Abstract
Following a request from the European Commission, EFSA was asked to deliver a scientific opinion on the safety and efficacy of Bovaer® 10 as a zootechnical additive for ruminants for milk production and reproduction. Systemic exposure or site of contact toxicity for the active substance 3‐nitrooxypropanol (3‐NOP), for which genotoxicity has not been fully clarified, in the target species, is unlikely based on ADME data available. Consequently, the FEEDAP Panel concluded that Bovaer® 10 was safe for dairy cows at the maximum recommended level. However, as a margin of safety could not be established, the FEEDAP Panel could not conclude on the safety of the additive for other animal species/categories. The FEEDAP Panel considered that the consumer was exposed to 3‐nitrooxypropionic acid (NOPA), which is one of the 3‐NOP metabolites. NOPA was not genotoxic based on the studies provided. The FEEDAP Panel concluded that the use of Bovaer® 10 in animal nutrition under the conditions of use proposed was of no concern for consumer safety and for the environment. The FEEDAP Panel concluded that the active substance 3‐NOP may be harmful if inhaled. It is irritant (but not corrosive) to skin, irritant to the eyes but it is not a skin sensitiser. As the genotoxicity of 3‐NOP is not completely elucidated, the exposure through inhalation of the additive may represent an additional risk for the user. The Panel concluded that the additive has a potential to be efficacious in dairy cows to reduce enteric methane production under the proposed conditions of use. This conclusion was extrapolated to all other ruminants for milk production and reproduction.
Keywords: 3‐nitrooxypropanol, 3‐NOP , 3‐nitrooxypropionic acid, NOPA, ruminants, methane reduction, zootechnical additive, environment
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from DSM Nutritional Products Ltd represented in the EU by DSM Nutritional Products Sp. Z o.o.2 for authorisation of the product Bovaer® 10 (3‐nitrooxypropanol), when used as a feed additive for ruminants for milk production and reproduction (category: zootechnical additive; functional group: substance which favourably affect the environment).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 23 October 2019.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product Bovaer® 10 (3‐nitrooxypropanol, 3‐NOP), when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
The feed additive Bovaer® 10 (3‐nitrooxypropanol) has not been previously assessed and is not authorised as a feed additive in the European Union.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier3 in support of the authorisation request for the use of Bovaer® 10 (3‐nitrooxypropanol) as a feed additive.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the Bovaer® 10 (3‐nitrooxypropanol) in animal feed. The Executive Summary of the EURL report can be found in Annex A.4
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of Bovaer® 10 (3‐nitrooxypropanol) is in line with the principles laid down in Regulation (EC) No 429/20085 and the relevant guidance documents: Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019).
3. Assessment
The product Bovaer® 10 (3‐nitrooxypropanol) is intended to be used as zootechnical additive (functional group: substances which affect favourably the environment) with the claim to reduce the emission of enteric methane (CH4) in ruminants for milk production and reproduction.
3.1. Characterisation
3.1.1. Characterisation of the additive
The additive Bovaer® 10 is a white, free‐flowing, fine granular powder. The active substance 3‐nitrooxypropanol (3‐NOP) is diluted in propylene glycol and adsorbed on silicic acid. The applicant provided the typical quantitative composition of the final formulation (3‐NOP 10.5%, propylene glycol 35.2% and silicic acid 54.3%). The minimum content of 3‐NOP is specified at 10%.
The content of the active substance (3‐NOP) was analysed in nine batches of the additive (mean: 10.98%, range: 10.32–11.3%).6 , ■■■■■ The results demonstrated that the additive complies with the proposed specification (3‐NOP minimum 10%).
According to the applicant, the propylene glycol and silicic acid used in Bovaer® 10, comply with Directive 2002/32/EC, with regard to the content of undesirable substances, but no data to support these statements were provided.
No data on possible presence of heavy metals and arsenic in the final formulation of the additive were provided.
