Abstract
Background
Drug overdoses surged during the COVID-19 pandemic, underscoring the need for expanded and accessible substance use disorder (SUD) treatment. Relatively little is known about the experiences of patients receiving treatment during the pandemic.
Methods
We worked with 21 harm reduction and drug treatment programs in nine states and the District of Columbia from August 2020 to January 2021. Programs distributed study recruitment cards to clients. Clients responded to the survey by calling a study hotline and providing a unique study identification number. Our survey included detailed questions about use of SUD treatment prior to and since the COVID-19 pandemic. We identified settings where individuals received treatment and, for those treated for opioid use disorder, we examined use of medications for opioid use disorder. Individuals also reported whether they had received telehealth treatment and pandemic related treatment changes (e.g., more take-home methadone). We calculated p-values for differences pre and since COVID-19.
Results
We interviewed 587 individuals of whom 316 (53.8%) were in drug treatment both before and during the COVID-19 pandemic. Individuals in treatment reported substantial reductions in in-person service use since the start of the pandemic, including a 27 percentage point reduction (p<.001) in group counseling sessions and 28 percentage point reduction in mutual aid group participation (p<.001). By contrast, individuals reported a 21 percentage point increase in receipt of overdose education (p<.001). Most people receiving medications for opioid use disorder reported taking methadone and had high continuity of treatment (86.1% received methadone pre-COVID and 87.1% since-COVID, p=.71). Almost all reported taking advantage of new policy changes such as counseling by video/phone, increased take-home medication, or fewer urine drug screens. Overall, respondents reported relatively high satisfaction with their treatment and with telehealth adaptations (e.g., 80.2% reported “I'm able to get all the treatment that I need”).
Conclusions
Accommodations to treatment made under the federal public health emergency appear to have sustained access to treatment in the early months of the pandemic. Since these changes are set to expire after the official public health emergency declaration, further action is needed to meet the ongoing need.
Keywords: Opioid use disorder, Substance use disorder, COVID-19, Methadone, Buprenorphine, Treatment, Access to care, Telehealth, Surveys, Policy
Abbreviations: OUD, opioid use disorder; SUD, substance use disorder; DEA, Drug Enforcement Administration; HHS, Health and Human Services
Fatal drug overdose has been rising in the United States since the early 2000s (Mattson et al., 2021), and accelerated during the COVID-19 pandemic (Faust et al., 2021). In 2020, more than 90,000 Americans died of an overdose, three-quarters of these deaths involved an opioid (Faust et al., 2021). Alongside an increasingly lethal drug supply dominated by synthetic opioids (i.e., fentanyl) (National Institute on Drug Abuse, 2021), poor access and quality of drug treatment is likely to be a major contributor to current overdose trends. Conversely, increasing utilization of drug treatment is a critical strategy for reducing substance use and improving health among people with substance use disorders (SUDs) (National Institute on Drug Abuse, 2018). Despite its effectiveness, only one-fifth of people with symptoms of an SUD used treatment in 2019 (Substance Abuse & Mental Health Services Administration, 2020b). Low utilization of evidence-based treatment is likely a major contributor to persistently high drug overdose rates.
SUD treatment was profoundly affected by the COVID-19 pandemic. Early in the pandemic, many programs opted to halt or reduce in-person services in order to prevent transmission of COVID-19 from occurring at treatment locations (Blanco, Compton, & Volkow, 2021; Kleykamp, Guille, Barth, & McClure, 2020). This had an impact on treatment offered in a variety of settings, including counseling and mutual support meetings that are integral to many SUD treatment programs. The challenge of safely delivering in-person treatment during the COVID-19 pandemic has been compounded by the elevated prevalence of health and social conditions that exacerbate COVID-19 risk and severity (e.g., virally unsuppressed HIV infection, cardiovascular disease, and unstable living conditions) among people with SUD (Allen et al., 2020; Wen, Barnett, & Saloner, 2020).
One area of particular concern has been treatment for opioid use disorder (OUD). Opioids – increasingly in combination with methamphetamines and cocaine – drove surging U.S. overdose deaths during 2020 (National Center for Health Statistics, 2021). Overdose deaths during the pandemic have risen most precipitously among Black, Latinx, and American Indian populations (Kaiser Family Foundation, 2021). Early in the pandemic when social distancing provisions were stringent and in-person clinical care was limited, a major concern was access to medications for opioid use disorder (MOUD). Buprenorphine and methadone are highly regulated by the federal government, and longstanding regulations have required in-person visits with a prescriber for buprenorphine patients and in-person visits to an opioid treatment program to receive dispensed methadone under supervision. Pre-pandemic guidelines from the US Department of Health and Human Services (HHS) and the Drug Enforcement Administration (DEA), substantially limited access to take-home methadone.
In March 2020, HHS enacted emergency regulations that allowed more widespread adoption of telehealth services. HHS, working with the DEA, waived the requirement for an initial in-person visit for buprenorphine prescription and increased the duration of take-home methadone to up to 28 days for the most stable patients and 14 days for less stable patients (Alexander, Stoller, Haffajee, & Saloner, 2020). “Stability” under the federal guidelines is determined based on factors such as the presence of recent history of substance use, regularity of clinical attendance, length of time in a program, and assurance that patients can safely store medications (Substance Abuse & Mental Health Services Administration, n.d.). In addition to the federal changes, many payers broadened reimbursement for certain telehealth services and increased rates to parity with in-person rates (Haque, 2021).
