Introduction
Functional abdominal pain (FAP) – persistent pain not associated with organic causes – is common in pediatric care [7,31,33]. Upon medical evaluation, most youth with FAP meet Rome criteria for a functional abdominal pain disorder, including irritable bowel syndrome, functional dyspepsia, and FAP not otherwise specified [8]. Given the importance of brain-gut interaction in functional gastrointestinal disorders (FGIDs) [41], a biopsychosocial approach is common and underlies treatments that target psychosocial factors in FAP [29]. Inconsistent results [1,29] may be partially due to failure of these treatments to address heterogeneity in patients’ psychosocial characteristics.
The Federal Pain Research Strategy (FPRS) [9] lists as a top priority the “identification of patient-specific factors associated with favorable versus poor responsiveness to specific treatments.” The FPRS calls for randomized controlled trials (RCTs) to identify patient subgroup characteristics that moderate treatment effects. Such moderators may reveal a treatment effect for one patient subgroup but not for others. Consistent with the goals of precision medicine, identification of treatment moderators such as patient subgroup may facilitate allocation of resources to patients most likely to benefit and, for those unlikely to benefit, inform the development of new treatments that address their particular needs.
In a large prospective study following pediatric FAP patients into late adolescence and young adulthood, we [48] statistically identified three patient subgroups (i.e., High Pain Dysfunctional [HPD]; High Pain Adaptive [HPA]; and Low Pain Adaptive [LPA]) based on assessment of pain-related psychological characteristics including pain cognitions, impairment, and affect. These patient subgroups predicted differences in FGIDs, chronic pain, and other outcomes at follow-up nearly a decade later, with HPD patients showing significantly worse outcomes. Patient subgrouping based on characteristics that predict outcomes can be useful in guiding individualized treatment decisions [21], although showing that these subgroups differentially predict treatment response is a critical next step.
The current RCT tested whether FAP patient subgroup moderated responses to psychological treatment. Cognitive behavioral therapy (CBT) for pain management shows promise as a FAP treatment [1,16,18,43]. When offered as a digital intervention, CBT reduces costs, increases patient access, and has benefits comparable to those of in-person CBT [3]. We modified an internet-delivered CBT intervention shown effective in other pediatric chronic pain conditions [24] for youth with FAP. Because parents can influence children’s pain [19,25,34,38,49], they were included.
Previously [48], we showed that HPD patients, compared to HPA and LPA ones, demonstrated significantly higher levels of maladaptive pain behavior, cognitions, and affect at their medical evaluation and significantly worse outcomes at long-term follow-up. These predictive factors are targeted by the CBT intervention developed by Palermo [24]. We hypothesized that youth in the HPD subgroup assigned to CBT, relative to those assigned to internet-delivered pain education (EDU), would exhibit greater reductions in gastrointestinal (GI) symptoms, abdominal pain, and pain interference during the trial. In contrast, youth in the HPA and LPA subgroups, with more adaptive pain characteristics, would benefit equally from CBT and EDU. We evaluated durability of treatment effects with 6- and 12-month follow-up assessments. We also measured treatment expectancies, engagement, and satisfaction.
Methods
Participants
Participants were consecutive new patients (ages 11–17 years) whose evaluation for abdominal pain at a pediatric gastroenterology clinic did not attribute the symptoms to organic causes. A parent also participated. Participants were enrolled in a double-blind, balanced (1:1), parallel group, RCT (ClinicalTrials.gov: NCT02327377) evaluating the efficacy of an 8-week internet-delivered CBT intervention (Web-based Management of Adolescent Pain [WebMAP],[24]) adapted for FAP patients in this study compared to an 8-week internet-delivered pain education control condition (EDU). Eligibility criteria included abdominal pain of at least 2 months’ duration (consistent with Rome criteria for duration of pediatric FAP disorders [8]), no comorbid chronic disease (e.g., inflammatory bowel disease, diabetes), no recent hospitalizations, English-speaking, and internet access.
The final sample comprised 278 parent-adolescent dyads. Youth were predominately female (66.2%, n = 184) and Caucasian (86.0%, n = 239) with mean age 14.62 years (SD = 1.88). Most parent participants were mothers (95.3%, n = 265).
Procedures
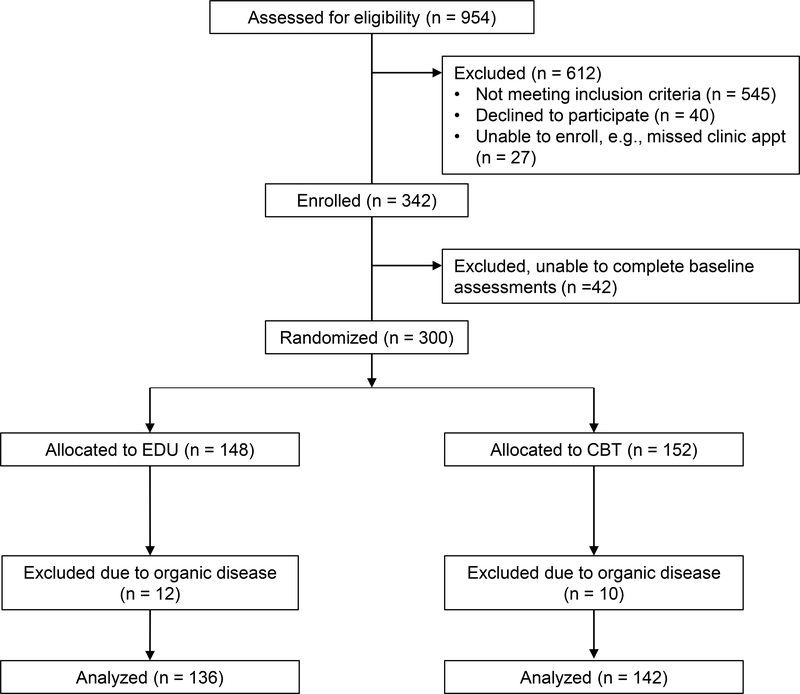
Vanderbilt University Medical Center’s Institutional Review Board approved study procedures. Recruitment began in November 2014 and ended in February 2018 when 300 participants had been randomized. To reduce selection bias in recruitment, consecutive new patients referred for evaluation of abdominal pain were identified prior to their clinic appointment at the Pediatric Gastroenterology Clinic at Monroe Carell Jr. Children’s Hospital at Vanderbilt University. When clinic staff contacted parents of these patients by telephone to remind them of their upcoming appointment, they offered information about the study. Parents who expressed interest in the study were prescreened regarding their child’s eligibility and asked to arrive early for their appointment to meet with the study recruiter. The recruiter confirmed eligibility and administered informed consent/assent procedures for parent and youth. In cases in which patient families could not be reached by telephone prior to their appointment, clinic staff offered families the opportunity to speak with the recruiter and learn about the study at clinic check-in. Baseline assessment was completed in the clinic prior to the medical evaluation and prior to randomization; assessment included questionnaires administered on REDCap, a secure online survey site [5,6]. See Figure 1 for participant flow.