The flowability of the additive is 5.8 sec/100 g while the bulk and tap densities are 550 and 630 kg/m3, respectively.■■■■■
The dusting potential and the particle size distribution■■■■■ of the additive were evaluated on three batches of the product. The dusting potential was measured using the Heubach test■■■■■ and ranged from 330 to 390 mg/m3. The particle size of the additive measured by laser light diffraction indicated that the fractions of particles below 10 μm and 50 μm were 0% and 0.4%, respectively. The average diameter was 290 μm.
3.1.2. Characterisation of the active substance
The active substance is 3‐NOP [International Union of Pure and Applied Chemistry (IUPAC) name: propan‐1,3‐diol‐mononitrate, Chemical Abstract Service (CAS) number 100502‐66‐7, chemical formula C3H7NO4, molecular weight 121.09 g/mol]. The chemical structure of 3‐NOP is shown in Figure 1.
Figure 1.
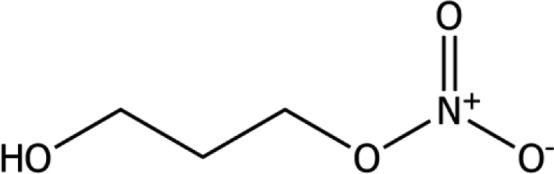
Chemical structure of the active substance 3‐NOP
The identity of the active substance (3‐NOP) was confirmed by analyses performed by ultraviolet–visible (UV‐VIS) spectroscopy,10 infrared (IR) spectroscopy,11 nuclear magnetic resonance (NMR, 1H‐ and 13C‐)11 and mass spectrometry (MS).11
The purity is specified to be > 98.0% 3‐NOP (w/w%). The content of 3‐NOP in three industrial batches of the active substance ranged between 99.1% and 99.4% (w/w%).■■■■■ However, certificates of analyses were not provided.
Specifications are set for substance‐related impurities ■■■■■ unidentified impurities (each ≤ 0.20%, area%), unspecified impurities (each ≤ 0.50%, area%), for the sum of all impurities (≤ 2.0%, area%) and for residual solvents ■■■■■ water ≤ 1.0%).■■■■■
The presence of these impurities as well as residual amount of solvents were tested on three batches of the active substance 3‐NOP as in‐process control parameters, and further in the diluted active substance ■■■■■ sum of PG‐dimers (0.04%)). The sum of all impurities was < 0.15%, with each single unidentified impurity accounting for ≤ 0.01%.■■■■■
3.1.3. Manufacturing process
■■■■■
3.1.4. Stability and homogeneity
3.1.4.1. Shelf‐life of the additive
Three batches of the additive (Bovaer® 10) were stored in closed, sealed aluminium bags for 18 months at 25°C (50% RH) and for 12 months at 30°C (65% RH).■■■■■ At the beginning of the experiment, all the batches contained a level of 3‐NOP ranging from 11% to 12%. The average retention after storage for 18 months at 25°C was 95.5% and 97.3% after 12 months at 30°C.
3.1.4.2. Stability in premixtures/feed
The stability of the additive in a mineral premixture and in a mineral complementary feed has been measured on three production batches.16 The inclusion level of the active substance (3‐NOP) was 6 g/kg, in both mineral premixture and mineral complementary feed. The samples were stored for 6 months at 25°C (50% RH). After 6 months, the recovery for mineral premixture ranged from 98.3% to 98.8% (mean 98.5%) and for the mineral complementary feed ranged from 67% to 69.5% (mean 68.2%).
The stability of three batches of the additive was measured also in a compound feed for ruminants when incorporated to reach a concentration of 60 mg of 3‐NOP/kg complementary feed.■■■■■ The samples were stored at 25°C (50% RH) for 3 months. After 3 months of storage, the recovery was 74%. The effects of pelleting were studied (80°C). The recovery after pelleting was 89%. After pelleting, the samples were stored for 3 months at the same conditions as described above and the recovery was 83.3%.