While these changes were all enacted to increase access to care, the experiences and challenges of people receiving SUD treatment during the COVID-19 pandemic are still relatively unknown, particularly among those who may have challenges such as homelessness or those who have active drug use. Existing studies using medical claims data indicate that telehealth provision related to mental health and substance use treatment rose dramatically in April 2020, while in-person encounters fell precipitously (Ziedan, Simon, & Wing, 2020). Because of the offsetting increase in telehealth, the overall volume of SUD-related care did not decline nearly as sharply as other forms of medical care (Ziedan et al., 2020). For example, prescriptions for buprenorphine held relatively steady overall, (Nguyen et al., 2020) though fewer new patients started treatment (Currie, Schnell, Schwandt, & Zhang, 2021; Huskamp et al., 2020). Further, a few studies have collected self-reported data from people who use opioids, (Krawczyk et al., 2021) but these surveys have been relatively small in scale or focused on specific cities or clinical systems (Jacka et al., 2021). Existing data from small geographic areas suggest that many, but not all, methadone patients have adapted to telehealth and take-home doses (Figgatt, Salazar, Day, Vincent, & Dasgupta, 2021). There have also been important innovations in the delivery of services, with some programs offering new mobile treatment or medication delivery (Samuels et al., 2020; Tracy, Wachtel, & Friedman, 2021). While these studies demonstrate that there have been adaptations in the delivery of SUD treatment during the pandemic, it is unclear whether the needs of patients are being adequately met during this period of heightened stress, particularly for those with limited connections to services.
The current study draws on a survey of clients of SUD treatment and harm reduction programs (i.e., programs that deliver services to promote the safety of people who use drugs, such as syringe services programs). There is a dearth of research that bridges treatment and harm reduction, despite the fact that harm reduction programs typically serve people with current drug use and therefore can provide additional insights into the needs of people who may be at the greatest risk of overdose. The study was fielded from late 2020 to early 2021. Data collection occurred in 9 states and the District of Columbia (DC), focused on areas with elevated drug overdose deaths. To our knowledge, this is the most comprehensive survey of individuals in SUD treatment during the COVID-19 pandemic. The primary aim of the study was to describe changing needs, substance use, and patterns of treatment among people with recent treatment experience. It also aimed to characterize treatment adaptations through telehealth and take-home methadone.
Methods
Data collection procedures
Study participants were recruited from a convenience sample of 21 drug treatment and harm reduction programs from DC, Maine, Maryland, Michigan, New Jersey, New Mexico, New York, Pennsylvania, Tennessee, and West Virginia. Most programs were from states participating in the Bloomberg Opioid Initiative, a campaign supported by Bloomberg Philanthropies to reduce overdose and were predominantly from regions with high overdose death rates. Programs that recruited participants for the study were nominated by partnering organizations involved in technical assistance efforts in these states, state health officials, or by other provider organizations. The technical assistance providers had typically previously interacted with clinicians or administrators at the recruiting sites, which enabled them to connect the sites to the study team. Sites served diverse populations, but were geographically skewed toward programs serving individuals in northeastern urban communities. As shown in the Appendix, compared to a nationally representative sample of people in substance use disorder treatment, the study sample was more likely to be older, African American, and to use opioids.
Interested programs were invited to an orientation phone call with a study coordinator and given an overview of study procedures. Each program needed to have staff available to assist with study card distribution. Staff at the programs were mailed 100–150 recruitment cards to distribute to their clients. The client recruitment card included the study logo, a study phone number, business hours for the study, and a unique study identifier, which reduced the possibility of non-recruited individuals participating in the study or repeat interviews from the same client.
People who expressed an interest in participating were instructed to call the study phone number during listed business hours to be screened for eligibility, provide informed consent, and take the survey. Interviewers had prior experience conducting surveys with vulnerable and hard-to-reach populations. Prior to data collection, all interviewers piloted the study survey instrument at least twice (once with another staff member and once with a client of a local service provider) to complete training. Eligibility criteria included being: (1) at least 18 years old; (2) currently a client of a referring organization; (3) able to provide informed consent; and (4) able to provide a valid, unused unique study identifier. A voicemail box was created that allowed individuals to leave a message requesting to take the survey if they either called after hours or when study phone lines were occupied. The survey took a median of 59 min to complete. Individuals who completed the survey received a $40 incentive payment, which was either mailed to an address of their choice or transmitted through the Venmo app.
Data collection commenced on August 19, 2020 and concluded on January 29, 2021. The peak data collection month was November 2020. Over this period, a total of 3200 cards were mailed to providers and 587 interviews were completed (i.e., 18.3% of all mailed cards led to a completed interview). The main analytic sample for the current study is 316 individuals who reported engaging in SUD treatment prior to and since COVID-19. We include individuals who indicated continuous SUD treatment regardless of whether they were recruited from a treatment or harm reduction program – clients of harm reduction programs represent an important, but often overlooked population in treatment. An additional 61 individuals exclusively reported engaging in treatment only prior to COVID-19 and 60 only since COVID-19 and were excluded from the main analysis; select outcomes for this larger sample are reported in the Appendix. The study was approved by the Johns Hopkins School of Public Health Institutional Review Board. Study protocols, including the survey instrument, were reviewed by an external advisory board comprised of service providers and national substance use experts.
