Abstract
As progress is made toward elimination of measles, the laboratory confirmation of measles becomes increasingly important. However, both false-positive and false-negative results can occur with the routinely used indirect measles immunoglobulin M (IgM) serology tests. The measles IgM capture assay is considered to be more specific, and therefore, its use is indicated for confirmatory testing, but its relative performance has not been fully assessed. Four commercial indirect measles IgM serology test kits (the Behring, Clark, Gull, and PanBio assays) and a commercial IgM capture assay (the Light Diagnostics assay) were evaluated for their abilities to detect measles virus-specific IgM antibody with a total of 308 serum samples from patients involved in a measles outbreak and with confirmed cases of measles and 454 samples from subjects without measles. The Centers for Disease Control and Prevention (CDC) IgM capture assay was also used in a part of the evaluation. Among the indirect assays, the overall sensitivities ranged from 82.8% (Clark assay) to 88.6% (Behring assay) and specificity ranged from 86.6% (PanBio assay) to 99.6% (Gull assay). These rates were 92.2 and 86.6%, respectively, for the Light Diagnostics capture assay and 87.0 and 94.8%, respectively, for the CDC capture assay. While the Light Diagnostics capture assay had the best detection rate (80%) with the acute-phase samples compared with those for the rest of the tests (CDC capture assay, 77%; Behring assay, 70%; Gull assay, 69%; PanBio assay, 58%; and Clark assay, 57%), all tests showed a significantly improved sensitivity in the range of 92% (Clark and PanBio assays) to 97% (Light Diagnostics and CDC capture assays) with the convalescent-phase samples, as expected. The best seropositivity rates (in the range of 92 to 100%) were observed with samples collected 6 to 14 days after the onset of symptoms. The Gull assay showed the highest positive predictive value (99.6%), followed by the Behring assay (97.8%) and the CDC capture assay (96.1%). Overall, the Gull and Behring assays were found to be as good as or better than the capture assays. In conclusion, laboratory diagnosis of measles based on IgM serology varies depending on the timing of specimen collection and the test used, and the case for the use of the IgM capture assay as the confirmatory test appears to be uncertain.
While the number of cases and deaths attributed to measles worldwide has declined substantially over the past two decades, measles remains one of the leading causes of childhood mortality in developing countries (4, 5, 21). Even countries that have achieved high levels of measles vaccination coverage have frequently witnessed large outbreaks of measles (1, 13, 17). However, with the implementation of new measles vaccination strategies, transmission of indigenous measles has been interrupted in the Americas and the United Kingdom (4–6, 9). Several other countries in Europe have either eliminated measles or are close to doing so (22). Following the success of global polio eradication strategies, there is now consensus that global measles eradication is technically feasible (5, 6). The Pan American Health Organization has targeted measles to be eliminated from the western hemisphere by the year 2000 (4). The European Advisory Group on Immunization for the World Health Organization (European Region) has recommended that measles be eliminated from Europe by the year 2007 (5). Accordingly, the current vaccination strategies in these regions aim to interrupt all chains of transmission and intensify surveillance for suspected cases of measles (4, 5, 7). As a part of this surveillance, recent consensus conferences on measles eradication have recommended that all isolated cases of measles and at least one case in each chain of transmission should be confirmed by laboratory tests (4, 5).
Measles-specific immunoglobulin M (IgM) serology is the standard test for the rapid laboratory diagnosis of measles, and IgM testing is now almost exclusively performed with commercial enzyme immunoassay (EIA) kits. These assays require the removal of IgG antibodies and rheumatoid factor through a pretreatment step to ensure optimal performance. Regardless, these assays can lead to both false-positive and false-negative results (10; S. A. Jenkerson, M. Beller, J. P. Middaugh, and D. D. Erdman, Letter, N. Engl. J. Med. 332:1103–1104, 1995). A measles IgM capture assay developed by the Centers for Disease Control and Prevention (CDC) does not require the removal of IgG antibodies and is considered to be more specific than the indirect EIAs for detection of measles IgM antibodies (8, 10, 11). As a result, the CDC capture assay has been recommended as the reference test for the laboratory confirmation of measles (4, 5, 10). Recently, a commercial version of the CDC measles IgM capture EIA has been developed (Light Diagnostics, Temecula, Calif.).
