Abstract
Background
Frequency, dimensions, management, and outcomes of the COVID-19 pandemic in children with endocrine disorders and diabetes were assessed.
Methods
A cross-sectional electronic survey was distributed to the global network of endocrine societies. Respondents’ professional and practice profiles, clinic sizes, their country of practice, and the impact of COVID-19 on endocrine diseases were investigated.
Results
Respondents from 131 pediatric endocrine centers in 51 countries across all continents completed the survey. Routine check-ups and education were altered in most pediatric endocrine clinics. Over 20% of clinics experienced a shortage of critical medications or essential supplies. ICU treatment was required for patients with diabetes and COVID-19 in 21.2% of centers. In diabetes, 44% of respondents reported increased diabetic ketoacidosis episodes in newly diagnosed cases and 30% in established cases. Biopsychosocial and behavioral changes were explicitly reported to be occurring among pediatric patients with endocrine disorders.
Conclusions
This large global survey conducted during the COVID-19 pandemic highlights that diabetes is more challenging to manage than any other pediatric endocrine disorder, with an increased risk of morbidity. Psychological distress due to COVID-19 needs to be recognized and addressed. The importance of close contact with healthcare professionals should be emphasized, and medical supplies should be readily available to all patients.
Keywords: COVID-19, children, diabetes, obesity and metabolic syndrome, adrenal, thyroid, growth, puberty
Highlights
A previous survey found increased risk of diabetic ketoacidosis at diabetes diagnosis.
Over 20% of clinics faced a shortage of critical medications or supplies.
Patients with diabetes needed intensive care in 21.2% centers.
Biopsychosocial concerns were highly reported, including attempted suicide. They must be recognized and addressed.
During the COVID-19 pandemic, management of diabetes appears to have been more challenging than other pediatric endocrine disorders.
1 Introduction
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is still having a severe global impact (1). While progress has been made in immunization, there are likely to be recurrent “waves” of infection over several years until a majority of the population achieves immunity either through infection or vaccination (2–4).
While children generally have milder disease than adults (5), occasional cases of a Kawasaki-like disease linked to SARS-CoV-2 infection is now a recognized complication of COVID-19 [multisystem inflammatory syndrome (MIS) in children (MIS-C)] (6–8). However, very little is known about the impact of COVID-19 on patients with chronic conditions like endocrine diseases, especially in children (9). Most recent studies have examined endocrine disorders in adult populations (10–13). This is despite healthcare professionals (HCPs) and families requiring guidance on the clinical management of children with endocrine disorders during the challenging circumstances of the COVID-19 pandemic, especially those with suspected or confirmed COVID-19.
Here we report the outcomes of a global survey of pediatric endocrine specialist HCPs registered in the International Consortium for Pediatric Endocrinology (ICPE) database. We report the clinical characteristics of patients managed during the pandemic and highlight the knowledge and practice of HCPs working in pediatric endocrinology and the specific challenges they have faced during the pandemic. Moreover, we discuss the gaps identified between a previous survey on diabetes conducted early in the pandemic (9) and the current survey to assess and explain the frequency, dimensions, management, viewpoints, and outcomes of the COVID-19 pandemic in children, adolescents, and young people living with chronic endocrine disease.
2 Methods
2.1 Study Design and Setting
This was a cross-sectional electronic survey conducted over an eight-week period from December 3, 2020 to February 5, 2021. The survey was conducted using Google Forms (Google LLC, Mountain View, CA, United States), which allows responses to be saved and subsequently analyzed in spreadsheet format. Details of the data collection and survey methodology are described in our previous publication (9).
The target population was identified from a global network of endocrine societies, under the umbrella of the ICPE, made up of the International Society for Pediatric and Adolescent Diabetes (ISPAD), European Society for Pediatric Endocrinology (ESPE), Global Pediatric Endocrinology and Diabetes (GPED), Latin American Society of Pediatric Endocrinology (SLEP), Australasian Pediatric Endocrine Group (APEG), Asia Pacific Pediatric Endocrine Society (APPES), African Society for Pediatric and Adolescent Endocrinology (ASPAE), Arab Society for Pediatric Endocrinology Diabetes (ASPED), Chinese Society of Pediatric Endocrinology and Metabolism (CSPEM), Indian Society for Pediatric and Adolescent Endocrinology (ISPAE), Japanese Society for Pediatric Endocrinology (JSPE), Pediatric Endocrinology Society of North America (PES), and Russian Pediatric Endocrinology Group (RAE). Previous participants of the societies’ conferences, training schools, or postgraduate courses were also included.
2.2 The Survey
The full version of the survey is available in Supplemental File 1 . Briefly, the survey questions were developed by six pediatric endocrinologists, and a direct web link and consent to participate in the survey was sent to ICPE members by email and social media platforms (Facebook, Twitter, and LinkedIn).
The survey questions were divided into fourteen sections that captured information on responders’ professional and practice profiles, sizes of their clinic and country of practice, and their management of the most common endocrine diseases.
The questions covered the practice and perceptions of HCPs with respect to the number of patients cared for, the organization of education sessions, the impact of the COVID-19 pandemic on daily routine, the availability of medications, frequency of acute complications, delays to diagnoses, deterioration of disease control, and the psychological impact on patients and their families. We included a few specific questions to characterize the profile of patients who tested positive for SARS-CoV-2 infection including their characteristics, clinical presentation, diagnosis, and treatment. The survey took about thirty minutes to complete.
2.3 Statistical Analysis
Data were analyzed using STATA 14.0 for Windows (College Station, TX, USA). The unit of analysis corresponded to a single center. Descriptive statistics were used to present demographic data and to evaluate the knowledge, attitudes, and perceptions of HCPs during the COVID-19 pandemic. Quantitative variables were described in the form of means and standard deviations (SD), and qualitative variables were described as numbers and percentages. The sum of some results exceeds the number of responses because of the option to answer as multiple choices. Some questions were open-ended and were analyzed using a coding technique, where similar answers are summarized by approximation into similar semantic content (14).
3 RESULTS
3.1 Responders’ Professional and Practice Profiles
A total of 136 responses were assessed; two responders declined to participate in the survey, and three responses were duplicates so were suppressed from analyses. A convenience sample of 131 pediatric endocrine centers from 51 countries across all continents participated in the study and were included in the final analysis. The country of origin of each responder and their professional background, center setting, and size are shown in Table 1 .
Table 1.
Endocrine clinical center characteristics and staff profiles.
| Characteristics (n respondents) | Respondents (%) |
|---|---|
| Centers by country (131) | |
| United Sates of America | 11 (8.4) |
| Spain | 9 (6.9) |
| Philippines | 8 (6.1) |
| Germany | 7 (5.3) |
| Egypt, Italy | 6 (4.6) each |
| Argentina, Brazil, United Kingdom | 5 (3.8) each |
| Canada, Greece | 4 (3.0) each |
| India, Japan, Netherlands, Portugal | 3 (2.3) each |
| Belgium, Bulgaria, Congo, Denmark, Indonesia, Iran, Malaysia, Mexico, New Zealand, Peru, Serbia and Montenegro, Sweden, Turkey | 2 (1.5) each |
| Australia, Austria, Bangladesh, Chile, Cyprus, Finland, Georgia, Hong Kong, Hungary, Iceland, Iraq, Ireland, Israel, Lebanon, Luxembourg, Malta, Netherlands Antilles, Poland, Romania, Slovenia, Sudan Taiwan, Ukraine | 1 (0.8) each |
| Current clinical role (131) | |
| Pediatric endocrinologist/diabetologist | 109 (83.2) |
| Pediatrician with interest in endocrinology | 15 (11.4) |
| Resident or fellow or trainee in pediatrics/pediatric endocrinology or diabetology or diabetes researcher | 4 (3.0) |
| Adult physician looking after pediatric or adolescent patients | 2 (1.5) |
| Nurse practitioner/registered nurse | 1 (0.8) |
| Clinical setting (131) | |
| University/academic hospital or clinic | 64 (48.9) |
| Public/governmental hospital or clinic | 41 (31.3) |
| Private hospital or clinic | 25 (19.1) |
| Primary care center | 1 (0.8) |
| Estimate case-mix, patients by endocrine disorders | |
| Type 1 diabetes | |
| <100 | 46 (40.7%) |
| 100-250 | 39 (34.5%) |
| 251-500 | 16 (14.2%) |
| >500 | 12 (10.6%) |
| Type 2 diabetes | |
| ≤50 | 45 (93.7%) |
| 51-100 | 1 (2.1%) |
| >100 | 3 (6.2%) |
| Other forms of diabetes | |
| ≤50 | 41 (91.1%) |
| 51-100 | 1 (2.2%) |
| >100 | 3 (6.7%) |
| Obesity and metabolic syndrome | |
| ≤50 | 37 (58.7%) |
| 51-100 | 8 (12.7%) |
| >100 | 18 (28.6%) |
| Hyperinsulinemic hypoglycemia | |
| ≤50 | 39 (97.5%) |
| >50 | 1 (2.5%) |
| Thyroid | |
| ≤50 | 49 (55.1%) |
| 51-100 | 16 (18.0%) |
| >100 | 24 (26.9%) |
| Adrenal | |
| ≤50 | 51 (79.7%) |
| 50-100 | 10 (15.6%) |
| >100 | 3 (7.8%) |
| Bone metabolism | |
| ≤50 | 26 (83.9%) |
| >50 | 5 (16.1%) |
| Pituitary and other CNS disorder | |
| ≤50 | 55 (90.2%) |
| >50 | 6 (9.8%) |
| Growth | |
| ≤50 | 44 (61%) |
| 51-100 | 515 (21%) |
| >100 | 13 (18%) |
| Pubertal | |
| ≤50 | 50 (73.5%) |
| 51-100 | 12 (17.6%) |
| >100 | 6 (8.8%) |
| Others: Gender dysphoria | |
| ≤50 | 1 (50%) |
| >50 | 1 (50%) |
3.2 Overall Outcomes From Pediatric Endocrine Disorders
During the COVID-19 pandemic, routine follow-up visits and education were altered in most pediatric endocrine centers, with care and disease literacy most often delivered face-to-face (F2F) using appropriate personal protective equipment (PPE) and to a lesser extent using telephone and video consultations. For diabetes care, F2F care was restricted to only one caregiver, while for hyperinsulinemic hypoglycemia (HH) and bone metabolism disorders, over one-fifth of centers maintained care as usual. Unsurprisingly, in most centers, contact with the diabetes or endocrine team was limited due to the fear of COVID-19 by the families themselves.
Over 20% of responder centers reported shortages of several supplies specific for diabetes, HH, adrenal, and bone metabolism disorders: glucose test stripes (14.1%), blood glucose sensors (12.4%), basal (11.5%) and bolus (10.6%) insulin, diazoxide (19.5%), hydrocortisone (25.7%), and calcitriol (9.4%). In addition, genetic testing and imaging were in part affected in centers managing monogenic diabetes (14.3%) and bone metabolism disorders (9.4%) ( Tables 2 , 3 ).
Table 2.
