Abstract
Introduction
Protein kinase C (PKC) is a promising drug target for various therapeutic areas. Natural products derived from plants, animals, microorganisms, and marine organisms have been used by humans as medicine from prehistoric times. Recently, several compounds derived from plants have been found to modulate PKC activities through competitive binding with ATP binding site, and other allosteric regions of PKC. As a result fresh race has been started in academia and pharmaceutical companies to develop an effective naturally derived small-molecule inhibitor to target PKC activities. Herein, in this review, we have discussed several natural products and their derivatives, which are reported to have an impact on PKC signaling cascade.
Methods
All information presented in this review article regarding the regulation of PKC by natural products has been acquired by a systematic search of various electronic databases, including ScienceDirect, Scopus, Google Scholar, Web of science, ResearchGate, and PubMed. The keywords PKC, natural products, curcumin, rottlerin, quercetin, ellagic acid, epigallocatechin-3 gallate, ingenol 3 angelate, resveratrol, protocatechuic acid, tannic acid, PKC modulators from marine organism, bryostatin, staurosporine, midostaurin, sangivamycin, and other relevant key words were explored.
Results
The natural products and their derivatives including curcumin, rottlerin, quercetin, ellagic acid, epigallocatechin-3 gallate, ingenol 3 angelate, resveratrol, bryostatin, staurosporine, and midostaurin play a major role in the management of PKC activity during various disease progression.
Conclusion
Based on the comprehensive literature survey, it could be concluded that various natural products can regulate PKC activity during disease progression. However, extensive research is needed to circumvent the challenge of isoform specific regulation of PKC by natural products.
Keywords: Natural products, Drug target, Cancer, Cardiac disease, Neurodegenerative diseases
Introduction
PKC is a ser/thr protein kinase that plays a vital role in the signal transduction by phosphorylation of target proteins to control numerous cellular functions. However, alteration in the normal activities of PKC causes progression of several deadly diseases like cancer, cardiac disease, autoimmune disease, metabolic disorders, and so on [1]. Therefore, precise control of PKC’s signal amplitude is necessary for the healthy condition of an organism. Therefore, the development of PKC modulators (activators and/or inhibitors) has been predicted to be a breakthrough in drug discovery [1, 2]. The search for PKC inhibitor from natural sources goes back to the 1980s with the isolation and assay of staurosporine from Streptomyces staurosporeus [3]. Naturally occurring compounds found in plants, animals, and microorganisms provide huge health benefits [4]. One of the mechanisms by which natural products (NPs) have shown their biological actions is by interfering with the PKC signaling pathway [5]. Currently, various natural compounds have been isolated and characterized for their PKC modulatory activity. Among them, curcumin, quercetin, resveratrol, bryostatin, staurosporine, and others have gone to clinical trials (https://clinicaltrials.gov/). In this review, different sources of natural compounds, biological activities, and their interaction with PKC have been described. Comprehensive knowledge about the association between NPs and PKC activity will provide an opportunity to design a specific and effective PKC modulator.
Methods
All information presented in this review article regarding the regulation of PKC by natural products has been acquired by a systematic search of various reliable and popular international scientific databases such as PubMed, Scopus, ScienceDirect, ResearchGate, Google Scholar, etc. The information was searched using the strings such as PKC and structure, PKC and function, natural products, curcumin and PKC, rottlerin and PKC, quercetin and PKC, ellagic acid and PKC, rottlerin and PKC, epigallocatechin-3 gallate and PKC, ingenol 3 angelate and PKC, resveratrol and PKC, protocatechuic acid and PKC, tannic acid and PKC, PKC modulators from marine organism, bryostatin and PKC, staurosporine, midostaurin, sangivamycin and PKC, and other relevant keywords. Clinicaltrial.gov was searched for upcoming data and trials of various NPs. After cross-referenced of a total of 178 relevant references are given here to depict the entire research related to PKC signaling and their regulation by natural compounds. Figure 1 represents the schematic diagram of PKC isoforms, Fig. 2 stands for the possible interaction of natural products to PKC during multiple disease conditions, Figs. 3, 4, 5 demonstrate a pictorial diagram of various natural products. Further, the mode of interaction of NPs to PKC presented in Table 1 and the past and present clinical trials using these NPs have been given in Table 2.
Fig. 1.
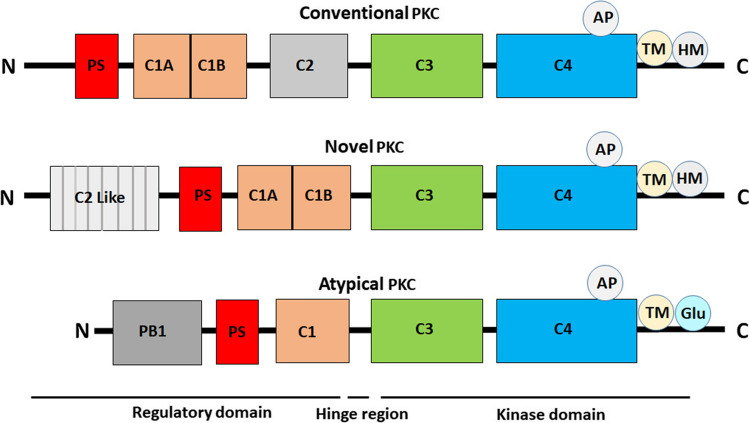
Schematic representation of the domain composition of the protein kinase C (PKC) family members. A regulatory region at N-terminal comprises two basic modules, C1 and C2 domain. The autoinhibitory pseudosubstrate sequence found at N -terminal. The highly conserved catalytic domain comprises the C3 domain act as the ATP-binding domain and the C4 domain is the substrate-binding kinase core
Fig. 2.
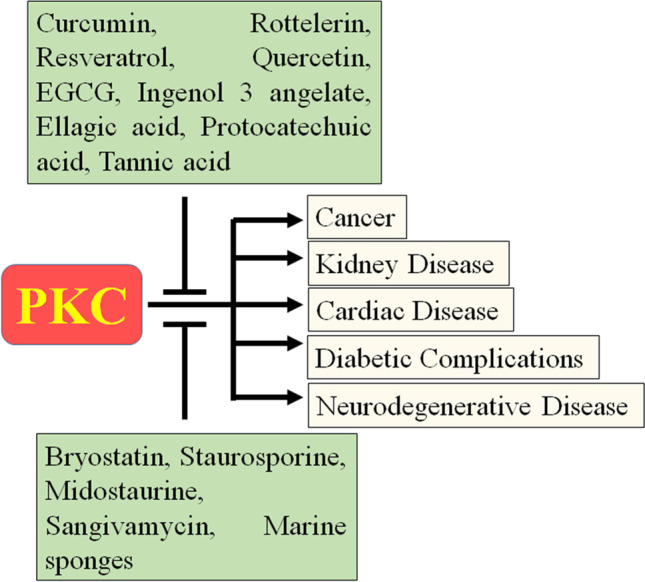
Diagrammatical representation of interaction of natural products to PKC during multiple disease conditions
Fig. 3.
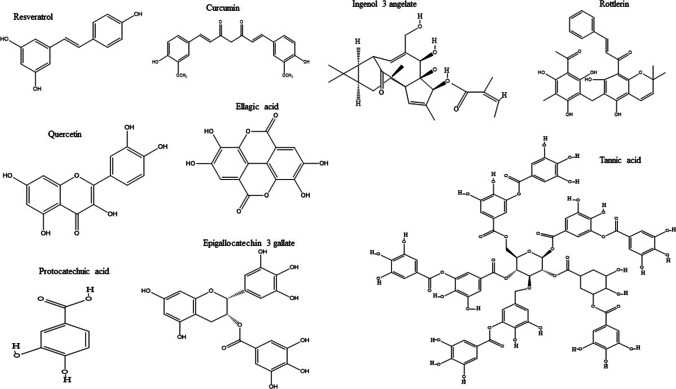
The Structure of natural products from terrestrial plant
Fig. 4.
The structure of natural product from marine origin
Fig. 5.
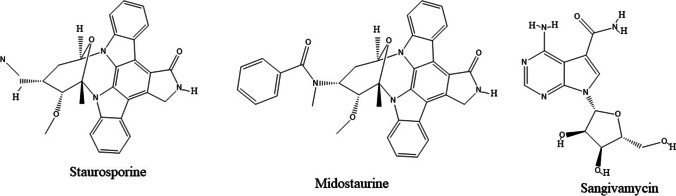
The structure of natural products from bacteria
Table 1.
The basic information of natural products and their interaction with PKC activity presented in this review
| Natural products | Sources | Natural product class | Binding to site | Target |
|---|---|---|---|---|
| Curcumin | curcumin longa | Polyphenol | C1 domain | PKCα |
| Rottlerin | Mallotus philippensis | Polyphenol | ----- | PKCδ |
| Quercetin | Apples, honey, onions, red grapes, and green leafy vegetables | Flavonoid | ----- | PKCδ,ε |
| Ellagic acid | Oak species, and mushroom | Polyphenol | ------ | PKCα |
| Epigallocatechin-3gallate | Dried leaf of tea | Polyphenol | ------ | PKC, PKCα,γ |
| Ingenol 3 angelate | Sap of the plantEuphorbia peplus | Diterpene ester | ------ | PKCδ |
| Resveratrol | Grapes, red wine, olive oil, and other foods | Polyphenol | C1 domain | PKCα,β, ε |
| Protocatechuic acid | Raspberry, Boswellia dalzielii, Cardiospermum halicacabum, and Diospyros melanoxylon | Phenolic acid | ------ | PKC |
| Tannic acid | Black tea, Caesalpinia spinosa, Rhus semialata, Quercus infectoria, and Terminalia chebula | Polyphenol | ------ | PKC |
| Bryostatin | Bryozoan Bugula neritina | Macrocyclic lactones | ------ | cPKC and nPKC |
| Staurosporine | Streptomyces Sp | Alkaloids | ATP binding site | PKC |
| Midostaurin | Staurosporine derivative | Alkaloids | ATP binding site | PKC |
| Sangivamycin | Streptomyces rimosus | Nucleoside analog | ------ | PKC |
Table 2.
