Abstract
Background:
Cognitive and behavioral sequelae of prenatal alcohol exposure (PAE) continue to be prevalent in the U.S. and worldwide. Because these sequelae are also common in other neurodevelopmental disorders, researchers have attempted to identify a distinct neurobehavioral profile to facilitate the differential diagnosis of fetal alcohol spectrum disorders (FASD). We used an innovative, individual participant meta-analytic technique to combine data from six large U.S. longitudinal cohorts to provide a more comprehensive and reliable characterization of the neurobehavioral deficits seen in FASD than can be obtained from smaller samples.
Methods:
Meta-analyses were performed on data from 2236 participants to examine effects of PAE (oz absolute alcohol/day (AA/day)) on IQ, four domains of cognition function (learning and memory, executive function, academic achievement), sustained attention, and behavior problems, after adjusting for potential confounders using propensity scores.
Results:
The effect sizes for IQ and the four domains of cognitive function were strikingly similar and did not differ at school age, adolescence, and young adulthood. Effect sizes were smaller in the more middle-class Seattle cohort and larger in the three cohorts that obtained more detailed and comprehensive assessments of AA/day. PAE effect sizes were somewhat weaker for parent- and teacher-reported behavior problems and not significant for sustained attention. A meta-analysis of five aspects of executive function showed the strongest effect on set-shifting.
Conclusions:
The similarity in the effect sizes for the four domains of cognitive function suggests that PAE affects an underlying component or components of cognition involving learning and memory and executive function that are reflected in IQ and academic achievement scores. The weaker effects in the more middle-class cohort may reflect a more cognitively stimulating environment, a different maternal drinking pattern (lower alcohol dose/occasion), and/or better maternal prenatal nutrition. These findings identify two domains of cognition—learning/memory and set-shifting—that are particularly affected by PAE, and one, sustained attention, which is apparently spared.
Keywords: meta-analysis, prenatal alcohol exposure, IQ, fetal alcohol spectrum disorders, cognitive development, behavior problems, multiple outcomes, causal analysis
Introduction
In the seminal paper that first identified fetal alcohol syndrome (FAS), Jones et al., (1973) reported a pattern of “craniofacial, limb, and cardiovascular defects associated with prenatal onset growth deficiency and developmental delay” in eight unrelated children born to mothers who were alcohol dependent. It was later determined that these children exhibited “deficient and often aberrant intellectual, motor, and behavioral performance” and that cognitive and behavioral impairment was also found in fetal alcohol-exposed children who lacked the growth restriction and craniofacial anomalies seen in FAS (Streissguth, 1976). In 1996, an Institute of Medicine panel introduced a classification system for fetal alcohol-related developmental disorders (Stratton et al., 1996), which was subsequently elaborated on in several diagnostic schemes (e.g., Astley, 2000; Hoyme et al., 2005, 2016; Cook et al., 2016) that used less medicalized and potentially stigmatizing terminology than was used when FAS was first identified in the early 1970’s. FAS, the most severe of the prenatal alcohol-related disorders, is characterized by a distinctive pattern of craniofacial dysmorphology (small palpebral fissures, flat philtrum, thin vermillion), pre- and/or postnatal growth restriction, and neurodevelopmental anomalies. Partial FAS (PFAS) is diagnosed in individuals with a confirmed history of prenatal alcohol exposure (PAE) who exhibit sentinel facial features and either growth restriction or neurodevelopmental impairment. Individuals with PAE who lack the characteristic FAS facial dysmorphology but exhibit neurobehavioral impairment are diagnosed with alcohol-related neurodevelopmental disorder (ARND).
Among the fetal alcohol spectrum disorders (FASD), ARND is the most prevalent (estimated in the US at 17.6–36.1/1000; May et al., 2020a, b) but also the most difficult to diagnose. The FAS and PFAS diagnoses are highly specific since the distinct pattern of facial features is rarely seen in other syndromes. By contrast, the neurobehavioral deficits that characterize FASD are frequently also seen in other learning and behavioral disorders. These deficits include lower IQ (Mattson et al. 1997; Streissguth et al., 1990) and poorer verbal learning and memory (e.g., Mattson et al. 1996; Lewis et al. 2015), executive function (Rasmussen 2005; Burden et al., 2005; McGee et al., 2008), arithmetic (Goldschmidt et al., 1996; Howell et al., 2006; J. Jacobson et al., 2011), language (Wyper and Rasmussen, 2011; Thorne, 2017), visuospatial skills (Kaemingk and Halverson, 2000; Coles et al., 1997), eyeblink conditioning (Coffin et al., 2005; S. Jacobson et al., 2008, 2011), and fine and gross motor function (Connor et al., 2006; Doney et al., 2014). Behavior problems are also often reported, particularly poor social skills and aggressive and delinquent behaviors (Carmichael Olson et al. 1998; Day et al. 2013; Dodge et al. 2014; Tsang et al., 2016), as are the inattention and hyperactivity/impulsivity problems that characterize attention deficit hyperactivity disorder (ADHD; Mick et al. 2002; J. Jacobson et al. 2011).
Because the cognitive and behavioral deficits seen in FASD are also common in other neurodevelopmental disorders, researchers have sought to identify a distinct neurobehavioral profile to facilitate the differential diagnosis of ARND. In two latent profile analyses comparing children with and without PAE, executive function and spatial processing were identified as important discriminating variables (Mattson et al., 2013, 2019). Studies comparing children with PAE to IQ-matched nonexposed controls found poorer learning and memory and higher levels of parent-rated behavior problems in the children with PAE (Vaurio et al., 2011) and poorer math skills (Crocker et al., 2015). Comparisons of children with PAE to non-exposed children with ADHD have noted greater problems in set-shifting, planning, verbal fluency, and working memory (Kingdon et al., 2016), numeric magnitude comparison (J. Jacobson et al., 2011), learning and memory (Crocker et al., 2011; Lewis et al., 2016), and social cognition (Greenbaum et al., 2009; Lindinger et al., 2016). Given the lack of consensus regarding the principal neurobehavioral sequelae of PAE, Mattson et al. (2019) suggested that future research should “consider using larger data sets in order to discern patterns not apparent in smaller studies.”
Much of the research on the neurodevelopmental sequelae of PAE has been conducted on cross-sectional samples, in which children and adolescents whose mothers are known to have used alcohol during pregnancy are compared with non-exposed controls (e.g., Mattson et al. 1997; Willoughby et al., 2008). Because the quantity of alcohol consumed during pregnancy is not known, these studies do not provide a reliable assessment of the effects of specific doses and patterns of exposure on developmental outcome. Between 1975 and 1994, the National Institutes of Health (NIH) funded six major U.S. cohort studies, in which mothers were interviewed about their alcohol use during pregnancy and their children were followed longitudinally through young adulthood. These studies, which were conducted independently, used different neuropsychological test batteries, but all assessed IQ and the same domains of neurobehavioral development, including learning and memory, executive function, academic achievement, sustained attention, and behavior problems.
The current study adapted cutting-edge individual participant meta-analytic methodology to integrate the data from the six longitudinal cohorts to examine effects of PAE on cognitive and behavioral development. To our knowledge, this prospective sample (N=2236) is the largest to examine the long-term effects of PAE on cognitive function and behavior. One distinct advantage of this meta-analysis was the availability of data regarding PAE provided by the mothers contemporaneously during pregnancy or immediately postpartum, which have been shown to be more reliable than retrospective reports obtained months or years later (Jacobson et al., 2002). Because the cognitive and behavioral outcomes and control variables were assessed in the six cohorts using different measures, it was not possible to integrate the variables into a single data set that could be examined using standard multivariate techniques. To address this issue we adopted a hierarchical meta-analytic approach developed by Ryan (2008), which was modified to accommodate the complexities of integrating these data (Akkaya-Hocagil et al., 2020). In traditional meta-analysis, effect size estimates from published studies are combined to provide an overall estimate of effect size. In the two-stage hierarchical meta-analytic approach developed by Akkaya-Hocagil et al., individual participant level data are used to estimate regression coefficients for the effect size of PAE on each of several outcomes after adjustment for confounders. At the second stage, the regression coefficients are pooled to obtain cohort-specific estimates of the PAE effect, which are then pooled across cohorts to obtain the final estimate.
Although IQ has been assessed in a large number of prospective longitudinal studies and in studies in which PAE was ascertained retrospectively, statistically significant effects of PAE on IQ have been reported in only three published papers (Streissguth et al., 1990; Larroque et al., 1995; Mattson et al., 1997). By contrast, there is extensive evidence documenting effects of PAE on specific domains of cognitive function, particularly learning and memory, arithmetic, and executive function. To investigate this inconsistency, we used Ryan’s (2008) hierarchical meta-analytic approach (1) to assess whether there is a consistent PAE-related effect on IQ and (2) to estimate this effect more precisely than can be done in smaller samples.
The term “executive function” refers to a set of inter-related higher-order cognitive processes (e.g., cognitive control, planning, response inhibition) that are involved in goal-directed behavior (Anderson, 2002). Cohen and collaborators (Botvinik et al., 2001; Miller and Cohen, 2001) distinguish between two specific aspects of cognitive control: “interference control,” defined as the ability to select a weaker task-relevant response in the face of an otherwise stronger but task-irrelevant one (as assessed in the Stroop Color-Word Test) and “conflict monitoring,” the ability to determine when task-relevant rules change. Whereas interference control tasks assess the ability to adhere to the goals and rules governing the current task, conflict monitoring (“set-shifting”) tasks assess the ability to alter one’s response when the goals and rules are altered during the course of the task. In neuroimaging studies, Cohen and his colleagues have demonstrated that interference control is mediated primarily by activity in the dorsolateral prefrontal cortex whereas cognitive monitoring is mediated primarily by activity in the anterior cingulate cortex.
Although FASD has frequently been linked to deficits in executive function (Kodituwakku et al., 2001; Mattson et al., 2019), it is not clear which aspects of executive function are particularly sensitive to PAE. In the only meta-analysis to compare effects of PAE on different facets of executive function, Kingdon et al. (2016) reported that deficits were most consistently found in set-shifting, verbal fluency, and planning; less so, in response inhibition. Among eight studies that examined multiple aspects of executive function, the seven that assessed set-shifting all found PAE-related deficits (Rasmussen and Bisanz, 2008; Connor et al., 2000; Mattson et al., 1999; Ware et al., 2012; Vaurio et al., 2008; Gautam et al, 2014; Glass et al., 2014). Similarly, all four studies that examined verbal fluency found deficits (Rasmussen and Bisanz, 2008; Ware et al., 2012; Vaurio et al., 2008: Glass et al., 2014). Among the four that assessed planning, two found significant deficits, one did not (Glass et al., 2014), and one found an effect that fell just short of significance (Mattson et al, 1999).
The current study used an innovative, individual participant hierarchical meta-analytic technique to combine data from the six longitudinal cohorts to provide a more comprehensive and reliable characterization of the neurobehavioral deficits seen in FASD than can be obtained from smaller samples. The aims were to: (1) derive robust and efficient estimates of the effects of PAE on overall cognitive function and on function within four cognitive domains (learning and memory, executive function, reading, and mathematics) at three developmental stages (school age, adolescence, and young adulthood); (2) derive estimates of the effects of PAE on visuospatial processing, attention, and behavior problems; (3) compare the PAE effect on a meta-analytically-derived composite measure of IQ with effects on IQ tests administered in single studies; and (4) compare the effects of PAE on five aspects of executive function: interference control, set-shifting, working memory, verbal fluency, and planning.
