Abstract
COVID-19 has become a major cause of global mortality and driven massive health and economic disruptions. Mass global vaccination offers the most efficient pathway towards ending the pandemic. The development and deployment of first-generation COVID-19 vaccines, encompassing mRNA or viral vectors, has proceeded at a phenomenal pace. Going forward, nanoparticle-based vaccines which deliver SARS-CoV-2 antigens will play an increasing role in extending or improving vaccination outcomes against COVID-19. At present, over 26 nanoparticle vaccine candidates have advanced into clinical testing, with ∼60 more in pre-clinical development. Here, we discuss the emerging promise of nanotechnology in vaccine design and manufacturing to combat SARS-CoV-2, and highlight opportunities and challenges presented by these novel vaccine platforms.
Keywords: nanoparticle vaccine, COVID-19 vaccine, SARS-CoV-2, neutralizing antibody, protein nanoparticle
1. SARS-CoV-2 and the generation of protective immunity by vaccination
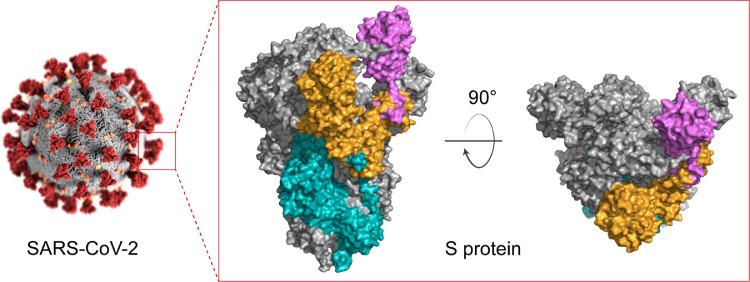
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The viral spike (S) protein mediates viral attachment, entry into host cells and serves as a critical target for vaccine design [1]. S exists as a homo-trimer on the virion surface and enables viral recognition of angiotensin-converting enzyme 2 (ACE2) on the host cell surface via the receptor binding domain (RBD) (Fig. 1). All 22 COVID-19 vaccines that are in use worldwide have included S or an S-derivative engineered for increased stability [2].
Fig. 1.
Structure of the SARS-CoV-2 spike (S) protein. S protein exists as homo-trimer. Each monomer is divided into S1 (yellow) and S2 (cyan) subunits. S1 contains receptor binding domain (RBD, purple). The SARS-CoV-2 structure was reprinted from CDC Public Health Image Library. The S protein structure was prepared using the PDB file ‘6VSB’ on PyMol.
Delivery of S protein or S-encoding nucleic acid as a vaccine induces neutralising antibodies and antiviral T and B cell memory [3], [4], [5], [6]. Induction of neutralising antibodies (nAbs) was recently demonstrated to be a robust correlate of protective efficacy for current COVID-19 vaccines [7]. After vaccine administration, vaccine antigen is trafficked to lymph nodes by lymphatic drainage or through immune cell-mediated transport, depending on routes of administration, vaccine antigen properties (e.g. molecular weight and dimensions) and adjuvants [8]. In the lymph node, antigen is introduced to the adaptive immune system. With the support of CD4+ T cells, antigen-specific B cells undergo activation, proliferation and selection for clones with mutations in their immunoglobulin genes that confer increased antigen affinity [9,10]. High-affinity B cells can differentiate into either antibody-producing plasma cells or memory B cells. In addition to direct neutralisation of free virus [7], Abs can also mediate clearance of virus-infected cells through Fc-mediated engagement with ‘killer’ immune cells [11]. Killing of virus-infected cells is also a primary function of CD8+ T cells, which develop rapidly after SARS-CoV-2 infection [12]. As vaccine-elicited antibody levels wane over time, ongoing protection against infection is likely to rely upon stable populations of memory B and T cells.
A major challenge in designing SARS-CoV-2 vaccines is the emergence of mutated viruses termed variants of concern (VOC) [13]. First generation COVID-19 vaccines were developed based on the genome of the original SARS-CoV-2 isolate, Wuhan-Hu-1 [14]. However, novel viral strains with multiple mutations in the spike protein have begun to circulate worldwide, and mutations (notably at the amino acids E484 and E417 in the RBD) can drive immune escape from vaccine elicited nAbs [13], with consequent reductions in vaccine efficacy [15]. Future proofing coronavirus vaccine strategies to protect human populations requires overcoming antibody-escape by emerging VOCs.
2. Nanoparticle vaccines for COVID-19 in development
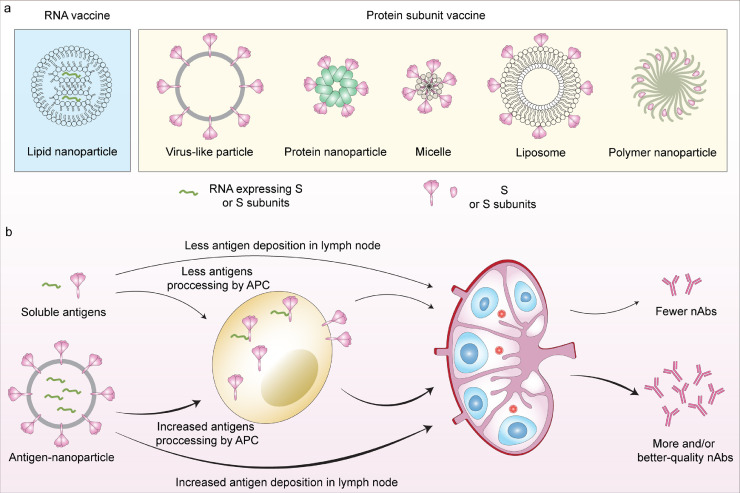
Besides traditional vaccine modalities (i.e. inactivated vaccines, live attenuated vaccines, and recombinant protein vaccines) and DNA and vector-based vaccines (which have been reviewed elsewhere [16]), nanoparticle vaccines offer a unique opportunity to advance vaccine science and provide tractable solutions to the current pandemic and beyond. Nanoparticles are loosely defined as nanoscale-sized and tunable particulate structures that mimic structural features of natural viruses. This adaptive design makes them highly promising platforms for next-generation vaccine development, providing pathways to drive strong nAb responses, or broader antibody-based immunity that might better account for variation and evolution of viral pathogens. At present, at least 26 nanoparticle-based vaccine candidates have advanced into human clinical trials (Table 1), with ∼60 additional candidates in varying stages of preclinical development. Such vaccines encompass diverse formats such as lipid nanoparticles (LNPs), virus-like particles (VLPs), protein nanoparticles, micelles, and other technologies (Fig. 2a). Potential advantages and disadvantages of different nanoparticle vaccine platforms are detailed in Table 2. Based on antigen loading strategies, nanoparticle vaccines can be divided into two groupings: (1) nanoparticles encapsulating vaccine antigens or nucleic acid cargos within their core and (2) nanoparticles that present vaccines antigens on their surface. A key feature of antigen-encapsulating nanoparticle vaccines is the protection and controlled release of cargo after immunisation, while nanoparticles displaying vaccine antigens are able to engage antigen-presenting cells (APCs) and/or efficiently promote B cell receptor (BCR) cross-linking, leading to potent immunogenicity [17].
Table 1.