3.1.4.3. Homogeneity
The capacity of Bovaer® 10 to homogenously distribute in feed for ruminants was assessed on three batches of the product (6 subsamples each batch).■■■■■ The additive was incorporated at a concentration of 60 mg 3‐NOP/kg complementary feed. The homogeneity was evaluated in the pelleted feed. The coefficient of variation (CV) ranged from 0.3% to 1.3%.
3.1.5. Conditions of use
The additive is intended to be used in feed for female ruminants (for milk production and reproduction) from first insemination to culling, to reduce enteric CH4 emission, at a proposed minimum recommended level of 60 mg 3‐NOP/kg dry matter (DM) and a maximum recommended level of 100 mg 3‐NOP/kg DM, which correspond to a minimum of 52.8 mg 3‐NOP and a maximum of 88 mg 3‐NOP/kg complete feed (with a DM content of 88%).
The additive can be incorporated into the total or partial mixed ration (T‐/P‐MR) via a mineral complementary feed or a concentrate complementary feed.
3.2. Safety
3.2.1. Absorption, distribution, metabolism and excretion
3.2.1.1. Experimental species and humans
In vitro studies
14C‐3‐NOP was incubated in liver fractions (S9, cytosol, microsomes) from several species/strains (Sprague–Dawley rat, Wistar Han rat, Beagle dog and humans) with or without nicotinamide adenine dinucleotide (NAD+).17 Aliquots of samples were removed at several time points, the proteins precipitated, and the supernatant analysed by liquid scintillation counting (LSC) and radio‐high‐performance liquid chromatography (HPLC).
After incubating 3‐NOP in S9 and cytosol of liver from Sprague–Dawley or Wistar rats, it was observed that the compound was oxidised to 3‐nitrooxypropionic acid (NOPA), mainly after the addition of NAD+ as a cofactor. In the cytosol, this oxidation reaction was very fast. In microsomes, NOPA was formed to a low extent and only after addition of NAD+. Sprague–Dawley cytosol showed to be more efficient to oxidise 3‐NOP to NOPA (half‐life 0.7 h). NOPA was also formed after incubation of 3‐NOP in S9 liver of dog added with NAD+, with a half‐life of 1.1 h.
Human S9 and cytosol liver fractions were able to metabolise 3‐NOP to NOPA, more efficiently in the presence of NAD+. This oxidation reaction was very rapid, with half‐lives of about 0.7 h. In human liver microsomes, the oxidation only happened after adding NAD+, being detected two additional minor metabolites.
This in vitro study showed that cytosol and S9 fractions of liver of all species are able to metabolise 3‐NOP to NOPA, more efficiently after addition of NAD+ as cofactor. In cytosol, this reaction was always faster, being 0.7 h the half‐life of 3‐NOP in both Sprague–Dawley rats and humans. This suggests that alcohol and aldehyde dehydrogenases can play an important role in the oxidation of 3‐NOP. There were no qualitative differences among species, although S9 and cytosol fractions of Sprague–Dawley rats and human were the most similar and faster to biotransform 3‐NOP to NOPA.
In vivo studies
Two in vivo toxicokinetic studies were performed under the OECD Test Guideline (TG) 417, by orally administering a single dose of 500 mg/kg body weight (bw) 14C‐3‐NOP to male Wistar rats.
In the first study,18 the animals were killed 48 h post‐dosing and the total radioactivity (TR) was determined in plasma, excreta and selected organs and tissues. The Cmax radioactivity in plasma was reached at 1 h after dosing, with about 600 μg 3‐NOP‐eq/g, decreasing to 27 μg 3‐NOP‐eq/g after 48 h.
Radioactivity recovered in urine in the first 24 h was 17.6% of the dose and only 0.3% in the interval 24‐48 h. In faeces, only 2.3% of the dose was excreted within 48 h. In total excreta at 48 h, the recovered radioactivity was 20% of the dose.