Assessment of pediatric diabetes care during the COVID-19 pandemic by clinical centers.
| Type 1 diabetes (n=113) | Type 2 diabetes (n=52) | Other forms of diabetes (n=47) | |
|---|---|---|---|
| Estimate proportion of delayed diagnose due to COVID-19 | 45.3% | 17.3% | 17.0% |
| Estimate perception of worsening disease management | 31.0% | 67.3% | 29.8% |
| Use of technologies among patients, % | |||
| • Insulin pump | N/A | N/A | |
| ○ Less than 10 | 39 (34.5%) | ||
| ○ 10-25 | 17 (15.0%) | ||
| ○ 26-50 | 23 (20.3%) | ||
| ○ 51-75 | 23 (20.3%) | ||
| ○ 76-100 | 11 (9.7%) | ||
| • CGMS | N/A | N/A | |
| ○ Less than 10 | 38 (33.6%) | ||
| ○ 10-25 | 28 (24.8%) | ||
| ○ 26-50 | 21 (18.6% | ||
| ○ 51-75 | 21 (18.6%) | ||
| ○ 76-100 | 5 (4.4%) | ||
| • Flash GMS | N/A | N/A | |
| ○ Less than 10 | 35 (31.0%) | ||
| ○ 10-25 | 31 (27.4%) | ||
| ○ 26-50 | 18 (15.9%) | ||
| ○ 51-75 | 19 (16.8%) | ||
| ○ 76-100 | 10 (8.8%) | ||
| Testing | |||
| • COVID-19 tests for newly diagnosed. | 71 (62.8%) | N/A | N/A |
| • Positivity | |||
| ○ No positives with standardized tests | 56 (49.6%) | N/A | N/A |
| ○ Less than 25% | 54 (47.8%) | ||
| ○ 26-50% | 1 (0.9%) | ||
| ○ More than 75% | 2 (1.8%) | ||
| • COVID-19 tests in DKA cases | 79 (69.9%) | N/A | N/A |
| • Positivity with standardized tests | |||
| ○ Less than 10% | |||
| Testing | |||
| • COVID-19 tests for newly diagnosed. | 71 (62.8%) | N/A | N/A |
| • Positivity | |||
| ○ No positives with standardized tests | 56 (49.6%) | N/A | N/A |
| ○ Less than 25% | 54 (47.8%) | ||
| ○ 26-50% | 1 (0.9%) | ||
| ○ More than 75% | 2 (1.8%) | ||
| • COVID-19 tests in DKA cases | 79 (69.9%) | N/A | N/A |
| • Positivity with standardized tests | |||
| ○ Less than 10% | 75 (87.2%) | N/A | N/A |
| ○ 10-25% | 6 (7.0%) | ||
| ○ 26-50% | 2 (2.3%) | ||
| ○ More than 75% | 3 (3.5%) | ||
| Diabetic ketoacidosis episodes | |||
| • Increase of newly-onset cases | 50 (44.2%) | N/A | N/A |
| • Increase in stablished cases | 34 (30.1%) | N/A | N/A |
| • Proportion of DKA episodes | |||
| ○ 0-25% | 66 (58.4%) | N/A | N/A |
| ○ 26-50% | 16 (14.1%) | ||
| ○ 51-75% | 20 (17.7%) | ||
| ○ 76-100% | 11 (9.7%) | ||
| • Proportion of mild DKA | N/A | N/A | |
| ○ 0-25% | 64 (56.6%) | ||
| ○ 26-50% | 31 (27.4%) | ||
| ○ 51-75% | 14 (2.4%) | ||
| ○ 76-100% | 4 (3.6%) | ||
| • Proportion of moderate DKA | N/A | N/A | |
| ○ 0-25% | 68 (60.2%) | ||
| ○ 26-50% | 33 (29.2%) | ||
| ○ 51-75% | 9 (8.0%) | ||
| ○ 76-100% | 3 (2.8%) | ||
| • Proportion of severe DKA | N/A | N/A | |
| ○ 0-25% | 81 (71.7%) | ||
| ○ 26-50% | 15 (13.3%) | ||
| ○ 51-75% | 15 (13.3%) | ||
| ○ 76-100% | 2 (1.8%) | ||
| • Perception of worsening episodes | 48 (42.5%) | N/A | N/A |
| Severe Hypoglycemia episodes | |||
| • Increase of SH episodes | 7 (6.2%) | N/A | N/A |
| Routine check-up | |||
| • As usual, no changes | 18 (15.9%) | 9 (17.3%) | 9 (19.2%) |
| • Sent SMS and emails for consultation. | 34 (30%) | 10 (19.2%) | 13 (27.6%) |
| • Apps | 19 (16.8%) | 6 (11.3%) | 12 (25.5%) |
| • Telephone consultations | 73 (64.6%) | 27 (51.9%) | 27 (57.4%) |
| • Video consultations | 50 (44.2%) | 23 (44.2%) | 18 (38.3%) |
| • Face to face consultation with appropriate personal protective equipment restricted to just one parent/caregiver | 75 (66.3%) | N/A | N/A |
| • Face to face consultation with appropriate personal protective equipment where all caregivers are allowed to attend | 15 (13.2%) | 34 (65.4%) | 28 (59.5%) |
| • No consultation during complete lockdown or postponing it to annual visits | 2 (1.7%) | 1 (1.9%) | 0 |
| • Limited contact with diabetes team because of COVID-19 fear | 85 (75.2%) | 39 (75.0%) | 34 (72.3%) |
| Daily routine | |||
| • Maintenance of physical activity | |||
| ○ Less than 10% | 23 (20.3%) | N/A | N/A |
| ○ 10-25% | 47 (41.6% | ||
| ○ 26-50% | 21 (18.6%) | ||
| ○ 51-75% | 12 (10.6%) | ||
| ○ 76-100% | 10 (8.8%) | ||
| • Worsening of dietary choices | |||
| ○ Less than 10% | 26 (23.0%) | N/A | |
| ○ 10-25% | 31 (27.4%) | ||
| ○ 26-50% | 37 (32.7%) | ||
| ○ 51-75% | 8 (7.1%) | ||
| ○ 76-100% | 11 (9.7%) | N/A | |
| • Increase of body weight | |||
| ○ Less than 10% | 23 (20.3%) | N/A | |
| ○ 10-25% | 28 (24.8%) | ||
| ○ 26-50% | 36 (31.9%) | ||
| ○ 51-75% | 18 (15.9%) | ||
| ○ 76-100% | 8 (7.1%) | ||
| • Extra dose of insulin | |||
| ○ Yes | |||
| ○ No | N/A | ||
| ○ Not on insulin therapy | N/A | N/A | |
| • Average glycemic control during pandemic | |||
| ○ Mostly improved | 21 (18.6%) | ||
| ○ Mostly maintained same level | 57 (50.4%) | ||
| ○ Mostly worsened | 35 (31.0%) | N/A | |
| • Regular use of anti-hypertensive | |||
| ○ ACE inhibitor | N/A | ||
| ○ B-blocker | |||
| ○ Ca channel blocker | 18 (34.6%) | N/A | |
| ○ Control with salt-free diet | 18 (34.6%) | ||
| ○ None | 16 (30.8%) | ||
| N/A | |||
| 26 (76.5%) | |||
| 1 (2.9%) | |||
| 2 (5.9%) | |||
| 1 (2.9%) | |||
| 5 (14.7%) | |||
| School activities | |||
| • Parental concerns to return to school activities | 90 (79.6%) | N/A | N/A |
| • Specific school guidelines during pandemic | 90 (79.6%) | ||
| Patient and family education | |||
| • As usual, no changes | 8 (7%) | 6 (11.5%) | 3 (6.4%) |
| • By telephone | 52 (46%) | 22 (42.3%) | 30 (63.8%) |
| • Video consultations | 49 (43%) | 20 (38.5%) | 18 (38.3%) |
| • Apps/digital platforms | 21 (19%) | 14 (26.9%) | 14 (29.8%) |
| • Face to face education wearing appropriate personal protective equipment | 97 (86%) | 41 (78.8%) | 39 (83.0%) |
| Supplies | |||
| • Refill prescription | N/A | N/A | |
| ○ Refill prescription every month | 12 (10.6%) | ||
| ○ Refill prescription every 3 months or less | 53 (46.7%) | ||
| ○ Refill prescription every 6 months or less | 14 (12.4%) | ||
| ○ Refill prescription every year or less | 9 (8.0%) | ||
| ○ As required by patient | 13 (11.5%) | ||
| ○ Automatic refill prescription from pharmacy | 5 (4.4%) | ||
| ○ I am not directly involved with prescription | 7 (6.2%) | ||
| • Shortage of supplies | |||
| ○ Yes | 25 (22.1%) | 7 (13.5%) | 6 (12.8%) |
| ○ No, everything was secured | 78 (69.0%) | 33 (63.5%) | 35 (74.5%) |
| ○ I am not aware of situation | 10 (8.9%) | 12 (23.1%) | 6 (12.8%) |
| • Item under shortage | |||
| ○ Basal insulin | 13 (11.5%) | 2 (12.5%) | 1 (7.1%) |
| ○ Bolus insulin | 12 (10.6%) | 0 | 0 |
| ○ Glucose test strips | 16 (14.1%) | 3 (18.7%) | 4 (28.6%) |
| ○ Blood glucose sensors | 14 (12.4%) | 0 | 3 (21.4%) |
| ○ Insulin pump supplies | 6 (5.3%) | 0 | 0 |
| ○ Ketone test strips | 8 (7.1) | 0 | 0 |
| ○ Oral medication | 0 | 1 (6.2%) | 1 (7.1%) |
| ○ Genetic tests | 0 | 1 (6.2%) | 2 (14.3%) |
| Characteristics of COVID-19 cases | |||
| • Estimate mean age, years | 11.4 (SD 3.6) (n=60) | 13.6 (SD 2.3) (n=9) | N/A |
| • Estimate % boys | 47.3 (SD 24.2) (n=46) | 53.4 (SD 24.9) (n=11) | |
| • Estimate % girls | 52.2 (SD 24.3) (n=46) | 46.6 (SD 25.0) (n=11) | |
| • Estimate mean duration of disease, years | 3.5 (SD 2.3) (n=55) | 1.2 (SD 1.4) (n=13) | |
| • Estimate mean HbA1c value, % | 8.5 (1.6) (n=50) | 7.7 (SD 3.2) (n=10) | |
| • BMI, Kg/m² | N/A | 30.2 (SD 3.0) (n=8) | |
| • Presence of comorbidities | |||
| ○ Asthma | 32 (28.3%) | 15 (28.8%) | 5 (10.6%) |
| ○ Cancer | 3 (2.6%) | 2 (3.8%) | 4 (8.5%) |
| ○ Obesity | 44 (38.9%) | 48 (92.3%) | 10 (21.3%) |
| ○ Hypertension | 19 (16.8%) | 23 (44.2%) | 1 (2.1%) |
| ○ Heart disease | 3 (2.6%) | 0 | 2 (4.2%) |
| ○ Kidney disease | 15 (13.3%) | 3 (5.8%) | 8 (17.0%) |
| ○ Neurological disease | 2 (1.7%) | 0 | 2 (4.2%) |
| ○ Celiac disease | 10 (8.8%) | 0 | 0 |
| ○ Hypothyroidism | 8 (7.1%) | 0 | 0 |
| ○ Cystic fibrosis or bronchial dysplasia | 11 (9.7%) | 2 (3.8%) | 12 (25.5%) |
| ○ Other: Allergy, other autoimmune disease, dyslipidemia, Polycystic ovary syndrome, Lupus, Anemia | 4 (3.5%) | 2 (3.8%) | 2 (4.2%) |
| ○ No | 32 (28.3%) | 4 (7.7%) | 18 (38.3%) |
| Psychological concerns | |||
| • Anxiety | 69 (61.1%) | 31 (59.6%) | 24 (51.1%) |
| • Parenting stress | 61 (54.0%) | 22 (42.3%) | 17 (36.2%) |
| • Depression | 42 (37.2%) | 24 (46.1%) | 14 (29.8%) |
| • Insomnia/hypersomnia or other sleep disruption | 36 (31.8%) | 13 (25.0%) | 5 (10.6%) |
| • Eating disorder | 25 (22.1%) | 15 (28.8%) | 5 (10.6%) |
| • Panic attacks | 17 (15.0%) | 11 (21.1%) | 7 (14.9%) |
| • Suicide attempt | 6 (5.3%) | 2 (3.8%) | 1 (2.1%) |
| • Patient or caregivers have improved the mood | 4 (3.5%) | 0 | 1 (2.1%) |
| • None have had psychological problems so far | 31 (27.4%) | 15 (28.8%) | 20 (42.5%) |
N/A, not applicable.
Table 3.
