Abstract
Organoid technology is a state-of-the-art cell culture tool that has revolutionized study of development, regeneration, and diseases. Human Liver Organoids (HLOs) are now derived from either adult stem/progenitors or pluripotent stem cells (PSCs), emulating cellular diversity and structural symphony akin to the human liver. With the rapid rise in decompensated liver disease conditions only treated by liver transplant therapy, HLOs represent an alternate source for transplantation to address the ongoing shortage of grafts. Although ongoing advancements in bioengineering technology have moved the organoid transplant approach to the next level, sustained survival of the transplanted tissue still eludes us towards functional organ replacement. Herein, we review the development of HLOs, and discuss promises and challenges on organoid transplant approaches.
Keywords: liver organoid, transplantation, transplantation sites, applications, safety, efficacy
Introduction
The incidence of diseases of civilization has accelerated astronomically over the past few decades [1]. Chronic liver disease is one such disease that accounts for approximately 2 million deaths annually due to factors such as imbalanced diet, excessive alcohol consumption, drug induced injury, viral infection and genetic mutations [2–4]. The rapid rise in these diseases has led to a shortage of donors for organ transplantations. Since 1983, orthotopic liver transplantation has been considered as the only treatment for patients who have lost their liver functions. As of 2020, there were still 107,603 patients on the waiting list, with 39,035 transplant recipients, and only 18,316 donors available at the time [5, 6]. Unfortunately, there was a 25% reduction in liver donors due to various reasons, leading to a crunch in overall liver transplantation [7]. Moreover, a majority of these chronic liver disease patients fail to meet the criteria in place for transplantation. Thus, there is a critical need to come up with alternative solutions to the shortage crisis and failure of acceptance affecting cadaveric transplants.
With a goal to supplement organ transplant needs, various cell culture methods have been formulated for developing renewable and transplantable hepatocytes [2]. For example, the discovery of epigenetic reprogramming has sparked significant advancements into understanding cell fate acquisition, thereby, turning the focus towards directed differentiation or direct reprogramming of adult stem/progenitors or PSCs into hepatocyte-like cells [8]. Another area of interest includes the development of three-dimensional miniature liver models, called human liver organoids, grown from adult or pluripotent stem cells, which have both structural and functional resemblance to in vivo organs (Fig. 1). Thus, hepatocytes generated in vitro, and organoids have attracted a great deal of attention, as reports have indicated their efficacious potential when transplanted. Organoid based approach is of particular interest here as the patient’s own cells can be used to generate unlimited amount of polarized hepatocytes existing in organized hepatic tissues accompanied by the emergence of secretory, metabolic and excretory functions. Additionally, recent advances of organoid technology can develop vasculature allowing them to engraft quickly and survive longer periods, thus supporting multi-faceted challenges informed by cell transplantation approaches [9, 10].
Figure 1. Types of human liver organoids.
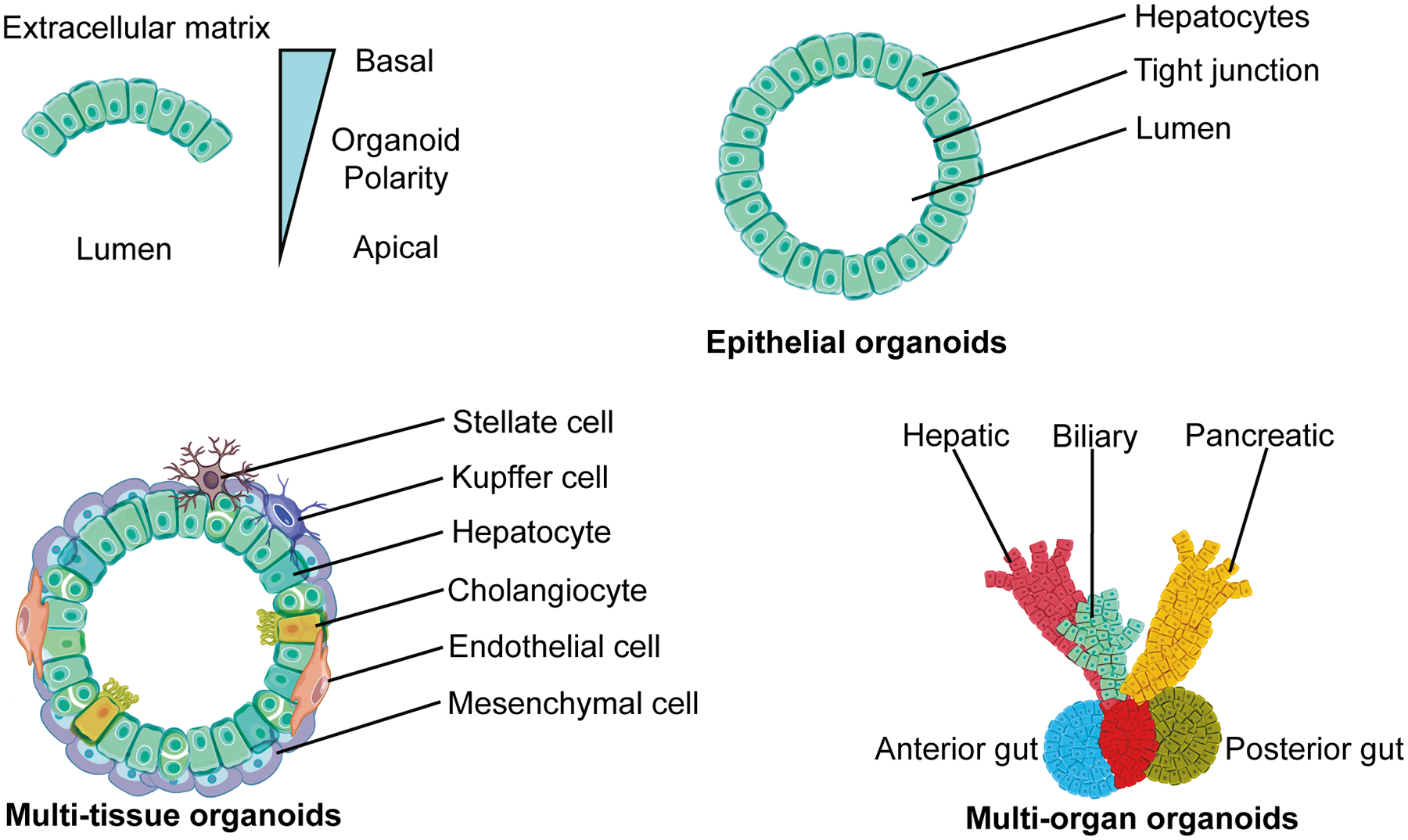
Epithelial organoids are derived from either adult stem/ progenitor cells or PSCs and differentiate into a single type of cell; these organoids only have basic functions of hepatocytes. Multi-tissue organoids are derived from either PSCs or combined with stromal cells to generate at least two germ layers; these organoids are capable of complicated functions of the liver and can vascularize to survive longer. Multi-organ organoids are the most complex and encompass multiple interconnected organ like structures that function separately; these organoids have been derived from PSCs only.
Organoids contain a collection of specific cell types that develop from stem cells or organ progenitors through self-organization by cell sorting and spatially restricted lineage commitment, in a manner similar to in vivo [11]. In 1907, Wilson first reported the phenomenon of isolating sponge cells self-assembled to form entire organisms [12]. In the 2000s, there were many reports of 3D generation of the intestine and cerebral cortex, which led to their dissemination in various clinical applications [13, 14]. Following these advancements the organoid field has never looked back, spreading to multiple organs such as the retina, kidney, lung, and liver [15]. Excitingly, in 2017 induced pluripotent stem cells (iPSC) derived retinal pigment epithelial (RPE) cells were transplanted in human patients with age-related macular degeneration (AMD). However, no organoids have been transplanted clinically with a goal to restore liver functions.
The liver is a complex organ with a variety of functions. Hepatocytes are the main epithelial component, accounting for 80% of the adult liver volume and over 60% of total cells in the liver; they are responsible for metabolism, protein secretion, detoxification, and bile production [16]. It has been known for some time that mature liver cells are extremely difficult to grow in vitro. Chick embryonic livers were reconstituted in 1960, yet hepatocytes were made from mouse and human embryonic stem cells only recently in 2004 [17, 18]. Reports of liver organoid generation quickly followed in the 2010s [19, 20]. Although the goal of organoid research is the elucidation of developmental processes and construction of pathological organs in vitro with hopes of developing and testing therapeutic options, there is also an increasing enthusiasm to utilize organoids in future clinical applications. The success of transplantation depends on many factors, including the location, the preconditioning method, and the use of immunosuppressive agents in the recipient liver. For example, administration through the portal vein, splenic arteries, and intraperitoneal sites have each shown therapeutic benefits, but the risk of increased portal pressure, gastric or splenic infarction, and microemboli in the lungs remain high [21]. Therefore, the next step is to resolve these shortcomings to turn organoid transplantation into reality.
