Abstract
Background
Interval debulking surgery (IDS), following induction or neoadjuvant chemotherapy, may have a role in treating advanced epithelial ovarian cancer (stage III to IV) where primary debulking surgery is not an option.
Objectives
To assess the effectiveness and complications of IDS for women with advanced stage epithelial ovarian cancer.
Search methods
We searched the Cochrane Gynaecological Cancer Group's Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 6, MEDLINE and EMBASE for the original review in to June 2012. We updated the searches in June 2009, 2012 and 2015 for the review updates.
Selection criteria
Randomised controlled trials (RCTs) comparing survival of women with advanced epithelial ovarian cancer, who had IDS performed between cycles of chemotherapy after primary surgery with survival of women who had conventional treatment (primary debulking surgery and adjuvant chemotherapy).
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. Searches for additional information from study authors were attempted. We performed meta‐analysis of overall and progression‐free survival (PFS), using random‐effects models.
Main results
Three RCTs randomising 853 women, of whom 781 were evaluated, met the inclusion criteria. Meta‐analysis of three trials for overall survival (OS) found no statistically significant difference between IDS and chemotherapy alone (hazard ratio (HR) = 0.80, 95% confidence interval (CI) 0.61 to 1.06, I² = 58%). Subgroup analysis for OS in two trials, where the primary surgery was not performed by gynaecologic oncologists or was less extensive, showed a benefit of IDS (HR = 0.68, 95% CI 0.53 to 0.87, I² = 0%). Meta‐analysis of two trials for PFS found no statistically significant difference between IDS and chemotherapy alone (HR = 0.88, 95% CI 0.57 to 1.33, I² = 83%). Rates of toxic reactions to chemotherapy were similar in both arms (risk ratio = 1.19, 95% CI 0.53 to 2.66, I² = 0%), but little information was available for other adverse events or quality or life (QoL).
Authors' conclusions
We found no conclusive evidence to determine whether IDS between cycles of chemotherapy would improve or decrease the survival rates of women with advanced ovarian cancer, compared with conventional treatment of primary surgery followed by adjuvant chemotherapy. IDS appeared to yield benefit only in women whose primary surgery was not performed by gynaecologic oncologists or was less extensive. Data on QoL and adverse events were inconclusive.
Keywords: Female; Humans; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Chemotherapy, Adjuvant; Chemotherapy, Adjuvant/mortality; Combined Modality Therapy; Combined Modality Therapy/methods; Induction Chemotherapy; Induction Chemotherapy/mortality; Neoadjuvant Therapy; Neoadjuvant Therapy/methods; Neoadjuvant Therapy/mortality; Ovarian Neoplasms; Ovarian Neoplasms/drug therapy; Ovarian Neoplasms/mortality; Ovarian Neoplasms/pathology; Ovarian Neoplasms/surgery; Quality of Life; Randomized Controlled Trials as Topic; Survival Rate; Tumor Burden; Tumor Burden/drug effects
Plain language summary
Interval debulking surgery for advanced epithelial ovarian cancer
Ovarian cancer frequently presents at an advanced stage so it may not be possible to remove all tumours during surgery. Several cycles of chemotherapy are generally given after primary surgery. Secondary surgery, performed after a few cycles of chemotherapy before further cycles of chemotherapy, is called interval debulking surgery (IDS). This review compares the survival of women with advanced epithelial ovarian cancer, who had IDS performed between cycles of chemotherapy after primary surgery, with survival of women who had conventional treatment (primary debulking surgery and adjuvant chemotherapy). It found similar survival rates in women who did and did not receive IDS. Not enough information about adverse effects was available. Information on quality of life of the women was also inconclusive.
Background
Ovarian cancer is the fourth most common gynaecologic cancer among women, and is the third leading cause of death in women with gynaecological malignancies. Approximately 238,700 new cases and 151,900 deaths of ovarian occurred worldwide in 2012 (Torre 2015). Primary surgery is the mainstay of treatment for ovarian cancer, followed by adjuvant chemotherapy to destroy any gross or microscopic residual tumour cells.
Primary ovarian cancer surgery is performed to achieve optimal cytoreduction, as the amount of residual tumour is one of the most important prognostic factors for survival of women with epithelial ovarian cancer (Griffiths 1975; Hoskin 1994; Bristow 2002). The definition of optimal debulking surgery has changed over the past 30 years from the residual tumour sized not more than 1 to 2 cms to no macroscopic disease (Griffiths 1975; Elattar 2011). An optimal surgical procedure required for advanced stage disease (III to IV) is not always possible, especially in women whose diseases are extensive. Such surgery can be complicated, requiring extensive bowel resection and major blood loss, with a high risk of morbidity. Another obstacle to extensive primary surgery lies in the women's medical condition, e.g. poor projected performance status or medical contraindications.
Induction chemotherapy can play an alternative role in these circumstances. The term generally describes the administration of chemotherapy to reduce tumour size, allowing further surgery. The term 'neoadjuvant chemotherapy' (NAC) is more specific in that it describes the administration of chemotherapy when primary debulking surgery is not feasible, and only a biopsy is done for histologic diagnosis. However, the two terms are sometimes used interchangeably. In this review, if chemotherapy administration does not fit the definition of NAC, we will use the term induction chemotherapy.
When a few cycles of chemotherapy are administered with some tumour response, secondary surgery may be possible before further chemotherapy is considered. This secondary surgery between the courses of chemotherapy is called interval debulking surgery (IDS). Although the optimal timing of IDS has not been agreed, it is usually performed after two to four cycles of chemotherapy. A longer interval between primary surgery and IDS (with more cycles of chemotherapy) could result in the chemotherapy selectively destroying chemosensitive tumour cells, leaving chemoresistant clones. Many retrospective or prospective non‐randomised trials report the beneficial effects of NAC or induction chemotherapy after inoperable advanced ovarian cancer or in those with gross residual diseases, respectively. Chemotherapy may increase the number of women suitable for secondary surgery (IDS); many authors report the rates of optimal resection in IDS after induction chemotherapy ranging from 77% to 94% (Lawton 1989; Jacob 1991; Surwit 1996; Ansquer 2001; Kuhn 2001; Chan 2003; Morice 2003; Giannopoulos 2006; Lee 2006).
Another potential benefit of IDS after NAC or induction chemotherapy, compared to aggressive primary debulking surgery, as reported in retrospective (Lawton 1989; Morice 2003) and prospective (Giannopoulos 2006) cohort studies, may be lower morbidity, e.g. less blood loss, requirement of intensive care unit admission, and duration of hospital stay due to the tumours being smaller. However, this was not found in another study (Kuhn 2001). The quality of life (QoL) of women treated with IDS after NAC was also reported in one study to be better than for those who had conventional treatment (primary debulking surgery followed by a complete and continual cycle of adjuvant chemotherapy) (Chan 2003). By removing the smaller size tumour masses induced by chemotherapy, IDS would facilitate the response of the residual tumours (if any) or of the microscopic lesions to subsequent chemotherapy.
Unlike the advantages for resectability and response rates which were demonstrated in most studies, there is still conflicting evidence from various studies regarding the survival benefit of IDS after chemotherapy compared to conventional treatment. Most studies of IDS after NAC or induction chemotherapy are non‐randomised and retrospective in nature. Many of them show that the survival rates of women who underwent IDS, after suboptimal primary surgery followed by chemotherapy, were similar to those of women who had primary debulking surgery (Jacob 1991; Surwit 1996; Schwartz 1999; Kayikçioglu 2001; Morice 2003; Shibata 2003; Loizzi 2005). Only a few studies reported significantly longer median survival of women who had IDS after chemotherapy than of those who had conventional treatment of primary surgery and adjuvant chemotherapy (Vergote 1998; Kuhn 2001), and even fewer studies showed an inferior result for IDS than for optimal primary cytoreduction (Fanfani 2003). This conflicting result on the survival benefit of IDS may depend on various characteristics of the women and their disease, e.g. extent of residual tumour after primary surgery or IDS, tumour response after induction chemotherapy and prior to IDS, etc. (Jacob 1991; Vergote 1998; Kuhn 2001; Fanfani 2003; Mazzeo 2003).
We are aware of three major randomised controlled trials (RCTs) (Redman 1994; Van der Burg 1995; Rose 2004) which have been conducted to evaluate the survival benefit of IDS in ovarian cancer. These trials did not agree on the benefit of survival outcomes for women with IDS. Redman 1994 and Rose 2004 showed similar survival rates between women who had IDS and those who had conventional treatment, while Van der Burg 1995 showed significantly longer survival in the IDS group which was still present after a 10‐year follow‐up.
One previous meta‐analytical study (Bristow 2006) and two systematic reviews (Bristow 2007; Morrison 2012) addressed the question of whether women with advanced ovarian cancer should have primary surgery before or after chemotherapy. The first meta‐analytical study reviewed the role of platinum‐based NAC and IDS for advanced ovarian cancer, involving 835 women from 51 studies (Bristow 2006). The result showed that the survival of women who had NAC after an attempt at primary surgery was inferior to those who had primary surgery. However, the review included only phase I to II and retrospective studies. The other systematic review of NAC or induction chemotherapy and IDS in advanced ovarian cancer was published in 2007 (Bristow 2007). The review included the three major RCTs, six non‐randomised studies, and another 26 retrospective and phase I or II studies. The authors categorised the studies into three groups according mainly to the survival outcomes of the women in the NAC or induction chemotherapy/IDS arm compared to the conventional arm: inferior survival outcome by NAC; no significant difference; and those with limited validation of inclusion criteria for NAC. The results from these studies were simply described and tabulated without a meta‐analysis for survival. A Cochrane systematic review was conducted on the role of NAC on overall survival of women with ovarian cancer (Morrison 2012), and included only one large and high quality randomised trial (Vergote 2010), of 632 eligible women with stage IIIC or IV ovarian cancer allocated either to NAC followed by IDS or to primary debulking surgery (PDS) followed by chemotherapy. Although the completely resection rate was higher in the NAC group (52% versus 20%), no significant differences in overall survival (OS) and progression‐free survival (PFS) were found between the study groups. The review authors concluded that NAC was a reasonable alternative in women with bulky stage IIIC to IV ovarian cancer.
The objective of these systematic reviews and meta‐analyses was to evaluate the use of NAC in lieu of primary surgery, which is different from the aim of our review which focuses on the role of repeated surgery (IDS) after primary surgery which had been attempted but resulted in suboptimal surgery.
