Abstract
Chidamide has demonstrated significant clinical benefits for patients with relapsed/refractory (R/R) PTCL in previous studies. This multi-center observational study was aimed to evaluate the objective response rate (ORR), overall survival (OS), and safety of chidamide. From February 2015 to December 2017, 548 patients with R/R PTCL from 186 research centers in China were included in the study. Among the 261 patients treated with chidamide monotherapy, ORR was 58.6% and 55 patients (21.1%) achieved complete response (CR). Among the 287 patients receiving chidamide-containing combination therapies, ORR was 73.2% and 73 patients (25.4%) achieved CR. The median OS of all patients was 15.1 months. The median OS of patients receiving chidamide monotherapy and combination therapies was 433 and 463 days, respectively. These results demonstrate a significant survival advantage of chidamide treatments as compared with international historical records. Common adverse effects (AEs) were hematological toxicities. Most AEs in both monotherapy and combined treatments were grade 1–2. No unanticipated AEs occurred. In conclusion, chidamide-based therapy led to a favorable efficacy and survival benefit for R/R PTCL. Future studies should explore the potential advantage of chidamide treatment combined with chemotherapy.
Keywords: lymphoma, T-cell, peripheral, histone deacetylase inhibitors, efficiency, safety, survival
Introduction
Peripheral T-cell lymphoma (PTCL) is a rare and heterogeneous group of clinically aggressive mature T- and natural killer (NK)-cell neoplasms associated with poor prognosis. Twenty-seven different types of PTCL are described in the 2016 revision of the World Health Organization classification of lymphoid neoplasms. PTCL represents 10–15% of non‐Hodgkin lymphomas (NHLs) in Western countries and accounts for about 25–30% of NHLs in China (1, 2). Moreover, the subtype distribution of PTCL is different between China and Western countries. The most common subtype of PTCL in China is extranodal NK/T-cell lymphoma (NKTCL), nasal type, followed by PTCL-not otherwise specified (PTCL-NOS), anaplastic large-cell lymphoma (ALCL), and angioimmunoblastic T-cell lymphoma (AITL) (2, 3).
For relapsed or refractory PTCL, conventional chemotherapy without intensification is usually associated with high treatment failure and disease relapse rates (3–5). Novel agents that target various pathways, such as histone deacetylase (HDAC) inhibitors, have been intensively studied and developed. Epigenetic therapies is also supported by identifying mutations of epigenetic genes in different PTCL subtypes, including TET2, IDH2, RHOA, DNMT3A, CD28, and FYN (6–10). Chidamide, a novel benzamide class of HDAC inhibitors, has been demonstrated to block the catalytic pocket of class I HDACs and selectively inhibit the activity of HDAC1, 2, 3, and 10 (11–17). For relapsed/refractory (R/R) PTCL, chidamide led to an overall response rate (ORR) of 28% in a phase II study (18) and an ORR of 39% in a real-world study (19). This study was a single arm, open-label, retrospective, post-marketing observational study of chidamide. The primary objective was to evaluate the safety, efficacy, and survival benefit of chidamide-containing therapy for relapsed or refractory (R/R) PTCL.
Methods
Patients and Study Design
The current study’s protocol was approved by the Institutional Review Board of all of the participating centers and was in accordance with the Declaration of Helsinki. Written informed consent was waived owing to the use of a deidentified data set.
From February 2015 to December 2017, patients with R/R PTCL from 186 research centers in China were enrolled in the study. The main inclusion criteria were as follows: PTCL subtypes being relapsed or refractory disease as defined by histologic pathology, and receiving chidamide-containing therapy with a duration more than six weeks. When monotherapy was chosen, a dose of 30 mg chidamide was orally administered twice weekly. When combined with other regimens, chidamide with a dose of 20–30 mg twice a week was given consecutively or according to physicians’ choices.
The response criteria was based on the Lugano classification recommendation for response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma (20). ORR was defined as the proportion of patients achieving complete remission (CR) and partial response (PR). OS was calculated from the initiation of chidamide until death or the final follow-up (June 2018). Safety assessment was graded according to the Common Toxicity Criteria for Adverse Events scale, v4.03 (CTCAEv4.03).