Figure 1.
CONSORT flow chart
Treatment allocation was concealed from participants and health care providers. Families were told they would be randomly assigned to one of two pain management websites. Differences between the websites were not described. Youth who completed baseline assessment received emails from Seattle Children’s Research Institute with login information linking them to CBT or EDU. Randomization was completed by research staff at Seattle Children’s Research Institute. The randomization assignment was generated four at a time using an online, free research randomizer (available at www.randomizer.org) and blacked out until a participant was ready to be randomized. Randomization was stratified by patient subgroup (i.e., High Pain Dysfunctional, High Pain Adaptive, and Low Pain Adaptive). Patient subgroups were generated by computer from baseline measures and were unknown by patients. A separate randomization table was created for each patient subgroup. The randomization schedule, including patient subgroups, was stored in a password-protected document accessible only to study staff responsible for randomization. Staff implementing the study at Vanderbilt Children’s Hospital did not have knowledge of patient treatment allocation. Standard care by each patient’s physician was not altered and physicians were not aware of patient treatment allocation or subtype. Youth and parents completed follow-up questionnaires through REDCap at mid-treatment, post-treatment, 6-month and 12-month follow-up.
Exclusions following study enrollment and randomization included two participants who enrolled in the study and were later found to be ineligible due to intellectual disabilities; they are included in the total of 545 ineligible participants. In addition, ten dyads decided not to continue in the study after being randomized (CBT: n = 8, EDU: n = 2). These dyads were marginally more likely to be assigned to CBT compared to EDU, X2(1) = 3.47, p = 0.06. Finally, data from twenty-two participants were excluded from analysis because review of medical records by a co-author (JA) revealed that the youth had been diagnosed with organic disease (e.g., Celiac disease, inflammatory bowel disease) during the course of medical evaluation subsequent to study enrollment.
Treatment Conditions
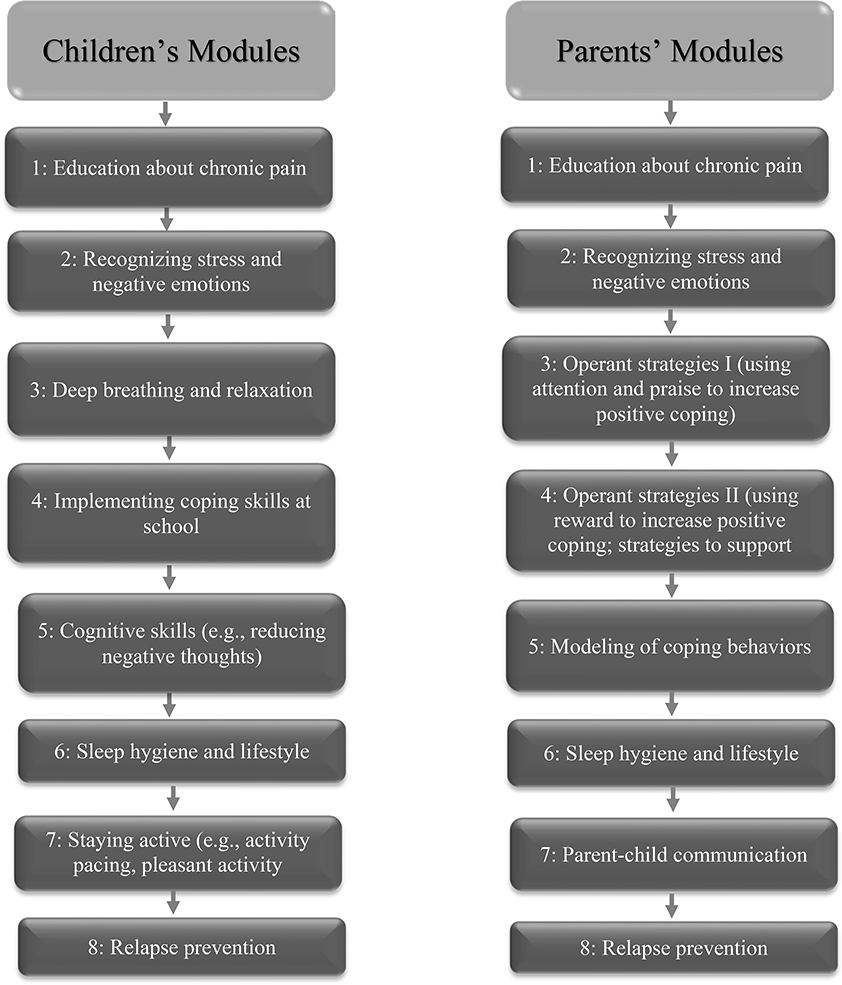
In order to ensure treatment fidelity, both conditions used standardized materials delivered on line. The CBT condition received an adapted version of WebMAP [24], a program originally developed for adolescents with mixed chronic pain conditions, which was modified for this study to be specific to FAP. The program includes 8 modules for youth to learn pain coping skills and 8 modules for parents to learn adaptive behavioral responses to pain. The program is interactive: participants complete behavioral assignments which are reviewed by a Health Coach providing standardized feedback. Health coaches were master’s degree psychology graduate students. To ensure treatment fidelity for the feedback that coaches provided, coaches completed a standard series of training tasks (readings, role play, and supervision) and used an online coaches’ manual [21] to standardize their responses to study participants. Coaches were supervised by co-author TP in their online responses to assignments and messages submitted by youth and parents. Coaches responded to each message sent by participants. The coaches’ manual included examples of standard responses to praise skills practice (e.g., “Nice job practicing guided imagery!”), strategies to overcome obstacles to skills practice (e.g., “Try practicing at the same time every day”), and content to build rapport (e.g., “Did you do anything fun over the weekend?”). Components of the WebMAP program are listed in Figure 2.
Figure 2.
Content of WebMAP Modules
The EDU condition served as a time and attention control treatment condition. It comprised eight modules of pain-related information (e.g., pain physiology, types of abdominal pain, assessment of chronic pain) from publicly available health websites, delivered to youth and parents as static content without skills training, assignments, or access to a health coach. EDU content was adapted from Palermo et al. [24] and is available in Supplementary Material (Table 1).
Table 1.