Past and present efforts to complement liver transplantation
Past and present cell therapies for liver diseases can be divided into four main categories: (i) hepatocyte transplantation (ii) non-parenchymal cell transplantation (iii) hepatic progenitor transplantation and (iv) bioartificial liver trials (Fig. 2 A). These techniques are extensively studied in animals and translated into clinics, providing a foundational lesson for shaping future organoid therapy.
Figure 2.
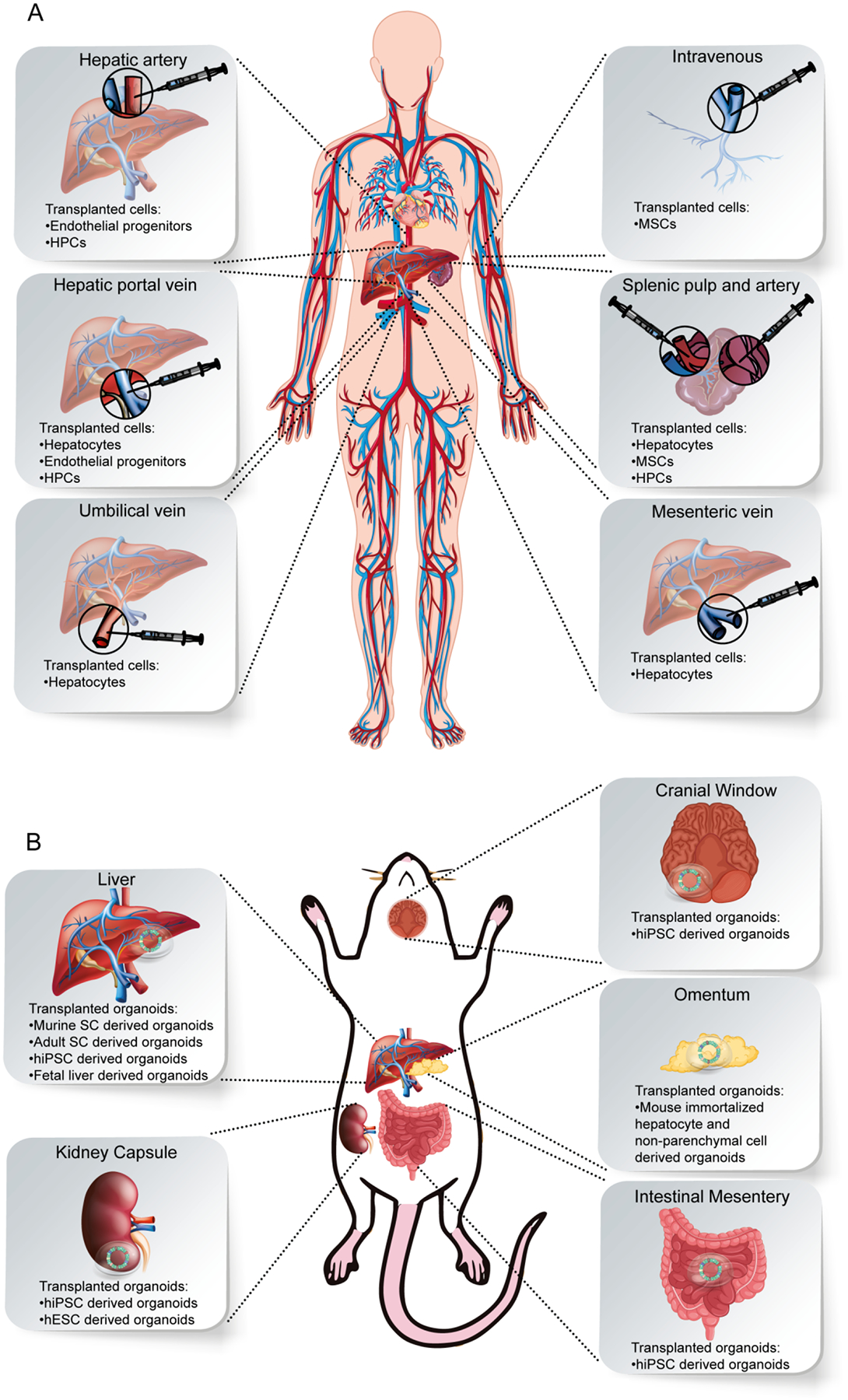
Hepatocyte and organoid based transplant approaches. A) Transplantation of hepatocytes in clinics. B) Transplantation of liver organoids in animals.
Hepatocyte transplantation
Hepatocyte transplantation (HTx) is a well-studied technique that has been applied both preclinically and clinically to treat diseases such as Crigler–Najjar syndrome (CNS), Urea cycle disorders (UCD), and Factor VII deficiency [22]. In this procedure hepatocytes are usually obtained from human deceased donors or partially resected liver samples using enzymatic perfusion techniques. The cells are transplanted immediately or cryopreserved for future use [23]. Mito et al. were the first to transplant hepatocytes in humans through the splenic pulp, however no clinical improvements were observed [24]. Approximately 109 to 1010 hepatocytes are usually transplanted into the liver through the portal vein; it is accessed either transhepatically, by an umbilical vein or by a peripheral mesenteric vein using a catheter or implantable device [25, 26]. HTx has been reported to ameliorate CNS by decreasing total bilirubin from 26.6 to 14 mg/dL, wherein transplanted hepatocytes were still viable after 11 months in the patient liver [27]. Numerous patients have undergone HTx for UCDs, and in some cases, reinstituted enzymatic activity led to a decrease in ammonia and an increase in urea levels [28]. Factor VII deficiency was also mitigated in two brothers using HTx, decreasing the need for Factor VII by 20% in these patients [29]. Following these advances, several clinical trials with HTx to treat liver-based metabolic deficiencies have been approved and are awaiting results (ClinicalTrials.gov, Identifier: NCT01345578 and NCT01465100). Overall, HTx trials have shown that hepatocyte based therapies has the potential to treat at least some hereditary liver diseases. However, the main drawback of HTx is the lack of engraftment and host immune responses that preclude its use in the clinics [23].
Non-parenchymal cell transplantation
Mesenchymal stem cell transplantations (MSC Tx) were initiated in the 1990s to modulate immune reactions. MSCs maintain specific ECM components and secrete soluble factors to control immune cells [30]. The MSCs are obtained from a variety of sources including the bone marrow and umbilical cord blood, and usually cultured for 4 generations before being transplanted or cryopreserved [31]. Shortly after that, this technique was suggested for the treatment of liver cirrhosis and fibrosis. MSCs in the range of 105 to 109 are infused by intravenous, intrahepatic, or intrasplenic injection, however it is unclear whether this therapy contributes to liver regeneration in the long period [32, 33]. In 2017, MSC Tx was reported to treat liver graft rejection; it was found that liver functions and allograft histology were improved in addition to Treg/ Th17 ratio, CD4+ T-cell activation [34]. Lin et al. also found improvements in survival rate, liver function, and reduction in infection when bone marrow derived MSCs were transplanted in acute-on-chronic liver disease patients [35]. More recently in 2018, liver cirrhosis patients exhibited enhancement of Child-Pugh scores, MELD (Model for End-Stage Liver Disease) scores and liver functions after intravenous injection of umbilical cord MSCs [36]. There are multiple clinical trials utilizing MSCs for liver disease treatment in different phases now showing promises [37]. Moroni et al. have shown the feasibility and efficacy of autologous macrophage therapy in liver cirrhosis patients. The clinicians injected 109 cells in 9 patients and reported a reduction in MELD scores over 90 days [38]. Several clinical trials are also underway to test the reversal of liver cirrhosis using autologous endothelial progenitor cells. The endothelial progenitor cell delivery studies utilize the hepatic artery and portal vein route for administration of CD133+ cells (ClinicalTrials.gov, Identifier: NCT01333228 and NCT03109236). Thus, non-parenchymal cell transplantation has established itself as a supporting technique to modulate immune function and hepatocyte survival in transplantations. That said, many clinical trials are yet to bear any substantially positive results due to the poorly defined nature of the MSCs [32].