Statistics
Data analysis was conducted using IBM SPSS for Windows software (Version 25.0; IBM Corp). A chi-square test was used for comparison of categorical variables, and a t test was used for comparison of continuous variables. Kaplan-Meier method was employed for survival analysis. Multivariate analysis for OS was performed using the Cox proportional hazards model.
Results
Patient Characteristics
A total of 548 patients with R/R PTCL were enrolled in the study. The baseline characteristics of the patients are summarized in Table 1 . The median age was 57 years (range, 18–89 years), with a male/female ratio of 1.6:1. More than one half of the patients received chidamide-containing combination treatments, in which a cytotoxic drug was predominant ( Supplement Table 1 ).
Table 1.
Baseline characteristics of 548 patients with relapsed or refractory PTCL.
| Characteristic | Number of patients (%) |
|---|---|
| Total | 548 |
| Sex | |
| Male | 341 (62.2) |
| Female | 207 (37.8) |
| Age | |
| ≤60 years | 332 (60.6) |
| >60 years | 216 (39.4) |
| ECOG PS | |
| 0–1 | 336 (61.3) |
| 2–4 | 212 (38.7) |
| Pathology type | |
| AITL | 177 (32.3) |
| PTCL-NOS | 220 (40.1) |
| ALCL | 41 (7.5) |
| ALK-positive | 12 (2.2) |
| ALK-negative | 11 (2.0) |
| ALK-unknown | 18 (3.3) |
| NKTCL | 66 (12.0) |
| Others | 44 (8.0) |
| IPI | |
| Low | 124 (22.6) |
| Low-intermediate | 173 (31.6) |
| High-intermediate | 157 (28.6) |
| High | 94 (17.2) |
| Treatment lines | |
| 2nd line | 224 (40.9) |
| 3rd line | 133 (24.3) |
| 4th line or beyond | 64 (11.7) |
| Data missing | 127 (23.2) |
| Stage | |
| I–II | 66 (12.1) |
| III- IV | 471 (85.9) |
| Data missing | 11 (2.0) |
| B symptoms | |
| With B symptoms | 169 (30.8) |
| Without B symptoms | 102 (18.6) |
| Data missing | 277 (50.5) |
PTCL, peripheral T-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; PS, performance status; AITL, angioimmunoblastic T-cell; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; ALCL, anaplastic large-cell lymphoma; ALK, anaplastic lymphoma kinase; NKTCL, natural killer/T-cell lymphoma; IPI, International Prognostic Index.
Efficacy
For the entire cohort, the ORR and CR rate were 66.2% and 23.4%, respectively. The best ORR was observed in AITL (75.1%), followed by ALCL (70.7%), PTCL-NOS (61.4%), and NKTCL (53.0%, Table 2 ). The CR rates varied from 20% to 30% according to different pathology, but was not statistically significant.
Table 2.
Efficacy of chidamide-based treatment stratified by baseline characteristics.
| CR | ORR | |||
|---|---|---|---|---|
| N (%) | P | N (%) | P | |
| Age | 0.031 | 0.842 | ||
| ≤60 | 88 (26.5) | 221 (66.6) | ||
| > 60 | 40 (18.5) | 142 (65.7) | ||
| Gender | 0.892 | 0.47 | ||
| Male | 79 (23.2) | 222 (65.1) | ||
| Female | 49 (23.7) | 141 (68.1) | ||
| ECOG PS | 0.177 | 0.004 | ||
| 0–1 | 85 (25.3) | 238 (70.8) | ||
| 2–5 | 43 (20.3) | 125 (59.0) | ||
| Stage | 124 (23.1) | 0.197 | 356 (66.3) | 0.426 |
| I–II | 16 (24.2) | 48 (72.7) | ||
| III- IV | 53 (22.9) | 131 (65.4) | ||
| Pathology | 0.55 | 0.006 | ||
| AITL | 53 (29.9) | 133 (75.1) | ||
| PTCL-NOS | 44 (20.0) | 135 (61.4) | ||
| ALCL | 10 (24.4) | 29 (70.7) | ||
| NKTCL | 16 (24.2) | 35 (53.0) | ||
| Others | 5 (11.4) | 31 (70.5) | ||
| IPI score | 0.391 | 0.115 | ||
| Low risk | 87 (70.2) | |||
| Low-intermediate risk | 39 (22.5) | 123 (71.1) | ||
| High-intermediate risk | 33 (21.0) | 97 (61.8) | ||
| High risk | 20 (21.3) | 56 (59.6) | ||
| Treatment line | 90 (21.4) | 0.672 | 276 (65.6) | 0.212 |
| 2nd line | 51 (22.8) | 155 (69.2) | ||
| 3rd line | 25 (18.8) | 80 (60.2) | ||
| ≥ 4th line | 4 (21.9) | 41 (64.1) | ||
CR, complete response; ORR, overall response rate; ECOG, Eastern Cooperative Oncology Group; PS, performance status; AITL, angioimmunoblastic T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; ALCL, anaplastic large-cell lymphoma; NKTCL, natural killer/T-cell lymphoma, IPI, International Prognostic Index.