Only 5.5% of the dose was recovered from tissues, organs and carcass and 0.2% remained in the gastrointestinal content. The highest concentrations were found in the liver (139.6 μg 3‐NOP‐eq/g), adrenal glands (113.9 μg 3‐NOP‐eq/g) and kidney (71.6 μg 3‐NOP‐eq/g). Low concentrations were found in muscle, brain and fat (27.1, 31.2 and 23.5 μg NOP‐eq/g). The mean overall recovery of the radioactivity applied was 28%.
The major urinary metabolite was 3‐hydroxy‐propionic acid (3‐HPA), representing 47.5% of the urine radioactivity or 7.2% of the dose administered. The major metabolic pathway of 3‐NOP in rat consisted in the oxidation of the alcohol group to NOPA, followed by hydrolysis of the nitrate group to 3‐HPA.
In the second study,19 500 mg/kg bw 14C‐3‐NOP were orally administered to male Wistar rats to evaluate the metabolic profile of 3‐NOP in the gut and plasma. One animal per time point was killed 1 h, 2 h and 3 h post‐dose, blood was collected and the contents of the stomach, small intestine, caecum and colon were removed for TR quantification.
One and 3 h after dosing, more than 80% of the radioactivity in the stomach content corresponded to the intact compound, and < 4% in the small intestine content. No 3‐NOP was detected in the caecum and colon. In plasma, radioactivity decreased from 713 μg 3‐NOP‐eq/g after 1 h to 522 and 156 μg 3‐NOP‐eq/g 2 h and 3 h post‐dose, respectively. A decrease in radioactivity from 1 h to 3 h post‐dosing in almost all the tissues was observed, being the lowest concentrations found in muscle and fat. The overall radioactivity recovered was 88%, 78% and 58% of the dose after 1 h, 2 h and 3 h, respectively.
Metabolites were characterised by NMR and/or liquid chromatography/mass spectrometry (LC/MS) analysis. No metabolites were detected in the stomach. 3‐HPA and 3‐NOP glucuronide were detected in the small intestine, and 3‐HPA, propionic acid and propane‐1,3‐diol in the caecum and colon. In plasma, 1 h after dosing, NOPA was the main metabolite, followed by 3‐HPA. At 2 h and 3 h after dosing, 3‐HPA was the major metabolite, followed by NOPA. Propane‐1,3‐diol was present at very low levels at the three time points. 3‐NOP was not detected in the liver; a mixture of putative metabolites was present, being not possible to elucidate their structure due to the complexity of the matrix. In the urine collected from 0 to 2 h of one animal, radioactivity was 13.7% of the applied dose, being the major urinary metabolites 3‐HPA followed by NOPA, and the glucuronide of 3‐NOP, corresponding to 58%, 20% and 11% of the urine radioactivity, respectively.
Some minor metabolites propane‐1,3‐diol glucuronide, 3‐HPA cysteine conjugate and 3‐HPA glutathione conjugate were identified in the small intestine. In urine, N‐acetylamino cysteine conjugate of propane‐1,3‐diol, N‐acetylamino cysteine conjugate of 3‐HPA, 3‐NOP sulfate, propane 1,3‐diol and the parent compound were at vestigial levels.
Data from both single dose studies showed that 3‐NOP orally administered to rats are stable in the stomach, partially metabolised in the intestine, rapidly absorbed and broadly distributed, and excreted as several metabolites, being the oxidation of 3‐NOP to NOPA and the hydrolysis of NOPA to 3‐HPA the major metabolic pathways.
A mass balance with volatile trapping and tissue distribution study was carried out under OECD TG 41720 using male Wistar rats orally administered with a single dose of 500 mg/kg bw 14C‐3‐NOP, to evaluate the formation of volatile compounds. The animals were housed individually in air‐tight glass metabolism cages and radioactive volatiles were adsorbed by trapping solutions from 0 to 8 h and 8 to 24 h post‐dosing. Urine and faeces were collected from 0 to 24 h.
