. J. Hum. Genet. 2004;49:182–186. doi: 10.1007/s10038-004-0129-4. [DOI] [PubMed] [Google Scholar]
- 39.Langenveld J, et al. Recurrence risk of a delivery before 34 weeks of pregnancy due to an early onset hypertensive disorder: A systematic review. Am. J. Perinatol. 2010;27:565–571. doi: 10.1055/s-0030-1248944. [DOI] [PubMed] [Google Scholar]
- 40.Kirkegaard H, et al. Maternal weight change from prepregnancy to 18 months postpartum and subsequent risk of hypertension and cardiovascular disease in Danish women: A cohort study. PLoS Med. 2021;18:e1003486. doi: 10.1371/journal.pmed.1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.