Abstract
Our wellness relies on continuous interactions between our brain and body: different organs relay their current state to the brain and are regulated, in turn, by descending visceromotor commands from our brain and by actions such as eating, drinking, thermotaxis, and predator escape. Human neuroimaging and theoretical studies suggest a key role for predictive processing in insular cortex in guiding these efforts to maintain bodily homeostasis. Here, we review recent studies recording and manipulating cellular activity in rodent insular cortex at timescales from seconds to hours. We argue that consideration of these findings in the context of predictive processing of future bodily states may reconcile several apparent discrepancies and offer a unifying, heuristic model for guiding future work.
Keywords: Insular cortex, insula, interoception, gustatory, mouse, rat, human, cellular level, circuit level, homeostasis, prediction, predictive coding, need states, hunger, thirst, motivation, salient cues
Livneh and Andermann review recent studies of cellular activity in insular cortex at timescales from seconds to hours. They discuss these findings in the context of predictive processing of future bodily states to provide a model for guiding future work.
Opening remarks
There is something for almost every neuroscientist in the insular cortex. Whether you are fascinated by decision-making, emotions, social interactions, sensory processing, bodily physiology, or have a more translational focus on addiction, chronic pain, anxiety, obesity, eating disorders or autism spectrum disorders, the insular cortex (InsCtx; also called ‘insula’) has probably come up on your radar. InsCtx is likely to play a prominent role in all these processes and many others. But how does one part of the cerebral cortex contribute to so many diverse processes? Does it have one underlying computation and function, or is it merely a hub connecting many distributed functional networks?
In this review, we present our perspective on InsCtx function. This review is not meant to be a comprehensive survey of the state of the InsCtx literature, as several excellent and thorough reviews of InsCtx exist (Craig, 2003a; Gogolla, 2017; Naqvi et al., 2014; Uddin, 2015). Rather, we will present a synthesis of older and newer work that together forms a conceptual working model of InsCtx function, in an attempt to synthesize diverse and seemingly disparate parts of the InsCtx literature, as well as to motivate future experiments and research directions. This working model is based on a combination of recent work from our lab and others using animal models, together with theoretical and cognitive neuroscience studies in humans. We start with an overview of the sensory function of InsCtx, which we view as important for interpretation of its activity in diverse contexts (Section 1). We then describe InsCtx activity on short and longer time-scales, and how it relates to the sensory function of InsCtx (Sections 2–3). Finally, we present a conceptual model linking activity patterns in InsCtx on short and long time-scales (Section 4).
1. InsCtx as a sensory cortex
1.1. Visceral and gustatory
Pioneering experiments stimulating the proximal end of the cut cervical vagus nerve, combined with electrophysiological local field potential recordings, revealed that InsCtx in monkeys, cats, and rats receives vagal afferent input (Bailey and Bremer, 1938; Ogawa et al., 1990; Saper, 2002; Yamamoto et al., 1980). This led to its designation as the vagal receptive cortex, or the primary visceral cortex. The seminal studies by Penfield and colleagues, using electrical stimulation to map the cortex of awake human neurosurgical patients, further confirmed the importance of InsCtx for conscious visceral sensations (Penfield and Faulk, 1955). These experiments revealed that electrical stimulation of different parts of InsCtx produces different sensations and autonomic changes (Fig. 1A). These include taste, oropharyngeal, esophageal, and gastrointestinal sensations, as well as changes in blood pressure, heart rate, and respiration. Later studies extensively replicated these results (Mazzola et al., 2017a; Mazzola et al., 2017b; Oppenheimer et al., 1992; Ostrowsky et al., 2002; Pugnaghi et al., 2011).
Figure 1: InsCtx as a Sensory Cortex – Function and Connectivity.
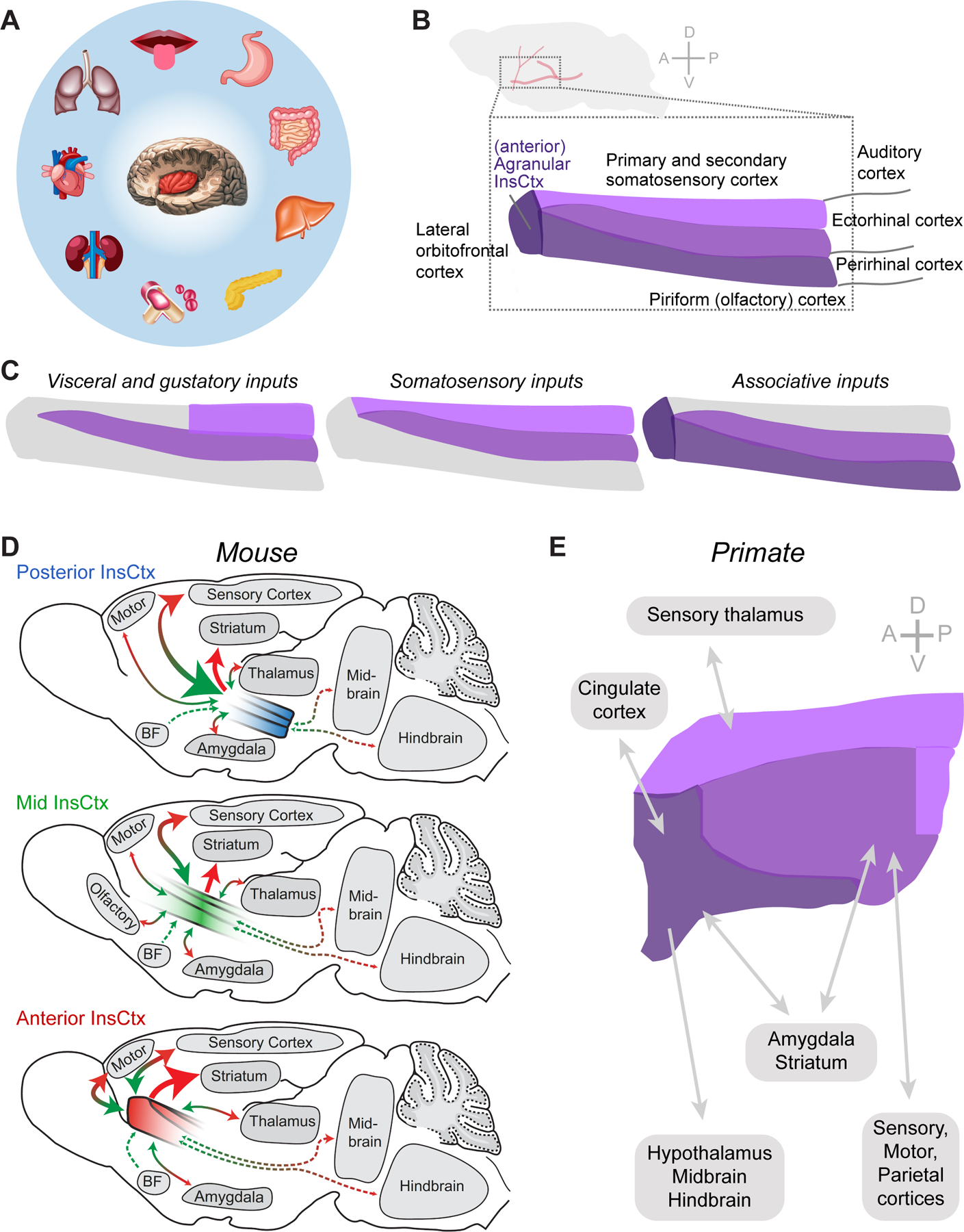
A. Illustration of some of the different organs and systems that are thought to provide direct or indirect sensory information to InsCtx (highlighted in red): mouth and tongue, stomach, intestines, liver, pancreas, blood, kidneys, heart, and lungs. Note that several additional relevant organs, such as the urinary bladder, esophagus, and genitals, are not shown.
B. Schematic side-view of the rodent brain, highlighting the different cytoarchitectural subdivisions of InsCtx (purple), and their neighboring cortical regions. A: anterior, P: posterior, D: dorsal, V: ventral.
C. Spatial organization of some of the prominent inputs to InsCtx. Purple and grey regions receive or lack these inputs, respectively.
D. Summary of the brain-wide connectivity of posterior, mid, and anterior InsCtx. Note increasing sensory cortical input and decreasing motor output from anterior to posterior regions. Modified with permission from Gehrlach et al., 2020.
E. Schematic view of the primate InsCtx and the general organization of its inputs. Based on Evrard, 2019. Connectivity in human InsCtx is considered to be very similar.
Subsequent anatomical work in animal models provided further support for InsCtx’s role as the primary gustatory and visceral sensory cortex. InsCtx encompasses three cytoarchitectonic subdivisions, distinguished by the presence, absence, or diffuse nature of cortical layer 4 (granular, agranular, or dysgranular cortex, respectively; Fig. 1B). The mid-posterior portions of granular and dysgranular InsCtx were found to be the main cortical sites that receive direct sensory input from the gustatory and visceral thalamic sensory nuclei – ventroposterior medial parvicellular nucleus (VPMpc) and ventroposterior lateral parvicellular nucleus (VPLpc; (Allen et al., 1991; Cechetto and Saper, 1987; Norgren and Wolf, 1975; Pritchard et al., 1986)). The more anterior parts of InsCtx have been suggested to function as a higher-order association sensory cortex, based on brain-wide and intra-InsCtx connectivity patterns ((Barrett and Simmons, 2015; Evrard, 2019; Mufson and Mesulam, 1982; Saper, 1982); Fig. 1C–D). Notably, the most anterior part of InsCtx transitions into the orbitofrontal cortex, and the distinction between these two regions remains somewhat ill-defined. Based on newer human functional imaging results, the view of anterior InsCtx as an association cortex has been recently refined to posit that it computes sensory predictions, while mid-posterior InsCtx integrates these predictions with incoming visceral and gustatory inputs to compute prediction errors ((Barrett and Simmons, 2015); see below).
The functional topography of insular cortex remains poorly understood. However, similar to other primary sensory cortical areas that demonstrate topographic organization of representations of the sensory epithelium (e.g., somatotopy, retinotopy, tonotopy), InsCtx is thought to be organized topographically. Specifically, sensory inputs from the tongue (tactile and chemosensory), esophagus, gastrointestinal tract, lungs, and cardiovascular system appear to be coarsely organized from anterior to posterior in InsCtx, albeit with substantial overlap between these representations in rodents (Aleksandrov et al., 1996; Bagaev and Aleksandrov, 2006; Cechetto and Saper, 1987; Hanamori et al., 1998), and potentially in humans (Avery et al., 2017). The observed overlap between visceral and gustatory representations in InsCtx suggests that there is no clear anatomical separation between the primary visceral and gustatory cortical regions in InsCtx.
Similar anterior-posterior organization has been suggested for representations of distinct gustatory features in InsCtx (Accolla et al., 2007; Chen et al., 2011), but this is still a matter of ongoing debate (Chen et al., 2021; Fletcher et al., 2017; Lavi et al., 2018; Levitan et al., 2019). High-resolution human neuroimaging studies have failed to find such topographic organization (Avery et al., 2020; Canna et al., 2019; Chikazoe et al., 2019). Moreover, results from InsCtx manipulation experiments in rodents across the anterior/posterior axis have suggested that this topographical organization should not be described in gustatory terms, but rather in terms of positive/negative valence or appetitive/aversive sensations (Gehrlach et al., 2019; Peng et al., 2015; Wang et al., 2018). These results are consistent with the view that InsCtx may be part of a larger brain system for determining valence (Tye, 2018).
We propose that at least some of this observed gustatory and valence topography may be explained by viscerosensory and visceromotor topography. More specifically, by the distinct autonomic and visceral changes anticipated from, or induced by, sensation of specific tastants. For example, the oral and post-oral effects of sweet vs. bitter substances may have distinct effects on anticipatory visceromotor functions such as gastric motility (bitter foods may be rejected after initial tasting, thereby precluding ‘cephalic phase’ gastrointestinal actions in preparation for digestion; e.g. (Avau et al., 2015; Eguchi et al., 2016; Harada et al., 2019; Waluga et al., 2018; Wicks et al., 2005); see Fig. 2E and related discussion below). Appetitive vs. aversive tastants also differentially modulate many other bodily processes including esophageal muscle contraction (Leow et al., 2007), heart rate and heart rate variability (Muroni et al., 2011), each of which can, in turn, influence distinct sets of InsCtx neurons. Furthermore, the aforementioned positive/negative valence effects observed upon manipulations of anterior/posterior InsCtx (Gehrlach et al., 2019; Peng et al., 2015; Wang et al., 2018), respectively, may result from the different visceral sensations that these InsCtx stimulations mimicked, and/or the visceral changes they caused. For example, electrical stimulation of InsCtx in animal models and humans can cause either a decrease or an increase in blood pressure and heart rate, depending on whether the stimulation is targeted to mid-anterior or posterior InsCtx, respectively (Chouchou et al., 2019; Yasui et al., 1991). The well-established activation of posterior InsCtx in pain perception (Craig, 2003c) could be partially explained by the associated autonomic and visceral changes induced by painful stimuli (Sharvit et al., 2019).
Figure 2: InsCtx Activity on Short and Long Time-Scales.