We evaluated the performance characteristics of four commercial measles IgM EIA kits which use the indirect format as well as those of the Light Diagnostics IgM capture EIA and the CDC IgM capture assay. The four commercial indirect EIA kits evaluated were those of Behring Enzygnost (Marburg, Germany), Clark Laboratories, Inc. (Jamestown, N.Y.), Gull Laboratories, Inc. (Salt Lake City, Utah), and PanBio (East Brisbane, Australia). We used three test panels comprising a total of 762 serum samples from patients involved in measles outbreaks and with confirmed cases of measles and subjects without measles for the evaluation.
MATERIALS AND METHODS
Test panel.
Three panels of sera were used for the study; two panels contained sera from patients involved in measles outbreaks (positive panels) and one contained sera from subjects without measles and included potentially troublesome specimens which were positive for other serological markers (negative panel). Positive panel I comprised single serum samples obtained from 108 patients who had clinically confirmed cases of measles and who were involved in the outbreaks in Ontario (1996), British Columbia (1997), and Newfoundland (1997), Canada. All 108 patients were school-aged children, and their symptoms met the measles clinical case definition (3, 20); 5 patients had cultured-confirmed cases of measles. The blood samples were obtained either at the time of the rash illness or within 2 weeks after rash onset. Positive panel II comprised paired acute-phase and convalescent-phase serum samples obtained from 100 patients with clinically confirmed cases of measles during a major measles outbreak in Quebec, Canada, in 1989 (16). The so-called acute-phase samples collected in this outbreak were not necessarily taken during the acute stage; rather, these represented the first specimen. All 100 patients showed at least a fourfold rise in measles antibody titers between the first and second serum samples by the complement fixation (CF) test and/or by the plaque reduction neutralization (PRN) test. The date of rash onset was not available for all 100 patients. When this was not available, the date of the first reported symptom (mostly fever) was used to calculate the time interval between the onset of symptoms and phlebotomy (16; G. Ozanne, personal communication). This could be determined for 60 of the 100 patients, as the date of specimen collection was not available for the remainder. The mean interval between the onset of symptoms and collection of the first specimens was 4.1 days (range, 0 to 17 days; median, 4 days). The corresponding interval for the collection of the second specimens was 18.2 days (range, 6 to 34 days; median, 18 days).
The negative panel comprised a total of 454 serum samples and included the following. Sixty-eight preimmunization serum samples from healthy 1-year-old children who had no history of measles and who tested negative for measles antibody by the PRN test, 47 serum samples from healthy adolescents and adults who had no known recent exposure to measles and who tested negative for measles antibody by the PRN test, 68 serum samples from patients who were suffering from inflammatory and autoimmune conditions such as rheumatoid arthritis, lupus, and infectious mononucleosis and who tested negative for measles antibody by the PRN test, and a total of 271 serum samples positive for a variety of other serological markers (parvovirus IgM, n = 144; Epstein-Barr virus viral capsid antigen IgM, n = 40; rubella IgM, n = 57; Mycoplasma pneumoniae IgM, n = 15; human herpesvirus 6, n = 5; cytomegalovirus IgM, n = 6; Chlamydia pneumoniae IgM, n = 2; antistreptolysin O, n = 2). Parvovirus IgM- and rubella IgM-positive serum samples were obtained from patients involved outbreaks in three Canadian provinces.
Laboratory test procedures.
All commercial indirect measles IgM EIA kits (the Behring Enzygnost, Clark, Gull, and PanBio assays) and Light Diagnostics IgM capture EIA were purchased from the respective manufacturers, the tests were carried out at the Newfoundland Public Health Laboratory, and the test results were interpreted according to the manufacturers' instructions. The CDC IgM capture assay was performed at the Laboratory Centre for Disease Control, Winnipeg, Manitoba, Canada, in accordance with the method developed at CDC (10, 11). The PRN test was carried out as an additional parameter of the present evaluation to substantiate or clarify the results of the CF test initially done with paired samples during the Quebec outbreak in 1989. The PRN test was performed at the Newfoundland laboratory as described previously (18), and the CF test was done at the Quebec public health laboratory by a micromethod (16). Culture for measles virus was done at the Newfoundland laboratory with the B95-8 cell line by a shell vial method (14, 20). Sera from the three test panels were assigned random code numbers, intermixed, and tested blindly throughout the study.
