Abstract
Objective:
In mechanically ventilated patients, deep sedation is often assumed to induce respirolysis, i.e. lyse spontaneous respiratory effort, while light sedation is often assumed to preserve spontaneous effort. This study was conducted to determine validity of these common assumptions, evaluating the association of respiratory drive with sedation depth and ventilator-free days in acute respiratory failure.
Design:
Prospective cohort study.
Setting:
Patients were enrolled during two month-long periods in 2016–2017 from five ICUs representing medical, surgical, and cardiac specialties at a U.S. academic hospital.
Patients:
Eligible patients were critically ill adults receiving invasive ventilation initiated ≤ 36 hours before enrollment. Patients with neuromuscular disease compromising respiratory function or expiratory flow limitation were excluded.
Interventions:
Respiratory drive was measured via P0.1, the change in airway pressure during a 0.1-second airway occlusion at initiation of patient inspiratory effort, every 12 ± 3 hours for 3 days. Sedation depth was evaluated via the Richmond agitation-sedation scale (RASS). Analyses evaluated the association of P0.1 with RASS (primary outcome) and ventilator-free days.
Measurements and Main Results:
Fifty-six patients undergoing 197 bedside evaluations across five intensive care units were included. P0.1 ranged between 0–13.3 (median 0.1, interquartile range 0.0–1.3) cm H2O. P0.1 was not significantly correlated with RASS (RSpearman 0.02, 95% CI −0.12 to 0.16; p = 0.80). Considering P0.1 terciles (range < 0.2, 0.2–1.0, and > 1.0 cm H2O), patients in the middle tercile had significantly more ventilator-free days than the lowest tercile (incidence rate ratio [IRR] 0.78, 95% CI 0.65–0.93; p < 0.01) or highest tercile (IRR 0.58, 95% CI 0.48–0.70; p < 0.01).
Conclusion:
Sedation depth is not a reliable marker of respiratory drive during critical illness. Respiratory drive can be low, moderate, or high across the range of routinely targeted sedation depth.
Keywords: anesthesia, analgesics, sedatives, mechanical ventilation, respiratory mechanics
INTRODUCTION
During invasive mechanical ventilation with critical illness, clinical practice guidelines recommend titrating analgesics and sedatives according to estimated pain and agitation/sedation, targeting light sedation where possible (1, 2).
Nevertheless, deep sedation sometimes is prescribed in attempt to facilitate lung-protective ventilation or eliminate patient-ventilator dyssynchrony (3–8). Patients with status asthmaticus, for example, may receive deep sedation in attempt to induce hypoventilation with permissive hypercapnia, thereby abating catastrophic dynamic hyperinflation (9). Similarly, in acute respiratory distress syndrome (ARDS), deep sedation may be chosen in attempt to maintain low tidal volumes and prevent certain patient-ventilator dyssynchronies (3, 10). In such cases, analgesics and sedatives are given not just for analgesic, anxiolytic, or sedating purposes, but also to suppress spontaneous respiratory effort, which we term their respirolytic effect. Efficacy of this practice is unknown.
While partial or complete respirolysis might help facilitate lung protection in select patients, in others it is an unintended side-effect of analgesics and sedatives. Prolonged, complete respirolysis may contribute to diaphragm disuse atrophy, impeding ventilator weaning (11–14). Better understanding of the relationship between sedation depth and respiratory drive is important to provide lung- and diaphragm-protective ventilation (15) and to minimize potentially harmful side-effects of sedation (16, 17).
Respiratory drive can be quantified easily at bedside using P0.1, the change in airway pressure during a brief (0.1 second) airway occlusion at the start of patient inspiratory effort (18). P0.1 quantifies motor output from the respiratory center at the beginning of inspiration and is a well-validated measure of drive available on most modern intensive care unit (ICU) ventilators (19–21).
Yet, respiratory drive is not monitored routinely in clinical practice. Rather, it is often assumed that deep sedation suppresses respiratory drive while light sedation preserves it (1, 3, 22–24). This common clinical assumption conflicts with our own experience at bedside, during which we have observed the effects of sedation on drive to be highly variable. Therefore, this study was conducted to evaluate the association of respiratory drive with sedation depth in patients with acute respiratory failure requiring invasive mechanical ventilation. Given the potential implications of respiratory drive for lung and diaphragm protection, we also evaluated its association with ventilator-free days.
METHODS
Patients
This prospective cohort included critically ill adults for whom invasive mechanical ventilation was newly initiated within the prior 36 hours. Patients were enrolled during two month-long periods in 2016–2017 from five ICUs (medical, surgical, and cardiac) at an academic hospital. Patients were excluded for neuromuscular disease that compromised respiratory function, obstructive airway disease causing expiratory flow limitation, chest tube with active air leak, tracheostomy, or ventilator lacking capability to measure P0.1. The UCSD review board approved the study (Project #181061XX) with consent waiver.
Measurement of P0.1
P0.1 was measured using a validated, automated maneuver on full-feature ICU ventilators (21, 25). During the maneuver, the ventilator inspiratory valve remains closed during the first 100 milliseconds (0.1 second) of the next patient-triggered inspiratory cycle, a period thought too short for detection, conscious or unconscious, by the patient (3). Change in airway pressure during this brief occlusion is measured by the ventilator as P0.1. After the 0.1-second occlusion, the inspiratory valve opens and flow is delivered. Although some ventilators estimate P0.1 during the trigger phase without an occlusion (supplement) (26), for this study, only ventilators that performed the conventional method of P0.1 measurement with a proper 0.1-second occlusion were used. For each assessment, P0.1 measurement was repeated three times consecutively and the average value reported (27, 28).
P0.1 measurement was performed every 12 ± 3 hours for three days (total of up to six assessments), immediately prior to bedside assessment of sedation depth, pain, and delirium. To ensure that data were representative of exposure, measurements were not performed during temporary spontaneous awakening and breathing trials. Periods during which enrolled patients received neuromuscular blockade were excluded from analyses.