Nanoparticle COVID-19 vaccine candidates in clinical development
| Types of nanoparticles | Developer | Types of vaccine candidates | Phase | Trial registration number |
|---|---|---|---|---|
| Lipid nanoparticles | Moderna, NIAID | mRNA-1273 (LNPs) | 4 | NCT04760132 |
| Pfizer/BioNTech, Fosun Pharma | BNT162 (3 LNP-mRNAs), also known as “Comirnaty” | 4 | NCT04760132 | |
| Moderna, NIAID | mRNA-1273.351 LNPs (mRNA encodes S proteins of the SARS-CoV-2 Beta variant) |
4 | EUCTR2021-000930-32 | |
| CureVac AG | CVnCoV mRNA vaccine | 3 | NCT04674189 | |
| Academy of Military Science (AMS), Walvax Biotechnology and Suzhou Abogen Biosciences | SARS-CoV-2 mRNA vaccine (ARCoV) | 3 | NCT04847102 | |
| Arcturus Therapeutics | ARCT-021 mRNA vaccine | 2 |
NCT04668339 NCT04728347 |
|
| Sanofi Pasteur and Translate Bio | MRT5500 mRNA vaccine | 2 | NCT04798027 | |
| Daiichi Sankyo Co., Ltd. | DS-5670a mRNA vaccine | 1/2 | NCT04821674 | |
| Elixirgen Therapeutics, Inc | EXG-5003, a temperature-sensitive self-replicating RNA vaccine expressing RBD | 1/2 | NCT04863131 | |
| GlaxoSmithKline | CoV2 SAM LNP (Self-amplifying mRNA in LNPs) + Spike antigen | 1 | NCT04758962 | |
| Imperial College London | LNP-nCoVsaRNA (self-amplifying RNA in LNPs) | 1 | ISRCTN17072692 | |
| Providence Therapeutics | PTX-COVID19-B, mRNA vaccine | 1 | NCT04765436 | |
| SENAI CIMATEC | HDT-301: Self-replicating mRNA-LNP vaccine | 1 | NCT04844268 | |
| ModernaTX, Inc. | mRNA-1283, a potentially refrigerator-stable LNP vaccine | 1 | NCT04813796 | |
| Chulalongkorn University | ChulaCov19 mRNA vaccine | 1 | NCT04566276 | |
| Shanghai East Hospital and Stemirna Therapeutics | mRNA-LNP COVID-19 vaccine | 1 | ChiCTR2100045984 | |
| MRC/UVRI and LSHTM Uganda Research Unit | LNP-nCoV saRNA-02 vaccine (Self-amplifying RNA (saRNA) encapsulated in LNPs) |
1 | NCT04934111 | |
| Virus like nanoparticles | Medicago Inc. | Coronavius-like particle (CoVLP) | 2/3 | NCT04636697 |
| The Scientific and Technological Research Council of Turkey | VLP vaccine | 2 | NCT04962893 | |
| Serum Institute of India, Accelagen Pty, SpyBiotech | RBD SARS-CoV-2 HBsAg VLP vaccine | 1/2 | ACTRN12620000817943 | |
| VBI Vaccine Inc. | VBI-2902a (Enveloped VLP of S protein) | 1/2 | NCT04773665 | |
| Radboud University | ABNCoV2 capsid VLP (cVLP) | 1 | NCT04839146 | |
| Protein nanoparticles | SK Bioscience Co., Ltd. | RBD-I53-50 nanoparticle | 3 | NCT05007951 |
| Walter Reed Army Institute of Research (WRAIR) | S protein-ferritin nanoparticle | 1 | NCT04784767 | |
| Micelles | Novavax | SARS-CoV-2 rS/Matrix M1-Adjuvant (Full length recombinant S protein-micelle nanoparticle adjuvanted with Matrix M1) NVX-CoV2373 |
3 |
NCT04611802 EUCTR2020-004123-16-GB NCT04583995 |
Fig. 2.
Nanoparticle COVID-19 vaccine platforms. a, Schematic illustration of different types of nanoparticle platforms exploited to design vaccines for COVID-19. b, Advances of nanoparticle vaccines in production of neutralising antibodies. Nanoparticle vaccines can drive increased antigen processing by APCs and antigen deposition in lymph nodes, eliciting more and/or better-quality neutralising antibodies than soluble antigens.
Table 2.
Potential advantages and disadvantages of nanoparticle vaccines in development as COVID-19 vaccines
| Antigen loading strategies | Types of nanoparticle vaccines | Size range | Vaccine antigens | Potential advantages | Potential disadvantages | References |
|---|---|---|---|---|---|---|
| Antigen encapsulated into nanoparticle core | Lipid nanoparticles | ∼ 100 nm | Nucleic acid (mRNA) | Fully synthetic manufacture, scalable Protection and controlled delivery of mRNA cargos Potently immunogenic, self-adjuvanting Readily tunable particle properties (i.e. size or surface charge) and lipid compositions |
Complicated cold-chain and handling requirements Relatively high cost Reports of rare myocarditis adverse events |
[3,4,20,28,29,83] |
| Polymer nanoparticles | 100 - 200 nm | RBD protein | Readily tunable particle properties (e.g. size, charge, shape) Potential co-delivery of adjuvants and targeting moieties Potential for intranasal administration by using mucoadhesive polymers |
Poor cargo loading efficiency, especially large proteins Encapsulation restricts immune recognition of protein cargo |
[34,84] | |
| Antigen presented onto nanoparticle surface | Virus-like nanoparticles | ∼ 100 nm | Structural proteins: S, M, E | Native-like presentation of viral proteins Rapidly scalable production via utilization of existing vaccine manufacturing infrastructure Simultaneous delivery of multiple viral proteins for expanded vaccine targets |
Manufacturing can require complicated helper viruses or cell lines Limited control of viral protein conformation, density or stoichiometry |
[37,38,54] |
| Protein nanoparticles | 10 – 50 nm | S and RBD | Enhanced immunogenicity compared to soluble protein subunit vaccines Allows presentation of high-order antigen arrays in native-like orientation Some control of particle size, antigen valency and orientation Simultaneous co-display of heterologous vaccine antigens |
Require co-formulation with adjuvants Scalable production currently unproven Require cell lines for manufacture, slowing development and regulatory approval |
[[62], [63], [64],[69], [70], [71], [72]] | |
| Micelle nanoparticles | 30 - 70 nm | S protein | Allows presentation of high-order arrays of antigens in native-like conformation | Poor physical stability in vivo Scalable production unproven |
[6,40] | |
| Liposomes | 100 - 150 nm | S and RBD | Fully synthetic manufacture, proven scalability Tunable particle properties (i.e. size or surface charge) Allow co-presentation and/or encapsulation of adjuvants |
Physical stability of liposomes might be affected by attached antigens In vivo stability of antigen-liposome coupling unproven |
[17,51,[85], [86], [87]] |
2.1. Antigen-encapsulated nanoparticles
2.1.1. Lipid nanoparticles
Lipid nanoparticles (LNPs) are used to deliver nucleic acid cargos (DNA and RNA) encoding vaccine antigens. Encapsulation within LNPs acts to both protect mRNA cargo from rapid degradation by RNases, and to facilitate cellular delivery of mRNA which is challenging due to size and charge constraints (reviewed in [18]). A key component of LNPs is an ionizable lipid, which is positively charged at low pH to allow encapsulation of negatively-charged mRNA, while being neutral at physiological pH [19]. LNPs enter cells via endocytosis and within the low pH of endosomes, the ionizable lipid becomes positively charged and can disrupt endosomal membranes, allowing mRNA cargo release into the cytoplasm for protein translation. LNPs often also contain a PEGylated lipid to minimise opsonization in vivo and various helper lipids and cholesterol to support particle formation. mRNA-LNPs are fully synthetic and manufactured without the requirement of living cells, allowing rapid production of vaccines at scale within weeks after the availability of a viral genome [20].