RESULTS
The sensitivities of the various measles IgM EIA kits were determined by using positive panels I and II. With positive panel I, the detection rate ranged from 98.1% (106 of 108) for the Clark and PanBio assays to 100% for the Gull assay. The Light Diagnostics capture assay detected 107 (99.1%) of the 108 cases of measles (Table 1). This panel included samples from five patients with culture-confirmed cases of measles. Serum samples from all five patients tested positive for IgM antibody by the Behring and Gull assays, but only four of the five tested positive by the Clark and PanBio assays as well as by the Light Diagnostics capture assay. In other words, one of the two samples missed by the PanBio assay and the single sample missed by both the Clark EIA and Light Diagnostics capture assay were from a patient with a culture-confirmed case of measles (Table 1). In accordance with the current guidelines, sera from the five patients with culture-confirmed cases were subsequently tested by the CDC capture assay. The sample which tested negative by the three commercial assays was also found to be negative by the CDC capture assay.
TABLE 1.
Detection of measles virus IgM antibody in single serum samples from 108 patients with measles, positive panel I
| Assay | No. (%) of samples
|
||
|---|---|---|---|
| Positive | Negative | Equivocal | |
| Behring | 107 (99.1) | 0 | 1 |
| Clark | 106 (98.1) | 1 | 1 |
| Gull | 108 (100) | 0 | 0 |
| PanBio | 106 (98.1) | 2 | 0 |
| Light Diagnostics | 107 (99.1) | 1 | 0 |
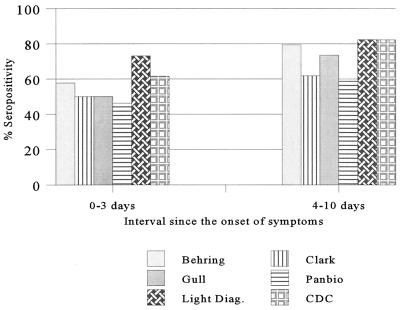
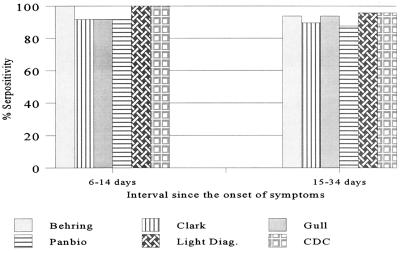
The evaluation of positive panel II, comprising paired acute- and convalescent-phase sera, included the CDC reference capture assay. With this test panel, the Light Diagnostics assay showed the best detection rate of 80% with the acute-phase samples compared to the rates for the other assays (Table 2). With the convalescent-phase samples, all assays showed a significantly improved sensitivity in the range of 92% (Clark and PanBio assays) to 97% (Light Diagnostics assay and CDC capture assay), as expected (Table 2). Measles IgM antibody was not detected in samples obtained from 56 of the 100 patients by at least one test. For 48 of the 56 patients, it involved the first (acute-phase) specimen, 43 of which had a CF titer of <8; for the remaining 5 patients the CF titers ranged from 8 to 256. For 40 of the 48 patients, the interval between the onset of symptoms and collection of the first specimen was 3.3 days (range, 0 to 17 days). For the remaining 8 of the 56 patients mentioned above, the lack of detection involved convalescent-phase specimens. In this instance, the mean interval between the onset of symptoms and phlebotomy was 17.7 days (range, 15 to 22 days). Although negative results most frequently occurred with the Clark and PanBio kits, both IgM capture assays failed to detect measles IgM in the convalescent-phase specimens from two patients, both of whom tested positive by the Gull assay. These convalescent-phase samples were collected 15 and 19 days after the onset of symptoms, respectively. Overall, the samples collected 6 to 14 days after the onset of symptoms showed the highest seropositivity rate, in the range of 92 to 100%. The effect of the timing of specimen collection on IgM seropositivity is shown in Fig. 1 and 2. On the basis of the results for all 108 samples in panel I and 100 acute-phase and 100 convalescent-phase paired samples in panel II, the overall IgM detection rate was 92.2% (284 of 308) for the Light Diagnostics assay and was 82.8% for the Clark assay, 88.3% for the Gull assay, and 88.6% for the Behring assay (Table 3). The sensitivity of the CDC capture assay was evaluated only with panel II samples, and on the basis of those results, the detection rate was found to be 87% (174 of 200) for this assay. Also, with this panel, there was an excellent correlation of the CF test results initially obtained during the outbreak investigation in 1989 with that of the PRN test results obtained during this study. The PRN test detected measles antibody at titers of >100 and >500 in 76 and 58 samples among the 100 acute-phase samples, respectively. In contrast, 74 of the 100 acute-phase samples had no detectable antibody (i.e., CF titer, <8) by the CF test.