Hepatic progenitor transplantation
Hepatic progenitor cells (HPCs), while their physiological nature is widely debated, can regenerate to produce hepatic parenchymal cells, hepatocytes, and/or bile ductular epithelial cells following injury. The progenitor cells are mainly obtained from resected liver samples by means of enzymatic digestion and cell separation techniques. The cells are then grown for a couple generations before being transplanted [39]. HPCs have advantages compared to adult hepatocyte because they can differentiate into hepatocytes and cholangiocytes [40, 41]. Several studies have also suggested that HPCs have a minimum risk of alloreactivity and can be directed towards the required lineage based on clinical needs [42, 43]. Khan et al. have reported transplanting HPCs through the hepatic artery to manage hyperbilirubinemia in biliary atresia. The authors observed a threefold drop in total bilirubin after just one month [44]. In 2019, human CD34+ progenitor cells were transplanted in five patients through the portal vein or hepatic artery, and these patients exhibited marked improvements in serum bilirubin and albumin levels [45]. Khan et al. have also supplemented their past efforts by transplanting human fetal liver-derived stem cells in 25 patients, reporting a significant decrease in bilirubin, creatinine, and MELD scores after 6 months [46]. Very few clinical trials have been approved for cirrhotic patient with human fetal liver cell transplantation. One trial used splenic artery injection under radiological guidance with up to 109 cells, which resulted in slightly improved Child-Pugh score and no serious adverse events (ClinicalTrials.gov, Identifier: NCT01013194). Promethera Bioscience has developed Hepastem, human adult liver progenitor cells, for phase II transplantation in CNS, UCD, and other chronic liver failure patients. The company has reported transplantation in over 40 patients with up to 73% survival rate who had minor complications [47, 48]. Hepatic progenitor cells can be effectively transplanted into the liver as was shown in hepatocyte therapy approach. Nonetheless, safety and efficacy remains the prime factor preventing HPCs from being used in the clinic as they play a major role in development of primary liver cancers [49].
Bioartificial liver (BAL) trials
A BAL device is an extracorporeal liver support system that performs regular functions and detoxification. Most BAL systems in clinical trials I to III have been reported to encapsulate clonal derivatives of hepatocellular carcinoma cells known as C3A cell line, or freshly isolated porcine hepatocytes in a hollow fiber and extracellular matrix or a bioreactor [50]. In 2002, molecular adsorbent re-circulating system (MARS) and plasma exchange was used to treat liver failure in 60 patients, showing only a 10% mortality rate in the patients [51]. Prometheus standard medical therapy (SMT) was used to treat 68 chronic liver patients, however no improvements in survival were observed [52]. Lee et al. treated 6 acute liver failure patients with the LifeLiver BAL system, and reported decrease in serum ammonia and MELD scores [53]. Several improvements were reported in 2020; Wu et al. reported the fabrication of a whole liver by integrating gelatin and hepatocytes in a decellularized matrix, while Hou et al. described a chitosan/gelatin scaffold that had higher hepatocyte viability (50 % increase) and mechanical strength [54, 55]. In 2018, scientists reported that Extracorporeal cellular therapy (ELAD), a hollow fiber based immortalized C3A human cell line system, was used on 96 alcoholic hepatitis patients with promising results. The results indicated that patients below 46.9 years and MELD < 28 had higher survivability with ELAD, however the study failed to show better results for the elderly [56]. Subsequently, ELAD was used in 78 patients, but the study failed after 600 days as survival dropped below 70% [57]. Additionally, human ESC (Embryonic Stem Cell) and iPSC derived cells have been considered for BAL devices for a long time [58]. Most recently, Takeishi et al. used hiPSC derived hepatocytes, endothelial cells, and biliary epithelial cells to repopulate a liver scaffold and transplanted it into immunocompromised rats [59]. There are several clinical trials underway now with ELAD, potentially advanced by organoid based system with more metabolic functionality. Yet, the myriad of adverse effects and the inability of BAL to improve survival over longer periods overshadow its potential [60].
Liver organoids
There has been remarkable progress in cell based liver disease treatments over the last decade, although no clinical trial has progressed to mainstream medical therapies. Most clinical trials have yet to increase survivability in elderly patients. This uncertainty has fueled the rise of human liver organoids (HLOs), 3D self-organizing assemblies derived from adult stem/ progenitor cells or PSCs that differentiate into single or multi-tissue structures. These organoids have shown encouraging developments over the years. The different types of these HLOs are discussed below (Fig. 1).
Epithelial organoids
Epithelial organoids are derived from a single germ layer, and small fragments of these organoids are capable of regenerating into entire organoids (Fig. 1) [61]. The first reported generation of a primitive hepatic organoid was using highly proliferative rat hepatocytes in 1999 by Mitaka et al. These pseudo-organoids did not proliferate or have complex functional capacities [62, 63]. Following this advance, Huch et al. in 2013 developed a culture system of leucine-rich-repeat-containing G-protein-coupled receptor 5+ (Lgr5+) murine bile duct stem cell derived organoids that grew indefinitely in vitro (Table 1) [19]. The investigators also reported that clonal hepatocytes were generated from human epithelial cellular adhesion molecule + (EpCAM+) cells in the periphery of the adult bile ducts [41]. The production methods of organoids continue to advance further. Due to the improved oxygenation in the spinner flasks, organoids derived from Lgr5+ adult human stem cells rapidly proliferated to reach an average 40-fold cell expansion after 2 weeks, compared with a 6-fold expansion in static cultures [64]. Hu et al. have successfully synthesized hepatocytes derived from fetal and adult cells with 3D culture by collagenase perfusion [65]. Yamamoto et al. reported on the development of induced hepatocytes from murine dermal fibroblasts that formed 3D aggregates, wherein re-aggregation was important for final maturation and was activated by Hippo signaling [66]. Further development in technique and culture media led Prior et al. to successfully isolate bipotent Lgr5+ human embryonic hepatoblasts which retain their ability to form hepatocytes or cholangiocyte organoids [67].
Table 1.
Post-transplant features of animal derived liver organoids in vivo.
| Database | Search term used | Hits | ||||
|---|---|---|---|---|---|---|
| PubMed | ((liver organoid) OR (liver bud)) AND (organoid transplant) | 72 | ||||
| Organoids | Recipient | Transplantatio n site | Purpose | Survival | Liver function | References |
| Mouse immortalized organoids | BALB/c nude mice | Subrenal capsule, omentum | Maturation and Engraftment | — | Albumin production+ | [84] |
| Mouse Lgr5+ stem cell derived organoids | FRG mice | Intrasplenic injection | Therapeutic | Improved | — | [19] |
| ROSA26 C57BL/6 mouse liver organoids | Wild-type C57BL/6 mice | Liver | Maturation and Engraftment | Limited | — | [74] |
| Mutant murine liver organoids | C57BL/6 and NSG mice | Subcutaneous injection | Disease Modeling | Worsened | — | [89] |
| Murine liver tumor organoids | NOG mice anti-cancer treated | Subcutaneous | Therapeutic | Improved | — | [92] |
| Murine hepatobiliary organoids | FRG mice | Vascularized chambers | Therapeutic | Improved | Albumin production+ | [100] |
| Canine liver organoid | COMMDI-deficient Beagle–Bedlington | Intrahepatic injection | Therapeutic | — | Cu excretion+ | [94] |
| Tumorigenic mouse liver organoids | NOD-SCID mice | Liver, subcutaneous | Disease Modeling | Worsened | — | [91] |
Multi-tissue and multi-organ organoids
Multi-tissue organoids are derived from co-culture of at least two germ layers or co-differentiation of PSCs, whereas multi-organ organoids are more complex, and incorporate multiple organs with interconnected structures (Fig. 1) [61]. In 2013, 3D transplantable and vascularized HLOs were first created from human iPSCs [20]. After inducing hepatoblasts from human iPSCs and mixing them with stromal cells such as mesenchymal cells and vascular endothelial cells, the cells self-aggregated and self-organized into a spherical liver bud [20]. Thus, the generated HLOs lacked long-term self-renewal, but the organoids efficiently vascularized within 48 hours and engrafted to produce albumin and were involved in the metabolism of drugs, bile, and cholesterol, resembling the fetal liver at around 2nd-3rd trimester stage. A single-cell RNA sequencing analysis of the iPSC derived HLOs revealed that each cell type had a distinct sign of maturation that depended on the endothelial-mesenchymal-endodermal communications [68]. In another method, Stevens et al. bio-fabricated seeds of human liver tissue, which are essentially multi-tissue organoids, by engineering human primary hepatocytes, endothelial cells, and fibroblasts based on cell signaling networks [69]. More recently, iPSC generated endoderm derived HLOs were shown to acquire more functional characteristics of mature hepatocytes in vitro [70]. Koike et al. have succeeded in constructing hepato-biliary-pancreatic (HBP) connections in hiPSC derived organoid culture by emulating the foregut-midgut boundary, a prospective region where HBP precursors segregate out to form individual organs [71]. Even with these advances, one limitation of using iPSCs is that they can become genetically unstable due to extensive proliferation and exposure to reprogramming factors [72]. However, proper monitoring and current Good Manufacturing Practice (cGMP) can be used to overcome this problem. Thus, organoids are the potential tools to address the current transplantation needs.