A summary of past and current clinical trials in different diseases conditions using natural products
| Drug | Other Drugs | Disease (S) | Phase | Status | Identifier | comments |
|---|---|---|---|---|---|---|
| Curcumin | Placebo | Prostate Cancer | 3 | Active and recruiting | NCT03769766 |
● Well tolerable and shown anticancer efficacy against variety of cancer. ● Ongoing clinical trial as anticancer agent, cardioprotective, and nephropretective agent. |
| ------- | Breast Cancer | 1 | Active and recruiting | NCT03980509 | ||
|
Placebo Nanoemulsion |
Breast Cancer Joint Pain |
----- | Active and recruiting | NCT03865992 | ||
| Placebo | Cervical Cancer, Stage IIB | 2 | Active and not yet recruiting | NCT04294836 | ||
| Placebo |
Cancer Cachexia, Head and Neck Cancer Head and Neck neoplasma |
2 | Active and recruiting | NCT04208334 | ||
| Placebo | Prostate cancer | 3 | Active and recruiting | NCT02064673 | ||
| Piperine Extract |
Bladder Spasm Malignant Neoplasm Pain Urinary Urgency |
1 | Active and recruiting | NCT02598726 | ||
| Ursolic Acid | Prostate Cancer | 1 | Active and not yet recruiting | NCT04403568 | ||
| Placebo | Neoplasm Cervix | 2 | Active and not yet recruiting | NCT04266275 | ||
|
Pembrolizumab Radiation: Radiation, Vitamin D, Aspirin, Lansoprazole, Cyclophosphamide |
Cervical Cancer Endometrial Cancer Uterine Cancer |
2 | Active and recruiting | NCT03192059 | ||
| ----- | Chronic Atrophic Gastritis | 2 | Active and recruiting | NCT02782949 | ||
| ------ |
Amyloidosis, Amyloid, Amyloid Neuropathies Familial Amyloid Cardiomyopathy Amyloid Primary Transthyretin Amyloidosis, AL Amyloidosis |
----- | Active and recruiting | NCT03431896 | ||
| ------ |
Obesity, High Blood Pressure, High Cholesterol, Type2 Diabetes Heart Diseses |
---- | Active and not yet recruiting | NCT03542240 | ||
| Placebo | Chronic Kidney Diseases | ---- | Active and recruiting | NCT03475017 | ||
| ------ |
Chronic Kidney Diseases Peritoneal Dialysis Hemodialysis |
---- | Active and not yet recruiting | NCT04413266 | ||
| Placebo |
Chronic Kidney Diseases Cognitive Decline |
2 | Active and recruiting | NCT03223883 | ||
| Placebo |
Chronic Kidney Diseases Blood Pressure Hyperemia Vasoconstriction |
2 | Active and not yet recruiting | NCT04132648 | ||
| Homotaurine, vitamin D3 | Diabetic Retinopathy | … | Completed | NCT04378972 | ||
| Quercetin | ------ |
Fanconi Anemia Squamous Cell Carcinoma |
2 | Active and recruiting | NCT03476330 |
● Not show effective anticancer property. ● Ongoing clinical trail in chornory artery disease, kidney disease and anemia pateints. |
| ------ |
Adenocarcinoma of the Prostate Recurrent Prostate Cancer Stage I Prostate Cancer Stage IIA Prostate Cancer Stage IIB Prostate Cancer Stage III Prostate Cancer Stage IV Prostate Cancer |
1 | Active and not yet recruiting | NCT01912820 | ||
| ------ | Coronary Artery Disease Progression | 3 | Active and recruiting | NCT03943459 | ||
| ------ | Chronic Kidney Disease | 2 | Active and recruiting | NCT02848131 | ||
| ------ | Fanconi Anemia | 1 | Active and recruiting | NCT01720147 | ||
| EGCG | ------ | Colon Cancer | 1 | Active and recruiting | NCT02891538 | ● Ongoing study in cancer patient. |
| ------ | Small Cell Lung Carcinoma | ---- | Active and recruiting | NCT01317953 | ||
| ------ |
Diabetic Nephropathy Hypertension |
2 | Completed | NCT01923597 | ||
| Ingenol 3 angelate or PEEP05 | ------ | Actinic Keratosis | 4 | Completed | NCT02446223 | ● Well tolerable and effective against actinic keratosis |
| ------ | Actinic Keratosis | 4 | Completed | NCT02866695 | ||
| Resveratrol | Pazopanib and Paclitaxel |
Stage III Melanoma Stage IV Melanoma Unresectable Melanoma |
2 | Completed | NCT01107665 |
● Not effective against cancer patients. ● Ongoing clinical trial in cardiac dieses and diabetic patients. |
| ------ | Chemoprevention | … | Active and not yet recruiting | NCT04266353 | ||
| Placebo | Congestive Heart Failure Chronic | 2 | Active and recruiting | NCT03525379 | ||
| Placebo | Dilated Cardiomyopathy | 3 | Active and recruiting | NCT01914081 | ||
| Placebo | Type 1 Diabetes | 1 | Active and recruiting | NCT04449198 | ||
| ----- |
Diabetes Mellitus, Type 2 Coronary Artery Disease |
…. | Active and recruiting | NCT03762096 | ||
| Bryostatin | ------ | Breast Cancer | 2 | completed | NCT00003205 | ● Not effective, and no any study is ongoing. |
| ------ | Lung Cancer | 2 | completed | NCT00005849 | ||
| Midostaurin | ------ | Core Binding Factor Acute Myeloid Leukemia (CBF-AML) | 2 | Active and recruiting | NCT03686345 | ● Effective against Acute Myeloid Leukemia |
| ------ | Acute Myeloid Leukemia | 3 | Active and recruiting | NCT03379727 | ||
| ------ |
Acute Myeloid Leukemia (AML) With FLT3 Mutation, Internal Tandem Duplication (ITD) or Tyrosine Kinase Domain (TKD) |
----- | Active and recruiting | NCT02624570 |
Result and discussion
PKC: Structure and regulation
The PKC enzymes were discovered by Nishizuka in 1977 as kinases that gets activated by proteolysis and later by diacylglycerol (DAG) [6, 7]. Soon after, in 1982 when it was found that phorbol ester (cancer-promoting agent) may activate intact PKC [8], raise a hope to target PKC in cancer primarily, and, later in other disease conditions. PKC family consists of 10 members, which is further divided into three groups depends on the virtue of their structure and co-factor requirement for activation [9]. Structurally, PKC comprises a single polypeptide structure with four conserved (C1-C4) regions which are interspersed by five (V1-V5) variable regions. Further, this polypeptide structure may be divided into two domains i.e. regulatory domain at N-terminal and a catalytic domain at C-terminal, linked via a short variable (V3) hinge region. The regulatory domain comprises C1 and C2 regions that interact with diacylglycerol (DAG), phosphatidylserine (PS), and Ca2+ thereby act as the membrane targeting module [9, 10]. The catalytic core comprises C3 for ATP binding and C4 domain for substrate-binding lobes to make the business end of the kinase. The C1 site contains two cysteine-rich zinc finger motif that comprises tandem C1A and C1B repeats that bind DAG and phorbol esters; by contrast, the C2 site is involved in Ca2+ dependent membrane binding. Classical PKCs (cPKCs: α, β and γ), consist of tandem C1 domains and C2 domain thus required DAG, PS, and Ca2+ for activation. Novel PKCs (nPKCs: δ, ε, η, θ) contain tandem C1 domain and a variant of the C2 (novel C2) domain that were observed not sensitive to Ca2+, therefore, nPKC activation only required DAG. Atypical PKCs (aPKCs: ι and ζ) have an atypical C1 domain that does not bind DAG and lack a C2 domain altogether, but instead, contain a Phox/Bem 1 (PB1) domain that mediates protein–protein interaction (Fig. 1) [10–12]. Additionally, it has been found that aPKCs and PKCα also contain a PDZ ligand at the C-terminal that mediates protein–protein interactions thereby scaffolding and localization of these isozymes. In addition, an auto-inhibitory pseudosubstrate sequence (similar in sequence to the optimal PKC substrate sequence, but lacks the serine/threonine phosphoacceptor residue) is found in all isozymes that maintain PKC in an inactive state by masking the substrate-binding cavity [13–15].
PKC: Activation and function
For PKC to become activated in response to second messengers the protein must first be undergone phosphorylation on several critical residues, a process called priming of PKC [16]. The activation of newly synthesized PKC within the cell relies on three molecular mechanisms (i) phosphorylation, (ii) cofactor binding, and (iii) intracellular translocation [12]. For all PKC isoforms, phosphorylation of the threonine residue in the activation loop (AP) [17–19], makes a conformational change and triggers subsequent phosphorylation at the turn motif (TM) and hydrophobic motif (HM). The final phosphorylation step releases the enzyme into the cytosol in a catalytically competent close-conformation in which the pseudosubstrate region masks the substrate-binding cavity [12]. The priming phosphorylation at AP, TM, and HM of PKC required Hsp90, Cdc34, and mTORC2 activity [12]. In response to extracellular agonists which may activate effector molecules, such as the membrane-associated phospholipase C (PLC), which hydrolyzes the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) to form two hydrolytic products: DAG and inositol 1,4,5-trisphosphate (IP3); a Ca2+ mobilizer. Accumulation of membrane-associated DAG provokes the translocation of inactive PKC to the membrane [11]. At plasma membrane binding of inactive PKC to phosphatidylserine and DAG (in the case of cPKCs and nPKCs) expel autoinhibitory pseudosubstrate from substrate binding cavity and make an active open-confirmation. Due to conformational changes, PKC isoforms could bind with their specific anchoring and scaffold proteins. This binding may determine the intracellular translocation and functional activity of PKC [10–12]. The finding that most PKC isozymes are ubiquitously expressed and activated by a plethora of signals in all tissues makes it difficult to define its functions. The expression profile represents that a high steady-state expression level of PKCα is present in the adult liver, heart, epithelium, and brain, in contrast, PKCβ and PKCγ are detectable but expressed at substantially lower levels in the heart [20, 21]. Additionally, PKCδ and PKCε are also present in the heart [22]. PKCε is present in the adult brain, heart, and liver, but not the adult kidney and lung. PKCζ is expressed in the fetal/neonatal than in the adult brain, lung, kidney, and heart. PKCδ is detected in vascular smooth muscle cells [5, 23]. PKCθ is predominantly found in the thymus and responsible for T cell maturation [24]. The cell-specific function of PKC may be conferred by binding with different anchoring proteins and translocation towards intracellular compartments. Functional analysis revealed that PKC isozymes have specific and sometimes opposing roles in the same cell type, depending on the place and time of activation and the nature of the substrate it acts on [10, 11]. Initially, phorbol ester and later isozyme specific pharmacological inhibitors and siRNAs demonstrated that PKC was found to regulate myriad cellular functions, including regulation of cell survival by apoptosis, proliferation, differentiation and autophagy, regulation of cell shape and migration, cell–cell contact, adhesion, and secretion, regulation of receptors and ion channels, regulation of metabolism, regulation of immune system [9, 10, 24–26]. PKC also plays a crucial role in normal physiological functions. PKC could regulate a variety of neural functions including neurotransmitter release [27], and synaptic plasticity [28], thereby responsible for muscle contraction, hippocampal learning, and memory [29]. In cardiac tissues, PKC particularly α isoform can phosphorylate several cardiac-specific substrates like troponin, PP1, α1C- subunit of the L-type calcium complex channel, and titin [30, 31], and regulates cardiac contractility and Ca2+ handling in myocytes [32]. Moreover, PKC activity also responsible for proper retinal function and thus essential for proper vision [33]. PKC plays a putative role in kidney functions [34, 35]. Therefore it is unsurprising that perturbation in PKC signaling cascade plays a major role in disease conditions including cardiac diseases like ischemic heart disease [36], heart failure [20], stroke [37], neurodegenerative disease like Alzheimer’s disease [38], Parkinson’s disease [39], bipolar disorder[40], amyotrophic lateral sclerosis [41], autoimmune disease [42], skin disease like psoriasis[43], lung disease [44], diabetic complications [45], ocular diseases [46], post-traumatic stress disorder [47] and others.