Bedside Assessment
Bedside assessments were performed by trained study personnel twice daily immediately after each P0.1 measurement. Sedation depth was assessed with the Richmond Agitation-Sedation Scale (RASS), pain with the Critical Care Pain Observation Tool (CPOT), and delirium with the Confusion Assessment Method for ICU (CAM-ICU) (supplement). Assessments were performed without knowledge of target RASS or nurse-charted value.
Vital signs, ventilator settings, analgesic/sedative exposure, and the most recent blood gas were recorded. Illness severity was assessed via baseline Acute Physiology and Chronic Health Evaluation-II (APACHE-II) and daily Sequential Organ Failure Assessment (SOFA).
Analgesic and Sedative Exposure
Analgesia and sedation were managed per usual care, consisting of nurse-driven titration to achieve a prescribed target RASS range (e.g. RASS 0 to −2; −1 to −3; −2 to −4; or −3 to −5) (29–32). Daily interruption of sedation and spontaneous breathing trials for extubation readiness screening were performed per hospital protocol, consistent with practice guidelines (2, 33). To quantify analgesic/sedative exposure, three time-intervals were considered: (1) the current dose of continuous infusion sedatives/analgesics at evaluation; (2) the total cumulative dose, including boluses, received during the hour immediately preceding bedside evaluation; and (3) the total dose, including boluses, received during the 12 hours immediately preceding bedside evaluation.
Clinical Outcomes
Patients were followed until discharge for vital status, ventilator-free days through day 28, duration of mechanical ventilation, and ICU/hospital lengths of stay. Ventilator-free days was computed as time between successful liberation from mechanical ventilation and day 28 for survivors, and zero days for non-survivors (34). Patients discharged alive before day 28 were presumed alive at day 28.
Statistical Analysis
For the primary outcome, Pearson and Spearman correlations evaluated the association of P0.1 with RASS for each of the six timepoints and for all observations in aggregate. Linear mixed models were constructed to evaluate the within-subject association of P0.1 with sedation depth over time (fixed effects) with random subject-specific intercept. Model marginal r2 quantified the proportion of variance explained by fixed effects. Sensitivity analyses are detailed in the supplement.
Linear mixed models with random intercept also were constructed to evaluate the association of P0.1 with pain, delirium, analgesic and sedative medication exposure, blood gas results, respiratory parameters, and illness severity over time.
To evaluate the association of respiratory drive with ventilator-free days, Poisson regression was conducted entering each patient’s average P0.1 over the available evaluations as the predictor of interest. P0.1 was entered as both a linear and quadratic term, with statistical significance for the quadratic term indicative of a nonlinear relationship warranting its retention in models. Multivariable models adjusted for APACHE-II, baseline PaO2:FiO2, and average RASS. To aid interpretability of a nonlinear relationship, unadjusted Poisson regression was rerun entering P0.1 as a categorical variable split into terciles. LOESS regression was performed to visualize data.
To evaluate association of respiratory drive with in-hospital mortality, logistic regression models were used with P0.1 entered as linear and quadratic terms as above.
Two-sided alpha of 0.05 was considered statistically significant.
RESULTS
Patients
Sixty-one patients met screening criteria, four were excluded for support with a ventilator lacking P0.1 capability, and one was excluded for neuromuscular disease that compromised respiratory function. Fifty-six patients were included in the final analysis. Accounting for early deaths, extubations, and neuromuscular blockade use, there were 204 eligible bedside observation periods during the 3-day measurement window (supplement). Seven observations (3.4%) were missing for study staff unavailability, leaving 197 bedside observations with P0.1 measurement for inclusion in the final analysis.
Patients were enrolled median [interquartile range] 19 [10.5–29] hours after intubation. Most patients (77%) were intubated for hypoxemic respiratory failure, and half of patients had ARDS at enrollment (Tables 1, E1). Patients were severely ill at enrollment, indicated by APACHE-II and SOFA scores predicting mortality of 33–50%. Observed in-hospital mortality was 33.9%.
Table 1.
Baseline Characteristics and Outcomes of Study Participants
| Characteristic | Value (n=56) |
|---|---|
| Age, years | 62.5 [53–75.5] |
| Female | 18 (32%) |
| Primary reason(s) for intubationa | |
| Hypoxemia | 43 (77%) |
| Hypercapnia | 5 (9%) |
| Airway protection | 11 (20%) |
| Time from intubation to enrollment, hours | 19 [10.5–29] |
| APACHE-IIb | 32 [28–36] |
| Acute respiratory distress syndrome | 28 (50%) |
| Vasopressor-dependent shock | 23 (41%) |
| Continuous infusion analgesia and sedation at enrollment | 50 (89%) |
| Propofol infusion | 40 (71%) |
| Infusion rate, mcg/kg/min | 35 [25–40] |
| Benzodiazepine infusion, n (%) | 5 (9%) |
| Midazolam-equivalent infusion rate, mg/hc | 4 [2–5] |
| Opioid infusion, n (%) | 25 (45%) |
| Fentanyl-equivalent infusion rate, mcg/hd | 50 [50–100] |
| Dexmedetomidine infusion | 1 (2%) |
| Infusion rate, mcg/kg/h | 0.6 |
| No continuous infusion | 6 (11%) |
| Richmond Agitation Sedation Scale (RASS) | −4 [−4 to −3] |
| Critical Care Pain Observation Tool score (CPOT) | 0 [0–0] |
| Delirium positive Confusion Assessment Method for ICU | 3 (5%) |
| Initial P0.1, cm H2O | 0.4 [0.0–1.2] |
| Initial ventilator management | |
| Volume-targeted pressure assist/control mode | 53 (95%) |
| Tidal volume (mL/kg predicted body weight) | 7.3 [6.5–8.3] |
| Airway driving pressure, cm H2O | 11 [9–14] |
| PEEP, cm H2O | 5 [5–9] |
| PaO2:FiO2 | 213 [168–312] |
| Patient outcomes | |
| Duration of mechanical ventilation, days | 4.5 [2.5–15.5] |
| Ventilator-free days | 12 [0–25] |
| ICU length of stay, days | 8.5 [5–21] |
| Hospital length of stay, days | 14 [6.5–22.5] |
| New tracheostomy prior to discharge | 7 (13%) |
| Death before hospital discharge | 19 (33.9%) |
Participants could have multiple reasons for intubation; categories are not mutually exclusive.