Assessment of pediatric endocrine care other than diabetes during COVID-19 pandemic by clinical centers.
| Obesity and Metabolic Syndrome (n=65) | Hyperinsulinemic hypoglycemia (n=41) | Thyroid disorders (n=91) | Adrenal disorders (n=66) | Bone metabolism disorders (n=32) | Pituitary and other CNS disorders (n=62) | Growth disorders (n=73) | Pubertal disorders (n=69) | |
|---|---|---|---|---|---|---|---|---|
| Estimated proportion of delayed diagnoses due to COVID-19 | 41.5% | 9.8% | 22.0% | 15.1% | 18.7% | 21.0% | 39.4% | 37.7% |
| Estimated proportion of increase in severity | N/A | 7.3% | N/A | 4.6% | N/A | N/A | N/A | N/A |
| Estimate perception of worsening disease management | 83.1% | 24.4% | 22.0% | 36.4% | 31.2% | 27.4% | 35.6% | 34.8% |
| Routine check-up | ||||||||
| • As usual, no changes | 11 (16.9%) | 12 (29.3%) | 17 (18.7%) | 9 (13.6%) | 7 (21.9%) | 11 (17.7%) | 10 (13.7%) | 13 (18.8%) |
| • Sent SMS and emails for consultation | 15 (23.1%) | 10 (24.4%) | 24 (26.4%) | 18 (27.3%) | 10 (31.2%) | 17 (27.4%) | 22 (30.1%) | 18 (26.1%) |
| • Apps | 5 (7.7%) | 7 (17.1%) | 7 (7.7%) | 7 (10.1%) | 7 (21.9%) | 5 (8.1%) | 8 (10.9%) | 8 (11.6%) |
| • Telephone consultations | 36 (55.4%) | 20 (48.8%) | 53 (58.2%) | 39 (59.1%) | 16 (50.0%) | 37 (59.7%) | 44 (60.3%) | 36 (52.2%) |
| • Video consultations | 19 (29.2%) | 9 (21.9%) | 27 (29.7%) | 21 (31.8%) | 10 (31.2%) | 20 (32.3%) | 23 (31.5%) | 22 (31.9%) |
| • Face to face consultation with appropriate personal protective equipment | 43 (66.1%) | 23 (56.1%) | 60 (65.9%) | 51 (77.3%) | 19 (59.4%) | 41 (66.1%) | 53 (72.6%) | 51 (73.9%) |
| • No consultation during complete lockdown or postponing it to annual visits | 1 (1.5%) | 0 | 1 (1.1%) | 1 (1.5%) | 0 | 1 (1.6%) | 1 (1.4%) | 0 |
| • Limited contact with endocrine team because of COVID-19 fear | 59 (90.7%) | 26 (63.4%) | 67 (73.6%) | 45 (68.2%) | 22 (68.7%) | 39 (62.9%) | 49 (67.1%) | 48 (69.6%) |
| Patient and family education | ||||||||
| • As usual, no changes | 3 (4.6%) | 3 (7.3%) | 7 (7.7%) | 3 (4.5%) | 2 (6.1%) | 2 (3.2%) | 5 (6.8%) | 4 (5.6%) |
| • By telephone | 34 (52.3%) | 25 (61.0%) | 53 (58.2%) | 36 (54.5%) | 20 (62.5%) | 35 (56.4%) | 39 (53.4%) | 34 (49.3%) |
| • Video consultations | 21 (32.3%) | 11 (26.8%) | 28 (30.8%) | 25 (37.9%) | 11 (34.4%) | 22 (35.5%) | 25 (34.2%) | 23 (33.3%) |
| • Apps/digital platforms | 10 (15.4%) | 8 (19.5%) | 12 (13.2%) | 11 (16.7%) | 8 (25.0%) | 9 (14.5%) | 12 (16.4%) | 11 (15.9%) |
| • Face to face education wearing appropriate personal protective equipment | 47 (72.3%) | 33 (80.5%) | 68 (74.2%) | 54 (81.8%) | 26 (81.2%) | 50 (80.7%) | 59 (80.8%) | 56 (81.2%) |
| Supplies | ||||||||
| • Shortage of supplies | ||||||||
| ○ Yes | 4 (6.1%) | 9 (21.9%) | 9 (9.9%) | 16 (24.2%) | 7 (21.9%) | 11 (17.7%) | 12 (16.4%) | 12 (17.4%) |
| ○ No, everything was secured | 48 (73.9%) | 26 (63.4%) | 72 (79.1%) | 41 (62.1%) | 20 (62.5%) | 40 (64.5%) | 53 (72.6%) | 48 (69.6%) |
| ○ I am not aware of situation | 13 (20.0%) | 6 (14.6%) | 10 (11.0%) | 9 (13.6%) | 5 (15.7%) | 11 (17.7%) | 8 (11.0%) | 9 (13.0%) |
| • Item under shortage | ||||||||
| ○ Oral/nasal medications (e.g., metformin, diazoxide, levothyroxine, methimazole, hydrocortisone, fludrocortisone, calcitriol, desmopressin, estrogen) | 2 (3.1%) | 8 (19.5%) | 9 (9.9%) | 17 (25.7%) | 3 (9.4%) | 8 (12.9%) | 0 | 1 (1.4%) |
| ○ Injectable medications (e.g., insulin, octreotide, glucagon, bisphosphonate, GnRHa, rhGHa) | 1 (1.5%) | 2 (4.9%) | 0 | 0 | 2 (6.2%) | 2 (3.2%) | 10 (13.7%) | 11 (15.9%) |
| ○ Topic medications (e.g., estrogen) | 0 | 0 | 0 | 0 | 0 | 1 (1.6%) | 0 | 0 |
| ○ Test strips | 1 (1.5%) | 1 (2.4%) | N/A | N/A | N/A | N/A | N/A | N/A |
| ○ Syringe | 0 | 1 (2.4%) | 0 | 0 | 0 | 0 | 1 (1.4%) | 0 |
| ○ Genetic testing /imaging | 0 | 1 (2.4%) | 1 (1.1%) | 1 (1.5%) | 3 (9.4%) | 2 (3.2%) | 3 (4.1%) | 2 (2.9%) |
| • Presence of comorbidities | ||||||||
| ○ Asthma | 27 (41.5%) | 2 (4.9%) | 15 (16.5%) | 6 (9.1%) | 1 (3.1%) | 4 (6.4%) | 16 (21.9%) | 10 (14.5%) |
| ○ Cancer | 2 (3.1%) | 0 | 5 (5.5%) | 2 (3.0%) | 1 (3.1%) | 11 (17.7%) | 4 (5.5%) | 5 (7.2%) |
| ○ Obesity | N/A | 6 (14.6%) | 27 (29.7%) | 11 (16.7%) | 9 (28.1%) | 29 (46.8%) | 21 (28.8%) | 25 (36.2%) |
| ○ Hypertension | 42 (64.6%) | 0 | 4 (4.4%) | 5 (7.5%) | 0 | 2 (3.2%) | 3 (4.1%) | 1 (1.4%) |
| ○ Heart disease | 4 (6.1%) | 0 | 5 (5.5%) | 2 (3.0%) | 0 | 3 (4.8%) | 9 (12.3%) | 3 (4.3%) |
| ○ Kidney disease | 5 (7.7%) | 3 (7.3%) | 2 (2.2%) | 1 (1.5%) | 10 (31.2%) | 3 (4.8%) | 10 (13.7%) | 6 (8.7%) |
| ○ Neurological disease | 0 | 2 (4.9%) | 0 | 0 | 1 (3.1%) | 0 | 0 | 2 (2.9%) |
| ○ Celiac disease | 0 | 0 | 6 (6.5%) | 0 | 0 | 0 | 0 | 1 (1.4%) |
| ○ Hypothyroidism | 2 (3.1%) | 0 | N/A | 1 (1.5%) | 0 | 1 (1.6%) | 0 | 1 (1.4%) |
| ○ Cystic fibrosis or bronchial dysplasia | 0 | 0 | 0 | 0 | 1 (3.1%) | 1 (1.6%) | 7 (9.6%) | 0 |
| ○ Other: allergy, dyslipidemia, polycystic ovary syndrome, lupus, anemia, obstructive sleep apnea, hepatic steatosis, bowel complication, other autoimmune or genetic disease | 7 (10.8) | 1 (2.4%) | 7 (7.7%) | 10 (15.1%) | 1 (3.1%) | 2 (3.2%) | 5 (6.8%) | 3 (4.3%) |
| ○ No | 10 (15.4%) | 28 (68.3%) | 44 (48.3) | 36 (54.5%) | 11 (34.4%) | 26 (41.9%) | 31 (42.5%) | 30 (43.5%) |
| Use of medications | ||||||||
| • Anti-hypertensive | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| ○ ACE inhibitors I/II | 32 (49.2%) | |||||||
| ○ Beta-blocker | 2 (3.1%) | |||||||
| ○ Ca channel blocker | 5 (7.7%) | |||||||
| ○ Salt-free diet | 2 (3.1%) | |||||||
| ○ No treatment | 25 (38.5%) | |||||||
| ○ Continuation of anti-hypertensive | 40 (61.5%) | |||||||
| ○ No complication with anti-hypertensive use | 40 (61.5%) | |||||||
| ○ Maintenance of treatment during COVID19 | 40 (61.5%) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| • Management of adrenal crisis | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| ○ Fluid and electrolyte resuscitation | 1 (1.5%) | |||||||
| ○ Ample doses of glucocorticoids | 48 (72.7%) | |||||||
| ○ Chronic glucocorticoid and mineralocorticoid replacement | 9 (13.6%) | |||||||
| ○ Treatment of the precipitating illness | 1 (1.5%) | |||||||
| Psychological concerns | ||||||||
| • Anxiety | 41 (63.1%) | 18 (43.9%) | 33 (36.3%) | 23 (34.8%) | 12 (37.5%) | 27 (43.5%) | 27 (37.0%) | 30 (43.5%) |
| • Parenting stress | 35 (53.8%) | 15 (36.6%) | 28 (30.8%) | 24 (36.4%) | 12 (37.5%) | 18 (29.0%) | 23 (31.5%) | 25 (36.2%) |
| • Depression | 32 (49.2%) | 6 (14.6%) | 17 (18.7%) | 12 (18.2%) | 7 (21.9%) | 12 (19.3%) | 11 (15.1%) | 8 (11.6%) |
| • Insomnia/hypersomnia or other sleep disruption | 22 (33.8%) | 5 (12.2%) | 14 (15.4%) | 6 (9.1%) | 5 (15.6%) | 9 (14.5%) | 13 (17.8%) | 11 (15.9%) |
| • Eating disorder | 32 (49.2%) | 4 (9.7%) | 17 (18.7%) | 5 (7.5%) | 3 (9.4%) | 10 (16.1%) | 14 (19.2%) | 12 (17.4%) |
| • Panic attacks | 7 (10.8%) | 5 (12.2%) | 8 (8.8%) | 4 (6.1%) | 3 (9.4%) | 7 (11.3%) | 4 (5.5%) | 8 (11.6%) |
| • Suicide attempt | 3 (4.6%) | 1 (2.4%) | 1 (1.1%) | 2 (3.0%) | 1 (3.1%) | 1 (1.6%) | 1 (1.4%) | 0 |
| • Patient or caregivers have improved the mood | 0 | 0 | 1 (1.1%) | 1 (1.5%) | 0 | 0 | 1 (1.4%) | 0 |
| • None have had psychological problems so far | 15 (23.1%) | 22 (53.6%) | 41 (45.5%) | 33 (50.0%) | 14 (43.7%) | 31 (50.0%) | 36 (49.3%) | 32 (46.4%) |
N/A, not applicable.
The percentages of centers that had patients affected by COVID-19 are shown in Table 2 for all forms of diabetes and in Table 3 for other endocrine disorders. Patients with diabetes were most affected by COVID-19, with more severe symptoms, probably due to a higher percentage of comorbidities ( Table 2 ) than patients with other endocrine diseases ( Table 3 ). Of note, patients with bone metabolism disorders also seemed to be more susceptible to COVID-19 infection due to concurrent kidney disease (31.2%).