Methods
Participant Recruitment
Participants were children from six prospective, longitudinal cohorts recruited in four U.S. cities: Detroit, Seattle, and two cohorts in Pittsburgh and in Atlanta. Table 1 shows the years when each cohort was recruited and summarizes the alcohol and drug use ascertainment protocols, recruitment criteria, racial composition, and ages at which the participants were assessed. In five of the cohorts, women were recruited and interviewed about their alcohol and drug use during pregnancy; in one, shortly after delivery. Four of the cohorts were recruited to over-represent moderate-to-heavy alcohol consumption during pregnancy; the two others oversampled for cocaine use but were included because a large proportion of the mothers drank moderate-to-heavy levels of alcohol. All six cohort studies obtained quantitative measures of alcohol consumption during pregnancy that were converted to three common metrics: average oz absolute alcohol (AA)/day (0.6 oz AA ≈ 1 standard drink; National Institute on Alcohol Abuse and Alcoholism (2010)), number of standard drinks/occasion, and frequency of drinking (days/month). Each oz of AA is equivalent to ≈ 1.67 standard drinks. Despite differences in the alcohol ascertainment protocols and recruitment criteria, average oz AA/day consumed during pregnancy was very similar for five of the cohorts and somewhat higher in Atlanta-1 (Table 2). Assessments for FAS were performed in infancy in the Seattle study and at 7.5 years in Detroit, and dysmorphology was assessed in all six cohorts. However, because these studies were completed before contemporary systems for FASD diagnosis were developed, FASD classification was not assessed in any of these cohorts.
Table 1.
Recruitment criteria and participant race and ages at the follow-up assessments
| Detroita (N = 377) | Pittsburgh-1b (N = 720) | Pittsburgh-2c (N = 268) | Atlanta-1d (N = 223) | Atlanta-2e (N = 138) | Seattlef (N = 510) | |
|---|---|---|---|---|---|---|
| Recruitment years | 1986–1989 | 1983–1986 | 1988–1993 | 1980–1986 | 1992–1994 | 1975–1976 |
| Alcohol and drug use ascertainment protocol | TLFB interviews at each antenatal visit relating to day-by-day alcohol consumption during the previous 2 wk. and frequency of illicit drug use; and at the 1st visit, relating to day-by-day drinking around time of conception. | Q-F-V interviews at the 4th or 5th mo. of gestation relating to early pregnancy and current alcohol, tobacco, and drug use; at the 7th mo, relating to the 2nd trimester; and at delivery, relating to the 3rd trimester. | Q-F-V interviews at the 4th or 5th mo. of gestation relating to early pregnancy and current alcohol, tobacco, and drug use; at the 7th mo, relating to the 2nd trimester; and at delivery, relating to the 3rd trimester. | Interview at 1st antenatal visit relating to frequency of alcohol consumption and drug use and number of drinks/occasion during pregnancy. | Interview shortly after delivery relating to frequency of alcohol consumption and drug use and number of drinks/occasion during pregnancy. | Q-F-V interview at the 4th or 5th mo. of gestation relating to alcohol, tobacco, and drug use during pregnancy. |
| Criterion for over-sampling | All women reporting ≥ 0.5 oz AA/day at conception | >3 standard drinks/week during the 1st trimesterg | Any use of crack/cocaine during the 1st trimester | All women reporting ≥ 1.0 oz AA/week during pregnancy | All women reporting alcohol or drug use during pregnancy | Based on an algorithm derived from maternal AA/day, alcohol use/occasion, volume variability, and frequency of intoxication |
| Criterion for enrollment as lightly or non-exposed | Random sample of those reporting ≥ 0.5 oz AA/day at conception (including abstainers)h | Random sample of light drinkers and abstainers | Random sample of non-users of crack/cocaine | Random sample of non-drinkers | Random sample of those who did not use alcohol or cocaine during pregnancy | Random sample of light drinkers and abstainers |
| Race | 100% African American | 47.5% Caucasian 52.5% African American |
52.5% Caucasian 47.5% African American |
4.0% Caucasian 96.0% African American |
3.7% Caucasian 96.3% African American |
86.3% Caucasian 7.3% African American 6.4% Other |
| Cohort n and (age at assessment in years)i | ||||||
| School age | 336 (7.7 ± 0.02) | 648 (6.5 ± 0.02) | 241 (7.4 ± 0.03) | 148 (7.6 ± 0.05) | 137 (8.2 ± 0.03) | 482 (7.4 ± 0.01) |
| 611 (10.5 ± 0.02) | 209 (10.7 ± 0.04) | |||||
| Adolescence | 291 (14.4 ± 0.04) | 528 (14.8 ± 0.02) | 184 (15.6 ± 0.05) | 190 (15.1 ± 0.05) | 464 (14.4 ± 0.01) | |
| Adulthood | 127 (19.4 ± 0.04) | 522 (22.8 ± 0.03) | 193 (21.3 ± 0.05) | 182 (23.2 ± 0.1) | 419 (21.7 ± 0.02) | |
N’s indicate number of cases in each cohort included in the data analysis. AA: Absolute alcohol. Q-F-V: Quantity-frequency-variability (Cahalan, et al., 1969). TLFB: Timeline follow-back (Jacobson et al., 2002).
Women who used marijuana ≥ twice/month in the first trimester were also over-represented in this sample.
To reduce the risk that alcohol might be confounded with cocaine exposure, 64 heavy cocaine (≥2days/wk), light alcohol (<7 drinks/wk) users were also included.
Values are cohort n (mean age ± standard error).
Table 2.
Sample characteristics (N = 2236)
| Detroit (N = 377) | Pittsburgh-1 (N = 720) | Pittsburgh-2 (N = 268) | Atlanta-1 (N = 223) | Atlanta-2 (N = 138) | Seattle (N = 510) | F or χ2 | |
|---|---|---|---|---|---|---|---|
| Demographic background | |||||||
| Maternal education (yr) at enrollmenta | 11.7 (1.6) | 11.8 (1.4) | 11.9 (1.3) | 11.2 (1.8) | 11.3 (1.4) | 13.6 (2.5) | 97.85 |
| Mothers ≥ 30 yr of age at deliveryb | 108 (28.8%) | 59 (8.3%) | 52 (19.9%) | 41 (17.1%) | 52 (38.0%) | 117 (22.6%) | 112.94 |
| Family SESc,d (infancy) | 20.2 (8.7) | 19.4 (7.6) | 21.7 (8.8) | --e | 20.6 (7.0) | 39.4 (15.9) | 310.77 |
| Annual family income ($)f,g | 35,348.3 (28413.7) | 31,299.6 (21873.2) | 33,828.9 (26323.0) | 10,450.0 (12033.5) | 20,786.4 (15603.1) | 81,966.7 (41032.1) | 258.39 |
| Biological mother child’s primary caregiverh | 238 (63.1%) | 655 (90.9%) | 214 (79.9%) | 172 (77.1%) | 98 (71.0%) | 479 (93.9%) | 202.46 |
| Foster carei | 0 (0.0%) | 7 (1.0%) | 7 (2.6%) | 4 (1.8%) | 4 (2.9%) | 3 (0.6%) | 15.98 |
| Prenatal exposures | |||||||
| Alcohol | |||||||
| AA/day (oz)j | 0.2 (0.6) | 0.2 (0.4) | 0.3 (0.6) | 1.0 (1.5) | 0.4 (1.2) | 0.3 (0.5) | 46.47 |
| Drinks/occasionk | 2.9 (3.4) | 2.5 (3.4) | 2.9 (3.9) | 3.6 (4.1) | 1.5 (3.8) | 1.8 (1.6) | 15.83 |
| Frequency (days/month)l | 3.4 (4.4) | 2.3 (3.5) | 3.2 (4.4) | 9.2 (10.0) | 4.9 (9.0) | 5.9 (8.1) | 53.90 |
| Smoking (cigarettes/day) m | 9.6 (11.5) | 8.5 (11.3) | 7.5 (8.9) | 8.0 (9.5) | 5.8 (7.7) | 6.2 (10.5) | 6.42 |
| Marijuana (days/month)n | 1.0 (2.5) | 2.9 (5.1) | 2.0 (4.2) | 1.4 (3.8) | 1.0 (3.6) | 1.2 (4.5) | 15.31 |
| Cocaine (days/month)o | 1.1 (3.0) | 0.1 (0.8) | 1.3 (2.9) | --e | 6.2 (10.3) | --e | 100.53 |
| History of alcohol abuse And/or dependencep | 79 (22.3%) | 133 (19.9%) | 62 (23.9%) | 57 (27.5%) | 21 (15.3%) | 23 (5.2%) | 73.46 |
| Child characteristics | |||||||
| Birth weight (g)q | 3096.8 (552.6) | 3202.2 (562.5) | 3264.7 (603.6) | 3027.2 (634.7) | 2532.5 (725.7) | 3499.4 (518.9) | 72.02 |
| Gestational age (weeks)r | 39.3 (1.7) | 39.8 (2.2) | 39.7 (2.1) | 39.5 (2.0) | 37.3 (3.3) | 39.9 (1.6) | 40.34 |
| Sex (male)s | 221 (58.9%) | 358 (50.3%) | 138 (52.9%) | 108 (43.7%) | 80 (58.4%) | 277 (53.5%) | 17.38 |
| Participant IQ t | |||||||
| School ageu | 84.2 (12.3) | 91.6 (14.1) | 91.1 (15.1) | 87.8 (12.8) | 80.5 (13.1) | 107.6 (14.5) | 47.21 |
| Adolescentv | 79.0 (13.0) | 88.6 (14.5) | --e | 76.4 (14.1) | --e | --e | 151.53 |
| Young adulthoodw | --e | --e | 94.8 (12.8) | 82.6 (13.5) | --e | 101.9 (12.5) | 261.03 |
Values are mean (standard deviation) or number (%). All F and χ2 tests are significant at p<0.001. Post hoc tests are given in the footnotes below. Biological mother child’s primary caregiver throughout infancy and childhood, foster care at any time during infancy and childhood, and history of alcohol abuse and/or dependence were coded No=0, Yes=1.
Seattle > all other cohorts (p<0.001); Detroit, Pittsburgh-1, Pittsburgh-2 > Atlanta-1, Atlanta-2 (p<0.05)
Atlanta-2, Detroit > all other cohorts (p<0.05); Seattle, Atlanta-1, Pittsburgh-2 > Pittsburgh-1 (p<0.05)
Socioeconomic status measured on Hollingshead (2011).
Seattle > all cohorts (p<0.001); Pittsburgh-2 > Pittsburgh-1 (p<0.05)
Not available for this cohort.
Reported by all mothers at adolescent follow-up and normalized to 2012 dollars based on the consumer price index (http://www.bls.gov/cpi).