An APACHE score of 30 corresponds to a predicted in-hospital mortality of approximately 50%.
Midazolam-equivalent dose calculated as midazolam 1 mg = lorazepam 0.5 mg.
Fentanyl-equivalent dose calculated as fentanyl 100 mcg = hydromorphone 2 mg = morphine 10 mg.
Sedation Depth, Pain, and Delirium
Patients were deeply sedated (RASS −4 or −5) during approximately half of evaluations (48.2%); most patients (75%) had at least one study assessment indicating deep sedation. The lightest sedation depth observed was RASS +1, corresponding to restless/anxious appearance without aggressive movements. Within-patient range in RASS during study observations was at least 1 level in 67.9% of patients, and at least 2 levels in 41.1% of patients (Table E2). Patients had behavioral findings suggestive of pain (CPOT > 2) during 6.1% of evaluations. Patients screened positive for delirium during 20.6% of eligible evaluations.
Respiratory Drive Assessed by P0.1
Across 197 separate evaluations, P0.1 ranged from 0 to 13.3 cm H2O, with median [IQR] value 0.1 [0.0–1.3] cm H2O (Figure E1). During only 38 of 197 measurements (19%) was P0.1 between 0.5 and 1.5 cm H2O, a range considered normal in healthy individuals at rest (20). P0.1 exceeded 1.5 cm H2O during 24.4% (48 of 197) of measurements. By contrast, P0.1 was zero, indicating no respiratory drive, during half (49.2%) of measurements.
Nearly half of all patients (41.1%) had at least one evaluation with P0.1 exceeding 1.5 cm H2O, including 44.4% of patients with ARDS. The average within-patient range of P0.1 during the study period was 1.3 cm H2O (minimum range 0 cm H2O, maximum range 12.2 cm H2O).
Respiratory Drive and Sedation Depth
Figure 1 presents P0.1 values according to RASS for all observations. No compelling association is conspicuous graphically.
Figure 1. P0.1 and Richmond Agitation-Sedation Scale (RASS).
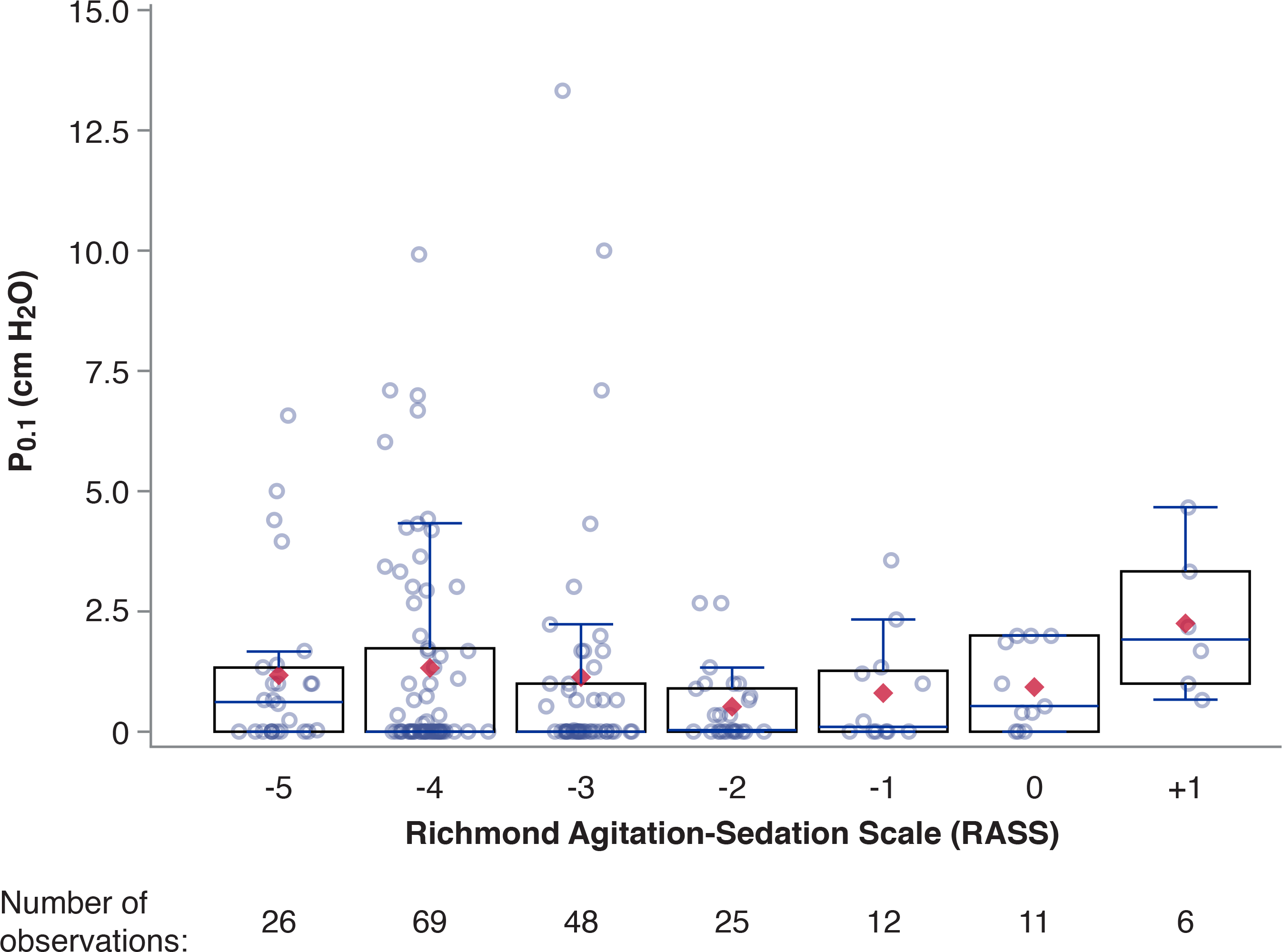
Overlaid box-and-whisker and scatter plot. Boxes represent median with interquartile range; whiskers extend 1.5 times the interquartile range beyond the first and third quartiles per the Tukey method. Diamonds represent mean values. Circles indicate individual observations; total of 197 observations were performed and all displayed. P0.1 values within each RASS level were randomly jittered to facilitate visualization. During the study period, the highest RASS observed was +1.