Chidamide-containing combination therapies exhibited a better ORR (73.2% vs. 58.6%, P < 0.001) as compared with chidamide monotherapy, but had similar CR (25.4% vs. 21.1%) rates. Among the 261 patients treated with chidamide monotherapy, 55 (21.1%) patients achieved CR, 98 (37.5%) achieved PR, and 80 (30.7%) achieved SD. Of the 287 patients receiving chidamide-containing combination therapies, 73 (25.4%) patients achieved CR, 137 (47.8%) achieved PR, and 49 (17.0%) achieved SD. The differences in either the CR rate or ORR between different combination regimens were not statistically significant.
Safety
The most common adverse events (AEs) were neutropenia (46.7%) in patients treated with chidamide monotherapy, and fatigue (89.2%) in those treated with chidamide-containing combination therapies. Neutropenia was the most common grade 3-4 AE. The incidences and severity of AEs were significantly higher in patients receiving combination treatments than in those receiving the monotherapy ( Table 3 ). There was no unanticipated AEs during the follow-up period.
Table 3.
Adverse events.
| Monotherapy | Combination therapy | |||
|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Neutropenia | 81 (31.0) | 41 (15.7) | 80 (27.9) | 106 (36.9) |
| Anemia | 68 (26.1) | 19 (7.3) | 113 (39.4) | 54 (18.8) |
| Thrombocytopenia | 82 (31.4) | 30 (11.5) | 93 (32.4) | 91 (31.7) |
| Fatigue | 89 (34.1) | 16 (6.1) | 167 (58.2) | 89 (31.0) |
| Fever | 31 (11.9) | 0 (0) | 58 (20.2) | 7 (2.4) |
| Nausea/vomiting | 59 (22.6) | 3 (1.1) | 99 (34.5) | 4 (1.4) |
| Diarrhea | 35 (13.4) | 2 (0.8) | 44 (15.3) | 3 (1.0) |
| Prolonged QTc period | 6 (2.3) | 1 (0.4) | 8 (2.8) | 0 (0) |
| Thromboembolism | 2 (0.8) | 0 (0) | 14 (4.9) | 0 (0) |
| Elevated ALT | 16 (6.1) | 4 (1.5) | 40 (13.9) | 2 (0.7) |
| Elevated AST | 14 (5.4) | 5 (1.9) | 29 (10.1) | 4 (1.4) |
| Elevated Creatinine | 7 (2.7) | 0 (0) | 11 (3.8) | 1 (0.3) |
| Proteinuria | 8 (3.1) | 0 (0) | 13 (4.5) | 0 (0) |
ALT, alanine transaminase; AST, aspartate transaminase; QTc, QT interval corrected by heart rate.