Twenty‐four hours after administration, 77.4% of the dose was recovered from the volatile traps, almost all as 14CO2; 9.8% and 1.2% in urine and faeces, respectively; 7.7% remained in the tissues and carcass; and 0.5% in the gastrointestinal tract content. The overall recovery of radioactivity was 99.3%. The metabolism to CO2 was very fast, being 64.3% of the radioactivity exhaled from 0 to 8 h and 13.0% from 8 to 24 h post‐dose.
The mean concentration of radioactive compounds in blood 24 h post‐dose corresponded to 48.7 μg 3‐NOP‐eq/g. In tissues, the highest concentrations were found in the liver (184.5 μg 3‐NOP‐eq/g), adrenal glands (159.3 μg 3‐NOP‐eq/g) and kidney (118.4 μg 3‐NOP‐eq/g). Low concentrations were found in fat (58.9 μg 3‐NOP‐eq/g), muscle (42.4 μg 3‐NOP‐eq/g) and brain (24.8 μg 3‐NOP‐eq/g).
All these results confirm to those obtained in the previous rat studies, where only about 30% of the radioactivity was present in urine, faeces, tissues and organs. In rat, 3‐NOP is predominantly exhaled as CO2 (about 70%).
Based on the above, it can be concluded that in rat 3‐NOP is predominantly exhaled as CO2, and the remaining is metabolised by oxidation and hydrolysis.
The ADME of 14C‐3‐NOP was studied in the rat following single or multiple oral administration.■■■■■ Four separate trials were carried out:
A single oral dose of 50 mg/kg bw of 14C‐3-NOP to four males and four females, and sacrifice 48 h after dosing
A single oral dose of 500 mg/kg bw of 14C‐3-NOP to four males and four females, and sacrifice 48 h after dosing
A single oral dose of 500 mg/kg bw of 14C‐3-NOP to two males and two females, and sacrifice 1 h after dosing
A daily oral dose of 50 mg/kg bw of 14C‐3-NOP to four males and four females, for five consecutive days and sacrifice 48 h after the last dose.
In trials (i) and (ii) (rats dosed with 50 mg/kg bw or 500 mg/kg bw of 14C‐3‐NOP), urine and faeces were collected at several time points and blood and tissues at sacrifice. Expired air was adsorbed for the periods of 0–8, 8–24 and 24–48 h post‐dose.
The mean levels of radioactivity in plasma 48 h after dosing was 7.0 in males and 4.2 μg 3‐NOP‐eq/mL in females for the low dose and 51.9 in males and 34.5 μg 3‐NOP‐eq/mL in females for the high dose. In males, after the single dose of 50 mg/kg bw, the highest mean concentration of TR was found in the adrenals, bone marrow, liver and kidneys (19.5, 14.3, 12.6 and 10.9 μg 3‐NOP‐eq/g, respectively). A very similar profile of distribution of radioactivity was found after the single dose of 500 mg/kg bw, being the values found about 8‐10 times higher than for the low dose.
In thymus, renal fat, prostate, spleen, pancreas and lungs, the mean values ranged from 8.97 to 7.23 μg 3‐NOP‐eq/g for the low dose and about 7–10 times higher for the high dose. All other tissues had values close to or lower than plasma at 48 h after dosing, 7.03 μg 3‐NOP‐eq/mL and 51.92 μg 3‐NOP‐eq/mL, respectively, for the low and high doses. In females, a very similar distribution was found. Other organs like the ovaries, spleen, thymus, lungs, pancreas and uterus had mean values from 7.37 to 4.64 μg 3‐NOP‐eq/g for the low dose and about 7–10 times higher for the high dose. All other tissues had values close to or lower than plasma at this time, 4.22 μg 3‐NOP‐eq/mL and 34.47 μg 3‐NOP‐eq/mL, for the low‐ and high‐dose levels. In carcass, radioactivity ranged from 3.1% to 4.4%, for both doses.