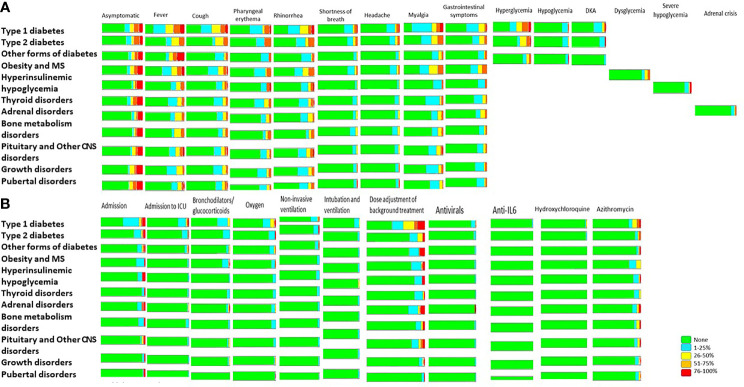
Among patients with COVID-19, most were asymptomatic or had mild to moderate symptoms ( Tables 4 , 5 ). However, in centers where patients with type 1 diabetes had a positive COVID-19 test, symptoms appeared to be more frequent and severe than in centers with patients with other endocrine conditions ( Figure 1A , Tables 4 , 5 ). No deaths were reported for any endocrine condition.
Table 4.
Symptoms, complications, and outcomes of pediatric diabetes cases during the COVID-19 pandemic by clinical center.
| Type 1 diabetes (n=113) | Type 2 diabetes (n=52) | Other forms of diabetes (n=47) | |
|---|---|---|---|
| Symptoms and/or complications among COVID-19 cases | |||
| • Asymptomatic | |||
| ○ None | 63 (55.7%) | 31 (59.6%) | 30 (63.8%) |
| ○ 1-25% | 20 (17.7%) | 6 (11.5%) | 3 (6.4%) |
| ○ 26-50% | 10 (8.8%) | 3 (5.8%) | 4 (8.5%) |
| ○ 51-75% | 11 (9.7%) | 8 (15.4%) | 4 (8.5%) |
| ○ 76-100% | 9 (8.0%) | 4 (7.7%) | 6 (12.8%) |
| • Fever | |||
| ○ None | 44 (38.9%) | 33 (63.5%) | 33 (70.2%) |
| ○ 1-25% | 26 (23%) | 5 (9.6%) | 7 (14.9%) |
| ○ 26-50% | 17 (15.1%) | 8 (15.4%) | 4 (8.5%) |
| ○ 51-75% | 14 (12.4%) | 6 (11.5%) | 3 (6.4%) |
| ○ 76-100% | 12 (10.6%) | 0 | 0 |
| • Cough | |||
| ○ None | 43 (38.05%) | 34 (65.4%) | 33 (70.2%) |
| ○ 1-25% | 28 (24.8%) | 3 (5.8%) | 9 (19.1%) |
| ○ 26-50% | 24 (21.2%) | 10 (19.2%) | 3 (6.4%) |
| ○ 51-75% | 11 (9.7%) | 5 (9.6%) | 2 (4.3%) |
| ○ 76-100% | 7 (6.2%) | 0 | 0 |
| • Pharyngeal erythema | |||
| ○ None | 66 (58.4%) | 37 (71.1%) | 38 (80.8%) |
| ○ 1-25% | 26 (23%) | 8 (15.4%) | 7 (14.9%) |
| ○ 26-50% | 5 (4.5%) | 0 | 0 |
| ○ 51-75% | 12 (10.6%) | 7 (13.5%) | 2 (4.3%) |
| ○ 76-100% | 4 (3.5%) | 0 | 0 |
| • Rhinorrhea | |||
| ○ None | 56 (49.6%) | 34 (65.4%) | 35 (74.5%) |
| ○ 1-25% | 23 (20.3%) | 9 (17.3%) | 9 (19.1%) |
| ○ 26-50% | 21 (18.6%) | 3 (5.8%) | 1 (2.1%) |
| ○ 51-75% | 7 (6.2%) | 6 (11.5%) | 2 (4.3%) |
| ○ 76-100% | 6 (5.3%) | 0 | 0 |
| • Shortness of breath | |||
| ○ None | 75 (66.4%) | 39 (75.0%) | 42 (89.4%) |
| ○ 1-25% | 27 (23.9%) | 7 (13.5%) | 4 (8.5%) |
| ○ 26-50% | 6 (5.3%) | 3 (5.8%) | 1 (2.1%) |
| ○ 51-75% | 3 (2.6%) | 3 (5.8%) | 0 |
| ○ 76-100% | 2 (1.8%) | 0 | 0 |
| • Headache | |||
| ○ None | 50 (44.2%) | 35 (67.3%) | 39 (83.0%) |
| ○ 1-25% | 35 (31.0%) | 8 (15.4%) | 4 (8.5%) |
| ○ 26-50% | 13 (11.5%) | 3 (5.8%) | 2 (4.3%) |
| ○ 51-75% | 9 (8.0%) | 6 (11.6%) | 2 (4.3%) |
| ○ 76-100% | 6 (5.3%) | 0 | 0 |
| • Myalgia | |||
| ○ None | 52 (46.0%) | 33 (63.5%) | 38 (80.8%) |
| ○ 1-25% | 34 (30.1%) | 9 (17.3%) | 5 (10.6%) |
| ○ 26-50% | 13 (11.5%) | 5 (9.6%) | 3 (6.4%) |
| ○ 51-75% | 7 (6.2%) | 5 (9.6%) | 1 (2.1%) |
| ○ 76-100% | 7 (6.2%) | 0 | 0 |
| • Gastrointestinal pain | |||
| ○ None | 56 (49.6%) | 34 (65.4%) | 37 (78.7%) |
| o1-25% | 33 (29.2%) | 9 (17.3%) | 7 (14.9%) |
| ○ 26-50% | 17 (15.0%) | 6 (11.5%) | 1 (2.1%) |
| ○ 51-75% | 5 (4.4%) | 3 (5.8%) | 2 (4.3%) |
| ○ 76-100% | 2 (1.8%) | 0 | 0 |
| • Hyperglycemia | |||
| ○ None | 59 (52.2%) | 36 (69.3%) | 38 (80.8%) |
| ○ 1-25% | 17 (15.0%) | 3 (5.8%) | 4 (8.5%) |
| ○ 26-50% | 15 (13.3%) | 8 (15.4%) | 3 (6.4%) |
| ○ 51-75% | 15 (13.3%) | 4 (7.7%) | 2 (4.3%) |
| ○ 76-100% | 7 (6.2%) | 1 (1.9%) | 0 |
| • Hypoglycemia | |||
| ○ None | 95 (84.1%) | 45 (86.5%) | 43 (91.5%) |
| ○ 1-25% | 15 (13.3%) | 6 (11.5%) | 3 (6.4%) |
| ○ 26-50% | 0 | 1 (1.9%) | 0 |
| ○ 51-75% | 3 (2.6%) | 0 | 0 |
| ○ 76-100% | 0 | 0 | 1 (2,1%) |
| • Diabetic ketoacidosis | |||
| ○ None | 80 (70.8%) | 44 (84.7%) | 45 (95.6%) |
| ○ 1-25% | 22 (19.5%) | 7 (13.5%) | 2 (4.4%) |
| ○ 26-50% | 6 (5.3%) | 0 | 0 |
| ○ 51-75% | 4 (3.5%) | 0 | 0 |
| ○ 76-100% | 1 (0.9%) | 1 (1.9%) | 0 |
| Outcomes for COVID-19 cases | |||
| • Admission | |||
| ○ None | 67 (59.3%) | 41 (78.8%) | 40 (85.1%) |
| ○ 1-25% | 32 (28.3%) | 7 (13.5%) | 4 (8.5%) |
| ○ 26-50% | 5 (4.4%) | 1 (1.9%) | 1 (2.1%) |
| ○ 51-75% | 2 (1.8%) | 1 (1.9%) | 1 (2.1%) |
| ○ 76-100% | 7 (6.2%) | 2 (3.8%) | 1 (2.1%) |
| • Admission to intensive care unit | |||
| ○ None | 89 (78.8%) | 45 (86.5%) | 43 (91.5%) |
| ○ 1-25% | 18 (15.9%) | 6 (11.5%) | 2 (4.3%) |
| ○ 26-50% | 2 (1.8%) | 0 | 1 (2.1%) |
| ○ 51-75% | 2 (1.8%) | 1 (1.9%) | 1 (2.1%) |
| ○ 76-100% | 2 (1.8%) | 0 | 0 |
| • Need for bronchodilators and glucocorticoids | |||
| ○ None | 85 (75.2%) | 45 (86.5%) | 44 (93.6%) |
| ○ 1-25% | 23 (20.4%) | 7 (13.5%) | 2 (4.3%) |
| ○ 26-50% | 5 (4.4%) | 0 | 1 (2.1%) |
| ○ 51-75% | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 |
| • Need for oxygen | |||
| ○ None | 81 (71.7%) | 44 (84.6%) | 40 (85.1%) |
| ○ 1-25% | 19 (16.8%) | 7 (13.5%) | 5 (10.6%) |
| ○ 26-50% | 9 (8.0%) | 0 | 1 (2.1%) |
| ○ 51-75% | 1 (0.9%) | 0 | 1 (2.1%) |
| ○ 76-100% | 3 (2.7%) | 1 (1.9%) | 0 |
| • Need for non-invasive ventilation | |||
| ○ None | 95 (84.1%) | 46 (88.5%) | 43 (91.5%) |
| ○ 1-25% | 14 (12.4%) | 5 (9.6%) | 3 (6.4%) |
| ○ 26-50% | 3 (2.7%) | 0 | 0 |
| ○ 51-75% | 1 (0.9%) | 0 | 1 (2.1%) |
| ○ 76-100% | 0 | 1 (1.9%) | 0 |
| • Need for intubation and ventilation | |||
| ○ None | 102 (90.3%) | 50 (96.1%) | 46 (97.9%) |
| ○ 1-25% | 10 (8.9%) | 2 (3.8%) | 1 (2.1%) |
| ○ 26-50% | 1 (0.9%) | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 |
| • No need for specific treatments | |||
| ○ None | 60 (53.1%) | 33 (63.5%) | 36 (76.6%) |
| ○ 1-25% | 10 (8.9%) | 3 (5.8%) | 3 (6.4%) |
| ○ 26-50% | 8 (7.1%) | 2 (3.8%) | 0 |
| ○ 51-75% | 13 (11.5%) | 5 (9.6%) | 4 (8.5%) |
| ○ 76-100% | 22 (19.5%) | 9 (17.3%) | 4 (8.5%) |
| • Increased insulin dosage/other treatment adjustment | |||
| ○ None | 49 (43.4%) | 38 (73.1%) | 34 (72.4%) |
| ○ 1-25% | 20 (17.7%) | 7 (13.5%) | 8 (17.0%) |
| ○ 26-50% | 22 (19.5%) | 5 (9.6%) | 0 |
| ○ 51-75% | 7 (6.2%) | 0 | 1 (2.1%) |
| ○ 76-100% | 15 (13.3%) | 2 (3.8%) | 4 (8.5%) |
| • Need for antivirals | |||
| ○ None | 103 (91.1%) | 50 (96.1%) | 46 (97.9%) |
| ○ 1-25% | 9 (8.0%) | 2 (3.8%) | 1 (2.1%) |
| ○ 26-50% | 0 | 0 | 0 |
| ○ 51-75% | 1 (0.9%) | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 |
| • Need for anti-IL6 therapy | |||
| ○ None | 111 (98.2%) | 52 (100%) | 47 (100%) |
| ○ 1-25% | 2 (1.8%) | 0 | 0 |
| ○ 26-50% | 0 | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 |
| • Need for hydroxychloroquine | |||
| ○ None | 109 (96.5%) | 51 (98.1%) | 47 (100%) |
| ○ 1-25% | 3 (2.6%) | 1 (1.9%) | 0 |
| ○ 26-50% | 1 (0.9%) | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 |
| • Need for azithromycin | |||
| ○ None | 86 (76.1%) | 46 (88.5%) | 43 (91.5%) |
| ○ 1-25% | 8 (7.1%) | 3 (5.8%) | 2 (4.3%) |
| ○ 26-50% | 10 (8.9%) | 1 (1.9%) | 1 (2.1%) |
| ○ 51-75% | 5 (4.4%) | 2 (3.8%) | 0 |
| ○ 76-100% | 4 (3.5%) | 0 | 1 (2.1%) |
| • Average glycemic control during the pandemic | |||
| ○ Mostly improved | 21 (18.6%) | N/A | N/A |
| ○ Mostly maintained same level | 57 (50.4%) | ||
| ○ Mostly worsened | 35 (31.0%) |
N/A, not applicable.