Survival
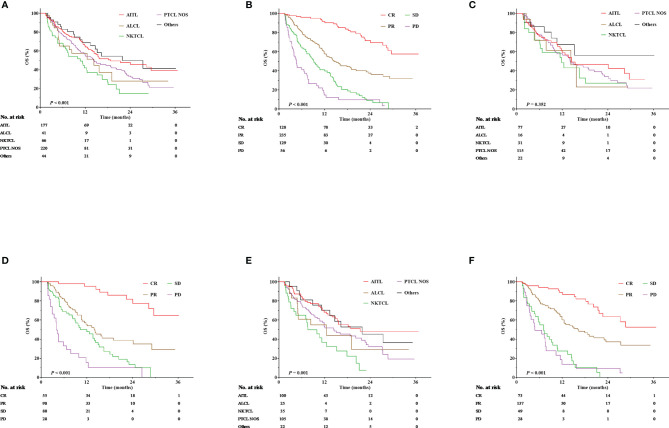
A total of 260 patients died during the follow-up period. The median OS was 15.1 months (range, 12.9–17.4 months), and the anticipated 1- and 2-year OS rates were 57.9% and 35.8%, respectively, for the entire cohort. In terms of pathological subtypes, the anticipated 1- and 2-year OS rates were 64.2% and 45.4%, respectively, for AITL; 50.7% and 27.7%, respectively, for ALCL; 41.8% and 14.5%, respectively, for NKTCL; 54.2% and 32.0%, respectively, for PTCL-NOS; and 65.4% and 41.4%, respectively, for other types (P < 0.001, Figure 1A ). The survival benefit varied according to treatment responses, with an anticipated 1- and 2-year OS rate of 90.4% and 69.4%, 58.1% and 36.1%, 39.7% and 8.7%, and 12.2% and 6.5% for patients achieving CR, PR, SD, and progression disease (PD), respectively (P < 0.001, Figure 1B ).
Figure 1.
Overall survival (OS) according to pathological subtypes and treatment responses. (A) OS according to pathological subtypes for the entire cohort. (B) OS according to treatment responses for the entire cohort. (C) OS according to pathological subtypes for those treated with chidamide monotherapy. (D) OS according to treatment responses for those treated with chidamide monotherapy. (E) OS according to pathological subtypes for those treated with chidamide-containing combined therapies. (F) OS according to treatment responses for those treated with chidamide-containing combined therapies.
The median follow-up was 4.9 months. Among patients treated with chidamide monotherapy, the expected 1- and 2-year OS rates were 58.0% and 36.5%, respectively, for all patients; 58.8% and 42.5%, respectively, for those with AITL; 46.0% and 23.0%, respectively, for those with ALCL; 48.5% and 27.0%, respectively, for those with NKTCL; 56.4% and 31.8%, respectively, for those with PTCL-NOS; and 67.2% and 56.0%, respectively, for those with other types (P = 0.352, Figure 1C ). In terms of treatment responses, the expected 1- and 2-year OS rates were 95.3% and 77.5%, 53.8% and 34.9%, 47.7% and 10.1%, and 10.3% and 0 for patients achieving CR, PR, SD, and PD, respectively (P < 0.001, Figure 1D ).
Among patients receiving chidamide-containing combination therapies, the expected 1- and 2-year OS rates were 57.3% and 35.2%, respectively, for all patients; 68.3% and 47.8%, respectively, for those with AITL; 43.2% and 28.8%, respectively, for those with ALCL; 32.2% and 7.4%, respectively, for those with NKTCL; 51.8% and 32.0%, respectively, for those with PTCL NOS; and 64.5% and 45.2%, respectively, for those with other types (P = 0.001, Figure 1E ). In terms of treatment responses, the expected 1- and 2-year OS rates were 86.7% and 63.4%, 60.2% and 37.1%, 27.5% and 0, and 13.9% and 4.6% for patients achieving CR, PR, SD, and PD, respectively (P < 0.001, Figure 1F ).
Discussion
The current large-scale, real-world study explored the safety, efficacy, and survival benefit of chidamide for R/R PTCL. Chidamide-containing therapy led to a satisfactory efficacy with a ORR of 73.2% and good tolerance without unanticipated AEs. Moreover, chidamide-containing therapy brought a survival advantage with a 2-year OS rate of 35.8%. Especially for those patients achieving CR, both chidamide monotherapy and combination therapy resulted in improved survival outcome with the 2-year OS of more than 60%.