Eighteen of the 20 highest P0.1 values, including all ten highest P0.1 values, were observed in patients with RASS −3 to −5.
In cross-sectional analysis, RASS was not significantly correlated with P0.1 at any evaluation time (Figure 2). Correlation coefficients were consistently near zero.
Figure 2. Respiratory drive is poorly correlated with sedation depth.
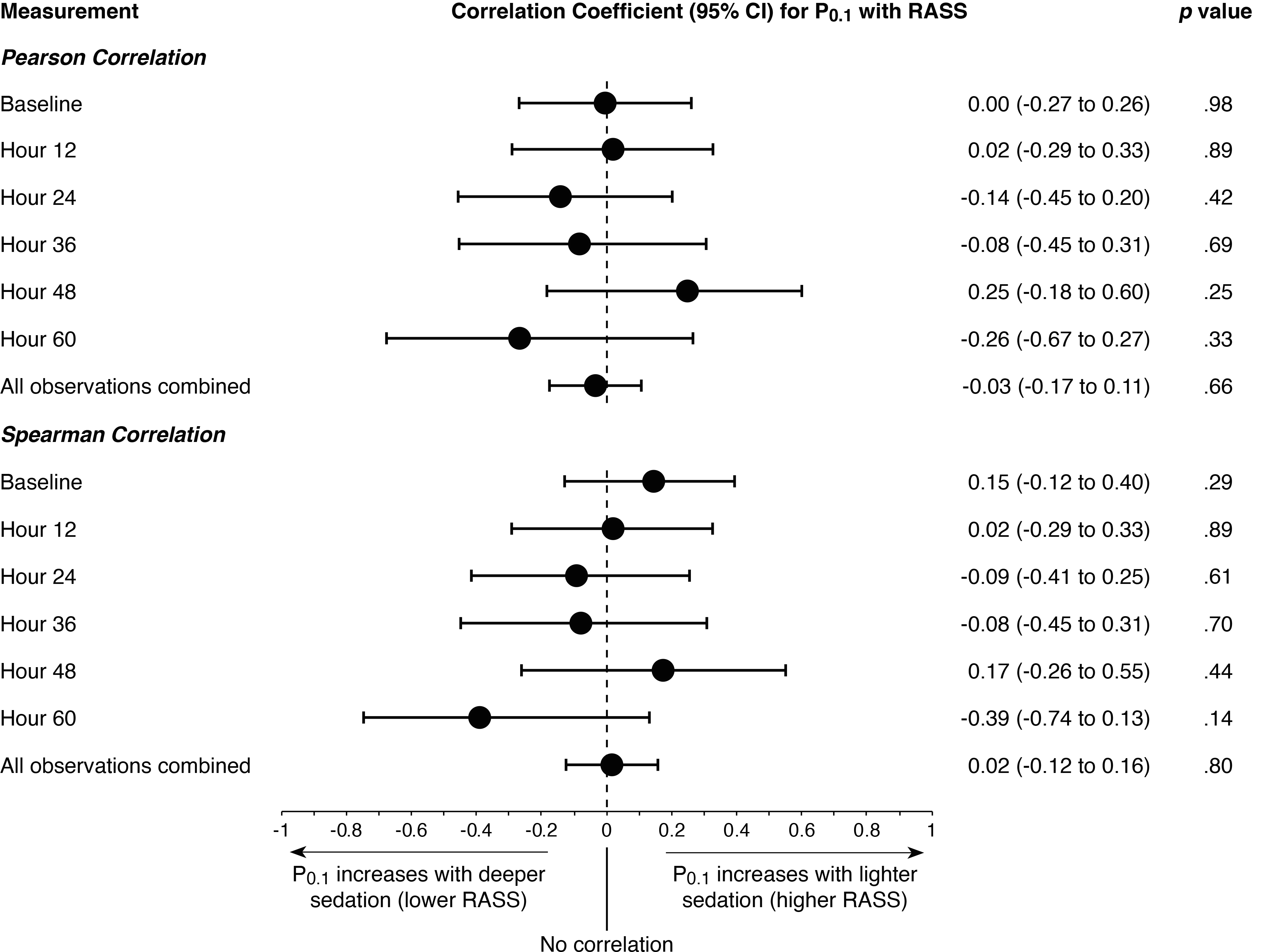
Pearson and Spearman correlations for cross-sectional relationship between P0.1 and sedation depth assessed via Richmond Agitation-Sedation Scale (RASS). Higher RASS corresponds to increased arousal (lighter sedation depth).
Considering average within-patient effects over time via linear mixed models, higher RASS was poorly correlated with higher P0.1 (marginal r2 = 2.4%; p = 0.02; Table E3). RASS was not significantly associated with P0.1 in sensitivity analyses adjusting for pain and delirium (Table E3).
Respiratory Drive and Pain
P0.1 exceeded 1.5 cm H2O during 21.6% of observations without pain. Sixteen of the 20 highest P0.1 values were observed in patients without apparent pain.
In linear mixed models evaluating within-patient associations, higher CPOT was poorly associated with higher P0.1 (marginal r2 = 4.0%) (Table E3). A similar lack of meaningful association was observed when entering CPOT as a dichotomous measure of pain (marginal r2 = 1.6%).