Table 5.
Symptoms, complications, and outcomes of pediatric endocrine cases other than diabetes during the COVID-19 pandemic by clinical center.
| Obesity and Metabolic Syndrome (n=65) | Hyperinsulinemic hypoglycemia (n=41) | Thyroid disorders (n=91) | Adrenal disorders (n=66) | Bone metabolism disorders (n=32) | Pituitary and other CNS disorders (n=62) | Growth disorders (n=73) | Puberty disorders (n=69) | |
|---|---|---|---|---|---|---|---|---|
| Symptoms and/or complications among COVID-19 cases | ||||||||
| • Asymptomatic | ||||||||
| ○ None | 40 (61.5%) | 32 (78.0%) | 57 (62.6%) | 48 (72.7%) | 24 (75.0%) | 43 (69.3%) | 46 (63.0%) | 45 (65.2%) |
| ○ 1-25% | 5 (7.7%) | 1 (2.5%) | 8 (8.8%) | 5 (7.6%) | 1 (3.1%) | 2 (3.2%) | 3 (4.1%) | 1 (1.4%) |
| ○ 26-50% | 7 (10.8%) | 1 (2.5%) | 5 (5.5%) | 3 (4.5%) | 1 (3.1%) | 6 (9.7%) | 8 (11.0%) | 6 (8.7%) |
| ○ 51-75% | 4 (6.1%) | 1 (2.5%) | 8 (8.8%) | 2 (3.0%) | 1 (3.1%) | 3 (4.8%) | 4 (5.5%) | 6 (8.7%) |
| ○ 76-100% | 9 (13.9%) | 6 (14.6%) | 13 (14.1%) | 8 (12.1%) | 5 (15.6%) | 8 (12.9%) | 12 (16.4%) | 11 (15.9%) |
| • Fever | ||||||||
| ○ None | 36 (55.4%) | 30 (73.2%) | 61 (67.0%) | 48 (72.7%) | 25 (78.1%) | 42 (67.7%) | 48 (65.7%) | 51 (73.9%) |
| ○ 1-25% | 12 (18.5%) | 4 (9.8%) | 18 (19.8%) | 6 (9.1%) | 1 (3.1%) | 9 (14.5%) | 15 (20.5%) | 8 (11.6%) |
| ○ 26-50% | 7 (10.8%) | 4 (9.8%) | 7 (7.7%) | 9 (13.6%) | 4 (12.5%) | 5 (8.1%) | 8 (11.0%) | 6 (8.7%) |
| ○ 51-75% | 7 (10.8%) | 3 (7.3%) | 5 (5.5%) | 3 (4.5%) | 2 (6.2%) | 4 (6.4%) | 2 (2.7%) | 3 (4.3%) |
| ○ 76-100% | 3 (4.6%) | 0 | 0 | 0 | 0 | 2 (3.2%) | 0 | 1 (1.4%) |
| • Cough | ||||||||
| ○ None | 35 (53.8%) | 31 (75.6%) | 61 (67.0%) | 50 (75.8%) | 25 (78.1%) | 42 (67.7%) | 49 (67.1%) | 50 (72.5%) |
| ○ 1-25% | 17 (26.1%) | 5 (12.2%) | 19 (20.9%) | 9 (13.6%) | 2 (6.2%) | 10 (16.1%) | 14 (19.2%) | 10 (14.5%) |
| ○ 26-50% | 7 (10.8%) | 2 (4.9%) | 7 (7.7%) | 5 (7.6%) | 3 (9.4%) | 6 (9.7%) | 7 (9.6%) | 5 (7.2%) |
| ○ 51-75% | 4 (6.1%) | 2 (4.9%) | 4 (4.4%) | 2 (3.0%) | 2 (6.2%) | 2 (3.2%) | 3 (4.1%) | 3 (4.3%) |
| ○ 76-100% | 2 (3.1%) | 1 (2.4%) | 0 | 0 | 0 | 2 (3.2%) | 0 | 1 (1.4%) |
| • Pharyngeal erythema | ||||||||
| ○ None | 44 (67.7%) | 33 (80.5%) | 70 (76.9%) | 53 (80.3%) | 27 (84.4%) | 49 (79.0%) | 53 (72.6%) | 53 (76.8%) |
| ○ 1-25% | 13 (20.0%) | 5 (12.2%) | 14 (15.4%) | 7 (10.6%) | 2 (6.2%) | 7 (11.3%) | 13 (17.8%) | 10 (14.5%) |
| ○ 26-50% | 2 (3.1%) | 0 | 4 (4.4%) | 4 (6.1%) | 1 (3.1%) | 4 (6.4%) | 3 (4.1%) | 4 (5.8%) |
| ○ 51-75% | 6 (9.2%) | 2 (4.9%) | 3 (3.3%) | 2 (3.0%) | 2 (6.2%) | 2 (3.2%) | 4 (5.5%) | 2 (2.9%) |
| ○ 76-100% | 0 | 1 (2.4%) | 0 | 0 | 0 | 0 | 0 | 0 |
| • Rhinorrhea | ||||||||
| ○ None | 41 (63.1%) | 30 (73.2%) | 65 (71.4%) | 49 (74.2%) | 27 (84.4%) | 43 (69.3%) | 50 (68.5%) | 52 (75.3%) |
| ○ 1-25% | 10 (15.4%) | 6 (14.6%) | 16 (17.6%) | 9 (13.6) | 2 (6.2%) | 10 (16.1%) | 14 (19.2%) | 9 (13.0%) |
| ○ 26-50% | 7 (10.8%) | 2 (4.9%) | 5 (5.5%) | 6 (9.1) | 1 (3.1%) | 5 (8.1%) | 5 (6.8%) | 4 (5.8%) |
| ○ 51-75% | 7 (10.8%) | 2 (4.9%) | 4 (4.4%) | 2 (3.0%) | 2 (6.2%) | 3 (4.8%) | 4 (5.5%) | 4 (5.8%) |
| ○ 76-100% | 0 | 1 (2.4%) | 1 (1.1%) | 0 | 0 | 1 (1.6%) | 0 | 0 |
| • Shortness of breath | ||||||||
| ○ None | 48 (73.8%) | 38 (92.7%) | 79 (86.8%) | 58 (87.9%) | 26 (81.2%) | 54 (87.1%) | 65 (89.0%) | 62 (89.9%) |
| ○ 1-25% | 12 (18.5%) | 2 (4.9%) | 11 (12.1%) | 5 (7.6%) | 4 (12.5%) | 6 (9.7%) | 6 (8.2%) | 5 (7.2%) |
| ○ 26-50% | 3 (4.6%) | 1 (2.4%) | 1 (1.1%) | 3 (4.5%) | 2 (6.2%) | 2 (3.2%) | 1 (1.4%) | 1 (1.4%) |
| ○ 51-75% | 2 (3.1%) | 0 | 0 | 0 | 0 | 0 | 1 (1.4%) | 0 |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.4%) |
| • Headache | ||||||||
| ○ None | 40 (61.5%) | 34 (82.9%) | 66 (72.5%) | 53 (80.3%) | 26 (81.2%) | 47 (75.8%) | 53 (72.6%) | 51 (73.9%) |
| ○ 1-25% | 8 (12.3%) | 4 (9.8%) | 16 (17.6%) | 6 (9.1%) | 3 (9.4%) | 7 (11.3%) | 12 (16.4%) | 9 (13.0%) |
| ○ 26-50% | 12 (18.5%) | 0 | 6 (6.6%) | 3 (4.5%) | 2 (6.2%) | 3 (4.8%) | 4 (5.5%) | 5 (7.2%) |
| ○ 51-75% | 5 (7.7%) | 2 (4.9%) | 3 (3.3%) | 4 (6.1%) | 1 (3.1%) | 5 (8.1%) | 4 (5.5%) | 4 (5.8%) |
| ○ 76-100% | 0 | 1 (2.4%) | 0 | 0 | 0 | 0 | 0 | 0 |
| • Myalgia | ||||||||
| ○ None | 39 (60.0%) | 34 (82.9%) | 64 (70.3%) | 53 (80.3%) | 26 (81.2%) | 46 (74.2%) | 52 (71.2%) | 51 (73.9%) |
| ○ 1-25% | 10 (15.4%) | 6 (14.6%) | 21 (23.1%) | 7 (10.6%) | 3 (9.4%) | 8 (12.9%) | 15 (20.5%) | 13 (18.8%) |
| ○ 26-50% | 10 (15.4%) | 0 | 3 (3.3%) | 4 (6.1%) | 2 (6.2%) | 5 (8.1%) | 4 (5.5%) | 4 (5.8%) |
| ○ 51-75% | 6 (9.2%) | 1 (2.4%) | 3 (3.3%) | 2 (3.0%) | 1 (3.1%) | 2 (3.2%) | 2 (2.7%) | 1 (1.4%) |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 1 (1.6%) | 0 | 0 |
| • Gastrointestinal pain | ||||||||
| ○ None | 39 (60.0%) | 32 (78.0%) | 63 (69.2%) | 48 (72.7%) | 26 (81.2%) | 47 (75.8%) | 52 (71.2%) | 52 (75.4%) |
| ○ 1-25% | 12 (18.5%) | 7 (17.1%) | 19 (20.9%) | 7 (10.6%) | 4 (12.5%) | 9 (14.5%) | 16 (21.9%) | 13 (18.8%) |
| ○ 26-50% | 7 (10.8%) | 0 | 7 (7.7%) | 8 (12.1%) | 1 (3.1%) | 4 (6.4%) | 2 (2.7%) | 2 (2.9%) |
| ○ 51-75% | 7 (10.8%) | 2 (4.9%) | 2 (2.2%) | 3 (4.5%) | 1 (3.1%) | 2 (3.2%) | 3 (4.1%) | 2 (2.9%) |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| • Other specific | [Dysglycemia] | [Severe hypoglycemia] | [Adrenal crisis] | |||||
| ○ None | 53 (81.5%) | 34 (82.9%) | N/A | 58 (87.9%) | N/A | N/A | N/A | N/A |
| ○ 1-25% | 4 (6.1%) | 4 (9.8%) | 5 (7.6%) | |||||
| ○ 26-50% | 5 (7.7%) | 1 (2.4%) | 2 (3.0%) | |||||
| ○ 51-75% | 3 (4.6%) | 0 | 1 (1.5%) | |||||
| ○ 76-100% | 0 | 2 (4.9%) | 0 | |||||
| Outcomes among COVID-19 cases | ||||||||
| • Admission | ||||||||
| ○ None | 54 (83.1%) | 35 (85.4%) | 86 (94.5%) | 56 (84.8%) | 28 (87.5%) | 56 (90.3%) | 69 (94.5%) | 66 (95.6%) |
| ○ 1-25% | 10 (15.4%) | 3 (7.3%) | 4 (4.4%) | 6 (9.1%) | 3 (9.4%) | 2 (3.2%) | 3 (4.1%) | 1 (1.4%) |
| ○ 26-50% | 0 | 0 | 0 | 0 | 0 | 2 (3.2%) | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 | 2 (3.0%) | 0 | 0 | 0 | 0 |
| ○ 76-100% | 1 (1.5%) | 3 (7.3%) | 1 (1.1%) | 2 (3.0%) | 1 (3.1%) | 2 (3.2%) | 1 (1.4%) | 2 (2.9%) |
| • Admission to intensive care unit | ||||||||
| ○ None | 63 (96.9%) | 40 (97.7%) | 88 (96.7%) | 64 (97.0%) | 30 (93.8%) | 60 (96.8%) | 72 (98.6%) | 68 (98.5%) |
| ○ 1-25% | 2 (3.1%) | 1 (2.4%) | 3 (3.3%) | 1 (1.5) | 2 (6.2%) | 2 (3.2%) | 1 (1.4%) | 1 (1.5%) |
| ○ 26-50% | 0 | 0 | 0 | 1 (1.5%) | 0 | 0 | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| • Need for bronchodilators and glucocorticoids | ||||||||
| ○ None | 55 (84.6%) | 40 (97.6%) | 85 (93.4%) | 62 (93.9%) | 31 (96.9%) | 59 (95.2%) | 70 (95.9%) | 69 (100%) |
| ○ 1-25% | 8 (12.3%) | 1 (2.4%) | 6 (6.6%) | 2 (3.0%) | 1 (3.1%) | 2 (3.2%) | 2 (2.7%) | 0 |
| ○ 26-50% | 0 | 0 | 0 | 1 (1.5%) | 0 | 1 (1.6%) | 1 (1.4%) | 0 |
| ○ 51-75% | 1 (1.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 76-100% | 1 (1.5%) | 0 | 0 | 1 (1.5%) | 0 | 0 | 0 | 0 |
| • Need for oxygen | ||||||||
| ○ None | 58 (89.2%) | 38 (92.7%) | 86 (94.5%) | 60 (90.9%) | 29 (90.6%) | 58 (93.6%) | 70 (95.9%) | 67 (97.1%) |