Natural products: As PKC modulators
NPs have been used for the treatment of various ailments dates back to the Neanderthal period. Natural products- compounds that are derived from natural sources like plants, microbial metabolites, and marine organisms [4]. Since ancient times, due to the mostly non-formal nature, and lack of knowledge, the utilities of many of these NPs are mainly anecdotal. Modern scientific practices provide a way to extract, identify, characterize and explore the functions of bioactive compounds which, in turn, has given insights into their activities on the human body [48]. Owing to the fact that NPs and their analogs could modulate basic cellular functions including survival, proliferation, effective immune system, and metabolism, therefore, it is not surprising that NPs possesses anti-proliferative, antioxidant, cardioprotective, hepatoprotective, antibacterial, anti-inflammatory, modulation of ion channels, and immunomodulatory effects [48–51]. Due to their incomparable structural diversity, and novel biological functions, NPs have become an important research area for drug discovery, particularly in the area of antibiotics and cancer treatment [48, 52, 53]. In 1805, German pharmacist Friedrich Wilhelm Sertürner, isolated morphine from opium, and it became both the first pure naturally derived medicine and the first to be commercialized, by Merck in 1826. This attracts pharmaceutical scientists to prefer purified natural compounds and make medicines. Subsequently, many natural compounds were isolated, purified, and characterized by various sources such as salicin, colchicine, caffeine, cocaine, etc. [54]. Since 2014, the search for an anti-cancer agent, mostly of anti-tumor drugs are closely related to natural products that were established [55]. Recent efforts by both academic and pharmaceutical researchers make some promising drug candidates such as huperzine A, triptolide, celastrol, capsaicin, vinca alkaloids, taxanes, camptothecins, ingenol mebutate, trabectedin, curcumin, and others [56, 57].It is noteworthy that aberrant PKC activity was noticed in pathological conditions. In recent years, due to their unique property (least side effects, structural diversity, and cost-effectiveness), NPs became an attractive target as kinase modulators [58, 59]. These compounds are isolated from a variety of natural sources and grouped into flavonoid, alkaloid, indolocarbazole, nucleoside analogous, and polyphenol compounds, which may modulate PKC activity (Fig. 2) [5, 59]. Further, subsequent studies have shown natural product’s therapeutic effect against various ailments including cancer [60], neurodegenerative disease [61], cardiac disease [62], diabetic complications [63], kidney disease [64], and food-borne illness [65] through PKC signaling pathway.
In 1982, it was shown that quercetin and related polyphenols selectively inhibited protein kinase CK2 [66], soon after in 1986 the discovery of staurosporine; an Indolocarbazoles isolated from Streptomyces staurosporeous, as PKC inhibitor shift paradigm in discovery and screening of natural compounds as potential PKC modulators [67]. Accordingly, some natural compounds have served as a resource for the discovery of next-generation potential PKC inhibitors that target the ATP binding site as well as other allosteric regions (Table 1). This section makes efforts to provide a detailed account of PKC modulators obtained from terrestrial plants and marine organisms.
PKC modulatory agents obtained from terrestrial plants
Historically, plant products have been used since ancient times in health care based upon the knowledge passed from generation to generation. Plant-derived products contributed 80% of total natural compounds [58]. It is also a vast source of protein kinase C modulators (Fig. 3). These compounds are found in a variety of fruits, vegetables, and medicinal plants with various therapeutic properties [5, 68].
Curcumin
Curcumin; also known as diferuloylmethane is extracted and obtained from rhizomes of curcumin longa. It comprises hydrophobic polyphenol molecular structure with many bioactivities such as anti-oxidant, anti-inflammatory, and antiproliferative [69, 70]. Curcumin selectively inhibited PKCα through interactions with the C1 domain [71]. Subsequent studies showed that curcumin regulates PKC signaling during various disease conditions including cancer. Curcumin inhibits invasion in lung cancer cells by lowering the expression of PKCα, P47phox, NOX2, ATF2 phosphorylation, and ROS generation [60]. Interferon-induced protein with tetratricopeptide repeats 2 (IFIT2) had property to modulate aPKC signaling in oral squamous cell carcinoma migration and invasion [72]. It has been found that curcumin induces apoptosis in U937 human leukemia cells through the upregulation of IFIT2 [73], augmented the role of curcumin in aPKC regulation indirectly. Moreover, an elegant study by Madhumita Roy et al. demonstrated that curcumin treatment could sensitize breast cancer cells to the chemotherapeutic drugs by downregulating the PKC, telomerase, NF-κB, and HDAC activity [74]. Although, the in vivo application of curcumin has been limited for its unsatisfactory pharmacokinetics like low efficacy, bioavailability, and selectivity [75, 76]. Different synthetic methods have been used to develop many curcumin analogs, among them C-150 (N-((E)-5-(3-hydroxyphenyl)-2-((E)-3-(3-hydroxyphenyl)acryloyl)-3-oxo-1-phenylpent-4-en-1-yl)acrylamide) which effectively downregulates the NF-κB and PKCα mediated glioblastoma, in vitro as well as in vivo study [77]. Further, a recent study demonstrated that another curcumin analogue 1,5-bis (4-hydroxy-3-((4-methylpiperazin-1-yl)methyl)phenyl)penta-1,4-dien-3-one attributed to cell cycle arrest and apoptosis induction in breast cancer cells through PI3K/AKT/mTOR/PKCθ signaling pathway [78]. Additionally, the therapeutic efficacy of curcumin was also observed in food-borne diseases. Recently, it has been found that curcumin suppressed the PKC pathway, thereby attenuates foodborne illness [65]. Moreover, curcumin regulates the PKC pathway and relaxes precontracted guinea pig gallbladder strips [79], attenuates diabetic nephropathy [64], suppresses diabetic cardiomyopathy [63], prevents hepatic insulin resistance [80], and oxidative stress in the liver with type1 diabetic mice [81]. Finally, curcumin is engaged in various stages of clinical trial studies in patients with prostate cancer, breast cancer, advanced stage head and neck cancer (stage III, IV), and chronic atrophic gastritis cancer (https://clinicaltrials.gov/). Besides various clinical trials are ongoing to validate the efficacy of curcumin in neurodegenerative diseases and metabolic syndrome (Table 2) (https://clinicaltrials.gov/).
Rottlerin
Rottlerin is obtained from Mallotus philippensis also known as Kamala tree. This compound has anti-oxidant, anti-proliferative, and cardioprotective properties [82]. Initially, it was observed that rottlerin selectively inhibited PKCδ, but, recent research demonstrates, it can inhibit a broad range of ser/ thr kinases [83]. Rottlerin inhibited PKCδ and decreased cell viability and induced apoptosis of HTLV-1-infected T cells [84]. Furthermore, rottlerin along with hypericin has shown synergism and induced apoptosis in U87 MG glioma cells via regulation of PKCδ [85]. PKCδ associated with modulation of collagen gene expression thereby pathogenesis of tissue fibrogenesis. It has been observed that PKCδ inhibition by rottlerin resulted in the downregulation of type-1 collagen expression and altered NF-κB gene transcription to attenuate fibrotic diseases [86].
Quercetin
Quercetin is a dietary bioflavonoid obtained from apples, honey, onions, red grapes, and green leafy vegetables. Quercetin has been shown to anti-oxidant, radio-protective, anti-proliferative, and hepato-protective properties [87]. Membrane-bound PKC was associated with histamine release and basophil activation. Quercetin has been shown to regulate basophil activity accompanied by PKC signaling [88]. Moreover, quercetin has shown its anticancer efficacy by modulating PKC activity against mouse melanoma [89], and Hepg2 cancer cells [90]. Neuroprotective effect of quercetin associated with the downregulation of the PKCε pathway. Mechanistically, quercetin and Allium cepa extract attenuates oxidative stress and protects neuronal cells by downregulation of PKCε [91]. Recently, Sanguine Byun et al. observed that quercetin can directly target PKCδ and JAK2 in the skin to elicit protective effects against UV-mediated skin aging and inflammation [92]. Clinical studies are ongoing to assess the anticancer efficacy of quercetin in patients with squamous cell carcinoma and prostate adenocarcinoma (Table 2). In addition, quercetin is engaged with dasatinib in phase1, phase2 clinical trial study in patients with Alzheimer's disease (Table 2). Quercetin has also proved its therapeutic effect and clinical trial study is ongoing in patients with coronary artery disease progression in phase 3, and chronic kidney disease (https://clinicaltrials.gov/).
Ellagic acid
Ellagic acid is present in oak species such as Quercus alba and Quercus robus, and can be extracted from mushroom Phellinus linteus. Mostly, in nature, they exist as complexes called ellagitannins and geranilins, which usually undergo hydrolysis in the gastrointestinal tract to form ellagic acid [93]. Subsequent studies proved its anti-diabetic [94] anti-inflammatory [95], anti-neoplastic [96] property. Initially, it was observed that ellagic acid inhibited PLCγ thus PKC cascade and thereby inhibited platelet aggregation [97]. Studies revealed that ellagic acid improves antioxidant activity, induces apoptosis, and inhibits tumor progression, via regulation of the PKC pathway in Dalton’s lymphoma cells [98–100]. Moreover, ellagic acid exerts a cardioprotective role and attenuates atherosclerosis formation by modulating PKCα/ ERK/ PPARy/ NF-κB pathway. The inhibition of this pathway resulted in decreased ROS production and ultimately inhibition of matrix metalloproteinases 1 (MMP1), and matrix metalloproteinases3 (MMP3) expression in human umbilical vein endothelial cells (HUVECs) [62].
Epigallocatechin-3 gallate
Epigallocatechin-3 gallate (EGCG) is an antioxidant polyphenol, mainly obtained from the dried leaf of tea [101]. Several observational studies have demonstrated that EGCG had therapeutic effects against metabolic syndrome, neurodegenerative disorders, inflammatory diseases, and cancer via regulation of many signaling pathways including PKC. It has been demonstrated that EGCG competitively inhibits the binding of ATP and TPA to PKC thereby suppressed PKC activity [102]. Several studies are showing that EGCG might play a neuroprotective role by regulating the PKC pathway. Molecular analysis reveals that EGCG attenuates amyloid β and 6-hydroxydopamine induced cell death in PC12 and SH-SY5Y cells accompanied through PKC activation [103, 104]. Accordingly, EGCG can activate PKC activity and promote the degradation of Bad protein, thereby increasing cell survival and provide neuroprotection [105]. Furthermore, it has been observed that EGCG restores neuronal integrity through PKCγ activation [106]. Recently, Zhao et al. demonstrated that EGCG could regulate extracellular signal-regulated kinase 1/2 (ERK1/2) and PKCα signaling pathways during stress-induced neuronal injury [107]. Moreover, EGCG downregulates the high glucose-induced PKCα and βII in glomerular epithelial cells and shown its nephroprotection role [108]. Further, EGCG attenuates high glucose-induced proliferation of vascular smooth muscle cells by inhibiting PKC and ERK1/2 pathway to attenuate diabetic vascular complications [109, 110]. Multiple clinical trial studies are ongoing to assess anticancer effects of EGCG in patients with colon cancer, small cell lung carcinoma, prostate cancer, and uterine fibroids (Table 2) (https://clinicaltrials.gov/). The clinical trial has demonstrated the efficacy of EGCG in patients with diabetic nephropathy, and hypertension [ClinicalTrials.gov Identifier: NCT01923597].
Ingenol 3 angelate
The diterpene ester ingenol 3 angelate (I3A) or PEEP005 is obtained from the sap of the plant Euphorbia peplus. Structurally, I3A is analogous to phorbol ester and potentially modulates PKC isozymes by binding with the C1 domain [111]. In human myeloid leukemia cancer cells, I3A activates PKCδ and induces cellular apoptosis [112]. Furthermore, Meria Serova et al. demonstrated that the treatment of I3A induces cell cycle arrest and apoptosis in colon cancer cells by induced the activation of PKCδ and reduced the expression of PKCα [113]. Moreover, I3A induces T cell survival by activation of PKCδ and simultaneously upregulation of mcl-1 and BclxL [114]. The compound has completed various phases of clinical trials in patients with actinic keratosis with great success (Table 2) (https://clinicaltrials.gov/).