Previous studies have shown that HDAC inhibitors have significant anticancer potential for R/R PTCL. In a phase II study involving 131 patients, romidepsin led to rapid response with a median time to objective response of 1.8 months, and resulted in an ORR of 25% and a CR rate of 15% (21). During the long-term follow-up period, the median DOR for all responders was 28 months, and 32% of patients achieving CR had a DOR of more than 24 months (22). In a real-world study, romidepsin resulted in an ORR of 33%, a CR rate of 12.5%, and a median DOR of 13.4 months (23). Similarly, a pivotal phase II study showed the ORR of belinostat led to an ORR of 25.8% with a CR rate of 10.8% (24). In the current study, the ORR of chidamide-containing therapy was 66.2% for the entire cohort. Notably, a relatively higher response rate was observed in AITL with an ORR of 75.1% and a CR rate of 29.9%. AITL is characterized by high frequencies of mutations in epigenetic modifiers in neoplastic T cells (9), which can partly explain the significant clinical benefits of chidamide. In addition, the efficacy of chidamide seemed to be higher than that of pralatrexate which led to an ORR of 29% with a CR rate of 11% for relapsed or refractory PTCL (25), but it was lower than that of Brentuximab vedotin which led to an ORR of 86% with a CR rate of 57% for ALCL (26).Therefore, future studies focusing on the impact of HDAC inhibitors on the survival benefit of specific subtypes are needed.
Survival expectations for patients with R/R PTCL treated with salvage chemotherapy is very poor. A retrospective study demonstrated that patients with first-time relapsed PTCL treated with chemotherapy only had a median OS of 6.5 months (27). In contrast, HDAC inhibitors showed a better survival advantage. Romidepsin resulted in a median DOR of 28 months and a median PFS of 29 months, of which a better survival benefit was observed in those who achieved CR for ≥ 12 months (22). In the current study, the overall median OS for all patients was 15.1 months, and the 2-year OS rate was 69.4% for patients achieving CR, suggesting a significantly improved long-term survival benefit of chidamide to patients with R/R PTCL.
Chidamide was generally well-tolerated in the current study. Most of the AEs were hematological toxicities of grades 1–2, including thrombocytopenia, neutropenia, and anemia. The incidence of AEs slightly increased in patients receiving chidamide-containing combination treatments, but all AEs were manageable. Transient prolongation of QT interval corrected by heart rate (QTc) period was observed, which was not associated with concurrent cardiac symptoms. Therefore, this study further confirmed the safety of chidamide both in monotherapy and along with other chemotherapies.
There was several limitations in the current study. First, the time to response was taken into account when the inclusion criteria was developed. The median time to objective response for romidepsin was 1.8 months (21), while chidamide led to a rapid response with 74% of all responses occurring within the first 6 weeks after treatment (18). Based on these reports, patients who received therapy with a duration more than six weeks were enrolled to explore the long-term survival benefit of chidamide in the current study. However, it resulted in a significant selection bias for the evaluation of efficacy, which led to a higher ORR (58.6%) than that reported in a previous real-world study (ORR was 51.2%) (19). Second, the optimal combined cytotoxic drugs were not determined due to the heterogeneous regimens during combined therapy, and data of salvage therapy after disease progression was not collected. Third, many baseline characteristics data including central pathology review, clinical manifestation, imaging examination methods for staging and response, and prognosis except international prognostic index was missing due to multicenter nature and enrollment, which made it difficult to select particular patient population who potentially benefitted from chidamide therapy.