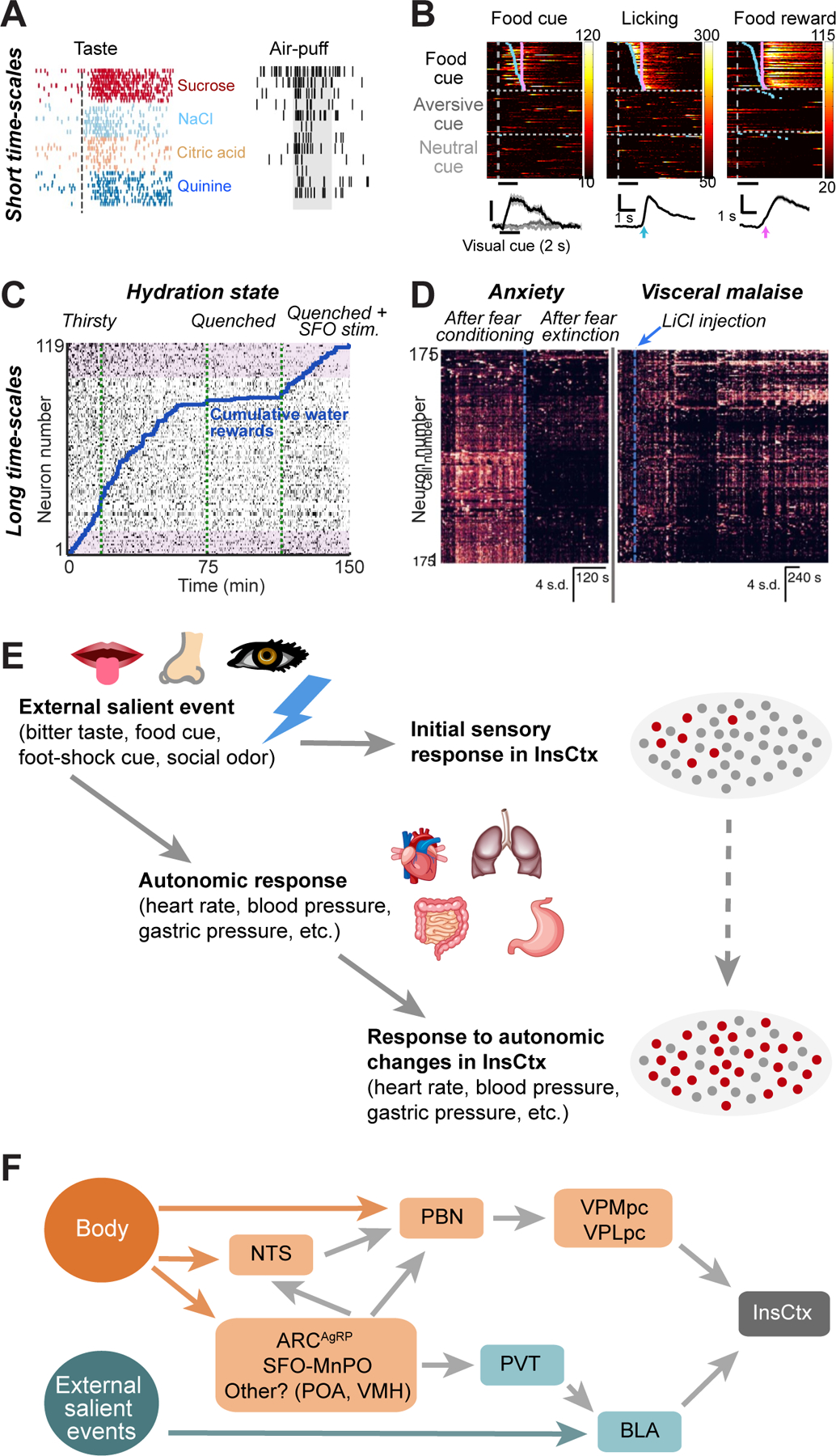
A-B. Examples of InsCtx activity on short time-scales.
A. Single-trial responses of a single InsCtx neuron (spike rasters) to intra-oral infusion of different tastants, and to an air-puff to the whisker region. Modified with permission from Vincis et al., 2016.
B. Top: Single-trial responses of 3 different InsCtx neurons (Ca2+ imaging of fluorescence changes) to a visual food cue (gray bar at bottom), lick bout onsets (white ticks) and liquid food reward (Ensure; pink ticks). Heatmaps sorted by lick bout onset. Bottom: average response. Modified with permission from Livneh et al., 2017.
C-D. Examples of InsCtx activity on long time-scales.
C. Ongoing activity of a population of simultaneously imaged InsCtx neurons (calcium imaging of fluorescence changes in a subset of inter-trial intervals, see Livneh et al., 2020 for details) during gradual quenching of thirst. After a quenched state was reached, the hypothalamic thirst system was stimulated to induce an artificial motivational drive. Note that despite restoration of behavior (e.g., resumed water consumption), InsCtx ongoing activity maintains a faithful representation of the true (isosmotic, euvolemic) hydration state. Modified with permission from Livneh et al., 2020.
D. Calcium activity of a population of simultaneously imaged InsCtx neurons. Left: activity after initial fear conditioning and then following extinction, potentially reflecting anxiety levels. Right: activity before and after induction of visceral malaise by systemic injection of LiCl. Modified with permission from Gehrlach et al., 2019.
E. Schematic model suggesting that the activity of InsCtx in different behavioral contexts could be explained by its interoceptive sensory function. A salient stimulus (bitter taste, appetitive/aversive predictive cue) first activates a small subset of InsCtx neurons. The salient stimulus then induces autonomic changes (heart rate, anticipatory gastric motility, etc.), which in turn activate a larger population of InsCtx neurons. From the experimenter’s perspective, interpretation of InsCtx activity is challenging in the absence of concurrent recording of other bodily signals.
F. Schematic model summarizing the pathways by which information from the body, as well as salient information from the outside world, affect InsCtx activity. MnPO: median preoptic area, POA: preoptic area, VMH: ventromedial hypothalamus.
Consistent with a role in representing visceral sensory stimuli, InsCtx activity on longer time-scales in humans and animal models has been shown to reflect bodily states such as hunger, thirst, and satiety (de Araujo et al., 2006; Egan et al., 2003; Gehrlach et al., 2019; Livneh et al., 2020; Meier et al., 2018; Tataranni et al., 1999). Gradual rehydration has been used in animal models and in humans to dissociate the physiological aspects of thirst from the motivational or subjective experience of thirst (Livneh et al., 2020; Meier et al., 2018). The dissociation between objective dehydration and the subjective sensation of thirst is possibly due to the fact that anticipatory signals of quenching (e.g., the presence of water in the mouth) reduce sensations of thirst long before the physiological state is restored by fluid absorption (Stricker and Hoffmann, 2007). We and others have found that mid-posterior InsCtx is a rare example of a cortical site whose activity tracks bodily physiology (in this case, degree of hydration), and not subjective sensations or motivation (i.e., “subjective water satiety”; (Livneh et al., 2020; Meier et al., 2018)). These representations of physiological states in InsCtx likely reflect multiple visceral, autonomic, as well as hormonal inputs that are differentially active across hunger, thirst, and satiety states (e.g., stomach stretch, blood pressure, and hormones such as cholecystokinin and angiotensin II). Nevertheless, it remains unclear how these patterns of cortical activity relate to peripheral sensory inputs – do they reflect integrated, highly processed sensory signals (integrated upstream of InsCtx), or a mixture of many ‘labeled lines’ of specific visceral sensory signals? Future studies relating InsCtx cellular activity to specific visceral changes could answer these questions.
In addition to the function of InsCtx as the primary gustatory and visceral sensory cortex, it also receives input from other sensory cortical areas, including the auditory, somatosensory, and olfactory cortices (Fig. 1C–E; (Augustine, 1996; Gehrlach et al., 2020)). Accordingly, many studies in humans and animal models have found responses to multi-modal sensory stimuli in InsCtx (de Araujo and Simon, 2009; Gogolla et al., 2014; Rodgers et al., 2008; Rolls, 2016; Small, 2012; Vincis and Fontanini, 2016). Based on these and many other observations, InsCtx has been suggested to be a central node in the “salience network”, identifying the most homeostatically relevant among multiple competing internal and external stimuli ((Uddin, 2015), see below). Such multi-sensory integration in InsCtx is also thought to play a central role in social behaviors (Rogers-Carter and Christianson, 2019), consistent with a role for InsCtx in representing bodily and visceral changes associated with territoriality, aggression, courtship, food-sharing, and other social behaviors.
1.2. Comparison with other sensory cortices
InsCtx microcircuit cortical connectivity (i.e., between layers and different neuronal subtypes) has not been studied as extensively as in other sensory cortices (Harris and Shepherd, 2015; Maffei et al., 2012). Furthermore, InsCtx local microcircuit connectivity is likely to vary across InsCtx cytoarchitectonic subdivisions (granular, agranular, and dysgranular), which also differ in their brain-wide connectivity (Evrard, 2019). Future work in genetically accessible animal models will enable the use of more precise molecular tools to target specific cell-types in each subdivision, thereby revealing how different patterns of connectivity give rise to distinct functions.
While InsCtx adheres to some of the hallmarks of sensory cortex connectivity, such as reciprocal connectivity with sensory thalamic nuclei (Allen et al., 1991; Holtz et al., 2015), its connectivity patterns also differ in interesting ways (Fig. 1D–E). For example, the cortico-striatal projections of InsCtx are mainly to the central amygdala (CeA, a striatal-like structure) and nucleus accumbens (NAc, in the ventral striatum) (Gehrlach et al., 2020; Schiff et al., 2018; Stern et al., 2021). Because CeA and NAc are key to consummatory and other motivated behaviors (reviewed, e.g., in (Fadok et al., 2018; Floresco, 2015)), this further emphasizes InsCtx’s role in directly regulating such behaviors. Additionally, InsCtx has strong reciprocal connectivity with the basolateral amygdala (BLA, a non-laminated cortical-like structure), a connection which we and others have shown to be important for regulating consummatory and other motivated behaviors (Kayyal et al., 2019; Lavi et al., 2018; Livneh et al., 2017; Livneh et al., 2020; Piette et al., 2012; Samuelsen et al., 2012; Schiff et al., 2018; Wang et al., 2018).
In summary, despite some unique properties of InsCtx, we suggest that considering it as a classical cortical module processing specific sensory information (i.e., gustatory and visceral) will be critical for understanding its function, and how local computations within InsCtx contribute to this function. Analyses of interoceptive processing in InsCtx should build on our current knowledge of feedforward and feedback processing in other better-understood exteroceptive sensory cortices, in combination with the currently more advanced understanding of gustatory (as opposed to visceral) sensory processing. Accordingly, we suggest that answering the following questions will be important for bettering our understanding of InsCtx function: Which interoceptive sensory inputs reach InsCtx, and into which layers? How is sensory information processed in the local cortical microcircuit (e.g., by distinct populations of interneurons in different layers)? How do long-range inputs into InsCtx interact with sensory processing and in which layers? How do different cortico-cortical, cortico-thalamic, cortico-striatal, and cortico-brainstem outputs of InsCtx mediate its function?
2. InsCtx activity on short and long time-scales
InsCtx neuronal activity has been shown to change in meaningful ways that reflect both salient changes in the external environment, as well as changes in the internal environment of the body. These changes occur on various time-scales, ranging from milliseconds to minutes and even hours. Below, we consider two broad categories that span this wide range of time-scales – short time-scales ranging from milliseconds to seconds, and long time-scales ranging from minutes to hours. The rationale for these two broad categories is that the short time-scale category encompasses fast changes in the external and internal environments (potentially reflecting predictions of bodily changes across longer time-scales, see below), while the longer time-scale category captures distinct internal processes such as changes in physiological bodily state (e.g., satiation), changes in motivation and arousal, and changes related to circadian rhythmicity.
2.1. Short time-scales
Consistent with a role in sensing of bodily changes, InsCtx neurons in animal models display short time-scale responses (~seconds; Fig. 2A–B). These include responses to gustatory and interoceptive sensory stimuli such as intra-oral tastants and intra-gastric pressure (Cechetto and Saper, 1987; Katz et al., 2001), as well as to learned cues that predict these stimuli (Gardner and Fontanini, 2014; Livneh et al., 2017; Samuelsen et al., 2012). Responses to tastants convey multiple types of information including tactile and chemosensory signals, as well as temperature and palatability (de Araujo and Simon, 2009; Jones et al., 2006; Kadohisa et al., 2005), which together comprise the multi-modal experience of flavor (Small, 2012). Additionally, consistent with its multi-modal saliency-related function, InsCtx neurons display short time-scale responses to other external sensory modalities including visual, auditory, and somatosensory, as well as licking-related and orofacial movement-related activity (de Araujo et al., 2006; Kadohisa et al., 2005; Livneh et al., 2017; Rodgers et al., 2008; Stapleton et al., 2006; Vincis and Fontanini, 2016; Yamamoto et al., 1988). Importantly, similar results have been observed in humans (reviewed, for example, in (de Araujo and Simon, 2009).
At the population level, InsCtx activity on short time-scales displays transitions in population activity patterns on the order of hundreds of milliseconds (Jones et al., 2007). These transitions likely result from recurrent local InsCtx connectivity (Mazzucato et al., 2019). Interestingly, tastants and learned distal sensory cues drive state transitions in population activity between different attractor states (Jones et al., 2007; Mazzucato et al., 2019; Sadacca et al., 2016). Such state transitions are necessary for behaviors such as gaping in response to an aversive tastant (Mukherjee et al., 2019).