Seattle > all cohorts (p<0.001); Detroit, Pittsburgh-1, Pittsburgh-2 > Atlanta-1, Atlanta-2 (p<0.01)
Seattle, Pittsburgh-1 > all other cohorts (p<0.001); Atlanta-1, Pittsburgh-2 > Detroit, Atlanta-2 (p<0.01); Atlanta-2 > Detroit (p<0.05)
Atlanta-2, Pittsburgh-2 > Pittsburgh-1, Seattle, Detroit (p<0.05); Atlanta-1 > Seattle, Detroit (p<0.05)
Atlanta-1 > all other cohorts (p<0.001); Atlanta-2 > Pittsburgh-1 (p<0.05)
Atlanta-1 > Pittsburgh-1, Seattle, Atlanta-2 (p<0.001); Pittsburgh-2, Detroit > Seattle, Atlanta-2 (p<0.05)
Atlanta-1 > all other cohorts (p<0.001); Seattle > Detroit, Pittsburgh-1, Pittsburgh-2 (p<0.001); Atlanta-2 > Pittsburgh-1 (p<0.01)
Detroit, Pittsburgh-1 > Seattle, Atlanta-2 (p<0.05)
Pittsburgh-1 > all other cohorts (p<0.01)
Atlanta-2 > all other cohorts (p<0.001); Detroit, Pittsburgh-2 > Pittsburgh-1 (p<0.001)
Seattle < all other cohorts (p<0.05)
Seattle > all other cohorts (p<0.001); Pittsburgh-1, Pittsburgh-2 > Detroit, Atlanta-1, Atlanta-2 (p<0.05); Detroit, Atlanta-1 > Atlanta-2 (p<0.001)
Atlanta-2 < all other cohorts (p<0.001); Detroit, Atlanta-1 < Seattle, Pittsburgh-1 (p<0.05)
Detroit, Seattle, Atlanta-2, Pittsburgh-2 > Atlanta-1 (p<0.05); Detroit, Atlanta-2 > Pittsburgh-1 (p<0.05)
IQ scores shown here are prior to transformation for the meta-analysis.
Seattle > all other cohort (p<0.001); Pittsburgh-1, Pittsburgh-2 > Detroit, Atlanta-2 (p<0.05); Atlanta-1 > Atlanta-2 (p<0.05)
Pittsburgh-1 > Detroit, Atlanta-1 (p<0.01)
Seattle > Atlanta-1, Pittsburgh-2 (p<0.01); Pittsburgh-2 > Atlanta-1 (p<0.05)
Cognitive and Behavioral Assessments
All the cohorts were followed up using laboratory-based assessments at school age, and five were also assessed during adolescence and young adulthood. All developmental outcome data were collected by research staff blind to prenatal exposure history.
IQ and four domains of cognitive function were assessed (see Supplementary Table 1). IQ and academic achievement were assessed using standardized tests. Reading (including spelling) and math achievement were examined separately. The learning and memory tests involved instructing the participant to encode verbal auditory or visual stimuli and to recall the information immediately and after a delay. Five aspects of executive function were assessed: (1) interference control, using the Stroop (1935) Color-Word paradigm, in which the participant is shown a series of color names printed in the wrong color font (e.g., the word “red” printed in blue) and is instructed to name the color, requiring inhibition of the propensity to read the word; (2) set-shifting, using tests in which categorization rules change during the task: the Wisconsin Card Sorting Test, the “switching” conditions in the D-KEFS (Delis et al., 2001) Color-Word Interference and Verbal Fluency Tests, the Trail Making Test (Reitan and Wolfson, 1993), and the D-KEFS Sorting Test; (3) verbal fluency, using tasks in which the participant lists as many items as possible in a given category (e.g., “name all the animals you know” or “all the words beginning with the letter ‘F’”); (4) planning, using variants of the Tower of London paradigm (Shallice, 1982); and (5) working memory, using the Wechsler Digit Span Backwards test. The only aspect of executive function not assessed in these studies was response inhibition. Limited information was available on visuospatial processing, which was assessed in four cohorts on a total of only 13 tasks, compared with cognitive and behavioral function, which were assessed extensively in all six cohorts, using 69 tasks and 45 rating scales, respectively.
Attention was assessed in all six cohorts using a continuous performance test paradigm. In this paradigm, which focuses on sustained attention, the child sees or hears a series of stimuli (usually letters or numbers) and is instructed to press a button each time a designated stimulus (e.g., the letter “X”) is presented, usually at relatively infrequent random intervals.
Behavior problems were assessed on the Achenbach parent-reported Child Behavior Checklist and/or Teacher Report Form in all six cohorts. Each of these instruments lists 113 descriptors of behavior problems (e.g., “gets into many fights,” “secretive, keeps things to self”), and the respondent is asked to indicate whether each statement is “not true, somewhat or sometimes true, or very or often true of the child.” The Achenbach items are then grouped into eight summary behavior problem scales, five of which comprise two summary categories: internalizing (anxious/depressed, withdrawn, somatic complaints) and externalizing (aggressive behavior, rule-breaking behavior). We analyzed the two summary categories and two behavior problem scales: attention problems and social problems. Thought problems were not included because they were reported too rarely. Parents and/or teachers in five of the cohorts also completed attention/hyperactivity disorder (ADHD) checklists, which were used to determine number of inattention and hyperactivity/impulsivity symptoms (from among those listed in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM)).
Control Variables
A broad range of control variables was assessed in each cohort (see Supplementary Table 2), including socioeconomic status (SES), maternal years of education, marital status, and family income; maternal smoking (cigarettes/day) and marijuana, cocaine, and other illicit drug use (days/month) during pregnancy; and child’s sex, race, and whether s/he was raised by his/her biological mother and/or was in foster care. In the three cohorts recruited during the late 1980’s and early 1990’s, when there was an epidemic of cocaine use in inner cities in the U.S. (e.g., Jacobson et al., 1996; Richardson et al., 1999), urine samples obtained from the mothers during pregnancy were analyzed for the presence of metabolites of cocaine, marijuana, and opiates. Maternal history of alcohol abuse or dependence was assessed as detailed in Supplementary Information.
Data Analysis
AA/day was highly skewed (skewness >3.0) and, therefore, subjected to log(X+1) transformation. Outcome measures for which the distribution of residuals was skewed were also subjected to log(X+1) transformation to ensure that the residuals were approximately symmetrically distributed. Each outcome measure was then rescaled to a mean of 100 (standard deviation=15) so the estimated effect sizes would be expressed in a common metric. Outcomes in which a low score is optimal (e.g., number of errors or reaction time) were multiplied by −1.0, so that a high score represented more optimal performance on all the measures. Outcome variables were excluded if their distributions were multimodal or showed strong ceiling or floor effects.
A two-stage meta-analytic approach was used to examine effects of PAE on cognitive function, visuospatial processing, attention, and behavior problems (Aims 1 and 2 described in the Introduction). PAE effects on four cognitive domains (learning and memory, executive function, reading achievement, math achievement) and five behavioral domains (internalizing, externalizing, attention problems, social problems, and ADHD symptoms) were examined. Three age periods were examined: school age (6–11 yr), adolescence (14–16 yr), and young adulthood (19–23 yr).
At the first stage of the analysis, regression coefficients were derived for each of the outcome measures from multiple regression analyses, in which the outcome was regressed on PAE (measured as log AA/day) and a propensity score (Imai and van Dyk, 2004). The propensity score approach makes it possible to adjust for a comprehensive set of potential confounders in the model without sacrificing precision due to inclusion of an excessive number of covariates. Propensity scores were derived separately for each cohort by regressing AA/day on the control variables listed in Supplementary Table 2 as detailed in Akkaya-Hocagil et al. (2021). We used multiple imputation by chained regression (MICE; van Buuren and Groothuis-Oudshoorn, 2011) to accommodate incomplete data on potential confounders and adapted it to adjust for two different kinds of incomplete data: failure to obtain a maternal report (where the data were presumably missing at random) and failure to obtain an accurate report on drug use due to maternal denial that was contradicted by a positive urine screen (see Supplementary Information).
At the second stage of the analysis, the regression coefficient estimates were synthesized in a meta-analysis procedure that we developed (Akkaya-Hocagil et al., 2020). Estimates synthesized in conventional meta-analyses are independent in that they come from different studies. Here, however, the regression coefficients for the different outcomes were not independent because each child provided multiple responses. We, therefore, used robust estimates of the pairwise covariance of estimators across outcomes to synthesize the Stage 1 estimates within cohorts, an approach that enables computation of robust standard errors when outcomes are correlated. We initially estimated pooled effect sizes for overall cognitive function and each domain and age period within each of the cohorts. The data were then pooled across cohorts to provide (1) a global estimate of the effect of PAE and (2) PAE effect sizes for each domain and each age period. Standardized regression coefficients were calculated by multiplying the raw regression coefficient by the ratio of the standard deviation of the outcome to the standard deviation of exposure, which was approximate because it was calculated by combining the exposure data from all cohorts. Omnibus χ2 tests were used to determine whether there were significant differences in estimated PAE effect size by cohort, domain, or age period. The PAE estimates and their associated 95% confidence intervals were displayed in forest plots for graphical analysis.
The two-stage meta-analytic models were subsequently re-run using robust regression, which adjusts for influential points by down-weighting data points with large residuals to reduce their influence. In addition, the models for cognitive and behavioral function were re-run in a series of sensitivity analyses, omitting outcome measures with different degrees of positive or negative skewness (e.g., skewness >0.5, 1.0, and 1.5).
We also examined regression coefficients estimating effects of PAE on each of the 14 IQ assessments obtained from the six cohorts, adjusted for propensity scores (Aim 3 described in the Introduction). These regression coefficients were compared with the effect of PAE derived by pooling IQ across the 14 assessments using the two-stage meta-analytic approach. A two-stage meta-analysis was also used to examine the effects of PAE on the five aspects of executive function (Aim 4).
Results
Demographic background, exposure levels, and IQ scores for each of the cohorts are summarized in Table 2. Cohort sample sizes ranged from 138 to 720 (median=318). Participant retention was good-to-excellent from childhood to adolescence (median=90.3%; range=86.4%−96.3%). Retention from adolescence to young adulthood was excellent (≥91.5%) in the Atlanta-1, Seattle, and two Pittsburgh cohorts. The Detroit young adult follow-up, which focused on neuroimaging, was funded to assess only a sub-sample (43.6%) of the cohort.
SES and maternal education were higher in Seattle than in the other cohorts. The Seattle cohort was predominantly middle class; 67.4% had Hollingshead scores in Levels I-III (business, professional, skilled craftsmen, clerical and sales workers), compared with only 10.7–20.8% in the other cohorts (median=14.4%). By contrast, large portions of the other cohorts (median=59.6%; range=52.6–72.9%) were economically disadvantaged, with Hollingshead scores in Level V (unskilled laborers and menial service workers), compared with only 14.5% in Seattle. Family income was higher in Seattle than the other cohorts and higher in the Detroit and Pittsburgh than the Atlanta cohorts.