We found only one previous meta‐analysis which reviewed the role of IDS after NAC in advanced ovarian cancer (Elit 1995). The authors of that review identified 33 publications and included three RCTs and three historical cohort trials. Homogeneity testing was not statistically significant by the Breslow‐Day method. Significant survival benefit from IDS was identified by a Mantel‐Haenszel odds ratio of 0.5 (P = 0.02).
Since there were potentially intrinsic biases of participant selection and variations in several factors, such as chemotherapeutic agents or cycles of administration in many retrospective or phase I and II studies, together with conflicting data from the RCTs, no definite conclusion about the advantage of IDS after attempted primary surgery could be drawn. Hence, a thorough systematic review of this subject is warranted, focusing only on high quality data or trials, to give a stronger assessment of the use of IDS in advanced epithelial ovarian cancers.
Objectives
To assess the effectiveness and complications of interval debulking surgery (IDS) for women with advanced stage epithelial ovarian cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with advanced stage epithelial ovarian cancer who have a confirmed pathological diagnosis from primary surgery which was suboptimal, with residual tumours of more than 1 to 2 cms.
Primary surgical procedures include tumour biopsy, tumour removal, or standard surgical staging for epithelial ovarian cancer.
Types of interventions
Treatment: Interval debulking surgery (IDS), defined as secondary surgery which is performed after two to four cycles of neoadjuvant chemotherapy (NAC) or induction chemotherapy, to remove the bulk of the tumour, and followed by adjuvant chemotherapy of the same type.
Control: Adjuvant chemotherapy only.
Types of outcome measures
Primary outcomes
Overall survival (OS): Survival until death from all causes. Survival was assessed from the time when women were enrolled in the study.
Secondary outcomes
Progression‐free survival (PFS).
Adverse events.
Quality of life (QoL), measured using a scale that had been validated through reporting of norms in a peer‐reviewed publication.
Search methods for identification of studies
We sought articles in all languages, and carried out translations where necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews. We ran searches on the following databases: Cochrane Gynaecological Cancer Group's Specialised Register (CGCSR) to June 2008, the Cochrane Central Register of Controlled Trials (CENTRAL) 2008, Issue 2, MEDLINE from January 1966 to June 2008, and EMBASE from January 1980 to June 2008. We extended the second wave of updated searches to July 2009, CENTRAL 2009, Issue 2, MEDLINE Ovid to June week 4 2009, EMBASE to 2009 week 27. We also extended the searches in June 2012 and again in June 2015 (see Appendix 1; Appendix 2; Appendix 3).
We identified all relevant articles found on PubMed, and used the 'related articles' feature to conduct a further search for newly published articles.
Searching other resources
Unpublished and grey literature
We searched Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov and www.cancer.gov/clinicaltrials and Gynaecologic Oncologists of Canada (http://www.g‐o‐c.org) for ongoing trials. We then contacted the main investigators of any relevant ongoing trials for further information, as well as the major co‐operative trials groups active in this area.
Reference lists and correspondence
We checked the citation lists of included trials to identify further study reports. We also contacted authors of all trials and/or reviews relevant to this topic to request information on any similar trials. We invited colleagues, collaborators and other experts in the field to identify missing or unreported trials.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (Endnote), where two review authors (ST and SM) removed duplicates and independently examined the remaining references. We excluded those studies which clearly did not meet the inclusion criteria, and obtained copies of the full text of potentially relevant references. We evaluated English abstracts of non‐English studies, and acquired full text versions of eligible studies and had them translated. The two authors (ST and SM) independently assessed the eligibility of all retrieved papers, resolving disagreements by discussion.
Data extraction and management
For included studies, we abstracted data as recommended in Chapter 7 of the Cochrane Handbook.
We collected data on authors, year of publication, journal citation, country, setting, inclusion and exclusion criteria, study design and methodology, study population (total number enrolled, participant characteristics, age, size and number of residual tumours after primary surgery, performance status, stage, histology, size and number of residual tumours before and after IDS), interventions (expertise of surgeons, type and schedule of chemotherapy, duration of the treatment), risk of bias, duration of follow‐up and outcomes (OS, PFS, QoL and adverse events). We also recorded the following information for each outcome of interest:
outcome definition;
unit of measurement (if relevant);
for scales: upper and lower limits, and whether high or low score is good;
results: number of participants allocated to each intervention group;
sample size; missing participants.
We extracted outcome data as follows:
for time to event (OS) data, we extracted the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmar 1998;
for dichotomous outcomes (e.g. adverse events), we extracted the number of participants in each group who experienced the outcome of interest and the number assessed at end point, in order to estimate a risk ratio (RR).
We recorded both unadjusted and adjusted statistics, if reported.
Where possible, all data extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants were analysed in the groups to which they were originally assigned.
We noted the time points at which outcomes were collected and reported.
Two review authors (ST and SM) independently extracted data, using a form specifically designed for this review. We resolved disagreements by discussion, or by recourse to a third author (PL).
Assessment of risk of bias in included studies
We assessed the risk of bias in the included RCTs using the Cochrane Collaboration's tool and the criteria specified in chapter 8 of the Cochrane Handbook. This includes assessment of:
sequence generation;
allocation concealment;
blinding (of outcome assessors only, since it was not possible to blind either participants or physicians to the assigned treatment);
incomplete outcome data: we coded the satisfactory level of loss to follow‐up for each outcome as
Yes, if fewer than 20% of women were lost to follow‐up and reasons for loss were similar in both treatment arms;
No, if more than 20% of women were lost to follow‐up or reasons for loss differed between treatment arms;
Unclear, if loss to follow‐up was not reported;
selective reporting of outcomes;
other possible sources of bias.
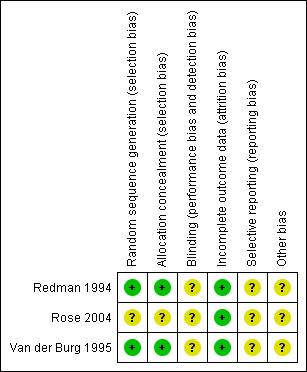
Two review authors (ST and SM) independently applied the risk of bias tool, resolving differences by discussion or by appeal to a third author (PL). Figure 1, Figure 2. We have interpreted the results of our meta‐analyses in the light of the findings of the risk of bias assessments.
1.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
We used the following measures of the effect of treatment:
for time to event data, we have used the hazard ratio (HR), where possible;
for dichotomous outcomes, we have used the risk ratio (RR).
Dealing with missing data
We attempted to extract data on the outcomes only among participants who were assessed at end point. We did not impute missing outcome data; if only imputed outcome data were reported, we contacted trial authors to request data on the outcomes only for participants who were actually assessed.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the I² statistic, i.e. the percentage of heterogeneity between trials which could not be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001), and if possible by subgroup analyses (see below). If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons.
Assessment of reporting biases
There were too few studies which met our inclusion criteria to allow us to assess reporting bias.
Data synthesis
We pooled the findings of the included studies in meta‐analyses, using adjusted summary statistics where available, and otherwise unadjusted results.
For time‐to‐event data, we produced and pooled HRs using the generic inverse variance facility of Review Manager 5.
For any dichotomous outcomes, we calculated the RR for each study and then pooled them.
We used random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses where possible, grouping trials by the expertise of the surgeons (gynaecological oncologist, gynaecologist, general surgeon). Factors such as age, stage, type of intervention, length of follow‐up, adjusted/unadjusted analysis were considered in interpretation of any heterogeneity.
Sensitivity analysis
There were too few included studies in this review to perform meaningful sensitivity analyses.
Results
Description of studies
Results of the search
We examined the titles and abstracts of 134 references identified by the original search, and considered that 18 studies were potentially relevant to this review. Two studies were readily excluded from the data in the abstracts (Evdokimova 1982; Kumar 2009). We obtained full text articles of 16 studies, and two authors (ST and SM) assessed them independently for eligibility. Thirteen of the 16 were excluded in this process. We present reasons for exclusion in the Characteristics of excluded studies table. Three randomised controlled trials (RCTs) met all the inclusion criteria (Redman 1994; Van der Burg 1995; Rose 2004). The updated search in 2010 identified 336 references, of which there were 17 possibly relevant articles. Only two of these were RCTs, and both were excluded studies (Onda 2009; Vergote 2010). The updated search in 2012 identified 836 references, some of which were duplicates. We identified 15 candidate articles, including two RCTs which were then excluded (Kumar 2009; Polcher 2009). We updated the search again in June 2015 and an additional 827 references were identified. The preliminary sift excluded 815 of theses and we identified 12 for further scrutiny. One RCT was identified but this was excluded (Madhuri 2014) (Figure 3).
3.
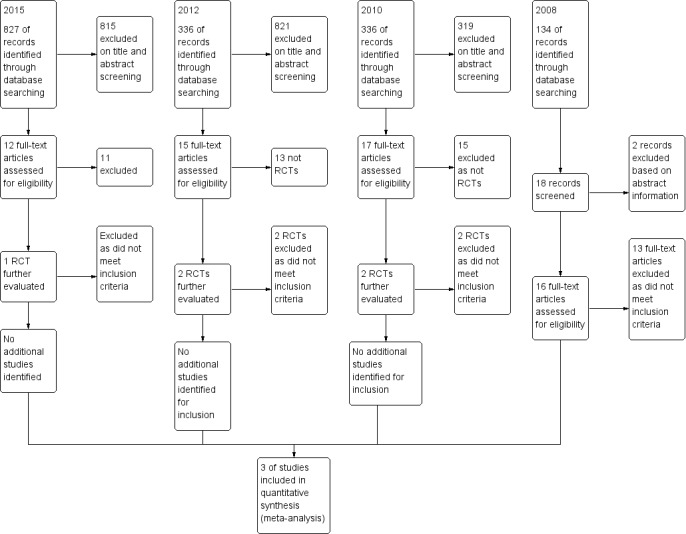
Study flow diagram.
Included studies
All three RCTs were multicentre studies: one from the United Kingdom (UK) involved four institutions which were the referral centres for cancer care (Redman 1994); one by the European Organisation for Research and Treatment of Cancer (EORTC) involved 14 participating institutions in Europe (Van der Burg 1995), and one by the Gynecologic Oncology Group (GOG) from the United States (USA) involved more than 42 cancer centres (Rose 2004).
All three of the included trials compared interval debulking surgery (IDS) plus chemotherapy with chemotherapy only.