14C‐3‐NOP was mainly excreted via the expired air within the first 8 h after dosing, with a mean of about 80% of the administered radioactivity recovered in males and females for the low dose and 73.5% and 61.7% in males and females, respectively, for the high dose. In the low dose, urinary excretion accounted for a mean of 6.3% in males and 4.8% in females, and in the high dose, 11.3% in males and 13.1% in females (roughly double for high dose). Faecal excretion was very low for both doses, from 1.1% to 3.2%. Including cage wash, tissues and carcass, the total mean recovery of administered radioactivity was for the low dose 95.9% and 91.1% in males and females, and for the high dose 93.2% and 85.3%, respectively.
In trial iii), two male and two female rats were orally given a single dose of 500 mg/kg bw of 14C‐3‐NOP and killed 1 h after dosing. The mean concentration of TR in plasma increased from 396 and 477 μg 3‐NOP‐eq/mL at 15 min to 512 and 629 μg 3‐NOP‐eq/mL at 45 min, decreasing to 467 and 583 μg 3‐NOP‐eq/mL at 1 h, in males and females, respectively. TR max was attained 45 min after dosing in all animals.
In males, the highest mean concentration of total radioactivity was found in the liver, kidneys, lungs and pancreas (655, 636, 494 and 471 μg 3‐NOP‐eq/g, respectively). All other tissues had values close to or lower than plasma at 1 h after dosing, 467 μg 3‐NOP‐eq/mL. In females, the Cmax TR was found in the kidneys and liver with values of 865 and 622 μg 3‐NOP‐eq/g, respectively. All other tissues had values close to or lower than plasma at 1 h after dosing, 583 μg 3‐NOP‐eq/mL.
In trial iv), rats were orally given, for five consecutive days, 50 mg/kg bw of 14C‐3‐NOP and killed 48 h after the last dose.
Radioactivity levels in plasma taken each day, 2 h before the after dosing, showed that the steady state was approached after the fourth dose, being 14.4 and 11.7 μg 3‐NOP‐eq/mL 48 h after the last dose, in males and females.
In males, 48 h after the last dose, the Cmax TR (μg 3‐NOP‐eq/g) was present in the adrenals (51.2), liver and kidneys (34.9, 34.8) and bone marrow (34.1). In the thymus, prostate, spleen, pancreas, lungs and epididymis, the mean values were 28.5, 24.6, 22.9, 20.0, 18.7 and 18.2 μg 3‐NOP‐eq/g, respectively. All other tissues had values close to or lower than plasma at this time, 14.4 μg 3‐NOP‐eq/mL. In females, 48 h after the last administration, the Cmax TR (μg 3‐NOP‐eq) was present in the adrenals (57.0), ovaries (35.7), renal fat (34.1), and liver and kidneys (32.3 and 30.0). Thymus, pancreas, bone marrow, lungs and spleen had mean values of 24.7, 21.6, 21.3, 20.7 and 20.1 μg 3‐NOP‐eq/g. All other tissues had values close to or lower than plasma at this time, 11.7 μg 3‐NOP‐eq/mL.
The major route of excretion after repeated dose administration was via the expired air, with a mean cumulative excretion at 48 h after the last dose of about 70% of the total administered radioactivity. Including urinary and faecal excretion, cage wash, tissues and carcass, the total mean recovery of the administered radioactivity was 86.4% and 83.1% in males and females, respectively. The carcass accounted for 3.5 and 3.6% in males and females, respectively. Daily excretion, expressed as μg 3‐NOP‐eq, indicated that excretion was almost complete in each 24 h period.
Identification and quantification of 3‐NOP and its metabolites in several tissues and organs (including epididymides and testes) was tentatively made after administration of a single dose of 500 mg/kg of 14C‐3‐NOP and sacrifice of rats 1 h after dosing (trial iii) as well as in some urine and plasma samples of animals of the other trials.