Alcohol use was particularly high in Atlanta with average maternal alcohol consumption during pregnancy (oz AA/day) higher in Atlanta-1 than the other cohorts and higher in Atlanta-2 than Pittsburgh-1. Alcohol dose/occasion (average oz AA/drinking day) was lower in the more middle-class Seattle cohort than in Atlanta-1, Detroit, and both Pittsburgh cohorts. Frequency of drinking was higher in Atlanta-1 than in the other cohorts and higher in Seattle than in Detroit and the two Pittsburgh cohorts. Birthweight was higher in Seattle than in the other cohorts and higher in the two Pittsburgh cohorts than in both Atlanta cohorts and Detroit. School age IQ scores were higher in Seattle than in the other cohorts and higher in the two Pittsburgh cohorts than in Detroit and Atlanta-2.
Cognitive Function
Table 3 shows the PAE effect size estimates derived by integrating data from the 134 regression coefficients relating AA/day (measured on the natural log scale) to cognitive function, after adjustment for propensity scores. The overall effect size on cognitive function was b=−4.5 s.e.=0.6.1 The estimate provided by robust regression analysis was virtually identical, indicating that the linear regression estimate was not unduly influenced by extreme observations. Sensitivity analyses (Supplementary Table 3) showed that the effects of PAE on overall cognitive function were virtually identical in analyses omitting outcome measures with skewness >0.5, 1.0, and 1.5.
Table 3.
Meta-analysis: Effect of prenatal alcohol exposure on cognitive function
| Number of outcome measures | Linear regression |
Robust regression |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| b | s.e. | β | P | b | s.e. | β | P | ||
| Overall cognitive function | 134 | −4.5 | 0.6 | −0.10 | <0.0001 | −4.2 | 0.7 | −0.09 | <0.0001 |
| Cohort | |||||||||
| Detroit | 24 | −5.6 | 1.1 | −0.10 | <0.0001 | −5.2 | 1.1 | −0.09 | <0.0001 |
| Pittsburgh-1 | 33 | −5.3 | 0.8 | −0.07 | <0.0001 | −5.5 | 0.8 | −0.07 | <0.0001 |
| Pittsburgh-2 | 25 | −6.3 | 1.1 | −0.13 | <0.0001 | −5.4 | 1.0 | −0.11 | <0.0001 |
| Atlanta-1 | 20 | −3.3 | 1.0 | −0.12 | 0.004 | −3.2 | 1.0 | −0.11 | 0.005 |
| Atlanta-2 | 9 | −4.4 | 1.5 | −0.12 | 0.019 | −4.3 | 1.6 | −0.11 | 0.027 |
| Seattle | 23 | −2.5 | 0.8 | −0.05 | 0.004 | −1.8 | 0.8 | −0.03 | 0.024 |
| Domain | |||||||||
| Learning and memory | 40 | −4.7 | 0.7 | −0.10 | <0.0001 | −4.5 | 0.9 | −0.10 | <0.0001 |
| Executive function | 49 | −4.3 | 1.0 | −0.09 | <0.0001 | −3.7 | 0.7 | −0.08 | <0.0001 |
| Achievement—reading | 25 | −3.8 | 1.1 | −0.08 | <0.0001 | −3.4 | 1.2 | −0.07 | 0.005 |
| Achievement—math | 20 | −5.2 | 1.1 | −0.11 | <0.0001 | −5.1 | 1.1 | −0.11 | <0.0001 |
| Age | |||||||||
| Childhood | 55 | −4.4 | 0.7 | −0.09 | <0.0001 | −4.2 | 0.8 | −0.09 | <0.0001 |
| Adolescence | 47 | −5.3 | 0.8 | −0.11 | <0.0001 | −5.3 | 0.8 | −0.11 | <0.0001 |
| Young adulthood | 32 | −3.1 | 0.8 | −0.07 | <0.0001 | −2.4 | 0.9 | −0.05 | 0.004 |
b (s.e.) is the raw regression coefficient (standard error) and β is the standardized regression coefficient for absolute alcohol per day during pregnancy, measured on the natural log scale, adjusted for potential confounders using propensity scores.
An omnibus test of homogeneity revealed significant differences in the cognitive effect size estimates among the six cohorts (χ2=12.67, p=0.027), but none of the pairwise between-cohort comparisons were significant based on the Tukey-Kramer test (Dubitzky et al., 2013). Nevertheless, the significant omnibus test suggests that other contrasts warrant consideration (Simon, 2009). Our prior knowledge about these cohorts suggested two exploratory contrasts that might explain this heterogeneity. The effect size for the predominantly middle-class Seattle cohort was smaller than the pooled effect size from the other, more economically disadvantaged cohorts (χ2=7.59, p=0.006). In addition, the pooled estimate for Detroit and the two Pittsburgh cohorts, in which maternal alcohol consumption was assessed on multiple occasions during pregnancy, was larger than the pooled estimate for Seattle and the two Atlanta cohorts, in which alcohol consumption was only assessed in a single maternal interview (χ2=10.80, p<0.001). These exploratory findings should be interpreted with caution, however, since they are not adjusted for multiple comparisons.
The effect sizes across the four domains of cognitive function (Table 3) were remarkably similar (χ2=1.07, p=0.785), suggesting that PAE affects an underlying component or components of cognitive function that influence multiple domains. Moreover, the assessments of cognitive function appear to be equally sensitive to PAE throughout the period from school age through young adulthood (χ2=4.16, p=0.125).
By contrast to the extensive data on learning and memory, executive function, and academic achievement obtained from these cohorts, limited data were available on visuospatial processing. Although the pooled estimate of effect of PAE on the visuospatial processing measures was not significant, (b=−1.8, s.e.=2.1, p=0.339), significant effects were seen during adolescence (b=−3.9, s.e.=1.6, p=0.012).
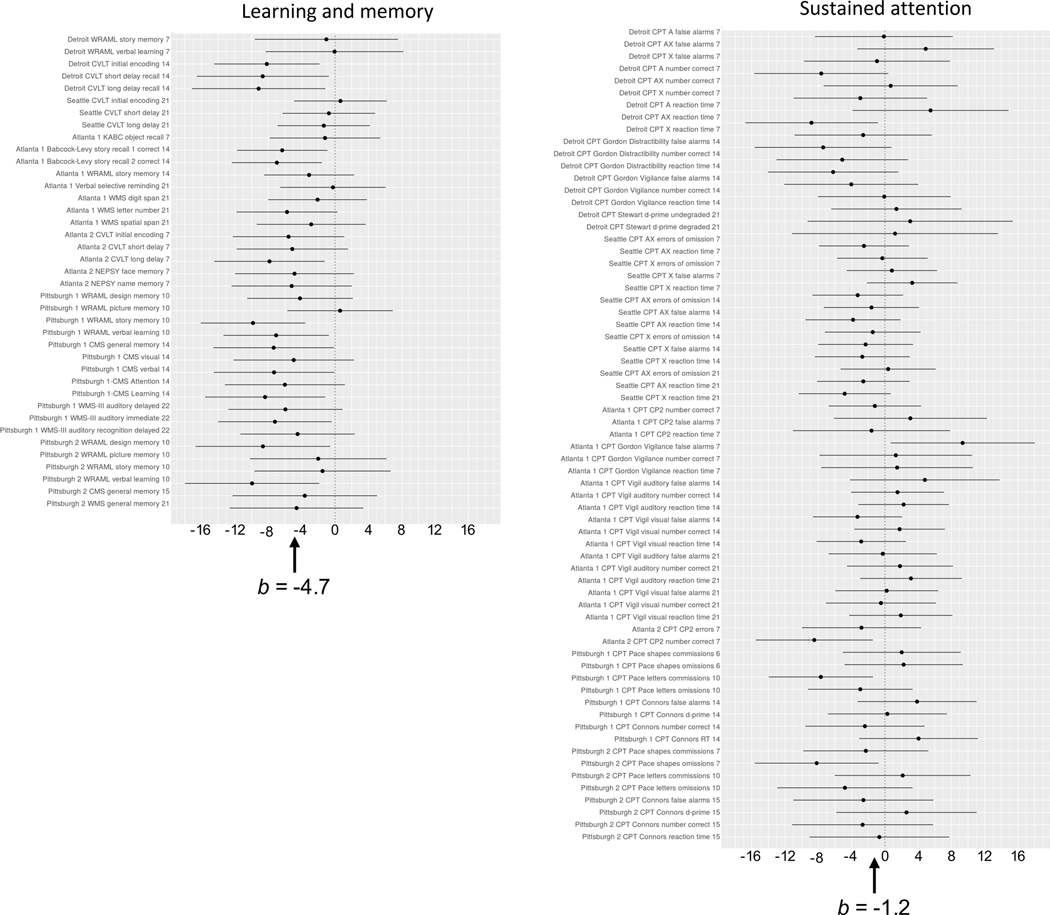
Attention
All six cohorts used continuous performance tests to assess sustained attention. When the sustained attention measures were examined in a two-stage hierarchical meta-analysis, neither the overall nor the individual cohort PAE effects were significant (Table 4). Figure 1 compares the forest plot for learning and memory with the plot for sustained attention. In the learning and memory plot, the effect sizes vary considerably, but higher PAE is inversely related to virtually all of the individual outcome measures. By contrast, visual inspection of the sustained attention forest plot shows that in the Seattle cohort, AA/day had a fairly consistent (albeit very weak) inverse association with sustained attention, and the data from the other cohorts showed no consistent effects within this domain.
Table 4.
Meta-analysis: Effect of prenatal alcohol exposure on sustained attention
| Number of outcome measures | Linear regression |
Robust regression |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| b | s.e. | β | P | b | s.e. | β | P | ||
| Overall sustained attention | 67 | −1.2 | 0.7 | −0.03 | 0.070 | −1.2 | 0.7 | −0.03 | 0.077 |
| Cohort | |||||||||
| Detroit | 17 | −2.2 | 1.2 | −0.04 | 0.090 | −2.5 | 1.1 | −0.04 | 0.037 |
| Pittsburgh-1 | 8 | −0.3 | 1.6 | −0.01 | 0.850 | −1.1 | 1.0 | −0.01 | 0.315 |
| Pittsburgh-2 | 8 | −2.3 | 1.3 | −0.05 | 0.123 | −2.4 | 1.2 | −0.05 | 0.092 |
| Atlanta-1 | 18 | 0.9 | 0.9 | 0.03 | 0.322 | 1.0 | 0.8 | 0.04 | 0.203 |
| Atlanta-2 | 2 | −5.7 | 3.0 | −0.15 | 0.308 | −5.7 | 2.9 | −0.15 | 0.297 |
| Seattle | 14 | −1.6 | 0.8 | −0.03 | 0.086 | −0.7 | 0.5 | −0.01 | 0.205 |
| Age | |||||||||
| Childhood | 30 | −1.2 | 1.0 | −0.03 | 0.242 | −1.4 | 0.9 | −0.03 | 0.113 |
| Adolescence | 26 | −1.0 | 0.7 | −0.02 | 0.145 | −0.5 | 0.5 | −0.01 | 0.363 |
| Young adulthood | 11 | −0.6 | 1.7 | −0.01 | 0.744 | −0.3 | 1.2 | −0.01 | 0.803 |
b (s.e.) is the raw regression coefficient (standard error) and β is the standardized regression coefficient for absolute alcohol per day during pregnancy, measured on the natural log scale, adjusted for potential confounders using propensity scores
Fig. 1.