The first two COVID-19 vaccines approved for use were LNPs delivering mRNA encoding engineered S [3,4]. Both Moderna and BioNTech/Pfizer mRNA-LNP vaccines demonstrate efficacy greater than 90% against Wuhan-Hu-1 SARS-CoV-2 after two vaccine doses in clinical trials. Similarly high effectiveness has been observed in mass vaccination settings such as Israel, where the BioNTech/Pfizer BNT162b2 mRNA vaccine (Comirnaty࣪) has been used to vaccinate more than 80% of the adult population with at least one dose [21]. Similarly, in the United States, the two mRNA-LNP vaccines were 94% effective against COVID-19 hospitalization among fully vaccinated older adults aged ≥65 years [22]. Neutralising antibody responses after LNP immunisation are durable, persisting past six months in all healthy adult participants in an ongoing phase 1 trial [23]. However, a reduction in effectiveness of both mRNA vaccines against VOCs has been observed [24], [25], [26], [27]. Currently, the Delta variant is the dominant SARS-CoV-2 VOC in circulation in many countries. A single dose of BNT162b2 was considerably less effective at preventing symptomatic disease caused by the Delta variant compared to the Alpha variant (30.7% compared to 48.7% effective) [27]. After two doses, BNT162b2 was 93.7% effective against Alpha and 88% effective against Delta. Thus, the next generation of reformulated mRNA-LNP vaccines aim to address emerging SARS-CoV-2 variants.
Both mRNA and lipid particles contain potent immunostimulatory properties [28,29], negating the need for additional formulation with adjuvants [3,4]. In fact, the intrinsic immunogenicity of mRNA can be problematic. Both the Moderna and BioNTech/Pfizer mRNA-LNP vaccines use mRNA containing modified nucleosides, designed to limit the immunogenicity of mRNA, allowing higher vaccine doses and enhancing translational capacity [30]. In contrast, the CureVac mRNA-LNP containing nucleosides lacking modification required lower doses to be delivered in clinical trial, and demonstrated only 47% efficacy against currently circulating variants [31]. In the development pipeline are numerous other vaccines comprised of LNPs encapsulating nucleic acids, not only conventional mRNA encoding S, but also self-amplifying RNA (saRNA) which enhances antigen expression at lower doses (ISRCTN17072692, NCT04758962), or mRNA cocktails encoding three structural proteins S, M, and E of SARS-CoV-2 (RQ3013) [32].
2.1.2. Polymer nanoparticles
Polymers (e.g. chitosan, poly(lactic-co-glycolic acid) (PLGA), or polyethylenimine (PEI)) have been widely used in the past to prepare nanoparticle vaccines for other coronaviruses (e.g. SARS-CoV, MERS-CoV, or hCoV) (reviewed by Medhi et al.) [33]. Chitosan, a natural polysaccharide, was utilised to encapsulate the SARS-CoV-2 RBD proteins [34]. Positively charged chitosan can electrostatically interact with negatively charged mucus sialic acid, facilitating nanoparticle adhesion in airway epithelial surfaces upon intranasal administration. In addition, the surface of the chitosan nanoparticles was decorated with mannose sugars. Innate immune recognition of mannose could lead to increased nanoparticle shuttling to the network of follicular dendritic cells and deposition in germinal centres of lymph nodes [35]. Although immunogenicity data for RBD-mannosylated chitosan nanoparticles have not yet been reported, a similar system was exploited to deliver swine influenza A viral antigens, which induced robust cross-reactive IgA and IgG antibody titers and protection in a viral challenge [34] .
2.2. Antigen-presenting nanoparticles
2.2.1. Virus-like particles
VLPs are nano-sized self-assemblies of viral structural proteins that can act as vaccine antigen delivery vehicles [36]. They mimic molecular and morphological features of native viral virions, while lacking genetic material rendering them non-infectious and non-replicating. Several SARS-CoV-2 VLP vaccines are in clinical and pre-clinical development, with CoVLP the most advanced in phase 2/3 clinical trials (NCT04636697). Developed by Medicago, CoVLP is produced by transient transfection of tobacco plants with Agrobacterium [37]. Enveloped vesicles spontaneously develop and display stabilized pre-fusion S trimers on the surface. In a phase 1 trial, vaccination with two doses of CoVLP formulated with AS03 adjuvant elicited potent nAb responses ∼10-50-fold higher than observed in COVID-19 convalescent individuals.
The quality and magnitude of antibody responses is greater following a single dose of a VLP vaccine compared to soluble antigen. A single dose of enveloped VLPs derived from murine leukemia virus and displaying prefusion S (VBI-2902a) induced robust nAb responses in mice and better protected hamsters from infectious SARS-CoV-2 challenge compared to recombinant prefusion S [38]. The VBI-2902a vaccine is currently in phase 1/2 clinical trials (NCT04773665), while trivalent VLPs displaying S from multiple coronavirus strains are currently in pre-clinical development (VBI-2901). Also in phase 1/2 clinical trials are VLPs consisting of RBD conjugated to Hepatitis B surface antigen (ACTRN12620000817943).
A candidate poised for rapid advancement is avian pathogen Newcastle disease virus-like particles (NDVLPs) used to display pre-fusion stabilized SARS-CoV-2 S ectodomain (S2P) [39]. The S2P transmembrane and cytoplasmic domains were replaced with those from NDV to create a fusion protein that when co-expressed with NDV matrix and nucleocapsid proteins form S2P-NDVLPs. Vaccination of mice with S2P-NDVLPs formulated with Sigma Adjuvant System elicited higher nAb titers at a lower antigen dose compared to soluble S2P. Importantly, the NDVLP technology can utilise existing influenza vaccine manufacturing capacity for rapid mass production of SARS-CoV-2 vaccines, thus speeding global vaccine deployment.
2.2.2. Micelles
Micelles are self-assembled amphiphilic structures that can deliver SARS-CoV-2 proteins, an example being the vaccine manufactured by Novavax currently in late-stage phase 3 clinical trial. Co-formulated with saponin-based Matrix-M1 adjuvant, this vaccine (NVX-CoV2373) uses the amphiphilic detergent polysorbate 80 (Tween 80) to drive self-assembly of micelles displaying pre-fusion stabilized S proteins [6,40]. The hydrophobic transmembrane domain of S interacts with the hydrophobic core of the micelle to form a protein-detergent rosette structure upon mixing. In clinical trial in the UK (NCT04583995), NVX-CoV2373 was reported to be 96% efficacious against wild-type SARS-CoV-2 and 86% against the Alpha VOC, protecting immunised subjects from mild, moderate, or severe disease outcomes [41]. In a phase 1/2 clinical trial, the addition of Matrix-M1 to the S-micelle vaccine was found to be antigen-dose sparing with a two 5 μg dose regime eliciting serum IgG and nAb titers markedly higher than those found in convalescent individuals [42]. However, in the phase 2b trial (NCT04533399) in South Africa, where the Beta VOC is dominantly circulating, the vaccine demonstrated a lower overall efficacy of 49.4% [43]. A reformulated vaccine that targets the Beta variant is under development.