Transplantation of Liver organoids at different sites
Preclinically, liver organoids have been transplanted into various sites in vivo (Fig. 2B) (Table 1 and 2). There are various sites of engraftment: liver, mesentery, kidney capsule, and omentum, but a complete replacement of liver function is yet to be reported. Numerous surgical techniques have been developed that could be applied to transplant organoids with varying regimes of drugs and immune-deficient organoids to prevent graft rejection. Therefore, various reports of successful transplantation have been recorded, and some of the transplantations are described below.
Table 2.
Post-transplant features of human liver organoids in vivo.
| Database | Search term used | Hits | ||||
|---|---|---|---|---|---|---|
| PubMed | ((human liver organoid) OR (human liver bud)) AND (organoid transplant) | 43 | ||||
| Organoids | Recipient | Transplantatio n site | Purpose | Survival | Liver function | References |
| Human iPSC (hiPSC) derived liver bud organoids | Ganciclovir treated TK-NOG mice | Mesentery, cranium, subrenal capsule | Maturation, Engraftment, and Therapeutic | Improved | Albumin production+ | [20, 78, 79, 83, 88] |
| Human EpCAM+ duct cell derived organoids | Retrorsine/CCl4-treated Balbc/nude mice | Intrasplenic injection | Maturation and Engraftment | — | Albumin production+ | [41] |
| 3D printed human LBs | Wild type Lewis rats | Liver | Maturation and Engraftment | — | Albumin production+ | [101] |
| hiPSC derived liver bud organoids | NOD/SCID mice | Cranium | Vascularization | — | Albumin production+ | [68] |
| hiPSC derived liver organoids | Diphtheria toxin-induced Alb-TRECK/SCID mice | Subrenal capsule | Therapeutic | Improved | Serum ALT and AST levels decreased | [85] |
| hiPSC derived liver organoids | Alb-TRECK/SCID mice | Subrenal capsule | Therapeutic | Improved | Albumin production+ | [95] |
| hiPSC derived liver organoids | Retrorsine treated FNRG mice | Intrasplenic injection | Therapeutic | — | Albumin production+ | [65] |
| hiPSC derived HBPOs | NSG mice | Subrenal capsule | Maturation and Engraftment | — | — | [71] |
| hiPSC derived liver organoids | NSG mice | Intrsplenic injection | Therapeutic | — | Albumin production+ | [70] |
| hiPSC derived liver organoids | Athymice nude rats | Intrsplenic injection | Therapeutic | Improved | Serum ALT and AST levels decreased | [93] |
| hiPSC derived liver organoids | Immunocompromised rats | Liver, intraportal | Therapeutic | Improved | Albumin production+ | [80] |
| hiPSC derived liver organoids | Piglets | Portal vein injection | Safety and Efficacy | — | Albumin production+ | [104] |
| Human amniotic mesenchymal stem cells | CCl4-treated CD1 mice | Intrsplenic injection | Therapeutic | Improved | Albumin production+ | [106] |
| hPSC derived liver organoids | NSG mice | Subrenal capsule | Maturation and Engraftment | — | Albumin production+ | [86] |
| hiPSC-derived cholangiocyte organoids | NSG mice, ex vivo human liver | Intrahepatic ducts | Therapeutic | Improved | Bile production+ | [81, 82] |
| HLA mismatched hiPSC liver bud organoids | NOG-HLA-A2Tg mice | Liver | Disease Modeling | — | Albumin production+ | [90] |
Orthotopic (Liver)
The methods for delivery into the liver are divided into three main routes: a splenic, or portal vein injection, or liver implantation. Huch et al reported that transplantation of Lgr5+ stem cell-derived murine liver organoid by splenic injection into FRG mice increased survival (Table 1) [19]. Researchers have also reported that human adult liver EpCAM+ cell derived HLOs showed albumin production in mice with liver injury (Table 2) [41]. Stevens et al. also transplanted human artificial liver “seeds” into mice experiencing liver failures and succeeded in expanding these multi-tissue organoids compared to randomly organized cells [69, 73]. HiPSC generated endoderm derived HLOs have also been efficiently engrafted in mice livers, and they expressed human albumin at even day 32 following a single intrasplenic injection [70]. Zhou et al. attempted to inject ROSA26 C57BL/6 murine single cells or organoids by splenic injection to implant them into the liver directly. The result showed that an organoid injected by direct orthotopic implantation showed limited signs of engraftment, and turned necrotic over time due to graft rejection [74]. Hu et al. injected HLOs derived from fetal liver as single cells into immunodeficient mice with a damaged liver, in the form of a splenic injection. Post-injection human albumin in the serum had risen 200-fold to more than 200 μg/ml on average, leading us to believe this therapeutical method can successfully repopulate damaged livers [65]. Other reports have shown that orthotopic administration of liver organoids from hESC, hiPSC, and canine hepatic progenitors to mice, rats, and dogs with liver damage respectively, reduced AST and ALT levels and improved liver function [75–77]. Our group has transplanted iPSC derived HLOs into the liver, kidney, and brain of immunodeficient mice, and extensive vascularization was observed leading to blood perfusion and eventual hepatocyte maturation. The transplantation of this liver bud into immunodeficient liver failure mice significantly improved their survival rate [20, 78, 79]. More recently, Tsuchida et al. posited that portal vein injection of fetal liver and hiPSC derived HLOs was safe and effective in treating rat chronic liver damage. They also reported about 70% replacement of the damaged liver after 120 days with an almost 100% survival rate [80]. The intravenous transplantation techniques are limited in their capacity for the treatment of intrahepatic injury, but the limitations can be overcome by techniques involving biliary reconstruction. Recently, it has been reported that primary cholangiocyte derived organoid replacement in the liver and bile ducts of mice with extrahepatic biliary injury has improved the prognosis [81, 82]. All in all, orthotopic liver transplantation has shed much needed light on the cellular and functional requirements needed for the treatment of liver diseases.
Ectopic
Mesentery
At the time of this publication, we are the only group to transplant hiPSC derived liver buds (LBs) into the mesentery. The human serum albumin levels in the blood 30 days after transplantation are higher when transplanted into the proximal mesentery (>1000 ng/ml) compared to the distal mesentery (<200 ng/ml) [78]. The mice exhibited human-specific drug metabolism after about 30 days. Additionally, the survival rate of ganciclovir-induced liver failure TK-NOG mice 30 days after the transplantation of LBs was >60% when compared to sham [20]. Thus, transplantation of liver organoids in the mesentery not only induces maturation but also adds therapeutic benefits.
Cranial window
Cranial window is a well-studied technique in brain physiology that has been repurposed to study vascular biology and engineering due to high vascularity and ease of optical access. Takebe et al. reported transplantation of hiPSC derived liver organoids into NOD/SCID mice to intravitally assess their vascularization potential. It was observed that the endothelial cells in transplanted organoids evolved functional anastomosis to recipient blood vessels at 48 hrs, increasing the chances of engraftment and maturation [83].
Kidney capsule
The transplantation of HLOs into the subrenal capsule is one of the most studied sites and technically the easiest for investigating vascularization, growth and maturation [84]. Furthermore, human albumin in the blood 30 days after transplantation was similar to those transplanted into the proximal mesentery (>800 ng/ml) [78, 83]. There have been reports claiming that both the survival rate and liver functions were improved after functional iPSC derived HLOs were transplanted into the renal capsule of mice with acute liver failure [85]. Almost 70% of the mice showed recovery from acute liver failure upon the HLO transplantation and human albumin was detected at the concentration of 1128±338.1 ng/ml and 988.2±660.3 ng/ml at 2 and 7 days after transplantation. Most recently, Harrison et al. showed a scalable method for PSC derived HLO production that was transplanted in the subrenal capsule with Matrigel and FGF2 [86].