Median length of follow‐up was reported in all three trials: 48 months (Redman 1994), 42 months (Van der Burg 1995), and.47 months (Rose 2004). Although Van de Burg and colleagues from the EORTC presented their long‐term follow‐up (10 years) as an oral presentation in the European Society of Gynaecological Oncology annual meeting in 2005 (Van der Burg 2005), the data were insufficient to include in our meta‐analysis.
Redman 1994 supplied data relating to non‐assignment of treatment as: death, disease progression, pulmonary embolus, and participant refusal. The other two trials reported only the numbers or percentages of those not undergoing surgery, but the reasons were not stated (Van der Burg 1995; Rose 2004).
All three trials reported hazard ratios (HRs) for overall survival (OS). Two trials (Van der Burg 1995; Rose 2004) used Cox regression to assess the prognostic significance of numerous covariates, including age, performance status, stage, tumour grade, response to induction chemotherapy, number of lesions, ascites, and residual diseases or size of tumours at three time points: after primary surgery, before IDS and after IDS. The definitions of optimal debulking or size of residual tumours after primary surgery varied among the trials. Optimal surgery was either defined as less than 2 cms (Redman 1994) or more than 1 cm (Van der Burg 1995; Rose 2004). Overall survival was variously calculated from the day of induction chemotherapy initiation at enrolment (Van der Burg 1995), or from the date of randomisation, which was either after primary surgery but before chemotherapy in Redman 1994, or after three cycles of chemotherapy in Rose 2004. Both the GOG and the EORTC trials reported HRs adjusted for prognostic factors; Redman 1994 also reported unadjusted HRs.
Rose 2004 reported the HR, adjusted for prognostic factors, for PFS data, and Van der Burg 1995 presented Kaplan‐Meier disease‐free survival curves, from which we used Parmar's method (Parmar 1998) to estimate the HR. The definitions of response and recurrence varied among the trials: Redman 1994 applied the International Union Against Cancer (UICC) criteria for response evaluation, using physical examination, imaging studies, but not CA125; the EORTC trial (Van der Burg 1995) used the World Health Organization (WHO) criteria allowing physical examination, imaging studies, and CA125; the GOG trial (Rose 2004) also used physical examination, imaging studies, and two CA125 levels two weeks apart, and defined progressive disease as an increase of at least 100 U/ml or a doubling of the nadir in those whose level did not return to baseline.
All three trials reported adverse events. However, Redman 1994 and Van der Burg 1995 described postoperative complications only in the IDS groups, while Rose 2004 compared general adverse effects between the IDS group and the chemotherapy only (control) group.
Quality of life (QoL) was assessed only in the GOG trial, with results reported subsequently by Wenzel 2005. The assessment tool used was the Functional Assessment of Cancer Therapy‐Ovarian (FACT‐O) questionnaire, and treatment‐specific supplemental questions at the third and sixth chemotherapy cycles and at six and 12 months after starting treatment.
Redman 1994
Redman 1994, from the UK, is the first known RCT of IDS for the management of epithelial ovarian cancer. From April 1986 to February 1990, the authors randomised 86 women with stage II to IV disease, who underwent primary surgery in 25 hospitals by 40 different surgeons and had residual disease greater than 2 cms. It was not clear how experienced the 40 surgeons were, but the primary surgery had to be performed with an attempt to remove as much tumour as possible. Stage IV included only malignant pleural effusion without other evidence of distant spread or unresectable diseases. The women received chemotherapy consisting of either a regimen of cisplatin and cyclophosphamide for eight cycles or a regimen of cisplatin, doxorubicin and bleomycin for three cycles followed by an escalated dose of cyclophosphamide for up to five cycles. Either regimen was given without detailed criteria for regimen selection. The control group had only chemotherapy after primary surgery. The intervention group had chemotherapy for one to four cycles and underwent IDS, which was performed by the primary surgeon, and then received further chemotherapy. Although our inclusion criteria for this review is IDS to be performed after chemotherapy for a minimum of two cycles, this study is included because only one out of 37 women in the IDS group had only one cycle of chemotherapy before IDS. Interval debulking surgery was not performed if there was progressive disease, stable disease, or insufficient response after three cycles.
Seven of the 86 randomised women were excluded after randomisation because primary surgery was not suboptimal. Of the remaining 79 women, 37 were in the IDS arm and 42 in the conventional arm. There were no significant differences between arms in participant or disease characteristics. Overall, 25 women (68%) in the intervention arm actually underwent IDS. The reasons for not performing IDS in 12 participants (32%) were: death or progressive disease, pulmonary embolism, and participant refusal. In the conventional arm, one woman (2%) had IDS upon request.
Adverse effects of IDS were reported as perioperative death and significant postoperative complications, including deep vein thrombosis, intestinal fistulae, chest or wound infections, or postoperative ileus. Adverse effects in the chemotherapy only group were not exhaustively reported. Toxicity was reported in both treatment groups.
Van der Burg 1995
Van der Burg 1995 enrolled 425 stage IIB to IV epithelial ovarian cancer patients between March 1987 and May 1993 who had undergone primary surgery and had residual disease greater than 1 cm. Their primary report described neither the extent nor the aim of primary surgery, nor the expertise of the surgeon. However, the authors provided additional data in their reply to a letter to the Editor of the New England Journal of Medicine (Kehoe 1995), and in their subsequent review article (Van der Burg 2003). They reported that the maximum effort to perform primary surgery was not attempted in all patients with different extents of debulking surgery, resulting in a high proportion of large residual tumours (more than 5 cms) after primary surgery (Van der Burg 2003).
All patients received three cycles of chemotherapy consisting of intravenous cisplatin and cyclophosphamide. Those who had response or stable diseases were randomised to undergo IDS or no IDS. Both groups would receive three more cycles of the same chemotherapy, with continuation after six cycles determined by institution policy.
Overall, 106 women were not randomised; this number consisted of 39 with progressive disease who were removed from the study, and also those who had contraindications to surgery, had died, had declined to participate in the study, were ineligible, or were lost to follow‐up, and those who were still receiving induction chemotherapy.
Of 319 women randomised, 278 were evaluated (140 women who underwent surgery and 138 who did not). The two groups were well balanced with respect to stage, histologic type and grade, number and size of lesions, peritoneal carcinomatosis, ascites, and response to induction chemotherapy. The rate of optimal debulking surgery (residual tumour less than 1 cm) was 64%.
The following peri‐ and postoperative adverse events were reported in the trial: bowel injury, urinary bladder injury, blood loss and postoperative fever, ileus, urinary tract infection, wound infection, deep vein thrombosis, and lung embolism.
Rose 2004
Rose 2004 enrolled 550 women from June 1994 to January 2001, with stage III to IV (malignant pleural effusion or a resected anterior abdominal wall tumour) who underwent primary surgery to remove as much tumour as possible, but who still had residual disease greater than 1 cm. However, after March 1996 when the EORTC trial (Van der Burg 1995) reported a greater benefit from secondary surgery after the exclusion of patients with stage IV diseases, only those with stage III disease were included. The primary surgeons were either fellowship‐trained or certified gynaecologic oncologists for 95% of participants. Those whose disease had not progressed and who had residual extraperitoneal tumour of less than 1 cm after three cycles of chemotherapy with paclitaxel and cisplatin were randomly assigned to secondary surgical cytoreduction (IDS) and further chemotherapy, or to chemotherapy alone. Overall, 102 women were not randomised; the most common reasons were: progressive disease or death in 40 women, while the remainder were either medically contra‐indicated, had declined, had extraperitoneal disease greater than 1 cm, had experienced excessive delay before randomisation, or for other unspecified reasons.
A total of 448 women were randomised: 226 were allocated to IDS and 222 to chemotherapy only. Participant characteristics were well balanced between the two groups. A considerable number of women in both groups had protocol violations, including: 7% in the IDS group did not have secondary surgery, versus 3% in the chemotherapy only arm who did receive surgery; 7% and 2% in the IDS and chemotherapy only arms respectively had fewer than three cycles of additional chemotherapy; and 10% and 13% in the IDS and chemotherapy only arms respectively had non‐protocol consolidation therapy before progressive disease. All randomised women were included in the analysis of OS and PFS, and were counted in the group comparisons.
We performed Cox regression for OS and PFS to evaluate the prognostic importance of: the maximal diameter of residual tumour (2.0 cms or less, 2.1 to 5.0 cms, or 5.0 cms or more) after initial surgery, age, performance status, the presence or absence of measurable disease before chemotherapy, and the size of residual tumour after IDS (less than 1 cm versus more than 1 cm).
Quality of life (QoL) of those who did and did not undergo IDS in the GOG study was evaluated and subsequently reported by Wenzel 2005. The self reported QoL was assessed in four settings, according to the Functional Assessment of Cancer Therapy‐Ovarian (FACT‐O), version 2‐questionnaire, which consists of 33 general questions for cancer patients and 12 questions specific to those with ovarian cancer. The first evaluation was at baseline after primary surgery and the third cycle of chemotherapy, but before allocation to IDS or chemotherapy only. The three subsequent evaluations were at the sixth cycle of chemotherapy, and at six and at 12 months after starting treatment. Completion rates for these questionnaires declined from 90% for the first questionnaire to 83%, 83%, and 80% for the second, third, and fourth questionnaires respectively. Nevertheless, lower completion rates were noted in the IDS group compared to the chemotherapy only group, especially at the second assessment, 77% and 89%, respectively (P < 0.001).
The included trials are described in detail below and in the table of Characteristics of included studies.
Excluded studies
After obtaining the full text, we excluded 18 studies for the following reasons:
ten references reported on non‐comparable controlled trials or non‐randomised studies (Kuhn 2001; Recchia 2001; Chan 2003; Ikeba 2004; Angioli 2006; ; Fuso 2006; Giannopoulos 2006; Lee 2006; Matulonis 2009; Onda 2009);
IDS was allowed in both arms of the trial in Park‐Simon 2006; Vergote 2010;
in Dutta 2005 survival outcomes were not analysed;
IDS was selectively performed in a subset of patients in Solomon 1988;
in Evdokimova 1982, women with ascites were not randomly assigned to interventions;
Kumar 2009 allowed either histology or cytology for a pathologic diagnosis of ovarian cancer, and there had been no attempt to do PDS in the NAC arm;
Liu 2004 was considered ineligible because NAC was given for only one cycle via intra‐arterial route before the IDS.
Polcher 2009 selected patients with ascites > 500 cc, without PDS, to have two or three cycles of NAC prior to surgery.