Resveratrol
Resveratrol is a polyphenol nonflavonoid compound obtained from grapes, red wine, olive oil, and other foods with anti-proliferative and anti-oxidant properties [115]. It inhibited PKCα and βI isoform at μM concentration, whereas ε and ζ isoform were unaffected. It may interact with the C1 domain of PKCα and thereby regulate associated signaling networks [116]. Resveratrol inhibited TPA induced PKC expression and attenuates metastasis in human cervical cancer cells [117], and human colon carcinoma cells [118]. It has been observed that PKCε activation resulted in protection from neuro-degeneration in both cortex and hippocampus. Morris-Blanco et al. revealed that resveratrol elicits neuroprotective effect via PKCε activation [119]. Nanoparticles are a causative factor for oxidative stress, inflammation, and apoptosis in lung cells. Carbon nanoparticles (CBNPs) induce inflammation in lung epithelial cells by upregulation of Nox2, iNOS, COX-2, NO, PGE, membrane expression of p67phox, increase of ROS production, and activation of PKCα. Recent research established that resveratrol attenuates CBNPs induced PKCα protein expression and inflammatory factors in lung epithelial cells [120]. A hyperglycemic condition associated with increased ROS production and vascular complications. Resveratrol treatment resulted in the downregulation of ROS production in human leukocytes cells through PKC inhibition during hyperglycemic conditions [121]. Phase2 clinical trial study of resveratrol in combination with Pazopanib and Paclitaxel were tried in stage III and IV melanoma. This compound is currently in phase 2 clinical trial for the treatment of congestive heart failure chronic, Phase 3 for dilated cardiomyopathy, and early phase 1 study for Type 1 diabetes (Table 2) (https://clinicaltrials.gov/).
Protocatechuic acid
Protocatechuic acid (PCA) is a type of phenolic acid that can be obtained from raspberry, Boswellia dalzielii, Cardiospermum halicacabum, and Diospyros melanoxylon [122, 123]. It has been shown to have anti-inflammatory, anti-diabetic and cognitive improving properties [122, 123]. PCA extensively interacts with PKCα and affects its translocation and reduces activity by 59% in mouse epidermal cells [124]. It also affects PKCζ, and PKCγ translocation to membrane fractions, however, PKCβI and βII remain unaffected. PCA is reported to be an anti-diabetic drug by suppressing the expression of PKCα and PKCβ activity [125]. It was also found that PCA could significantly attenuates inflammation in the myocardial tissue via inhibition of PKC/ PARP/ NF-κB signaling pathway [126].
Tannic acid
Tannic acid, a type of polyphenol, can be found in black tea, Caesalpinia spinosa, Rhus semialata, Quercus infectoria, and Terminalia chebula [5, 127]. Multiple studies have shown tannic acid to exert anti-tumor, anti-bacterial, anti-viral, anti-hypersensitive, neuroprotective and hepatoprotective properties [5, 128, 129]. Tannic acid reported being decreased PKCα, βI and βII activities in mouse epidermal cell lines [124]. Mechanistically, tannic acid increased the levels of PKCα, βI, and βII in the cytosolic fraction and decreased their activity in the membrane fraction of mouse epidermis [124]. Tannic acid exerts anti-tumor activities by inhibiting PKC activity in human carcinoma cells [130].
Miscellaneous
Other NPs from terrestrial plants have shown modulatory effects on PKC activity. Caffeic acid isolated from coffee, blueberry, pears and apple was reported to be a non-competitive inhibitor of PKC [131]. Apegenin, belongs to flavone polyphenol subclass, which acts as a competitive inhibitor of ATP to PKC [132], and induced apoptosis in leukemia cells via up-regulation of the PKCδ [133]. A study demonstrated that verbascoside competitively and non-competitively inhibits PKC [134]. At lower micromolar concentration viniferin inhibits PKCα, βII, γ, δ, and ε in non-competitive manner. Furthermore, a group of flavonoids such as fistenluteolin, morin, and rutin has been reported to inhibit PKC[135, 136]. It has been also observed that hydroxytyrosol, possesses vascular protective effects via inhibition of PKC α and βI activity [137].
PKC modulators from marine organism
Oceans and seas with 70% of the earth’s surface and approximately 80% of all leaving organisms are the largest ecosystems [138]. According to the MarinLit database (http://pubs.rsc.org/marinlit), more than 28,000 marine products have been reported after being isolated from a variety of marine sources; such as algae, bryozoa, corals, microorganisms, sea squirts, sponges, etc. [139]. Although, the majority of the current natural compound-derived drugs are associated with the terrestrial origin; however, the marine organism has attracted researchers due to inherent property to produce incomparable chemical and biological novelties [59]. A comparative study showed that marine isolated natural compounds are superior to terrestrial natural products in terms of chemical novelty [140]. Marine sources as kinase modulators are vast [59]. In recent years, a variety of marine organisms have also provided important PKC modulators such as bryostatin-1, from the marine bryozoan Bugula neritina [141]. Further, Marine sponges are also a great source of PKC inhibitors [142].
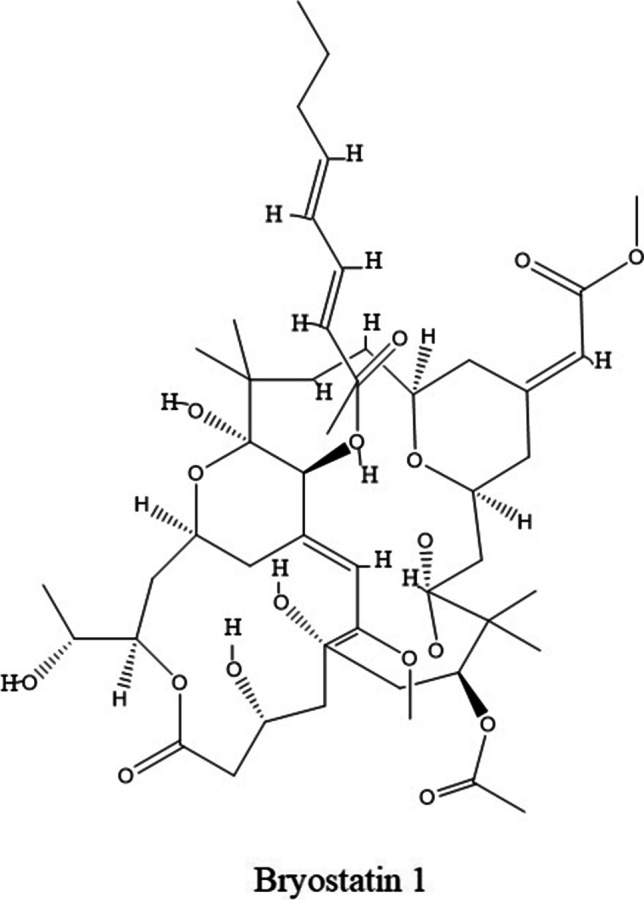
Bryostatin
Bryostatins are a family of at least 20 macrocyclic lactones extracted from bryozoan Bugula neritina since the late 1960s [141]. Bryostatin-1 is a prototype member of this family (Fig. 4.). Bryostatin-1 can bind to PKC isozymes at nanomolar concentration and modulate the activity of cPKC and nPKC [143]. It was observed that bryostatin 1 has the highest affinity for PKCα and ε isoform [144]. Most of the pharmacological and clinical research established bryostatin1 as an anticancer and neuroprotective agent. Several pharmacological and clinical studies have shown that bryostatin exhibits growth-inhibiting, anti-angiogenic effects on a variety of cancer [145]. Conversely, the anti-apoptotic action of bryostatin1 was observed in prostate cancer cells. Molecular study reveals that bryostatin1 inhibits PMA induced PKCδ translocation and release of TNFα and thereby apoptosis in prostate cancer cells [146]. Tumor cells have impaired T cell functions including IFN-γ production and MHCII functions. Interestingly, bryostatin1 possesses immunomodulatory effects and abrogate cancer progression. It has been demonstrated that bryostatin with IL2 synergistically control transcription and post the transcription of IFNγ by p38 mitogen-activated protein kinase-dependent pathway, independent of PKC pathway [147]. Later, it has been found that the introduction of PKC agonist bryostatin1 and PMA may improve IFNγ mediated signaling cascade thereby MHCII function in colorectal carcinoma cells [148]. Further, bryostatin reactivates HIV1 latent expression in NHA and U87 cells via the upregulation of PKC mainly α and δ isoforms[149]. Nevertheless, it abrogates acute infection in a receptor-independent manner and exerts its antiviral property. Mechanistically, bryostatin particularly modulates nPKC involving stress-induced AMPK, compound C partially ablated viral reactivation effect [150]. As previously described bryostatin can modulate T cell functions during cancer progression might be a strategy to target HIV infection. Furthermore, bryostatin 1 may modulate PKC activity and provide substantial benefit in the treatment of Alzheimer's disease, and acute ischemic stroke. It was shown that repeated bryostatin1 introduction improves survival rate, reduced lesion volume salvaged tissue infarcted hemisphere by reducing inflammation in acute cerebral ischemia rat by changes in PKCα, and PKCε expression in neurons [151]. Alzheimer's disease associated with loss of PKC activity, mainly α and ε isoforms, and accumulation of β- amyloid. Bryostatin1 treatments may restores PKCα and ε activity and thus abrogate the level of soluble β- amyloid to prevents and/or reverses the loss of hippocampal synapses, and improve the memory [152]. Therefore, bryostatin was studied in several phases I and II clinical studies to check the efficacy, tolerability, and assessing safety against moderate to severe Alzheimer’s disease patients [153]. Reports have been suggested that it was a preferable dose up to 20 μg with safety, and effectiveness. Further, Bryostatin is involved in epithelial barrier function through phosphorylation of the occludin and recruitment of tight junction proteins like claudin-1 and ZO-2, by PKC dependent pathway [154]. In phase I clinical study bryostatin has shown anticancer efficacy against metastatic renal cell carcinoma and soft tissue sarcoma, synergizing with temsirolimus; an mTORC inhibitor. Moreover, bryostatin has displayed some clinical activity as a single agent and was able to potentiate the anti-tumor activity of some of the clinically-used cytotoxins (Taxol® and doxorubicin) (https://clinicaltrials.gov/). Currently, there is no clinical study on the therapeutic value of individual PKC isozymes in terms of bryostatin efficacy.
Marine sponges as PKC modulators
Marine Sponges are the richest source of PKC regulators including xestocyclamine A from Xestospongia sp [155]; (Z)-Axinohydantoin and debromo-Z-axinohydantoin from Stylotella aurantium [156]; frondosins A–E from Dysidea frondosa [157], BRS1 from class Calcarea [158], nakijiquinones A–D from Okinawan [159], sesterpenes spongianolides from genus Spongia [160], lasonolide from Caribbean sponge Forcepia sp. [161], penazetidine A from the Indo-Pacific marine sponge Penares sollasi [162], corallidictyals A and B, from Aka (= Siphonodictyon) coralliphaga. Among them Frondosins A–E were also reported to target interleukin-8 [157] and ( −)-frondosins A (6) and D (9) have shown comparable activity against the HIV virus [163]. nakijiquinones G–I derived from Okinawan marine sponge has shown anticancer activity against various cancer cell lines with inhibitory effects on multiple kinases [164].
PKC modulators from bacteria
Staurosporine
It is the first reported ATP competitive PKC inhibitor, isolated from Streptomyces Sp with an anti-proliferative property [165]. However, despite its cytotoxicity activity staurosporine is less selective towards PKC isozymes. Therefore various compounds were designed based on parental staurosporine structure including midostaurin, enzastaurin, ruboxistaurin which have been evaluated in various disease conditions (Fig. 5.) [68, 166].