Forest plots of effects of prenatal alcohol exposure (oz AA/day) on learning and memory (left column) and sustained attention (right column). Regression coefficients (indicating effect sizes after adjustment for potential confounders) are shown on the x-axis. Numbers in the outcome variable names on the y-axis indicate age group at time of testing (7 = school age; 14 = adolescence; 21 = young adulthood). Overall effect sizes (b) are shown at the bottom of the figures. WRAML = Wide Range Assessment of Memory and Learning. CVLT = California Verbal Learning Test. KABC = Kaufman Assessment Battery for Children. WMS = Wechsler Memory Scale. NEPSY = A Developmental NEuroPSYchological Assessment. CMS = Children’s Memory Scale. CPT = continuous performance test. RT = reaction time. X = press the button whenever the letter X appears on the screen; AX = press to the X only if is preceded by the letter A.
Behavior Problems
PAE effect size was significant for overall parent- and teacher-reported behavior problems and three of the five domains (Table 5) and generally weaker than for cognitive function. By contrast to cognitive function, the estimates of the effects on behavior problems did not differ between the cohorts (χ2=2.53, p=0.771). Nor did they differ between the five behavioral domains (χ2=4.80, p=0.308) or between childhood and adolescence, the two age periods when behavior problems were assessed (χ2=0.04, p=0.851). The robust regression and sensitivity (Supplementary Table 4) analyses indicate that the estimates of the PAE effects on behavior problems were not unduly influenced by extreme observations or skewness.
Table 5.
Meta-analysis: Effect of prenatal alcohol exposure on behavior problems
| Number of outcome measures | Linear regression |
Robust regression |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| b | s.e. | β | P | b | s.e. | β | P | ||
| Overall behavior problems | 108 | −2.6 | 0.5 | −0.06 | <0.0001 | −2.6 | 0.5 | −0.06 | <0.0001 |
| Cohort | |||||||||
| Detroit | 20 | −4.5 | 1.5 | −0.08 | 0.009 | −4.3 | 1.5 | −0.07 | 0.008 |
| Pittsburgh-1 | 32 | −1.6 | 1.1 | −0.02 | 0.145 | −2.0 | 0.9 | −0.03 | 0.034 |
| Pittsburgh-2 | 28 | −2.7 | 1.3 | −0.05 | 0.056 | −2.6 | 1.2 | −0.05 | 0.041 |
| Atlanta-1 | 14 | −2.7 | 1.6 | −0.10 | 0.116 | −2.4 | 1.5 | −0.08 | 0.147 |
| Atlanta-2 | 8 | −2.4 | 1.4 | −0.06 | 0.114 | −2.4 | 1.2 | −0.06 | 0.093 |
| Seattle | 6 | −3.2 | 1.9 | −0.06 | 0.144 | −2.9 | 1.5 | −0.05 | 0.123 |
| Domain | |||||||||
| Internalizing | 23 | −2.1 | 0.8 | −0.04 | 0.012 | −2.3 | 0.8 | −0.05 | 0.003 |
| Externalizing | 23 | −3.0 | 0.8 | −0.06 | 0.0002 | −2.8 | 0.7 | −0.06 | <0.0001 |
| Attention problems | 23 | −4.2 | 0.8 | −0.09 | <0.0001 | −3.9 | 0.8 | −0.08 | <0.0001 |
| Social problems | 23 | −2.2 | 1.3 | −0.05 | 0.075 | −2.5 | 1.0 | −0.05 | 0.017 |
| ADHD symptoms | 16 | −1.9 | 1.1 | −0.04 | 0.101 | −1.8 | 1.0 | −0.04 | 0.089 |
| Age | |||||||||
| Childhood | 68 | −2.5 | 0.7 | −0.05 | 0.0002 | −2.5 | 0.6 | −0.05 | <0.0001 |
| Adolescence | 40 | −2.7 | 1.2 | −0.06 | 0.025 | −2.7 | 0.9 | −0.06 | 0.005 |
b (s.e.) is the raw regression coefficient (standard error) and β is the standardized regression coefficient for absolute alcohol per day during pregnancy, measured on the natural log scale, adjusted for potential confounders using propensity scores.
IQ Scores
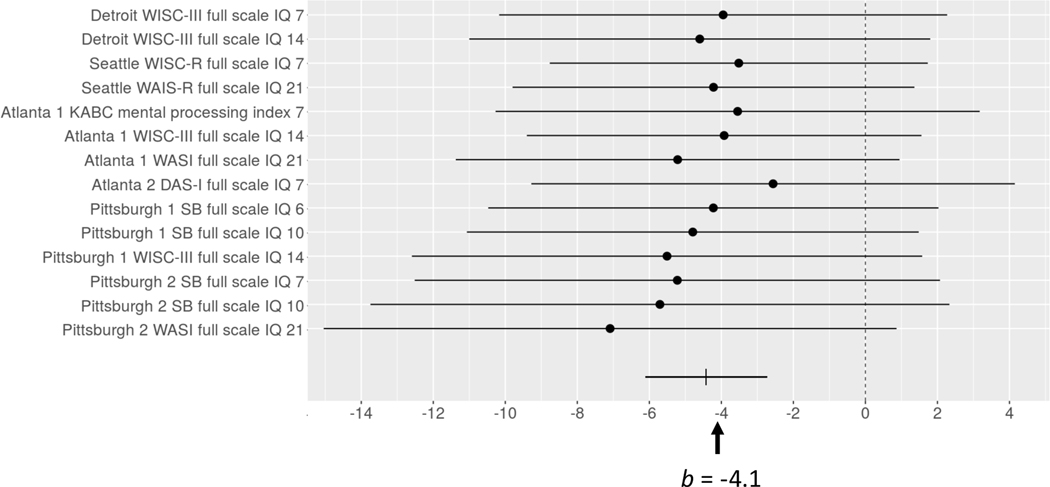
Table 6 shows the effects of PAE on each of the 14 IQ measures obtained from the six cohorts after adjustment for propensity scores. Although PAE effects were detected in two of the tests administered during young adulthood, none of the others were statistically significant. Nevertheless, Table 6 and Figure 2 show a very consistent pattern of adverse effect on IQ. In most cases, the standardized regression coefficients range between −0.06 and −0.11, and the overall PAE effect size on IQ was −0.09. These data show the classic pattern in which results are suggestive but non-significant for individual studies, yet highly significant based on meta-analysis. The meta-analysis benefits from the increased sample size obtained by pooling across cohorts, while taking into account any cohort-specific variation. It is of interest that the effect size for overall IQ (b=−4.1, s.e.=0.6) was similar to the effect size measure for overall cognitive function shown in Table 3.
Table 6.
Effects of prenatal alcohol exposure on each of the IQ measures obtained for this meta-analysis and on the estimate for overall IQ, after adjustment for potential confounders
| N | b | s.e. | β | p | |
|---|---|---|---|---|---|
| Detroit | |||||
| WISC IQ 7 | 336 | −4.0 | 3.1 | −0.08 | 0.287 |
| WISC IQ 14 | 288 | −4.6 | 3.2 | −0.09 | 0.145 |
| Pittsburgh-1 | |||||
| SB IQ 6 | 648 | −4.2 | 3.1 | −0.06 | 0.131 |
| SB IQ 10 | 611 | −4.8 | 3.4 | −0.06 | 0.143 |
| WISC IQ 15 | 528 | −5.5 | 3.5 | −0.07 | 0.118 |
| Pittsburgh-2 | |||||
| SB IQ 7 | 241 | −5.2 | 3.6 | −0.11 | 0.087 |
| SB IQ 10 | 209 | −5.7 | 4.0 | −0.11 | 0.098 |
| WASI IQ 21 | 193 | −7.1 | 4.0 | −0.14 | 0.036 |
| Atlanta-1 | |||||
| Kaufman MPI 7 | 122 | −3.5 | 3.4 | −0.13 | 0.324 |
| WISC IQ 15 | 189 | −3.9 | 2.7 | −0.14 | 0.165 |
| WASI IQ 21 | 179 | −5.2 | 3.1 | −0.19 | 0.024 |
| Atlanta-2 | |||||
| DAS IQ 8 | 138 | −2.6 | 3.4 | −0.07 | 0.493 |
| Seattle | |||||
| WISC IQ 7 | 478 | −3.5 | 2.6 | −0.07 | 0.237 |
| WAIS IQ 21 | 401 | −4.2 | 2.8 | −0.10 | 0.141 |
| Overall IQa | 2219 | −4.1 | 0.6 | −0.09 | <0.001 |
b (s.e.) is the raw regression coefficient (standard error) and β is the standardized regression coefficient for absolute alcohol per day during pregnancy, measured on the natural log scale, adjusted for potential confounders using propensity scores. DAS: Differential Ability Scales; Kaufman MPI: Kaufman Mental Processing Index; SB: Stanford-Binet; WAIS: Wechsler Adult Intelligence Scale; WASI: Wechsler Abbreviated Scales of Intelligence; WISC: Wechsler Intelligence Scales for Children. p-values based on the Wald statistic.
Fig. 2.
Forest plot of effects of prenatal alcohol exposure (oz AA/day) on IQ tests administered in each of the cohorts. Regression coefficients (indicating effect sizes after adjustment for potential confounders) are shown on the x-axis. Numbers in the outcome variable names on the y-axis indicate age group at time of testing (7 = school age; 14 = adolescence; 21 = young adulthood). Overall effect size (b) is shown at the bottom of the figure. WAIS = Wechsler Adult Intelligence Scale. WISC = Wechsler Intelligence Scale for Children. KABC = Kaufman Assessment Battery for Children. WASI = Wechsler Abbreviated Scales of Intelligence. DASI = Differential Ability Scales 1st edition; SB = Stanford-Binet.
Executive Function
Table 7 shows the effects of PAE on the five aspects of executive function examined in these cohorts. The omnibus test for cohort was significant (χ2=17.04, p=0.004). As in the analysis of PAE effects on overall cognitive function, the effect was stronger in the Detroit and Pittsburgh cohorts than in the Seattle and Atlanta cohorts (χ2=5.70, p=0.017).
Table 7.