For further details of all the excluded studies see the table Characteristics of excluded studies.
Risk of bias in included studies
Two trials (Redman 1994; Van der Burg 1995) were at moderate risk of bias: they satisfied three of the criteria that we used to assess risk of bias. Rose 2004 was considered to be at high risk of bias as it satisfied only one of the criteria (see Figure 1; Figure 2).
Redman 1994 and Van der Burg 1995 reported the method of generation of the sequence of random numbers used to allocate women to treatment arms. They also reported concealment of the allocation sequence from participants and healthcare professionals involved in the trial. Rose 2004 reported neither the method of sequence generation nor concealment of allocation. None of the trials reported whether the outcome assessors were blinded. It was not clear whether all three trials reported all the outcomes that they assessed, and it was unclear whether any other biases may have been present. At least 87% of the women who were enrolled were assessed at end point in the three trials.
There were too few trials to support a funnel plot, so the possibility of reporting bias could not be explored.
Effects of interventions
IDS versus chemotherapy only
Survival
Overall survival (OS) (Analysis 1.1)
Meta‐analysis of three trials (Redman 1994; Rose 2004; Van der Burg 1995), assessing 781 participants, found no statistically significant difference in the risk of death between IDS with chemotherapy and chemotherapy alone (hazard ratio (HR) = 0.80, 95% confidence interval (CI) 0.61 to 1.06). The percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may represent substantial heterogeneity (I² = 58%). Hence, we explored the sources of heterogeneity from the clinical factor of the expertise of the surgeon in the primary surgery, and subsequently performed subgroup meta‐analysis for OS based on this factor.
The conclusions above were not robust to subgroup analyses examining women who received surgery from general surgeons (or had less extensive primary surgery) separately from gynaecologic oncologists. Meta‐analysis of two trials (Redman 1994; Van der Burg 1995) assessing 357 women who received surgery from a general surgeon found that IDS with chemotherapy was associated with a statistically significant decrease in the risk of death compared with chemotherapy alone (HR = 0.68, 95% CI 0.53 to 0.87, I² = 0%).
Rose 2004, assessing 424 participants, found no statistically significant difference in the risk of death between IDS with chemotherapy and chemotherapy alone (HR = 0.99, 95% CI 0.79 to 1.24. Analysis 1.1).
1.1. Analysis.
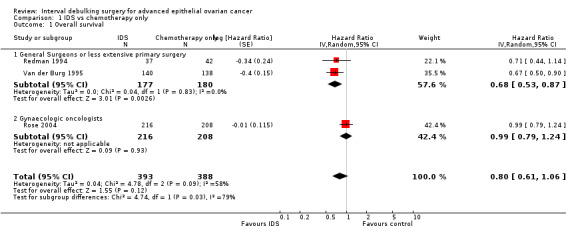
Comparison 1 IDS vs chemotherapy only, Outcome 1 Overall survival.
Progression‐free survival (PFS) (Analysis 1.2)
Meta‐analysis of two trials (Van der Burg 1995; Rose 2004), assessing 781 participants, found no statistically significant difference in the risk of disease progression between IDS with chemotherapy and chemotherapy alone (HR = 0.88, 95% CI 0.57 to 1.33; Analysis 1.2 ). The percentage of the variability in effect estimates that is due to heterogeneity rather than chance may represent considerable heterogeneity (I² = 83%).
1.2. Analysis.
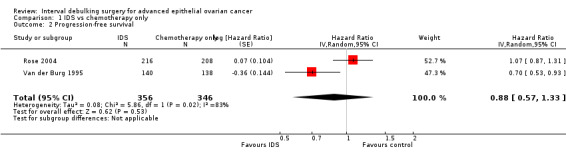
Comparison 1 IDS vs chemotherapy only, Outcome 2 Progression‐free survival.
Adverse events
Toxic reactions were the only adverse events which could be meta‐analysed. They were reported in both treatment groups in Redman 1994 and Van der Burg 1995. It was not possible to meta‐analyse other adverse events as they were not reported in sufficient detail for both treatment arms.
Toxic reactions to chemotherapy (Analysis 1.3)
Meta‐analysis of two trials (Redman 1994; Van der Burg 1995), assessing 357 participants, found no statistically significant difference in the risk of disease progression between IDS with chemotherapy and chemotherapy alone (HR = 1.19, 95% CI 0.53 to 2.66; Analysis 1.3). The percentage of the variability in effect estimates that is due to heterogeneity rather than to chance is insignificant (I² = 0%).
1.3. Analysis.
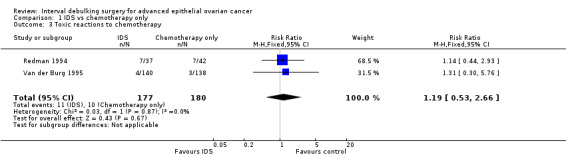
Comparison 1 IDS vs chemotherapy only, Outcome 3 Toxic reactions to chemotherapy.
The following adverse events were reported in Rose 2004:
Peripheral neuropathy of grade 2 or higher (Analysis 1.4)
Women who received IDS with chemotherapy for treatment of advanced epithelial ovarian cancer had a significantly higher risk of high grade peripheral neuropathy than women who received chemotherapy alone (risk ratio (RR) = 0.62, 95% CI 0.43 to 0.91. Analysis 1.4).
1.4. Analysis.
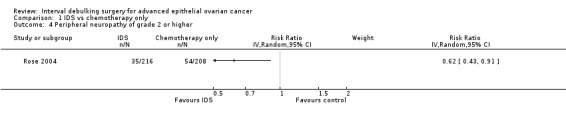
Comparison 1 IDS vs chemotherapy only, Outcome 4 Peripheral neuropathy of grade 2 or higher.
Grade 3 or 4 gastrointestinal adverse events (Analysis 1.5)
There was no statistically significant difference in the risk of a high grade gastrointestinal adverse event between IDS with chemotherapy and chemotherapy alone (RR = 1.81, 95% CI 0.78 to 4.17. Analysis 1.5).
1.5. Analysis.
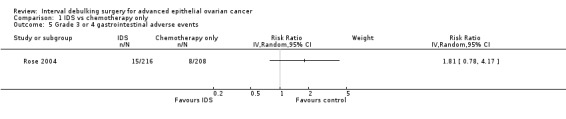
Comparison 1 IDS vs chemotherapy only, Outcome 5 Grade 3 or 4 gastrointestinal adverse events.
Grade 4 pulmonary adverse events (Analysis 1.6)
There was no statistically significant difference in the risk of a grade 4 pulmonary adverse event between IDS with chemotherapy and chemotherapy alone. There were only two observed events in the IDS group and no events in the chemotherapy only group. Analysis 1.6.
1.6. Analysis.
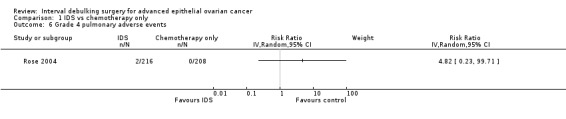
Comparison 1 IDS vs chemotherapy only, Outcome 6 Grade 4 pulmonary adverse events.
Grade 4 cardiovascular adverse events (Analysis 1.7)
There was no statistically significant difference in the risk of a grade 4 cardiovascular adverse event between IDS with chemotherapy and chemotherapy alone (RR = 2.89, 95% CI 0.30 to 27.55. Analysis 1.7).
1.7. Analysis.
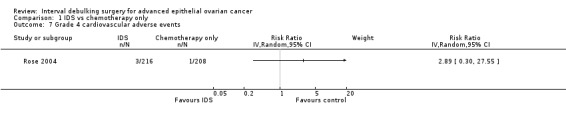
Comparison 1 IDS vs chemotherapy only, Outcome 7 Grade 4 cardiovascular adverse events.
Quality of life (QoL)
Only Rose 2004 evaluated QoL, which was subsequently reported by Wenzel 2005. At six months after starting treatment, significantly more women who had only chemotherapy experienced persistent numbness or tingling than those who had IDS (54% versus 38%; P = 0.01). Otherwise, QoL was not significantly different in the two treatment groups at any time point.
Discussion
Our systematic review includes three randomised controlled trials (RCTs), which evaluated 781 women out of 853 randomised, and a further RCT in abstract form which randomised 718 women, but it was unclear how many of these were evaluated. These four RCTs provide inconclusive evidence as to whether interval debulking surgery (IDS) improves or worsens the overall survival rate of patients. The random‐effects model showed substantial heterogeneity between the results of the three trials. Similarly, meta‐analysis of two RCTs which evaluated 702 women out of 767 randomised, provides inconclusive evidence about whether IDS improves or worsens progression‐free survival; again, there was substantial heterogeneity between the trials.
There are potential reasons for the inconclusive evidence so far. Firstly, the small number of included studies, evaluating 781 women, may not have had adequate statistical power to detect a small effect. Furthermore, the studies had different characteristics, which may explain the heterogeneity in their results.
A major difference between these trials was the expertise and/or the level of effort given by the surgeons performing the primary debulking surgical procedures in the participating institutions in each study, which may partly explain the heterogeneity between trials. The majority of operations in the Gynecological Oncology Group (GOG) trial were performed by gynaecologic oncologists or fellowship‐trained surgeons in various cancer centres (Rose 2004), while the surgical procedures in Redman 1994 were performed mostly by general surgeons or gynaecologists in various hospitals. In the European Organisation for Research and Treatment of Cancer (EORTC) trial, where the expertise of the surgeon was not specified (Van der Burg 1995) but was subsequently revealed in other publications (Kehoe 1995; Van der Burg 2003), the maximum effort to perform primary surgery was not attempted in all patients with different extents of debulking surgery. Since there is substantial evidence from many retrospective studies that the extent of optimal surgery affects the survival of patients with epithelial ovarian cancer (Griffiths 1975; Hoskin 1994; Bristow 2002), the maximum primary surgical efforts in the optimal surgical setting in the GOG trial might indicate that their primary surgery was sufficient and that subsequent surgical attempts would not further affect survival. Compared to Redman 1994 and Van der Burg 1995, in which primary surgery was performed in suboptimal settings without gynaecologic oncologists (Redman 1994), without maximum effort (with less extensive primary debulking surgery) resulting in a high proportion of large residual tumours (Kehoe 1995; Van der Burg 1995; Van der Burg 2003), so the secondary surgery (IDS) after the tumours were down‐sized by chemotherapy appeared worthwhile, although significant only in the EORTC trial. Our subgroup meta‐analysis confirmed that IDS had benefits in this particular subgroup of women. Nevertheless, this should be interpreted with caution because our subgroup analysis was based on only two trials (Redman 1994; Van der Burg 1995).