Treatment utilization outcomes
Individuals were first asked whether they had received any drug or alcohol treatment prior to the onset of the COVID-19 pandemic (before March 2020). If yes, they identified settings where they had received treatment, services received, and frequency of treatment received prior to the pandemic. Patients were asked if they were undergoing treatment for OUD, and if yes, were asked whether they were receiving any of the three approved MOUDs. If applicable, individuals were also asked about changes in take-home methadone or length of buprenorphine prescriptions. Finally, they were asked about whether their treatment provider had adopted telehealth and other safety precautions since the pandemic.
Covariates
Covariates included several socio-demographic and structural vulnerability factors including age, sex, self-reported race and ethnicity, employment status, insurance coverage, current homelessness, and food insecurity (i.e., going to bed hungry at least once per week). Respondents also answered detailed questions about recent drug use (e.g., types of drugs used, frequency, and route of administration) and changes in self-reported substance use since the COVID-19 pandemic. These questions were modeled on other studies of people who use drugs (Allen et al., 2019; Sherman et al., 2019, 2021).
Analysis
We calculated the mean percentage of the sample reporting each outcome. For questions where individuals were queried on changes before and since the pandemic, we calculated t-tests and indicate whether the difference is significantly different between pre versus post differences (p<.05). We also calculated t-tests to compare differences in means between treatment and harm reduction clients in Table 1 .
Table 1.
Demographic and socioeconomic status of study sample by referring provider type.
| Referring Provider Type |
||
|---|---|---|
| Harm Reduction | Treatment Services | |
| Demographics | ||
| Sex | ||
| Male | 50.9 | 57.7 |
| Female | 49.1 | 42.3 |
| Other | 0 | 1.03 |
| Age | ||
| age 20–39 | 26.0⁎⁎⁎ | 50.5 |
| age 40–50 | 26.9 | 22.7 |
| age 51–75 | 47.0⁎⁎⁎ | 26.8 |
| Race/Ethnicity | ||
| Hispanic | 22.1 | 22.7 |
| NH Black | 35.2⁎⁎⁎ | 17.5 |
| NH White | 42.0 | 57.7 |
| NH Other | 1.37 | 4.12 |
| Health Status | ||
| Fair/poor | 35.0 | 28.9 |
| Serious health condition | 52.8* | 42.3 |
| Socioeconomic Status | ||
| Education | ||
| Less than HS | 35.8⁎⁎⁎ | 18.6 |
| HS graduate | 39.4 | 45.4 |
| Some/college graduate | 24.8⁎⁎ | 36.1 |
| Health Insurance | ||
| Medicaid | 53.4 | 55.7 |
| Other health insurance | 40.2 | 35.1 |
| Uninsured | 6.39 | 9.28 |
| Social Risk Factors | ||
| Currently Homeless | 17.8 | 20.8 |
| Currently Food Insecure | 18.7* | 28.9 |
Notes: Sample restricted to individuals who said that they were referred from harm reduction only services (N = 219) and treatment services (N = 97). P-value is calculated from t-tests between each of the groups. NH=Non-Hispanic, HS=high school.
P<.05.
P<.01.
P<.001.
Results
Table 1 displays characteristics of people with SUD treatment experience both pre and since COVID-19 overall, and stratified by whether the individual was recruited from a primarily harm reduction program (N = 219) versus a treatment program (N = 97). The main differences between the two groups is that individuals who were recruited from harm reduction versus treatment programs were more likely to be over age 50, to be non-Hispanic black, to have a serious health condition, to have less education, but less likely to be under age 40 and have current food insecurity.
Across the full sample, 63.9% of people receiving treatment reported any drug use in the past month. Table 2 summarizes current substance use among people who reported using any drugs in the month they took the survey. The most common route of administration was smoking a substance, followed by injection, snorting, and swallowing. Among those using drugs, 67.8% were using opioids, 65.7% were using marijuana, 34.7% were using cocaine, 15.8% were using methamphetamines, and 31.2% were using some other drug (e.g., prescription sedatives or hallucinogens). For all routes of administration, more people said that they were using more often since the COVID-19 pandemic. The difference was largest for injection (38.5% more often versus 24.2% less often).
Table 2.
Drug use since the COVID-19 pandemic among people in treatment pre- and since-COVID.
| Use Route? | Type of Drug Currently Used by Route (Among those with Any Use) |
Frequency of Route Since COVID-19 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Opioid | MJ | Cocaine | Meth | Other | About the same | Less often | More often | ||
| Any drug use | 63.9 | 67.8 | 65.7 | 34.7 | 15.8 | 31.2 | |||
| Inject | 29.7 | 93.6 | – | 22.3 | 23.4 | 7.4 | 37.4 | 24.2 | 38.5 |
| Smoke | 46.5 | 10.2 | 80.3 | 38.1 | 14.3 | – | 49 | 22.4 | 28.7 |
| Snort | 25.6 | 79 | – | 12.3 | 12.3 | 16 | 41.3 | 29.3 | 29.3 |
| Swallow | 18.4 | 24.1 | – | – | – | 84.5 | 45.1 | 23.5 | 31.4 |
Notes: Sample restricted to individuals who said that they were in treatment both pre and since the COVID-19 pandemic, N = 316. Each route of administration was asked about separately. Opioids include heroin, fentanyl, prescription opioids, and buprenorphine. Cocaine includes crack. “Other” drugs include non-opioid prescription medications (e.g., sedatives, tranquilizers, stimulants), and hallucinogens. MJ=marijuana and meth=methamphetamines. Cells are blank if the drug is not relevant to the route of administration.