2.2.3. Self-assembling protein nanoparticles
Protein-based nanoparticle platforms have also been developed to deliver SARS-CoV-2 subunit protein vaccines (S and RBD). A self-assembling SARS-CoV-2 S ferritin nanoparticle (SpFN) is currently in a phase 1 clinical trial (NCT04784767). To produce SpFN, a gene encoding prefusion-stabilized S ectodomain was genetically fused to a gene encoding Helicobacter pylori ferritin, a ubiquitous, iron-storage protein that consists of 24 subunits [44]. Upon expression in mammalian cells, SpFN self-assembles into a nanoparticle, with 8 vertices with 3-fold symmetry facilitating the ordered display of trimeric S proteins. In preclinical testing, vaccination of rhesus macaques with two doses of 50 μg SpFN co-formulated with a liposomal adjuvant elicited robust nAb titers, and protected animals against intranasal and intratracheal SARS-CoV-2 challenge. Reduced viral replication was reported in the lower and upper airways, as well as reduced pulmonary pathology.
In a parallel study, SpFN was compared to immunisation with RBD-ferritin nanoparticles (RFN) in mice and macaques [45,46]. After two doses in mice, RFN elicited equivalent neutralising titers as a single immunisation of SpFN, which were more than 20-fold higher than titres in convalescent donor serum. Passive transfer of purified IgG from either SpFN- and RFN-vaccinated mice induced robust protection for K18-hACE2 transgenic mice from a lethal SARS-CoV-2 virus challenge. Moreover, immunisation of rhesus macaques with two doses of RFN co-formulated with a liposomal adjuvant elicited ∼10-50-fold greater nAb titer relative to those observed in NHP studies of several authorised COVID-19 vaccines. Furthermore, vaccination inhibited viral replication in the upper and lower airways following high-dose SARS-CoV-2 respiratory challenge.
SARS-CoV-2 protein antigens can also be covalently conjugated onto a protein nanoparticle core using the SpyTag/SpyCatcher system. SpyTag peptide (13 amino acids) and SpyCatcher proteins (116 amino acids) are derived from Streptococcus pyogenes and spontaneously form isopeptide bonds upon mixing [47]. Either SpyTag or SpyCatcher can be fused to vaccine antigens or to protein nanoparticle platforms, facilitating rapid covalent linkage upon mixing. Compared to direct fusion of antigens onto protein platforms, SpyTag/SpyCatcher can increase expression yields or facilitate high throughput testing of a range of vaccine antigens. Such a strategy was used to construct ferritin nanoparticles displaying the SARS-CoV-2 RBD (ferritin-NP-RBD) [48], which elicits potent antibody responses approximately 100-fold higher than observed after immunisation with soluble RBD-SpyTag. Antibody responses after ferritin-NP-RBD vaccination were durable, lasting for at least 7 months and were significantly higher than observed with the soluble protein vaccine, suggesting particulate antigen display drives durable antibody immunity.
Alongside ferritin, other self-assembling protein nanoparticle platforms are under development to deliver SARS-CoV-2 protein immunogens. For example, a 60-subunit Aquifex aeolicus lumazine synthase (LuS) displaying S via SpyTag/SpyCatcher is potently immunogenic in mice, with 0.08 µg of S-LuS nanoparticle eliciting comparable neutralizing responses to 2.0 µg of a prototypic S protein vaccine, a substantial dose-sparing effect [49]. Similarly, construction of bacteriophage capsid-like particles (RBD-CLP) using the SpyTag/SpyCatcher system allows unidirectional and high density display of RBD vaccine antigens [50]. Mice vaccinated with a single dose of RBD-CLP vaccine formulated with squalene-water-emulsion adjuvant elicited nAb titers higher than vaccination with soluble RBD and comparable to COVID-19 convalescent human plasma, with further titre improvements following a booster dose.
2.2.4. Liposomes
Liposomes are nanostructured assemblies of amphipathic phospholipids with one or multiple lipid bilayers forming a membrane which, unlike LNPs, encapsulate an aqueous core. Liposomes have been used to deliver SARS-CoV-2 vaccine antigens. In a preclinical trial, RBD subunits were attached to the surface of liposomes to form RBD-liposomal vaccines [51]. By simply mixing histidine-tagged RBDs with liposomes containing cobalt porphyrin-phospholipid (CoPoP), chelating bonds between cobalt ion and histidine residues formed, resulting in serum-stable and conformationally intact display of RBD on the liposome surface. RBD-liposomes elicited robust antibody titers in vaccinated mice that inhibited live virus replication. RBD-liposomes also displayed enhanced antigen uptake by APCs and increased immune cell recruitment to draining lymph nodes. Liposomes have also been further upgraded to increase their biomimetic properties. Intranasal administration of liposomes encapsulating Toll-like receptor agonist Poly(I:C) and coated with a pulmonary surfactant in addition to the display of RBD on the surface induced stronger mucosal immunity than intramuscular or subcutaneous administration in mice [52].
3. Application of nanotechnology to address vaccine challenges
3.1. Maximising protective nAbs with tunable nanoparticle design
Nanoparticle-based vaccines can confer more robust protective antibody responses against SARS-CoV-2 compared to soluble or non-particulate vaccine antigens (reviewed in [53,54]). Mechanistically, efficient uptake by APCs and improved lymph node drainage drives enhanced antigen deposition in lymph nodes and consequently increased nAb production (Fig. 2b). More importantly, multimerisation of antigens on the surface of antigen-presented nanoparticle vaccines can enhance B cell activation via direct engagement and cross-linking of BCRs [55]. Here we discuss recent advances of nanoparticles with surface display of vaccine antigens, which aim to maximize productions of nAbs.