2.2. Long time-scales
Longer times-scale changes in InsCtx activity that are not directly linked to immediate motor actions (herein termed ‘ongoing activity’) have been shown in our studies and others to represent slow changes in the physiological state of the body (Fig. 2C–D; (de Araujo et al., 2006; Egan et al., 2003; Gehrlach et al., 2019; Livneh et al., 2020; Meier et al., 2018; Tataranni et al., 1999)). Additionally, longer time-scale changes in ongoing InsCtx activity have been associated with specific positive and negative emotional states (Fig. 2D; (Dolensek et al., 2020; Gehrlach et al., 2019)), though it remains unclear whether the InsCtx activity patterns explicitly reflect valence or whether they reflect sensory representations that then acquire valence via recruitment of downstream amygdalar and striatal circuits (Klawonn et al., 2018; Wang et al., 2018). Accordingly, InsCtx inhibition experiments have also pointed to an important role for InsCtx ongoing activity on longer time-scales in feeding, anxiety, and drug addiction (Contreras et al., 2007; Gehrlach et al., 2019; Stern et al., 2020; Wu et al., 2020).
Behavioral effects of InsCtx inhibition have often been interpreted in our studies and others as a consequence of disruption of short time-scale responses in InsCtx (e.g., (Livneh et al., 2017; Peng et al., 2015; Schiff et al., 2018)). Importantly, however, the methods we and others employed in these studies lack the temporal precision to determine whether the observed behavioral effects were due to effects on short and/or long time-scale activity. In contrast, other studies have elegantly demonstrated that temporally precise inhibition of InCtx can yield different results when applied during different behavioral epochs (Kusumoto-Yoshida et al., 2015; Mukherjee et al., 2019; Vincis et al., 2020). Thus, in light of recent work demonstrating robust long time-scale activity (Gehrlach et al., 2019; Livneh et al., 2020), we suggest that inferences on behavioral roles of InsCtx will greatly benefit from experiments involving precisely timed silencing. More specifically, transient optogenetic perturbations should reveal whether the aforementioned behavioral effects of sustained disruption of InsCtx are due to perturbations of short and/or long time-scale activity. However, even transient inhibition of InsCtx activity could alter ongoing activity patterns after inhibition has ended. Thus, combined optogenetic perturbations and cellular-level recordings of neural activity in vivo will be crucial for proper interpretation.
2.3. Relating InsCtx activity across short and long time-scales
One of the most consistent findings regarding InsCtx is its importance for learning and remembering to avoid a novel taste associated with visceral malaise. This is modeled behaviorally using ‘conditioned taste aversion’ (CTA; reviewed in (Yiannakas and Rosenblum, 2017), see also (Schier et al., 2014)). CTA has provided a useful model system for investigating the molecular mechanisms of associative learning and memory (Bermudez-Rattoni, 2004). From the perspective of short and long time-scales of InsCtx activity, CTA presents an interesting challenge. Short time-scale InsCtx activity patterns in response to the novel taste become associated with a delayed and prolonged change in InsCtx activity, driven by subsequent induction of visceral malaise (Fig. 2D; (Aguilar-Rivera et al., 2020; Gehrlach et al., 2019)), beginning as long as 30 minutes after the taste experience. This taste-malaise association is then remembered for many weeks. This learning process modifies the InsCtx response to the malaise-associated tastant such that it becomes more similar to innately aversive tastants (Accolla and Carleton, 2008; Grossman et al., 2008; Lavi et al., 2018). It remains unclear whether a pattern of persistent activity exists in InsCtx that bridges the extremely long gap in time between the taste and the subsequent malaise. However, several neuromodulatory systems potentially affect InsCtx (e.g., (Ju et al., 2020; Rogers-Carter et al., 2018)), and some have been directly implicated in CTA learning (e.g., acetylcholine, norepinephrine, and dopamine, (Berman and Dudai, 2001); reviewed in (Yiannakas and Rosenblum, 2017)). Neuromodulatory systems may bridge this long gap in time, as recently shown for delayed tone-shock conditioning (Guo et al., 2019). Alternatively or in concert with neuromodulatory actions, offline reactivations of cortical activity patterns associated with recent novel sensory experiences (e.g. (Sugden et al., 2020)) may overlap with the activity patterns driven by subsequent induction of malaise, thereby providing a substrate for plasticity between temporally disparate events.
Associating short time-scale sensory stimuli with longer time-scale post-ingestive events guides our everyday decisions regarding food consumption. CTA is but one extreme example of this phenomenon. Specifically, our food choices are governed not only by conscious perception of flavor, but also by unconscious associations between a flavor and the post-ingestive consequences of consumption of associated nutrients (reviewed in (de Araujo et al., 2020)). These consequences include gut-driven striatal dopamine release, which reinforces future consumption of a certain flavor if it is associated with post-ingestive absorption of calories in animal models and in humans (de Araujo et al., 2008; Thanarajah et al., 2019). Furthermore, such associations are important for maintaining normal peripheral metabolism (Dalenberg et al., 2020). The visceral neural underpinnings of post-ingestive reward are beginning to be revealed (Han et al., 2016; Han et al., 2018; Kaelberer et al., 2018; Tan et al., 2020). Importantly, InsCtx is necessary for using these visceral signals for behavioral conditioning (Oliveira-Maia et al., 2012). As such, InsCtx is likely a central site in which conscious flavor perception is associated with unconscious post-ingestive events, whether good (e.g. calories) or bad (e.g. malaise), to guide future behavior ((Dalenberg et al., 2020; de Araujo et al., 2020); see below). The precise neural mechanisms by which these associations occur remain to be revealed.
2.4. Relating ongoing activity to ongoing behavior in InsCtx and throughout the brain
While Section 1 described the specific visceral and limbic inputs to InsCtx, this region also receives dense connectivity from other cortical, neuromodulatory and subcortical inputs (Fig. 1D). To investigate potential representations of ongoing physiological states in InsCtx of behaving animals, it is important to first consider more global contributions to ongoing cortical activity. Recent advances in large-scale, cellular-resolution recording technologies (both imaging and electrophysiology) have led to the observation that coordinated changes in ongoing neural activity are ubiquitous throughout the mammalian brain, including InsCtx. These observations are in line with many previous investigations of “resting state” activity using human neuroimaging (Northoff et al., 2010). However, these advances also highlight long-standing challenges in the interpretation of neural activity in behaving animals. For example, do changes in ongoing activity throughout the brain reflect physiological states, emotional states, arousal, unobserved motor actions, and/or other unobserved ill-defined ‘internal states’? Recent studies have started to shed light on this issue by combining large-scale extracellular electrophysiology recordings with concurrent measurements of other behavioral and physiological parameters, such as orofacial movements, pupil diameter (as a proxy for arousal), whisking, and locomotion. Ongoing activity throughout the brain was found to be largely associated with spontaneous and goal-directed motor actions, which may collectively reflect so-called “internal states” or “behavioral states” (Allen et al., 2019; Grundemann et al., 2019; Musall et al., 2019; Salkoff et al., 2019; Stringer et al., 2019). Importantly, cortical action-related activity throughout sensory cortex may not be necessary for executing concurrent behaviors (Zatka-Haas et al., 2020), particularly when the behaviors and associated contexts have become routine. This suggests that the function of motor-related activity across the cortex and associated brain regions might be to create sensory-motor associations, thereby contextualizing sensory information. These can then guide future behavior based on generation of more accurate sensory-motor predictions and prediction error signals (Keller and Mrsic-Flogel, 2018).
Recently, we were able to dissociate InsCtx representations of slow changes in bodily physiology from the aforementioned activity patterns related to motor actions, motivation, and arousal. To achieve this goal, we combined neural and behavioral measurements with manipulations of bodily physiology, as well as bidirectional manipulations of distinct hypothalamic neurons that drive hunger or thirst. We found that InsCtx ongoing activity contains faithful and specific representations of distinct bodily states associated with caloric deficiency (hunger) or fluid deficiency (thirst). These representations were independent of concurrent behavior or arousal levels (Livneh et al., 2020). We speculate that the function of these representations may be to provide a specific interoceptive context in which a cue-outcome association is learned (Euston et al., 2012). They may also be used for regulation of bodily functions via descending projections to vagal motor neurons and other brainstem targets (Shipley, 1982).
This discussion touches upon an important open issue regarding the neurobiological investigation of interoception and emotions in animal models: how do we distinguish between the neural underpinnings of such internal states and those of their autonomic and motor correlates (see e.g., (Barrett et al., 2007; Salzman and Fusi, 2010))? As mentioned above, InsCtx ongoing activity has been associated with anxiety and other emotion states (see also recent related work on the BLA; (Grundemann et al., 2019; Lee et al., 2017)). The visceral and multi-modal sensory nature of InsCtx, together with the aforementioned motor-related brain-wide activity, raises an important issue regarding interpretations of InsCtx activity patterns correlated with anxiety-like behaviors. Is it possible that such InsCtx activity actually reflects concurrent changes in co-varying physiological parameters such as heart rate, blood pressure, and motor actions such as orofacial changes and locomotion (Fig. 2E)? For example, heart rate varies with anxiety-like behavior in the elevated plus maze, a well-established assay for anxiety in rodents (and potentially in humans; (Biedermann et al., 2017; Okonogi et al., 2018). As such, does related activity in InsCtx represent an emotion state, a sensory readout of changes in heart rate and other autonomic changes, or descending regulation of autonomic tone? Can these two alternatives even be dissociated (Cannon, 1987)? Furthermore, autonomic sensory and descending motor information, conveyed via the vagus nerve, may be necessary for expression of anxiety-like behaviors in rodents (Klarer et al., 2014). The crux of the matter is that the nested set of brain-body sensorimotor loops is not easily untangled. While these questions have yet to be answered, recent work, based on a cross-species, neurobiologically-motivated conceptual framework (Anderson and Adolphs, 2014) has made important progress in this direction (Dolensek et al., 2020). The tools now exist to make substantial advances in overcoming this challenge and addressing these questions by using large-scale neural recordings and manipulations in combination with tracking and modeling of a much larger number of physiological and behavioral parameters.
3. Circuit mechanisms for short and long time-scale activity in InsCtx
3.1. Mechanisms of short time-scale changes
Our understanding of the circuit mechanisms of InsCtx activity has significantly advanced in recent years, especially that of short time-scale activity. As mentioned above, gustatory and visceral sensory responses arise from dedicated thalamic inputs (Saper, 2002). Recent work has also revealed circuit mechanisms of InsCtx responses to learned salient external cues (e.g., auditory or visual cues; Fig. 2F). Specifically, we and others used pharmacology and pathway-specific chemogenetics, combined with electrophysiology and somatic and axonal calcium imaging, to show that BLA is a central source of salient learned cue responses for InsCtx (Livneh et al., 2017; Livneh et al., 2020; Samuelsen et al., 2012). Further work demonstrated the dissociation between the sources driving InsCtx responses to tastants vs. responses to learned taste-predicting cues. Specifically, InsCtx learned cue responses arise from BLA inputs and are independent of gustatory thalamic inputs. In contrast, InsCtx chemosensory taste responses are largely independent of BLA and arise from gustatory thalamic inputs (Samuelsen et al., 2012, 2013). Notably, InsCtx palatability encoding (occurring ~1 sec after chemosensory coding; (Katz et al., 2001)) is dependent on BLA inputs (Piette et al., 2012). At the synaptic level, a subset of InsCtx neurons receive direct/indirect input from both BLA and from gustatory thalamus (Stone et al., 2020). Intriguingly, for the subset of InsCtx neurons that are responsive to both learned predictive cues and their predicted tastants, both types of responses depend on intact BLA inputs (Samuelsen et al., 2013). In these experiments, liquid tastants were voluntarily consumed by thirsty animals. This suggests that the subset of BLA-dependent taste responses may actually reflect reward encoding, rather than taste per se. This further supports the idea that multiple types of information are represented by InsCtx neurons, including sensory cues and more general information regarding saliency and valence.
The saliency of learned cues depends on the animal’s need state. For example, a food-predicting cue is more salient during hunger, while a water-predicting cue is more salient during thirst. We have recently used a combined imaging and circuit-mapping approach to investigate the neural circuits that gate biased responses to salient cues in InsCtx (Livneh et al., 2017; Livneh et al., 2020). We leveraged the recent discoveries of molecularly distinct hypothalamic neurons that drive either hunger or thirst (reviewed in (Andermann and Lowell, 2017; Augustine et al., 2020; Sternson and Eiselt, 2017)), and used them as specific entry points for the investigation of the underlying neural circuits. We uncovered a functionally relevant neural pathway from hypothalamic hunger and thirst neurons to InsCtx via paraventricular thalamus (PVT) and BLA (Fig. 2F; (Livneh et al., 2017)). Interestingly, individual PVT→BLA neurons in this pathway received convergent inputs from both hunger neurons and thirst neurons (Livneh et al., 2020). PVT is also a site of convergence of other hypothalamic populations that promote specific behaviors during distinct motivational drives, such as temperature regulation, aggression and parental behavior (Hashikawa et al., 2017; Kohl et al., 2018; Tan et al., 2016). Thus, the PVT may be a major hub that routes and/or integrates information from multiple hypothalamic populations to modulate processing of need-relevant predictive cues in InsCtx and other cortical areas (Fig. 2F). This modulation may scale with the relative urgency of various need states (Sutton and Krashes, 2020).