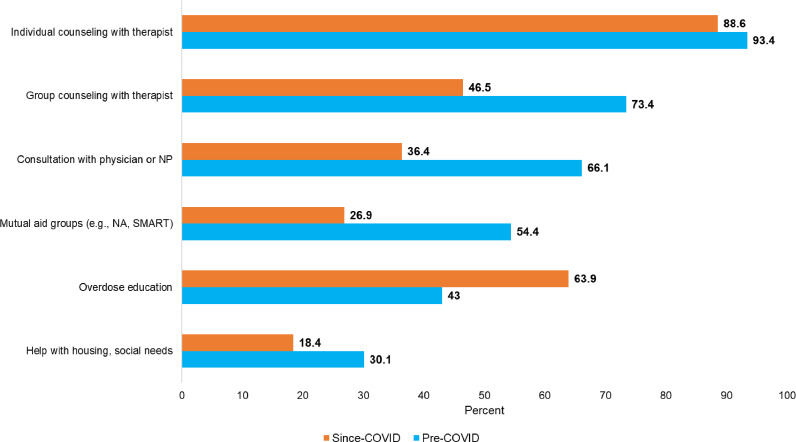
Fig. 1 displays the overall differences in types of non-medication psychosocial services received among individuals who were in treatment before and since the COVID-19 pandemic. The most commonly received psychosocial service pre-COVID was individual counseling with a therapist, which decreased from 93.4% to 88.6% (p=.037 for difference). Group counseling with a therapist decreased from 73.4% to 46.5% (p<.001), consultation with a physician or nurse practitioner for a substance use disorder decreased from 66.1% to 36.4% (p<.001), and attendance at mutual aid groups (e.g., SMART Recovery or Narcotics Anonymous) decreased from 54.4% to 26.9% (p<.001). Help with housing and social needs provided by a drug treatment program decreased from 30.1% to 18.4% (p<.001). The only service that increased was overdose education, which increased from 43.0% to 63.9% (p<.001).
Fig. 1.
Types of Non-Medication Substance Use Disorder Services Received Pre- and Since COVID-19 Pandemic.
Notes: Sample restricted to individuals who said that they were in treatment both pre and since the COVID-19 pandemic N = 316. All differences between bars were statistically significant at the p<.05 level.
Table 3 shows that among people in treatment for OUD pre and since COVID-19 (N = 287), the majority consistently received some MOUD. The most common medication received was methadone: 86.1% pre COVID-19 and 87.1% since COVID-19 (p=.71). Other medications were less common: buprenorphine, 12.5% pre COVID-19 and 10.5% since COVID-19 (p=.43) and naltrexone, 1.4% pre COVID-19 and 0.7% since (p=.41).
Table 3.
Use of medications for opioid use disorder.
| Opioid Use Disorder Treatment | Pre-COVID | Since-COVID | p-value |
|---|---|---|---|
| No medication | 2.4 | 1.7 | 0.56 |
| Methadone | 86.1 | 87.1 | 0.71 |
| Buprenorphine | 12.5 | 10.5 | 0.43 |
| Naltrexone | 1.4 | 0.7 | 0.41 |
Notes: Restricted to individuals treated for opioid use disorder pre and since-COVID (N = 287). Columns sum to more than 100% because individuals could endorse multiple medications.
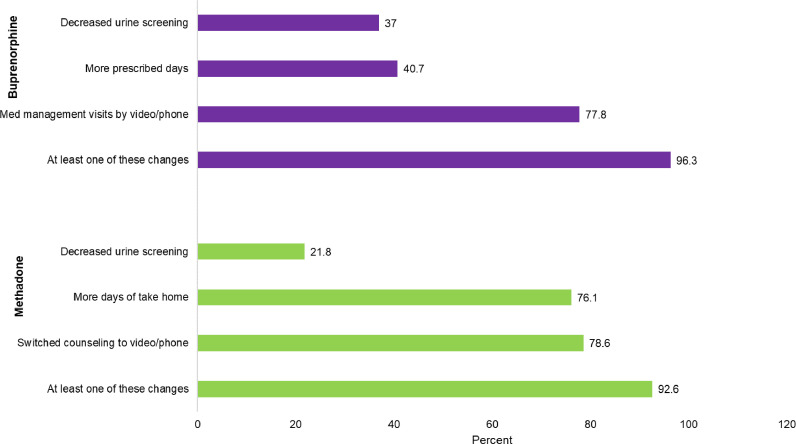
Fig. 2 reflects changes in treatment for individuals who reported currently receiving methadone or buprenorphine. Among persons receiving methadone: 78.6% reported counseling was switched to video or phone, 76.1% reported more take-home days, 21.8% decreased urine drug testing. Overall, 92.6% reported at least one of these changes to their methadone treatment. Among persons receiving buprenorphine, 77.8% reported more medication management visits by phone or video, 40.7% more prescribed days, and 37.0% had decreased urine drug screenings.
Fig. 2.