Omentum
Saito et al. described transplantation of murine liver organoids into a pocket under the kidney capsule and the omentum of BALB/cAJcl mice. These organoids were generated by mouse-immortalized hepatocytes and nonparenchymal cells in a radial flow bioreactor. The expression of albumin mRNA increased at 8 weeks after the transplantation of the murine organoids in the omentum and kidney [84]. Therefore, the omentum serves as a site for maturation of liver organoids.
Ectopic transplantation of HLOs has taught us a lot regarding the niche, vascularization, and cellular support required for proper engraftment. However, from a biological perspective, a perfect site for transplantation of HLOs to complement the liver is yet to be discovered. For clinical application, we must also consider less invasive routes for administration of the organoids and safety of patients with poor systemic conditions. However, it is noteworthy that intra-portal administration is common in some hepatocyte transplantation studies [87]. The omentum is another alternative candidate for transplantation site since it has ample blood vessels for vascularization, and its proximity and unique microenvironment is amenable to liver development.
Applications of transplanted HLOs
Organoid transplantation has opened up new avenues of research in hopes of supporting disease treatment and liver transplantation (Fig. 3). Extensive experimentations in the field have borne many facets of utility:
Figure 3.
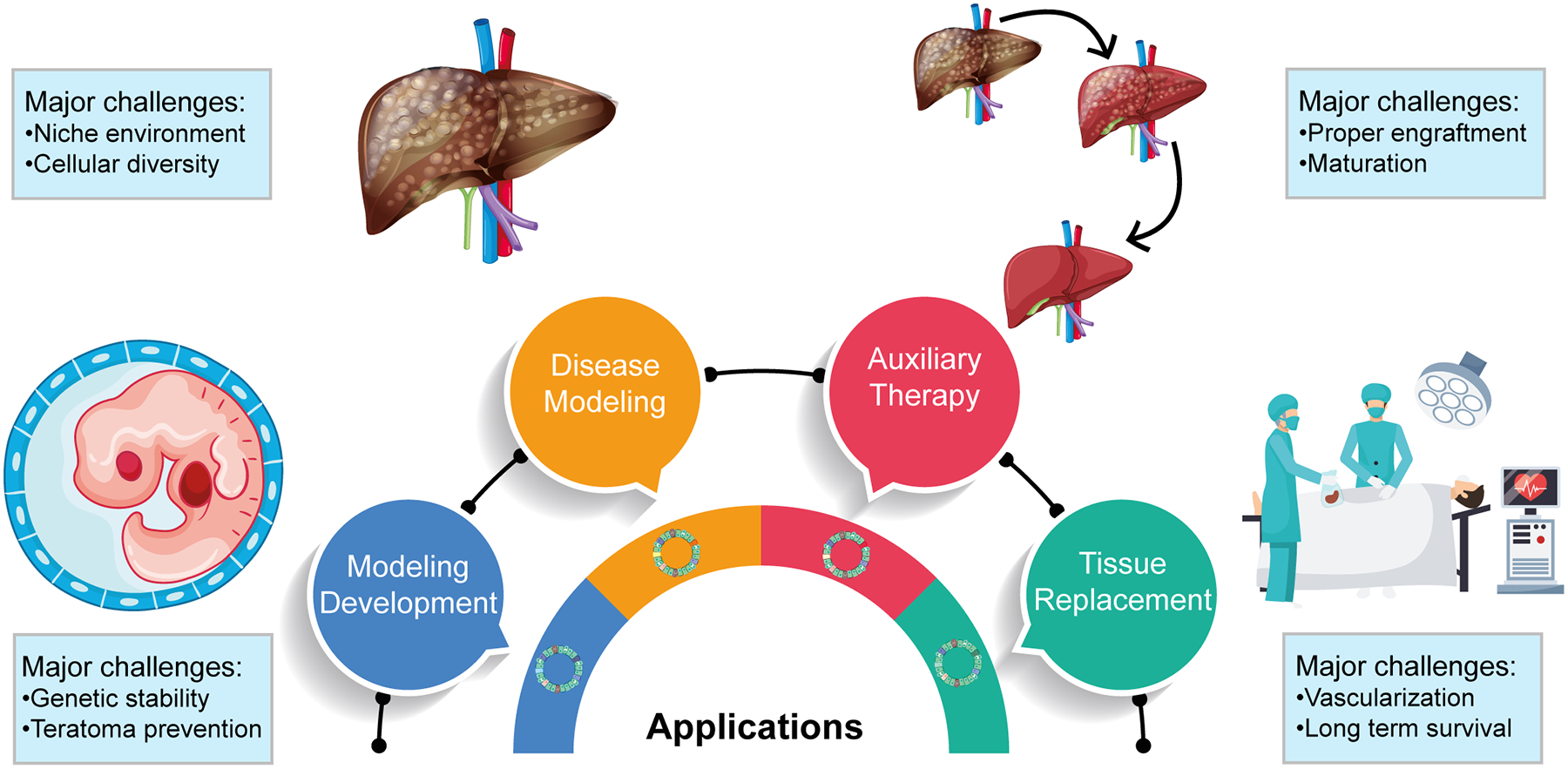
Applications of transplanted human liver organoids derived from hPSCs, and their major challenges.
Modeling development
Most HLOs are fetal in nature and express immature liver markers present in fetal development, therefore organoids are the perfect tool to understand the developmental process by tracking their maturation. For example, we recently applied single cell RNA sequencing technology to analyze human iPSC derived multi-cellular organoids over time, highlighting a unique role of inter-cellular communication during liver development, otherwise inaccessible in human. Increasing evidence indicate that the inclusion of stromal population is essential for post-transplant development of hepatic vascularization and function [20, 78, 83, 88]. Nie et al. reported that endothelial and mesenchymal cells played a major role in liver development and regeneration. The authors’ data suggests that endothelial and mesenchymal cells provide a unique microenvironment to promote hepatic gene expression, and thereby regeneration in acute liver failure mice [85]. Transplanted iPSC derived HLOs indicated that VEGF crosstalk potentiates the endothelial network thereby promoting hepatoblast differentiation [68]. Recent developments in organoid technology and transplantation techniques have led to a better understanding of the multilineage communication inherent to liver development.
Disease modeling
Kras mutant murine liver organoids have been used to model Cholangiocarcinoma (CCA). The p3 gene was deleted in these organoids and transplanted subcutaneously in mice. The organoids formed tumors that bore a striking resemblance to CCAs [89]. Mori et al. transplanted HLA-A2 human hematopoietic stem cells and HLA mismatched human liver bud organoids into humanized mice to model alloimmune response. The researchers found that human T cells invaded the HLOs to induce graft rejection [90]. Finally, genetically modified tumorigenic murine liver organoids were transplanted in NOD-SCID mice to study tumor development. The resulting neoplastic tissue spread to form cholangiocarcinoma, however dual blockade of FF-Erk signaling significantly reduced tumor volume [91]. Additionally, our group has found human specific metabolism of ketoprofen or debrisoquine in HLOs transplanted in TK-NOG mice [20]. Even though HLOs are being used in drug screening, these transplantation procedures present an opportunity to study the systemic effects of various drugs in trial. Combined, these studies demonstrate the utility of liver organoids in the study of human diseases and investigation of therapeutic targets.
Auxiliary therapy
Organoids are already being used in different organs for multiple clinical trials as an auxiliary therapy to treat metabolic diseases by complementing as little as 1% of functional tissue [73]. Additionally, Cao et al. devised a method to transplant tumorigenic murine liver organoids into NOG mice by subcutaneous implantation. The scientists tested anti-cancer drugs on the organoids and showed that they reduce proliferation [92]. Another group generated hepatocyte-like organoids from hiPSCs and transplanted them by intrasplenic route in athymic nude rats. The liver-failure rats had higher survival rates due to human albumin secretion and improvements in ALT and AST levels [93]. In 2020, COMMD1-Deficient canine liver organoids were genetically restored using lentiviral transduction. The canine organoids were transplanted in an intrahepatic injury model using a portal catheter, which resulted in long-term survival of the organoids and led to improved excretion of copper in bile [94].