The second minor difference between the trials is the timing of randomisation. Redman 1994 was the only trial which randomised participants into two groups (to have or not to have IDS) at the start of the trial, and only 67% of those in the IDS group actually underwent surgery, because the remainder had disease progression or died before IDS. The other two larger trials randomised only the patients who showed some response to induction chemotherapy (Van der Burg 1995; Rose 2004); this resulted in a high percentage of the women (approximately 93% in both trials) who were randomised actually undergoing IDS. This difference may affect the results of each trial, based on an intention‐to‐treat analysis.
We explored the women's disease characteristics as another potential reason for different effects of IDS on survival outcomes: Redman 1994 and Rose 2004 did not show any advantage of IDS, while Van der Burg 1995 showed significant survival improvement with IDS. Redman 1994 evaluated only 79 of the total of 86 women included in the meta‐analysis of OS and, therefore had limited weight in the results of the meta‐analysis. The difference in outcomes from Rose 2004 and Van der Burg 1995 may be due to the different proportion of women who had a poor response to induction chemotherapy, and who generally had poorer prognoses than those who showed some response: approximately 52% of women in the GOG trial had residual diseases of more than 1 cm after induction chemotherapy (Rose 2004), compared to 44% in the EORTC trial (Van der Burg 1995). This might be interpreted as the GOG trial having a higher proportion of participants with more aggressive tumours who would not benefit from any treatment, even optimal IDS. However, this mechanism of tumour aggression and prognosis may not solely explain the women's ultimate outcome, because the women in the EORTC trial who had a tumour greater than 1 cm after induction chemotherapy but which was reduced to less than 1 cm had better survival rates than any other group of women in the trial. This might suggest that IDS may play some role in survival improvement.
To emphasize the importance of the sensitivity of response to chemotherapy before IDS, we would draw attention to the findings from the GOG and EORTC trials which showed that the women whose tumour masses were reduced by chemotherapy to less than 1 cm before the IDS (Van der Burg 1995; Rose 2004) had better survival than the other groups. This response to chemotherapy might be used as a selection criterion for the women who are most likely to gain survival benefit from an IDS procedure.
As we have mentioned in the results, meta‐analysis of adverse events was not possible due to different formats of presentation of data in each RCT. The only obvious advantage appears to be fewer neurologic complications in the IDS group than in the chemotherapy only group in the GOG trial (Rose 2004). However, this adverse effect may be related to a particular drug and might not be experienced in other settings using different chemotherapeutic regimens.
For the quality of life (QoL) comparison, which was only assessed by the GOG trial (Wenzel 2005), women in the chemotherapy only arm had a higher rate of neurotoxicity than did those in the IDS arm at one of the four time points at which they were assessed, despite a similar total dose of chemotherapy exposures. The authors ascribe this finding to intermission from chemotherapy exposure allowing a certain degree of recovery, but it may be a chance finding, as 48 tests of possible differences in QoL were made. Although other aspects of QoL were similar in the two groups of women at every time point, definitive conclusions cannot be drawn, because a significantly lower proportion of women in the IDS group completed the QoL questionnaires in the second assessment compared to those in the chemotherapy only group, and it is possible that this lower questionnaire completion rate may be associated with QoL.
Authors' conclusions
Implications for practice.
The heterogeneity of the results in our review precludes any definitive guidance or recommendations for clinical practice. Without strong evidence to support the superiority of interval debulking surgery (IDS) in combination with chemotherapy over conventional primary surgery and chemotherapy, a clinician may remain unconvinced of the benefit of IDS instead of aggressive primary surgery for a woman with advanced ovarian cancer. The choice of extensive primary surgery or upfront chemotherapy followed by IDS must be individualized to each patient. Since we found a benefit of IDS in the subgroup of women whose primary surgery had not been performed under optimal conditions by the oncologic surgeons or without maximum surgical effort, we suggest that IDS may improve patient survival in this setting. However, if the primary surgery has already been performed by the oncologic surgeons or with maximal surgical effort, IDS may not yield any further benefit for survival. Nevertheless, in a situation when there is evidence that primary surgery would be impossible or that morbidity from surgery would outweigh the benefit, induction chemotherapy followed by IDS may have a role to play.
Implications for research.
The theoretical benefit of IDS needs further well‐designed randomised controlled trials to generate evidence which may resolve the equivocal findings of this review.
These studies should focus on a comparison of current conventional primary surgery with adjuvant chemotherapy versus neoadjuvant chemotherapy (without any attempt to remove the bulk of tumours except a biopsy for histologic diagnosis) followed by IDS, and then by further chemotherapy.
The level and expertise of the surgeon or the effort in performing the primary surgery should be standardised as far as possible, in order to obtain the best surgical outcome. The particular subgroup of women with their specific disease characteristics should be a set criterion for IDS, e.g. patients with only small tumours after induction or neoadjuvant chemotherapy.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2016 | Amended | Additional reference amended. |
| 4 January 2016 | New search has been performed | Searches updated in June 2015. |
| 4 January 2016 | New citation required but conclusions have not changed | We did not identified any new studies for inclusion. |
| 4 January 2016 | Amended | CRG funding acknowledgment added. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 5 March 2013 | New search has been performed | Two RCTs were identified and subsequently excluded. |
| 5 March 2013 | New citation required but conclusions have not changed | The search was updated to June 2012. |
| 19 August 2010 | Amended | Searches re‐run in July 2009. Seventeen possible relevant articles were identified. Only two RCTs were found; one RCT of a Japanese study and the EORTC 55971 trial. However, the Japanese study referred to a study that was excluded in our primary review and EORTC 55971 trial allowed IDS in both arms at discretion of physician. |
| 10 February 2009 | Feedback has been incorporated | Feedback regarding new trial incorporated. |
| 10 November 2008 | New citation required but conclusions have not changed | Errors in reporting HR and RR corrected. Explanation regarding surgical expertise included. |
Acknowledgements
We gratefully acknowledge the support and advice of Dr. J Green, Dr. R Naik, Dr. S Kehoe, Ms S Pniauskas, Dr. HO Dickinson and Dr. Y Tanapat, We are also grateful to Anne Oestmann, Jane Hayes, Gail Quinn and Jo Morrison from the Cochrane Gynaecological Cancer Group.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. MEDLINE Ovid
1 exp Ovarian Neoplasms/ 2 (ovar* adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp. 3 1 or 2 4 exp Surgical Procedures, Operative/ 5 surg*.mp. 6 surgery.fs. 7 4 or 5 or 6 8 (interval or debulk* or cytoreduc* or secondary).mp. 9 3 and 7 and 8 10 randomized controlled trial.pt. 11 controlled clinical trial.pt. 12 randomized.ab. 13 randomly.ab. 14 trial.ab. 15 groups.ab. 16 10 or 11 or 12 or 13 or 14 or 15 17 9 and 16
key: mp = title, original title, abstract, name of substance word, subject heading word fs = floating subheading pt = publication type ab = abstract sh = subject heading
Appendix 2. EMBASE
1 exp ovary tumor/ 2 (ovar* adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp. 3 1 or 2 4 exp surgery/ 5 surg*.mp. 6 su.fs. 7 4 or 5 or 6 8 (interval or debulk* or cytoreduc* or secondary).mp. 9 7 and 8 10 exp cytoreductive surgery/ 11 9 or 10 12 3 and 11 13 exp controlled clinical trial/ 14 randomized.ab. 15 randomly.ab. 16 trial.ab. 17 groups.ab. 18 13 or 14 or 15 or 16 or 17 19 12 and 18
key: mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name fs = floating subheading ab = abstract
Appendix 3. CENTRAL
#1 MeSH descriptor Ovarian Neoplasms explode all trees #2 ovar* near/5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*) #3 (#1 OR #2) #4 MeSH descriptor Surgical Procedures, Operative explode all trees #5 surg* #6 Any MeSH descriptor with qualifier: SU #7 (#4 OR #5 OR #6) #8 interval or debulk* or cytoreduc* or secondary #9 (#3 AND #7 AND #8)
Data and analyses
Comparison 1. IDS vs chemotherapy only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | 781 | Hazard Ratio (Random, 95% CI) | 0.80 [0.61, 1.06] |
| 1.1 General Surgeons or less extensive primary surgery | 2 | 357 | Hazard Ratio (Random, 95% CI) | 0.68 [0.53, 0.87] |
| 1.2 Gynaecologic oncologists | 1 | 424 | Hazard Ratio (Random, 95% CI) | 0.99 [0.79, 1.24] |
| 2 Progression‐free survival | 2 | 702 | Hazard Ratio (Random, 95% CI) | 0.88 [0.57, 1.33] |
| 3 Toxic reactions to chemotherapy | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.53, 2.66] |
| 4 Peripheral neuropathy of grade 2 or higher | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5 Grade 3 or 4 gastrointestinal adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6 Grade 4 pulmonary adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7 Grade 4 cardiovascular adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Redman 1994.
| Methods | Study duration: April 1986 to February 1990. Type of trial: Multicentre RCT, intention‐to‐treat analysis. | |
| Participants | Stage II‐IV (except unresectable stage IV diseases).
Performance status ECOG: at least 2.
Histology proven, with exclusion of borderline tumour.
Primary surgery must be performed through an appropriate incision/ a maximal attempt to remove tumours.
Residual disease status > 2 cm.