Respiratory Drive and Delirium
P0.1 exceeded 1.5 cm H2O during 21.8% of observations without delirium. Thirteen of the 20 highest P0.1 values were observed in patients not meeting criteria for delirium.
Delirium was weakly correlated with higher P0.1 in linear mixed models evaluating within-patient associations (marginal r2 = 5.8%) (Table E3).
Respiratory Drive and Sedative Exposure
Higher propofol dose over the prior hour was weakly associated with a modest decrease in P0.1 (Table 2). Benzodiazepine and opioid titrations were not significantly associated with P0.1.
Table 2.
Association of P0.1 with Analgesic and Sedative Exposure Over Timea
| Medication | Median [IQR] Dose | ß (95% CI) | P | Marginal r2 for fixed effectsb | Conditional r2 for fixed and random effectsb |
|---|---|---|---|---|---|
| Infusion rate at time of evaluation | |||||
| Propofol, mcg/kg/min | 20 [0–40] | −0.015 (−0.031, 0.001) | 0.07 | 2.7% | 57.4% |
| Midazolam-equivalent, mg/hc | 0 [0–0] | 0.014 (−0.068, 0.097) | 0.74 | ||
| Fentanyl-equivalent, mcg/hd | 25 [0–50] | 0.003 (−0.003, 0.010) | 0.31 | ||
| Total dose over prior 1 h | |||||
| Propofol, mcg/kg | 1200 [0–2400] | −3.3×10−4 (−5.8×10−4, −0.7×10−4) |
0.01 | 3.6% | 58.1% |
| Midazolam-equivalent, mg | 0 [0–0] | 0.012 (−0.070, 0.094) | 0.77 | ||
| Fentanyl-equivalent, mcg | 25 [0–75] | 0.002 (−0.004, 0.009) | 0.50 | ||
| Total dose over prior 12 h | |||||
| Propofol, mcg/kg | 14,400 [0–25,200] | −1.8×10−5 (−4.2×10−5, 6.2×10−6) |
0.14 | 1.8% | 56.4% |
| Midazolam-equivalent, mg | 0 [0–0] | 3.3×10−4 (−6.8×10−3, 7.4×10−3) |
0.93 | ||
| Fentanyl-equivalent, mcg | 300 [0–825] | 2.2×10−4 (−3.9×10−4, 8.3×10−4) |
0.48 |
Analyses included 197 bedside evaluations among 56 patients. Reported regression coefficient (95% CI) and p-value were calculated from linear mixed effects model with all three classes of medications and time entered as fixed effects and random subject effect (intercept). The resulting regression coefficients report the change in P0.1 per 1-unit change in sedative dose, adjusting for the effects of the other sedatives and time. Separate models were run for infusion rate at time of evaluation and total dose over prior 1 h. Negative values indicate decrease in P0.1 with higher medication dose.
Marginal and conditional r2 were computed per the method of Nakagawa and Schielzeth. Marginal r2 quantifies the proportion of variance explained by model fixed effects only, while conditional r2 quantifies the proportion of variance explained by fixed and random effects. A low marginal r2 and high conditional r2 together indicate that most model variance is explained by the random effect (subject intercept).
Midazolam-equivalent dose calculated as midazolam 1 mg = lorazepam 0.5 mg.
Fentanyl-equivalent dose calculated as fentanyl 100 mcg = hydromorphone 2 mg = morphine 10 mg.
Respiratory Drive and Other Factors
Within-patient associations of P0.1 with other factors were evaluated to reconfirm face-validity of P0.1 as a measure of respiratory drive. Lower pH, higher respiratory rate, and higher minute-volume were associated with higher P0.1 (Tables E4, E5). After adjusting for sedation depth, greater illness severity (SOFA) also was associated with higher P0.1.
Clinical Outcomes
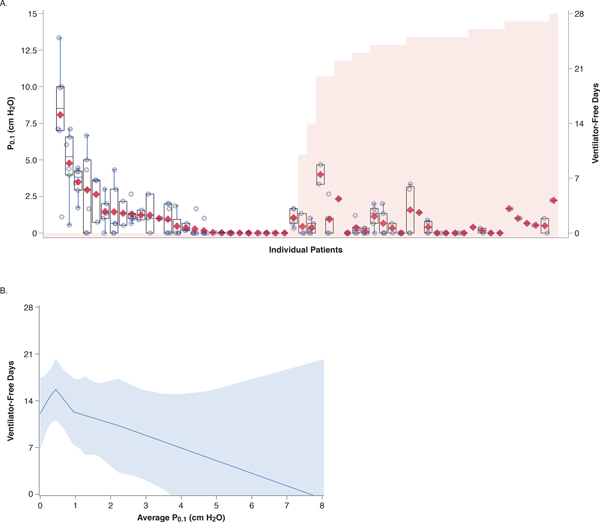
Average P0.1 exhibited a significant, non-linear association with ventilator-free days (unadjusted Poisson model with coefficients: VFD = −0.0801 P0.12 + 0.1166 P0.1 + 2.5686; p < 0.01 for P0.12 term). This association remained statistically significant and qualitatively unchanged in a multivariable model adjusting for baseline APACHE-II, baseline PaO2:FiO2, and average RASS. Rerunning the unadjusted model entering P0.1 tercile (range 0.0–0.1, 0.2–1.0, and 1.1–8.1) as a class variable with the middle tercile as the reference group demonstrated a similar relationship. Patients in the middle tercile had significantly more ventilator-free days compared to patients in either the lowest tercile (incidence rate ratio [IRR] 0.78, 95% CI 0.65–0.93; p < 0.01) or highest tercile (IRR 0.58, 95% CI 0.48–0.70; p < 0.01). The same relationship was evident graphically with LOESS regression: patients with moderate respiratory drive on average had more ventilator-free days than patients with either no drive or high drive (Figure 3).
Figure 3. Both high and low respiratory drive, compared to moderate drive, are associated with fewer ventilator-free days.