| ○ 1-25% | 6 (9.2%) | 3 (7.3%) | 5 (5.6%) | 5 (7.6%) | 3 (9.4%) | 4 (6.4%) | 2 (2.7%) | 1 (1.4%) |
| ○ 26-50% | 0 | 0 | 0 | 1 (1.5%) | 0 | 0 | 1 (1.4%) | 0 |
| ○ 51-75% | 1 (1.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.4%) |
| • Need for non-invasive ventilation | ||||||||
| ○ None | 62 (95.4%) | 38 (92.7%) | 87 (95.6%) | 64 (97.0%) | 31 (96.9%) | 60 (96.8%) | 71 (97.3%) | 68 (98.5%) |
| ○ 1-25% | 3 (4.6%) | 3 (7.3%) | 4 (4.4%) | 2 (3.0%) | 1 (3.1%) | 2 (3.2%) | 2 (2.7%) | 1 (1.5%) |
| ○ 26-50% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| • Need for intubation and ventilation | ||||||||
| ○ None | 63 (96.9%) | 40 (97.6%) | 88 (96.7%) | 65 (98.5%) | 31 (96.9%) | 61 (98.4%) | 72 (98.6%) | 69 (100%) |
| ○ 1-25% | 2 (3.1%) | 0 | 3 (3.3%) | 1 (1.5%) | 0 | 1 (1.6%) | 1 (1.4%) | 0 |
| ○ 26-50% | 0 | 1 (2.4%) | 0 | 0 | 1 (3.1%) | 0 | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| • No need for specific treatments | ||||||||
| ○ None | 44 (67.7%) | 33 (80.5%) | 75 (82.4%) | 56 (84.8%) | 24 (75.0%) | 50 (80.7%) | 57 (78.1%) | 55 (79.7%) |
| ○ 1-25% | 4 (6.1%) | 1 (2.4%) | 3 (3.3%) | 2 (3.0%) | 2 (6.2%) | 2 (3.2%) | 2 (2.7%) | 1 (1.4%) |
| ○ 26-50% | 4 (6.1%) | 0 | 4 (4.4%) | 0 | 2 (6.2%) | 3 (4.8%) | 3 (4.1%) | 3 (4.3%) |
| ○ 51-75% | 1 (1.5%) | 1 (2.4%) | 1 (1.1%) | 2 (3.0%) | 0 | 2 (3.2%) | 2 (2.7%) | 0 |
| ○ 76-100% | 12 (18.5%) | 6 (14.6%) | 8 (8.8%) | 6 (9.1%) | 4 (12.5%) | 5 (8.1%) | 9 (12.3%) | 10 (14.5%) |
| • Dose adjustment of background treatment | ||||||||
| ○ None | 50 (76.9%) | 34 (82.9%) | 75 (82.4%) | 47 (71.2%) | 27 (84.4%) | 48 (77.4%) | 64 (87.7%) | 65 (94.2%) |
| ○ 1-25% | 11 (16.9%) | 5 (12.2%) | 12 (13.2%) | 11 (16.7%) | 3 (9.4%) | 7 (11.3%) | 6 (8.2%) | 3 (4.3%) |
| ○ 26-50% | 1 (1.5%) | 0 | 3 (3.3%) | 2 (3.0%) | 1 (3.1%) | 3 (4.8%) | 1 (1.4%) | 0 |
| ○ 51-75% | 1 (1.5%) | 0 | 0 | 1 (1.5%) | 0 | 2 (3.2%) | 1 (1.4%) | 0 |
| ○ 76-100% | 2 (3.1%) | 2 (4.9%) | 1 (1.1%) | 5 (7.6%) | 1 (3.1%) | 2 (3.2%) | 1 (1.4%) | 1 (1.4%) |
| • Need for antivirals | ||||||||
| ○ None | 63 (96.9%) | 40 (97.6%) | 90 (98.9%) | 65 (98.5%) | 31 (96.9%) | 60 (96.8%) | 71 (97.3%) | 68 (98.5%) |
| ○ 1-25% | 2 (3.1%) | 1 (2.4%) | 1 (1.1%) | 0 | 1 (3.1%) | 2 (3.2%) | 2 (2.7%) | 1 (1.5%) |
| ○ 26-50% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 | 1 (1.5%) | 0 | 0 | 0 | 0 |
| • Need for anti-IL6 therapy | ||||||||
| ○ None | 65 (100%) | 41 (100%) | 91 (100%) | 66 (100%) | 32 (100%) | 62 (100%) | 73 (100%) | 69 (100%) |
| ○ 1-25% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 26-50% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| • Need for hydroxychloroquine | ||||||||
| ○ None | 65 (100%) | 41 (100%) | 91 (100%) | 66 (100%) | 32 (100%) | 62 (100%) | 73 (100%) | 69 (100%) |
| ○ 1-25% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 26-50% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 51-75% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ○ 76-100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 01 |
| • Need for azithromycin | ||||||||
| ○ None | 50 (76.9%) | 35 (85.4%) | 79 (86.8%) | 55 (83.3%) | 28 (87.5%) | 54 (87.1%) | 65 (89.0%) | 62 (89.9%) |
| ○ 1-25% | 8 (12.3%) | 4 (9.8%) | 9 (9.9%) | 6 (9.1%) | 1 (3.1%) | 4 (6.5%) | 5 (6.9%) | 4 (5.8%) |
| ○ 26-50% | 7 (10.8%) | 1 (2.4%) | 3 (3.3%) | 2 (3.0%) | 2 (6.2%) | 3 (4.8%) | 1 (1.4%) | 2 (2.9%) |
| ○ 51-75% | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.4%) | 1 (1.4%) |
| ○ 76-100% | 0 | 1 (2.4%) | 0 | 3 (4.5%) | 1 (3.1%) | 1 (1.6%) | 1 (1.4%) | 0 |
N/A, not applicable.
Figure 1.
Proportion of general and specific symptomatology (A) and requirement for specific therapeutic management (B) in COVID-19 patients attending pediatric endocrine centers. MS, metabolic syndrome; CNS, central nervous system; DKA, Diabetic ketoacidosis; ICU, Intensive Care Unit; IL-6, Interleukin-6.
General therapeutic measures for COVID-19 were usually unnecessary. In most centers, patients did not need to be admitted in hospital, and most of did not need intensive care unit (ICU) beds, except for patients with diabetes (21.2%) ( Figure 1B , Tables 4 , 5 ). The proportion of patients who needed bronchodilators and glucocorticoids more frequently was higher in type 1 diabetes (24.8%), type 2 diabetes (13.5%), and obesity (15.4%), than patients with other endocrine conditions tested positive for COVID-19. They also needed oxygen (28.3%), non-invasive ventilation (15.9%), intubation and ventilation (9.7%), and other therapies (e.g., antibiotics in 23.9%, antiviral agents in 8.9%) more often than in other endocrine conditions ( Tables 4 , 5 ).
Although specific therapeutic measures for endocrine management were only infrequently needed in patients with COVID-19, background treatment dose adjustment was common: in 56.6% of centers, patients with type 1 diabetes needed insulin adjustments and in 26.9% of centers, patients with type 2 diabetes added insulin or adjusted oral medications. Similar adjustments to specific therapies were reported in 27.6% of centers for other forms of diabetes, 23.1% for obesity, 28.8% for adrenal disorders, and 26.6% for pituitary and other CNS disorders ( Tables 4 , 5 ).
3.3 Disease-Related Outcomes From Pediatric Endocrine Disorders
3.3.1 Diabetes (All Forms)
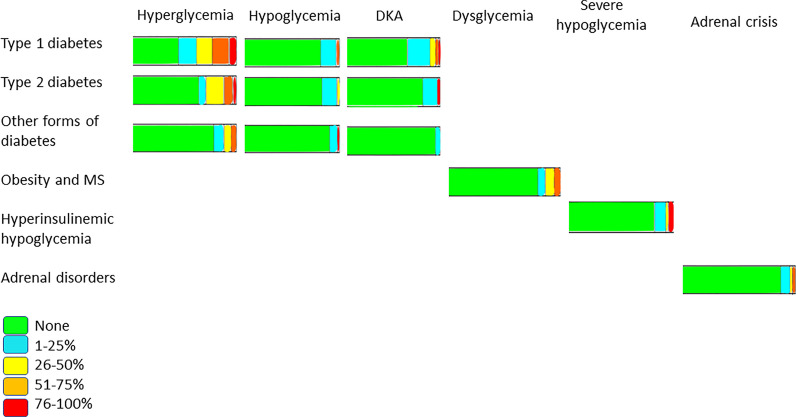
Newly diagnosed mild to severe diabetic ketoacidosis (DKA) and new episodes in established cases were increased in 44% and 30% of centers, respectively ( Table 2 ). In contrast, only 6% of centers reported severe hypoglycemia ( Figure 2 ) .
Figure 2.
Proportion of specific symptoms in COVID-19 patients among pediatric endocrine centers.
Most HCPs performed COVID-19 testing in newly diagnosed patients (62.8%) and for DKA episodes in established patients (69.9%), which were negative in 49.6% of centers ( Table 2 ).
3.3.2 Other Endocrine Disorders
For most investigated endocrine disorders, there was some delay in new diagnoses as well as a worsening of the management. For patients who were diagnosed with COVID-19, most had asymptomatic or mild to moderate symptoms ( Tables 3 , 4 , 5 , Figure 1 ).
Remarkably, in patients with obesity or metabolic syndrome, ACE inhibitors, the most used antihypertensive drug (49.2%), were continued without interruption and with no complications reported to date ( Figure 1B ). Of note, in patients with HH, fewer than 10% reported at least one episode of severe hypoglycemia ( Figure 2 ).
Two pediatric endocrine centers specialized in pediatric patients with gender dysphoria. Most patients were followed-up and educated by telephone consultation or F2F wearing appropriate PPE. In both centers, contact with the endocrine team was limited due the fear of the pandemic, and sometimes the diagnosis was possibly delayed but without perceived worsening of the management. Patients with gender dysphoria were not more likely to suffer from COVID-19, as seen for most other endocrine disorders.