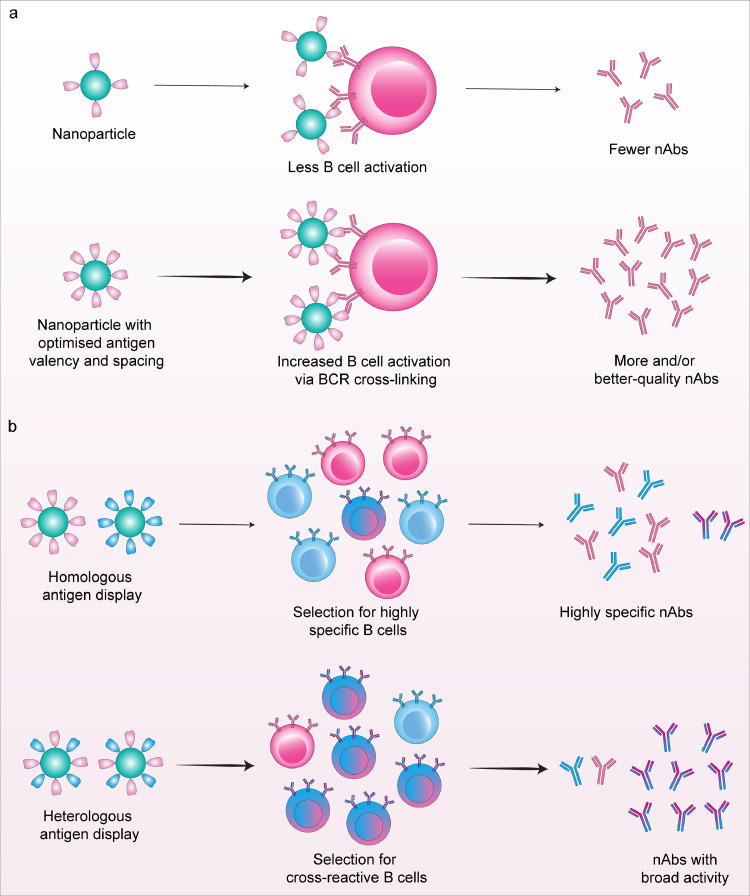
The modular nature of nanoparticle platforms enables particle characteristics such as size, antigen valency and spacing to be modified to further optimise the elicitation of protective antibody responses (Fig. 3a). However the tunability of self-assembling protein nanoparticles is limited by the small number of naturally occurring scaffolds whose structural properties are amenable for use as a vaccine. Advances in computational protein modelling and design algorithms have allowed the design of synthetic protein building blocks with low-energy protein-protein interfaces to drive self-assembly of nanomaterials with atomic-level control of particle structure [56]. For example, mi3 and I3-01 are computationally designed scaffolds based on the 2-keto-3-deoxy-phosphogluconate (KDPG) aldolase from Thermotoga maritima with mutations to promote assembly into a higher order dodecahedral 60-mer nanocages, which allows display of 60 monomeric or 20 trimeric antigens on their surface [57], [58], [59]. Self-assembling 24-mer ferritin (12.2 nm), 60-mer E2p (23.2 nm) and computationally designed 60-mer I3-01 (25.0 nm) were used to display 24 – 60 copies of RBD or 8 - 20 copies of stabilized S on a single particle [58]. E2p and I3-01 nanoparticle vaccines with a larger particle size and higher antigen valency induced increased antigen-specific binding and nAb titres in vaccinated mice as compared to those immunised with ferritin nanoparticles or soluble antigens. The I3-01 vaccine elicited not only the highest neutralising activity at 8 weeks post vaccination, but also potent T cell responses. In another study, combining computationally-designed mi3 with the SpyTag/SpyCatcher system allowed multivalent presentation of RBDs [60]. Vaccination of mice and pigs with two doses of adjuvanted RBD-mi3 induced strong nAb responses equivalent to convalescent sera, and 5-10-fold higher than soluble RBD alone. Importantly, this vaccine was also thermostable following repeated freeze/thaw cycles and able to be lyophilized, removing the cold-chain distribution requirement of current COVID-19 vaccines.
Fig. 3.
Mechanisms of immune response modulation by nanoparticle-based vaccines. a, Tuning features such as particle size or antigen valency can optimise the presentation of vaccine antigens on nanoparticle vaccines, which can drive increased B cell activation via efficient B cell receptor cross-linking. This can in turn elicit higher levels and/or better-quality neutralising antibodies. b, Mosaic design sees the display of heterologous vaccine antigens on the surface of a single nanoparticle. This can act to favour the selection of cross-reactive B cells into the vaccine-elicited immune response, thereby broadening range of pathogens recognised by neutralising antibodies.
Computationally designed protein nanoparticles need not be assembled from a single identical subunit, but can utilise multiple distinct protein subunits. Multicomponent protein nanoparticles allow greater control of antigen spacing, the assembly process, and the possibility of cargo encapsulation within the nanoparticle core [61]. For instance, a two-component computationally-designed I53-50 scaffold, which constitutes 20 trimeric (I53-50A) and 12 pentameric (I53-50B) subunits, self-assembles in vitro into a icosahedral particle with a diameter of ∼30 nm [62]. This platform can be constructed to attach either 60 [63] or 120 [64] protein monomers or 20 trimers [62] on their surface. Self-assembling ferritin, one-component computationally-designed mi3 and two-component computationally-designed I53-50 were used to display 24, 60, and 120 copies of SARS-CoV-2 RBDs respectively using the SpyTag/SpyCatcher system [64]. Vaccination of mice with the RBD-nanoparticles co-formulated with either AddaVax or Sigma Adjuvant System exhibited 8-120-fold greater neutralising activity against both pseudo- and authentic viruses than those immunized with monomeric RBD. Increased valency of RBD presented on the nanoparticle surface was positively associated with elicitation of neutralising antibodies.
In another study, I53-50 scaffolds were designed to attach 20 copies of SARS-CoV-2 S protein with 15 nm spacing [62]. These S-I53-50 nanoparticles were able to trigger direct B cell activation in vitro through BCR crosslinking and elicit potent nAbs in vaccinated mice, rabbits, and cynomolgus macaques. Vaccination with S-I53-50 protected macaques from a high-dose viral infection, with reduced viral replication in the upper and lower airways [62]. The I53-50 cage was also used to display 60 copies of monomeric SARS-CoV-2 RBD, which is currently in phase 1/2 clinical trials (NCT04742738 and NCT04750343). In preclinical testing, RBD-I53-50 nanoparticles protected vaccinated mice from infectious viral challenge and elicited 10-fold higher nAb titers in mice relative to animals immunised with prefusion-stabilized S proteins, despite a 5-fold lower dose [63]. In a further study, RBD-I53-50 nanoparticle vaccination of rhesus macaques generated robust nAb titers maintained for at least 180 days and conferred significant protection against SARS-CoV-2 infection, with viral loads reduced in the pharynges, nares, and in the bronchoalveolar lavage [65].
3.2. Using nanotechnology to combat viral variants and ongoing evolution
Existing nanoparticle vaccine platforms have been rapidly updated to combat emerging SARS-CoV-2 variants. With first generation mRNA-LNPs exhibiting a reduced efficacy against the VOCs [24], [25], [26], [27], Moderna has developed mRNA-1273.351 with mRNA cargo updated to express S from the Beta VOC. It is noteworthy that compared to traditional methods (e.g. thin film hydration, reverse-phase evaporation, etc.), novel microfluidic mixer approach currently used to manufacture mRNA-LNP vaccines allows rapid and large-scale production of LNPs with high mRNA encapsulation efficiency [66]. This vaccine is currently in a phase 2 clinical trial (NCT04405076), with mRNA-1273.351 booster immunisation of trial participants previously vaccinated with the wildtype mRNA-1273 eliciting markedly increased neutralising antibody titres against Beta and Gamma variants [67]. Boosting with mRNA-1273.351 appeared more effective at increasing neutralization against Beta compared to boosting with mRNA-1273. Encouragingly, immunisation of macaques with protein nanoparticles displaying RBD and adjuvanted with 3M-052 and alum induced cross-neutralising antibodies against bat coronaviruses, SARS-CoV and multiple SARS-CoV-2 variants [68].
Nanoparticles which allow co-display of heterologous vaccine antigens on a single particle might favour the selection of cross-reactive B cells into the vaccine-elicited immune response, thus broadening the range of variants or pathogens recognised by nAbs (Fig. 3b) [69,70]. The computationally-designed protein nanoparticle platform mi3 employing the SpyTag/SpyCatcher system was used to form mosaic nanoparticles displaying four to eight distinct RBDs from SARS-CoV-2 and zoonotic betacoronaviruses [71]. Mice immunised with mosaic RBD-mi3 nanoparticles elicited superior cross-reactive antibody recognition and neutralisation activity against heterologous virus strains, compared to those vaccinated with SARS-CoV-2-RBD nanoparticles or COVID-19 convalescent human plasma. In another approach, the two-component computationally designed protein scaffold I53-50 was constructed to deliver RBDs from four distinct sarbecoviruses - SARS-CoV-1, SARS-CoV-2 and two bat coronaviruses WIV1 and RaTG13 [72]. Immunisation of mice with either a mosaic (four RBDs co-displayed on the same nanoparticle) or as a cocktail (four nanoparticles each expressing a single type of RBD) was able to elicit broad neutralising activity against diverse sarbecoviruses including the SARS-CoV-2 Beta variant.