Meta-analysis: Effects of prenatal alcohol exposure on five aspects of executive function
| Number of outcome measures | Linear regression |
Robust regression |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| b | s.e. | β | P | b | s.e. | β | P | ||
| Overall executive function | 39 | −3.7 | 1.4 | −0.08 | 0.009 | −3.1 | 1.2 | −0.07 | 0.008 |
| Cohort | |||||||||
| Detroit | 13 | −5.8 | 1.4 | −0.10 | 0.001 | −5.3 | 1.4 | −0.09 | 0.002 |
| Pittsburgh-1 | 8 | −2.8 | 1.2 | −0.04 | 0.062 | −3.3 | 1.2 | −0.04 | 0.030 |
| Pittsburgh-2 | 7 | −7.5 | 1.8 | −0.15 | 0.005 | −5.3 | 2.0 | −0.11 | 0.041 |
| Atlanta-1 | 2 | −8.5 | 5.1 | −0.30 | 0.345 | −10.1 | 5.2 | −0.36 | 0.304 |
| Atlanta-2 | 1 | 2.3 | 3.3 | 0.06 | 0.889 | 2.4 | 3.5 | 0.06 | 0.876 |
| Seattle | 8 | −1.0 | 1.2 | −0.02 | 0.445 | −0.4 | 1.1 | −0.01 | 0.710 |
| Domain | |||||||||
| Interference control | 8 | −2.4 | 3.0 | −0.05 | 0.417 | −2.3 | 2.7 | −0.05 | 0.406 |
| Set shifting | 18 | −4.5 | 0.9 | −0.10 | <0.0001 | −4.2 | 1.1 | −0.09 | 0.0002 |
| Verbal fluency | 5 | −5.4 | 3.2 | −0.11 | 0.095 | −4.5 | 3.1 | −0.10 | 0.137 |
| Working memory | 4 | −3.2 | 2.7 | −0.07 | 0.242 | −2.5 | 2.0 | −0.05 | 0.214 |
| Planning | 4 | −1.4 | 1.8 | −0.03 | 0.458 | 0.4 | 1.7 | −0.01 | 0.818 |
| Age | |||||||||
| Childhood | 12 | −3.1 | 2.1 | −0.07 | 0.142 | −3.2 | 1.9 | −0.07 | 0.086 |
| Adolescence | 14 | −5.1 | 1.1 | −0.11 | <0.0001 | −5.1 | 1.0 | −0.11 | <0.0001 |
| Young adulthood | 13 | −1.8 | 1.2 | −0.04 | 0.120 | −0.4 | 1.0 | −0.01 | 0.731 |
b (s.e.) is the raw regression coefficient (standard error) and β is the standardized regression coefficient for absolute alcohol per day during pregnancy, measured on the natural log scale, adjusted for potential confounders using propensity scores.
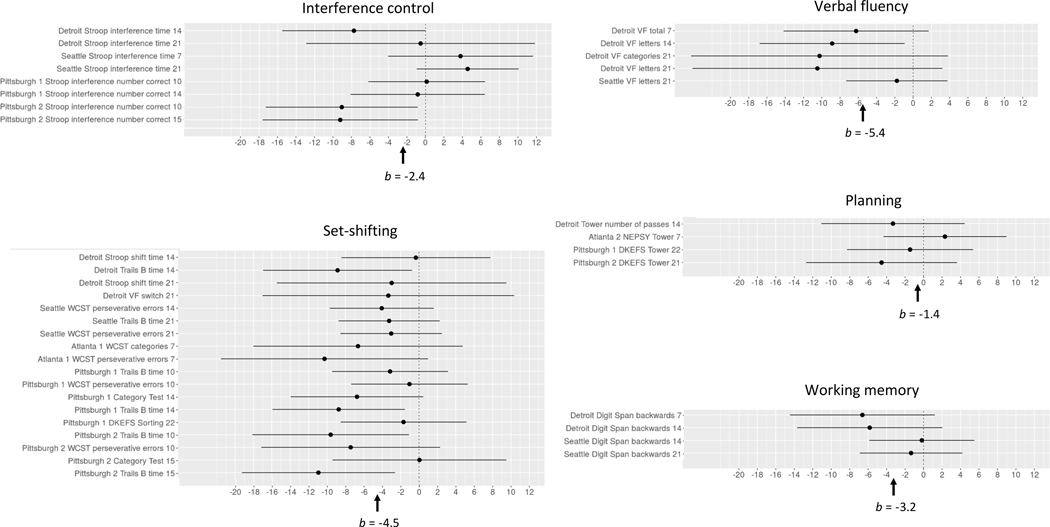
Among the five subdomains of executive function, only the effect on set-shifting was significant. As can be seen in Figure 3, the effects of PAE on virtually all of the 18 set-shifting measures were negative. By contrast, the forest plot shows no consistent evidence of adverse effect on interference control; effects on two measures of interference control were positive, three were negative, and three were essentially zero. All the effects of PAE on verbal fluency were negative, but the pooled estimate in Table 7 fell short of significance, likely because only five measures were available for that domain. The effects of PAE on working memory and planning were mostly negative, but no clear inferences can be drawn because so few measures of these domains were available. As with cognitive function, the effects of PAE on executive function did not differ across the three age periods (χ2=4.45, p=0.108).
Fig. 3.
Forest plots of effects of PAE on five aspects of executive function. Regression coefficients (indicating effect sizes after adjustment for confounders) are shown on the x-axis. Numbers in the outcome variable names on the y-axis indicate age group at time of testing (7 = school age; 14 = adolescence; 21 = young adulthood). Overall effect sizes (b) are shown at the bottom of the figures. DKEFS = Delis-Kaplan Executive Function System. NEPSY = Developmental NEuroPSYchological Assessment. VF = Verbal Fluency. WCST = Wisconsin Card Sorting Test.
Discussion
This study used an innovative two-stage hierarchical meta-analytic approach to integrate outcome data from six prospective longitudinal cohorts to examine the effects of PAE on cognitive and behavioral function spanning the period from early school age through young adulthood. The strongest effects were seen on data pooled across 134 measures of cognitive function derived from assessments of learning and memory, executive function, and reading and math academic achievement. The reliability of the effect size estimates was confirmed by robust regression and by sensitivity analyses that showed that inclusion of variables with skewed distributions had little effect on the effect sizes.
The sample included participants from a range of sociodemographic backgrounds. The Seattle cohort was higher in SES, family income, maternal education, birthweight, and child IQ than the other five cohorts. The Atlanta cohorts were lowest in family income; the Detroit and Pittsburgh cohorts were intermediate. Although five of the cohorts were predominantly economically disadvantaged, the sample as a whole was demographically diverse in that the more middle-class Seattle cohort provided 23.0% of the cases. It is noteworthy that, with the exception of the more middle-class Seattle sample, which was recruited in the mid-1970’s before the risks associated with drinking during pregnancy were well known, socioeconomically disadvantaged, lower SES African American and Caucasian mothers were over-represented in these cohorts, leading to a higher prevalence of stressful life events and depression, conditions that increase the risk of alcohol-exposed pregnancies.
Alcohol consumption averaged across pregnancy (AA/day) was higher in Atlanta-1 and similar in the other five cohorts, two of which had been recruited by oversampling for cocaine use. Drinks/occasion and drinking frequency were highest in Atlanta-1 and intermediate in the Detroit and Pittsburgh cohorts. Notably, the more middle-class Seattle cohort showed a different drinking pattern—fewer drinks/occasion (mean=1.5, compared with a median of 2.5 for the other five cohorts)—but higher frequency (5.9 days/month compared with a median of 3.4).
The PAE effect sizes on the four domains of cognitive function were strikingly similar, suggesting that PAE may affect an underlying component or components of cognitive function involving learning and memory and executive function that are also reflected in academic achievement. Using terminology from structural equation modeling, this underlying aspect of cognitive function can be considered a latent variable that our data show to be inversely related to PAE (see Supplementary Information regarding possible underlying brain structural and/or functional processes that may mediate the effect on overall cognitive function). The similarity in effect size for our overall cognitive function measure and overall IQ suggests that these measures likely reflect similar underlying components of cognition. One advantage of our cognitive function measure is that it permits us to examine the impact of PAE on distinct domains of intellectual function.
The smaller effect size of PAE on cognitive function in the Seattle cohort is likely attributable, in part, to the higher prevalence of more middle-class families in that cohort, which may have partially mitigated the adverse effects of PAE by providing more optimal intellectual stimulation and less socioeconomically-driven stress. In addition, the lower alcohol dose/occasion in the Seattle cohort may have provided additional protection. Laboratory animal studies have shown that ingestion of a given dose of alcohol over a short time period generates a higher peak blood alcohol concentration and greater neuronal (Bonthius and West, 1990) and behavioral impairment (Goodlett et al., 1987) than the same (or even a larger) dose ingested more gradually over several days. Several human behavioral and neuroimaging studies have shown that prenatal exposure to more concentrated doses of alcohol (i.e., binge drinking) is related to poorer cognitive and brain function (e.g., Jacobson et al., 1998; Jacobson et al., 2008; Carter et al., 2005; Robertson et al., 2015; Lewis et al., 2021). Children in the Seattle cohort may also have been protected in utero by better maternal nutrition. A growing body of human and animal studies has demonstrated that prenatal nutrition can modify teratogenic effects of PAE (Carter et al., 2017; Jacobson et al., 2018; Huebner et al., 2018; Thomas et al., 2009).
The PAE effect on cognitive function was strongest and most reliable in the cohorts that provided the most detailed and comprehensive assessments of maternal alcohol consumption during pregnancy. In the Detroit cohort, a 2-week day-by-day timeline follow-back interview was administered to the mother at each prenatal clinic visit (mean=5.2 interviews; range=1–14). In the Pittsburgh cohorts, detailed quantity-frequency-variability interviews assessed alcohol consumption during each trimester of pregnancy. By contrast, in the Seattle and Atlanta cohorts, oz AA/day was based on the mother’s response to two questions during a single interview administered in mid-pregnancy or postpartum. Thus, less extensive ascertainment of PAE levels may account, in part, for the smaller effect sizes in those cohorts.
It is noteworthy that the PAE effect sizes on cognitive function did not differ by age at time of assessment. Although IQ and achievement tests are modified to make them more challenging as children grow older, the learning and memory and executive function tests are not. In a recent study of 96 children with heavy PAE and 59 controls from the same community, who were evaluated longitudinally for PAE-related dysmorphology and somatic growth at ages 5, 9, 13, and 16 years, we found that the anthropometric features used to identify FAS and PFAS evolve and change with age, such that the craniofacial dysmorphology and growth restriction required for a diagnosis of FAS are most reliably detected between age 5 years and puberty (Jacobson et al., 2021). By contrast, the data reported here indicate that the cognitive impairment associated with PAE can be reliably assessed from school age through adulthood. PAE has also been linked to poorer performance on several narrow band tests of cognition during infancy that are early precursors and moderately predictive of childhood cognitive function (e.g., S. Jacobson et al., 1993; Kable and Coles, 2004; Jacobson and Jacobson, 2017). However, it seems unlikely that a reliable assessment of the deficit in cognitive function that emerged from this meta-analysis can be obtained prior to school age, given that the core elements of learning and memory and executive function that comprise this deficit are not fully manifest or readily measurable before 6 years of age.
The learning and memory forest plot illustrates the degree to which the effects of PAE on cognitive function may vary from study to study. It is impressive that, despite this variability, PAE is inversely related to virtually every measure from this domain. Given the broad range in effect size among the learning and memory measures both between and within the cohorts, the forest plot makes clear that the “true” effect size for a given domain cannot be reliably determined from any one measure obtained from a single sample at a single age. The sustained attention forest plot demonstrates that, although an effect may be observed fairly consistently in a single cohort (in this case, Seattle), meta-analytic data may subsequently make clear that the observed effect is not reliable. One important implication of these data is that a single failure to replicate a finding should not be considered sufficient to negate a reported effect and that repeated assessments will often be needed to determine its reliability. This caveat holds particularly for the type of relatively subtle deficits like those seen in PAE studies of cognitive function.