Midostaurin (PKC412 or n-benzoylstaurosporine)
A semisynthetic derivative of staurosporine that recognized as multiple kinases inhibitor including PKC with modest isozyme selectivity. Midostaurin has shown broad anticancer activity against solid tumor [167], melanoma cells [168], therefore already have been approved by the FDA for the treatment of acute myelocytic leukemia (AML) with Fms-like tyrosine kinase 3 (FLT3)-mutant subtype [169]. In preclinical studies, midostaurin has potentiated the cytotoxic effect of various chemotherapeutic drugs against various cancer types [170, 171]. Midostaurin was shown to have induced antiapoptotic activity of rituximab in Burkitt’s lymphoma cells by reducing the phosphorylation of PKC and consequently downstream molecules such as Bad, Bcl-2, and NF-κB [172]. Midostaurin was well-tolerated in phase I/II study with few side effects and prolonged the survival of patients with newly diagnosed Flt3-mutated AML [173]. Additionally, midostaurin improved the overall survival and relapse-free survival in patients with advanced systemic mastocytosis compared with historical controls. Besides, midostaurin was found to improve mast cell mediator-related symptoms and quality of life [174]. Moreover, this compound is currently being employed in multiple phase clinical trials for AML with or without FLT3 mutation (Table 2) (https://clinicaltrials.gov/).
Sangivamycin
4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine or sangivamycin; a naturally occurring deaza purine nucleoside analog originally isolated from the culture broth of Streptomyces rimosus, which is known as an antitumor antibiotic [175]. It is a potent PKCs inhibitor competes with the binding of ATP at catalytic site [176]. Sangivamycin and its derivatives have been evaluated as anticancer agents against various cancer types. This drug can induce apoptosis in breast cancer cells via Protein Kinase Cδ and JNK activation [177]. Moreover, in a comprehensive study, it was found that ARC (ARC (NSC 188,491, SMA-491), 4-amino-6-hydrazino-7-β-d-ribofuranosyl-7H-pyrrolo-(2,3-d)-pyrimidine-5-carboxamide) and Sangivamycin exhibit identical activity in terms of cytotoxicity, cell cycle arrest, inhibition of positive transcription elongation factor b (P-TEFb), inhibition of VEGF secretion, and simultaneous inhibition of PKC activity [178]. The antineoplastic behavior of sangivamycin is interesting given that several reports exist of Phase I trials of sangivamycin in patients with a range of malignancies [166].
PKC pharmacology: Future aspects
The frequently observed perturbation of PKC signalling in various pathological conditions, generated interest in the designing of PKC-based drugs with isoform-specificity and therapeutic efficacy [1]. The most common modulators that target PKC include; ATP-binding site modulators, C1 domain binding modulators, peptides that disrupt PKC interaction with binding partners, and PKC-isoform specific antisense oligonucleotides [10]. Despite promising preclinical results, PKC modulators failed to produce therapeutic effects during clinical trials. However, with the continuous emergence of the non-isoform specific target of PKC modulators, unforeseen side effects, inadequate therapeutic efficacy, the development of PKC-modulators become very challenging. [1]. Further, the development of isozyme selective drugs became very difficult due to the structural homology, and antagonistic biological effect [1, 11]. Therefore, natural products, which are an important source of drugs, continue to rise in the development of new PKC modulators. The search for PKC modulator from natural sources has proven effective with the advent of curcumin [71], rottlerin [84], quercetin [89], ellagic acid [98], epigallocatechin-3 gallate [102], ingenol 3 angelate [111], resveratrol [116], protocatechuic acid [124], tannic acid [124], staurosporine [165]. Indeed, the involvements of PKC in life threatening diseases and unavailability of PKC isoform specific modulators warrants research in PKC biology. Although scanty literature on the NPs as PKC modulators are available, the modern technology brings to access previously unexplored NPs, it is certain that many more metabolites with incomparable structures and potent PKC modulatory activities will be discovered in the future.
Perspective and conclusion
The involvement of PKC in the regulation of both normal physiology and disease condition makes it an enticing target for drug development. In light of the above study, this article provides few highlights in terms of NPs derived PKC modulators such as (a) NPs and their derivatives can modulate PKC signaling cascade during various pathological conditions, (b) NPs could regulate PKC in isoform and context-dependent manner (c) Few of them have proved their clinical efficacy against various disease conditions (d) Many NPs can bind to various kinases in a single cell or tissue type.Since NPs and their derivatives play a critical role as therapeutic regimes and that approximately 70% of anti-cancer drugs are derived from natural products, and possess unique structural features, NPs have become an important research area for drug discovery. Although the NPs have been extensively studied in various disease conditions in vitro, clinical efficacy is still under debate. The development of NPs as a PKC modulator is expected to access unexplored new biological targets with novel structure and potent PKC modulatory activities. A systematic and comprehensive study of isoform-selective PKC NPs, determining the sites of action of NPs on PKC, so that new molecules could be developed based on these sites. Furthermore, as our understanding of the mechanism and regulation of PKC continues to grow, NPs-derived kinase inhibitors are destined to play an expanding role in the treatment and prevention of various diseases.
Acknowledgements
We are grateful to the CSIR-UGC, New Delhi for the financial support to RKS for his research work.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discovery. 2012;11(12):937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konopatskaya O, Poole AW. Protein kinase Cα: disease regulator and therapeutic target. Trends Pharmacol Sci. 2010;31(1):8–14. doi: 10.1016/j.tips.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omura S, Iwai Y, Hirano A, Nakagawa A, Awaya J, Tsuchiya H, et al. A new alkaloid AM-2282 of Streptomyces origin taxonomy, fermentation, isolation and preliminary characterization. The J Antibio. 1977;30(4):275–82. [DOI] [PubMed]
- 4.Harvey AL. Natural products in drug discovery. Drug Discovery Today. 2008;13(19–20):894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Das J, Ramani R, Suraju MO. Polyphenol compounds and PKC signaling. Biochimica et Biophysica Acta (BBA)-General Subjects. 2016;1860(10):2107–21. [DOI] [PMC free article] [PubMed]
- 6.Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252(21):7603–9. [PubMed]
- 7.Takai Y, Kishimoto A, Kikkawa U, Mori T, Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- 8.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257(13):7847–7851. [PubMed] [Google Scholar]
- 9.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270(48):28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 10.Singh RK, Kumar S, Gautam PK, Tomar MS, Verma PK, Singh SP, et al. Protein kinase C-α and the regulation of diverse cell responses. Biomol Concepts. 2017;8(3–4):143–153. doi: 10.1515/bmc-2017-0005. [DOI] [PubMed] [Google Scholar]
- 11.Isakov N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Seminars in cancer biology: Elsevier; 2018. p. 36–52. [DOI] [PubMed]
- 12.Newton AC. Protein kinase C: poised to signal. Am J Physiol-Endocrinol and Metabol. 2010. [DOI] [PMC free article] [PubMed]
- 13.House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa K, Toker A, Johannes F-J, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272(2):952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 15.Ono Y, Fujii T, Igarashi K, Kuno T, Tanaka C, Kikkawa U, et al. Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci. 1989;86(13):4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeley M, Kelleher D, Long A. Regulation of protein kinase C function by phosphorylation on conserved and non-conserved sites. Cell Signal. 2011;23(5):753–762. doi: 10.1016/j.cellsig.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Dutil EM, Toker A, Newton AC. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1) Curr Biol. 1998;8(25):1366–1375. doi: 10.1016/s0960-9822(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 18.Cenni V, Döppler H, Sonnenburg ED, Maraldi N, Newton AC, Toker A. Regulation of novel protein kinase C ε by phosphorylation. Biochemical Journal. 2002;363(3):537–545. doi: 10.1042/0264-6021:3630537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen C-S, et al. Regulation of protein kinase C ζ by PI 3-kinase and PDK-1. Curr Biol. 1998;8(19):1069–1078. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Molkentin JD. Protein kinase Cα as a heart failure therapeutic target. J Mol Cell Cardiol. 2011;51(4):474–478. doi: 10.1016/j.yjmcc.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pass JM, Gao J, Jones WK, Wead WB, Wu X, Zhang J, et al. Enhanced PKCβII translocation and PKCβII-RACK1 interactions in PKCε-induced heart failure: a role for RACK1. Am J Physiol-Heart and Circul Physiol. 2001;281(6):H2500–H2510. doi: 10.1152/ajpheart.2001.281.6.H2500. [DOI] [PubMed] [Google Scholar]
- 22.Duquesnes N, Lezoualc'h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers? J Mol Cell Cardiol. 2011;51(5):665–673. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg M, Steinberg SF. Tissue-specific developmental regulation of protein kinase C isoforms. Biochem Pharmacol. 1996;51(8):1089–1093. doi: 10.1016/0006-2952(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 24.Altman A, Kong K-F. Protein kinase C enzymes in the hematopoietic and immune systems. Annu Rev Immunol. 2016;34:511–538. doi: 10.1146/annurev-immunol-041015-055347. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Liu C, Jia L. The roles of PKCs in regulating autophagy. J Cancer Res Clin Oncol. 2018;144(12):2303–2311. doi: 10.1007/s00432-018-2731-4. [DOI] [PubMed] [Google Scholar]
- 26.Clemens M, Trayner I, Menaya J. The role of protein kinase C isoenzymes in the regulation of cell proliferation and differentiation. J Cell Sci. 1992;103(4):881–887. doi: 10.1242/jcs.103.4.881. [DOI] [PubMed] [Google Scholar]