3.2. Mechanisms of long time-scale changes: visceral-sensory
Much less is known regarding the circuit mechanisms underlying long time-scale activity in InsCtx. Ongoing visceral sensory inputs from the thalamic sensory nuclei VPMpc and VPLpc may relay real-time changes in physiological parameters such as heart rate, blood pressure, gastric nutrients, gastric stretch, and temperature (Fig. 2F; (Saper, 2002)). However, the nature of the central sensory representation of these stimuli is currently largely unknown. For example, do sensory responses adapt quickly and mostly report changes? If so, what is the threshold for detecting such changes and does it vary, for example, based on the current state of caloric and fluid balance? Furthermore, intrinsic local InsCtx circuitry likely underlies some level of ongoing activity, and long-range inputs may thus shift the activity between different attractor states (Mazzucato et al., 2019). The understanding of the gustatory system, from the sensory periphery to InsCtx and beyond, has greatly advanced in the last two decades (Jones et al., 2006; Simon et al., 2006; Vincis and Fontanini, 2019; Yarmolinsky et al., 2009). A similar investigation of peripheral, brainstem, thalamic and InsCtx visceral sensory properties would shed new light on the role of ongoing visceral sensory inputs in driving ongoing activity in InsCtx, and would thus help answer the above questions. Recent work has begun to make important progress in this direction (Bai et al., 2019; Campos et al., 2016; Han et al., 2018; Kim et al., 2020; Tan et al., 2020; Williams et al., 2016). It will be important to overcome two major challenges to make rapid mechanistic progress. First, the study of slow time-scale activity in InsCtx will benefit from methods that can track activity in many neurons across hours and days with greater precision, such as long-term electrophysiological recordings from the same population of neurons (Okun et al., 2016; Steinmetz et al., 2021). Second, recording of InsCtx activity concurrent with precise tracking and manipulation of (i) activity in specific viscerosensory vagal and spinal afferent neurons, and (ii) hormonal signals, will be important for linking slow-timescale changes in the cortex and periphery.
Notably, additional mechanisms also likely regulate activity at slow timescales. For example, as discussed above, arousal and motor actions affect neural activity throughout the brain, including in InsCtx (Allen et al., 2019; Livneh et al., 2020; Musall et al., 2019; Salkoff et al., 2019; Stringer et al., 2019). Brain-wide neuromodulatory systems, such as acetylcholine and norepinephrine, underlie some of these affects in other sensory cortices (McCormick et al., 2020). Similar mechanisms may act in InCtx to shape long time-scale activity.
3.3. Mechanisms of long time-scale changes: hypothalamus to cortex
Several genetically distinct populations of hypothalamic neurons that sense and drive distinct need states have been identified. Two prominent examples are hypothalamic hunger-promoting neurons expressing agouti-related peptide (AgRP neurons), and thirst-promoting glutamatergic subfornical organ (SFO) neurons. These neurons are commonly referred to as “hunger neurons” or “thirst neurons”, as they are thought to act both as sensors of physiological imbalances and as actuators of relevant behavioral and physiological counter-regulatory responses (Andermann and Lowell, 2017; Augustine et al., 2018b; Sternson and Eiselt, 2017; Zimmerman and Knight, 2020). We and others have recently shown that artificial activation of these neurons mimics the natural deficiency state not only behaviorally, but also in the pattern of neural responses to need-relevant cues throughout the brain, including in InsCtx (Allen et al., 2019; Livneh et al., 2017; Livneh et al., 2020). Notably, hypothalamic neurons could potentially also modulate the feed-forward flow of gustatory and visceral sensory information to InsCtx via direct projections to the paraventricular thalamus (Livneh et al., 2017; Livneh et al., 2020), to the hindbrain (e.g., nucleus of the solitary tract and parabrachial nucleus), and to other hypothalamic populations that modulate hindbrain activity (Fig. 2F; (Saper and Lowell, 2014)). Indeed, recent work has shown that direct and indirect hypothalamic projections to the hindbrain can regulate appetite, taste, visceral malaise, peripheral glucose homeostasis, and pain (e.g., (Alhadeff et al., 2018; Brandt et al., 2018; Campos et al., 2016; Cheng et al., 2020; Essner et al., 2017; Fu et al., 2019; Garfield et al., 2015; Kim et al., 2020; Steculorum et al., 2016; Wu et al., 2012)). As such, hypothalamic modulation of visceral sensory inputs to InsCtx could potentially occur for both short and long time-scales of activity in InsCtx.
Despite the above examples of hypothalamic modulation of visceral processing in the hindbrain, it appears that at least some aspects of the visceral sensory inputs to InsCtx are not regulated by the hypothalamus. Specifically, we have recently discovered that hypothalamic neurons that drive hunger or thirst bidirectionally control learned cue responses, but not the ongoing activity representation of physiological need states (i.e., caloric or fluid deficiency), in InsCtx (Fig. 2C; (Livneh et al., 2020)). In contrast, rehydration by peripheral injection of isotonic saline did modify ongoing activity in thirsty mice (Livneh et al., 2020). Thus, hypothalamus may control the sensitivity of InsCtx neurons to motivational signals but not to visceral signals of fluid and energy balance. Future experiments could address these questions directly by combining InsCtx neural recordings with carefully controlled visceral stimulation and manipulations of relevant brainstem and hypothalamic populations. This would allow dissection of the different routes for context-dependent flow of visceral sensory information to InsCtx.
3.4. Mechanisms of short time-scale changes: cortex to hypothalamus
One of the recent developments in hypothalamic and interoception research is the discovery that hypothalamic neurons controlling various aspects of bodily physiology are not only sensitive to physiological changes but also sensitive to feedforward anticipatory signals such as cues predicting food or water availability (Allen et al., 2017; Augustine et al., 2018a; Betley et al., 2015; Chen et al., 2015; Gizowski et al., 2016; Mandelblat-Cerf et al., 2017; Mandelblat-Cerf et al., 2015; Zimmerman et al., 2016). This confirms earlier ideas inspired by the theory of adaptive control systems (Carpenter, 2004; Somjen, 1992; Woods and Ramsay, 2007), and suggests that forebrain control of motivated behaviors acts not only in parallel with but also through the hypothalamus. Subsequent work has made progress in revealing the different mechanisms of feed-forward regulation (Augustine et al., 2019; Beutler et al., 2017; Garfield et al., 2016; Su et al., 2017). Nevertheless, the “top-down” sources of these cognitively processed, learned responses have yet to be discovered. We recently investigated the pattern of InsCtx population activity in response to feed forward signals of food or water availability (i.e., food/water cues, and their immediate ingestion). Intriguingly, we found that these patterns of activity (but not average levels of activity) share some prominent features with the feedforward learned cue responses of hypothalamic neurons, including their timing and sensitivity to current physiological state (Chen et al., 2015; Livneh et al., 2017; Livneh et al., 2020). Therefore, we speculate that hypothalamic feed-forward signals might originate, in part, from InsCtx and/or from the BLA, which is also a key source of predictive cue inputs to InsCtx (Livneh et al., 2017; Livneh et al., 2020; Samuelsen et al., 2012). Such signals could reach the hypothalamus via inputs from InsCtx and other cortical areas to targets such as the lateral hypothalamus (Wu et al., 2020), which has a well-established role in the regulation of cue-driven motivated approach behaviors including feeding (Petrovich, 2018; Stuber and Wise, 2016). Nevertheless, it is as yet unclear how the state-dependent specificity of this top-down regulation is achieved (e.g., selective effects of food cues vs. water cues on hypothalamic hunger vs. thirst circuits). Future work combining precise cortical manipulations with recordings of hypothalamic neurons could reveal the specific circuit mechanisms underlying distinct hypothalamic feedforward signals.
4. Towards a unifying framework: short time-scale activity as a prediction of long time-scale activity in InsCtx
The above sections have considered InsCtx activity across a range of paradigms and time-scales, with a focus on recent cellular-level studies in animal models. We have argued that InsCtx activity tracks slow changes in the current state of the body across long time-scales, above and beyond arousal-related changes that affect all cortical areas. We have also reviewed evidence that at least some short time-scale activity in InsCtx, such as responses to learned, motivationally salient visual or auditory cues, is relayed by limbic inputs rather than bottom-up viscerosensory sources. Can findings regarding these two time-scales be incorporated into a unified heuristic model of InsCtx function? Below, we review recent progress in this area, and consider how ideas from human cognitive neuroscience models of predictive coding in InsCtx might provide a unifying framework for understanding various and sometimes conflicting studies of rodent InsCtx.
4.1. Interoceptive predictions
In recent decades, theories and empirical evidence increasingly suggest that a key role of cortex in sensory perception and motor behaviors is in building associations that are then used to predict changes in the external world and in our musculoskeletal movements. An early application of these theories involved perception of visual and other external sensory stimuli (Barrett and Simmons, 2015; Friston, 2010; Keller and Mrsic-Flogel, 2018; Rao and Ballard, 1999). It was argued that high-level cortical areas predict the current state of the external sensory environment and relay predictions back to neurons in earlier sensory cortical areas that may be involved in detailed filling-in of the percept of the sensory world. If this prediction matches the external sensory input, then the resulting activity pattern does not change. However, if a sensory stimulus unexpectedly appears or disappears, then a prediction error signal is generated. In this way, only unexpected sensory signals are then propagated forward to higher-order cortical areas to drive learning and updating of predictions.
There have been compelling arguments for applying similar theories of predictive coding to understanding the activity patterns in insular cortex and its role in interoception. Indeed, predictions of action-driven changes in the future state of body organs may be particularly valuable to the organism. This is because the delay between current actions (e.g. eating) and visceral outcomes (e.g. arrival and digestion of nutrients) can be several minutes (much longer than for most other sensorimotor loops). Further, interoceptive predictions about slow changes in the body may sit atop a hierarchy of predictions regarding any given sensory state (Kiebel et al., 2008), as most sensory predictions may ultimately serve to preserve current and future bodily homeostasis and survival.
As reviewed above, descending projections from insular cortex neurons can have potent effects on visceromotor functions involving control of smooth muscles and glands, such as regulation of gastric motility, heart rate, breathing, and salivation (Aleksandrov et al., 1996; Bagaev and Aleksandrov, 2006; Cechetto and Saper, 1987; Chouchou et al., 2019; Maeda et al., 2014; Yasui et al., 1991). Several cognitive neuroscience groups have posited that collaterals from neurons in agranular InsCtx generate interoceptive predictions and send these to granular and dysgranular regions of InsCtx where they are compared to ascending viscerosensory inputs from spinal, vagal, and possibly humoral pathways (for an overview, see (Barrett and Simmons, 2015)). Such theories have garnered empirical support, for example, from well-controlled neuroimaging studies of anticipated and actual pain stimuli (Fazeli and Buchel, 2018; Geuter et al., 2017). Specifically, these studies reveal that activity in more anterior agranular regions of human InsCtx more strongly reflects anticipation of expected pain, whereas activity in posterior granular regions more strongly reflects the actual intensity of painful stimuli. Notably, these studies provide evidence that these predictions are modality-specific and involve expectation of pain per se, rather than a general expectation of an aversive outcome. This further supports a role for InsCtx in anticipation of specific bodily sensations, rather than general valence. Below, we discuss how this consideration of InsCtx through the lens of predictive coding may help unify many of the apparently conflicting observations from the cellular-level studies of insular cortex reviewed above.
4.2. Anticipation of future physiological states in InsCtx
We recently linked long and short time-scale activity patterns in InsCtx using two-photon calcium imaging in behaving mice. As discussed above, we found that InsCtx activity patterns gradually changed across tens of minutes as food-restricted mice became sated (Livneh et al., 2020). By tracking the same neurons across sessions, we observed a largely similar hunger-related pattern of ongoing activity across multiple days, with similar transitions to a satiety-related activity pattern across days (Livneh et al., 2020). Notably, dehydration-related patterns of ongoing activity differed from those during food restriction, and gradually transitioned to a quenched pattern following repeated ingestion of drops of water. While these three slow time-scale patterns reflecting calorie restriction, dehydration, and satiety were composed of activity of hundreds of neurons, the patterns could be represented as three points in a two-dimensional space (Fig. 3A) with one axis reflecting water deficit/surfeit, and the other axis reflecting calorie deficit/surfeit. Note that while these axes are distinct, they are not necessarily orthogonal in reality, due to shared encoding of interoceptive changes that are common across different deviations from homeostasis (e.g. those reflecting increased arousal, blood pressure or heart rate).
Figure 3: Short Time-Scale Activity as a Prediction of Long Time-Scale Activity in InsCtx.