Demographic Factors and Clinical Characteristics by FAP Patient Subgroup
| Demographic Factor | High Pain Dysfunctional (n = 109) |
High Pain Adaptive (n = 114) |
Low Pain Adaptive (n = 55) |
|---|---|---|---|
| Adolescent Age, M ± SD | 15.08 ± 1.89a | 14.48 ± 1.82b | 14.01 ± 1.79b |
| Adolescent Sex, % (n) | |||
| Female | 79.8% (87)a | 66.7% (76)a | 38.2% (21)b |
| Male | 20.2% (22)a | 33.3% (38)a | 61.8% (34)b |
| Adolescent Race, % (n) | |||
| Caucasian | 80.7% (89) | 88.6% (101) | 92.6% (50) |
| Minority Group* | 19.3% (21) | 11.4% (13) | 7.4% (4) |
| Parent who participated in study, % (n) | |||
| Mother or grandmother | 93.6% (102) | 95.6% (109) | 96.4% (53) |
| Father | 6.4% (7) | 4.4% (5) | 3.6% (2) |
| Parent employment, % (n) | |||
| Employed | 66.7% (72) | 67.5% (77) | 61.8% (34) |
| Unemployed | 33.3% (36) | 32.5% (37) | 38.2% (21) |
| Parent education, % (n) | |||
| High school or less | 26.6% (29) | 16.7% (19) | 21.8% (12) |
| Vocational school or some college | 32.1% (35) | 36.8% (42) | 40.0% (22) |
| Four-year college | 31.2% (34) | 32.5% (37) | 25.5% (14) |
| Graduate or professional school | 10.1% (11) | 14.0% (16) | 12.7% (7) |
| Parent marital status, % (n) | |||
| Married or partnered | 69.7% (76) | 71.9% (82) | 78.2% (43) |
| Single, Divorced, or Separated | 30.3% (33) | 21.1% (32) | 21.8% (12) |
| Baseline GI symptom severity (CSSI), M ± SD | 2.05 ± .67a | 1.50 ± .62b | .92 ± .56c |
| Baseline abdominal pain severity (API), M ± SD | 2.75 ± .75a | 2.24 ± .75b | 1.33 ± .48c |
| Baseline pain interference (PROMIS), M ± SD | 57.16 ± 7.24a | 50.92 ± 6.79b | 43.21 ± 5.82c |
Note. Adolescent age and sex differed significantly by patient subgroup (p < .05). Within rows, differing superscripts indicate significant differences between subgroups at p < .05 level.
Due to the low frequency of some racial groups, races identified by the National Institutes of Health as minority groups were collapsed into a single category. Percentages do not always add up to 100% due to missing data for some demographic variables. GI = Gastrointestinal, CSSI = Children’s Somatic Symptoms Inventory, API = Abdominal Pain Index, PROMIS = Patient-Reported Outcomes Measurement Information System
Measures
Subtype Classification
Using a previously validated classification algorithm [35,48], youth were classified into three patient subgroups (HPD, HPA, LPA) comprised of youth-reported measures of pain-related psychological characteristics obtained at baseline assessment prior to randomization. Measures used in the classification algorithm include the Abdominal Pain Index (API, [15]); GI and non-GI symptom subscales of the Children’s Somatic Symptoms Inventory – 24 (CSSI-24, [36,45,46]); the Catastrophizing subscale of the Pain Response Inventory (PRI, [44]); the 26-item version of the Children’s Depression Inventory (CDI, [12]); the Functional Disability Inventory (FDI, 10-item version, [47]); and subscales of the Pain Beliefs Questionnaire-Short Form (PBQ-SF, [37]), including Pain Threat, Problem-Focused Coping Efficacy (PFCE), and Emotion-Focused Coping Efficacy (EFCE). The API, CSSI-24, PRI, PBQ-SF, and FDI are scored on a 0–4 scale. CDI total scores were converted to a 0–4 scale to match the scaling of the other measures by summing the items, dividing by 52, and then multiplying by 4. All measures exhibited adequate internal consistency (α’s ranged from 0.73–0.90). Additional information regarding these measures and application of the classification algorithm to the current sample has been presented elsewhere [35,48].
Briefly, the HPD subgroup was characterized by high levels of abdominal pain severity, GI and non-GI symptom severity, pain catastrophizing, negative affect, functional disability, and pain threat appraisal, as well as low levels of emotion focused and problem focused pain coping efficacy. The HPA subgroup, compared to the HPD subgroup, was characterized by similarly high pain frequency with slightly lower severity than the HPD subgroup and more adaptive psychosocial characteristics compared to the HPD subgroup (i.e., lower GI and non GI somatic symptom severity, pain catastrophizing, negative affect, functional disability, pain threat appraisals, and higher problem-focused and emotion-focused pain coping efficacy). The LPA subgroup, compared to both the HPD and HPA subgroups was characterized by the lowest levels of physical symptoms (e.g., abdominal pain, GI and non-GI symptom severity) and had adaptive psychosocial features including low levels of negative affect, pain catastrophizing, and pain threat appraisal. The LPA subgroup also exhibited the lowest levels of functional impairment and the highest emotion-focused and problem-focused pain coping efficacy. See Stone et al. [35] for further details regarding characteristics of youth and their parents in each subgroup.
Outcome Measures
The primary outcome measure was youth report of GI symptom severity assessed by the GI Symptom Subscale of the Children’s Somatic Symptoms Inventory (CSSI-24, [36,45,46]). Participants rated how much they were bothered by each symptom on a 5-point Likert scale ranging from (0) not at all to (4) a whole lot. The GI subscale comprises 7 items which assess nausea, constipation, diarrhea, stomach aches, vomiting, feeling bloated or gassy, and food making you sick. Items are averaged to yield a mean score (range 0 – 4) with higher scores indicating greater GI symptom severity. Alpha reliability for the GI symptoms subscale was adequate across the 5 timepoints (baseline: 0.72, mid-treatment: 0.80, post-treatment: 0.80, 6 months: 0.84, 12 months: 0.85).
Secondary outcome measures were youth report of abdominal pain severity on the Abdominal Pain Index (API, [15]) and youth report of pain interference on the PROMIS Pain Interference scale [42]. The Abdominal Pain Index (API) represents a composite of abdominal pain frequency, duration, and intensity [15]. The frequency of abdominal pain episodes during the previous 2 weeks is rated on a 6-point scale ranging from not at all (0) to every day (5). The typical daily frequency of abdominal pain episodes is assessed on a 6-point scale ranging from none (0) to constant during the day (5). The typical duration of pain episodes is rated on a 9-point scale ranging from none (0) to all day (8). The typical intensity of abdominal pain in the past 2 weeks is rated on an 11-point scale ranging from no pain (0) to the most pain possible (10). A composite score for the API was computed by placing each item on a (0–5) six-point scale, and then converting the mean of these items to a (0–4) five-point scale to put this measure on the same scale as other self-reported measures of pain characteristics (e.g., CSSI-24, PBQ-SF). Higher scores on the API indicate greater abdominal pain severity. Alpha reliability for the API was adequate across the 5 timepoints (baseline: 0.79, mid-treatment: 0.87, post-treatment: 0.88, 6 months: 0.89, 12 months: 0.92). Note that internal consistency of the API increased as pain decreased given the number of individuals who reported no pain.