No evidence of progressive disease, extraperitoneal tumour > 1 cm, or had excessive delay after induction chemotherapy and before randomisation. Baseline characteristics (N = 86): 24 women aged <50, 25 between 50 ‐ 60, and 30> 60 years. 50 women had performance status 0 or 1, 20 status 2 and 9 women had status 3 or 4. 6 women were diagnosed with FIGO stage IIB, 62 stage III and 11 had stage IV disease. 39 women had residual disease between 2 ‐ 5 cm, 29 between 5 ‐ 10 cm and 11 >10 cm. Histology type was as follows: Serous 32, mucinous 6, endometroid 13, clear cell 4, undifferentiated 6 and unspecified 20. Histology differentiation was as follows: Poor 37, moderate 27, well 6 and unspecified 9. |
|
| Interventions | Intervention: IDS: after 1 ‐ 4 cycles of induction chemotherapy consisting of IV cisplatin 75 mg/m² + cyclophosphamide 750 mg/m² or cisplatin 75 mg/m² + doxorubicin 50 mg/m² + bleomycin 50 mg/m² followed by escalated dose of cyclophosphamide (0.5 g/m²‐2.5 g/m²) up to 5 cycles. Chemotherapy cycles were repeated every 3 weeks. Control: No IDS: the same regimen of chemotherapy was given in a row every 3 weeks. | |
| Outcomes | Overall survival (OS). Perioperative complications: primary haemorrhage, blood transfusion, deep vein thrombosis, intestinal fistula, postoperative febrile morbidity, postoperative ileus. | |
| Notes | Randomised at entry (within 4 weeks after primary surgery). Number ineligible: 7 (primary surgery was not suboptimal). Only 9% of primary surgeons were gynaecologic oncologists. IDS performed by the primary surgeon. Response evaluation by physical examination, imaging studies (CA125 not used). OS was measured from the date of entry. Rate of complications was described only in the IDS group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation sheets were produced by the random permuted blocks methods utilising the NAG Fortran Library subroutine to generate random numbers" |
| Allocation concealment (selection bias) | Low risk | "These preprinted sheets were kept at the trial's office and medical personnel were given no access to them" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N analysed: 79/86 (92%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Rose 2004.
| Methods | Study duration: June 1994 to January 2001. Type of trial: Multicentre RCT, intention‐to‐treat basis. | |
| Participants | Stage III‐IV (malignant pleural effusion or a resected anterior abdominal wall tumour) (exclusion of stage IV after March 1996 after EORTC reported a greater benefit from secondary surgery after the exclusion of such patients from the analyses). Age < 75 years Performance status ACOG: 0 ‐ 2 with life expectancy of at least 8 weeks. Primary surgery performed within 6 weeks before chemotherapy. Primary surgery aimed to remove as much as possible. Histology proven, with central pathologic review. Residual disease status > 1 cm. Had no delay of chemotherapy treatment > 2 weeks. Baseline characteristics (N = 448): Median age 58.1 (range: 25.4 ‐ 81.6 years) in the IDS group and 57 (range: 27‐81.6 years) in the no IDS group. 166 women had GOG performance status 0, 227 had status 1 and 31 women had status 2. 400 women were diagnosed with FIGO stage III and 24 had stage IV disease. 297 women had measurable disease. 53 women had residual disease between 1 ‐ 2 cm, 183 between 2.1 ‐ 5 cm, 150 between 5.1 ‐ 10 cm and 38 >10 cm. Histology type was as follows: Serous 324, mucinous 3, endometroid 28, clear cell 7, mixed epithelial 37, adenocarcinoma (unspecified) 12 and undifferentiated or other 13. Histology grade was as follows: 1: 40, 2: 167, 3 or clear cell: 217 | |
| Interventions | Intervention: IDS: after 3 cycles of chemotherapy consisting of IV paclitaxel 135 mg/m² + cisplatin 75 mg/m² every 3 weeks. Three more cycles of the same chemotherapy regimen were given after IDS. No IDS: the same regimen of chemotherapy was given in a row every 3 weeks for 6 cycles. | |
| Outcomes | Overall survival. Progression‐free survival. Adverse effects: peripheral neuropathy, haematologic effects, gastrointestinal events, pulmonary events, cardiovascular events, and cause of death. Quality of life. | |
| Notes | Randomised after 3 cycles of chemotherapy. Number ineligible: 24 Majority of surgeons for primary and secondary surgery (IDS) were gynaecologic oncologists. Response evaluation by physical examination, imaging studies, and CA125. Consolidation chemotherapy was allowed. Overall and progression‐free survival were measured from the date of randomisation and also from the date of enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N analysed: 424/448 (95%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Van der Burg 1995.
| Methods | Study duration: March 1987 to May 1993. Type of trial: Multicentre RCT, intention‐to‐treat analysis. | |
| Participants | Stage IIB‐IV. Age of participants < 75 years. Performance status WHO: 0‐2. Primary surgery performed within 6 weeks before induction chemotherapy. Histology proven, with central pathologic review. Residual disease status > 1 cm. Have some response or stable disease after induction chemotherapy without evidences of progressive diseases or had contraindication to surgery. Baseline characteristics (N = 319): Median age was 59 years in both groups (range: 32 ‐ 74). 68 women had performance status 0, 99 status 1, and 33 status 2. 10 women were diagnosed with FIGO stage IIB, 146 stage III and 44 had stage IV disease. 10 women had residual disease between 1 ‐ 2 cm, 45 between 2 ‐ 5 cm, 44 between 5 ‐ 10cm, 60 >10 cm and 41 unknown but > 2 cm. Histology type was as follows: Serous 115, mucinous 12, endometroid 17, clear cell 5, and unclassified 51. Histology grade was as follows: 1: 17, 2: 59, 3: 105 and unknown: 9. | |
| Interventions | Intervention: IDS: after 3 cycles of induction chemotherapy composing of IV cyclophosphamide 750 mg/m² + IV cisplatin 75 mg/m² every 3 weeks. Three more cycles of the same chemotherapy regimen were given after IDS. No IDS: the same regimen of chemotherapy was given in a row every 3 weeks for 6 cycles. | |
| Outcomes | Overall survival. Progression‐free survival. Perioperative complications: bowel injury, urinary bladder injury, blood loss, postoperative fever, ileus, urinary tract infection, wound infection, deep vein thrombosis, lung embolism. Clinical response rate after 6 cycles of chemotherapy. | |
| Notes | Randomised after 3 cycles of induction chemotherapy at Central EORTC data centre, after stratification with a minimization technique to account for institution, performance status, and clinical response. Number ineligible: 4 Response evaluation by WHO response criteria. Consolidation chemotherapy after 6 cycles was allowed based on institutions policy. Survivals were measured from the first date of chemotherapy (after enrolment). Rate of complications was detailed only in the IDS group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done ... after stratification with a minimization technique to account for institution, performance status, and clinical response" |
| Allocation concealment (selection bias) | Low risk | "Randomization was done centrally at the EORTC Data Center" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N analysed: 278/319 (87%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angioli 2006 | This study was evaluated as a non‐comparable controlled clinical trial. NAC followed by IDS was selectively given to the women based on initial laparoscopic findings of inoperability. |
| Chan 2003 | This study was evaluated as an uncontrolled clinical trial. NAC was given to the women without a control group. The women were selected by their inoperability based on CT scan without primary operation being attempted. Either histology or only cytology was allowed for a pathologic diagnosis of ovarian cancer. |
| Dutta 2005 | This was an RCT of ovarian cancer patients to have either 3 cycles of NAC followed by IDS and further chemotherapy versus 6 cycles of NAC before surgery. Main objective was to study proapoptotic and antiapoptotic proteins in ovarian cancer tissue without survival outcome evaluation. |
| Evdokimova 1982 | This was an RCT comparing survival of women with ovarian carcinoma who had NAC/IDS versus conventional treatment of surgery then chemotherapy. However, those without ascites were selected to have primary surgery followed by chemotherapy while those with ascites had either NAC/IDS or conventional treatment. |
| Fuso 2006 | This uncontrolled prospective study was to assess the feasibility of triple chemotherapy composed of gemcitabine, carboplatin, and paclitaxel as first‐line drugs in advanced ovarian cancer. The women were selectively assigned to have two different types of treatment. After laparoscopic biopsy in all participants, those who were judged to be operable underwent primary surgery followed by chemotherapy, while the inoperable women were given NAC followed by IDS. However, few who had primary surgery also had IDS. |
| Giannopoulos 2006 | This study was evaluated as a non‐comparable controlled clinical trial. NAC and IDS was selectively given to the women who were deemed to be inoperable based on CT scan and initial laparoscopic findings. Either histology or cytology was allowed for a pathologic diagnosis of ovarian cancer. |
| Ikeba 2004 | This study was evaluated as an uncontrolled clinical trial. NAC was given to all, without a control group. |
| Kuhn 2001 | This study was evaluated as a non‐comparable controlled clinical trial. NAC was selectively given to women in poor general health for primary surgery. The control group consisted of those who did not agree to the study protocol or were ineligible for treatment for psychological reasons. |
| Kumar 2009 | This RCT allowed either histologic or cytologic diagnosis. NAC was given to the study arm without an attempt to perform a PDS. |
| Lee 2006 | This study was evaluated as a non‐comparable controlled clinical trial. NAC was selectively given to those who agreed to undergo the NAC treatment protocol. NAC cycles ranged from three to six. Some women had secondary surgery which was not in an interval setting. |
| Liu 2004 | This RCT was considered as ineligible because NAC was given only for one cycle via intra‐arterial route before the IDS. |
| Madhuri 2014 | RCT comparing the efficacy of neutral argon plasma to standard surgery during PDS or IDS |
| Matulonis 2009 | This prospective study was a non‐randomised study. The women aged 75 ‐ 86 years with stage II ‐ IV disease to have PDS followed by adjuvant chemotherapy or to have NAC if they had poor performance status or inoperable metastatic disease. All five women who had NAC were not able to undergo surgery. |
| Onda 2009 | This was a prospective single arm study to evaluate the role of diagnostic laparoscopy in those with a diagnosis of Mullerian carcinoma. |
| Onda 2014 | This RCT compared PDS or NAC then IDS followed more cycles of chemotherapy. IDS was performed in both arms: 31.3% in the PDS arm and 86.8% in the IDS arm. |
| Park‐Simon 2006 | This RCT compared survivals of ovarian cancer patients who had ascites > 500 cc who were given NAC for either two versus three cycles, but followed by IDS in both arms. |
| Polcher 2009 | This RCT randomised women with ascites > 500 cc to have two or three cycles of NAC without an attempt to do PDS. |
| Recchia 2001 | This study was evaluated as an uncontrolled clinical trial. NAC was given to all without a control group. Either histology or only cytology was allowed for a pathologic diagnosis of ovarian cancer. |
| Solomon 1988 | This study was presented in conjunction with the prior RCT comparing combination versus sequential chlorambucil and cisplatin. IDS in this study was selectively performed only in the subset of women who had not been debulked at primary surgery. Second‐look surgery or surgical re‐exploration were also performed on those with clinical complete remission or on those with complications requiring surgery, respectively. |
| Tiersten 2009 | This was a prospective single arm study when the women must have had no aggressive PDS before receiving intraperitoneal chemotherapy followed by surgery. |
| Vergote 2010 | This RCT compared the role of PDS followed by adjuvant chemotherapy in the control arm with NAC before IDS followed by additional chemotherapy in the study arm. However, IDS was also allowed in the PDS arm too, at the discretion of the physician. Furthermore, either histologic or cytologic diagnosis (with tumour marker and imaging criteria to exclude cancers of other non‐Mullerian origins) was allowed. |
IDS: interval debulking surgery NAC: neoadjuvant chemotherapy RCT: randomised controlled trial
Differences between protocol and review
None of the trials reported continuous outcomes such as quality of life (QoL), or had multiple treatment groups. We had originally specified the following in various sections of the protocol.