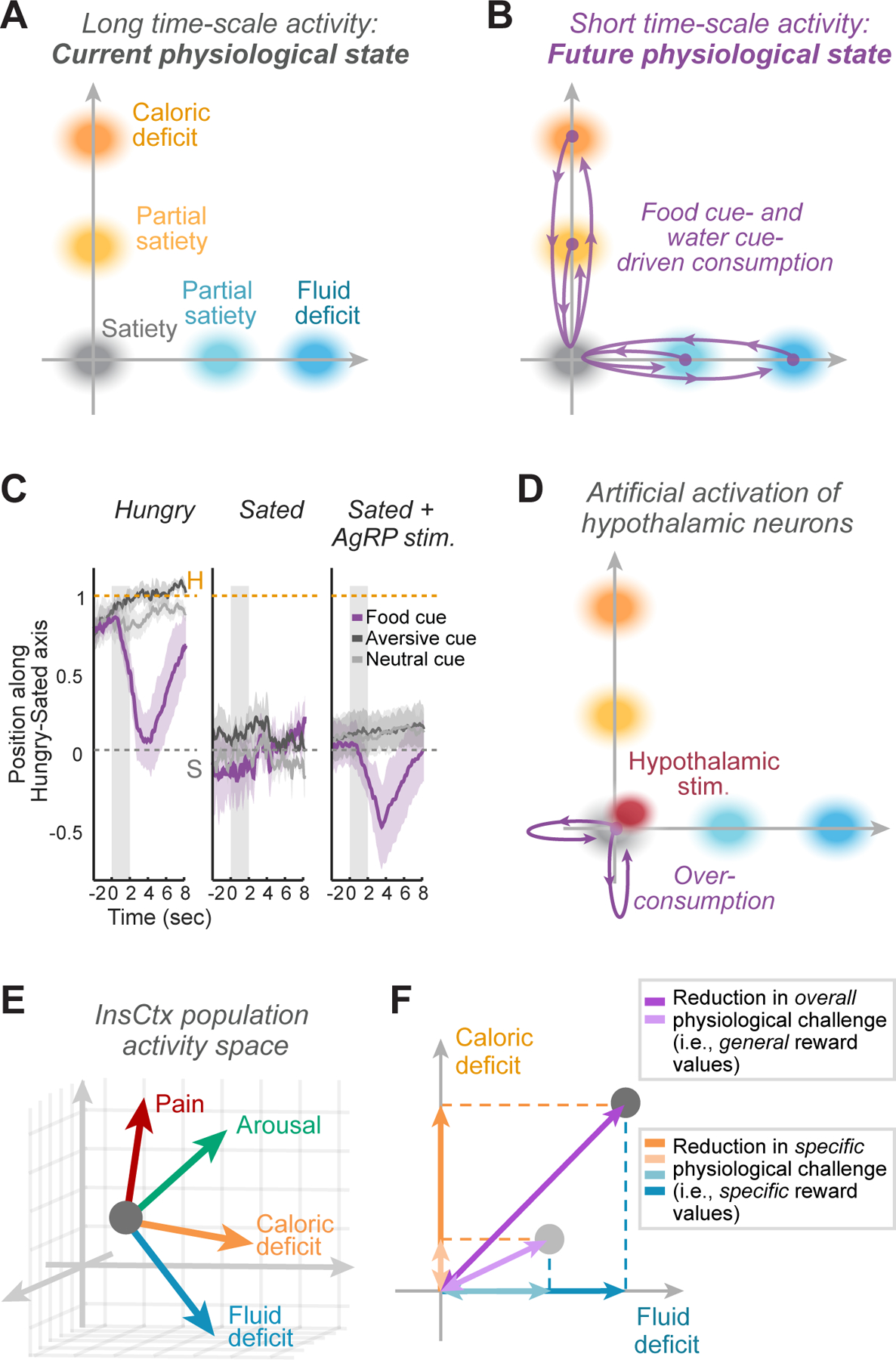
A. InsCtx population activity patterns are multi-dimensional. Within this multi-dimensional activity space, activity can move along many different axes of deficit/surfeit (e.g. axes in panel ‘E’, below). We first consider only 2 axes of activity in the multi-dimensional activity space: caloric and fluid deficit.
B. Similar to ‘A’, but for short time-scale activity. During caloric or fluid deficit, cue-driven consumption of a very small food/water reward (0.1–1% of the amount needed for satiety) transiently drives activity patterns along the relevant deficit axis towards the sated state. This potentially reflects a transient prediction of the future satiety state that will be reached in 1–2 hrs following repeated consumption of such rewards.
C. Experimental data supporting the model in ‘A’ and ‘B’. Modified with permission from Livneh et al., 2020. Note that artificial activation of hypothalamic “hunger neurons” in sated animals drives cue-driven consumption, but does not affect InsCtx ongoing activity. During AgRP activation, cue driven consumption drives activity patterns along the caloric deficit axis beyond the pattern associated with satiety. This potentially reflects a transient prediction of the future state associated with over-consumption.
D. Similar to ‘B’, but now showing the results of activation of hypothalamic “hunger neurons” and “thirst neurons” (which evoked no change in activity pattern on their own) and of the dynamics of InsCtx activity patterns following food or water cue presentation (purple trajectory; cf. panel C).
E. Schematic of InsCtx multi-dimensional population activity space, illustrated along three axes for simplicity. Activity can move within this space along different axes, simultaneously representing the broad range of visceral physiological states.
F. Example states caused by two physiological perturbations (gray circles) that each induced varying levels of both caloric and fluid deficiency. A cue predicting the availability of both food and water, and thus a return to the homeostatic ‘set point’, will have a general reward value that is proportional to its net distance from the ‘set point’. Additionally, this cue will have specific reward values along the two deficiency axes. Note that consumption of dry, salty food might have negative general reward values in these example states, as it pushes activity further away from the eucaloric/euhydrated state.
This deliberately simplified representation of two axes representing states of hydration and energy balance, respectively, provides a useful heuristic framework for reconsidering how fast cue response patterns may relate to slow changes in activity in InsCtx. Specifically, we found that when a mouse detected a learned cue predicting water or food, and began to drink a single drop of water or food, the InsCtx activity pattern transiently changed to the pattern reflecting the state of satiety that was only experienced by the mouse tens of minutes later, after repeated ingestion (Fig. 3B; (Livneh et al., 2020)). As discussed in greater detail below, we suggest that this satiety-related pattern may reflect a kind of ‘set point’ reflecting the state of the animal after completing all voluntary ingestion of available water and food. In the sated and quenched state, we did not observe fast InsCtx responses to food or water cues (Livneh et al., 2017; Livneh et al., 2020), consistent with many reports in human insular cortex (Becker et al., 2015; Cornier et al., 2009; de Araujo et al., 2003; LaBar et al., 2001). This lack of responsiveness is consistent with the idea that any shift in the InsCtx pattern from that associated with the sated state would reflect a prediction of a deviation away from homeostasis, and would thus not occur. In other words, once the ongoing InsCtx activity pattern indicates that the body is at or near its set point, actions involving ingestion of food or water and associated preparation of the body for ingestion are unlikely to be pursued.
We then considered what would happen if sated mice were compelled experimentally to ingest water or food via stimulation of hypothalamic SFO glutamatergic neurons or AgRP neurons, respectively (Fig. 3C–D). We had found that these hypothalamic manipulations did restore water- and food-cue responses in InsCtx, but did not restore the slow time-scale ongoing activity patterns to those observed during dehydration or food restriction. A unified explanation for the above findings emerged when we considered them in the context of this predictive coding framework: InsCtx coding of slow time-scale visceral sensory signals is not restored by this hypothalamic stimulation, and thus the InsCtx ongoing activity pattern should remain at the current interoceptive set point. However, because the mouse is artificially compelled to ingest more water or food, this drives an InsCtx prediction that renewed ingestive behaviors will push the state of the body from the current set point to a new state reflecting a surplus of water (i.e. a hypervolemic/hypoosmotic state) or of food (e.g. high stomach distension and glucose concentration, etc.; Fig. 3C–D). Below, we consider previous studies in rodents in the context of this framework of InsCtx representations of distributed interoceptive predictions.
The notion that multiple need states are represented by distinct patterns of activity in insular and other cortical regions (e.g. orbitofrontal and cingulate cortex) has been modeled in recent theoretical studies of ‘homeostatic reinforcement learning’ (Juechems and Summerfield, 2019; Keramati and Gutkin, 2014), based on extensions of the classic drive reduction theory of Hull (Hull, 1943). These studies posit that anticipated increases in proximity of the state of the body to the homeostatic set point should drive decisions and actions. In the two-axis plot in Fig. 3A, this theory predicts that anticipated decreases in negative energy balance or in dehydration should guide decision-making and reinforce actions that enable the seeking and consumption of food or water, as well as the preemptive reduction of compensatory mechanisms of homeostasis such as gluconeogenesis by the liver (Diaz-Munoz et al., 2000) or the restriction of water excretion from kidney to bladder (Mandelblat-Cerf et al., 2017).
This theory is readily extendable beyond hunger, thirst, and other ingestive drives to other perturbations from homeostasis including deviations in respiratory rate and resistance, air hunger, heart rate and blood pressure, body temperature, bladder volume, and visceral pain (Fig. 3E). The increases in heart rate and blood pressure during states of anxiety or fear may also be seen as perturbations from body homeostasis, albeit adaptive ones (Nadine Gogolla’s lab, unpublished data, https://www.crowdcast.io/e/nadine-gogollas/1). If, as in the case of calorie restriction vs. dehydration (Livneh et al., 2020), these other axes of deviation from homeostasis are also represented by the relative firing rates of distributed but partially intermingled neurons in InsCtx, this could facilitate decision making in situations where actions have multiple consequences that push the body towards homeostasis along some axes but potentially away from homeostasis along others. For example, a hungry yet quenched individual that prepares to consume a cold, salty, high-calorie drink might predict a decrease in caloric deficit but also departures from homeostasis in the form of a decrease in body temperature and an increase in blood osmolarity.
This geometric model of InsCtx population activity allows one to empirically measure different axes and then project InsCtx activity onto them. This then allows one to describe quantitatively how population activity represents multiple physiological parameters simultaneously. Equally important, it allows one to describe specific predictions along these empirically defined axes. This is an important advantage of this model, as it allows a concrete description of multi-dimensional population activity in relation to multiple physiological parameters simultaneously. Of note, future work using such a framework could also reveal whether these representations map onto InsCtx topography and/or cytoarchitectonic subdivisions.
Another potential use of this multi-dimensional geometric representation is that it allows testing of whether reinforcement signals in the brain, such as release of dopamine in ventral striatum, will increase if there is a net increase in the proximity of the state of the body to the homeostatic set point, when considered across multiple axes of homeostatic perturbation. Conversely, actions or experiences that involve unexpected departures from homeostasis should actually drive aversion and a decrease in such reinforcement signals. Chronic recordings should allow testing of these hypotheses, via mapping of these various axes of homeostatic perturbation in the same InsCtx neurons across days, together with concurrent monitoring of reinforcement signals. Several disynaptic pathways could link neurons encoding predictions in InsCtx to reinforcement-related dopamine neurons in VTA and SNc, including pathways via vBNST (Girven et al., 2020), lateral hypothalamus (Wu et al., 2020), central amygdala (Kim et al., 2018), orbitofrontal cortex (Namboodiri et al., 2019), and parabrachial nucleus (Boughter et al., 2019).
The above theory suggests that downstream brain regions can extract either general or need-specific reward values from InsCtx activity patterns. General value is extracted as the overall reduction in net distance from the set point following a given course of action, whereas need-specific values are extracted as the reductions in distance from the set point along specific need-related axes (Fig. 3F). Specific values could be read out by different subsets of hypothalamic neurons, such as those controlling fluid balance and energy balance, which respond predominantly to the need-specific rewards (Allen et al., 2017; Augustine et al., 2018a; Betley et al., 2015; Chen et al., 2015; Gizowski et al., 2016; Mandelblat-Cerf et al., 2017; Mandelblat-Cerf et al., 2015; Zimmerman et al., 2016). As mentioned above, general value could be read out, for example, by VTA dopamine neurons that project to ventral striatum.
Interoceptive predictions in InsCtx could be used for both model-free and model-based reinforcement learning (Drummond and Niv, 2020). In situations involving high uncertainty (a highly palatable food that in some contexts might also cause extreme malaise, e.g., if not prepared from fresh ingredients), InsCtx might represent distinct interoceptive predictions (e.g., gastric, cardiovascular, respiratory, etc.), which could then be used for fine-grained model-based learning and decision-making. In situations involving less uncertainty (e.g., a candy bar that always generates the same taste and interoceptive outcome), InsCtx interoceptive predictions could “merge” or “average” into a single integrated interoceptive value that can guide model-free learning. This merging or averaging of expected interoceptive outcomes into a single prediction could involve anterior agranular InsCtx, which receives specific information from mid/posterior granular InsCtx. Alternatively, this merging could involve downstream effector nuclei such as the CeA or NAc that might receive convergent input from InsCtx neurons representing specific predictions.
A different yet related question is whether and when InsCtx interoceptive predictions reach consciousness to form explicit conscious interoceptive predictions. Predictive processing theories, applied to interoception (Barrett and Simmons, 2015; Owens et al., 2018), have postulated that predictions themselves usually do not reach consciousness, unless they are extremely precise and reliable, and that conscious interoceptive sensations usually arise from strong prediction error signals (Quadt et al., 2018). While most InsCtx interoceptive predictions are likely unconscious, we speculate that InsCtx does form explicit conscious interoceptive predictions in certain circumstances. One example is interoceptive mental imagery (Wilson-Mendenhall et al., 2019), which could be used for decision-making and action selection (Nummenmaa et al., 2018). In the following sections, we consider how the distributed nature of putative representations of predictions across InsCtx may help us better understand previous neural recordings and stimulation experiments in rodent InsCtx.