Organ replacement
Organ replacement is being attempted in multiple studies to replace whole parts of organs as a functional unit and to treat diseases. Zhang et al. reported hiPSC derived PFG that formed HLOs. The authors transplanted the HLOs in Alb-TRECK/SCID mice and showed that this recued the mice from liver failure [95]. In 2010, another group showcased the regenerative effect of fetal liver and iPSC HLOs by portal vein injections in a rat Retrorsine/Partial Hepatectomy model. The transplantation results exhibited an incredible 70% replacement of the damaged liver. The authors were also able to detect albumin 14 days post-transplantation verifying the method as a potential treatment for chronic liver disease [80]. More recently, it was shown that human cholangiocyte derived organoids could reconstruct the extrahepatic biliary tree. When these organoids were transplanted in extrahepatic biliary injury mouse model, the cells from the organoid replaced the native bile duct and resulted in increased bile excretion [81]. The same group also succeeded in transplanting the organoids in an ex vivo human liver from deceased donors undergoing normothermic perfusion. The organoid grafted livers had repaired intrahepatic ducts and were maintained for up to 100 hours without any major complications [82].
Challenges ahead
As with many new technologies, organoids have many limitations and challenges that need to be overcome before moving to a clinical setting. Clinical safety, efficacy and ethical guidelines have yet to be established for liver organoid transplantation, but effective interdisciplinary collaboration between clinicians, researchers, and pharmaceutical industries will facilitate the proper protocol development. There are also fears of HLOs undergoing genetic instability leading to the formation of teratomas, however that has largely been assuaged even with long term cultures [41]. The results of hepatocytes transplantation for many metabolic liver diseases such as CNS Type 1, glycogen storage disease type 1a have been encouraging, but persistent recovery of at least ~1 to 5% of total liver function remains a major challenge, which is thought to be sufficient for clinical improvement. The transplanted hepatocytes engrafted into the liver and continued to confer functional augmentation for a few months at best [87]. Another key limitation of organoids is the lack of innate cellular diversity, and their global maturation over time. Proper environment and support which include differential and incremental exposure to hormones, oxygen, nutrients, and growth for differentiation and proliferation is an equally important issue, but this too can be addressed by mixing in specific cells that provide a unique niche to confer precise trophic effects [96]. Stromal cells aid in this context by forming connective tissue [97]. Furthermore, there is a crucial need to develop more robust and well-defined biomaterials for proper maturation of organoids [98]. Ye et al. have made great strides in this regard by developing a chemically define hydrogel that is capable of promoting proliferation and differentiation to induce specific liver functions in HLOs [99].
One of the most important aspects is mitigating hypoxia that becomes more and more difficult with the size and complexity of organoids. Recent data suggest that liver sinusoidal cells can promote vascularization and thus support survival over long periods of time [100]. Yanagi et al. attempted transplantation of 3D printed scaffold free LBs in ligated and transected liver parenchyma. The authors reported that the LBs did not survive long due to non-viable parenchyma and lack of vasculature [101]. However, there have been great strides in addressing the deficiency of angiogenetic features and implantation over the years [102]. In 2018, a microsurgical method enabled bleeding free engraftment of 3D printed LBs in rat liver [103]. Another major challenge is translating the therapeutic experiments to the clinic since results do not always conform between species. Nevertheless, Sampaziotis et al. have shown much promise by transplanting cholangiocyte organoids to repair bile ducts in deceased human transplant donor livers, similar to mice, ex vivo using fluoroscopic guidance [82]. Although there is a dire need for transplants, the cost, safety and ethical concerns of HLOs have not been addressed in the long run. Even though safety is a prime issue, recent developments in several models have shown time and again that when controlled properly liver organoids can be safely transplanted in a clinical setting without any risk of integration at extrahepatic sites [104].
Conclusion
HLOs have become a versatile tool for understanding the development and pathology of the liver with a wide range of potential clinical applications including personalized medicine and cell therapy. For this purpose, the cooperation of physicians, biologists, bioengineers, and specialists, has become necessary [105]. Clinical applications and basic research with HLOs have the potential to grow tremendously over the next decade, especially in liver transplantation. Organoid therapy may be a powerful adjunctive or alternative to deceased donor or living donor liver transplantation in the future because of its ability to suppress liver damage. This therapy may be effective for the recipient of liver transplantation, or patients who are ineligible for liver transplantation, or even as organ preservation in donor livers to inhibit damage or to promote regeneration.
Acknowledgement
The authors wish to acknowledge Drs. Kyle Lewis, Takuma Iguchi, and Kentaro Iwasawa for critically reviewing the article. We would also like to thank all other Takebe lab members for their support.
Funding
This work was supported by Cincinnati Children’s Research Foundation grant, the Falk Catalyst Research Awards Program, NIH Director’s New Innovator Award (DP2 DK128799-01) and CREST (20gm1210012h0001) grant from Japan Agency for Medical Research and Development (AMED) to TT. This work was also supported by an NIH grant UG3/UH3 DK119982, Cincinnati Center for Autoimmune Liver Disease Fellowship Award, PHS Grant P30 DK078392 (Integrative Morphology Core and Pluripotent Stem Cell and Organoid Core) of the Digestive Disease Research Core Center in Cincinnati, Takeda Science Foundation award, Mitsubishi Foundation award and AMED grants JP18fk0210037h0001, JP18bm0704025h0001, JP21gm1210012h0002, JP21bm0404045h0003, and JP21fk0210060h0003, JST Moonshot JPMJMS2022-10 and JPMJMS2033-12, and JSPS KAKENHI Grant JP18H02800, 19K22416. TT is a New York Stem Cell Foundation – Robertson Investigator.
Footnotes
Conflict of interest
The authors have declared that no conflicts of interest exist.
References
- 1.Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. The American Journal of Clinical Nutrition. 2005; 81: 341–54. [DOI] [PubMed] [Google Scholar]
- 2.Akbari S, Arslan N, Senturk S, Erdal E. Next-Generation Liver Medicine Using Organoid Models. Front Cell Dev Biol. 2019; 7: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019; 70: 151–71. [DOI] [PubMed] [Google Scholar]
- 4.Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014; 20: 7312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Network for Organ Sharing. Current state of donation and transplantation.