TABLE 2.
Detection of measles virus IgM antibody in paired serum samples from 100 patients with serologically confirmed cases of measles, positive panel IIa
| Assay | No. of samples
|
|||||
|---|---|---|---|---|---|---|
| Acute phase
|
Convalescent phase
|
|||||
| Positive | Negative | Equivocal | Positive | Negative | Equivocal | |
| Behring | 70 | 22 | 8 | 96 | 3 | 1 |
| Clark | 57 | 40 | 3 | 92 | 7 | 1 |
| Gull | 69 | 30 | 1 | 95 | 4 | 1 |
| PanBio | 58 | 30 | 12 | 92 | 5 | 3 |
| Light Diagnostics | 80 | 14 | 6 | 97 | 2 | 1 |
| CDC | 77 | 16 | 7 | 97 | 2 | 1 |
A total of 200 samples were tested.
FIG. 1.
Effect of timing of sample collection on IgM seropositivity on the basis of results for acute-phase samples from 60 patients.
FIG. 2.
Effect of timing of sample collection on IgM seropositivity on the basis of results for convalescent-phase samples from 60 patients.
TABLE 3.
Relative overall sensitivity, specificity, and predictive values of measles virus IgM antibody tests
| Assay | Sensitivity (%)a | Specificity (%)b | PPV (%) | NPV (%)c |
|---|---|---|---|---|
| Behring | 88.6 (85.1, 92.1)d | 96.7 (95.1, 98.3) | 97.8 (96.1, 99.5) | 94.6 (92.5, 96.7) |
| Clark | 82.8 (76.6, 87.0) | 97.1 (95.6, 98.6) | 95.9 (93.5, 98.3) | 90.2 (87.6, 92.8) |
| Gull | 88.3 (84.7, 91.9) | 99.6 (99.0, 100) | 99.6 (98.9, 100) | 93.0 (90.7, 95.3) |
| PanBio | 83.1 (78.9, 87.3) | 86.6 (83.5, 89.7) | 97.7 (95.9, 99.5) | 91.4 (88.8, 94.0) |
| Light Diagnostics | 92.2 (89.2, 95.2) | 86.6 (83.5, 89.7) | 88.2 (84.7, 91.7) | 95.9 (94.0, 97.8) |
| CDC | 87.0 (82.3, 91.7) | 94.8 (92.4, 96.8) | 96.1 (93.3, 98.9) | 95.7 (93.5, 97.5) |
Data are based on results for a total of 308 samples tested by all assays except the CDC capture assay, which was used to test 200 samples.
Data are based on results for a total of 454 samples tested by all assays except the CDC capture assay, which was used to test 423 samples.
NPV, negative predictive value.
Values in parentheses are the 95% confidence intervals.