4.2. Neuronal recordings in InsCtx through the lens of predictive coding of interoceptive states
As discussed above, previous studies have shown that InsCtx responses to initially pleasant tastes become more similar to responses to aversive cues and outcomes following conditioned taste aversion (CTA; (Accolla and Carleton, 2008; Grossman et al., 2008; Lavi et al., 2018)). We hypothesize that this involves taste-evoked anticipatory activation of the InsCtx activity patterns predicted to occur during actual states of sickness or malaise. This likely involves changes in many organs (e.g. GI tract, breathing, heart rate) and thus is expected to be broadly distributed across millimeters of rodent InsCtx representing various body organs, not just the subregion receiving gustatory thalamic input. Indeed, it has long been recognized that tastes can act as distal cues (Woods, 2009) that predict different changes throughout the body in preparation for ingestion of a given substance (e.g. anticipatory changes in gut motility, blood glucose) or bodily changes related to anticipated removal of the tastant (e.g. salivation during gape responses, vomiting during sickness). Thus, during learned food-seeking behaviors in awake animals, predictive circuits become engaged such that different tastants should drive distinct yet highly distributed patterns of activity throughout InsCtx. The distributed nature of neural response to various tastes across diverse regions of insular cortex is consistent with observations from several groups (Chen et al., 2021; Fletcher et al., 2017; Lavi et al., 2018; Levitan et al., 2019). In contrast, other studies in anesthetized animals have suggested much more spatially segregated responses to different tastants, predominantly in the gustatory thalamo-recipient regions of InsCtx (Accolla et al., 2007; Chen et al., 2011). Thus, differences in the degree of distributed vs. topographically restricted responses to tastes may reflect state-related differences in the degree of subsequent anticipatory activation of representations of the upcoming state of various organs.
4.3. Neuronal stimulation of InsCtx and reinforcement learning through the lens of predictive coding of interoceptive states
Several recent studies have shown that bulk stimulation of broad regions of posterior InsCtx is aversive (Gehrlach et al., 2019; Peng et al., 2015; Wang et al., 2018), while stimulation of broad regions of anterior InsCtx appears to be rewarding (Dolensek et al., 2020; Gehrlach et al., 2019) (Wang et al., 2018 ADD Gogolla REF). Below, we offer a possible interpretation of these studies in the context of predictive coding. The anterior regions of InsCtx that were stimulated contain not only portions of gustatory cortex with potential biases towards representations of sweet tastants, but also representations of the GI tract (see Section 1.1). Thus, following the logic from the previous section (Section 4.2), voluntary tasting of a sweet substance is typically followed by swallowing, and thus should also drive changes in activity of GI-tract representations in anterior InsCtx that reflect predicted changes in the GI tract (e.g. gut stretch and nutrient absorption). In contrast, tasting of a bitter substance is typically followed by rejection, and thus should not drive predictions of major changes in the state of the GI tract. In rare cases, predictions of deviations of the state of the GI tract away from homeostasis may occur in this more anterior region of InsCtx (e.g. during food poisoning or following CTA). However, standard home-cage housed mice experience a lifetime of reliable daily associations between food ingestion (e.g. mouse chow) and confirmation by interoceptive feedback signals that the ingested food was nutritive, restored energy balance, and did not cause malaise. Thus, we speculate that in home-cage housed mice, the dominant prediction in anterior gustatory and GI tract representations is one in which food availability and consumption will ultimately drive an increase in calories, thereby addressing current energy deficits. If bulk stimulation of anterior InsCtx activates this common prediction of relief of energy deficit more than other predictions, it stands to reason that this manipulation should be rewarding. This hypothesis may also apply to stimulation of anterior InsCtx in a sated mouse, as sated mice will nevertheless consume highly palatable sweet foods such as chocolate (Chen et al., 2015), thereby addressing not only current but also anticipated energy deficits.
In contrast to bulk stimulation of anterior InsCtx, stimulation of specific subsets of neurons in posterior InsCtx neurons – those encoding predicted departures away from homeostasis – may drive aversive responses. Further, bulk stimulation of posterior InsCtx neurons in a sated mouse in the absence of imminent threats is aversive (Gehrlach et al., 2019; Wang et al., 2018). The posterior regions of InsCtx that were stimulated contain not only portions of gustatory cortex with potential biases towards representations of bitter tastants, but also neurons sensitive to painful stimuli and perturbations in breathing and heart rate (see Section 1.1). Thus, aversive reactions to stimulation of this region are consistent with the framework of InsCtx predictions of future bodily states. Random deviations in the pattern of InsCtx activity from the pattern associated with a current homeostatic state, along axes of breathing, heart rate, and/or pain (cf. Fig. 3E–F), are all expected to be aversive. In contrast, if an animal is already in an extremely aversive state, then certain random patterns of bulk stimulation may indeed become appetitive (Nadine Gogolla, unpublished observations, https://www.crowdcast.io/e/nadine-gogollas/1), as such patterns may represent states that are more proximal to the homeostatic state.
4.4. Implications for disease research
Several excellent reviews discuss implications of predictive coding models and the role of InsCtx in substance abuse and various psychiatric and neurological disorders (Barrett and Simmons, 2015; Craig, 2003b; Critchley and Harrison, 2013; de Araujo et al., 2020; Khalsa et al., 2018). As our own studies on predictions in InsCtx focused on energy balance and hydration, we briefly consider how aberrant predictions may contribute to these processes going awry.
A key cause of greater obesity in modern societies is the increase in energy intake (Swinburn et al., 2009). One driver of this behavior is our obesogenic environment, which provides affordable access to processed, high-calorie foods and beverages. Here, food-related sensory cues may not allow accurate prediction of caloric outcomes (Block et al., 2013; Franckle et al., 2016). Critically, the notion of a set point pattern of activity in InsCtx need not reflect what the optimal state of the body should be, but rather what the typical state of the body is after meal consumption is complete. It is worth considering this idea in the context of the “active interoceptive inference” framework proposed by Friston and colleagues. Here, the state of overconsumption in obese individuals would become the most expected state, and thus the “goal state” (Pezzulo et al., 2018). Similarly, in eating disorders such as anorexia nervosa, there might be a shift in the expected post-meal “set point” in the opposite direction. Such shifts in set point may, in turn, drive overly strong or weak motivation to consume food. In the context of active inference over previously experienced interoceptive states, the “set point” representation in InsCtx may be more analogous to anticipation of a homeostatic settling point (Lowell et al., 2021). This is because settling points passively reflect the steady state achieved after countering homeostatic deficits, but will rarely reflect an optimal state in which all bodily threats are removed (Pezzulo et al., 2018). Using cellular-level manipulations in insular cortex, it should be possible to test whether modification of prediction-related activity can help normalize aberrant behaviors across a range of animal models of compromised motivational drives.
Acknowledgements
We thank members of the Andermann lab for helpful discussions. Authors were supported by a research grant from the Center for New Scientists at the Weizmann Institute of Science, the Alon Scholarship from the Israeli Council for Higher Education (Y.L.); NIH grants DP2 DK105570, DP1 AT010971, R01 DK109930, the McKnight Foundation, and the Klarman Family Foundation (M.L.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accolla R, Bathellier B, Petersen CC, and Carleton A (2007). Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci 27, 1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accolla R, and Carleton A (2008). Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc Natl Acad Sci U S A 105, 4010–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Rivera M, Kim S, Coleman TP, Maldonado PE, and Torrealba F (2020). Interoceptive insular cortex participates in sensory processing of gastrointestinal malaise and associated behaviors. Sci Rep 10, 21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrov VG, Bagaev VA, Nozdrachev AD, and Panteleev SS (1996). Identification of gastric related neurones in the rat insular cortex. Neurosci Lett 216, 5–8. [DOI] [PubMed] [Google Scholar]
- Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, and Betley JN (2018). A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell 173, 140–152 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, and Cechetto DF (1991). Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 311, 1–16. [DOI] [PubMed] [Google Scholar]
- Allen WE, Chen MZ, Pichamoorthy N, Tien RH, Pachitariu M, Luo L, and Deisseroth K (2019). Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen WE, DeNardo LA, Chen MZ, Liu CD, Loh KM, Fenno LE, Ramakrishnan C, Deisseroth K, and Luo L (2017). Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357, 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, and Lowell BB (2017). Toward a Wiring Diagram Understanding of Appetite Control. Neuron 95, 757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, and Adolphs R (2014). A framework for studying emotions across species. Cell 157, 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22, 229–244. [DOI] [PubMed] [Google Scholar]
- Augustine V, Ebisu H, Zhao Y, Lee S, Ho B, Mizuno GO, Tian L, and Oka Y (2019). Temporally and Spatially Distinct Thirst Satiation Signals. Neuron 103, 242–249 e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine V, Gokce SK, Lee S, Wang B, Davidson TJ, Reimann F, Gribble F, Deisseroth K, Lois C, and Oka Y (2018a). Hierarchical neural architecture underlying thirst regulation. Nature 555, 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine V, Gokce SK, and Oka Y (2018b). Peripheral and Central Nutrient Sensing Underlying Appetite Regulation. Trends Neurosci 41, 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine V, Lee S, and Oka Y (2020). Neural Control and Modulation of Thirst, Sodium Appetite, and Hunger. Cell 180, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avau B, Rotondo A, Thijs T, Andrews CN, Janssen P, Tack J, and Depoortere I (2015). Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci Rep 5, 15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Gotts SJ, Kerr KL, Burrows K, Ingeholm JE, Bodurka J, Martin A, and Kyle Simmons W (2017). Convergent gustatory and viscerosensory processing in the human dorsal mid-insula. Hum Brain Mapp 38, 2150–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Liu AG, Ingeholm JE, Riddell CD, Gotts SJ, and Martin A (2020). Taste Quality Representation in the Human Brain. J Neurosci 40, 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagaev V, and Aleksandrov V (2006). Visceral-related area in the rat insular cortex. Auton Neurosci 125, 16–21. [DOI] [PubMed] [Google Scholar]
- Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, et al. (2019). Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 179, 1129–1143 e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P, and Bremer F (1938). A SENSORY CORTICAL REPRESENTATION OF THE VAGUS NERVE: WITH A NOTE ON THE EFFECTS OF LOW BLOOD PRESSURE ON THE CORTICAL ELECTROGRAM. J Neurophysiol 1, 405–412. [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, and Gross JJ (2007). The experience of emotion. Annu Rev Psychol 58, 373–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, and Simmons WK (2015). Interoceptive predictions in the brain. Nat Rev Neurosci 16, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CA, Schmalzle R, Flaisch T, Renner B, and Schupp HT (2015). Thirst and the state-dependent representation of incentive stimulus value in human motive circuitry. Soc Cogn Affect Neurosci 10, 1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DE, and Dudai Y (2001). Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291, 2417–2419. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F (2004). Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci 5, 209–217. [DOI] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, and Sternson SM (2015). Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler LR, Chen Y, Ahn JS, Lin YC, Essner RA, and Knight ZA (2017). Dynamics of Gut-Brain Communication Underlying Hunger. Neuron 96, 461–475 e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann SV, Biedermann DG, Wenzlaff F, Kurjak T, Nouri S, Auer MK, Wiedemann K, Briken P, Haaker J, Lonsdorf TB, et al. (2017). An elevated plus-maze in mixed reality for studying human anxiety-related behavior. BMC Biol 15, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block JP, Condon SK, Kleinman K, Mullen J, Linakis S, Rifas-Shiman S, and Gillman MW (2013). Consumers’ estimation of calorie content at fast food restaurants: cross sectional observational study. BMJ 346, f2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter JD Jr., Lu L, Saites LN, and Tokita K (2019). Sweet and bitter taste stimuli activate VTA projection neurons in the parabrachial nucleus. Brain Res 1714, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Nolte H, Henschke S, Engstrom Ruud L, Awazawa M, Morgan DA, Gabel P, Sprenger HG, Hess ME, Gunther S, et al. (2018). Food Perception Primes Hepatic ER Homeostasis via Melanocortin-Dependent Control of mTOR Activation. Cell 175, 1321–1335 e1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Schwartz MW, and Palmiter RD (2016). Parabrachial CGRP Neurons Control Meal Termination. Cell Metab 23, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canna A, Prinster A, Cantone E, Ponticorvo S, Russo AG, Di Salle F, and Esposito F (2019). Intensity-related distribution of sweet and bitter taste fMRI responses in the insular cortex. Hum Brain Mapp 40, 3631–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB (1987). The James-Lange theory of emotions: a critical examination and an alternative theory. By Walter B. Cannon, 1927. Am J Psychol 100, 567–586. [PubMed] [Google Scholar]
- Carpenter RH (2004). Homeostasis: a plea for a unified approach. Advances in physiology education 28, 180–187. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, and Saper CB (1987). Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 262, 27–45. [DOI] [PubMed] [Google Scholar]