- 27.Lanuza MA, Santafe MM, Garcia N, Besalduch N, Tomàs M, Obis T, et al. Protein kinase C isoforms at the neuromuscular junction: localization and specific roles in neurotransmission and development. J Anat. 2014;224(1):61–73. doi: 10.1111/joa.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu X, Yasuda R, Colgan LA. Rac1 is a downstream effector of PKCα in structural synaptic plasticity. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-58610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim C-S, Nam HJ, Lee J, Kim D, Choi JE, Kang SJ, et al. PKCα-mediated phosphorylation of LSD1 is required for presynaptic plasticity and hippocampal learning and memory. Sci Rep. 2017;7(1):1–15. doi: 10.1038/s41598-017-05239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrocco V, Bogomolovas J, Ehler E, dos Remedios CG, Yu J, Gao C, et al. PKC and PKN in heart disease. J Mol Cell Cardiol. 2019;128:212–226. doi: 10.1016/j.yjmcc.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kooij V, Zhang P, Piersma SR, Sequeira V, Boontje NM, Wijnker PJ, et al. PKCα-specific phosphorylation of the troponin complex in human myocardium: a functional and proteomics analysis. PLoS ONE. 2013;8(10):e74847. doi: 10.1371/journal.pone.0074847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, et al. PKC-α regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10(3):248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 33.Wakeham CM, Wilmarth PA, Cunliffe JM, Klimek JE, Ren G, David LL, et al. Identification of PKCα-dependent phosphoproteins in mouse retina. J Proteomics. 2019;206:103423. doi: 10.1016/j.jprot.2019.103423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaff IL, Wagner H-J, Vallon V. Immunolocalization of protein kinase C isoenzymes α, βI and βII in rat kidney. J Am Soc Nephrol. 1999;10(9):1861–1873. doi: 10.1681/ASN.V1091861. [DOI] [PubMed] [Google Scholar]
- 35.Serlachius E, Svennilson J, Schalling M, Aperia A. Protein kinase C in the developing kidney: isoform expression and effects of ceramide and PKC inhibitors. Kidney Int. 1997;52(4):901–910. doi: 10.1038/ki.1997.411. [DOI] [PubMed] [Google Scholar]
- 36.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70(2):222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Chou W-H, Messing RO. Protein kinase C isozymes in stroke. Trends Cardiovasc Med. 2005;15(2):47–51. doi: 10.1016/j.tcm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Alfonso SI, Callender JA, Hooli B, Antal CE, Mullin K, Sherman MA, et al. Gain-of-function mutations in protein kinase Cα (PKCα) may promote synaptic defects in Alzheimer’s disease. Sci Signaling. 2016;9(427):ra47-ra. [DOI] [PMC free article] [PubMed]
- 39.Zhang D, Anantharam V, Kanthasamy A, Kanthasamy AG. Neuroprotective effect of protein kinase Cδ inhibitor rottlerin in cell culture and animal models of Parkinson's disease. J Pharmacol Exp Ther. 2007;322(3):913–922. doi: 10.1124/jpet.107.124669. [DOI] [PubMed] [Google Scholar]
- 40.Hahn CG, Friedman E. Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord. 1999;1(2):81–86. doi: 10.1034/j.1399-5618.1999.010204.x. [DOI] [PubMed] [Google Scholar]
- 41.Tury A, Tolentino K, Zou Y. Altered expression of atypical PKC and Ryk in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Dev Neurobiol. 2014;74(8):839–850. doi: 10.1002/dneu.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cirillo N, Lanza A, Prime SS. Induction of hyper-adhesion attenuates autoimmune-induced keratinocyte cell–cell detachment and processing of adhesion molecules via mechanisms that involve PKC. Exp Cell Res. 2010;316(4):580–592. doi: 10.1016/j.yexcr.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Skvara H, Dawid M, Kleyn E, Wolff B, Meingassner JG, Knight H, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Investig. 2008;118(9):3151–3159. doi: 10.1172/JCI35636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee IT, Yang CM. Inflammatory signalings involved in airway and pulmonary diseases. Media of Inflamm. 2013;2013. [DOI] [PMC free article] [PubMed]
- 45.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zong Y, Yuan Y, Qian X, Huang Z, Yang W, Lin L, et al. Small Molecular-Sized Artesunate Attenuates Ocular Neovascularization via VEGFR2. PKCα and PDGFR Targets Scientific reports. 2016;6(1):1–12. doi: 10.1038/srep30843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominique J-F, Kolassa I-T, Ackermann S, Aerni A, Boesiger P, Demougin P, et al. PKCα is genetically linked to memory capacity in healthy subjects and to risk for posttraumatic stress disorder in genocide survivors. Proc Natl Acad Sci. 2012;109(22):8746–8751. doi: 10.1073/pnas.1200857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong J. Role of natural product diversity in chemical biology. Curr Opin Chem Biol. 2011;15(3):350–354. doi: 10.1016/j.cbpa.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA)-General Subjects. 2013;1830(6):3670–95. [DOI] [PMC free article] [PubMed]
- 51.Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25(3):475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 52.Zhu F, Ma XH, Qin C, Tao L, Liu X, Shi Z, et al. Drug discovery prospect from untapped species: indications from approved natural product drugs. PLoS ONE. 2012;7(7):e39782. doi: 10.1371/journal.pone.0039782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winter JM, Tang Y. Synthetic biological approaches to natural product biosynthesis. Curr Opin Biotechnol. 2012;23(5):736–743. doi: 10.1016/j.copbio.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji HF, Li XJ, Zhang HY. Natural products and drug discovery: can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009;10(3):194–200. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 56.Cragg GM, Pezzuto JM. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract. 2016;25(Suppl. 2):41–59. doi: 10.1159/000443404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meiyanto E, Hermawan A. Natural products for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian Pacific journal of cancer prevention: APJCP. 2012;13(2):427. doi: 10.7314/apjcp.2012.13.2.427. [DOI] [PubMed] [Google Scholar]
- 58.Guerra B, Issinger O-G. Natural compounds and derivatives as Ser/Thr protein kinase modulators and inhibitors. Pharmaceuticals. 2019;12(1):4. doi: 10.3390/ph12010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bharate SB, Sawant SD, Singh PP, Vishwakarma RA. Kinase inhibitors of marine origin. Chem Rev. 2013;113(8):6761–6815. doi: 10.1021/cr300410v. [DOI] [PubMed] [Google Scholar]
- 60.Fan Z, Duan X, Cai H, Wang L, Li M, Qu J, et al. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol Rep. 2015;34(2):691–698. doi: 10.3892/or.2015.4044. [DOI] [PubMed] [Google Scholar]
- 61.Chen S-Q, Wang Z-S, Ma Y-X, Zhang W, Lu J-L, Liang Y-R, et al. Neuroprotective effects and mechanisms of tea bioactive components in neurodegenerative diseases. Molecules. 2018;23(3):512. doi: 10.3390/molecules23030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuo M-Y, Ou H-C, Lee W-J, Kuo W-W, Hwang L-L, Song T-Y, et al. Ellagic acid inhibits oxidized low-density lipoprotein (OxLDL)-induced metalloproteinase (MMP) expression by modulating the protein kinase C-α/extracellular signal-regulated kinase/peroxisome proliferator-activated receptor γ/nuclear factor-κB (PKC-α/ERK/PPAR-γ/NF-κB) signaling pathway in endothelial cells. J Agric Food Chem. 2011;59(9):5100–5108. doi: 10.1021/jf1041867. [DOI] [PubMed] [Google Scholar]
- 63.Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Mito S, Harima M, et al. Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: possible involvement of PKC–MAPK signaling pathway. Eur J Pharm Sci. 2012;47(3):604–614. doi: 10.1016/j.ejps.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 64.Soetikno V, Watanabe K, Sari FR, Harima M, Thandavarayan RA, Veeraveedu PT, et al. Curcumin attenuates diabetic nephropathy by inhibiting PKC-α and PKC-β1 activity in streptozotocin-induced type I diabetic rats. Mol Nutr Food Res. 2011;55(11):1655–1665. doi: 10.1002/mnfr.201100080. [DOI] [PubMed] [Google Scholar]
- 65.Kim J-Y, Lee Y-M, Kim D-W, Min T, Lee S-J. Nanosphere Loaded with Curcumin Inhibits the Gastrointestinal Cell Death Signaling Pathway Induced by the Foodborne Pathogen Vibrio vulnificus. Cells. 2020;9(3):631. doi: 10.3390/cells9030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cochet C, Feige J, Pirollet F, Keramidas M, Chambaz E. Selective inhibition of a cyclic nucleotide independent protein kinase (G type casein kinase) by quercetin and related polyphenols. Biochem Pharmacol. 1982;31(7):1357–1361. doi: 10.1016/0006-2952(82)90028-4. [DOI] [PubMed] [Google Scholar]
- 67.Tamoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid calcium dependent protein kinase. Biochem Biophys Res Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 68.Matias D, Bessa C, Simões MF, Reis CP, Saraiva L, Rijo P. Natural products as lead protein kinase c modulators for cancer therapy. Studies in natural products chemistry. Elsevier; 2016. p. 45–79.
- 69.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hewlings SJ, Kalman DS. Curcumin: a review of its’ effects on human health. Foods. 2017;6(10):92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pany S, You Y, Das J. Curcumin Inhibits Protein Kinase Cα Activity by Binding to Its C1 Domain. Biochemistry. 2016;55(45):6327–6336. doi: 10.1021/acs.biochem.6b00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lai K, Liu C, Chang K, Lee T. Depleting IFIT2 mediates atypical PKC signaling to enhance the migration and metastatic activity of oral squamous cell carcinoma cells. Oncogene. 2013;32(32):3686–3697. doi: 10.1038/onc.2012.384. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Kong Y, Liu S, Zeng L, Wan L, Zhang Z. Curcumin induces apoptosis in human leukemic cell lines through an IFIT2-dependent pathway. Cancer Biol Ther. 2017;18(1):43–50. doi: 10.1080/15384047.2016.1276129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roy M, Mukherjee S, Sarkar R, Biswas J. Curcumin sensitizes chemotherapeutic drugs via modulation of PKC, telomerase, NF-κB and HDAC in breast cancer. Ther Deliv. 2011;2(10):1275–1293. doi: 10.4155/tde.11.97. [DOI] [PubMed] [Google Scholar]
- 75.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 76.Schiborr C, Kocher A, Behnam D, Jandasek J, Toelstede S, Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol Nutr Food Res. 2014;58(3):516–527. doi: 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]
- 77.Hackler L, Jr, Ózsvári B, Gyuris M, Sipos P, Fábián G, Molnár E, et al. The curcumin analog C-150, influencing NF-κB, UPR and Akt/Notch pathways has potent anticancer activity in vitro and in vivo. PLoS ONE. 2016;11(3):e0149832. doi: 10.1371/journal.pone.0149832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Badr G, Gul HI, Yamali C, Mohamed AA, Badr BM, Gul M, et al. Curcumin analogue 1, 5-bis (4-hydroxy-3-((4-methylpiperazin-1-yl) methyl) phenyl) penta-1, 4-dien-3-one mediates growth arrest and apoptosis by targeting the PI3K/AKT/mTOR and PKC-theta signaling pathways in human breast carcinoma cells. Bioorg Chem. 2018;78:46–57. doi: 10.1016/j.bioorg.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Kline LW, Karpinski E. Curcumin Relaxes Precontracted Guinea Pig Gallbladder Strips via Multiple Signaling Pathways. Gastroenterology Res. 2015;8(5):253. doi: 10.14740/gr689w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Zhang B, Huang F, Liu B, Xie Y. Curcumin inhibits lipolysis via suppression of ER stress in adipose tissue and prevents hepatic insulin resistance. J Lipid Res. 2016;57(7):1243–1255. doi: 10.1194/jlr.M067397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie Z, Zeng X, Li X, Wu B, Shen G, Wu Q, et al. Curcumin attenuates oxidative stress in liver in Type 1 diabetic rats. Open Life Sciences. 2017;12(1):452–459. [Google Scholar]