Changes to Buprenorphine and Methadone Treatment.
Notes: Sample restricted to individuals who said that they were in treatment both pre and since the COVID-19 pandemic who used either buprenorphine (N = 27) or methadone (N = 243).
Source: Authors’ analysis of the COVID HARTS survey.
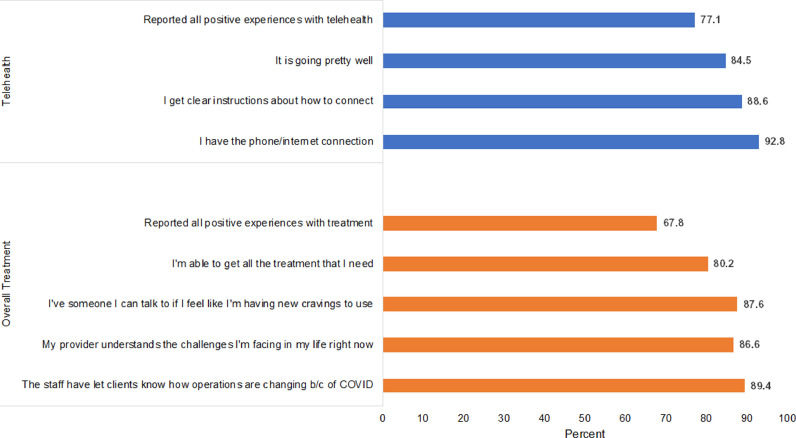
Fig. 3 displays perceptions of treatment for all people receiving SUD treatment and perceptions of telehealth for those receiving any telehealth. Patients generally had positive perceptions of treatment: 89.4% reported that the staff let clients know how operations changed, 87.6% that they had someone to talk to about if they had new cravings, 86.6% that the provider “understands the challenges I'm facing in my life right now”, and 80.2% that they were able to “get all the treatment I need right now.” Overall, 67.8% endorsed all positive responses to all these questions. Patients using telehealth also had positive perceptions of telehealth. For example, 92.8% said that they had the internet/phone connection they needed, 88.6% said they got clear instructions about how to connect, and 84.5% said “it is going pretty well.”
Fig. 3.
Experiences with Treatment Overall and with Telehealth.
Notes: Sample restricted to individuals who said that they were in treatment pre and since the COVID-19 pandemic (N = 286) and those who had recent experience with telehealth (N = 238).
Source: Authors’ analysis of the COVID HARTS survey.
Discussion
This study documents the impact of COVID-19 on SUD treatment among people receiving treatment in nine U.S. states and the District of the Columbia during the first year of the pandemic. Participants reported decreases in the use of a variety of in-person services, particularly group counseling, consultation with a clinician, and mutual aid groups. Despite these decreases, for people in OUD treatment, access to MOUD remained relatively stable, and most reported that they were able to take advantage of new flexibilities offered under the pandemic such as increased days of take-home methadone. Overall, participants reported relatively high satisfaction with their current treatment and those using telehealth modalities were likewise relatively satisfied with how the technology was working.
Participants reported that many programs adapted their service delivery model to the necessities of social distancing during the pandemic. Reported changes, particularly shifts to telehealth and declines in in-person visits, have also been identified in other studies. For example, studies using administrative data have also shown that there has been continuity in medication treatment for opioid use disorder (Currie et al., 2021; Nguyen et al., 2020) similar to what we show. Adaptations such as greater use of telehealth likely improved continuity of treatment for the individuals in our study. Concerns have been raised that telehealth, especially those requiring smart phone technology, could leave behind vulnerable populations, such as lower-income, older, publicly-insured, and less educated populations (Ramsetty & Adams, 2020; Wang et al., 2021). However, it is notable that these changes were generally reported to be successful among our sample respondents, a group with large proportions over the age of 50, homeless, Medicaid enrollment, and low levels of education. During the COVID-19 pandemic, programs serving low-income individuals undertook efforts to bridge the lower levels of digital literacy and technology access challenges of their populations, and it is possible that these efforts supported individuals in our study (Wang et al., 2021). Extending the benefits of technology will require reaching groups that may have disconnected from treatment during the pandemic (and were therefore not in our study), including people previously served by programs that may have terminated operations rather than adapting care.
Furthermore, it is unclear whether treatment is adequately addressing newly arising changes in substance use and overdose risk during COVID-19. On net, individuals in the study sample were reporting more frequent drug use since the start of the pandemic. These findings should be examined in the broader context of heightened overdose risk since the start of the pandemic (Faust et al., 2021; Friedman, Beletsky, & Schriger, 2021). Overdose deaths surged to unprecedented levels in 2020, a complex situation that has likely been exacerbated by the conditions of isolation, rising fentanyl presence in the illicit drug supply, and increased economic insecurity arising during the pandemic. Programs likely undertook efforts to counteract this increase in overdose risk. Indeed, the only service that individuals reported receiving more frequently since the pandemic was overdose education, which may reflect targeted efforts by service providers to address the instability many of their patients are facing during the pandemic. Further, it is likely that naloxone distribution accompanied take-home methadone, which is a proven harm reduction strategy recommended for opioid treatment programs (Katzman et al., 2020; Substance Abuse & Mental Health Services Administration, 2020a). As shown, drug use often continued among people in drug treatment. Programs can address ongoing health risks by adopting harm reduction principles, partnering with harm reduction programs or offering harm reduction services directly, especially naloxone distribution, which is sometimes provided to clients of methadone programs. Ensuring that patients have adequate medication dosage can also reduce drug use and overdose risk by reducing the likelihood of uncontrolled cravings or withdrawal (Fareed, Vayalapalli, Casarella, & Drexler, 2012).