- Chen K, Kogan JF, and Fontanini A (2021). Spatially Distributed Representation of Taste Quality in the Gustatory Insular Cortex of Behaving Mice. Curr Biol 31, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gabitto M, Peng Y, Ryba NJ, and Zuker CS (2011). A gustotopic map of taste qualities in the mammalian brain. Science 333, 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW, and Knight ZA (2015). Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Gonzalez I, Pan W, Tsang AH, Adams J, Ndoka E, Gordian D, Khoury B, Roelofs K, Evers SS, et al. (2020). Calcitonin Receptor Neurons in the Mouse Nucleus Tractus Solitarius Control Energy Balance via the Non-aversive Suppression of Feeding. Cell Metab 31, 301–312 e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Lee DH, Kriegeskorte N, and Anderson AK (2019). Distinct representations of basic taste qualities in human gustatory cortex. Nat Commun 10, 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchou F, Mauguiere F, Vallayer O, Catenoix H, Isnard J, Montavont A, Jung J, Pichot V, Rheims S, and Mazzola L (2019). How the insula speaks to the heart: Cardiac responses to insular stimulation in humans. Hum Brain Mapp 40, 2611–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, and Torrealba F (2007). Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318, 655–658. [DOI] [PubMed] [Google Scholar]
- Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, and Tregellas JR (2009). The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE 4, e6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2003a). Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13, 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003b). Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13, 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003c). Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 26, 1–30. [DOI] [PubMed] [Google Scholar]
- Critchley HD, and Harrison NA (2013). Visceral influences on brain and behavior. Neuron 77, 624–638. [DOI] [PubMed] [Google Scholar]
- Dalenberg JR, Patel BP, Denis R, Veldhuizen MG, Nakamura Y, Vinke PC, Luquet S, and Small DM (2020). Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans. Cell Metab 31, 493–502 e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A Jr., Nicolelis MA, and Simon SA (2006). Neural ensemble coding of satiety states. Neuron 51, 483–494. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, and McGlone F (2003). Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol 90, 1865–1876. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, and Simon SA (2008). Food reward in the absence of taste receptor signaling. Neuron 57, 930–941. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Schatzker M, and Small DM (2020). Rethinking Food Reward. Annu Rev Psychol 71, 139–164. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, and Simon SA (2009). The gustatory cortex and multisensory integration. Int J Obes 33 Suppl 2, S34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Munoz M, Vazquez-Martinez O, Aguilar-Roblero R, and Escobar C (2000). Anticipatory changes in liver metabolism and entrainment of insulin, glucagon, and corticosterone in food-restricted rats. Am J Physiol Regul Integr Comp Physiol 279, R2048–2056. [DOI] [PubMed] [Google Scholar]
- Dolensek N, Gehrlach DA, Klein AS, and Gogolla N (2020). Facial expressions of emotion states and their neuronal correlates in mice. Science 368, 89–94. [DOI] [PubMed] [Google Scholar]
- Drummond N, and Niv Y (2020). Model-based decision making and model-free learning. Curr Biol 30, R860–R865. [DOI] [PubMed] [Google Scholar]
- Egan G, Silk T, Zamarripa F, Williams J, Federico P, Cunnington R, Carabott L, Blair-West J, Shade R, McKinley M, et al. (2003). Neural correlates of the emergence of consciousness of thirst. Proc Natl Acad Sci U S A 100, 15241–15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi K, Kashima H, Yokota A, Miura K, Yamaoka Endo M, Hirano H, Tsuji T, and Fukuba Y (2016). Acute effect of oral sensation of sweetness on celiac artery blood flow and gastric myoelectrical activity in humans. Auton Neurosci 197, 41–45. [DOI] [PubMed] [Google Scholar]
- Essner RA, Smith AG, Jamnik AA, Ryba AR, Trutner ZD, and Carter ME (2017). AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons. J Neurosci 37, 8678–8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, and McNaughton BL (2012). The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC (2019). The Organization of the Primate Insular Cortex. Front Neuroanat 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Markovic M, Tovote P, and Luthi A (2018). New perspectives on central amygdala function. Curr Opin Neurobiol 49, 141–147. [DOI] [PubMed] [Google Scholar]
- Fazeli S, and Buchel C (2018). Pain-Related Expectation and Prediction Error Signals in the Anterior Insula Are Not Related to Aversiveness. J Neurosci 38, 6461–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Ogg MC, Lu L, Ogg RJ, and Boughter JD Jr. (2017). Overlapping Representation of Primary Tastes in a Defined Region of the Gustatory Cortex. J Neurosci 37, 7595–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB (2015). The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66, 25–52. [DOI] [PubMed] [Google Scholar]
- Franckle RL, Block JP, and Roberto CA (2016). Calorie Underestimation When Buying High-Calorie Beverages in Fast-Food Contexts. Am J Public Health 106, 1254–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2010). The free-energy principle: a unified brain theory? Nat Rev Neurosci 11, 127–138. [DOI] [PubMed] [Google Scholar]
- Fu O, Iwai Y, Narukawa M, Ishikawa AW, Ishii KK, Murata K, Yoshimura Y, Touhara K, Misaka T, Minokoshi Y, et al. (2019). Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat Commun 10, 4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MP, and Fontanini A (2014). Encoding and tracking of outcome-specific expectancy in the gustatory cortex of alert rats. J Neurosci 34, 13000–13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. (2015). A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci 18, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, et al. (2016). Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci 19, 1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrlach DA, Dolensek N, Klein AS, Roy Chowdhury R, Matthys A, Junghanel M, Gaitanos TN, Podgornik A, Black TD, Reddy Vaka N, et al. (2019). Aversive state processing in the posterior insular cortex. Nat Neurosci 22, 1424–1437. [DOI] [PubMed] [Google Scholar]
- Gehrlach DA, Weiand C, Gaitanos TN, Cho E, Klein AS, Hennrich AA, Conzelmann KK, and Gogolla N (2020). A whole-brain connectivity map of mouse insular cortex. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Boll S, Eippert F, and Buchel C (2017). Functional dissociation of stimulus intensity encoding and predictive coding of pain in the insula. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girven KS, Aroni S, Navarrete J, Marino RAM, McKeon PN, Cheer JF, and Sparta DR (2020). Glutamatergic input from the insula to the ventral bed nucleus of the stria terminalis controls reward-related behavior. Addict Biol, e12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizowski C, Zaelzer C, and Bourque CW (2016). Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature 537, 685–688. [DOI] [PubMed] [Google Scholar]
- Gogolla N (2017). The insular cortex. Curr Biol 27, R580–R586. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Takesian AE, Feng G, Fagiolini M, and Hensch TK (2014). Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron 83, 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, and Katz DB (2008). Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci 28, 2864–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundemann J, Bitterman Y, Lu T, Krabbe S, Grewe BF, Schnitzer MJ, and Luthi A (2019). Amygdala ensembles encode behavioral states. Science 364. [DOI] [PubMed] [Google Scholar]
- Guo W, Robert B, and Polley DB (2019). The Cholinergic Basal Forebrain Links Auditory Stimuli with Delayed Reinforcement to Support Learning. Neuron 103, 1164–1177 e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Niu J, Medina S, Ferreira TL, Zhang X, Su J, Tong J, Schwartz GJ, van den Pol A, et al. (2016). Striatal Dopamine Links Gastrointestinal Rerouting to Altered Sweet Appetite. Cell Metab 23, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, et al. (2018). A Neural Circuit for Gut-Induced Reward. Cell 175, 887–888. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, and Kannan H (1998). Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol 79, 2535–2545. [DOI] [PubMed] [Google Scholar]
- Harada Y, Koseki J, Sekine H, Fujitsuka N, and Kobayashi H (2019). Role of Bitter Taste Receptors in Regulating Gastric Accommodation in Guinea Pigs. J Pharmacol Exp Ther 369, 466–472. [DOI] [PubMed] [Google Scholar]
- Harris KD, and Shepherd GM (2015). The neocortical circuit: themes and variations. Nat Neurosci 18, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa Y, Hashikawa K, Falkner AL, and Lin D (2017). Ventromedial Hypothalamus and the Generation of Aggression. Front Syst Neurosci 11, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz SL, Fu A, Loflin W, Corson JA, and Erisir A (2015). Morphology and connectivity of parabrachial and cortical inputs to gustatory thalamus in rats. J Comp Neurol 523, 139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CL (1943). Principles of behavior: an introduction to behavior theory. (Appleton-Century; ). [Google Scholar]
- Jones LM, Fontanini A, and Katz DB (2006). Gustatory processing: a dynamic systems approach. Curr Opin Neurobiol 16, 420–428. [DOI] [PubMed] [Google Scholar]
- Jones LM, Fontanini A, Sadacca BF, Miller P, and Katz DB (2007). Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proc Natl Acad Sci U S A 104, 18772–18777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju A, Fernandez-Arroyo B, Wu Y, Jacky D, and Beyeler A (2020). Expression of serotonin 1A and 2A receptors in molecular- and projection-defined neurons of the mouse insular cortex. Mol Brain 13, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juechems K, and Summerfield C (2019). Where Does Value Come From? Trends Cogn Sci 23, 836–850. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls ET, and Verhagen JV (2005). Neuronal representations of stimuli in the mouth: the primate insular taste cortex, orbitofrontal cortex and amygdala. Chem Senses 30, 401–419. [DOI] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, and Bohorquez DV (2018). A gut-brain neural circuit for nutrient sensory transduction. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DB, Simon SA, and Nicolelis MA (2001). Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci 21, 4478–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayyal H, Yiannakas A, Kolatt Chandran S, Khamaisy M, Sharma V, and Rosenblum K (2019). Activity of Insula to Basolateral Amygdala Projecting Neurons is Necessary and Sufficient for Taste Valence Representation. J Neurosci 39, 9369–9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GB, and Mrsic-Flogel TD (2018). Predictive Processing: A Canonical Cortical Computation. Neuron 100, 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramati M, and Gutkin B (2014). Homeostatic reinforcement learning for integrating reward collection and physiological stability. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, Feusner JD, Garfinkel SN, Lane RD, Mehling WE, et al. (2018). Interoception and Mental Health: A Roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 3, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel SJ, Daunizeau J, and Friston KJ (2008). A hierarchy of time-scales and the brain. PLoS Comput Biol 4, e1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Yoon S, Nakajima R, Lee HJ, Lim HJ, Lee YK, Choi JS, Yoon BJ, Augustine GJ, and Baik JH (2018). Dopamine D2 receptor-mediated circuit from the central amygdala to the bed nucleus of the stria terminalis regulates impulsive behavior. Proc Natl Acad Sci U S A 115, E10730–E10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Heo G, Kim M, Kim H, Jin JA, Kim HK, Jung S, An M, Ahn BH, Park JH, et al. (2020). A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580, 376–380. [DOI] [PubMed] [Google Scholar]
- Klarer M, Arnold M, Gunther L, Winter C, Langhans W, and Meyer U (2014). Gut vagal afferents differentially modulate innate anxiety and learned fear. J Neurosci 34, 7067–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawonn AM, Fritz M, Nilsson A, Bonaventura J, Shionoya K, Mirrasekhian E, Karlsson U, Jaarola M, Granseth B, Blomqvist A, et al. (2018). Motivational valence is determined by striatal melanocortin 4 receptors. J Clin Invest 128, 3160–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, Miyamishi K, Zweifel LS, Luo L, Uchida N, et al. (2018). Functional circuit architecture underlying parental behaviour. Nature 556, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto-Yoshida I, Liu H, Chen BT, Fontanini A, and Bonci A (2015). Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc Natl Acad Sci U S A 112, 1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, and Mesulam MM (2001). Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral neuroscience 115, 493–500. [DOI] [PubMed] [Google Scholar]
- Lavi K, Jacobson GA, Rosenblum K, and Luthi A (2018). Encoding of Conditioned Taste Aversion in Cortico-Amygdala Circuits. Cell Rep 24, 278–283. [DOI] [PubMed] [Google Scholar]
- Lee SC, Amir A, Haufler D, and Pare D (2017). Differential Recruitment of Competing Valence-Related Amygdala Networks during Anxiety. Neuron 96, 81–88 e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow LP, Huckabee ML, Sharma S, and Tooley TP (2007). The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses 32, 119–128. [DOI] [PubMed] [Google Scholar]
- Levitan D, Lin JY, Wachutka J, Mukherjee N, Nelson SB, and Katz DB (2019). Single and population coding of taste in the gustatory cortex of awake mice. J Neurophysiol 122, 1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, et al. (2017). Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Sugden AU, Madara JC, Essner RA, Flores VI, Sugden LA, Resch JM, Lowell BB, and Andermann ML (2020). Estimation of Current and Future Physiological States in Insular Cortex. Neuron 105, 1094–1111 e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Swanson LW, and Horn JP (2021). The Hypothalamus: Autonomic, Hormonal, and Behavioral Control of Survival. In Principles of Neural Science Kandel E, J. Koester, Mack S, and Siegelbaum S, eds. [Google Scholar]
- Maeda N, Kobashi M, Mitoh Y, Fujita M, Minagi S, and Matsuo R (2014). Differential involvement of two cortical masticatory areas in submandibular salivary secretion in rats. Brain Res 1543, 200–208. [DOI] [PubMed] [Google Scholar]
- Maffei A, Haley M, and Fontanini A (2012). Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol 22, 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Kim A, Burgess CR, Subramanian S, Tannous BA, Lowell BB, and Andermann ML (2017). Bidirectional Anticipation of Future Osmotic Challenges by Vasopressin Neurons. Neuron 93, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, and Andermann ML (2015). Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola L, Mauguiere F, and Isnard J (2017a). Electrical Stimulations of the Human Insula: Their Contribution to the Ictal Semiology of Insular Seizures. J Clin Neurophysiol 34, 307–314. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Royet JP, Catenoix H, Montavont A, Isnard J, and Mauguiere F (2017b). Gustatory and olfactory responses to stimulation of the human insula. Ann Neurol 82, 360–370. [DOI] [PubMed] [Google Scholar]