The specificities of the indirect IgM EIAs and the IgM capture assays were determined by using the negative panel of 454 specimens. Specificity ranged from 86.6% for the Light Diagnostics capture assay and the PanBio assay to 99.6% for the Gull assay, and the latter result was statistically significant (P < 0.01) from the rest of the results (Table 4). The Gull assay also had the highest positive predictive value (PPV; 99.6%) (Table 3), which, with the exception of the Behring EIA, was significantly different (P < 0.02) from the PPVs for the rest of the assays. All samples yielding false-positive or indeterminate reactions were those that were reactive for other serological markers. The distribution of false-positive or equivocal results with the various test kits are shown in Table 5.
TABLE 4.
Specificity of measles virus IgM serology tests: negative panela
| Assay | No. (%) of samples
|
||
|---|---|---|---|
| Negative | Positive | Equivocal | |
| Behring | 439 (96.7) | 6 (1.3) | 9 (2.0) |
| Clark | 441 (97.1) | 11 (2.2) | 2 (0.4) |
| Gull | 452 (99.6) | 1 (0.2) | 1 (0.2) |
| PanBio | 393 (86.6) | 6 (1.3) | 55 (12.1) |
| Light Diagnostics | 393 (86.6) | 38 (8.4) | 23 (5.1) |
| CDC | 401 (94.8) | 7 (1.7) | 15 (3.5) |
A total of 454 serum samples were tested, but only 423 of the 454 serum samples were tested by the CDC assay.
TABLE 5.
Distribution of false-positive or equivocal results
| Assay | No. (%a) of samples positive or equivocal for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Parvovirus (n = 142) | Rubella virus (n = 57) | Epstein-Barr virus (n = 40) | Cytomegalovirus (n = 6) | Human herpesvirus 6 (n = 5) | Mycoplasma (n = 15) | Other (n = 68) | Total | |
| Behring | 3 (2.1) | 1 (1.8) | 0 | 2 (33.3) | 1 (20) | 1 (6.7) | 6 (8.8) | 14 (3.1)b |
| Clark | 2 (1.4) | 1 (1.8) | 3 (7.5) | 1 (16.7) | 0 | 0 | 6 (8.8) | 13 (2.9)b |
| Gull | 0 | 0 | 0 | 0 | 1 (20) | 0 | 1 (1.5) | 2 (0.4)b |
| PanBio | 45 (31.7) | 5 (8.8) | 8 (20.0) | 2 (33.3) | 1 (20) | 0 | 0 | 61 (13.4)b |
| Light Diagnostics | 40 (28.2) | 9 (15.8) | 4 (10) | 1 (16.7) | 1 (20) | 2 (13.3) | 4 (5.9) | 61 (13.4)b |
| CDC | 10 (7.0) | 2 (3.5) | 0 | 0 | 0 | 1 (6.7) | 9 (13.2) | 22 (5.2)c |
Percentage of the total in each serological marker category.
Percentage is based on a total of 454 samples tested.
Percentage is based on a total of 423 samples tested.
Many of the specimens in panels I and II were tested more than once with one of the assay kits at different times. For example, the Behring EIA was performed for routine diagnostic purposes in Toronto (1996) and Quebec (1989) during the initial outbreak investigations and was repeated in St. John's, Newfoundland, during the course of the current evaluation of positive panels I and II. Similarly, the Light Diagnostics capture assay was used independently in Toronto and St. John's in 1998 to test the samples from positive panel I. In all instances similar results, including range of optical densities and interrun and intersite run reproducibilities, were obtained with the same kit.
DISCUSSION
The ultimate goal of a measles control program is to stop the indigenous circulation of measles virus. Monitoring of the success of such programs requires a sensitive surveillance system. With the time for measles elimination in the Americas set for the year 2000, enhanced surveillance based on laboratory confirmation of suspected cases of measles becomes increasingly important. Measles IgM serology allows the testing of a single serum specimen and is diagnostic if the result is positive. However, as the number of true measles cases declines, the positive predictive value of the same tests applied to a population with a low disease prevalence will decrease, and consequently, the rate of false-positive IgM serology will progressively increase. In addition, lack of sensitivity by the tests used will result in missed cases. In this regard, our data provide useful information on the relative performances of some commercial indirect measles IgM serology test kits and measles IgM capture assays.