The maintenance of long-term immune protection against VOCs might similarly be achieved by driving increased T cell immunity. It has been established that the peptide epitopes recognized by anti-viral CD4 and CD8 T cells are relatively conserved in the viral spike, allowing T cell responses to be better maintained in the face of mutations present in VOC compared with antibody responses [73]. Nanoparticle vaccines that can elicit strong T cell memory responses might therefore provide a pathway to durable protection against current and future VOCs. Notably, presentation of RBD on protein nanoparticles elicited greater cytotoxic CD8+ T cell responses compared to monomeric antigen in mice [74]. However, optimal T cell immunity would likely be localised to the site of infection in the form of resident memory T cells. Therefore, the utility of delivering nanoparticle vaccines directly to mucosal sites requires further investigation.
The availability of multiple types of nanoparticle vaccines could support the deployment of heterologous prime-boost strategies, which can reduce the impact of supply-chain disruptions and allow greater flexibility in vaccination programmes [5,75]. So called “mix-and-match” strategies have demonstrated that exceptionally potent immune responses can be generated by combining two or more existing vaccine platforms [76], in particular, boosting adenoviral vaccines primed subjects with mRNA-LNP vaccines [77]. Maximising immunity in the population is likely critical for vaccine protection to allow better control of emerging viral variants. In addition, diminished vaccine immunogenicity due to increasing anti-vector immunity seen after repeated dosing with live virus-based vaccines suggests multiple non-redundant vaccine platforms will be required for boosters into the future [76].
4. Future of nanoparticle vaccines for this pandemic and the next
The SARS-CoV-2 pandemic has heralded a coming-of-age for vaccine nanotechnology. Decades of groundwork optimizing new vaccine platforms allowed clinical trials with nanoparticle vaccine candidates to begin within 2 months of the SARS-CoV-2 genome sequence being made publicly available. The unprecedented swiftness of this response and the high degree of efficacy afforded by nanoparticle COVID-19 vaccines now approved has saved many lives and significantly advanced the science of vaccinology. However, how nanoparticle-based vaccine technologies will evolve for the next phase of the SARS-CoV-2 pandemic, or to combat emerging pandemic threats from other pathogens is not yet clear.
Current COVID-19 vaccine deployment is not globally equitable with selected high- and middle-income countries approaching high vaccine coverage while vaccine roll-out has barely begun in many low-income countries [2]. mRNA-LNP vaccines are more expensive than traditional vaccines, with both the Pfizer/BioNTech and Moderna vaccines costing ∼$USD 15-30 per dose compared to ∼$USD 2 for a less efficacious, whole-inactivated virus vaccine Covaxin, developed in India [78]. Novel mRNA-LNP and protein nanoparticle vaccine platforms require specialised manufacturing facilities and highly skilled workforces, a major challenge in many resource-limited settings. An additional drawback of mRNA vaccines is the current requirement of ultra-cold chain storage, which complicates the logistics of vaccine manufacturing, storage and distribution. A next generation COVID-19 mRNA vaccine candidate (mRNA-1283) developed by Moderna is refrigerator-stable, facilitating easier distribution and administration in a wider range of settings and has entered a phase 1 clinical trial (NCT04813796). Protein-based nanoparticle platforms face extensive challenges also. In particular, the stringent requirements of GMP-grade cell-line development to facilitate manufacturing, along with relatively complicated purification, formulation and stability challenges can complicate vaccine scale-up efforts, potentially leading to significant delays in gaining regulatory approval [79]. There is an urgent need for technology transfer, and research into advanced manufacturing technologies to spread the benefits of nanoparticle vaccine technologies more widely and ensure an adequate global supply of vaccines for durable immune control over the virus.
The rapid and widespread use of new classes of nanoparticle vaccines has raised safety concerns, with evidence of extremely rare but severe anaphylaxis and myocarditis following vaccinations with mRNA-LNP [80]. A lipid component in mRNA-LNP vaccine formulation which contains polyethylene glycol (PEG) 2000 has been believed to cause this anaphylactic response, especially in people with pre-existing anti-PEG antibodies [81]. This phenomenon also raises safety concerns to other PEG derivatives such as polysorbates which are formulated with other nanoparticle vaccines, for examples S protein-micelle vaccines manufactured by Novavax [82]. Thus, further investigations into specific mechanisms and/or genetic/epigenetic pre-disposing factors for such reactions, not only related to PEG but also other new materials/carriers need to be urgently performed in order to avert the risk of increased vaccine hesitancy and improve public acceptance of novel vaccine technologies.
5. Outstanding questions
Finally, we need more information to guide the rational design of next generation vaccines to combat SARS-CoV-2 and other emerging pathogens. In particular, which vaccine platforms strike the optimal balance between efficacy and manufacturability for global scale use? Can combinations of multiple nanoparticle vaccine platforms in a heterologous prime-boost approach confer superior immunity? And will mosaic vaccines allow universal protection against all SARS-CoV-2 strains, or at least reduce the impact of emerging variants that escape antibody-based neutralisation?
6. Search strategy and selection criteria
Data for this Review were identified by searches of PubMed and references from relevant articles using the search terms “nanoparticle”, “COVID-19”, “SARS-CoV-2” and “vaccine”. Non-peer reviewed summary data was also collected from the WHO website and submitted but not yet peer-reviewed articles were also collected from bioRxiv and medRxiv. Only articles published in English between 2019 and 2021 were included.
Contributors
M.N.V. and H.G.K. wrote the original draft, S.J.K. and A.K.W. revised and edited the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
This work was supported by the Australian Research Council (ARC) Centre of Excellence in Convergent Bio-Nano Science and Technology (Project No. CE140100036). M.N.V. acknowledges the financial support from Monash Graduate Scholarship (MGS) and Monash International Postgraduate Research Scholarship (MIPRS). SJK and AKW are supported by NHMRC fellowships. Funding sources were not involved in the decision to submit this review for publication or in the writing of this review.
Contributor Information
Stephen J. Kent, Email: skent@unimelb.edu.au.
Adam K. Wheatley, Email: a.wheatley@unimelb.edu.au.