The study also makes an important methodological contribution, by illustrating the potential of a novel approach to rapid data collection with a vulnerable population during a pandemic where face-to-face data collection was infeasible. The study recruitment card approach and remote study hotline had the advantage of being accessible to a multi-state population and was successful in reaching people who are typically difficult to recruit to surveys. Notably, active drug use was highly prevalent among this group of people currently in treatment (63.9%), which may reflect the inclusion of low-threshold treatment programs. While the multi-state study design was not nationally representative, it does include participants from many communities, including areas where there may have been more versus less COVID-19 related disruptions to services.
The study does have important limitations, however. First, as compared to surveys with a defined sampling frame, it is difficult to gauge how respondents may have differed from non-respondents. Individuals who called the survey hotline may have had more reliable phone access, greater self-efficacy, and higher levels of trust in research than non-respondents, though the survey could also have skewed toward people who were more financially precarious and seeking incentive payments. As compared to a traditional survey with a defined sampling frame, we are unable to assess the potential biases of our select sample. The survey strategy also necessarily excludes people who were disconnected from any services at the time of the study. As such, study findings can only be generalized to people who were retained in treatment during the pandemic, and do not address the challenges and concerns of people who chose to leave treatment or otherwise lost access to care. Second, some study measures have not been specifically validated using psychometric testing. Measuring care satisfaction in surveys in susceptible to “ceiling effects”, particularly as patients often generously rate their health care providers (Voutilainen, Pitkäaho, Vehviläinen-Julkunen, & Sherwood, 2015). Finally, the study is limited by the cross-sectional design, which asks individuals to self-report their current substance use and treatment utilization, and how these changed since the pandemic. These changes may be subject to recall and social desirability bias. COVID-19 lockdowns occurred at different times in the study states, and the survey did not provide anchors for time periods (e.g., “prior to COVID-19”). This could lead to differences in how respondents interpreted and responded to questions about changes in behavior.
Conclusion
Ensuring access to treatment for substance use disorders during the COVID-19 pandemic has been a major policy and logistical challenge, especially as overdose rates have reached historically high levels. In a multistate sample, we find that patients accessing treatment through accommodations to federal regulations made for the public health emergency are generally satisfied with their care. These accommodations are slated to be phased out after the federal public health emergency, however, there are opportunities to continue these policies through further adaptations to the regulations that could be accomplished without passing new federal legislation (Connolly, McBournie, & Doyle, 2021). Continuation of these regulations could be combined with efforts to further tailor treatment to the emerging risk factors confronting people who use drugs, such as unstable housing or greater isolation. Further, there is important work to be done focusing on harm reduction for people who may be engaging in continued use while in treatment, such as regular access to safer drug use supplies and naloxone. All of these changes could have substantial public health benefits as the U.S. seeks to recover from the COVID-19 pandemic and beyond.
Funding
The study was supported by Bloomberg Philanthropies. STA is also supported by the National Institutes of Health (K01DA046234). The funders were not involved in the collection of study data, the drafting of the manuscript, or the decision to submit the study for publication.
Declarations of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge the assistance of colleagues at Vital Strategies and Pew Charitable Trusts, the study advisory board, and programs that helped distribute client cards.
Appendix
Table A1.
Demographic and socioeconomic status of study sample by current treatment status.
| Individual was Participating in Treatment: |
|||
|---|---|---|---|
| Pre-COVID Only | Pre- and Since-COVID | Since-COVID Only | |
| Demographics | |||
| Sex | |||
| Male | 37.7 | 52.7⁎⁎ | 53.3* |
| Female | 60.7 | 47* | 46.7 |
| Other | 1.6 | 0.3 | 0 |
| Age | |||
| age 20–39 | 55.7 | 33.5⁎⁎⁎ | 53.3 |
| age 40–50 | 21.3 | 25.6 | 18.3 |
| age 51–75 | 23 | 40.8⁎⁎⁎ | 28.3 |
| Race/Ethnicity | |||
| Hispanic | 19.7 | 22.3 | 16.7 |
| NH Black | 21.3 | 29.7 | 25 |
| NH White | 54.1 | 46.8 | 56.7 |
| NH Other | 4.9 | 2.2 | 5 |
| Health Status | |||
| Fair/poor | 42.6 | 33.1 | 42.4 |
| Serious health condition | 49.2 | 49.5 | 43.3 |
| Socioeconomic Status | |||
| Education | |||
| Less than HS | 23 | 30.5 | 20 |
| HS graduate | 41 | 41.3 | 40 |
| Some/college graduate | 36.1 | 28.3 | 40 |
| Health Insurance | |||
| Medicaid | 63.9 | 54.1 | 53.3 |
| Other health insurance | 23 | 38.6⁎⁎ | 33.3 |
| Uninsured | 13.1 | 7.3 | 13.3 |
| Social Risk Factors | |||
| Currently Homeless | 36.1 | 18.7⁎⁎⁎ | 25.4 |
| Currently Food Insecure | 29.5 | 21.8 | 28.3 |
Notes: Sample restricted to individuals who said that they were in treatment pre or since the COVID-19 pandemic N = 61 pre only, N = 316 pre and since, and N = 60 since only. P-value is calculated from pairwise t-tests between each of the groups relative to the pre-COVID only group.