- Mazzucato L, La Camera G, and Fontanini A (2019). Expectation-induced modulation of metastable activity underlies faster coding of sensory stimuli. Nat Neurosci 22, 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Nestvogel DB, and He BJ (2020). Neuromodulation of Brain State and Behavior. Annu Rev Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier L, Federspiel A, Jann K, Wiest R, Strik W, and Dierks T (2018). Thirst-Dependent Activity of the Insular Cortex Reflects its Emotion-Related Subdivision: A Cerebral Blood Flow Study. Neuroscience 383, 170–177. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, and Mesulam MM (1982). Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol 212, 23–37. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Wachutka J, and Katz DB (2019). Impact of precisely-timed inhibition of gustatory cortex on taste behavior depends on single-trial ensemble dynamics. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroni P, Crnjar R, and Barbarossa IT (2011). Emotional Responses to Pleasant and Unpleasant Oral Flavour Stimuli. Chemosens Percept 4. [Google Scholar]
- Musall S, Kaufman MT, Juavinett AL, Gluf S, and Churchland AK (2019). Single-trial neural dynamics are dominated by richly varied movements. Nat Neurosci 22, 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namboodiri VMK, Otis JM, van Heeswijk K, Voets ES, Alghorazi RA, Rodriguez-Romaguera J, Mihalas S, and Stuber GD (2019). Single-cell activity tracking reveals that orbitofrontal neurons acquire and maintain a long-term memory to guide behavioral adaptation. Nat Neurosci 22, 1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, and Bechara A (2014). The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci 1316, 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R, and Wolf G (1975). Projections of thalamic gustatory and lingual areas in the rat. Brain Res 92, 123–129. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, and Nakao T (2010). Rest-stimulus interaction in the brain: a review. Trends Neurosci 33, 277–284. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Hari R, Hietanen JK, and Glerean E (2018). Maps of subjective feelings. Proc Natl Acad Sci U S A 115, 9198–9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Ito S, Murayama N, and Hasegawa K (1990). Taste area in granular and dysgranular insular cortices in the rat identified by stimulation of the entire oral cavity. Neurosci Res 9, 196–201. [DOI] [PubMed] [Google Scholar]
- Okonogi T, Nakayama R, Sasaki T, and Ikegaya Y (2018). Characterization of Peripheral Activity States and Cortical Local Field Potentials of Mice in an Elevated Plus Maze Test. Front Behav Neurosci 12, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M, Lak A, Carandini M, and Harris KD (2016). Long Term Recordings with Immobile Silicon Probes in the Mouse Cortex. PLoS ONE 11, e0151180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, de Araujo IE, Monteiro C, Workman V, Galhardo V, and Nicolelis MA (2012). The insular cortex controls food preferences independently of taste receptor signaling. Front Syst Neurosci 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, and Hachinski VC (1992). Cardiovascular effects of human insular cortex stimulation. Neurology 42, 1727–1732. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, and Mauguiere F (2002). Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex 12, 376–385. [DOI] [PubMed] [Google Scholar]
- Owens AP, Allen M, Ondobaka S, and Friston KJ (2018). Interoceptive inference: From computational neuroscience to clinic. Neurosci Biobehav Rev 90, 174–183. [DOI] [PubMed] [Google Scholar]
- Penfield W, and Faulk ME Jr. (1955). The insula; further observations on its function. Brain 78, 445–470. [DOI] [PubMed] [Google Scholar]
- Peng Y, Gillis-Smith S, Jin H, Trankner D, Ryba NJ, and Zuker CS (2015). Sweet and bitter taste in the brain of awake behaving animals. Nature 527, 512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD (2018). Lateral Hypothalamus as a Motivation-Cognition Interface in the Control of Feeding Behavior. Front Syst Neurosci 12, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Rigoli F, and Friston KJ (2018). Hierarchical Active Inference: A Theory of Motivated Control. Trends Cogn Sci 22, 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette CE, Baez-Santiago MA, Reid EE, Katz DB, and Moran A (2012). Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses. J Neurosci 32, 9981–9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, and Norgren R (1986). Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol 244, 213–228. [DOI] [PubMed] [Google Scholar]
- Pugnaghi M, Meletti S, Castana L, Francione S, Nobili L, Mai R, and Tassi L (2011). Features of somatosensory manifestations induced by intracranial electrical stimulations of the human insula. Clin Neurophysiol 122, 2049–2058. [DOI] [PubMed] [Google Scholar]
- Quadt L, Critchley HD, and Garfinkel SN (2018). The neurobiology of interoception in health and disease. Ann N Y Acad Sci 1428, 112–128. [DOI] [PubMed] [Google Scholar]
- Rao RP, and Ballard DH (1999). Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci 2, 79–87. [DOI] [PubMed] [Google Scholar]
- Rodgers KM, Benison AM, Klein A, and Barth DS (2008). Auditory, somatosensory, and multisensory insular cortex in the rat. Cereb Cortex 18, 2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, and Christianson JP (2019). An insular view of the social decision-making network. Neurosci Biobehav Rev 103, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, and Christianson JP (2018). Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci 21, 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2016). Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn 110, 4–19. [DOI] [PubMed] [Google Scholar]
- Sadacca BF, Mukherjee N, Vladusich T, Li JX, Katz DB, and Miller P (2016). The Behavioral Relevance of Cortical Neural Ensemble Responses Emerges Suddenly. J Neurosci 36, 655–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff DB, Zagha E, McCarthy E, and McCormick DA (2019). Movement and Performance Explain Widespread Cortical Activity in a Visual Detection Task. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman CD, and Fusi S (2010). Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci 33, 173–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Gardner MP, and Fontanini A (2012). Effects of cue-triggered expectation on cortical processing of taste. Neuron 74, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Gardner MP, and Fontanini A (2013). Thalamic contribution to cortical processing of taste and expectation. J Neurosci 33, 1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB (1982). Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol 210, 163–173. [DOI] [PubMed] [Google Scholar]
- Saper CB (2002). The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25, 433–469. [DOI] [PubMed] [Google Scholar]
- Saper CB, and Lowell BB (2014). The hypothalamus. Curr Biol 24, R1111–1116. [DOI] [PubMed] [Google Scholar]
- Schier LA, Hashimoto K, Bales MB, Blonde GD, and Spector AC (2014). High-resolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning. Proc Natl Acad Sci U S A 111, 1162–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff HC, Bouhuis AL, Yu K, Penzo MA, Li H, He M, and Li B (2018). An Insula-Central Amygdala Circuit for Guiding Tastant-Reinforced Choice Behavior. J Neurosci 38, 1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharvit G, Vuilleumier P, and Corradi-Dell’Acqua C (2019). Sensory-specific predictive models in the human anterior insula. F1000Res 8, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT (1982). Insular cortex projection to the nucleus of the solitary tract and brainstem visceromotor regions in the mouse. Brain Res Bull 8, 139–148. [DOI] [PubMed] [Google Scholar]
- Simon SA, de Araujo IE, Gutierrez R, and Nicolelis MA (2006). The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci 7, 890–901. [DOI] [PubMed] [Google Scholar]
- Small DM (2012). Flavor is in the brain. Physiol Behav 107, 540–552. [DOI] [PubMed] [Google Scholar]
- Somjen GG (1992). The Missing Error Signal—Regulation Beyond Negative Feedback. Physiology 7, 184–185. [Google Scholar]
- Stapleton JR, Lavine ML, Wolpert RL, Nicolelis MA, and Simon SA (2006). Rapid taste responses in the gustatory cortex during licking. J Neurosci 26, 4126–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engstrom Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, et al. (2016). AgRP Neurons Control Systemic Insulin Sensitivity via Myostatin Expression in Brown Adipose Tissue. Cell 165, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Aydin C, Lebedeva A, Okun M, Pachitariu M, Bauza M, Beau M, Bhagat J, Bohm C, Broux M, et al. (2021). Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern SA, Azevedo EP, Pomeranz LE, Doerig KR, Ivan VJ, and Friedman JM (2021). Top-down control of conditioned overconsumption is mediated by insular cortex Nos1 neurons. Cell Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern SA, Doerig KR, Azevedo EP, Stoffel E, and Friedman JM (2020). Control of non-homeostatic feeding in sated mice using associative learning of contextual food cues. Mol Psychiatry 25, 666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, and Eiselt AK (2017). Three Pillars for the Neural Control of Appetite. Annu Rev Physiol 79, 401–423. [DOI] [PubMed] [Google Scholar]
- Stone ME, Fontanini A, and Maffei A (2020). Synaptic Integration of Thalamic and Limbic Inputs in Rodent Gustatory Cortex. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker EM, and Hoffmann ML (2007). Presystemic signals in the control of thirst, salt appetite, and vasopressin secretion. Physiol Behav 91, 404–412. [DOI] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, and Harris KD (2019). Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, and Wise RA (2016). Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 19, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Alhadeff AL, and Betley JN (2017). Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Rep 21, 2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden AU, Zaremba JD, Sugden LA, McGuire KL, Lutas A, Ramesh RN, Alturkistani O, Lensjo KK, Burgess CR, and Andermann ML (2020). Cortical reactivations of recent sensory experiences predict bidirectional network changes during learning. Nat Neurosci 23, 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AK, and Krashes MJ (2020). Integrating Hunger with Rival Motivations. Trends Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Lo SK, Westerterp KR, Rush EC, Rosenbaum M, Luke A, Schoeller DA, DeLany JP, Butte NF, et al. (2009). Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr 89, 1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, and Knight ZA (2016). Warm-Sensitive Neurons that Control Body Temperature. Cell 167, 47–59 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HE, Sisti AC, Jin H, Vignovich M, Villavicencio M, Tsang KS, Goffer Y, and Zuker CS (2020). The gut-brain axis mediates sugar preference. Nature 580, 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, and Ravussin E (1999). Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A 96, 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanarajah SE, Backes H, DiFeliceantonio AG, Albus K, Cremer AL, Hanssen R, Lippert RN, Cornely OA, Small DM, Bruning JC, et al. (2019). Food Intake Recruits Orosensory and Post-ingestive Dopaminergic Circuits to Affect Eating Desire in Humans. Cell Metab 29, 695–706 e694. [DOI] [PubMed] [Google Scholar]
- Tye KM (2018). Neural Circuit Motifs in Valence Processing. Neuron 100, 436–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Vincis R, Chen K, Czarnecki L, Chen J, and Fontanini A (2020). Dynamic Representation of Taste-Related Decisions in the Gustatory Insular Cortex of Mice. Curr Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincis R, and Fontanini A (2016). Associative learning changes cross-modal representations in the gustatory cortex. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincis R, and Fontanini A (2019). Central taste anatomy and physiology. Handbook of clinical neurology 164, 187–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waluga M, Jonderko K, Domoslawska E, Matwiejszyn A, Dzielicki M, Krusiec-Swidergol B, and Kasicka-Jonderko A (2018). Effects of taste stimulation on gastric myoelectrical activity and autonomic balance. Saudi J Gastroenterol 24, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gillis-Smith S, Peng Y, Zhang J, Chen X, Salzman CD, Ryba NJP, and Zuker CS (2018). The coding of valence and identity in the mammalian taste system. Nature 558, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks D, Wright J, Rayment P, and Spiller R (2005). Impact of bitter taste on gastric motility. Eur J Gastroenterol Hepatol 17, 961–965. [DOI] [PubMed] [Google Scholar]
- Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, and Liberles SD (2016). Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 166, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Mendenhall CD, Henriques A, Barsalou LW, and Barrett LF (2019). Primary Interoceptive Cortex Activity during Simulated Experiences of the Body. J Cogn Neurosci 31, 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC (2009). The control of food intake: behavioral versus molecular perspectives. Cell Metab 9, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, and Ramsay DS (2007). Homeostasis: beyond Curt Richter. Appetite 49, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, and Palmiter RD (2012). Deciphering a neuronal circuit that mediates appetite. Nature 483, 594–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Chen C, Chen M, Qian K, Lv X, Wang H, Jiang L, Yu L, Zhuo M, and Qiu S (2020). The anterior insular cortex unilaterally controls feeding in response to aversive visceral stimuli in mice. Nat Commun 11, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, and Kawamura Y (1980). Localization of cortical gustatory area in rats and its role in taste discrimination. J Neurophysiol 44, 440–455. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Kiyomitsu Y, and Kitamura R (1988). Sensory inputs from the oral region to the cerebral cortex in behaving rats: an analysis of unit responses in cortical somatosensory and taste areas during ingestive behavior. J Neurophysiol 60, 1303–1321. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, and Ryba NJ (2009). Common sense about taste: from mammals to insects. Cell 139, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, and Cechetto DF (1991). Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303, 355–374. [DOI] [PubMed] [Google Scholar]
- Yiannakas A, and Rosenblum K (2017). The Insula and Taste Learning. Front Mol Neurosci 10, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatka-Haas P, Steinmetz NA, Carandini M, and Harris KD (2020). A perceptual decision requires sensory but not action coding in mouse cortex. bioRxiv, 501627. [Google Scholar]
- Zimmerman CA, and Knight ZA (2020). Layers of signals that regulate appetite. Curr Opin Neurobiol 64, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, Lin YC, Leib DE, Guo L, Huey EL, Daly GE, Chen Y, and Knight ZA (2016). Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 537, 680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]