(A) Overlaid box-and whisker and scatter plots of all P0.1 values for each patient; red diamonds represent mean values, and circles indicate values from individual observations. Boxes represent median with interquartile range; whiskers extend 1.5 times the interquartile range beyond the first and third quartiles per the Tukey method. Shaded vertical bars indicate number of ventilator-free days for each patient (secondary Y-axis). Patients are sorted by number of ventilator free days and average P0.1 value to aid interpretation. (B) Locally estimated scatterplot smoothing (LOESS) regression line with 95% confidence bands for model associating P0.1 with ventilator-free days.
P0.1, entered into logistic models as either a linear or quadratic term, was not significantly associated with mortality in univariable or multivariable models.
DISCUSSION
This study demonstrates that sedation depth is a poor surrogate of respiratory drive, as measured by P0.1, in critically ill adults with acute respiratory failure. Across the range of routinely targeted sedation depth, respiratory drive could be low, moderate, or high. Deep sedation often did not suppress respiratory drive, light sedation did not ensure respiratory effort was preserved, and many non-sedated awake patients had normal respiratory drive. Patients with moderate respiratory drive experienced more ventilator-free days than patients in whom drive was either high or completely extinguished.
Clinical practice guidelines addressing ICU sedation advocate using validated tools for the identification, treatment and prevention of pain, agitation/anxiety, and delirium (2). While absent from these guidelines, monitoring respiratory drive could be useful to evaluate whether the intended clinical effect is achieved, and to recognize an undesirable side-effect when sedation is prescribed for other indications. If deep sedation is prescribed for respirolysis but fails to decrease drive, it exposes patients to potential harm from excessive sedatives/analgesics (35) with no benefit. Sedation prescribed to alleviate pain or anxiety may cause occult respirolysis, risking disuse atrophy of respiratory muscles (11, 13).
Critically ill patients with acute respiratory failure have a multitude of factors that might contribute to high respiratory drive: pain, anxiety, systemic and cerebral inflammation, infection, pulmonary mechanical perturbations, acidemia, hypercapnia, and hypoxemia, among others (36). Medications that solely alleviate pain or anxiety are unlikely to achieve respirolysis in the setting of other contributors to drive. Common tools for assessing pain and anxiety/sedation, CPOT and RASS, include “fighting the ventilator” as a marker of both pain and anxiety/agitation. Our data indicate that high drive, a proximate cause to patient-ventilator dyssynchronies that might be construed as “fighting the ventilator,” is often not responsive to analgesia or anxiolysis and frequently occurs absent agitation.
Many sedatives and analgesics can modulate respiratory pattern directly. Prior studies in non-critically ill populations have found propofol depresses inspiratory muscle effort and induces a rapid shallow breathing pattern (37–39). A similar effect has been reported with midazolam, which also may blunt ventilatory response to carbon dioxide (40–42). Dexmedetomidine, in contrast, is thought to have little effect on respiratory drive or pattern (43). Opioids may cause bradypnea with less effect on drive, and can produce a unique respiratory pattern characterized by prolonged active exhalation with abdominal muscle contraction (44). In the present study, higher propofol dose was associated with a modest decrease in P0.1, while no discernible within-patient effect was observed with benzodiazepines or opioids. Clinician rationale for a particular sedative or sedation depth was not recorded in this study, and there are several reasons why a particular sedation depth may be targeted in a given patient. Nevertheless, our data suggest dose-response relationships described in healthy volunteers or surgical patients are inconsistently reproduced during critical illness at routinely prescribed doses.
P0.1 does have limitations as a measure of respiratory drive. P0.1 measures motor output from the respiratory center and can be discordant with neural drive due to muscle weakness, fatigue, or other disruption to neuromechanical coupling. This study enrolled patients early in their ventilator course and excluded patients with known neuromuscular weakness to mitigate this concern. Breath-to-breath variability in P0.1 requires averaging multiple consecutive measures to obtain representative values (20, 28), an approach used in this study. Expiratory flow limitation with intrinsic PEEP can introduce a phase delay between inspiratory muscle effort and pressure change at the airway opening. Patients with overt flow limitation were excluded from this study to negate this potential concern, although prior data suggest P0.1 remains valid despite intrinsic PEEP (27, 45). Measurement technique differs across ventilators, and resulting imprecision could impede clinical applications. Reassuringly, reported values appear reasonably similar on newer ventilators regardless of technique performed (21, 46).
Alternative measures of respiratory drive, including electrical activity of the diaphragm, esophageal pressure, and transdiaphragmatic pressure, are technically challenging to perform and not widely available. By comparison, P0.1 is well-validated over decades, near-ubiquitous on modern ICU ventilators, often completely automated requiring no technical expertise, and can be monitored breath to breath continuously over time. P0.1 has not been studied previously for monitoring sedation effects but has been used to titrate assistance during pressure support ventilation (47–49) and evaluate extubation readiness (50).
The association of P0.1 extremes (high or low) with fewer ventilator-free days may not be causal. Both high drive and complete pharmacologic respirolysis could be markers of underlying pathology that contributes to morbidity independent of drive. Yet, the association of P0.1 with ventilator-free days remained significant after adjusting for APACHE-II, PaO2:FiO2, and RASS. In at-risk patients, high drive could prolong ventilator weaning by (i) exacerbating lung injury (high tidal volumes, breath stacking, pendelluft, atelectrauma (10, 51)); (ii) exacerbating diaphragm fatigue and injury (under-assisted or dyssynchronous eccentric contractions (52)); and (iii) prompting administration of additional sedatives/analgesics with accompanying adverse effects (3). Low drive might prolong ventilator weaning via respiratory muscle atrophy and excess sedative/analgesic exposure (11, 13).