This study was a simple comparison of different tests performed with a set of serum specimens under identical conditions. It should also be noted that throughout the study all samples were tested under code. The differences observed in the performance of individual tests may be attributed to the design and relative sensitivities of the tests and the level of IgM antibody present in the samples at the time of collection. Among the four indirect EIA kits evaluated, both the Behring and Gull assays were found to be better performers than the Clark and PanBio assays. Also, the former assays were found to be as good as or better than the CDC capture assay (Table 3). While the Light Diagnostics capture assay showed the highest level of sensitivity, specificity was poor, at 86.6%. It is also significant that, with positive panel I, both capture assays missed IgM in a specimen from a patient with a culture-confirmed case of measles that was found to be positive by both the Behring and Gull assays. This sample was from a 21-year-old male who was exposed to children involved in the Newfoundland measles outbreak in 1997 and who had a clinically confirmed case of measles. Measles virus was cultured from his nasopharyngeal specimen; this specimen and the single serum sample tested were collected 3 days after the onset of rash and fever. Furthermore, both IgM capture assays failed to detect measles virus IgM in the convalescent-phase specimens (collected 15 and 19 days, respectively, after rash onset) from two other patients with confirmed cases of measles, both of whom tested positive by the Gull assay. Collection of specimens between 3 and 28 days after rash onset is generally recommended for IgM detection (10). In our evaluation series, the samples from 56 patients with confirmed cases of measles in positive panel II tested IgM negative or indeterminate by at least one assay. These results reflect the significant impact of the timing of sample collection for IgM detection, and the greatly improved rate of detection of measles virus in the convalescent-phase specimens for all tests supports this observation. A Canadian study indicated that the IgM positivity rate increased from 40 to 90% for samples collected from 1 to 7 days after the onset of symptoms and reached 100% for samples taken later than 15 days after the onset of symptoms (16), and a U.S. study reported the IgM seropositivity rate to be 56% for samples collected within 5 days of rash onset (15). Our data indicate that the best detection rate is achieved with samples taken 6 to 14 days after the onset of symptoms (Fig. 1 and 2). A slight drop in the positivity rate for samples taken 15 to 34 days after the onset of symptoms may reflect a decline in the level of or the disappearance of IgM antibody. These findings emphasize the importance of the timing of sample collection for measles IgM serology and reiterate that testing of only an acute-phase sample may not be adequate to confirm or rule out measles, particularly in settings of sporadic measles activity (15, 16).
Among the samples in the negative panel of 454 serum samples used to assess test specificity, false-positive results occurred with all assays, with the Light Diagnostics capture assay showing the highest rate of false positivity. More importantly, false-positive results were observed for patients with other exanthema such as parvovirus and rubella infections, which can also clinically mimic measles. Therefore, there is potential for such rash illnesses to be misdiagnosed as measles not only by clinical examination but also by laboratory testing even if the capture assay is used for confirmatory testing. Both the Gull and Behring assays had higher PPVs than the rest of the assays. A low PPV of measles diagnostic methods has been noted (9), and this has important consequences from the standpoint of measles surveillance. Additional studies are required to examine this issue further.
The Gull assay is more practical in that the IgG-absorbent material is incorporated in the specimen diluent, hence avoiding a separate pretreatment step for the removal of IgG. The Gull assay further permits serum dilutions to be performed in microtiter plates, which can easily be transferred to test plates with a multichannel pipette. In contrast, the Light Diagnostics capture assay requires serum dilutions to be made in test tubes followed by transfer of the diluted samples individually to a microtiter test plate. Otherwise, the hands-on times were similar for each of the assays with the exception of the Behring assay, which requires more time. Also, while a run can typically be completed in about 2 h with the other kits, the Behring assay requires about 4 h. We found all products with the exception of the Light Diagnostics capture assay to be competitively priced; the Light Diagnostics assay costs considerably more than the rest of the assays. It is significant that both the Behring EIA and the Light Diagnostics capture assay yielded identical results in tests carried out in different laboratories and over a considerable time interval. This reveals the high levels of interassay precision of these two test kits. We also observed an excellent correlation of the results of the CF test with those of the PRN test; this provided an additional validation of the results for the positive panel and attested to the exquisite sensitivity of the PRN test for detection of measles antibody (2).