PAE-related adverse effects were also seen on parent- and teacher-reported behavior problems. These effects were weaker than for cognitive function, possibly because no direct observational assessments of behavior were available other than for sustained attention. Although our data suggest that sustained attention is spared in FASD, the effects on parent- and teacher-reported attention problems suggest difficulties in other aspects of attention, such as focused, selective, or divided attention, that warrant further examination. Very high rates of comorbidity of ADHD with FASD have been reported in clinic-referred samples (Fryer et al. 2007), but the PAE effects on the inattention and hyperactivity/impulsivity symptoms used to diagnose ADHD fell short of statistical significance in these prospectively-recruited cohorts and were notably weaker than for cognitive function and parent- and teacher-reported externalizing and attention problems. The higher comorbidity with ADHD in the clinic-referred samples is likely attributable to over-representation of behaviorally oppositional children in those samples, compared with general population samples of PAE. It is of interest that the effects of PAE on parent- and teacher-reported behavior problems were virtually identical in magnitude whether obtained during childhood or adolescence.
Although IQ has been assessed in several longitudinal and case-control studies, few studies have reported effects of PAE on Full-Scale IQ. Among the 14 IQ tests administered to the six cohorts, most of the effect sizes ranged from −0.06 to −0.11 and were not significant. The much larger effective sample size in our meta-analysis yielded an overall effect size of −0.09 with a much smaller standard error, providing greater precision and demonstrating a small but highly reliable effect of PAE on IQ. Our finding that the only significant effects on IQ measures examined individually were from assessments during young adulthood suggests that the effects of PAE on IQ may be more readily detectable in adults.
This study also provided an opportunity to examine whether specific aspects of executive function are differentially affected by PAE. The meta-analysis focusing on executive function provided clear evidence of adverse effects of PAE on set-shifting, whereas interference control (assessed on the Stroop Color-Word test) was not reliably affected. These findings are consistent with executive function data from a meta-analysis of 46 studies that found markedly stronger PAE effects on set-shifting compared with two other aspects of executive function: inhibitory control and working memory (Khoury et al., 2015). Our findings further suggest that verbal fluency is likely affected by PAE. The data are also consistent with previous reports of adverse effects on working memory and planning, but too few measures of those aspects of executive function were available to confirm those effects statistically.
One limitation of this study relates to the challenges involved in recalling alcohol consumption accurately. Noting the concern that women might underreport their drinking during pregnancy due to stigma, Jacobson et al. (2002) compared timeline follow-back maternal reports of alcohol consumption obtained during pregnancy with reports obtained from the same mothers retrospectively at 1-year postpartum. Consistent with previous studies (e.g., Ernhart et al., 1988), the mothers reported higher levels of pregnancy alcohol consumption retrospectively than they had during pregnancy. However, the correlations of PAE with the infant outcomes were stronger for the antenatal measures, supporting the greater accuracy of the contemporaneous pregnancy interviews, which, as noted above, predicted a broad range of adverse outcomes in these cohorts from infancy through young adulthood.
A second limitation relates to outcomes for which only limited data were available. For example, no clear inference can be drawn regarding the effect of PAE on visuospatial function, which, although significant during adolescence, was not significant for the pooled estimate of the effect size based on all 13 measures obtained for this domain. New diagnostic criteria for FASD were proposed in the fifth edition of the DSM, which recognized “Neurodevelopmental Disorder Associated with Prenatal Alcohol Exposure (ND-PAE)” as a “condition in need of further study” (APA 2013; Kable et al., 2015). In addition to cognitive impairment, an ND-PAE diagnosis requires evidence of impairment in self-regulation (i.e., emotional regulation, impulse control) and adaptive function (i.e., social communication and interaction, daily living and motor skills). We were not able to examine effect sizes for PAE on these other domains of neurobehavioral function due to the limited data collected in our cohorts relating to these domains.
A third limitation relates to the generalizability of the findings across social class and historical time period. As noted above, there is a suggestion in the data that more optimal intellectual stimulation and lower levels of socioeconomically-induced stress in the more middle-class Seattle cohort may have reduced the magnitude of the PAE effect size on cognitive function to some degree. With regard to historical time period, alcohol consumption during pregnancy is less prevalent in the middle class today than during the 1970’s, when the risks of PAE were not yet widely known. Similarly, prenatal cocaine exposure was comorbid with PAE only in the cohorts that were recruited during the period when cocaine use was prevalent.
In this meta-analytic study, we applied hierarchical meta-analytic modeling to pool linear regression coefficients assessing effects of PAE (after adjustment for confounders) to determine which domains of cognitive and behavioral function are most reliably affected. Although the effect sizes were modest, they were robust and remarkably consistent across domain, cohort, and age. It is important to recognize that these findings do not provide a full dose-response analysis of the effect of PAE but constitute a critical first step in examining dose-response; namely, assessing the presence of an effect and identification of the relevant outcomes. In subsequent analyses, we plan to characterize dose-response relationships more fully by exploring possible non-linearities and the differential impact of alcohol dose/occasion at different levels of drinking frequency. This next step will require an approach that is different from the hierarchical meta-analysis techniques described here.
The strongest effects in this study were seen on cognitive function, the outcome that has been studied most extensively in FASD. The effect sizes for cognitive function were remarkably similar, whether measured by standardized IQ and achievement tests or by narrow band tests that focus specifically on learning and memory and executive function. By contrast, although effects on sustained attention have been reported in a few studies, our meta-analysis showed that sustained attention is not reliably affected in FASD. The forest plots revealed impressive consistency in effect size across a large number of IQ tests administered across this age period and showed that, within the domain of executive function, set-shifting is particularly affected. Parent- and teacher-reported behavior problems were also affected, although the effect sizes were weaker than for cognition, possibly because parents are not objective, trained observers and vary in their expectations for their children’s behavior, and middle and high school teachers do not observe their students in a broad range of contexts. One challenge in the diagnosis of ARND, where PAE is confirmed but craniofacial dysmorphology and growth restriction are not found, is identifying the specific aspects of neurobehavioral function that are most sensitive to fetal alcohol exposure. The findings from this meta-analytic study suggest that, when an individual with PAE presents with deficits in learning/memory and set-shifting but sustained attention in the normal range, the clinician can have greater confidence in making a diagnosis of ARND. However, given the diversity of the cognitive deficits linked to PAE, it would not be appropriate to rule out an ARND diagnosis in individuals with PAE who manifest a different pattern of cognitive impairment.
Supplementary Material
Acknowledgments
We appreciate the pioneering work of Ann P. Streissguth, Ph.D., who initiated and directed the first prospective, longitudinal cohort study on the neurobehavioral sequelae of PAE and inspired us and many others in the field. We are grateful to the individuals and families who participated in the six cohort studies without whom this unique and valuable research would not have been possible and for the contributions of our dedicated collaborators and research staff.
Funding: This research was supported by Grant No. R01 AA025905 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA)/National Institutes of Health (NIH) and the Lycaki-Young Fund from the State of Michigan. Louise Ryan received support from the Australian Research Council Centre of Excellence for Mathematical and Statistical Frontiers (Grant No. CE140100049). Richard J. Cook was supported by Natural Sciences and Engineering Research Council of Canada grants RGPIN 155849 and RGPIN 04207. Data collection for the Detroit cohort was supported by NIAAA/NIH grants R01 AA06966, R01 AA09524, and P50 AA07606 and National Institute on Drug Abuse (NIDA)/NIH grant R21 DA021034; for the Pittsburgh cohorts, by NIAAA/NIH grants R01 AA06390, R01 AA06666, R01 AA14215, R01 AA18116, National Institute on Child Health and Human Development/NIH grant HD036890, and NIDA/NIH grants R01 DA00090, R01 DA03209, R01 DA03874, and R01 DA17786, R01 DA05460, R01 DA06839, R01-DA08916, and R01 DA12401; for the Atlanta cohorts, by NIAAA/NIH grants R01 AA08105, R01 AA10108, and R01 AA13272, NIDA/NIH grants R01 DA0737362 and R01 DA0737362, and Georgia State Contract DHR 427 93 05050762; and for the Seattle cohort, by NIAAA/NIH grant R01 AA01455.
Footnotes
The authors declare no competing financial interests.
As noted above, all outcomes had been standardized to a mean of 100, standard deviation of 15, so that the estimated effect sizes can be considered comparable.