References
- 1.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 3.Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillus D, Schwarz T, Tober-Lau P, Vanshylla K, Hastor H, Thibeault C, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021 doi: 10.1016/s2213-2600(21)00357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian JH, Patel N, Haupt R, Zhou H, Weston S, Hammond H, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021;12:1–14. doi: 10.1038/s41467-020-20653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 8.Moyer TJ, Zmolek AC, Irvine DJ, Moyer TJ, Zmolek AC, Irvine DJ. Beyond antigens and adjuvants : formulating future vaccines. J Clin Invest. 2016;126:799–808. doi: 10.1172/JCI81083.the. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vu MN, Kelly HG, Tan H, Juno JA, Esterbauer R, Davis TP, et al. Hemagglutinin Functionalized Liposomal Vaccines Enhance Germinal Center and Follicular Helper T Cell Immunity. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly HG, Tan HX, Juno JA, Esterbauer R, Ju Y, Jiang W, et al. Self-assembling influenza nanoparticle vaccines drive extended germinal center activity and memory B cell maturation. JCI Insight. 2020;5 doi: 10.1172/jci.insight.136653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WS, Selva KJ, Davis SK, Wines BD, Reynaldi A, Esterbauer R, et al. Decay of Fc-dependent antibody functions after mild to moderate COVID-19. Cell Reports Med. 2021;2 doi: 10.1016/j.xcrm.2021.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996–1012. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Chen J, Gao K, Wei G-W. Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, South Africa, and other COVID-19-devastated countries. Genomics. 2021;113:2158–2170. doi: 10.1016/j.ygeno.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The COVID-19 candidate vaccine landscape and tracker. Date Accessed 1 July 2021 n.d. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 15.Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 17.Bale S, Goebrecht G, Stano A, Wilson R, Ota T, Tran K, et al. Covalent Linkage of HIV-1 Trimers to Synthetic Liposomes Elicits Improved B Cell and Antibody Responses. J Virol. 2017;91 doi: 10.1128/JVI.00443-17. e00443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thanh T, Thi H, Suys EJA, Lee JS, Nguyen DH, Park KD, et al. Lipid-Based Nanoparticles in the Clinic and Clinical Trials : From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines. 2021;9:359. doi: 10.3390/vaccines9040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Editoral. Let's talk about lipid nanoparticles. Nat Rev Mater. 2021;6 doi: 10.1038/s41578-021-00281-4. [DOI] [Google Scholar]
- 20.Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. Npj Vaccines. 2020;5:3–8. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perchik S, Katz MA, Hernán MA, Lipsitch M, Phil D, Reis B, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenforde MW, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, et al. Neutralizing Activity of BNT162b2-Elicited Serum. N Engl J Med. 2021;384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379–1384. doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu K, Werner AP, Moliva JI, Koch M, Choi A, Stewart-Jones GBE, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. BioRxiv. 2021 doi: 10.1101/2021.01.25.427948. [DOI] [Google Scholar]
- 27.Bernal JL, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalpke AH, Helm M. RNA mediated Toll-like receptor stimulation in health and disease. RNA Biol. 2012;9:828–842. doi: 10.4161/rna.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrie Y, Crofts F, Devitt A, Griffiths HR, Kastner E, Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Adv Drug Deliv Rev. 2016;99:85–96. doi: 10.1016/j.addr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CureVac. CureVac Provides Update on Phase 2b/3 Trial of First-Generation COVID-19 Vaccine Candidate, CVnCoV n.d. https://www.curevac.com/en/2021/06/16/curevac-provides-update-on-phase-2b-3-trial-of-first-generation-covid-19-vaccine-candidate-cvncov/(accessed October 21, 2021).
- 32.Lu J, Lu G, Tan S, Xia J, Xiong H, Yu X, et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020;30:936–939. doi: 10.1038/s41422-020-00392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medhi R, Srinoi P, Ngo N, Tran H, Lee TR. Nanoparticle-Based Strategies to Combat COVID-19. ACS Appl Nano Mater. 2020;3:8557–8580. doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- 34.Renu S, Feliciano-ruiz N, Patil V. Immunity and Protective Efficacy of Mannose Conjugated Chitosan-Based Influenza Nanovaccine in Maternal Antibody Positive Pigs. Front Immunol. 2021;12:516. doi: 10.3389/fimmu.2021.584299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JYH, et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science (80-) 2019;363:649–654. doi: 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortega-Rivera OA, Shin MD, Chen A, Beiss V, Moreno-Gonzalez MA, Lopez-Ramirez MA, et al. Trivalent Subunit Vaccine Candidates for COVID-19 and Their Delivery Devices. J Am Chem Soc. 2021;143:14748–14765. doi: 10.1021/jacs.1c06600. [DOI] [PubMed] [Google Scholar]
- 37.Ward BJ, Gobeil P, Séguin A, Atkins J, Boulay I, Charbonneau PY, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med. 2021;27:1071–1078. doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fluckiger AC, Ontsouka B, Bozic J, Diress A, Ahmed T, Berthoud T, et al. An enveloped virus-like particle vaccine expressing a stabilized prefusion form of the SARS-CoV-2 spike protein elicits highly potent immunity. Vaccine. 2021;39:4988–5001. doi: 10.1016/j.vaccine.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Shi W, Abiona OM, Nazzari A, Olia AS, Ou L, et al. Newcastle disease virus-like particles displaying prefusion-stabilized sars-cov-2 spikes elicit potent neutralizing responses. Vaccines. 2021;9:73. doi: 10.3390/vaccines9020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bangaru S, Ozorowski G, Turner HL, Antanasijevic A, Huang D, Wang X, et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science (80-) 2020;370:1089–1094. doi: 10.1126/science.abe1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials n.d.:Date accessed 23 March 2021. https://ir.novavax.com/news-releases/news-release-details/novavax-confirms-high-levels-efficacy-against-original-and-0.
- 42.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021;384:1899–1909. doi: 10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joyce MG, King HAD, Naouar IE, Ahmed A, Peachman KK, Cincotta CM, et al. Efficacy of a Broadly Neutralizing SARS-CoV-2 Ferritin Nanoparticle Vaccine in Nonhuman Primates. BioRxiv. 2021 doi: 10.1101/2021.03.24.436523. [DOI] [Google Scholar]
- 45.Joyce MG, Chen W, Sankhala RS, Hajduczki A, Paul V, Choe M, et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. BioRxiv 2021. 10.1101/2021.05.09.443331. [DOI] [PMC free article] [PubMed]
- 46.King HAD, Joyce MG, Naouar IE, Cincotta CM, Subra C, Peachman KK, et al. Efficacy and breadth of adjuvanted SARS-CoV-2 receptor-binding domain nanoparticle 2 vaccine in macaques. BioRxiv 2021. 10.1101/2021.04.09.439166. [DOI] [PMC free article] [PubMed]