Our data show that at least some commercially available indirect EIAs are sensitive and specific and that the Behring and Gull EIAs are as good as or better than both of the capture assays. Although the capture assay format provides performance at least equivalent to those of the indirect EIAs, the capture assays are unlikely to enhance the reliability of IgM serology results if tests such as the Behring or Gull indirect EIAs are used for routine laboratory diagnosis of measles. Furthermore, the IgM capture assays are also unlikely to resolve the indeterminate results obtained by indirect EIAs. The testing of a second (convalescent-phase) specimen will remain the only means of confirmation as well as the only means for resolving indeterminate results either by repeat IgM testing or, more importantly, by observing changes in IgG titers by the CF or PRN test. This underscores the importance and capacities of these time-honored tests to serve as confirmatory tests in this setting. The other alternative is measles virus detection by culture or detection of the viral RNA by reverse transcription-PCR (RT-PCR), which can be accomplished with nasopharyngeal or throat swabs or urine specimens (12, 20). As fewer and fewer cases of measles are encountered, viral culture, in fact, might be very helpful for a definitive diagnosis. Measles viral culture is also indicated to facilitate genotyping of measles virus isolates for molecular epidemiological surveillance (19). From the practical standpoint, however, measles viral culture service as well as viral RNA detection by RT-PCR would be limited to select reference centers. In addition, the duration of measles virus shedding is short, 4 and 7 days after rash onset for nasopharyngeal aspirates and throat swabs and for urine, respectively. Therefore, virus detection via culture or RT-PCR, while useful, is somewhat limited for routine diagnostic application in the context of global measles laboratory surveillance programs.
The conclusions as outlined above, as a matter of fact, form the basis of the current Canadian recommendations for measles diagnosis from the standpoint of measles surveillance as a part of the measles elimination program in Canada. The Canadian recommendations (recommendations of the Working Group on Measles Elimination in Canada) for measles diagnosis are as follows. The definition of a clinical case of measles (in the absence of recent [1 to 14 days] immunization with measles-containing vaccine) is fever (temperature, ≥38°C), generalized maculopapular rash for ≥3 days, and cough, coryza, or conjunctivitis. A case of measles is considered to be laboratory confirmed when clinical measles occurs in a patient epidemiologically linked to a patient with a laboratory-confirmed case of measles, when a case of measles occurs in a patient who has had a recent travel history to an area with known measles activity and who is positive for measles IgM serology by a recommended assay or virus isolation, or when clinical measles occurs in a patient with no epidemiological link or recent travel history but with measles virus isolation or a demonstrated rise in IgG titer between acute- and convalescent-phase sera. Regardless, in regions where significant progression toward measles elimination is taking place, complete evaluation of the methods used for laboratory diagnosis is essential to define the optimal characteristics of laboratory assays for the confirmation of measles.
ACKNOWLEDGMENTS
We thank Elizabeth Oates and Vivian Moulton, Newfoundland Public Health Laboratory, St. John's, and Tina Orchard, Ontario Central Public Health Laboratory, Toronto, for technical assistance, and Margaret Litt, Laboratory Centre for Disease Control, Ottawa, for statistical analysis. We also thank Darrel Cook, British Columbia Provincial Public Health Laboratory, Vancouver; Kevin Fonseca, Southern Alberta Public Health Laboratory, Calgary; Magdi Dawood, Cadham Provincial Laboratory, Winnipeg; Spencer Lee, Nova Scotia Public Health Laboratory, Halifax; and Rosanna Peeling, Laboratory Centre for Disease Control, Winnipeg, for providing serum specimens for the study.
This study was partly supported by a grant from the Division of Immunization, Laboratory Centre for Disease Control, Ottawa.