References
- Akkaya-Hocagil TA, Ryan LM, Cook RJ, Richardson GA, Day NL, Coles CD, Olson HC, Jacobson SW, Jacobson JL (2020) A hierarchical meta-analysis for settings involving multiple outcomes across multiple cohorts. arXiv:2009.01323 [stat.ME] (available at http://arxiv.org/abs/2009.01323). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaya-Hocagil T, Cook RJ, Jacobson SW, Jacobson JL, Ryan LM(2021) Propensity score analysis for a semi-continuous exposure variable: A study of gestational alcohol exposure and childhood cognition. J Royal Stat Soc Series A. 10.1111/rssa.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. 5th ed. APA, Arlington, VA. [Google Scholar]
- Anderson P (2002) Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8:71–82 [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK (2000) Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol 35:400–410. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR (1990) Alcohol‐induced neuronal loss in developing rats: Increased brain damage with binge exposure. Alcohol Clin Exp Res 14:107–118. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Carter CS, Barch DM, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108:624–652. [DOI] [PubMed] [Google Scholar]
- Brown JV, Bakeman R, Coles CD, Sexson WR, Demi AS (1998) Maternal drug use during pregnancy: Are preterm and full-term infants affected differently? Dev Psychol 34:540. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL (2005) Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res 29:443–452. [DOI] [PubMed] [Google Scholar]
- Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL (1998) Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res 22:1998–2012. [PubMed] [Google Scholar]
- Carter RC, Jacobson SW, Molteno CD, Chiodo LM, Viljoen D, Jacobson JL (2005) Effects of prenatal alcohol exposure on infant visual acuity. J Pediatr 147:473–479. [DOI] [PubMed] [Google Scholar]
- Carter RC, Senekal M, Molteno CD, Duggan CD, Dodge NC, Meintjes EM, Jacobson JL, Jacobson SW (2017) Poor maternal caloric intake and weight gain during pregnancy exacerbates fetal alcohol growth restriction in humans. FASt Data Presentation at the Research Society on Alcoholism, Denver, CO. [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O’Neill J (2005) Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex 41:389–98. [DOI] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A (1991) Effects of prenatal alcohol exposure at school age. I. physical and cognitive development. Neurotoxicol Teratol 13:357–367. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind‐Hood CL, Brown RT, Falek A, Smith IE (1997) A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res 21:150–161. [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL Barr HM, Streissguth AP (2000) Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol 18:331–354. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM (2006) Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia 44:744–751. [DOI] [PubMed] [Google Scholar]
- Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, Chudley AE, Conry JL, LeBlanc N, Loock CA, Lutke J, Mallon BF (2016) Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. CMAJ 188:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN (2011) Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention‐deficit/hyperactivity disorder. Alcohol Clin Exp Res 35:1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Riley EP, Mattson SN (2015) Visual-spatial abilities relate to mathematics achievement in children with heavy prenatal alcohol exposure. Neuropsychol 29:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Richardson GA, Geva D, Robles N (1994) Alcohol, marijuana, and tobacco: effects of prenatal exposure on offspring growth and morphology at age six. Alcohol Clin Exp Res 18:786–794. [DOI] [PubMed] [Google Scholar]
- Day NL, Helsel A, Sonon K, Goldschmidt L (2013) The association between prenatal alcohol exposure and behavior at 22 years of age. Alcohol Clin Exp Res 37:1171–1178. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JK (2001) Delis-Kaplan Executive Function System. San Antonio, TX. [Google Scholar]
- Dodge NC, Jacobson JL, Jacobson SW (2014) Protective effects of the alcohol dehydrogenase-ADH1B* 3 allele on attention and behavior problems in adolescents exposed to alcohol during pregnancy. Neurotoxicol Teratol 41:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doney R, Lucas BR, Jones T, Howat P, Sauer K, Elliott EJ (2014) Fine motor skills in children with prenatal alcohol exposure or fetal alcohol spectrum disorder. J Dev Behav Pediatr 35:598–609. [DOI] [PubMed] [Google Scholar]
- Dubitzky W, Wolkenhauer O, Cho KH, Yokota H (2013) Tukey-Kramer method, in Encyclopedia of Systems Biology (Dubitzky W, Wolkenhauer O, Cho KH, Yokota H eds). Springer, New York. [Google Scholar]
- Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S (1988) Underreporting of alcohol use in pregnancy. Alcohol Clin Exp Res 12:506–511. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SM (2007) Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics 119:e733–e741. [DOI] [PubMed] [Google Scholar]
- Glass L, Ware AL, Mattson SN (2014) Neurobehavioral, neurologic, and neuroimaging characteristics of fetal alcohol spectrum disorders. Handbook Clin Neurol 125:435–462. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Stoffer DS, Geva D, Day NL (1996) Prenatal alcohol exposure and academic achievement at age six: a nonlinear fit. Alcohol Clin Exp Res 20:763–770. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Kelly SJ, West JR (1987) Early postnatal alcohol exposure that produces high blood alcohol levels impairs development of spatial navigation learning. Psychobiol 15:64–74. [Google Scholar]
- Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J (2009) Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: a comparison with attention deficit hyperactivity disorder. Alcohol Clin Exp Res 33:1656–1670. [DOI] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD (2006) Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J Pediatr Psychol 31:116–126. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK (2005) A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatr 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T (2016) Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatr 138:e20154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner SM, Helfrich KK, Saini N, Blohowiak SE, Cheng AA, Kling PJ, Smith SM (2018) Dietary iron fortification normalizes fetal hematology, hepcidin, and iron distribution in a rat model of prenatal alcohol exposure. Alcohol Clin Exp Res 42:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, van Dyk DA(2004). Causal inference with general treatment regimes. J Am Stat Assoc 99:854–866. [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Kaplan-Estrin MG (1993) Teratogenic effects of alcohol on infant development. Alcohol Clin Exp Res 17:174–183. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Ager JW (1998) Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcohol Clin Exp Res 22:345–351. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Dodge NC, Burden MJ, Klorman R, Jacobson SW (2011) Number processing in adolescents with prenatal alcohol exposure and ADHD: differences in the neurobehavioral phenotype. Alcohol Clin Exp Res 35:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW (1993) Prenatal alcohol exposure and infant information processing ability. Child Dev 64:1706–1721. [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL (2002) Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatr 109:815–825. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM (1996) New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr 129:581–590. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R (2004) Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5‐year intellectual function. Alcohol Clin Exp Res 28:1732–1745. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2008) Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res 32:365–372. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2011) Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res 35:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL (2017) Cardiac orienting response as an early indicator of impairment in fetal alcohol spectrum disorders. Alcohol Clin Exp Res 41:262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, Lewis CE, Dodge NC, Hoyme HE, Zeisel SH, Meintjes EM, Duggan CP, Jacobson JL (2018) Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: A randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res 42:1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Hoyme HE, Molteno CD, Meintjes EM, Carter RC, Jacobson JL (2021). Evolution of the physical phenotype of fetal alcohol spectrum disorders from childhood through adolescence. Alcohol Clin Exp Res, 45:395–408. doi: 10.1111/acer.14534. [DOI] [PubMed] [Google Scholar]
- Jones K, Smith D (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet 302:999–1001. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD (2004) The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res 28:489–496. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Lynch ME, Platzman K (2008) Physiological responses to social and cognitive challenges in 8 year olds with a history of prenatal cocaine exposure. Dev Psychobiol 50:251–265. [DOI] [PubMed] [Google Scholar]
- Kable JA, O’Connor MJ, Carmichael Olson H, Paley B, Mattson SN, Anderson SM, Riley EP (2015) Neurobehavioral Disorder associated with Prenatal Alcohol Exposure (ND-PAE): Proposed DSM-5 diagnosis. Child Psychiatr Human Dev 46:493–642. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Halverson P (2000) Spatial memory following prenatal alcohol exposure: More than a material specific memory deficit. Child Neuropsychol 6:115–128. [DOI] [PubMed] [Google Scholar]
- Khoury JE, Milligan K, Girard TA (2015) Executive functioning in children and adolescents prenatally exposed to alcohol: A meta-analytic review. Psychol Rev 25:149–170. [DOI] [PubMed] [Google Scholar]
- Kingdon D, Cardoso C, McGrath JJ (2016) Research review: Executive function deficits in fetal alcohol spectrum disorders and attention‐deficit/hyperactivity disorder–a meta‐analysis. J Child Psychol Psychiatr 57:116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku PW, Wendy K, May PA (2001) The effects of prenatal alcohol exposure on executive functioning. Alcohol Res Health 25:192–198. [PMC free article] [PubMed] [Google Scholar]
- Larroque B, Kaminski M, Dehaene P, Subtil D, Delfosse MJ, Querleu D (1995) Moderate prenatal alcohol exposure and psychomotor development at preschool age. Am J Public Health 85:1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Thomas KG, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW (2015) Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Thomas KGF, Molteno CD, Kliegel M, Meintjes EM, Jacobson JL, Jacobson SW (2016) Prospective memory impairment in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 40:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Thomas KGF, Ofen N, Warton CMR, Lindinger NM, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW (2021) An fMRI investigation of neural activation during memory formation in children with fetal alcohol spectrum disorders. NeuroImage: Clin 3:102532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger NM, Malcolm-Smith S, Dodge NC., Molteno CD, Thomas KGF, Meintjes EM, Jacobson JL, Jacobson SW (2016) Theory of mind in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 40:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL (1996) Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res 20:810–816. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL (1997) Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr 131:718–721. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis CD, Riley EP (1999) Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL (2013) Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 37:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA, Doyle LR (2019) Fetal alcohol spectrum disorders: a review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol Clin Exp Res 43:1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hasken JM, Bozeman R, Jones JV, Burns MK, Goodover J, Kalberg WO, Buckley D, Brooks M, Ortega MA, Elliot AJ, Hedrick DM, Tabachnik BG, Abdul-Rahman O, Adam MP, Jewett T, Robinson LK, Manning MA, Hoyme HE (2020a) Fetal alcohol spectrum disorders in a rocky mountain region city: child characteristics, maternal risk traits, and prevalence. Alcohol Clin Exp Res 44:900–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hasken JM, Baete A, Russo J, Elliott AJ, Kalberg WO, Buckley D, Brooks M, Ortega MA, Hedrick DM, Tabachnik BG, Abdul-Rahman O, Adam MP, Jewett T, Robinson LK, Manning MA, Hoyme HE (2020b) Fetal alcohol spectrum disorders in a midwestern city: Child characteristics, maternal risk traits, and prevalence. Alcohol Clin Exp Res 44:919–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Schonfeld AM, Roebuck-Spencer TM, Riley EP, Mattson SN (2008) Children with heavy prenatal alcohol exposure demonstrate deficits on multiple measures of concept formation. Alcohol Clin Exp Res 32:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S (2002) Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolescent Psychiatr 41:378–385. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Ann Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2010) What is a standard drink? [NIAAA Web site]. Available at https://www.niaaa.nih.gov/alcohols-effects-health/overview-alcohol-consumption/what-standard-drink. Accessed June 8, 2021.
- Rasmussen C (2005) Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res 29:1359–1367. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Bisanz J (2009) Executive functioning in children with fetal alcohol spectrum disorders: profiles and age-related differences. Child Neuropsychol 15:201–215. [DOI] [PubMed] [Google Scholar]
- Reiten RM, Wolfson D (1993) The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2nd ed. Neuropsychology Press, South Tucson, AZ. [Google Scholar]
- Richardson GA, Hamel SC, Goldschmidt L, Day NL (1999) Growth of infants prenatally exposed to cocaine/crack: comparison of a prenatal care and a no prenatal care sample. Pediatrics 104:e18. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L (2002) Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol 24:309–320. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Larkby C, Day NL (2015) Effects of prenatal cocaine exposure on adolescent development. Neurotoxicol Teratol 49:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson FC, Narr KL, Molteno CD, Jacobson JL, Jacobson SW, Meintjes EM (2015) Prenatal alcohol exposure is associated with regionally thinner cortex during the preadolescent period. Cerebral Cortex 26:3083–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L (2008) Combining data from multiple sources, with applications to environmental risk [DOI] [PubMed] [Google Scholar]
- Shallice T (1982) Specific impairments of planning. Philosoph Trans Royal Soc London B 298:199–209. [DOI] [PubMed] [Google Scholar]
- Simon S (2009) When the F test is significant, but Tukey is not. Available at: http://www.pmean.com/05/TukeyTest.html. [Google Scholar]
- Stratton K, Howe C, Battaglia FC (1996) Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. National Academies Press, Washington, DC. [Google Scholar]
- Streissguth AP (1976) Psychologic handicaps in children with fetal alcohol syndrome. Ann NY Acad Sci 273:140–145. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Martin DC, Martin JC, Barr HM (1981) The Seattle longitudinal prospective study on alcohol and pregnancy. Neurobehav Toxicol Teratol 3:223–233. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD (1990) Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res 14:662–669. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Carmichael Olson H, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF (1994) Maternal drinking during pregnancy and attention and short-term memory in 14-year-old offspring: a longitudinal prospective study. Alcohol Clin Exp Res 18:202–218. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662. [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD (2009) Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol 31:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JC (2017) Accentuate the negative: Grammatical errors during narrative production as a clinical marker of central nervous system abnormality in school-aged children with fetal alcohol spectrum disorders. J Speech Language Hearing Res 60:3523–3537. [DOI] [PubMed] [Google Scholar]
- Tsang TW, Lucas BR, Carmichael Olson H, Pinto RZ, Elliott EJ (2016) Prenatal alcohol exposure, FASD, and child behavior: A meta-analysis. Pediatrics 137:e20152542. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K (2011) MICE: multivariate imputation by chained equations in R. J Stat Software 45:1–67. [Google Scholar]
- Vaurio L, Riley EP, Mattson SN (2008) Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc 14:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN (2011) Neuropsychological comparison of children with heavy prenatal alcohol exposure and an IQ-matched comparison group. J Int Neuropsychol Soc 17: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN, CIFASD (2012) Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res 36:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J (2008) Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc 14:1022–1033. [DOI] [PubMed] [Google Scholar]
- Wyper KR, Rasmussen CR (2011) Language impairments in children with fetal alcohol spectrum disorder. J Population Therapeutics Clin Pharmacol 18:1–13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.