The PROMIS Pediatric Pain Interference-Short Form 8a [42] comprised eight items which assessed self-reported consequences of pain on adolescent’s life, including social, cognitive, emotional, and physical domains over the past seven days (e.g., “It was hard for me to pay attention with I had pain.”, “It was hard to have fun when I had pain.”). Adolescents responded to each item on a five-point scale ranging from (0) never to (4) always. Item responses were summed yielding a total score ranging from 0 to 32 and then converted to T-scores using the PROMIS scoring manual. Higher scores indicate greater interference in activities due to pain. Alpha reliability for the measure was adequate across the 5 timepoints (baseline: 0.85, mid-treatment: 0.91, post-treatment: 0.90, 6 months: 0.90, 12 months: 0.92).
Treatment expectancies, engagement, and satisfaction were also assessed. At the baseline assessment, teens were asked to rate how likely it is that the WebMAP program would be useful for them and their parent(s), and how likely it is that the program would be helpful overall with managing their pain, using a 5-point scale ranging from (0) not at all likely to (4) extremely likely [39], Treatment satisfaction was assessed at post-treatment with six items adapted from the Treatment Evaluation Inventory, short form [10,11,24]. Patients rated their agreement with the statements on a 5-point scale ranging from (0) not at all to (4) extremely. Treatment engagement, serving as a measure of adherence, was calculated as the sum of the number of modules completed by each youth and parent dyad and could range from 0 to 16.
Statistical Analyses
Data analysis based on intention-to-treat used R Version 3.5.3 and R Studio Version 1.0.143. Longitudinal data were analyzed using linear mixed effects (LME) models [28], with 5 time points nested within each individual (Pre-treatment, Mid-treatment, Post-treatment, 6-month, 12-month follow-up). Within this framework, we designated a piecewise analysis of time, which allows for the representation of discrete time periods by modeling separate variables (and therefore separate coefficients and slopes) for conceptually meaningful periods of the RCT in the same model. Consistent with typical trends in treatment studies where the greatest effects occur by the end of treatment and change levels off during follow-up [24], we specified two conceptually meaningful time periods, or “pieces”: Piece 1 examined changes across the treatment period (Pre-, Mid, to Post-treatment), and Piece 2 modeled changes across the follow-up period (Post-, 6-month FU, to 12-month FU). Of note, this piecewise approach yielded significantly improved fit across analyses and reduced risk of inflated Type I error that would occur with use of a series of separate analyses.
Separate piecewise models were specified for each outcome. To assess whether treatment response was moderated by patient subgroups, we evaluated the Treatment x Subgroup x Time (Piece 1) interaction, which focuses on differential treatment outcomes during the active treatment period. Subsequently, we evaluated the Treatment x Subgroup x Time (Piece 2) interaction to assess treatment durability, that is, whether slopes maintained a significantly flat or decreasing trajectory throughout the follow-up period, within both subgroup and treatment condition. All models contained these three-way interactions and their component two-way interactions and main effects.
Pairwise comparisons of predicted marginal means at each timepoint were also probed to further eludicate the nature of observed effects. Initial models included age, sex, and treatment engagement as covariates. Treatment engagement was not significant in any model and was dropped in all final models, leaving age and sex as covariates. Rates of completion of at least 80% of the assessment measures were achieved by 76% of the CBT condition and 82% of the EDU condition. Participants with and without missing data did not differ significantly on age, sex, treatment engagement, baseline abdominal pain, or treatment condition. We note that linear mixed effects models are robust to missing data through full maximum likelihood estimation.
Finally, to fortify our findings regarding the significant Treatment x Subgroup x Piece 1 interaction effects, we assessed the utility of patient subgroup as a composite moderator over and above baseline symptom severity. All models were re-evaluated substituting baseline symptom severity in lieu of patient subgroup as a potential moderator of treatment outcome; for all outcomes, the three-way Treatment x Baseline Symptom Severity x Time (Piece 1) interactions were not significant.
Power analysis.
To determine whether the study was adequately powered to detect a clinically meaningful effect, power analyses were conducted prospectively using the minimum detectable effect size based on a feasible sample size [14]. We computed estimated power based on a sample size of 255, which anticipated a total enrollment of 300 participants with 4% drop out at each time point and 10% additional missing data at each time point. To achieve 80% power with p < .05 two-tailed alpha, differences between CBT and EDU would need to reach an effect size of at least 0.278 (Cohen’s d). Moderation analyses were powered to detect “medium” effects (Cohen’s d close to 0.5) for comparing the HPD and LPA patient subgroups.
Model specification.
Separate linear mixed effects (LME) models were conducted for each outcome variable in R [27] using the nlme package [26]. This approach offers advantages compared to more traditional methods for analyzing repeated measures data (e.g., repeated measures ANOVA), including accommodation of missing values to avoid listwise deletion, treating time as a continuous variable to yield more accurate estimates of change in the outcome over time (i.e., treatment response), and more flexible selection of covariance structures of the data. Of note, in the LME framework, calculation of significance values may vary based on software due to differences in the specification of degrees of freedom and unclear consensus regarding the appropriate null distribution to estimate effects of interest. The nlme package provides a standardized t-value equal to β/SE(β) where β is the regression parameter, and associated p-values for each effect.
For each model, fixed effects included age, sex, time, treatment condition, patient subgroup, and the three-way Treatment Condition x Patient subgroup x Time interaction. Subject was treated as a random effect to account for the correlation of repeated outcome measures. Subject-specific random effects were determined using restricted maximum likelihood estimation (REML) and population-level fixed effects with full maximum likelihood estimation. We specified a compound symmetric autoregressive structure to model the residual errors and used the Akaike Information Criterion [2] to determine the appropriate autoregressive structure. The unit of measurement at Level 1 was time and the unit of measurement at Level 2 was the individual. We specified a piecewise analysis of time where Piece 1 examined changes during the treatment period (Pre-, Mid-, to Post-treatment), and Piece 2 modeled changes during the follow-up period (Post-, 6-month FU, to 12-month FU). Each timepoint was coded using the median number of weeks from baseline of module completion for the full sample.
To build the models, random effects for variation at the intercept and in the slope for Piece 1 and Piece 2 were first entered into each model and retained when significant. At the second step, we entered fixed effects for Piece 1, Piece 2, treatment condition, patient subgroup and the Treatment x Subgroup x Piece 1 and Treatment x Subgroup x Piece 2 interactions, into the model. Of note, the statistical significance of the three-way interaction of primary interest did not change when treatment engagement, representing adherence, was included as a covariate.
Results
Sample Characteristics at Baseline
The clustering algorithm [35,48] was applied to baseline data and classified 39% (n =109), 41% (n = 114), and 20% (n = 55) of youth into the HPD, HPA, and LPA subgroups, respectively. Characteristics of the three subgroups are described elsewhere.[35] The subgroups differed significantly on youth age, F(2, 277) = 6.72, p = 0.001, and sex, χ2(2) = 28.33, p < 0.001. Table 1 presents demographic and baseline clinical characteristics by subgroup.