REFERENCES
- 1.Agocs M M, Markowitz L E, Straub I, Dômôk I. The 1988–1989 measles epidemic in Hungary: assessment of vaccine failure. Int J Epidemiol. 1992;21:1007–1013. doi: 10.1093/ije/21.5.1007. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht P, Herrmann K, Burns G R. The role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981;3:251–260. doi: 10.1016/0166-0934(81)90062-8. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. Case definitions for public health surveillance. Morbid Mortal Weekly Rep. 1990;33:23. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Measles eradication: recommendations from a meeting cosponsored by the World Health Organization, the Pan American Health Organization, and CDC. Morbid Mortal Weekly Rep. 1997;46(RR-11):1–20. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Advances in global measles control and elimination: summary of the 1997 International Meeting. Morbid Mortal Weekly Rep. 1998;47:1–23. [PubMed] [Google Scholar]
- 6.de Quadros C A. Global eradication of poliomyelitis and measles: another quiet revolution. Ann Intern Med. 1997;127:156–158. doi: 10.7326/0003-4819-127-2-199707150-00012. [DOI] [PubMed] [Google Scholar]
- 7.de Quadros C A, Olivé J M, Hersh B S, Strassburg M A, Henderson D A, Bennett D B, Alleyne G A O. Measles elimination in the Americas. Evolving strategies. JAMA. 1996;275:224–229. doi: 10.1001/jama.275.3.224. [DOI] [PubMed] [Google Scholar]
- 8.Erdman D D, Anderson L J, Adams D R, Stewart J A, Markowitz L E, Bellini W J. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J Clin Microbiol. 1991;29:1466–1471. doi: 10.1128/jcm.29.7.1466-1471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gay N, Ramsay M, Cohen B, Hesketch L, Capner P M, Brown D, Miller E. The epidemiology of measles in England and Wales since the 1994 vaccination campaign. Commun Dis Rep CDR Rev. 1997;7:R17–R21. [PubMed] [Google Scholar]
- 10.Helfand R F, Heath J L, Anderson L J, Maes E F, Guris D, Bellini W J. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J Infect Dis. 1997;175:195–199. doi: 10.1093/infdis/175.1.195. [DOI] [PubMed] [Google Scholar]
- 11.Hummel K B, Erdman D D, Health J, Bellini W J. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol. 1992;30:2874–2880. doi: 10.1128/jcm.30.11.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin L, Richards A, Brown D W G. Development of a dual target-PCR for detection and characterization of measles virus in clinical specimens. Mol Cell Probes. 1996;10:191–200. doi: 10.1006/mcpr.1996.0027. [DOI] [PubMed] [Google Scholar]
- 13.Kambarami R A, Nathoo K J, Nkrumah F K, Pirie D J. Measles epidemic in spite of high measles immunization coverage rates in Harare, Zimbabwe. Bull W H O. 1991;69:213–219. [PMC free article] [PubMed] [Google Scholar]
- 14.Kobune F, Sakata H, Sugiura A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990;64:700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo D R, Brennan T, Cormier D P, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol. 1991;29:2865–2867. doi: 10.1128/jcm.29.12.2865-2867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozanne G, D'Halewyn M-A. Performance and reliability of the Enzygnost measles enzyme-linked immunosorbent assay for detection of measles virus-specific immunoglobulin M antibody during a large measles epidemic. J Clin Microbiol. 1992;30:564–569. doi: 10.1128/jcm.30.3.564-569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan American Health Organization. Update: measles in Canada, 1995. 1996. EPI Newsl. 18:3–4.
- 18.Ratnam S, Gadag V, West R, Burris J, Oates E, Stead F, Bouilianne N. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J Clin Microbiol. 1995;33:811–815. doi: 10.1128/jcm.33.4.811-815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tipples G. Laboratory aspects of the measles elimination program in Canada. Can Commun Dis Rep. 1999;25:45–47. [PubMed] [Google Scholar]
- 20.Working Group on Measles Elimination in Canada. Measles surveillance: guidelines for laboratory support. Can Commun Dis Rep. 1998;24:33–44. [PubMed] [Google Scholar]
- 21.World Health Organization. Expanded programme on immunization—accelerated measles strategies. Weekly Epidemiol Rec. 1994;69:229–234. [PubMed] [Google Scholar]
- 22.World Health Organization. EPI information system global summary, August 1997. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]