For continuous outcomes (e.g. QoL measures), we will extract the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (if trials measured outcomes on the same scale) or standardised mean differences (if trials measured outcomes on different scales) between treatment arms and its standard error.
For continuous outcomes, we will use the mean difference between treatment arms.
For continuous outcomes, the mean differences between the treatment arms at the end of follow‐up will be pooled if all trials measured the outcome on the same scale, otherwise standardised mean differences will be pooled.
If any trials have multiple treatment groups, the ‘shared’ comparison group will be divided into the number of treatment groups and comparisons between each treatment group and the split comparison group will be treated as independent comparisons.
Where possible, indirect comparisons, using the methods of Bucher 1997 will be used to compare competing interventions that have not been compared directly with each other.
We had planned to compute funnel plots, but this was not possible due to an insufficient number of included trials in the review. The protocol stated the following:
Funnel plots corresponding to meta‐analysis of the primary outcome will be examined for evidence of small study effects. If such evidence exists, publication bias and other possible explanations will be considered. If funnel plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random‐effects model, sensitivity analyses will be performed using fixed‐effect models.
We planned to perform sensitivity analyses to evaluate whether the pooled effect sizes were robust across components of methodological quality. However, only one trial reported adequate concealment of allocation (Redman 1994) and other components of quality were similar across studies, so these sensitivity analyses were not performed.
Contributions of authors
ST and SM: protocol development, searching for and determining the relevance of trials for the review, assessing their methodological quality, extracting data, and review development. ML: protocol development, data analysis, and review development. PL: protocol development, providing methodological advice throughout the review, arbitration over any disagreements, advising on the content and presentation of the review. AB: methodological and statistical support.
Sources of support
Internal sources
Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Thailand.
Faculty of Medicine, Khon Kaen University, Thailand.
Faculty of Public Health, Khon Kaen University, Thailand.
External sources
Thailand Research Fund (Outstanding Research Professor Award), Thailand.
Cochrane Thailand, Thailand.
Declarations of interest
There is no known conflict of interest among the authors of this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Redman 1994 {published data only}
- Redman CW, Warwick J, Luesley DM, Varma R, Lawton FG, Blackledge GR. Intervention debulking surgery in advanced epithelial ovarian cancer. British Journal of Obstetrics and Gynaecology 1994;101:142‐6. [DOI] [PubMed] [Google Scholar]
- Varma R, Blackledge G, Redman C, Luesley D, Chan KK, Mould J. A randomised trial of intervention debulking surgery and the duration of cis‐platinum combination chemotherapy in advanced epithelial ovarian cancer (EOC). Annals of Oncology. 1990; Vol. 1 (Suppl 9):4.
Rose 2004 {published data only}
- Rose PG, Nerenston S, Brady MF, Clarke Pearson D, Olt G, Rubin SC, et al. Operative morbidity following primary and interval debulking surgery for advanced ovarian cancer. Proceedings of the American Society of Clinical Oncology. 2004; Vol. 23:450.
- Rose PG, Nerenstone S, Brady M, Clarke Pearson D, Olt G, Rubin SC, et al. A phase III randomised study of interval secondary cytoreduction in patients with advanced stage ovarian carcinoma with suboptimal residual disease: a Gynecologic Oncology Group study. Proceedings of the American Society of Clinical Oncology. 2002; Vol. 21 (Pt 1):201a.
- Rose PG, Nerenstone S, Brady MF, Clarke‐Pearson D, Olt G, Rubin SC, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. The New England Journal of Medicine 2004;351:2489‐97. [DOI] [PubMed] [Google Scholar]
Van der Burg 1995 {published data only}
- Burg ME, Coens C, Lent M, Kobierska A, Colombo N, Favalli G, et al. After ten years follow‐up interval debulking surgery remains a significant prognostic factor for survival and progression free survival for advanced ovarian cancer: the EORTC Gynaecological Cancer Group study. International Journal of Gynecological Cancer. 2004; Vol. 14 (Suppl 1):3.
- Burg ME, Lent M, Kobierska A, Colombo N, Favalli G, Lacave AJ, et al. Intervention debulking surgery (IDS) does improve survival in advanced epithelial ovarian cancer (EOC); an EORTC gynecological cancer cooperative group (GCCG) study. Proceedings of the American Society of Clinical Oncology. 1993; Vol. 12:258.
- Burg ME, Lent M, Kobierska A, Colombo N, Favalli G, Lacave AJ, et al. Intervention debulking surgery (IDS) does increase survival in advanced epithelial ovarian cancer (OC) ‐ an EORTC Gynecological Cancer Cooperative Group (GCCG) study. European Journal of Cancer. 1993; Vol. 29 (Suppl 6):S132.
- Burg MEL, Coens C, Lent M, Kobierska A, Colombo M, Favalli G, et al. The survival benefit of interval debulking surgery (IDS) in advanced ovarian cancer is maintained during ten years; the EORTC GCG 55865 study. International Journal of Gynecologic Cancer. 2005; Vol. 15 (supplement 2):79.
- Burg MEL, Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the EORTC. The New England Journal of Medicine 1995;332(10):629‐34. [DOI] [PubMed] [Google Scholar]
- Burg MEL, Lent M, Buyse M, Kobierska A, Maggioni A, Favalli G, et al. The role of intervention debulking surgery in advanced epithelial ovarian cancer: An EORTC Gynecological Cancer Cooperative Group study. International Journal of Gynecological Cancer 1996;6 (5 Suppl 1):30‐8. [Google Scholar]
- Burg MEL, Lent M, Kobierska A, Colombo N, Favalli G, Lacave AJ, et al. Secondary tumor debulking itself does not increase survival in advanced epithelial ovarian cancer (EOC); an EORTC Gynecological Cancer Cooperative (GCCG) study. International Journal of Gynecological Cancer. 1993; Vol. 3 (Suppl 1):52.
- Burg MEL, Lent M, Kobierska A, Pitelli MR, Favalli G, Lacave AJ, et al. After 6 years follow‐up intervention debulking surgery remains an independent prognostic factor for survival in advanced epithelial ovarian cancer: an EORTC Gynecological Cancer Cooperative Group study. International Journal of Gynecological Cancer. 1997; Vol. 7 (Suppl 2):28.
References to studies excluded from this review
Angioli 2006 {published data only}
- Angioli R, Palaia I, Zullo MA, Muzii L, Manci N, Calcagno M, et al. Diagnostic open laparoscopy in the management of advanced ovarian cancer. Gynecologic Oncology 2006;100:455‐61. [DOI] [PubMed] [Google Scholar]
Chan 2003 {published data only}
- Chan YM, Ng TY, Ngan HY, Wong LC. Quality of life in women treated with neoadjuvant chemotherapy for advanced ovarian cancer: a prospective longitudinal study. Gynecologic Oncology 2003;88:9‐16. [DOI] [PubMed] [Google Scholar]
Dutta 2005 {published data only}
- Dutta T, Sharma H, Kumar L, Dinda AK, Kumar S, Bhatla N, et al. Neoadjuvant chemotherapy for epithelial ovarian cancer‐‐role of apoptosis. Cancer Chemotherapy and Pharmacology 2005;56(4):427‐35. [DOI] [PubMed] [Google Scholar]
Evdokimova 1982 {published data only}
- Evdokimova NI, Grigorova TM. Comparative study of 2 combined treatment regimens in stage‐III to ‐IV ovarian cancer. Voprosy onkologii 1982;28(7):28‐34. [PubMed] [Google Scholar]
Fuso 2006 {published data only}
- Fuso L, Amant F, Neven P, Berteloot P, Vergote I. Gemcitabine‐carboplatin‐paclitaxel combination as first‐line therapy in advanced ovarian carcinoma: A single institution phase II study in 24 patients. International Journal of Gynecological Cancer 2006;16 (Suppl 1):60‐7. [DOI] [PubMed] [Google Scholar]
Giannopoulos 2006 {published data only}
- Giannopoulos T, Butler‐Manuel S, Taylor A, Ngeh N, Thomas H. Clinical outcomes of neoadjuvant chemotherapy and primary debulking surgery in advanced ovarian carcinoma. European Journal Gynaecologic Oncology 2006;27:25‐8. [PubMed] [Google Scholar]
Ikeba 2004 {published data only}
- Ikeba K, Okubo M, Takeda S, Kinoshita K, Maeda H. Five‐year results of cyclic semi‐high dose neoadjuvant chemotherapy supported by autologous peripheral blood stem‐cell transplantation in patients with advanced ovarian cancer. International Journal Clinical Oncology 2004;9:113‐9. [DOI] [PubMed] [Google Scholar]
Kuhn 2001 {published data only}
- Kuhn W, Rutke S, Spathe K, Schmalfeldt B, Florack G, Hundelshausen B, et al. Neoadjuvant chemotherapy followed by tumor debulking prolongs survival for patients with poor prognosis. Cancer 2001;92:2585‐91. [DOI] [PubMed] [Google Scholar]
Kumar 2009 {published data only}
- Janga D, Kumar L, Kumar S, Shukla NK, Thulkar S, Singh R. Neoadjuvant chemotherapy (CT) followed by debulking surgery vs upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma (EOC): a prospective, randomized study. Proceedings of the American Society of Clinical Oncology 2003. 2007; Vol. 22:487:Abstract 1957.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Thulkar S, Vijayaraghavan M, et al. Neoadjuvant chemotherapy followed by interval debulking surgery versus upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma: a prospective randomized study ‐ interim results. Proceeding of the ASCO Annual Meeting 2007, Part I. 2007; Vol. 28, issue 18 (Suppl):Abstract 5531.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Thulkar S, Shukla NK . Neo‐adjuvant chemotherapy in advanced epithelial ovarian cancer (eoc): A prospective, randomized study. Indian Journal of Medical and Paediatric Oncology 2009;30(1):15. [NCT00715286] [Google Scholar]
Lee 2006 {published data only}
- Lee SJ, Kim BG, Lee JW, Park CS, Lee JH, Bae DS. Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epithelial ovarian cancer who are inadequate for optimum primary surgery. Journal of Obstetric and Gynaecological Research 2006;32:99‐106. [DOI] [PubMed] [Google Scholar]
Liu 2004 {published data only}
- Liu EL, Mi RR. Neoadjuvant intraarterial chemotherapy and embolization in treatment of advanced ovarian epithelial carcinoma. Chinese Medical Journal 2004;117:1547‐51. [PubMed] [Google Scholar]
Madhuri 2014 {published data only}
- Madhuri TK, Ashbourne W, Butler‐Manuel SA, Tailor A. RCT to evaluate utility and efficacy of neutral argon plasma as new technology in achieving complete cytoreduction of advanced epithelial ovarian carcinoma‐initial feasibility study. 15th Biennial Meeting of the International Gynecologic Cancer Society Melbourne, VIC Australia.. 2014; Vol. 245.