- 82.Clements RT, Cordeiro B, Feng J, Bianchi C, Sellke FW. Rottlerin increases cardiac contractile performance and coronary perfusion through BKCa++ channel activation after cold cardioplegic arrest in isolated hearts. Circul. 2011;124(11_suppl_1):S55-S61. [DOI] [PMC free article] [PubMed]
- 83.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochemical Journal. 2000;351(1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mori N, Ishikawa C, Senba M. Activation of PKC‑δ in HTLV‑1‑infected T cells. Int J Oncol. 2015;46(4):1609–18. [DOI] [PubMed]
- 85.Misuth M, Horvath D, Miskovsky P, Huntosova V. Synergism between PKCδ regulators hypericin and rottlerin enhances apoptosis in U87 MG glioma cells after light stimulation. Photodiagn Photodyn Ther. 2017;18:267–274. doi: 10.1016/j.pdpdt.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 86.Wermuth PJ, Addya S, Jimenez SA. Effect of protein kinase C delta (PKC-δ) inhibition on the transcriptome of normal and systemic sclerosis human dermal fibroblasts in vitro. PLoS ONE. 2011;6(11):e27110. doi: 10.1371/journal.pone.0027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelly GS. Monograph-Quercetin. Altern Med Rev. 2011;16(2):172. [PubMed] [Google Scholar]
- 88.Chirumbolo S, Marzotto M, Conforti A, Vella A, Ortolani R, Bellavite P. Bimodal action of the flavonoid quercetin on basophil function: an investigation of the putative biochemical targets. Clinical and Molecular Allergy. 2010;8(1):1–12. doi: 10.1186/1476-7961-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X-M, Huang S-P, Xu Q. Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother Pharmacol. 2004;53(1):82–88. doi: 10.1007/s00280-003-0702-0. [DOI] [PubMed] [Google Scholar]
- 90.Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol Biol Rep. 2015;42(9):1419–1429. doi: 10.1007/s11033-015-3921-7. [DOI] [PubMed] [Google Scholar]
- 91.Lee BK, Jung Y-S. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via PKC-ε inactivation/ERK1/2 activation. Oxidative Med and Cell Longevity. 2016;2016. [DOI] [PMC free article] [PubMed]
- 92.Shin EJ, Lee JS, Hong S, Lim T-G, Byun S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int J Mol Sci. 2019;20(21):5262. doi: 10.3390/ijms20215262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larrosa M, García-Conesa MT, Espín JC, Tomás-Barberán FA. Ellagitannins, ellagic acid and vascular health. Mol Aspects Med. 2010;31(6):513–539. doi: 10.1016/j.mam.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 94.Kesavan R, Ganugula R, Avaneesh T, Kumar U, Reddy GB, Dixit M. Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats. J Nutr Biochem. 2013;24(11):1830–1839. doi: 10.1016/j.jnutbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 95.Mo J, Panichayupakaranant P, Kaewnopparat N, Songkro S, Reanmongkol W. Topical anti-inflammatory potential of standardized pomegranate rind extract and ellagic acid in contact dermatitis. Phytother Res. 2014;28(4):629–632. doi: 10.1002/ptr.5039. [DOI] [PubMed] [Google Scholar]
- 96.Chung YC, Lu LC, Tsai MH, Chen YJ, Chen YY, Yao SP, et al. The inhibitory effect of ellagic acid on cell growth of ovarian carcinoma cells. Evidence-Based Complement and Altern Med. 2013;2013. [DOI] [PMC free article] [PubMed]
- 97.Chang Y, Chen W-F, Lin K-H, Hsieh C-Y, Chou D-S, Lin L-J, et al. Novel bioactivity of ellagic acid in inhibiting human platelet activation. Evidence-Based Complement and Altern Med. 2013;2013. [DOI] [PMC free article] [PubMed]
- 98.Mishra S, Vinayak M. Ellagic acid inhibits PKC signaling by improving antioxidant defense system in murine T cell lymphoma. Mol Biol Rep. 2014;41(7):4187–4197. doi: 10.1007/s11033-014-3289-0. [DOI] [PubMed] [Google Scholar]
- 99.Mishra S, Vinayak M. Ellagic acid checks lymphoma promotion via regulation of PKC signaling pathway. Mol Biol Rep. 2013;40(2):1417–1428. doi: 10.1007/s11033-012-2185-8. [DOI] [PubMed] [Google Scholar]
- 100.Mishra S, Vinayak M. Role of ellagic acid in regulation of apoptosis by modulating novel and atypical PKC in lymphoma bearing mice. BMC Complement Altern Med. 2015;15(1):1–8. doi: 10.1186/s12906-015-0810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82(12):1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitano K, Nam K-Y, Kimura S, Fujiki H, Imanishi Y. Sealing effects of (−)-epigallocatechin gallate on protein kinase C and protein phosphatase 2A. Biophys Chem. 1997;65(2–3):157–164. doi: 10.1016/s0301-4622(96)02254-5. [DOI] [PubMed] [Google Scholar]
- 103.Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Aβ toxicity and PKC-dependent release of non-amyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. FASEB J. 2003;17(8):1–23. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- 104.Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002;277(34):30574–30580. doi: 10.1074/jbc.M202832200. [DOI] [PubMed] [Google Scholar]
- 105.Kalfon L, Youdim MB, Mandel SA. Green tea polyphenol (–)-epigallocatechin-3-gallate promotes the rapid protein kinase C-and proteasome-mediated degradation of Bad: implications for neuroprotection. J Neurochem. 2007;100(4):992–1002. doi: 10.1111/j.1471-4159.2006.04265.x. [DOI] [PubMed] [Google Scholar]
- 106.Menard C, Bastianetto S, Quirion R. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front Cell Neurosci. 2013;7:281. doi: 10.3389/fncel.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao X, Liu F, Jin H, Li R, Wang Y, Zhang W, et al. Involvement of PKCα and ERK1/2 signaling pathways in EGCG’s protection against stress-induced neural injuries in Wistar rats. Neuroscience. 2017;346:226–237. doi: 10.1016/j.neuroscience.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park SJ, Jeong JM, Jeong H-S, Park J-S, Kim N-H. Effects of Epigallocatechin-3-Gallate on the Expression of TGF-β1, PKC α/βII, and NF-κB in High-Glucose-Stimulated Glomerular Epithelial Cells. Chonnam Med J. 2011;47(2):116–121. doi: 10.4068/cmj.2011.47.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang J, Han Y, Chen C, Sun H, He D, Guo J, et al. EGCG attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-κB signaling in human umbilical vein endothelial cells. Life Sci. 2013;92(10):589–597. doi: 10.1016/j.lfs.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 110.Yang J, Han Y, Sun H, Chen C, He D, Guo J, et al. (−)-Epigallocatechin gallate suppresses proliferation of vascular smooth muscle cells induced by high glucose by inhibition of PKC and ERK1/2 signalings. J Agric Food Chem. 2011;59(21):11483–11490. doi: 10.1021/jf2024819. [DOI] [PubMed] [Google Scholar]
- 111.Kedei N, Lundberg DJ, Toth A, Welburn P, Garfield SH, Blumberg PM. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Can Res. 2004;64(9):3243–3255. doi: 10.1158/0008-5472.can-03-3403. [DOI] [PubMed] [Google Scholar]
- 112.Hampson P, Chahal H, Khanim F, Hayden R, Mulder A, Assi LK, et al. PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood. 2005;106(4):1362–1368. doi: 10.1182/blood-2004-10-4117. [DOI] [PubMed] [Google Scholar]
- 113.Serova M, Ghoul A, Benhadji KA, Faivre S, Le Tourneau C, Cvitkovic E, et al. Effects of protein kinase C modulation by PEP005, a novel ingenol angelate, on mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling in cancer cells. Mol Cancer Ther. 2008;7(4):915–922. doi: 10.1158/1535-7163.MCT-07-2060. [DOI] [PubMed] [Google Scholar]
- 114.Lee WY, Hampson P, Coulthard L, Ali F, Salmon M, Lord JM, et al. Novel antileukemic compound ingenol 3-angelate inhibits T cell apoptosis by activating protein kinase Cθ. J Biol Chem. 2010;285(31):23889–98. [DOI] [PMC free article] [PubMed]
- 115.Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J Agric Food Chem. 2002;50(3):431–435. doi: 10.1021/jf010812u. [DOI] [PubMed] [Google Scholar]
- 116.Slater SJ, Seiz JL, Cook AC, Stagliano BA, Buzas CJ. Inhibition of protein kinase C by resveratrol. Biochimica et Biophysica Acta (BBA)-Mole Basis of Dis. 2003;1637(1):59–69. [DOI] [PubMed]
- 117.Woo J-H, Lim JH, Kim Y-H, Suh S-I, Chang J-S, Lee YH, et al. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC δ signal transduction. Oncogene. 2004;23(10):1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]
- 118.Fang J-Y, Li Z-H, Li Q, Huang W-S, Kang L, Wang J-P. Resveratrol affects protein kinase C activity and promotes apoptosis in human colon carcinoma cells. Asian Pac J Cancer Prev. 2012;13(12):6017–6022. doi: 10.7314/apjcp.2012.13.12.6017. [DOI] [PubMed] [Google Scholar]
- 119.Morris-Blanco KC, Dave KR, Saul I, Koronowski KB, Stradecki HM, Perez-Pinzon MA. Protein kinase C epsilon promotes cerebral ischemic tolerance via modulation of mitochondrial Sirt5. Sci Rep. 2016;6:29790. doi: 10.1038/srep29790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hsu H-T, Tseng Y-T, Wong W-J, Liu C-M, Lo Y-C. Resveratrol prevents nanoparticles-induced inflammation and oxidative stress via downregulation of PKC-α and NADPH oxidase in lung epithelial A549 cells. BMC Complement Altern Med. 2018;18(1):1–13. doi: 10.1186/s12906-018-2278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gonzaga R, Volpe C, Villar-Delfino P, Anjos P, Gomes P, NOGUEIRA-MACHADO J. Resveratrol inhibits the production of reactive oxygen species in phorbol ester-and toll-like receptor-stimulated granulocytes from diabetic patients. J Diabetes Metab Disord Control. 2016;3(7):147–52.
- 122.Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. Int Scholar Res Notices. 2014;2014. [DOI] [PMC free article] [PubMed]
- 123.Semaming Y, Pannengpetch P, Chattipakorn SC, Chattipakorn N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evidence-Based Complement and Altern Med. 2015;2015. [DOI] [PMC free article] [PubMed]
- 124.Szaefer H, Kaczmarek J, Rybczyńska M, Baer-Dubowska W. The effect of plant phenols on the expression and activity of phorbol ester-induced PKC in mouse epidermis. Toxicology. 2007;230(1):1–10. doi: 10.1016/j.tox.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 125.Lin C-Y, Tsai S-J, Huang C-S, Yin M-C. Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice. J Agric Food Chem. 2011;59(9):5117–5124. doi: 10.1021/jf200103f. [DOI] [PubMed] [Google Scholar]
- 126.Bhattacharjee N, Dua TK, Khanra R, Joardar S, Nandy A, Saha A, et al. Protocatechuic acid, a phenolic from Sansevieria roxburghiana leaves, suppresses diabetic cardiomyopathy via stimulating glucose metabolism, ameliorating oxidative stress, and inhibiting inflammation. Front Pharmacol. 2017;8:251. doi: 10.3389/fphar.2017.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hussain G, Huang J, Rasul A, Anwar H, Imran A, Maqbool J, et al. Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: An updated review. Molecules. 2019;24(12):2213. doi: 10.3390/molecules24122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmad T. Reviewing the tannic acid mediated synthesis of metal nanoparticles. Journal of Nanotechnology. 2014;2014.
- 129.Mittal DK, Joshi D, Shukla S. Antioxidant and Hepatoprotective Effects of Polygonum Bistorta Linn. and Tannic Acid on Carbon Tetrachloride-treated Rats. IJP; 2011.