The optimal level of respiratory drive in critical illness is unknown and likely depends on potentially conflicting risks of lung injury, diaphragm injury, and the side-effect profile of interventions designed to modulate drive. Adjusting the ventilator to optimize patient-ventilator interactions and treating causes underlying high drive should be attempted before considering pharmacologic interventions solely for respirolysis. In less critically ill patients without severe lung injury, acceptance of heightened drive and permissive dyssynchrony (53) is likely preferable to added sedative, analgesic, or paralytic exposure, while the converse might be true in select patients at extremely high risk of lung injury. Whether attempting to use sedatives and analgesics for respirolysis affords benefit to this narrow highest-risk population is unclear, but risks of deep sedation (16, 17, 33) and respiratory muscle inactivity (11–14) are well established.
CONCLUSION
Sedation depth is a poor marker of respiratory drive and should not be used as a surrogate of drive during critical illness. Extremes of respiratory drive (high and low) were observed across the range of routinely targeted sedation depth and were independently associated with fewer ventilator-free days. Whether measuring respiratory drive to guide clinical decision-making can improve outcomes is unknown and warrants evaluation.
Supplementary Material
Acknowledgments
Funding: U.S. National Heart, Lung, and Blood Institute (K23-HL133489). The NHLBI had no role in the design or conduct of the study, the collection, analysis, or interpretation of the data, the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Disclosures: Dr. Beitler has received consulting fees from Sedana Medical and Hamilton Medical unrelated to this work. All other authors report no potential conflicts of interest to disclose.
REFERENCES
- 1.Reade MC, Finfer S: Sedation and delirium in the intensive care unit. N Engl J Med 2014; 370:444–454 [DOI] [PubMed] [Google Scholar]
- 2.Devlin JW, Skrobik Y, Gélinas C, et al. : Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 3.Chanques G, Kress JP, Pohlman A, et al. : Impact of ventilator adjustment and sedation-analgesia practices on severe asynchrony in patients ventilated in assist-control mode. Crit Care Med 2013; 41:2177–2187 [DOI] [PubMed] [Google Scholar]
- 4.Hanidziar D, Bittner EA: Sedation of mechanically ventilated COVID-19 patients: challenges and special considerations. Anesth Analg 2020; 131:e40–e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payen JF, Chanques G, Mantz J, et al. : Current practices in sedation and analgesia for mechanically ventilated critically Ill patients. Anesthesiology 2007; 106:687–695 [DOI] [PubMed] [Google Scholar]
- 6.Mehta S, Burry L, Fischer S, et al. : Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med 2006; 34:374–380 [DOI] [PubMed] [Google Scholar]
- 7.Hansen-Flaschen JH: Use of sedating drugs and neuromuscular blocking agents in patients requiring mechanical ventilation for respiratory failure: a national survey. JAMA 1991; 266:2870–2875 [PubMed] [Google Scholar]
- 8.Rhoney DH, Murry KR: National survey of the use of sedating drugs, neuromuscular blocking agents, and reversal agents in the intensive care unit. J Intensive Care Med 2003; 18:139–145 [DOI] [PubMed] [Google Scholar]
- 9.Marini JJ: Dynamic hyperinflation and auto-positive end-expiratory pressure: lessons learned over 30 years. Am J Respir Crit Care Med 2011; 184:756–762 [DOI] [PubMed] [Google Scholar]
- 10.Beitler JR, Sands SA, Loring SH, et al. : Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med 2016; 42:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine S, Nguyen T, Taylor N, et al. : Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008; 358:1327–1335 [DOI] [PubMed] [Google Scholar]
- 12.Jaber S, Petrof BJ, Jung B, et al. : Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011; 183:364–371 [DOI] [PubMed] [Google Scholar]
- 13.Goligher EC, Fan E, Herridge MS, et al. : Evolution of diaphragm thickness during mechanical ventilation: impact of inspiratory effort. Am J Respir Crit Care Med 2015; 192:1080–1088 [DOI] [PubMed] [Google Scholar]
- 14.Reynolds SC, Meyyappan R, Thakkar V, et al. : Mitigation of ventilator-induced diaphragm atrophy by transvenous phrenic nerve stimulation. Am J Respir Crit Care Med 2017; 195:339–348 [DOI] [PubMed] [Google Scholar]
- 15.Goligher EC, Dres M, Patel BK, et al. : Lung and diaphragm-protective ventilation. Am J Respir Crit Care Med 2020; 202:950–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shehabi Y, Bellomo R, Reade MC, et al. : Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med 2012; 186:724–731 [DOI] [PubMed] [Google Scholar]
- 17.Treggiari MM, Romand J-A, Yanez ND, et al. : Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med 2009; 37:2527–2534 [DOI] [PubMed] [Google Scholar]
- 18.Whitelaw WA, Derenne JP, Milic-Emili J: Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol 1975; 23:181–199 [DOI] [PubMed] [Google Scholar]
- 19.Whitelaw WA, Derenne JP: Airway occlusion pressure. J Appl Physiol 1993; 74:1475–1483 [DOI] [PubMed] [Google Scholar]
- 20.Telias I, Damiani F, Brochard L: The airway occlusion pressure (P0.1) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not-so-new problem. Intensive Care Med 2018; 44:1532–1535 [DOI] [PubMed] [Google Scholar]
- 21.Telias I, Junhasavasdikul D, Rittayamai N, et al. : Airway occlusion pressure as an estimate of respiratory drive and inspiratory effort during assisted ventilation. Am J Respir Crit Care Med 2020; 201:1086–1098 [DOI] [PubMed] [Google Scholar]
- 22.Slutsky AS, Villar J: Early paralytic agents for ARDS? Yes, No, and Sometimes. N Engl J Med 2019; 380:2061–2063 [DOI] [PubMed] [Google Scholar]