A multivariate analysis of variance found no significant differences in primary and secondary outcome variables between treatment conditions at baseline (GI Symptoms: F[1, 278] = 0.36, p=0.55, d=0.05; Abdominal Pain: F[1, 278] = 0.092, p=0.76, d=0.03; Pain Interference: F[1, 278] = 0.10, p=0.76, d=0.06). Treatment conditions did not differ significantly on youth age, sex, or race, or on parental sex, education, employment, or marital status (Table 1).
Treatment Expectancies, Engagement, and Satisfaction
Youth assigned to the CBT and EDU treatment conditions reported comparable baseline treatment expectancies (all p’s > 0.25). Treatment conditions differed significantly on participant engagement; dyads in the EDU condition completed more modules (Mean = 13.01, SD = 4.71) than dyads in the CBT condition (Mean = 10.93, SD = 5.24), t(276) = 3.61, p< 0.001, d = 0.43. Satisfaction with treatment was significantly higher for CBT (Mean = 20.96, SD = 5.63) compared to EDU (Mean = 17.24, SD = 5.76, t[223] = 4.88, p< 0.001, d = 0.65).
Overall Treatment Effects
Across all outcomes, there was a main effect of FAP subgroup and time during Piece 1 (all p’s < 0.05), such that overall levels of abdominal pain, GI symptoms, and pain interference were highest for the HPD subgroup, followed by the HPA and LPA subgroups (HPD > HPA > LPA), a pattern of effects that was maintained across the full length of the RCT. The significant main effect of time during Piece 1 also indicated that, across all participants and irrespective of subgroup, levels of the outcome variables decreased (i.e., slopes were significantly negative) throughout the treatment period. In contrast, the main effect for Piece 2 was not significant across outcome variables, indicating stable slopes that were not significantly different from 0 during the follow-up period. Finally, the main effect of treatment was not significant in all models (all p’s > 0.05), indicating that both CBT and EDU were comparable in their overall treatment effect when participants were not distinguished by subgroup.
Patient Subgroup as a Moderator of Treatment Outcomes
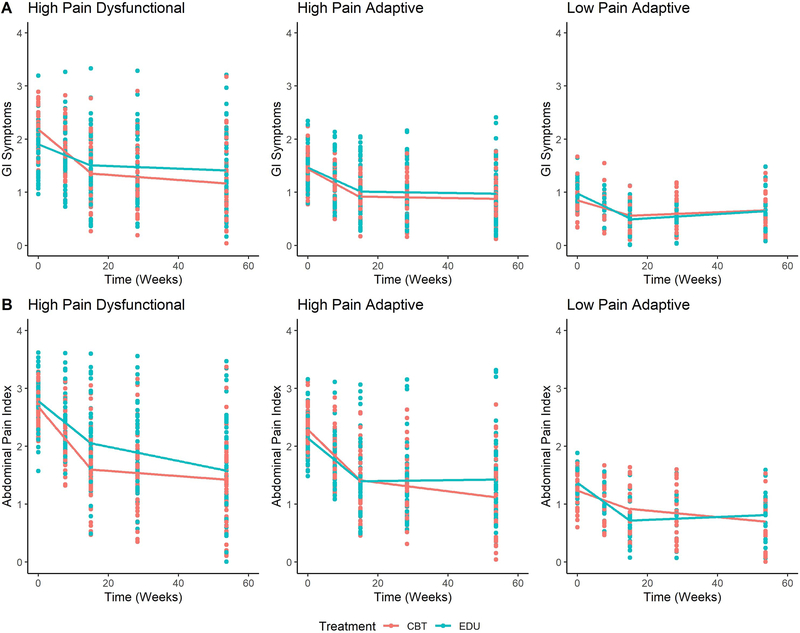
The goal of the study was to quantify the utility of an a priori composite patient subgroup moderator in predicting differential treatment response. Significant Treatment X Subgroup X Time (Piece 1) interaction effects indicated significant moderation of treatment outcomes by subgroup for both GI symptoms (t[853] = −2.93, p = 0.003) and abdominal pain (t[844] = −2.14, p=0.03), but not for pain interference (t[850] = −0.91, p = 0.36). In other words, treatment response differed based on FAP subgroup when predicting changes in GI symptoms and abdominal pain over the treatment period. Figure 3 presents the Treatment x Subgroup x Time (Piece 1) interactions for GI Symptoms (Panel A) and abdominal pain (Panel B). As shown in Figure 3 and Tables 2 and 3, the significant three-way interaction indicates that change in GI symptoms and abdominal pain during the treatment period depended on both treatment condition and patient subgroup. Specifically, within the HPD subgroup, youth assigned to CBT demonstrated significantly greater reductions in GI symptoms compared to youth in EDU (t[853] = −3.24, p = 0.001, d = −0.22). Furthermore, among all patients within the CBT treatment condition, HPD youth demonstrated significantly greater reductions in GI symptoms compared to LPA youth (t[853] = −3.89, p = 0.003, d = −0.27) and HPA youth (t[853] = −2.47, p=0.04, d = −0.17); and greater reductions in abdominal pain compared to LPA youth (t[844] = −3.63, p = 0.004, d = −0.25). Among patients assigned to CBT, HPA youth also showed greater reduction in abdominal pain compared to LPA youth (t[844] = −2.79, p=0.01, d = −0.19). Pairwise mean comparisons indicated that HPD youth in CBT also reported significantly lower levels of abdominal pain at post-treatment than did HPD youth in EDU (t[270] = −2.12, p = 0.03, d = −0.26).
Figure 3.
Effect of treatment on Gastrointestinal (GI) symptoms (Panel A) and abdominal pain (Panel B) by patient subgroup
Table 2.