P<.05.
P<.01.
P<.001.
Table A2.
Comparing COVID HARTS and NSDUH treatment samples.
| NSDUH 2019 | COVID HARTS | |
|---|---|---|
| N | 459 | 316 |
| Sex | ||
| Female | 40.65 | 47 |
| Male | 59.35 | 52.7 |
| Other | 0.00 | 0.3 |
| Age | ||
| 18–34 | 51.05 | 18.7 |
| 35–49 | 29.82 | 35.7 |
| 50+ | 19.10 | 46.2 |
| Race/Ethnicity | ||
| NH white | 70.98 | 46.8 |
| NH black | 11.70 | 29.7 |
| Hispanic | 12.56 | 22.3 |
| Other race | 4.76 | 2.2 |
| Health Status | ||
| Fair/poor | 21.70 | 33.1 |
| Drugs Currently Used | ||
| Opioid | 36.57 | 67.8 |
| MJ | 62.22 | 65.7 |
| Cocaine | 25.67 | 34.7 |
| Meth | 23.22 | 15.8 |
Notes: NSDUH sample represents respondents to the 2019 National Survey on Drug Use and Health who said that they had received substance use disorder treatment in the prior year.
References
- Alexander G.C., Stoller K.B., Haffajee R.L., Saloner B. An epidemic in the midst of a pandemic: Opioid use disorder and COVID-19. Annals of Internal Medicine. 2020;173(1):57–58. doi: 10.7326/M20-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B., El Shahawy O., Rogers E.S., Hochman S., Khan M.R., Krawczyk N. Association of substance use disorders and drug overdose with adverse COVID-19 outcomes in New York City: January-October 2020. Journal of Public Health. 2020;43(3):462–465. doi: 10.1093/pubmed/fdaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S.T., O'Rourke A., White R.H., Schneider K.E., Kilkenny M., Sherman S.G. Estimating the number of people who inject drugs in a rural county in Appalachia. American Journal of Public Health. 2019;109(3):445–450. doi: 10.2105/AJPH.2018.304873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C., Compton W.M., Volkow N.D. Opportunities for research on the treatment of substance use disorders in the context of COVID-19. JAMA Psychiatry. 2021;78(4):357. doi: 10.1001/jamapsychiatry.2020.3177. [DOI] [PubMed] [Google Scholar]
- Connolly, E., McBournie, A., & Doyle, S. (2021,. April 22). More flexible methadone access should continue post-pandemic. https://www.pewtrusts.org/en/research-and-analysis/articles/2021/04/22/more-flexible-methadone-access-should-continue-post-pandemic.
- Currie J.M., Schnell M.K., Schwandt H., Zhang J. Prescribing of opioid analgesics and buprenorphine for opioid use disorder during the COVID-19 pandemic. JAMA Network Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed A., Vayalapalli S., Casarella J., Drexler K. Effect of buprenorphine dose on treatment outcome. Journal of Addictive Diseases. 2012;31(1):8–18. doi: 10.1080/10550887.2011.642758. [DOI] [PubMed] [Google Scholar]
- Faust J.S., Du C., Mayes K.D., Li S.-.X., Lin Z., Barnett M.L., et al. Mortality from drug overdoses, homicides, unintentional injuries, motor vehicle crashes, and suicides during the pandemic, March-August 2020. Journal of the American Medical Association. 2021;326(1):84–86. doi: 10.1001/jama.2021.8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figgatt M.C., Salazar Z., Day E., Vincent L., Dasgupta N. Take-home dosing experiences among persons receiving methadone maintenance treatment during COVID-19. Journal of Substance Abuse Treatment. 2021;123 doi: 10.1016/j.jsat.2021.108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Beletsky L., Schriger D.L. Overdose-related cardiac arrests observed by emergency medical services during the US COVID-19 epidemic. JAMA Psychiatry. 2021;78(5):562. doi: 10.1001/jamapsychiatry.2020.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S.N. Telehealth beyond COVID-19. Psychiatric Services. 2021;72(1):100–103. doi: 10.1176/appi.ps.202000368. [DOI] [PubMed] [Google Scholar]
- Huskamp H.A., Busch A.B., Uscher-Pines L., Barnett M.L., Riedel L., Mehrotra A. Treatment of opioid use disorder among commercially insured patients in the context of the covid-19 pandemic. Journal of the American Medical Association. 2020;324(23):2440. doi: 10.1001/jama.2020.21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka B.P., Janssen T., Garner B.R., Yermash J., Yap K.R., Ball E.L., et al. Impacts of the COVID-19 pandemic on healthcare access among patients receiving medication for opioid use disorder. Drug and Alcohol Dependence. 2021;221 doi: 10.1016/j.drugalcdep.2021.108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation. (2021,. August 12). Drug overdose deaths rose during the COVID-19 pandemic, particularly among black and American Indian/Alaska native people. https://www.kff.org/coronavirus-covid-19/press-release/drug-overdose-deaths-rose-during-the-covid-19-pandemic-particularly-among-black-and-american-indian-alaska-native-people/.