- 23.Huang DT, Angus DC, Moss M, et al. : Design and rationale of the reevaluation of systemic early neuromuscular blockade trial for acute respiratory distress syndrome. Ann Am Thorac Soc 2017; 14:124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sottile PD, Albers D, Higgins C, et al. : The association between ventilator dyssynchrony, delivered tidal volume, and sedation using a novel automated ventilator dyssynchrony detection algorithm. Crit Care Med 2018; 46:e151–e157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhlen R, Hausmann S, Pappert D, et al. : A new method for P0.1 measurement using standard respiratory equipment. Intensive Care Med 1995; 21:554–560 [DOI] [PubMed] [Google Scholar]
- 26.Kuhlen R, Mohnhaupt R, Slama K, et al. : Validation and clinical application of a continuous P0.1 measurement using standard respiratory equipment. Technol Health Care 1996; 4:415–424 [PubMed] [Google Scholar]
- 27.Conti G, Cinnella G, Barboni E, et al. : Estimation of occlusion pressure during assisted ventilation in patients with intrinsic PEEP. Am J Respir Crit Care Med 1996; 154:907–912 [DOI] [PubMed] [Google Scholar]
- 28.Kera T, Aihara A, Inomata T: Reliability of airway occlusion pressure as an index of respiratory motor output. Respir Care 2013; 58:845–849 [DOI] [PubMed] [Google Scholar]
- 29.Pandharipande PP, Pun BT, Herr DL, et al. : Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007; 298:2644–2653 [DOI] [PubMed] [Google Scholar]
- 30.Riker RR, Shehabi Y, Bokesch PM, et al. : Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009; 301:489–499 [DOI] [PubMed] [Google Scholar]
- 31.Shehabi Y, Howe BD, Bellomo R, et al. : Early sedation with dexmedetomidine in critically ill patients. N Engl J Med 2019; 380:2506–2517 [DOI] [PubMed] [Google Scholar]
- 32.Kawazoe Y, Miyamoto K, Morimoto T, et al. : Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: a randomized clinical trial. JAMA 2017; 317:1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barr J, Fraser GL, Puntillo K, et al. : Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41:263–306 [DOI] [PubMed] [Google Scholar]
- 34.Schoenfeld DA, Bernard GR, ARDS Network: Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002; 30:1772–1777 [DOI] [PubMed] [Google Scholar]
- 35.Kress JP, Hall JB: The changing landscape of ICU sedation. JAMA 2012; 308:2030–2031 [DOI] [PubMed] [Google Scholar]
- 36.Spinelli E, Mauri T, Beitler JR, et al. : Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med 2020; 46:606–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaschetto R, Cammarota G, Colombo D, et al. : Effects of propofol on patient-ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med 2014; 42:74–82 [DOI] [PubMed] [Google Scholar]
- 38.Khamiees M, Amoateng-Adjepong Y, Manthous CA: Propofol infusion is associated with a higher rapid shallow breathing index in patients preparing to wean from mechanical ventilation. Respir Care 2002; 47:150–153 [PubMed] [Google Scholar]
- 39.Goodman NW, Black AMS, Carter JA: Some ventilatory effects of propofol as sole anesthetic agent. Br J Anaesth 1987; 59:1497–1503 [DOI] [PubMed] [Google Scholar]
- 40.Forster A, Gardaz JP, Suter PM, et al. : Respiratory depression by midazolam and diazepam. Anesthesiology 1980; 53:494–497 [DOI] [PubMed] [Google Scholar]
- 41.Berggren L, Eriksson I, Mollenholt P, et al. : Changes in respiratory pattern after repeated doses of diazepam and midazolam in healthy subjects. Acta Anaesthesiol Scand 1987; 31:667–672 [DOI] [PubMed] [Google Scholar]
- 42.Morel DR, Forster A, Bachmann M, et al. : Effect of intravenous midazolam on breathing pattern and chest wall mechanics in human. J Appl Physiol 1984; 57:1104–1110 [DOI] [PubMed] [Google Scholar]
- 43.Conti G, Ranieri VM, Costa R, et al. : Effects of dexmedetomidine and propofol on patient-ventilator interaction in difficult-to-wean, mechanically ventilated patients: a prospective, open-label, randomised, multicentre study. Crit Care 2016; 20:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drummond GB, Duncan MK: Abdominal pressure during laparoscopy: effects of fentanyl. Br J Anaesth 2002; 88:384–388 [PubMed] [Google Scholar]
- 45.Murciano D, Aubier M, Bussi S, et al. : Comparison of esophageal, tracheal, and mouth occlusion pressure in patients with chronic obstructive pulmonary disease during acute respiratory failure. Am Rev Respir Dis 1982; 126:837–841 [DOI] [PubMed] [Google Scholar]
- 46.Beloncle F, Piquilloud L, Olivier P-Y, et al. : Accuracy of P0.1 measurements performed by ICU ventilators: a bench study. Ann Intensive Care 2019; 9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alberti A, Gallo F, Fongaro A, et al. : P0.1 is a useful parameter in setting the level of pressure support ventilation. Intensive Care Med 1995; 21:547–553 [DOI] [PubMed] [Google Scholar]
- 48.Perrigault PF, Pouzeratte YH, Jaber S, et al. : Changes in occlusion pressure (P0.1) and breathing pattern during pressure support ventilation. Thorax 1999; 54:119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iotti GA, Brunner JX, Braschi A, et al. : Closed-loop control of airway occlusion pressure at 0.1 second (P0.1) applied to pressure-support ventilation: algorithm and application in intubated patients. Crit Care Med 1996; 24:771–779 [DOI] [PubMed] [Google Scholar]
- 50.Sklar MC, Burns K, Rittayamai N, et al. : Effort to breathe with various spontaneous breathing trial techniques: a physiological meta-analysis. Am J Respir Crit Care Med 2017; 195:1477–1485 [DOI] [PubMed] [Google Scholar]
- 51.Yoshida T, Torsani V, Gomes S, et al. : Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013; 188:1420–1427 [DOI] [PubMed] [Google Scholar]
- 52.Goligher EC, Brochard LJ, Reid WD, et al. : Diaphragmatic myotrauma: a mediator of prolonged ventilation and poor patient outcomes in acute respiratory failure. Lancet Respir Med 2019; 7:90–98 [DOI] [PubMed] [Google Scholar]
- 53.Telias I, Beitler JR: Reverse triggering, the rhythm dyssynchrony: potential implications for lung and diaphragm protection. Am J Respir Crit Care Med 2021; 203:5–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.