In conclusion, the current large-scale study demonstrated that chidamide had a favorable efficacy and a tolerable safety profile for patients with R/R PTCL. In addition, the current study demonstrated the potential survival benefit of chidamide for patients with R/R PTCL when combined with chemotherapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of all of the participating centers. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
WpL conceived and designed the study, analyzed the data, and drafted and revised the paper. DLZ prepared and analyzed the data. JuZ, JM, and ZS conceptualized and designed the study. All authors provided critical comments to the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JH declared a shared affiliation, with WZ and a past co-authorship with JJ, TL, LQ, WC, JH, and PL to the handling editor at the time of the review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the patients, their families, and all investigators involved in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.750323/full#supplementary-material
References
- 1. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu W, Liu J, Song Y, Zeng X, Wang X, Mi L, et al. Burden of Lymphoma in China, 2006-2016: An Analysis of the Global Burden of Disease Study 2016. J Hematol Oncol (2019) 12(1):115. doi: 10.1186/s13045-019-0785-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu W, Ji X, Song Y, Wang X, Zheng W, Lin N, et al. Improving Survival of 3760 Patients With Lymphoma: Experience of an Academic Center Over Two Decades. Cancer Med (2020) 9:3765–74. doi: 10.1002/cam4.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu X, Yang M, Wu M, Zheng W, Xie Y, Zhu J, et al. A Retrospective Study of the CHOP, CHOPE, and CHOPE/G Regimens as the First-Line Treatment of Peripheral T-Cell Lymphomas. Cancer Chemother Pharmacol (2019) 83:443–9. doi: 10.1007/s00280-018-3744-z [DOI] [PubMed] [Google Scholar]
- 5. Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe RS, MacLennan KA, et al. Peripheral T-Cell Lymphoma, Not Otherwise Specified: A Report of 340 Cases From the International Peripheral T-Cell Lymphoma Project. Blood (2011) 117:3402–8. doi: 10.1182/blood-2010-09-310342 [DOI] [PubMed] [Google Scholar]
- 6. Ye Y, Ding N, Mi L, Shi Y, Liu W, Song Y, et al. Correlation of Mutational Landscape and Survival Outcome of Peripheral T-Cell Lymphomas. Exp Hematol Oncol (2021) 10(1):9. doi: 10.1186/s40164-021-00200-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palomero T, Couronné L, Khiabanian H, Kim M-Y, Ambesi-Impiombato A, Perez-Garcia A, et al. Recurrent Mutations in Epigenetic Regulators, RHOA and FYN Kinase in Peripheral T Cell Lymphomas. Nat Genet (2014) 46:166–70. doi: 10.1038/ng.2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quivoron C, Couronné L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, et al. TET2 Inactivation Results in Pleiotropic Hematopoietic Abnormalities in Mouse and is a Recurrent Event During Human Lymphomagenesis. Cancer Cell (2011) 20:25–38. doi: 10.1016/j.ccr.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 9. Odejide O, Weigert O, Lane AA, Toscano D, Lunning MA, Kopp N, et al. A Targeted Mutational Landscape of Angioimmunoblastic T-Cell Lymphoma. Blood (2014) 123:1293–6. doi: 10.1182/blood-2013-10-531509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cairns RA, Iqbal J, Lemonnier F, Kucuk C, de Leval L, Jais JP, et al. IDH2 Mutations are Frequent in Angioimmunoblastic T-Cell Lymphoma. Blood (2012) 119:1901–3. doi: 10.1182/blood-2011-11-391748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie A, Liao C, Li Z, Ning Z, Hu W, Lu X, et al. Quantitative Structure-Activity Relationship Study of Histone Deacetylase Inhibitors. Curr Med Chem Anticancer Agents (2004) 4:273–99. doi: 10.2174/1568011043352948 [DOI] [PubMed] [Google Scholar]
- 12. Ning ZQ, Li ZB, Newman MJ, Shan S, Wang XH, Pan DS, et al. Chidamide (CS055/HBI-8000): A New Histone Deacetylase Inhibitor of the Benzamide Class With Antitumor Activity and the Ability to Enhance Immune Cell-Mediated Tumor Cell Cytotoxicity. Cancer Chemother Pharmacol (2012) 69:901–9. doi: 10.1007/s00280-011-1766-x [DOI] [PubMed] [Google Scholar]