Matulonis 2009 {published data only}
- Matulonis UA, Krag KJ, Krasner CN, Atkinson T, Horowitz NS, Lee H, et al. Phase II prospective study of paclitaxel and carboplatin in older patients with newly diagnosed Mullerian tumors. Gynecologic Oncology 2009;112(2):394‐399. [DOI] [PubMed] [Google Scholar]
Onda 2009 {published data only}
- Onda T, Kobayashi H, Nakanishi T, Hatae M, Iwasaka T, Konishi I, et al. Feasibility study of neoadjuvant chemotherapy followed by interval debulking surgery for stage III/IV ovarian, tubal, and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0206. Gynecologic Oncology 2009;113(1):57‐62. [DOI] [PubMed] [Google Scholar]
Onda 2014 {published data only}
- Onda T, Yoshikawa H, Shibat a T, Nakamura K, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in phase III randomized trial: JCOG0602. Journal of Clinical Oncology. 2014; Vol. 32:suppl; abstr 5508.
Park‐Simon 2006 {published data only}
- Park‐Simon T, Jänicke F, Ortmann O, Hilfrich J, Breitbach G, Höss C, et al. A randomized multicenter phase II study on neoadjuvant chemotherapy with carboplatin and docetaxel in advanced ovarian carcinomas (PRIMOVAR). Journal of Clinical Oncology: 2006 ASCO Annual meeting proceedings 2006;24(18S (June 20 Supplement)):Abstract 15025. [Google Scholar]
Polcher 2009 {published data only}
- Polcher M, Mahner S, Ortmann O, Hilfrich J, Diedrich K, Breitbach GP, et al. Neoadjuvant chemotherapy with carboplatin and docetaxel in advanced ovarian cancer‐‐a prospective multicenter phase II trial (PRIMOVAR). Oncology Reports 2009;22(3):605‐613. [DOI] [PubMed] [Google Scholar]
Recchia 2001 {published data only}
- Recchia F, Filippis S, Rosselli M, Saggio G, Carta G, Rea S. Primary chemotherapy in stage IV ovarian cancer. A prospective phase II study. European Journal of Gynaecologic Oncology 2001;22:287‐91. [PubMed] [Google Scholar]
Solomon 1988 {published data only}
- Solomon J. The role of surgical reexploration following chemotherapy in ovarian cancer. Australian and New Zealand Journal of Obstetrics and Gynaecology 1988;28:121‐6. [DOI] [PubMed] [Google Scholar]
Tiersten 2009 {published data only}
- Tiersten AD, Liu PY, Smith HO, Wilczynski SP, Robinson WR III, Markman M, et al. Phase II evaluation of neoadjuvant chemotherapy and debulking followed by intraperitoneal chemotherapy in women with stage III and IV epithelail ovarian, fallopian tube or primary peritoneal cancer: Soutwest Oncology Group Study S0009. Gynecologic Oncology 2009;112:444‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergote 2010 {published data only}
- EORTC 55971. A randomized phase III study comparing upfront debulking surgery vs neo‐adjuvant chemotherapy in patients with stage IIIc or IV epithelial ovarian carcinoma. IGCS 12th Biennial Meeting. 2008.
- Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. European Organization for Research and Treatment of Cancer‐Gynaecological Cancer Group, NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New England Journal of Medicine 2010;363(10):943‐53. [DOI] [PubMed] [Google Scholar]
Additional references
Ansquer 2001
- Ansquer Y, Leblanc E, Clough K, Morice P, Dauplat J, Mathevet P, et al. Neoadjuvant chemotherapy for unresectable ovarian carcinoma. Cancer 2001;91:2329‐34. [PubMed] [Google Scholar]
Bristow 2002
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta‐analysis. Journal of Clinical Oncology 2002;20:1248‐59. [DOI] [PubMed] [Google Scholar]
Bristow 2006
- Bristow RE, Chi DS. Platinum‐based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta‐analysis. Gynecologic Oncology 2006;103(3):1070‐6. [DOI] [PubMed] [Google Scholar]
Bristow 2007
- Bristow RE, Eisenhauer EL, Santillan A, Chi DS. Delaying the primary surgical effort for advanced ovarian cancer: a systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecologic Oncology 2007;104(2):480‐90. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). BMJ Publication Group, London, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Elattar 2011
- Elattar A, Bryant A, Winter‐Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD007565.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elit 1995
- Elit A, Carey M. The role of interval debulking surgery in advanced ovarian cancer: a meta‐analysis. International Journal of Gynecological Cancer. 1995; Vol. 5 (Suppl 1):37 (abstract).
Fanfani 2003
- Fanfani F, Ferrandina G, Corrado G, Fagotti A, Vito Zakut H, Mancuso S, et al. Impact of interval debulking surgery on clinical outcome in primary unresectable FIGO stage IIIc ovarian cancer patients. Oncology 2003;65:316‐22. [DOI] [PubMed] [Google Scholar]
Griffiths 1975
- Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Journal of the National Cancer Institute Monographs 1975;42:101‐4. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoskin 1994
- Hoskins WJ, McGuire WP, Brady MF, Homsley HD, Creasman WT, Berman M, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. American Journal of Obstetrics and Gynecology 1994;170:974‐80. [DOI] [PubMed] [Google Scholar]
Jacob 1991
- Jacob JH, Gerhenson DM, Morris M, Copeland LJ, Burke TW, Wharton JT. Neoadjuvant chemotherapy and interval debulking for advanced epithelial ovarian cancer. Gynecologic Oncology 1991;42:146‐50. [DOI] [PubMed] [Google Scholar]
Kayikçioglu 2001
- Kayikçioglu F, Köse MF, Boran N, Caliskan E, Tulunay G. Neoadjuvant chemotherapy or primary surgery in advanced ovarian carcinoma.. International Journal of Gynecological Cancer 2001;11(6):446‐70. [DOI] [PubMed] [Google Scholar]
Kehoe 1995
- Kehoe S, Shafi M, Luesley D, Neijt JP, Cannistra SA, Burg MEL, et al. Interval cytoreduction in ovarian cancer. New England Journal of Medicine 1995;333:254‐5. [PubMed] [Google Scholar]
Lawton 1989
- Lawton FG, Redman CW, Luesley DM, Chan KK, Blackledge G. Neoadjuvant (cytoreductive) chemotherapy combined with intervention debulking surgery in advanced, unresected epithelial ovarian cancer. Obstetrics and Gynecology 1989;73:61‐5. [PubMed] [Google Scholar]
Loizzi 2005
- Loizzi V, Cormio G, Resta L, Rossi CA, Gilio AR, Cuccovillo A, et al. Neoadjuvant chemotherapy in advanced ovarian cancer: a case‐control study. International Journal of Gynecological Cancer 2005;15:217‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mazzeo 2003
- Mazzeo F, Berliere M, Kerger J, Squifflet J, Duck L, D'Hondt V, et al. Neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy in patients with primarily unresectable, advanced‐stage ovarian cancer. Gynecologic Oncology 2003;90:163‐9. [DOI] [PubMed] [Google Scholar]
Morice 2003
- Morice P, Dubernard G, Rey A, Atallah D, Pautier P, Pomel C, et al. Results of interval debulking surgery compared with primary debulking surgery in advanced stage ovarian cancer. Journal of the American College of Surgeons 2003;197:955‐63. [DOI] [PubMed] [Google Scholar]
Morrison 2012
- Morrison J, Haldar K, Kehoe S, Lawrie TA. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD005343.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Schwartz 1999
- Schwartz PE, Rutherford TJ, Chambers JT, Kohorn EI, Thiel RP. Neoadjuvant chemotherapy for advanced ovarian cancer:long term survival. Gynecologic Oncology 1999;72:93‐9. [DOI] [PubMed] [Google Scholar]
Shibata 2003
- Shibata K, Kikkawa F, Mika M, Suzuki Y, Kajiyama H, Ino K, et al. Neoadjuvant chemotherapy for FIGO stage III or IV ovarian cancer: Survival benefit and prognostic factors. International Journal of Gynecological Cancer 2003;13:587‐92. [DOI] [PubMed] [Google Scholar]
Surwit 1996
- Surwit E, Childers J, Atlas I, Nour M, Hatch K, Hallum A, et al. Neoadjuvant chemotherapy for advanced ovarian cancer. International Journal of Gynecological Cancer 1996;6:356‐61. [Google Scholar]
Torre 2015
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics 2012. CA: a Cancer Journal for Clinicians 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
Van der Burg 2003
- Burg ME, Vergote I, Gynecological Cancer Group of the EORTC. The role of interval debulking surgery in ovarian cancer. Current Oncology Reports 2003;5:473‐81. [DOI] [PubMed] [Google Scholar]
Van der Burg 2005
- Burg MEL, Coens C, Lent M, Kobierska A, Colombo M, Favalli G, et al. The survival benefit of interval debulking surgery (IDS) in advanced ovarian cancer is maintained during ten years; the EORTC GCG 55865 study. International Journal of Gynecologic Cancer. 2005; Vol. 15 (supplement 2):79.
Vergote 1998
- Vergote I, Wever I, Tjalma W, Gramberen M, Decloedt J, Dam P. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecologic Oncology 1998;71:431‐6. [DOI] [PubMed] [Google Scholar]
Wenzel 2005
- Wenzel L, Huang HQ, Monk BJ, Rose PG, Cella D. Quality‐of‐life comparisons in a randomized trial of interval secondary cytoreduction in advanced ovarian carcinoma: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2005;23:5605‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]