Estimated Slopes (change) from Pre- to Post-treatment (Piece 1) and Effect Sizes (Cohen’s d) for Pairwise Slope Comparisons of Treatment Condition by Patient Subgroup
| Measure | FAP Subgroup | Treatment Condition | Slope from Pre- to Post-Treatment (SE) [95% CI] |
Pairwise Contrast (SE) Effect Size (Cohen’s d) |
|---|---|---|---|---|
| GI Symptoms | HPD | CBT | −0.06 (0.007) [−0.07, −0.04] | −0.03 (0.009) d = −0.22*** |
| EDU | −0.03 (0.006) [−0.04, −0.01] | |||
| HPA | CBT | −0.03 (0.006) [−0.05, −0.02] | −0.004 (0.009) d = −0.03 | |
| EDU | −0.03 (0.006) [−0.04, −0.02] | |||
| LPA | CBT | −0.01 (0.009) [−0.03, 0.005] | 0.02 (0.01) d = 0.09 | |
| EDU | −0.03 (0.01) [−0.05, −0.01] | |||
| Abdominal Pain | HPD | CBT | −0.07 (0.009) [−0.09, −0.06] | −0.02 (0.01) d = −0.12 |
| EDU | −0.05 (0.008) [−0.07, −0.03] | |||
| HPA | CBT | −0.06 (0.009) [−0.08, −0.04] | −0.01 (0.01) d = −0.06 | |
| EDU | −0.05 (0.008) [−0.06, −0.03] | |||
| LPA | CBT | −0.02 (0.01) [−0.04, 0.008] | 0.02 (0.01) d = 0.09 | |
| EDU | −0.04 (0.01) [−0.07, −0.02] |
p < 0.001
Effect sizes were calculated using the following formula: where t represents the t ratio corresponding to the pairwise estimate yielded by the lstrends function in the lsmeans R package.[17] CBT = Cognitive behavioral therapy, EDU = Education, GI = Gastrointestinal, HPD = High Pain Dysfunctional, HPA = High Pain Adaptive, LPA = Low Pain Adaptive
Table 3.
Estimated Slopes from Pre- to Post-treatment (Piece 1) and Effect Sizes (Cohen’s d) for Pairwise Slope Comparisons between Patient Subgroups within Treatment Condition
| Measure | Treatment Condition | FAP Subgroup | Slope from Pre- to Post-Treatment (SE) [95% CI] |
Pairwise Contrast (SE) Effect Size (Cohen’s d) |
||
|---|---|---|---|---|---|---|
| HPD vs. LPA | HPD vs. HPA | HPA vs. LPA | ||||
| GI Symptoms | CBT | HPD | −0.06 (0.01) [−0.07, −0.04] |
−0.04 (0.01) d = −0.27*** |
−0.02 (0.01) d = −0.17* |
−0.02 (0.01) d = −0.13 |
| HPA | −0.03 (0.01) [−0.05, −0.02] |
|||||
| LPA | −0.01 (0.01) [−0.03, 0.01] |
|||||
| EDU | HPD | −0.02 (0.01) [−0.04, −0.01] |
0.003 (0.01) d = 0.02 |
0.003 (0.01) d = 0.02 |
0.0002 (0.01) d = 0.001 |
|
| HPA | −0.03 (0.01) [−0.04, −0.02] |
|||||
| LPA | −0.03 (0.01) [−0.05, −0.01] |
|||||
| Abdominal Pain | CBT | HPD | −0.07 (0.01) [−0.09, −0.06] |
−0.06 (0.02) d = −0.25*** |
−0.01 (0.01) d = −0.07 |
−0.04 (0.02) d = −0.19** |
| HPA | −0.06 (0.01) [−0.08, −0.04] |
|||||
| LPA | −0.02 (0.01) [−0.04, 0.01] |
|||||
| EDU | HPD | −0.05 (0.01) [−0.07, −0.03] |
−0.009 (0.02) d = −0.04 |
−0.002 (0.01) d = −0.01 |
−0.007 (0.02) d = −0.03 |
|
| HPA | −0.05 (0.01) [−0.06, −0.03] |
|||||
| LPA | −0.04 (0.01) [−0.07, −0.02] |
|||||
p<0.05
p<0.01
p<0.001
Effect sizes were calculated using the following formula: where t represents the t ratio corresponding to the pairwise estimate yielded by the lstrends function in the lsmeans R package.[17] CBT = Cognitive behavioral therapy, EDU = Education, GI = Gastrointestinal, HPD = High Pain Dysfunctional, HPA = High Pain Adaptive, LPA = Low Pain Adaptive
Throughout the follow-up period (Piece 2), estimated slopes within each subgroup and treatment condition were not significantly different from zero, except for a significantly decreasing slope indicating continued decreases in abdominal pain in the CBT condition (slope = −0.006, se = 0.003, 95% CI of the slope = [−0.01, −0.001]). The output from the full LME models and means and standard deviations of outcome measures across timepoints by treatment condition are available in Supplementary Material (Tables 2 & 3).
Regarding covariates, greater age was associated with significantly higher GI symptoms (t[270] = 3.30, p = 0.001, d = .40) and female sex was associated with significantly higher abdominal pain (t[270] = 1.97, p = 0.05, d = 0.24) and pain interference (t[270] = 2.90, p = 0.004, d = 0.35). Interaction effects of treatment condition and patient subgroup with age and sex were not significant (all p’s > .05).
Adverse Events
No adverse events were reported.
Discussion
The most important, novel finding of this RCT was that subgrouping based on pain-related psychological characteristics moderated patients’ responses to psychological treatment for persistent abdominal pain. Among patients in the HPD subgroup – characterized by high pain, maladaptive pain characteristics, and poor long-term prognosis [35,48] – those assigned to CBT exhibited significantly greater reductions in GI symptoms (the primary outcome) during the treatment period as compared to those assigned to the EDU control condition. Moreover, these patients maintained symptom reductions at 6- and 12-month follow-up, providing more evidence of the efficacy of internet-delivered CBT for the HPD subgroup. In contrast, youth in the HPA and LPA subgroups – characterized by more adaptive pain characteristics – showed significant GI symptom reduction that was no greater in CBT than EDU.
The superiority of CBT over EDU for the HPD subgroup but not for the other subgroups may be explained by differences in subgroup characteristics. As reported elsewhere [35,48], the HPD subgroup, compared to both the HPA and LPA subgroups, exhibited significantly higher levels of pain catastrophizing, poorer pain self-efficacy, and greater impairment in daily activities. CBT’s focus on pain coping skills (involving weekly skills practice) may have matched these patients’ needs. Moreover, CBT parent modules focused on effective responding to children’s pain and were a good match for the HPD subgroup in that parents in the HPD subgroup, compared to those in the HPA and LPA subgroups, exhibited significantly higher levels of catastrophizing about their child’s pain, responding solicitously to their child’s pain behavior, and displaying their own pain behavior that can serve as a model for children’s pain-related impairment [35]. In contrast to the HPD subgroup, while patients in the HPA subgroup had frequent pain they also had significantly higher pain self-efficacy and their parents had more adaptive behavioral responses to their children’s pain [35]. Thus, youth and parents in the HPA subgroup may have been prepared to benefit as much from low intensity EDU pain education as from CBT. Finally, youth in the LPA subgroup – with the lowest pain and most adaptive pain-related characteristics – may have had little need for adjunctive psychological treatment. This is supported by findings that among all patients randomized to CBT, HPD youth displayed significantly greater reductions in both GI symptoms and abdominal pain (secondary outcome) than did LPA youth, consistent with floor effects in the LPA group.