- 6.Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives. Einstein (Sao Paulo). 2015; 13: 149–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merola J, Schilsky ML, Mulligan DC. The Impact of COVID-19 on Organ Donation, Procurement, and Liver Transplantation in the United States. Hepatology Communications. 2021; 5: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131: 861–72. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y, Sekine K, Kin T, Takebe T, Taniguchi H. Self-Condensation Culture Enables Vascularization of Tissue Fragments for Efficient Therapeutic Transplantation. Cell Rep. 2018; 23: 1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takebe T, Enomura M, Yoshizawa E, et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell. 2015; 16: 556–65. [DOI] [PubMed] [Google Scholar]
- 11.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014; 345: 1247125. [DOI] [PubMed] [Google Scholar]
- 12.Wilson HV. A NEW METHOD BY WHICH SPONGES MAY BE ARTIFICIALLY REARED. Science. 1907; 25: 912–5. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009; 459: 262–5. [DOI] [PubMed] [Google Scholar]
- 14.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008; 3: 519–32. [DOI] [PubMed] [Google Scholar]
- 15.Willyard C. The boom in mini stomachs, brains, breasts, kidneys and more. Nature. 2015; 523: 520–2. [DOI] [PubMed] [Google Scholar]
- 16.Stanger BZ. Cellular homeostasis and repair in the mammalian liver. Annu Rev Physiol. 2015; 77: 179–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss P, Taylor AC. RECONSTITUTION OF COMPLETE ORGANS FROM SINGLE-CELL SUSPENSIONS OF CHICK EMBRYOS IN ADVANCED STAGES OF DIFFERENTIATION. Proc Natl Acad Sci U S A. 1960; 46: 1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirahashi H, Wu J, Yamamoto N, et al. Differentiation of human and mouse embryonic stem cells along a hepatocyte lineage. Cell Transplant. 2004; 13: 197–211. [DOI] [PubMed] [Google Scholar]
- 19.Huch M, Dorrell C, Boj SF, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013; 494: 247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013; 499: 481–4. [DOI] [PubMed] [Google Scholar]
- 21.Anderson TN, Zarrinpar A. Hepatocyte transplantation: past efforts, current technology, and future expansion of therapeutic potential. Journal of Surgical Research. 2018; 226: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorns C, Ellis EC, Nowak G, et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. Journal of Internal Medicine. 2012; 272: 201–23. [DOI] [PubMed] [Google Scholar]
- 23.Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatric Research. 2018; 83: 232–40. [DOI] [PubMed] [Google Scholar]
- 24.Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992; 24: 3052–3. [PubMed] [Google Scholar]
- 25.Horslen SP, Fox IJ. HEPATOCYTE TRANSPLANTATION. Transplantation. 2004; 77: 1481–6. [DOI] [PubMed] [Google Scholar]
- 26.Darwish AA, Sokal E, Stephenne X, Najimi M, de Goyet Jde V, Reding R. Permanent access to the portal system for cellular transplantation using an implantable port device. Liver Transpl. 2004; 10: 1213–5. [DOI] [PubMed] [Google Scholar]
- 27.Fox IJ, Chowdhury JR, Kaufman SS, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998; 338: 1422–6. [DOI] [PubMed] [Google Scholar]
- 28.Najimi M, Defresne F, Sokal EM. Concise Review: Updated Advances and Current Challenges in Cell Therapy for Inborn Liver Metabolic Defects. Stem Cells Transl Med. 2016; 5: 1117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhawan A, Mitry RR, Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. Journal of inherited metabolic disease. 2006; 29: 431–5. [DOI] [PubMed] [Google Scholar]
- 30.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010; 12: 87–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Zhang C, Gu J, et al. A Randomized, Placebo-Controlled Trial of Human Umbilical Cord Blood Mesenchymal Stem Cell Infusion for Children With Cerebral Palsy. Cell Transplantation. 2018; 27: 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Meng Y, Han Z, Ye F, Wei L, Zong C. Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell & Bioscience. 2020; 10: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen A, Newsome PN. Mesenchymal Stromal Cells, a New Player in Reducing Complications From Liver Transplantation? Frontiers in Immunology. 2020; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi M, Liu Z, Wang Y, et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl Med. 2017; 6: 2053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bl Lin, Jf Chen, Wh Qiu, et al. Allogeneic bone marrow–derived mesenchymal stromal cells for hepatitis B virus–related acute ‐ on ‐ chronic liver failure: a randomized controlled trial. Hepatology. 2017; 66: 209–19. [DOI] [PubMed] [Google Scholar]
- 36.Fang X, Liu L, Dong J, et al. A study about immunomodulatory effect and efficacy and prognosis of human umbilical cord mesenchymal stem cells in patients with chronic hepatitis B-induced decompensated liver cirrhosis. J Gastroenterol Hepatol. 2018; 33: 774–80. [DOI] [PubMed] [Google Scholar]
- 37.Lee C-W, Chen Y-F, Wu H-H, Lee OK. Historical Perspectives and Advances in Mesenchymal Stem Cell Research for the Treatment of Liver Diseases. Gastroenterology. 2018; 154: 46–56. [DOI] [PubMed] [Google Scholar]
- 38.Moroni F, Dwyer BJ, Graham C, et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nature Medicine. 2019; 25: 1560–5. [DOI] [PubMed] [Google Scholar]
- 39.Weiss TS, Lichtenauer M, Kirchner S, et al. Hepatic progenitor cells from adult human livers for cell transplantation. Gut. 2008; 57: 1129–38. [DOI] [PubMed] [Google Scholar]
- 40.Messina A, Luce E, Hussein M, Dubart-Kupperschmitt A. Pluripotent-Stem-Cell-Derived Hepatic Cells: Hepatocytes and Organoids for Liver Therapy and Regeneration. Cells. 2020; 9: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huch M, Gehart H, van Boxtel R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015; 160: 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao M-S, Khan A-A, Parveen N, Habeeb M-A, Habibullah C-M, Pande G. Characterization of hepatic progenitors from human fetal liver during second trimester. World journal of gastroenterology. 2008; 14: 5730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katoonizadeh A, Poustchi H, Malekzadeh R. Hepatic progenitor cells in liver regeneration: current advances and clinical perspectives. Liver International. 2014; 34: 1464–72. [DOI] [PubMed] [Google Scholar]
- 44.Khan AA, Parveen N, Mahaboob VS, et al. Management of Hyperbilirubinemia in Biliary Atresia by Hepatic Progenitor Cell Transplantation Through Hepatic Artery: A Case Report. Transplantation Proceedings. 2008; 40: 1153–5. [DOI] [PubMed] [Google Scholar]
- 45.Gordon MY, Levičar N, Pai M, et al. Characterization and Clinical Application of Human CD34+ Stem/Progenitor Cell Populations Mobilized into the Blood by Granulocyte Colony-Stimulating Factor. STEM CELLS. 2006; 24: 1822–30. [DOI] [PubMed] [Google Scholar]
- 46.Khan AA, Shaik MV, Parveen N, et al. Human Fetal Liver-Derived Stem Cell Transplantation as Supportive Modality in the Management of End-Stage Decompensated Liver Cirrhosis. Cell Transplantation. 2010; 19: 409–18. [DOI] [PubMed] [Google Scholar]
- 47.Smets F, Dobbelaere D, McKiernan P, et al. Phase I/II Trial of Liver-derived Mesenchymal Stem Cells in Pediatric Liver-based Metabolic Disorders: A Prospective, Open Label, Multicenter, Partially Randomized, Safety Study of One Cycle of Heterologous Human Adult Liver-derived Progenitor Cells (HepaStem) in Urea Cycle Disorders and Crigler-Najjar Syndrome Patients. Transplantation. 2019; 103: 1903–15. [DOI] [PubMed] [Google Scholar]
- 48.Nevens F, Gustot T, Laterre PF, et al. A phase II study of human allogeneic liver-derived progenitor cell therapy for acute-on-chronic liver failure and acute decompensation. JHEP Reports. 2021: 100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo RC-L, Ng IO-L. Hepatic progenitor cells: their role and functional significance in the new classification of primary liver cancers. Liver Cancer. 2013; 2: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens KR, Schwartz RE, Ng S, Shan J, Bhatia SN. Chapter 46 - Hepatic Tissue Engineering. In: Lanza R, Langer R, Vacanti J, eds. Principles of Tissue Engineering (Fourth Edition). Academic Press, Boston, 2014: 951–86. [Google Scholar]
- 51.Huang YK, Tan DM, Xie YT, et al. Randomized controlled study of plasma exchange combined with molecular adsorbent re-circulating system for the treatment of liver failure complicated with hepatic encephalopathy. Hepatogastroenterology. 2012; 59: 1323–6. [DOI] [PubMed] [Google Scholar]
- 52.Kribben A, Gerken G, Haag S, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012; 142: 782–9.e3. [DOI] [PubMed] [Google Scholar]
- 53.Lee S, Lee J-H, Lee D-H, et al. Phase 1/2a Trial of a Bioartificial Liver Support System (LifeLiver) for Acute Liver Failure Patients. Transplantation. 2018; 102. [Google Scholar]
- 54.Wu G, Wu D, Lo J, et al. A bioartificial liver support system integrated with a DLM/GelMA-based bioengineered whole liver for prevention of hepatic encephalopathy via enhanced ammonia reduction. Biomaterials Science. 2020; 8: 2814–24. [DOI] [PubMed] [Google Scholar]
- 55.Hou Y-T, Hsu C-C. Development of a 3D porous chitosan/gelatin liver scaffold for a bioartificial liver device. Journal of Bioscience and Bioengineering. 2020; 129: 741–8. [DOI] [PubMed] [Google Scholar]
- 56.Thompson J, Jones N, Al-Khafaji A, et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2018; 24: 380–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pyrsopoulos NT, Hassanein T, Subramanian R, et al. THU-272-A study investigating the effect of extracorporeal cellular therapy with C3A cells on the survival of alcoholic hepatitis designed along the guidelines of the NIAAA. Journal of Hepatology. 2019; 70: e282. [Google Scholar]