- 130.Yang EB, Wei L, Zhang K, Chen YZ, Chen WN. Tannic acid, a potent inhibitor of epidermal growth factor receptor tyrosine kinase. J Biochem. 2006;139(3):495–502. doi: 10.1093/jb/mvj050. [DOI] [PubMed] [Google Scholar]
- 131.Nardini M, Scaccini C, Packer L, Virgili F. In vitro inhibition of the activity of phosphorylase kinase, protein kinase C and protein kinase A by caffeic acid and a procyanidin-rich pine bark (Pinus marittima) extract. Biochimica et Biophysica Acta (BBA)-General Subjects. 2000;1474(2):219–25. [DOI] [PubMed]
- 132.Huang Y-T, Kuo M-L, Liu J-Y, Huang S-Y, Lin J-K. Inhibitions of protein kinase C and proto-oncogene expressions in NIH 3T3 cells by apigenin. Eur J Cancer. 1996;32(1):146–151. doi: 10.1016/0959-8049(95)00540-4. [DOI] [PubMed] [Google Scholar]
- 133.Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced-apoptosis is mediated by the activation of PKCδ and caspases in leukemia cells. Biochem Pharmacol. 2006;72(6):681–692. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 134.Herbert J, Maffrand J, Taoubi K, Augereau J, Fouraste I, Gleye J. Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J Nat Prod. 1991;54(6):1595–1600. doi: 10.1021/np50078a016. [DOI] [PubMed] [Google Scholar]
- 135.End D, Look R, Shaffer N, Balles E, Persico F. Non-selective inhibition of mammalian protein kinases by flavinoids in vitro. Res Commun Chem Pathol Pharmacol. 1987;56(1):75–86. [PubMed] [Google Scholar]
- 136.Ferriola PC, Cody V, Middleton E., Jr Protein kinase C inhibition by plant flavonoids: kinetic mechanisms and structure-activity relationships. Biochem Pharmacol. 1989;38(10):1617–1624. doi: 10.1016/0006-2952(89)90309-2. [DOI] [PubMed] [Google Scholar]
- 137.Scoditti E, Nestola A, Massaro M, Calabriso N, Storelli C, De Caterina R, et al. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis. 2014;232(1):17–24. doi: 10.1016/j.atherosclerosis.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 138.Shang J, Hu B, Wang J, Zhu F, Kang Y, Li D, et al. Cheminformatic insight into the differences between terrestrial and marine originated natural products. J Chem Inf Model. 2018;58(6):1182–1193. doi: 10.1021/acs.jcim.8b00125. [DOI] [PubMed] [Google Scholar]
- 139.Blunt JW, Copp BR, Hu W-P, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2008;25(1):35–94. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- 140.Bernan V, Greenstein M, Maiese W. Marine microorganisms as a source of new natural products. Advances in applied microbiology. Elsevier; 1997. p. 57–90. [DOI] [PubMed]
- 141.Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. Isolation and structure of bryostatin 1. J Am Chem Soc. 1982;104(24):6846–6848. [Google Scholar]
- 142.Skropeta D, Pastro N, Zivanovic A. Kinase inhibitors from marine sponges. Mar Drugs. 2011;9(10):2131–2154. doi: 10.3390/md9102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun MK, Alkon DL. Bryostatin-1: Pharmacology and Therapeutic Potential as a CNS Drug. CNS Drug Rev. 2006;12(1):1–8. doi: 10.1111/j.1527-3458.2006.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim H, Han S, Quan H, Jung Y-J, An J, Kang P, et al. Bryostatin-1 promotes long-term potentiation via activation of PKCα and PKCε in the hippocampus. Neuroscience. 2012;226:348–355. doi: 10.1016/j.neuroscience.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 145.Kollár P, Rajchard J, Balounová Z, Pazourek J. Marine natural products: bryostatins in preclinical and clinical studies. Pharm Biol. 2014;52(2):237–242. doi: 10.3109/13880209.2013.804100. [DOI] [PubMed] [Google Scholar]
- 146.von Burstin VA, Xiao L, Kazanietz MG. Bryostatin 1 inhibits phorbol ester-induced apoptosis in prostate cancer cells by differentially modulating protein kinase C (PKC) δ translocation and preventing PKCδ-mediated release of tumor necrosis factor-α. Mol Pharmacol. 2010;78(3):325–332. doi: 10.1124/mol.110.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Curiel RE, Garcia CS, Farooq L, Aguero MF, Espinoza-Delgado I. Bryostatin-1 and IL-2 synergize to induce IFN-γ expression in human peripheral blood T cells: implications for cancer immunotherapy. J Immunol. 2001;167(9):4828–4837. doi: 10.4049/jimmunol.167.9.4828. [DOI] [PubMed] [Google Scholar]
- 148.Kudinov Y, Wiseman CL, Kharazi AI. Phorbol myristate acetate and Bryostatin 1 rescue IFN-gamma inducibility of MHC class II molecules in LS1034 colorectal carcinoma cell line. Cancer Cell Int. 2003;3(1):1–16. doi: 10.1186/1475-2867-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Díaz L, Martínez-Bonet M, Sánchez J, Fernández-Pineda A, Jiménez JL, Muñoz E, et al. Bryostatin activates HIV-1 latent expression in human astrocytes through a PKC and NF-ĸB-dependent mechanism. Sci Rep. 2015;5:12442. doi: 10.1038/srep12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, et al. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS ONE. 2010;5(6):e11160. doi: 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tan Z, Turner RC, Leon RL, Li X, Hongpaisan J, Zheng W, et al. Bryostatin improves survival and reduces ischemic brain injury in aged rats after acute ischemic stroke. Stroke. 2013;44(12):3490–3497. doi: 10.1161/STROKEAHA.113.002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hongpaisan J, Sun M-K, Alkon DL. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer's disease transgenic mice. J Neurosci. 2011;31(2):630–643. doi: 10.1523/JNEUROSCI.5209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Farlow MR, Thompson RE, Wei L-J, Tuchman AJ, Grenier E, Crockford D, et al. A randomized, double-blind, placebo-controlled, phase II study assessing safety, tolerability, and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer’s disease. J Alzheimers Dis. 2019;67(2):555–570. doi: 10.3233/JAD-180759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yoo J, Nichols A, Mammen J, Calvo I, Song JC, Worrell RT, et al. Bryostatin-1 enhances barrier function in T84 epithelia through PKC-dependent regulation of tight junction proteins. Am J Physiol Cell Physiol. 2003;285(2):C300–C309. doi: 10.1152/ajpcell.00267.2002. [DOI] [PubMed] [Google Scholar]
- 155.Rodriguez J, Peters BM, Kurz L, Schatzman RC, McCarley D, Lou L, et al. An alkaloid protein kinase C inhibitor, xestocyclamine A, from the marine sponge Xestospongia sp. J Am Chem Soc. 1993;115(22):10436–10437. [Google Scholar]
- 156.Patil AD, Freyer AJ, Killmer L, Hofmann G, Johnson RK. Z-Axinohydantoin and debromo-Z-axinohydantoin from the sponge Stylotella aurantium: Inhibitors of protein kinase C. Nat Prod Lett. 1997;9(3):201–207. [Google Scholar]
- 157.Patil AD, Freyer AJ, Killmer L, Offen P, Carte B, Jurewicz AJ, et al. Frondosins, five new sesquiterpene hydroquinone derivatives with novel skeletons from the sponge Dysidea frondosa: Inhibitors of interleukin-8 receptors. Tetrahedron. 1997;53(14):5047–5060. [Google Scholar]
- 158.Willis R, De Vries D. BRS1, a C30 bis-amino, bis-hydroxy polyunsaturated lipid from an Australian calcareous sponge that inhibits protein kinase C. Toxicon. 1997;35(7):1125–1129. doi: 10.1016/s0041-0101(96)00218-8. [DOI] [PubMed] [Google Scholar]
- 159.Shigemori H, Madono T, Sasaki T, Mikami Y, Kobayashi Ji. Nakijiquinones A and B, new antifungal sesquiterpenoid quinones with an amino acid residue from an Okinawan marine sponge. Tetrahedron. 1994;50(28):8347–54.
- 160.He H, Kulanthaivel P, Baker BJ. New cytotoxic sesterterpenes from the marine sponge Spongia sp. Tetrahedron Lett. 1994;35(39):7189–7192. [Google Scholar]
- 161.Longley RE, Harmody D. A rapid colorimetric microassay to detect agonists/antagonists of protein kinase C based on adherence of EL-4. IL-2 cells. The J Antibio. 1991;44(1):93–102. [DOI] [PubMed]
- 162.Alvi KA, Jaspars M, Crews P, Strulovici B, Oto E. Penazetidine A, an alkaloid inhibitor of protein kinase C. Bioorg Med Chem Lett. 1994;4(20):2447–2450. [Google Scholar]
- 163.Hallock YF, Cardellina JH, Boyd MR. (-)-Frondosins A and D, HIV-Inhibitory Sesquiterpene Hydroquinone Derivatives from Euryspongia sp. Nat Prod Lett. 1998;11(2):153–160. [Google Scholar]
- 164.Takahashi Y, Kubota T, Ito J, Mikami Y, Fromont J, Kobayashi Ji. Nakijiquinones GI, new sesquiterpenoid quinones from marine sponge. Bioorganic & med chem. 2008;16(16):7561–4. [DOI] [PubMed]
- 165.Takahashi I, Kobayashi E, Asano K, Yoshida M, Nakano H. UCN-01, a selective inhibitor of protein kinase C from Streptomyces. J Antibiot. 1987;40(12):1782–1784. doi: 10.7164/antibiotics.40.1782. [DOI] [PubMed] [Google Scholar]
- 166.Marengo B, De Ciucis C, Ricciarelli R, Pronzato MA, Marinari UM, Domenicotti C. Protein kinase C: an attractive target for cancer therapy. Cancers. 2011;3(1):531–567. doi: 10.3390/cancers3010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Propper D, McDonald A, Man A, Thavasu P, Balkwill F, Braybrooke J, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19(5):1485–1492. doi: 10.1200/JCO.2001.19.5.1485. [DOI] [PubMed] [Google Scholar]
- 168.Selzer E, Okamoto I, Lucas T, Kodym R, Pehamberger H, Jansen B. Protein kinase C isoforms in normal and transformed cells of the melanocytic lineage. Melanoma Res. 2002;12(3):201–209. doi: 10.1097/00008390-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 169.Rasko JE, Hughes TP. First approved kinase inhibitor for AML. Cell. 2017;171(5):981. doi: 10.1016/j.cell.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 170.Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A, et al. PKC412-a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des. 2000;15(1):17–28. [PubMed] [Google Scholar]
- 171.Fabbro D, Buchdunger E, Wood J, Mestan J, Hofmann F, Ferrari S, et al. Inhibitors of protein kinases: CGP 41251, a protein kinase inhibitor with potential as an anticancer agent. Pharmacol Ther. 1999;82(2–3):293–301. doi: 10.1016/s0163-7258(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 172.Ge X, Chen J, Li L, Ding P, Wang Q, Zhang W, et al. Midostaurin potentiates rituximab antitumor activity in Burkitt’s lymphoma by inducing apoptosis. Cell Death Dis. 2018;10(1):1–12. doi: 10.1038/s41419-018-1259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tvedt TH, Nepstad I, Bruserud Ø. Antileukemic effects of midostaurin in acute myeloid leukemia–the possible importance of multikinase inhibition in leukemic as well as nonleukemic stromal cells. Expert Opin Investig Drugs. 2017;26(3):343–355. doi: 10.1080/13543784.2017.1275564. [DOI] [PubMed] [Google Scholar]
- 174.Valent P, Akin C, Hartmann K, George TI, Sotlar K, Peter B, et al. Midostaurin: a magic bullet that blocks mast cell expansion and activation. Ann Oncol. 2017;28(10):2367–2376. doi: 10.1093/annonc/mdx290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Osada H, Sonoda T, Tsunoda K, Isono K. A new biological role of sangivamycin; inhibition of protein kinases. J Antibiot. 1989;42(1):102–106. doi: 10.7164/antibiotics.42.102. [DOI] [PubMed] [Google Scholar]
- 176.Loomis C, Bell R. Sangivamycin, a nucleoside analogue, is a potent inhibitor of protein kinase C. J Biol Chem. 1988;263(4):1682–1692. [PubMed] [Google Scholar]
- 177.Lee SA, Jung M. The nucleoside analog sangivamycin induces apoptotic cell death in breast carcinoma MCF7/adriamycin-resistant cells via protein kinase Cδ and JNK activation. J Biol Chem. 2007;282(20):15271–15283. doi: 10.1074/jbc.M701362200. [DOI] [PubMed] [Google Scholar]
- 178.Stockwin LH, Sherry XY, Stotler H, Hollingshead MG, Newton DL. ARC (NSC 188491) has identical activity to Sangivamycin (NSC 65346) including inhibition of both P-TEFb and PKC. BMC Cancer. 2009;9(1):1–13. doi: 10.1186/1471-2407-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]