Although the HPD subgroup in this RCT benefitted more from CBT than from EDU, all three subgroups assigned to the EDU control condition made significant gains, likely due in part to attention, parent involvement, and other nonspecific treatment effects. Moreover, pain education has demonstrated significant benefit as an active intervention in other RCT’s [18,24] and shows potential for treatment of pediatric chronic pain [30]. Our results imply that pain education might be sufficient for HPA and LPA patient subgroups, although a comparison to a treatment-as-usual control condition would be needed to support that assertion.
RCT’s comparing CBT to an active control condition may miss a significant moderating effect of patient subgroup if they only test for treatment main effects. In this RCT comparing CBT to EDU, CBT did not have a significant treatment main effect: average, group-level effects indicated similar benefits for CBT and EDU. However, the moderating effect of patient subgroup demonstrated that CBT was superior to EDU for nearly 40% of the sample – those in the HPD subgroup. Other RCTs tested only treatment main effects and found minimal effects of CBT for pediatric pain, but may have missed the moderating effect of patient subgroup. For example, comparisons of CBT versus Intensive Medical Care [43] and Disease Education [4] found no main effects of treatment for pediatric pain and a comparison to Nutrition Education for youth with FAP [18] found a main effect of CBT on abdominal pain by parent but not youth report and no effect on functional disability. Of note, however, Palermo et al. [24] found a small but significant main effect of WebMAP CBT versus education on activity limitations in her sample of adolescents from specialty pain clinics -- patients whose high levels of pain severity and disability may have been similar to those of the HPD patient subgroup in the present study that benefitted significantly more from CBT than from EDU.
A recent RCT comparing internet-delivered CBT to treatment-as-usual, rather than an active control condition, found that CBT yielded significantly greater reductions in GI symptoms for children with FAP [16], averaged across all patients. Whether patient subgroup moderated the effect of CBT, that is, whether a particular patient subgroup accounted for the significant overall treatment effect, was not evaluated. This is a critical question as decisions regarding allocation of health care resources must consider which patients are likely to experience significantly better outcomes with a resource-intensive treatment such as CBT.
Very few studies have evaluated moderators of psychological treatments for pediatric pain. In a recent exception, evaluation of several potential demographic and psychological moderators in a mixed group of patients with chronic pain demonstrated that the WebMAP intervention was more effective in younger (versus older) adolescents and those whose parents had lower (versus higher) levels of emotional distress [20]. The present study builds upon and advances these results by testing for moderation using profiles or composites based on several relevant psychological characteristics rather than examining each characteristic individually. Strategic use of subgroup profiles may be a more efficient and powerful way to identify patients who are most likely to benefit from a treatment.
Strengths of the current study include a large sample; randomization to treatment condition stratified by patient subgroup; an active treatment condition; concealment of treatment allocation; high treatment expectancies and engagement in both treatment conditions; recruitment of consecutive new patients without selection biases associated with self- or physician referral; pre-specified primary and secondary outcome measures with strong psychometric properties; control for patient age and sex in all analyses; and assessment of outcomes at mid-treatment, post-treatment, 6 months, and 12 months.
Study findings must be interpreted in the context of study limitations. The sample was drawn from a tertiary care setting of adolescents who were primarily Caucasian; whether our classification of patient subgroups and RCT findings are relevant to other FAP populations is unknown. The majority of participating parents were mothers and the extent to which they shared online material with their spouses/partners is also unknown. Several methodologies are emerging for assessment of clinically meaningful outcomes following psychological treatment for pediatric pain [23]; these methods will be compared in a forthcoming paper assessing the clinical significance of treatment outcomes reported here.
This RCT was unique among pediatric pain RCTs in that randomization was stratified by patient subgroup, a composite moderator [13,22,40] representing several pain-related psychosocial factors. The observed moderating effect of patient subgroup on treatment outcomes suggests that subgrouping may inform treatment allocation and optimize treatment response, and merits further attention in pediatric pain research. Important unanswered questions are whether the FAP patient subgroup classification characterizes other pediatric pain conditions and whether it can be adapted for clinical use to tailor patient treatment and thereby maximize clinical efficacy while reducing treatment costs. To optimize clinical use of our classification algorithm, some of our future work will seek to reduce the number of components in the algorithm to identify the most salient items for moderating treatment response.
Supplementary Material
Acknowledgements
Funding/Support: This work was supported by grants from the National Institutes of Health (NIH) R01 HD076983 (PI: Walker), P30 HD15052 (Vanderbilt Kennedy Center), DK058404 (Vanderbilt Digestive Disease Research Center), T32 MH018921 (PI: Garber), and T32 GM 108554 (A.L.S.).
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Disclosures: None reported.
References
- [1].Abbott RA, Martin AE, Newlove-Delgado TV, Bethel A, Thompson-Coon J, Whear R, Logan S. Psychosocial interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev 2017. doi: 10.1002/14651858.CD010971.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Akaike H Information Theory and an Extension of the Maximum Likelihood Principle. In: Parzen E, Tanabe K, Kitagawa G, editors. Selected Papers of Hirotugu Akaike. Springer Series in Statistics. New York, NY: Springer, 1998. pp. 199–213. doi: 10.1007/978-1-4612-1694-0_15. [DOI] [Google Scholar]
- [3].Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlöf E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn Behav Ther 2018;47:1–18. [DOI] [PubMed] [Google Scholar]
- [4].Connelly M, Schanberg LE, Ardoin S, Blakley M, Carrasco R, Chira P, Hayward K, Ibarra M, Kimura Y, Kingsbury DJ, Klein-Gitelman MS, Lawson E, Stinson J. Multisite randomized clinical trial evaluating an online self-management program for adolescents with juvenile idiopathic arthritis. J Pediatr Psychol 2019;44:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hoekman DR, Rutten JMTM, Vlieger AM, Benninga MA, Dijkgraaf MGW. Annual costs of care for pediatric irritable bowel syndrome, functional abdominal pain, and functional abdominal pain syndrome. J Pediatr 2015;167:1103–1108.e2. [DOI] [PubMed] [Google Scholar]
- [8].Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016;150:1456–1468.E2. [Google Scholar]
- [9].Interagency Pain Research Coordinating Committee. Federal Pain Research Strategy. Baltimore, MD: National Institutes of Health, 2017. 8–9 pp. Available: https://www.iprcc.nih.gov/sites/default/files/iprcc/FPRS_Research_Recommendations_Final_508C.pdf. Accessed 17 Nov 2020. [Google Scholar]