- 58.Pan X-P, Li L-J. Advances in cell sources of hepatocytes for bioartificial liver. Hepatobiliary & Pancreatic Diseases International. 2012; 11: 594–605. [DOI] [PubMed] [Google Scholar]
- 59.Takeishi K, Collin de l’Hortet A, Wang Y, et al. Assembly and Function of a Bioengineered Human Liver for Transplantation Generated Solely from Induced Pluripotent Stem Cells. Cell reports. 2020; 31: 107711–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chamuleau RA, Poyck PP, Van De Kerkhove M-P. Bioartificial Liver: Its Pros and Cons. Therapeutic Apheresis and Dialysis. 2006; 10: 168–74. [DOI] [PubMed] [Google Scholar]
- 61.Marsee A, Roos FJM, Verstegen MMA, et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell. 2021; 28: 816–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitaka T, Sato F, Mizuguchi T, Yokono T, Mochizuki Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999; 29: 111–25. [DOI] [PubMed] [Google Scholar]
- 63.Mitaka T. Reconstruction of hepatic organoid by hepatic stem cells. Journal of Hepato-Biliary-Pancreatic Surgery. 2002; 9: 697–703. [DOI] [PubMed] [Google Scholar]
- 64.Schneeberger K, Sánchez-Romero N, Ye S, et al. Large-Scale Production of LGR5-Positive Bipotential Human Liver Stem Cells. Hepatology. 2020; 72: 257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu H, Gehart H, Artegiani B, et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018; 175: 1591–606.e19. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto J, Udono M, Miura S, Sekiya S, Suzuki A. Cell Aggregation Culture Induces Functional Differentiation of Induced Hepatocyte-like Cells through Activation of Hippo Signaling. Cell Rep. 2018; 25: 183–98. [DOI] [PubMed] [Google Scholar]
- 67.Prior N, Hindley CJ, Rost F, et al. Lgr5(+) stem and progenitor cells reside at the apex of a heterogeneous embryonic hepatoblast pool. Development. 2019; 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Camp JG, Sekine K, Gerber T, et al. Multilineage communication regulates human liver bud development from pluripotency. Nature. 2017; 546: 533–8. [DOI] [PubMed] [Google Scholar]
- 69.Stevens KR, Scull MA, Ramanan V, et al. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci Transl Med. 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akbari S, Sevinç GG, Ersoy N, et al. Robust, Long-Term Culture of Endoderm-Derived Hepatic Organoids for Disease Modeling. Stem Cell Reports. 2019; 13: 627–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koike H, Iwasawa K, Ouchi R, et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature. 2019; 574: 112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tapia N, Schöler HR. Molecular Obstacles to Clinical Translation of iPSCs. Cell Stem Cell. 2016; 19: 298–309. [DOI] [PubMed] [Google Scholar]
- 73.Sun L, Hui L. Progress in human liver organoids. Journal of Molecular Cell Biology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou VX, Lolas M, Chang TT. Direct orthotopic implantation of hepatic organoids. J Surg Res. 2017; 211: 251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Wang X, Tan Z, et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019; 29: 1009–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pettinato G, Lehoux S, Ramanathan R, et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci Rep. 2019; 9: 8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kruitwagen HS, Oosterhoff LA, van Wolferen ME, et al. Long-Term Survival of Transplanted Autologous Canine Liver Organoids in a COMMD1-Deficient Dog Model of Metabolic Liver Disease. Cells. 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takebe T, Zhang RR, Koike H, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014; 9: 396–409. [DOI] [PubMed] [Google Scholar]
- 79.Takebe T, Enomura M, Yoshizawa E, et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell. 2015; 16: 556–65. [DOI] [PubMed] [Google Scholar]
- 80.Tsuchida T, Murata S, Matsuki K, et al. The Regenerative Effect of Portal Vein Injection of Liver Organoids by Retrorsine/Partial Hepatectomy in Rats. Int J Mol Sci. 2019; 21: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sampaziotis F, Justin AW, Tysoe OC, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017; 23: 954–63. [DOI] [PubMed] [Google Scholar]
- 82.Sampaziotis F, Muraro D, Tysoe OC, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021; 371: 839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takebe T, Sekine K, Kimura M, et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Reports. 2017; 21: 2661–70. [DOI] [PubMed] [Google Scholar]
- 84.Saito R, Ishii Y, Ito R, et al. Transplantation of liver organoids in the omentum and kidney. Artif Organs. 2011; 35: 80–3. [DOI] [PubMed] [Google Scholar]
- 85.Nie YZ, Zheng YW, Ogawa M, Miyagi E, Taniguchi H. Human liver organoids generated with single donor-derived multiple cells rescue mice from acute liver failure. Stem Cell Res Ther. 2018; 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrison SP, Siller R, Tanaka Y, et al. Scalable production of tissue-like vascularised liver organoids from human PSCs. bioRxiv. 2020: 2020.12.02.406835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nussler A, Konig S, Ott M, et al. Present status and perspectives of cell-based therapies for liver diseases. J Hepatol. 2006; 45: 144–59. [DOI] [PubMed] [Google Scholar]
- 88.Sekine K, Ogawa S, Tsuzuki S, et al. Generation of human induced pluripotent stem cell-derived liver buds with chemically defined and animal origin-free media. Scientific Reports. 2020; 10: 17937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saborowski A, Wolff K, Spielberg S, et al. Murine Liver Organoids as a Genetically Flexible System to Study Liver Cancer In Vivo and In Vitro. Hepatology communications. 2019; 3: 423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mori A, Murata S, Tashiro N, et al. Establishment of Human Leukocyte Antigen-Mismatched Immune Responses after Transplantation of Human Liver Bud in Humanized Mouse Models. Cells. 2021; 10: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cristinziano G, Porru M, Lamberti D, et al. FGFR2 fusion proteins drive oncogenic transformation of mouse liver organoids towards cholangiocarcinoma. Journal of Hepatology. 2021. [DOI] [PubMed] [Google Scholar]
- 92.Cao W, Liu J, Wang L, et al. Modeling liver cancer and therapy responsiveness using organoids derived from primary mouse liver tumors. Carcinogenesis. 2018; 40: 145–54. [DOI] [PubMed] [Google Scholar]
- 93.Pettinato G, Lehoux S, Ramanathan R, et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Scientific reports. 2019; 9: 8920–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kruitwagen HS, Oosterhoff LA, van Wolferen ME, et al. Long-Term Survival of Transplanted Autologous Canine Liver Organoids in a COMMD1-Deficient Dog Model of Metabolic Liver Disease. Cells. 2020; 9: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang R-R, Koido M, Tadokoro T, et al. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Reports. 2018; 10: 780–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen C, Soto-Gutierrez A, Baptista PM, Spee B. Biotechnology Challenges to In Vitro Maturation of Hepatic Stem Cells. Gastroenterology. 2018; 154: 1258–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu J, Li P, Wang L, et al. Cancer-Associated Fibroblasts Provide a Stromal Niche for Liver Cancer Organoids That Confers Trophic Effects and Therapy Resistance. Cell Mol Gastroenterol Hepatol. 2021; 11: 407–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim SK, Kim YH, Park S, Cho S-W. Organoid engineering with microfluidics and biomaterials for liver, lung disease, and cancer modeling. Acta Biomaterialia. 2021. [DOI] [PubMed] [Google Scholar]
- 99.Ye S, Boeter JWB, Mihajlovic M, et al. A Chemically Defined Hydrogel for Human Liver Organoid Culture. Advanced Functional Materials. 2020; 30: 2000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yap KK, Gerrand Y-W, Dingle AM, Yeoh GC, Morrison WA, Mitchell GM. Liver sinusoidal endothelial cells promote the differentiation and survival of mouse vascularised hepatobiliary organoids. Biomaterials. 2020; 251: 120091. [DOI] [PubMed] [Google Scholar]
- 101.Yanagi Y, Nakayama K, Taguchi T, et al. In vivo and ex vivo methods of growing a liver bud through tissue connection. Scientific reports. 2017; 7: 14085–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grebenyuk S, Ranga A. Engineering Organoid Vascularization. Frontiers in Bioengineering and Biotechnology. 2019; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eiji K, Shin E. Liver bud transplantation in rats. Magyar Sebészet (Hungarian Journal of Surgery) MaSeb. 2018; 71: 163–9. [DOI] [PubMed] [Google Scholar]
- 104.Tsuchida T, Murata S, Hasegawa S, et al. Investigation of Clinical Safety of Human iPS Cell-Derived Liver Organoid Transplantation to Infantile Patients in Porcine Model. Cell transplantation. 2020; 29: 963689720964384-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takebe T, Wells JM, Helmrath MA, Zorn AM. Organoid Center Strategies for Accelerating Clinical Translation. Cell Stem Cell. 2018; 22: 806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang H, Tian Y, Li X, Yang M, Yan Y. Amniotic mesenchymal stem cells derived hepatocyte-like cells attenuated liver fibrosis more efficiently by mixed-cell transplantation. Int J Physiol Pathophysiol Pharmacol. 2020; 12: 11–24. [PMC free article] [PubMed] [Google Scholar]