- 13. Gong K, Xie J, Yi H, Li W. CS055 (Chidamide/HBI-8000), a Novel Histone Deacetylase Inhibitor, Induces G1 Arrest, ROS-Dependent Apoptosis and Differentiation in Human Leukaemia Cells. Biochem J (2012) 443:735–46. doi: 10.1042/BJ20111685 [DOI] [PubMed] [Google Scholar]
- 14. Yao Y, Zhou J, Wang L, Gao X, Ning Q, Jiang M, et al. Increased PRAME-Specific CTL Killing of Acute Myeloid Leukemia Cells by Either a Novel Histone Deacetylase Inhibitor Chidamide Alone or Combined Treatment With Decitabine. PLoS One (2013) 8:e70522. doi: 10.1371/journal.pone.0070522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan DS, Yang QJ, Fu X, Shan S, Zhu JZ, Zhang K, et al. Discovery of an Orally Active Subtype-Selective HDAC Inhibitor, Chidamide, as an Epigenetic Modulator for Cancer Treatment. Med Chem Commun (2014) 5:1789–96. doi: 10.1039/C4MD00350K [DOI] [Google Scholar]
- 16. Zhou Y, Pan DS, Shan S, Zhu JZ, Zhang K, Yue XP, et al. Non-Toxic Dose Chidamide Synergistically Enhances Platinum-Induced DNA Damage Responses and Apoptosis in Non-Small-Cell Lung Cancer Cells. BioMed Pharmacother (2014) 68:483–91. doi: 10.1016/j.biopha.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 17. Dong M, Ning ZQ, Xing PY, Xu JL, Cao HX, Dou GF, et al. Phase I Study of Chidamide (CS055/HBI-8000), a New Histone Deacetylase Inhibitor, in Patients With Advanced Solid Tumors and Lymphomas. Cancer Chemother Pharmacol (2012) 69:1413–22. doi: 10.1007/s00280-012-1847-5 [DOI] [PubMed] [Google Scholar]
- 18. Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, et al. Results From a Multicenter, Open-Label, Pivotal Phase II Study of Chidamide in Relapsed or Refractory Peripheral T-Cell Lymphoma. Ann Oncol (2015) 26:1766–71. doi: 10.1093/annonc/mdv237 [DOI] [PubMed] [Google Scholar]
- 19. Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, et al. Chidamide in Relapsed or Refractory Peripheral T Cell Lymphoma: A Multicenter Real-World Study in China. J Hematol Oncol (2017) 10:69. doi: 10.1186/s13045-017-0439-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an International Workshop to Standardize Response Criteria for non-Hodgkin's Lymphomas. J Clin Oncol (1999) 17:1244. doi: 10.1200/JCO.1999.17.4.1244 [DOI] [PubMed] [Google Scholar]
- 21. Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results From a Pivotal, Open-Label, Phase II Study of Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma After Prior Systemic Therapy. J Clin Oncol (2012) 30(6):631–6. doi: 10.1200/JCO.2011.37.4223 [DOI] [PubMed] [Google Scholar]
- 22. Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Romidepsin for the Treatment of Relapsed/Refractory Peripheral T-Cell Lymphoma: Pivotal Study Update Demonstrates Durable Responses. J Hematol Oncol (2014) 7:11. doi: 10.1186/1756-8722-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimony S, Horowitz N, Ribakovsky E, Rozovski U, Avigdor A, Zloto K, et al. Romidepsin Treatment for Relapsed or Refractory Peripheral and Cutaneous T-Cell Lymphoma: Real-Life Data From a National Multicenter Observational Study. Hematol Oncol (2019) 37(5):569–77. doi: 10.1002/hon.2691 [DOI] [PubMed] [Google Scholar]
- 24. O'Connor OA, Horwitz S, Masszi T, Hoof AV, Brown P, Doorduijn J, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol (2015) 33(23):2492–9. doi: 10.1200/JCO.2014.59.2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, et al. Pralatrexate in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results From the Pivotal PROPEL Study. J Clin Oncol (2011) 29(9):1182–9. doi: 10.1200/JCO.2010.29.9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab Vedotin (SGN-35) in Patients With Relapsed or Refractory Systemic Anaplastic Large-Cell Lymphoma: Results of a Phase II Study. J Clin Oncol (2012) 30(18):2190–6. doi: 10.1200/JCO.2011.38.0402 [DOI] [PubMed] [Google Scholar]
- 27. Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, et al. Survival of Patients With Peripheral T-Cell Lymphoma After First Relapse or Progression: Spectrum of Disease and Rare Long-Term Survivors. J Clin Oncol (2013) 31:1970–6. doi: 10.1200/JCO.2012.44.7524 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.