- 47.Reddington SC, Howarth M. Secrets of a covalent interaction for biomaterials and biotechnology : SpyTag and SpyCatcher. Curr Opin Chem Biol. 2015;29:94–99. doi: 10.1016/j.cbpa.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Huang B, Zhu Y, Tan W, Zhu M. Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice. Cell Mol Immunol. 2021;18:749–751. doi: 10.1038/s41423-021-00643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang B, Chao CW, Tsybovsky Y, Abiona OM, Hutchinson GB, Moliva JI, et al. A platform incorporating trimeric antigens into self ‑ assembling nanoparticles reveals SARS ‑ CoV ‑ 2 ‑ spike nanoparticles to elicit substantially higher neutralizing responses than spike alone. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-74949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fougeroux Cyrielle, Goksøyr Louise, Idorn Manja, Soroka Vladislav, Myeni Sebenzile K., Dagil Robert, Janitzek Christoph M., Søgaard Max, Aves Kara-Lee, Horsted Emma W., Erdoğan Sayit Mahmut, Gustavsson Tobias, Dorosz Jerzy, Clemmensen Stine. Laurits Freds AS& AFS. Capsid-like particles decorated with the SARS- CoV-2 receptor-binding domain elicit strong virus neutralization activity. Nat Commun. 2021;12:1–11. doi: 10.1038/s41467-020-20251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang WC, Zhou S, He X, Chiem K, Mabrouk MT, Nissly RH, et al. SARS-CoV-2 RBD Neutralizing Antibody Induction is Enhanced by Particulate Vaccination. Adv Mater. 2020;32 doi: 10.1002/adma.202005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng B, Peng W, Guo M, Huang M, Gu Y, Wang T, et al. Inhalable nanovaccine with biomimetic coronavirus structure to trigger mucosal immunity of respiratory tract against COVID-19. Chem Eng J. 2021;418 doi: 10.1016/j.cej.2021.129392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 54.Kelly HG, Kent SJ, Wheatley AK. Immunological basis for enhanced immunity of nanoparticle vaccines. Expert Rev Vaccines. 2019;18:269–280. doi: 10.1080/14760584.2019.1578216. [DOI] [PubMed] [Google Scholar]
- 55.Villar RF, Patel J, Weaver GC, Kanekiyo M, Wheatley AK, Yassine HM, et al. Reconstituted B cell receptor signaling reveals carbohydrate-dependent mode of activation. Sci Rep. 2016:36298. doi: 10.1038/srep36298. Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King NP, Bale JB, Sheffler W, McNamara DE, Gonen S, Gonen T, et al. Accurate design of co-assembling multi-component protein nanomaterials. Nature. 2014;510:103–108. doi: 10.1038/nature13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruun TUJ, Andersson AC, Draper SJ, Howarth M. Engineering a Rugged Nanoscaffold To Enhance Plug-and-Display Vaccination. ACS Nano. 2018;12:8855–8866. doi: 10.1021/acsnano.8b02805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He L, Lin X, Wang Y, Abraham C, Sou C, Ngo T, et al. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci Adv. 2021;7:1–18. doi: 10.1126/sciadv.abf1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsia Y, Bale JB, Gonen S, Shi D, Sheffler W, Fong KK, et al. Design of a hyperstable 60-subunit protein icosahedron. Nature. 2016;535:136–139. doi: 10.1038/nature18010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan TK, Rijal P, Rahikainen R, Keeble AH, Schimanski L, Hussain S, et al. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. Nat Commun. 2021;12:1–16. doi: 10.1038/s41467-020-20654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bale JB, Gonen S, Liu Y, Sheffler W, Ellis D, Thomas C, et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science (80-) 2016;353:389–394. doi: 10.1126/science.aaf8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brouwer PJM, Brinkkemper M, Maisonnasse P, Dereuddre-Bosquet N, Grobben M, Claireaux M, et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell. 2021;184:1188–1200. doi: 10.1016/j.cell.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walls AC, Fiala B, Schäfer A, Wrenn S, Pham MN, Murphy M, et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell. 2020;183:1367–1382. doi: 10.1016/j.cell.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang YF, Sun C, Zhuang Z, Yuan RY, Zheng Q, Li JP, et al. Rapid Development of SARS-CoV-2 Spike Protein Receptor-Binding Domain Self-Assembled Nanoparticle Vaccine Candidates. ACS Nano. 2021;15:2738–2752. doi: 10.1021/acsnano.0c08379. [DOI] [PubMed] [Google Scholar]
- 65.Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021:1–10. doi: 10.1038/s41586-021-03530-2. [DOI] [PubMed] [Google Scholar]
- 66.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021:1–17. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu K, Choi A, Koch M, Ma L, Hill A, Nunna N. Preliminary Analysis of Safety and Immunogenicity of a SARS-CoV-2 Variant Vaccine Booster. MedRxiv 2021. 10.1101/2021.05.05.21256716. [DOI] [PMC free article] [PubMed]
- 68.Saunders KO, Lee E, Parks R, Martinez DR, Li D, Chen H, et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature. 2021;594:553–559. doi: 10.1038/s41586-021-03594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol. 2019;20:362–372. doi: 10.1038/s41590-018-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyoglu-Barnum S, Ellis D, Gillespie RA, Hutchinson GB, Park Y-J, Moin SM, et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature. 2021;592:623–628. doi: 10.1038/s41586-021-03365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen AA, Gnanapragasam PNP, Lee YE, Hoffman PR, Ou S, Kakutani LM, et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science (80-) 2021;741:735–741. doi: 10.1126/science.abf6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walls AC, Miranda MC, Pham MN, Schäfer A, Greaney A, Prabhu S, et al. Elicitation of broadly protective sarbecovirus immunity by receptor- binding domain nanoparticle vaccines. BioRxiv 2021. https://doi.org/10.1101/2021.03.15.435528. [DOI] [PMC free article] [PubMed]
- 73.Jordan SC, Shin BH, Gadsden TAM, Chu M, Petrosyan A, Le CN, et al. T cell immune responses to SARS-CoV-2 and variants of concern (Alpha and Delta) in infected and vaccinated individuals. Cell Mol Immunol. 2021 doi: 10.1038/s41423-021-00767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma X, Zou F, Yu F, Li R, Yuan Y, Zhang Y, et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity. 2020;53:1315–1330. doi: 10.1016/j.immuni.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ewen Callaway. Mixing Covid Vaccines Triggers Potent Immune Response. Nature. 2021;593:491. doi: 10.1038/d41586-021-01359-3. [DOI] [PubMed] [Google Scholar]
- 76.Chiu NC, Chi H, Tu YK, Huang YN, Tai YL, Weng SL, et al. To mix or not to mix? A rapid systematic review of heterologous prime–boost covid-19 vaccination. Expert Rev Vaccines. 2021:1–10. doi: 10.1080/14760584.2021.1971522. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, et al. Heterologous SARS-CoV-2 Booster Vaccinations – Preliminary Report. MedRxiv 2021:2021. 10.10.21264827. https://doi.org/10.1101/2021.10.10.21264827.
- 78.Dyer O. Covid-19: Countries are learning what others paid for vaccines. BMJ Bristish Med J. 2021;372:n281. doi: 10.1136/bmj.n281. [DOI] [PubMed] [Google Scholar]
- 79.Pollet J, Chen WH, Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv Drug Deliv Rev. 2021;170:71–82. doi: 10.1016/j.addr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blumenthal KG, Phadke NA, Bates DW. Safety Surveillance of COVID-19 mRNA Vaccines Through the Vaccine Safety Datalink. JAMA. 2021;326:1375–1377. doi: 10.1001/jama.2021.14808. [DOI] [PubMed] [Google Scholar]
- 81.McSweeney MD, Mohan M, Commins SP, Lai SK. Anaphylaxis to Pfizer/BioNTech mRNA COVID-19 Vaccine in a Patient With Clinically Confirmed PEG Allergy. Front Allergy. 2021;2:1–5. doi: 10.3389/falgy.2021.715844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castells MC, Philips EJ. Maintaining Safety with SARS-CoV-2 Vaccines. N Engl J Med. 2021;384:e37. doi: 10.1056/nejmc2100766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwendener RA. Liposomes as vaccine delivery systems: A review of the recent advances. Ther Adv Vaccines. 2014;2:159–182. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bose RJ, Kim M, Chang JH, Paulmurugan R, Moon JJ, Koh WG, et al. Biodegradable polymers for modern vaccine development. J Ind Eng Chem. 2019;77:12–24. doi: 10.1016/j.jiec.2019.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu L, Liu Z, Chen H, Liu H, Gao Q, Cong F, et al. Subunit Nanovaccine with Potent Cellular and Mucosal Immunity for COVID-19. ACS Appl Bio Mater. 2020;3:5633–5638. doi: 10.1021/acsabm.0c00668. [DOI] [PubMed] [Google Scholar]
- 86.Marqués-Gallego P, De Kroon AIPM. Ligation strategies for targeting liposomal nanocarriers. Biomed Res Int. 2014 doi: 10.1155/2014/129458. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Z, Moon JJ, Cheng W. Quantitation and Stability of Protein Conjugation on Liposomes for Controlled Density of Surface Epitopes. Bioconjug Chem. 2018;29:1251–1260. doi: 10.1021/acs.bioconjchem.8b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]