3.4 Biopsychosocial and Behavior Outcomes From Pediatric Endocrine Disorders
In patients with type 1 diabetes, a lack of physical activity (41.6%) and poorer dietary choices (32.7%) with resulting increase in body weight (31.7%) were observed. Eighty percent of respondents reported high parental concerns about returning to school activities ( Table 2 ). However, reassuringly, the majority (80%) had received specific school guidelines to keep children and teachers safe at school in the context of the COVID-19 pandemic. Moreover, general psychosocial and behavioral changes were commonly reported in patients with pediatric endocrine disorders including all forms of diabetes. The most commonly reported problems were anxiety, depression, parenting stress, sleep disruption, and eating disorders. Indeed, suicide attempts were seen in every condition except for puberty disorders ( Tables 2 , 3 ).
4 Discussion
This survey shows that the proportion of patients with endocrine disorders testing positive for COVID-19 is low enough to not consider pediatric endocrine disorders as a poor prognostic factor for COVID-19. As previously reported, children and adolescents with any endocrine condition were not at increased risk of COVID-19 than children without endocrine conditions (15). However, comparing the proportion of patients with diabetes and COVID-19 in our previous survey with those reported here [only diabetes was assesses previously (9)], there was a significant increase in the number of children and adolescents with type 1 diabetes or other forms of diabetes who tested positive for COVID-19 (9). This is likely to be due to far more COVID-19 testing performed as the pandemic progressed compared to during the early phase of the pandemic (January-September 2020) and the reopening of schools in many countries.
While most pediatric patients with endocrine disorders affected by COVID-19 have asymptomatic or mild symptoms (16) it is important to note that patients with type 1 or type 2 diabetes were not only more likely to suffer from COVID-19 but also experience moderate to severe symptoms, especially when other comorbidities were present (17, 18). As a result, the number of patients with diabetes admitted to the ICU also increased compared to our previous survey (9), reaching a higher proportion of centers which reported admission for non-invasive ventilation (15.9%) and intubation and ventilation (9.7%) in comparison to other endocrine conditions in our survey (6% and 3%, respectively). Luckily, no deaths have been reported so far; diabetes and obesity are both risk factors for increased morbidity and mortality in adult patients with COVID-19 (19–21), but it is reassuring that in patients below 25 years the mortality rate approaches zero even when diabetic or obese.
While there are some data in adult patients (10–13), little is known about the impact of COVID-19 on other endocrine diseases in the pediatric population, and to our knowledge this is the first real-world and global study of this topic. The current experience of pediatric endocrinology HCPs suggests that the management of diabetes is much more demanding and complex than other endocrine disorders. There is a two-way association between COVID-19 and diabetes mellitus. In adults, uncontrolled diabetes is related to the severity of COVID-19. Moreover, severe metabolic complications, including DKA, have been observed in patients with COVID-19, either at onset (22, 23) or in pre-existing diabetes (24). While pediatric patients with diabetes do not appear to be at higher risk of infection by SARS-CoV-2, it is still prudent to avoid being infected and to adopt all preventive measures possible (25). It has also been hypothesized that SARS-CoV-2 might itself be diabetogenic, like in patients with SARS coronavirus 1 pneumonia (26). However, this association has yet to be confirmed and requires further study in both adults and children.
The present survey confirms that the COVID-19 pandemic delayed hospital admissions for diabetes and other endocrine diseases, resulting, for example, in a higher proportion of severe DKA cases, as observed elsewhere (22, 23, 27). A secure non-COVID-19 path through pediatric emergency departments is essential to help and reassure parents who wish to bring their children to hospital in a timely manner and avoid unnecessary complications in diabetes and other endocrine disorders (22).
Once endocrine disease is established, it is important to maintain an uninterrupted link with HCPs, as recommended by many endocrine societies (9, 28, 29), especially with telemedicine to avoid crowded waiting rooms. However, in the present survey, we observed that despite the need to minimize unnecessary hospital visits during the pandemic using dedicated platforms or video calls, text messaging, and emails, routine F2F visits remained the most common method of consultation. Improved knowledge of telemedicine might help families and patients gain trust in this way of delivering care. Prioritization of guidance on care management and accelerated innovation in telehealth is necessary to avoid complications in these patients, especially in limited resource settings. Video platforms have been implemented in many institutions, especially for education, although not all allow telehealth for inpatient care and the efficacy of telemedicine for education remains uncertain [30-32].
Many parents were concerned about the safety of sending their children with endocrine disorders, especially diabetes, back to school during the COVID-19 pandemic, believing that they had greater likelihood of contracting coronavirus. Reassuringly, however, the majority were familiar with school guidelines and ensured that a disease care plan was in place (25, 30, 31).
Ready access to endocrine and diabetes care medications and supplies, already a problem in large parts of the world before the pandemic, is of vital importance. With important infrastructure such as outpatient clinics and public transport severely limited by the pandemic, access issues have been exacerbated, even though daily self-management, sick day management, and survival depends on the availability of medical supplies. Fortunately, in the present survey, relatively few (6-22% according to different endocrine disorders) centers experienced a shortage of supplies.
We observed that COVID-19 was related to endocrine disease and comorbidities, with obesity and hypertension the most commonly reported in all endocrine diseases. Children and adolescents who needed intensive care often had comorbidities. Therefore, it is essential to understand which modifiable risk factors might play a role in increasing the severity of COVID-19 (32–36). Indeed, some children suffered from more serious COVID-19, but the reasons remain unclear; comorbidities are less frequent in young patients that in adults, which might explain why children are less vulnerable to COVID-19 but why some still fall critically ill. Given the recent rise in type 2 diabetes and obesity in youth, there could be a significant number of children at higher risk.
The COVID-19 pandemic has exacerbated mental health comorbidities throughout society, not least in patients with diabetes and other endocrine disorders (37, 38). Children are a particularly vulnerable group, as their nervous systems, endocrine systems, and hypothalamic-pituitary-adrenal axes are not well developed. Psychological crises often result in feelings of abandonment, despair, incapacity, and exhaustion in children, and even raise the risk of suicide. Of note, our survey revealed reports of suicide attempts during the pandemic in children with a wide range of different endocrine conditions. Psychosocial support for children and their families, especially those with chronic health conditions, must be a part of the health response to disaster and disaster recovery. Timely and appropriate protections are needed to prevent psychological and behavioral problems. Emerging digital applications and health services such as telehealth, social media, mobile health, and remote interactive online education may help bridge the social distance and support mental and behavioral health for children (39).
The SARS-CoV-2 virus has multiple pathophysiologic interconnections with endocrine systems with the potential to cause disturbances in pituitary, adrenal and thyroid function, and mineral metabolism. Data regarding the risks of SARS-CoV-2 infection in individuals with underlying endocrine disorders have primarily been described in adults (40). However, the limited existing data are generally favorable in terms of endocrine complications of COVID-19 in the pediatric population (41), as confirmed in our survey, where children with well-managed endocrine conditions did not seem to be at increased risk of getting infected or becoming severely ill with COVID-19.
Prior studies showed successful use of telemedicine for pediatric obesity, but it can be challenging to translate this process into telemedicine for other endocrine conditions (42).
Currently, there are no data indicating increased risk of acquiring SARS-CoV-2 infection or altered disease course in children and adolescents with underlying thyroid disorders. However, it is important to keep in mind that patients with Graves’ disease treated with anti-thyroid drug therapy are at higher risk of agranulocytosis and secondary infections (43). This is particularly important as data from one study showed that half of COVID-19 non-survivors experienced a secondary infection (44). Additionally, like other infections, COVID-19 may precipitate thyroid storm in patients with poorly controlled hyperthyroidism (41). Underlying thyroid disease, including hypothyroidism, does appear to be a risk factor for a more severe disease course in adults with COVID-19 (45–47). Children with metabolic bone disease or a skeletal dysplasia resulting in respiratory insufficiency due to altered chest wall structure may be at increased risk of COVID-19 complications (48).
Patients with primary adrenal insufficiency (e. g., congenital adrenal hyperplasia) are slightly more susceptible to infections in general, due to the impaired natural immunity function characterized by a defective action of neutrophils and natural killer cells, which is known to be associated with primary adrenal insufficiency (49). Furthermore, susceptibility to infections may also be explained by an insufficient increase of the hydrocortisone dosage at the beginning of an infection. Therefore, recommendations suggest that, if asymptomatic, children should remain on regular replacement doses of hydrocortisone and not increased doses. If symptoms suggestive of COVID-19, it is recommended to immediately increasing the hydrocortisone doses until the fever has subsided and adding an extra doubled dose.
Children diagnosed with hypopituitarism are also not at increased risk for COVID-19. As a significant percentage of these patients have secondary adrenal insufficiency, the same recommendations apply as for children with adrenal insufficiency (50). Hyperinsulinemic hypoglycemia side effects of the medications used to treat hyperinsulinemic hypoglycemia (e. g., diazoxide: water retention and pulmonary hypertension; somatostatin analogues: cardiac arrhythmias and cardiac conduction disorders) should be taken into consideration in the case of COVID-19. During this pandemic, children should follow management for hypoglycemia, which include close monitoring of glucose levels, adequate hydration, ensuring availability of medications and emergency regime. However, it is reassuring to note that survey data show that all these endocrine disorders did not cause any adjunctive distress to patients and the only serious issue to face is the possible shortage of medicines and/or supplies.
Majority of endocrine data are coming from recommendations issued by various health organizations and endocrine associations for the management of pediatric endocrine conditions during the pandemic. Adhering to the specific “sick day management rules” and undelayedly seeking for medical advice are only needed in most of the cases, as most children with endocrine disorders do not represent a high-risk population for contamination or severe presentation of COVID-19 (51).
Although it is difficult to analyze the effects of COVID-19 on endocrine disorders in children due to lack of studies and relatively less severe cases as compared to adults (52–54), looking at the data collected with the present survey, it appears that diabetes is still more challenging to manage than any other pediatric endocrine disorder with an increased risk of morbidity.
This study has some limitations. We received fewer overall responses than in the first survey, which focused on diabetes (51 vs. 75 countries and 131 endocrine centers vs. 215 diabetes centers, respectively). We hypothesize that HCPs perceive diabetes as more influenced by COVID-19 both directly because diabetes is a risk factor for COVID-19-related mortality and morbidity and indirectly because the pandemic can influence its management and supply availability. Other reasons for non-response might include workloads due to COVID-19, survey fatigue during the pandemic, insufficient reach of possible responders through email, perceived stress, and work-related burnout. Nevertheless, this is the first global study of the impact of COVID-19 in all pediatric endocrinology conditions. Most of the pediatric endocrinologists that responded to the survey were based in countries severely impacted by COVID-19 and worked in university/academic centers, strengthening the data and its global reach.
In conclusion, here we show that diabetes has been a particular management challenge during the COVID-19 pandemic and has an increased risk of morbidities including DKA. Specific strategies are essential to educate and reassure parents about maintaining close contact with their HCPs and about timely attendance at the emergency department when children develop symptoms unrelated to COVID-19. The global supply of essential medicines must always be maintained. Telemedicine requires strengthening and should be routine in every center. The mental health needs of children and adolescents with endocrine disorders must be addressed. International recommendations to reduce the severity of the impact of COVID-19 on pediatric patients with diabetes and other endocrine disorders must be devised with a special emphasis on its psychological impact.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
NE drafted, revised, and approved the survey, analyzed data, drafted and discussed the manuscript. TS drafted, revised, and approved the survey, analyzed data, drafted and discussed the manuscript. CB drafted, revised, and approved the survey, and discussed the manuscript. EW, drafted, revised, and approved the survey, and discussed the manuscript. AP, drafted, revised, and approved the survey, and discussed the manuscript. AS drafted, revised, and approved the survey, drafted and discussed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express their gratitude to all survey respondents who shared their expertise and experience by participating in the survey and making this study possible. We also thank the International Consortium for Pediatric Endocrinology (ICPE) and the societies participating in this survey.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.735554/full#supplementary-material
References
- 1. WHO Coronavirus Dashboard. Available at: https://covid19.who.int (Accessed April 20, 2021).