- Katzman J.G., Takeda M.Y., Greenberg N., Moya Balasch M., Alchbli A., Katzman W.G., et al. Association of take-home naloxone and opioid overdose reversals performed by patients in an opioid treatment program. JAMA Network Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2020.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleykamp B.A., Guille C., Barth K.S., McClure E.A. Substance use disorders and COVID-19: The role of telehealth in treatment and research. Journal of Social Work Practice in the Addictions. 2020;20(3):248–253. doi: 10.1080/1533256X.2020.1793064. [DOI] [Google Scholar]
- Krawczyk N., Bunting A.M., Frank D., Arshonsky J., Gu Y., Friedman S.R., et al. How will I get my next week's script?” Reactions of Reddit opioid forum users to changes in treatment access in the early months of the coronavirus pandemic. International Journal of Drug Policy. 2021;92 doi: 10.1016/j.drugpo.2021.103140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson C.L., Tanz L.J., Quinn K., Kariisa M., Patel P., Davis N.L. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR. Morbidity and Mortality Weekly Report. 2021;70(6):202–207. doi: 10.15585/mmwr.mm7006a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics . 2021. Provisional drug overdose death counts. [Google Scholar]; https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
- National Institute on Drug Abuse . National Institute on Drug Abuse.; 2018. Principles of drug addiction treatment: A research-based guide (Third addition)https://www.drugabuse.gov/download/675/principles-drug-addiction-treatment-research-based-guide-third-edition.pdf?v=74dad603627bab89b93193918330c223 [Google Scholar]
- National Institute on Drug Abuse . 2021. Opioid overdose crisis. [Google Scholar]; https://www.drugabuse.gov/drug-topics/opioids/opioid-overdose-crisis.
- Nguyen T.D., Gupta S., Ziedan E., Simon K.I., Alexander G.C., Saloner B., et al. Assessment of filled buprenorphine prescriptions for opioid use disorder during the coronavirus disease 2019 pandemic. JAMA Internal Medicine. 2020;181(4):562–565. doi: 10.1001/jamainternmed.2020.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsetty A., Adams C. Impact of the digital divide in the age of COVID-19. Journal of the American Medical Informatics Association. 2020;27(7):1147–1148. doi: 10.1093/jamia/ocaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels E.A., Clark S.A., Wunsch C., Jordison Keeler L.A., Reddy N., Vanjani R., et al. Innovation during COVID-19: Improving addiction treatment access. Journal of Addiction Medicine. 2020;14(4):e8–e9. doi: 10.1097/ADM.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S.G., Park J.N., Galai N., Allen S.T., Huettner S.S., Silberzahn B.E., et al. Drivers of HIV infection among cisgender and transgender female sex worker populations in Baltimore city: Results from the SAPPHIRE study. Journal of Acquired Immune Deficiency Syndromes. 2019;80(5):513–521. doi: 10.1097/QAI.0000000000001959. (1999) [DOI] [PubMed] [Google Scholar]
- Sherman S.G., Tomko C., White R.H., Nestadt D.F., Silberzahn B.E., Clouse E., et al. Structural and environmental influences increase the risk of sexually transmitted infection in a sample of female sex workers. Sexually Transmitted Diseases. 2021;48(9):648–653. doi: 10.1097/OLQ.0000000000001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (n.d.). SAMHSA/OTP/FDA colleague letter. Substance abuse and mental health services administration. Retrieved May 27, 2021, from https://www.samhsa.gov/sites/default/files/programs_campaigns/medication_assisted/dear_colleague_letters/2008-colleague-letter-unsupervised-take-home-doses-opioid-treatment.pdf.
- Substance Abuse and Mental Health Services Administration . Substance Abuse and Mental Health Services Administration; 2020. COVID-19 and opioid treatment programs FAQ. [Google Scholar]; https://www.samhsa.gov/sites/default/files/sample-otp-covid-19-faqs.pdf.
- Substance Abuse and Mental Health Services Administration . Substance Abuse and Mental Health Services Administration; 2020. Key substance use and mental health indicators in the United States: Results from the 2019 national survey on drug use and health. [Google Scholar]
- Tracy K., Wachtel L., Friedman T. The impact of COVID-19 on opioid treatment program (OTP) services: Where do we go from here? Journal of Substance Abuse Treatment. 2021;131 doi: 10.1016/j.jsat.2021.108394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen A., Pitkäaho T., Vehviläinen-Julkunen K., Sherwood P.R. Meta-analysis: Methodological confounders in measuring patient satisfaction. Journal of Research in Nursing. 2015;20(8):698–714. doi: 10.1177/1744987115619209. [DOI] [Google Scholar]
- Wang L., Weiss J., Ryan E.B., Waldman J., Rubin S., Griffin J.L. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. Journal of Substance Abuse Treatment. 2021;124 doi: 10.1016/j.jsat.2020.108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H., Barnett M.L., Saloner B. Clinical risk factors for COVID-19 among people with substance use disorders. Psychiatric Services. 2020;71(12):1308. doi: 10.1176/appi.ps.202000215. [DOI] [PubMed] [Google Scholar]
- Ziedan E., Simon K., Wing C. National Bureau of Economic Research; 2020. Effects of state COVID-19 closure policy on non-COVID-19 health care utilization. (No. w27621) [DOI] [Google Scholar]