- [10].Kazdin AE. Acceptability of alternative treatments for deviant child behavior. J Appl Behav Anal 1980;13:259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kelley ML, Heffer RW, Gresham FM, Elliott SN. Development of a modified treatment evaluation inventory. J Psychopathol Behav Assess 1989;11:235–247. [Google Scholar]
- [12].Kovacs M Children’s depression inventory (CDI). Multi-Health System Toronto, 2003 p. [Google Scholar]
- [13].Kraemer HC. Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Stat Med 2013;32:1964–1973. [DOI] [PubMed] [Google Scholar]
- [14].Kraemer HC, Blasey CM. How Many Subjects?: Statistical Power Analysis in Research. Second edition. Los Angeles: SAGE Publications, Inc, 2015 p. [Google Scholar]
- [15].Laird KT, Sherman AL, Smith CA, Walker LS. Validation of the Abdominal Pain Index using a revised scoring method. J Pediatr Psychol 2015;40:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lalouni M, Ljótsson B, Bonnert M, Ssegonja R, Benninga M, Bjureberg J, Högström J, Sahlin H, Simrén M, Feldman I, Hedman-Lagerlöf E, Serlachius E, Olén O. Clinical and cost effectiveness of online cognitive behavioral therapy in children with functional abdominal pain disorders. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2019;17:2236–2244.e11. [DOI] [PubMed] [Google Scholar]
- [17].Lenth RV. Least-squares means: The R package lsmeans. J Stat Softw 2016;69:1–33. [Google Scholar]
- [18].Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, DuPen MM, Feld AD, Ballard SA, Welsh EM, Jeffery RW, Young M, Coffey MJ, Whitehead WE. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. Am J Gastroenterol 2010;105:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Levy RL, Whitehead WE, Walker LS, Von Korff M, Feld AD, Garner M, Christie D. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol 2004;99:2442–2451. [DOI] [PubMed] [Google Scholar]
- [20].Murray CB, de la Vega R, Loren DM, Palermo TM. Moderators of Internet-delivered cognitive-behavioral therapy for adolescents with chronic pain: Who benefits from treatment at long-term follow-up? J Pain 2020;21:603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ng MY, Weisz JR. Building a science of personalized intervention for youth mental health. J Child Psychol Psychiatry 2016;57:216–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Niles AN, Loerinc AG, Krull JL, Roy-Byrne P, Sullivan G, Sherbourne CD, Bystritsky A, Craske MG. Advancing personalized medicine: Application of a novel statistical method to identify treatment moderators in the Coordinated Anxiety Learning and Management Study. Behav Ther 2017;48:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Palermo TM, Kashikar-Zuck S, Friedrichsdorf SJ, Powers SW. Special considerations in conducting clinical trials of chronic pain management interventions in children and adolescents and their families: PAIN Rep 2019;4:e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Palermo TM, Law EF, Fales J, Bromberg MH, Jessen-Fiddick T, Tai G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: a randomized controlled multicenter trial. Pain 2016;157:174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Palermo TM, Valrie CR, Karlson CW. Family and parent influences on pediatric chronic pain: a developmental perspective. Am Psychol 2014;69:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. 2020 p. Available: https://CRAN.R-project.org/package=nlme.
- [27].R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2018 p. Available: https://www.R-project.org/. [Google Scholar]
- [28].Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Thousand Oaks: Sage Publications, 2002 p. [Google Scholar]
- [29].Reed-Knight B, Maddux MH, Deacy AD, Lamparyk K, Stone AL, Mackner L. Brain–gut interactions and maintenance factors in pediatric gastroenterological disorders: Recommendations for clinical care. Clin Pract Pediatr Psychol 2017;5:93–105. [Google Scholar]
- [30].Robins H, Perron V, Heathcote L, Simons L. Pain neuroscience education: State of the art and application in pediatrics. Children 2016;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rouster AS, Karpinski AC, Silver D, Monagas J, Hyman PE. Functional gastrointestinal disorders dominate pediatric gastroenterology outpatient practice. J Pediatr Gastroenterol Nutr 2016;62:847–851. [DOI] [PubMed] [Google Scholar]
- [32].Rutten JMTM, Korterink JJ, Venmans LMAJ, Benninga MA, Tabbers MM. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics 2015;135:522–535. [DOI] [PubMed] [Google Scholar]
- [33].Saps M, Velasco-Benitez CA, Langshaw AH, Ramírez-Hernández CR. Prevalence of functional gastrointestinal disorders in children and adolescents: Comparison between Rome III and Rome IV criteria. J Pediatr 2018;199:212–216. [DOI] [PubMed] [Google Scholar]
- [34].Stone AL, Bruehl S, Smith CA, Garber J, Walker LS. Social learning pathways in the relation between parental chronic pain and daily pain severity and functional impairment in adolescents with functional abdominal pain. Pain 2018;159:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stone AL, Han GT, Bruehl S, Garber J, Smith CA, Palermo TM, Walker LS. Subgroups of pediatric patients with functional abdominal pain: Replication, parental characteristics, and health service use. Clin J Pain 2020;36:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stone AL, Walker LS, Heathcote LC, Hernandez JM, Basch MC, Wilson AC, Simons LE. Somatic symptoms in pediatric patients with chronic pain: Proposed clinical reference points for the Children’s Somatic Symptoms Inventory (formerly the Children’s Somatization Inventory). J Pain 2019;20:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stone AL, Walker LS, Laird KT, Shirkey KC, Smith CA. Pediatric Pain Beliefs Questionnaire: Psychometric properties of the short form. J Pain 2016;17:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Stone AL, Wilson AC. Transmission of risk from parents with chronic pain to offspring: an integrative conceptual model. Pain 2016;157:2628–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tsao JC, Meldrum M, Bursch B, Jacob MC, Kim SC, Zeltzer LK. Treatment expectations for CAM interventions in pediatric chronic pain patients and their parents. Evid Based Complement Alternat Med 2005;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tukey JW. Tightening the clinical trial. Control Clin Trials 1993;14:266–285. [DOI] [PubMed] [Google Scholar]
- [41].Van Oudenhove L, Crowell MD, Drossman DA, Halpert AD, Keefer L, Lackner JM, Murphy TB, Naliboff BD, Levy RL. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Varni JW, Stucky BD, Thissen D, DeWitt EM, Irwin DE, Lai J-S, Yeatts K, DeWalt DA. PROMIS Pediatric Pain Interference Scale: An item response theory analysis of the pediatric pain item bank. J Pain 2010;11:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van der Veek SMC, Derkx BHF, Benninga MA, Boer F, de Haan E. Cognitive behavior therapy for pediatric functional abdominal pain: a randomized controlled trial. Pediatrics 2013;132:e1163–1172. [DOI] [PubMed] [Google Scholar]
- [44].Walker LS, Baber KF, Garber J, Smith CA. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Walker LS, Beck JE, Garber J, Lambert W. Children’s Somatization Inventory: Psychometric properties of the revised form (CSI-24). J Pediatr Psychol 2009;34:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Walker LS, Garber J. Manual for the Children’s Somatic Symptoms Inventory. 2018.
- [47].Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol 1991;16:39–58. [DOI] [PubMed] [Google Scholar]
- [48].Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 2012;153:1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani TA. Parent attention versus distraction: Impact on symptom complaints by children with and without chronic functional abdominal pain. Pain 2006;122:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.