- 2. Saunders-Hastings PR, Krewski D. Reviewing the History of Pandemic Influenza: Understanding Patterns of Emergence and Transmission. Pathogens (2016) 5(4):66. doi: 10.3390/pathogens5040066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albery GF, Eskew EA, Ross N, Olival KJ. Predicting the Global Mammalian Viral Sharing Network Using Phylogeography. Nat Commun (2020) 11:2260. doi: 10.1038/s41467-020-16153-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the Transmission Dynamics of SARS-CoV-2 Through the Postpandemic Period. Science (2020) 368(6493):860–8. doi: 10.1126/science.abb5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 Infection in Children. N Engl J Med (2020) 382(17):1663–5. doi: 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schvartz A, Belot A, Kone-Paut I. Pediatric Inflammatory Multisystem Syndrome and Rheumatic Diseases During SARS-CoV-2 Pandemic. Front Pediatr (2020) 8:605807. doi: 10.3389/fped.2020.605807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory Shock in Children During COVID-19 Pandemic. Lancet (2020) 395(10237):1607–8. doi: 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Girona-Alarcon M, Bobillo-Perez S, Sole-Ribalta A, Hernandez L, Guitart C, Suarez R, et al. The Different Manifestations of COVID-19 in Adults and Children: A Cohort Study in an Intensive Care Unit. BMC Infect Dis (2021) 21(1):87. doi: 10.1186/s12879-021-05786-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elbarbary NS, Dos Santos TJ, de Beaufort C, Agwu JC, Calliari LE, Scaramuzza AE. COVID-19 Outbreak and Pediatric Diabetes: Perceptions of Health Care Professionals Worldwide. Pediatr Diabetes (2020) 21(7):1083–92. doi: 10.1111/pedi.13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lundholm MD, Poku C, Emanuele N, Emanuele MA, Lopez N. SARS-CoV-2 (COVID-19) and the Endocrine System. J Endocr Soc (2020) 4(11):bvaa144. doi: 10.1210/jendso/bvaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marazuela M, Giustina A, Puig-Domingo M. Endocrine and Metabolic Aspects of the COVID-19 Pandemic. Rev Endocr Metab Disord (2020) 21(4):495–507. doi: 10.1007/s11154-020-09569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID-19 on the Thyroid Gland: An Update. Rev Endocr Metab Disord (2020) 1–13. doi: 10.1007/s11154-020-09615-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agarwal S, Agarwal SK. Endocrine Changes in SARS-CoV-2 Patients and Lessons From SARS-CoV. Postgrad Med J (2020) 96(1137):412–6. doi: 10.1136/postgradmedj-2020-137934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lavrakas P. ed. Encyclopedia of Survey Research Methods. Sage Publications, SAGE Publications: Thousand Oaks, California: (2008). [Google Scholar]
- 15. Cianfarani S. Pediatric Endocrinology in the Time of the COVID-19 Pandemic. Horm Res Paediatr (2019) 92(6):345–6. doi: 10.1159/000507703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ludvigsson JF. Systematic Review of COVID-19 in Children Shows Milder Cases and a Better Prognosis Than Adults. Acta Paediatr (2020) 109(6):1088–95. doi: 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho C, Ng NBH, Lee YS. Caring for Pediatric Patients With Diabetes Amidst the Coronavirus Disease 2019 Storm. J Pediatr (2020) 223:186–7. doi: 10.1016/j.jpeds.2020.04.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dayal D. We Urgently Need Guidelines for Managing COVID-19 in Children With Comorbidities. Acta Paediatr (2020) 109(7):1497–8. doi: 10.1111/apa.15304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med (2020) 382(18):1708–20. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. SARS-CoV-2 Surveillance Group . Characteristics of SARS-CoV-2 Patients Dying in Italy: Report Based on Available Data on January 27th, 2021. Available at: https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_27_january_2021.pdf.
- 21. Razzaghi H, Wang Y, Lu H, Marshall KE, Dowling NF, Paz-Bailey G, et al. Estimated County-Level Prevalence of Selected Underlying Medical Conditions Associated With Increased Risk for Severe COVID-19 Illness — United States, 2018. MMWR Morb Mortal Wkly Rep (2020) 69(29):945–50. doi: 10.15585/mmwr.mm6929a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A. Has COVID-19 Delayed the Diagnosis and Worsened the Presentation of Type 1 Diabetes in Children? Diabetes Care (2020) 43(11):2870–2. doi: 10.2337/dc20-1321 [DOI] [PubMed] [Google Scholar]
- 23. Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, et al. Ketoacidosis in Children and Adolescents With Newly Diagnosed Type 1 Diabetes During the COVID-19 Pandemic in Germany. JAMA (2020) 324:801. doi: 10.1001/jama.2020.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic Ketoacidosis Precipitated by COVID-19: A Report of Two Cases and Review of Literature. Diabetes Metab Syndr (2020) 14(5):1459–62. doi: 10.1016/j.dsx.2020.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scaramuzza AE, Rabbone I, Maffeis C, Schiaffini R. Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Seasonal Flu and COVID-19 Recommendations for Children, Adolescents and Young Adults With Diabetes. Diabetes Med (2021) 38(1):e14427. doi: 10.1111/dme.14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-Onset Diabetes in Covid-19. N Engl J Med (2020) 383(8):789–90. doi: 10.1056/NEJMc2018688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawrence C, Seckold R, Smart C, King BR, Howley P, Feltrin R, et al. Increased Paediatric Presentations of Severe Diabetic Ketoacidosis in an Australian Tertiary Centre During the COVID-19 Pandemic. Diabetes Med (2021) 38(1):e14417. doi: 10.1111/dme.14417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wake DJ, Gibb FW, Kar P, Kennon B, Klonoff DC, Rayman G, et al. Endocrinology in the Time of COVID-19: Remodelling Diabetes Services and Emerging Innovation. Eur J Endocrinol (2020) 183(2):G67–77. doi: 10.1530/EJE-20-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and Endocrine Diseases. A Statement From the European Society of Endocrinology. Endocrine (2020) 68(1):2–5. doi: 10.1007/s12020-020-02294-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coronavirus Infection (COVID-19)-II ISPAD Summary, March 25, 2020. Available at: https://www.ispad.org/page/CoronavirusinfectionCOVID-19-IIISPADSummary (Accessed April 12, 2021).
- 31. American Diabetes Association . Diabetes and Coronavirus (2020). Available at: https://www.diabetes.org/coronavirus-covid-19 (Accessed April 12, 2021).
- 32. Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes Care (2020) 43(7):e72–4. doi: 10.2337/dc20-0682 [DOI] [PubMed] [Google Scholar]
- 33. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors Associated With Hospital Admission and Critical Illness Among 5279 People With Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ (2020) 369:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, Clinical Course, and Outcomes of Critically Ill Adults With COVID-19 in New York City: A Prospective Cohort Study. Lancet (2020) 395(10239):1763–70. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr (2020) 174(9):868–73. doi: 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr (2020) 174(10):e202430. doi: 10.1001/jamapediatrics.2020.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duffus SH, Cooper KL, Agans RP, Jain N. Mental Health and Behavioral Screening in Pediatric Type 1 Diabetes. Diabetes Spectr (2019) 32(2):171–5. doi: 10.2337/ds18-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Butwicka A, Frisén L, Almqvist C, Zethelius B, Lichtenstein P. Risks of Psychiatric Disorders and Suicide Attempts in Children and Adolescents With Type 1 Diabetes: A Population-Based Cohort Study. Diabetes Care (2015) 38(3):453–9. doi: 10.2337/dc14-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Di Carlo F, Sociali A, Picutti E, Pettorruso M, Vellante F, Verrastro V, et al. Telepsychiatry and Other Cutting-Edge Technologies in COVID-19 Pandemic: Bridging the Distance in Mental Health Assistance. Int J Clin Pract (2021) 75(1):e13716. doi: 10.1111/ijcp.13716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cianfarani S. Pediatric Endocrinology in the Time of the COVID-19 Pandemic. Horm Res Paediatr (2019) 92(6):345–6. doi: 10.1159/000507703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller R, Ashraf AP, Gourgari E, Gupta A, Kamboj MK, Kohn B, et al. SARS-CoV-2 Infection and Paediatric Endocrine Disorders: Risks and Management Considerations. Endocrinol Diabetes Metab (2021) 4(3):e00262. doi: 10.1002/edm2.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Regelmann MO, Conroy R, Gourgari E, Gupta A, Guttmann-Bauman I, Heksch R, et al. PES Drug and Therapeutics Committee and Practice Management Committee. Pediatric Endocrinology in the Time of COVID-19: Considerations for the Rapid Implementation of Telemedicine and Management of Pediatric Endocrine Conditions. Horm Res Paediatr (2020) 93(6):343–50. doi: 10.1159/000513060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid (2016) 26(10):1343–421. doi: 10.1089/thy.2016.0229 [DOI] [PubMed] [Google Scholar]
- 44. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boelaert K, Visser WE, Taylor PN, Moran C, Léger J, Persani L. Endocrinology in the Time of Covid-19: Management of Hyperthyroidism and Hypothyroidism. Eur J Endocrinol (2020) 183(1):G33–9. doi: 10.1530/EJE-20-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dworakowska D, Grossman AB. Thyroid Disease in the Time of COVID-19. Endocrine (2020) 68(3):471–4. doi: 10.1007/s12020-020-02364-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical Characteristics of 140 Patients Infected With SARS-CoV-2 in Wuhan, China. Allergy (2020) 75(7):1730–41. doi: 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 48. Brizola E, Adami G, Baroncelli GI, Bedeschi MF, Berardi P, Boero S, et al. Providing High-Quality Care Remotely to Patients With Rare Bone Diseases During COVID-19 Pandemic. Orphanet J Rare Dis (2020) 15(1):228. doi: 10.1186/s13023-020-01513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bancos I, Hazeldine J, Chortis V, Hampson P, Taylor AE, Lord JM, et al. Eur J Primary Adrenal Insufficiency Is Associated With Impaired Natural Killer Cell Function: A Potential Link to Increased Mortality. Endocrinol (2017) 176:471–80. doi: 10.1530/EJE-16-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. European Society for Paediatric Endocrinology (ESPE) . Coronavirus Disease (COVID-19) Information for Children and Adolescents Living With Endocrine Conditions, Including Type 1 Diabetes Mellitus. Internet, Cited 23 Mar 2020. Available at: https://www.eurospe.org/news/item/14064/COVID-19-information-for-children-and-adolescents-living-with-endocrine-conditions-including-type-1-diabetes-mellitus.
- 51. Kostopoulou E, Güemes M, Shah P. COVID-19 in Children and Adolescents With Endocrine Conditions. Horm Metab Res (2020) 52(11):769–74. doi: 10.1055/a-1227-6635 [DOI] [PubMed] [Google Scholar]
- 52. Regelmann MO, Conroy R, Gourgari E, Gupta A, Guttmann-Bauman I, Heksch R, et al. Pediatric Endocrinology in the Time of COVID-19: Considerations for the Rapid Implementation of Telemedicine and Management of Pediatric Endocrine Conditions. Horm Res Paediatr (2020) 93(6):343–50. doi: 10.1159/000513060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jones MS, Goley AL, Alexander BE, Keller SB, Caldwell MM, Buse JB. Inpatient Transition to Virtual Care During COVID-19 Pandemic. Diabetes Technol Ther (2020) 22(6):444–8. doi: 10.1089/dia.2020.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peters AL, Garg SK. The Silver Lining to COVID-19: Avoiding Diabetic Ketoacidosis Admissions With Telehealth. Diabetes Technol Ther (2020) 22(6):449–53. doi: 10.1089/dia.2020.0187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.