In the stomach and caecum of rats dosed with 500 mg/kg of 14C‐3‐NOP, only the parent compound was identified (83% and 70% of administered dose in males and females). Other minor compounds were detected but the structure was not elucidated. In the small intestine, besides the parent compound, 3‐HPA (at the higher level 34.8% and 46.5% of total residual radioactivity (TRR) in males and females) and NOPA were also present, and other minor compounds were not identified. In colon, the parent compound and NOPA were present at low levels.
In the liver, only the parent compound was identified at very low levels (5.7 and 3.5% of TRR), being detected five other compounds, the most representative being 3‐HPA. In kidneys and muscle, only NOPA was identified. Other compounds, not identified, were present, some at higher levels than NOPA. In the epididymides and testes, 3‐HPA and NOPA were present being 3‐HPA the major compound, from 60 to 84% TRR in epididymis and testes, respectively. In all these organs, some other compounds were detected but not identified.
In plasma of rats dosed with single or repeated doses of 3‐NOP at 50 mg/kg, 3‐HPA, NOPA and the parent compound were present, being NOPA at the highest levels.
NOPA was detected in urine of rats dosed single doses of 50 or 500 mg/kg, 8 h post‐dose. In the repeated dose protocol, no metabolites were detected in the urine 128 h post‐first dose.
From the results obtained in the four trials carried out in this study, it can be concluded that 3‐NOP is stable in the stomach, partially metabolised in the small intestine, rapidly absorbed and broadly distributed. Tissue concentrations in the high‐dose group were approximately 8–10 times higher than in the low‐dose group demonstrating linearity between the dose and tissues levels. The urinary excretion was about 2–3 times higher in the high‐dose groups, similar in both sexes.
TRR in tissues following repeated dose administration is more than double that seen after a single low‐dose administration, indicating incorporation of the test item‐related material. There is no evidence of sex difference following any of the dosing regimens, being liver, kidney, adrenals and bone marrow the tissues with the highest concentrations.
The urinary excretion after administration of 50 mg/kg bw for five consecutive days was similar to that following a single dose, indicating that the rate of excretion remains constant.
Conclusions on ADME in experimental species and humans
Overall, from the in vivo experiments carried out in rats, it can be concluded that 3‐NOP orally administered is stable in the stomach and partially metabolised in the bsmall intestine. 3‐NOP and the metabolites formed in intestine are rapidly and extensively absorbed and further metabolised in the liver. An extensive metabolism of 3‐NOP was consistently verified, mainly to 14CO2 (about 70% of the dose) and oxidation to NOPA and subsequent hydrolysis to 3‐HPA. 3‐NOP and its metabolites are broadly distributed in tissues and organs, the major part of the radioactivity detected in the adrenals, kidneys and liver and also an appreciable amount in bone marrow. Excretion happens mainly in expired air as CO2, and a small portion in urine and faeces as metabolites. The compound and its metabolites seem not to accumulate in organism, and the difficulty of identifying the structure of polar compounds chromatographically detected indicates that the low portion of radioactivity present in tissues and organs may correspond to incorporation in endogenous compounds.
3.2.1.2. Ruminants
In vitro studies
Three in vitro trials were conducted with rumen fluids using similar testing procedures.
In two separate experiments, samples of (3‐14C)‐3‐NOP (concentrations: 23 or 2.2 mg/L) were incubated in vitro with cow rumen fluid in combination with feed up to 24 h at 38°C under anaerobic conditions at a pH of about 6.2.22 In the trapped gas phases, ≤ 0.1% of the total radioactivity was detected. Only a minor proportion of the radioactivity (7% or ≤ 5.5%, respectively) remained in the non‐dissolved fractions after separation and centrifugation of the incubated samples. The liquid phases were analysed by radio‐HPLC. 3‐NOP concentration decreased from 100% at t = 0 to < 0.1% or 0%, respectively, at t = 24 h. After 6 h and 12 h, the concentration of 3‐NOP had decreased to 54% and 6%, respectively. There was a concomitant increase of one major metabolite, identified by 13C‐ and 1H‐NMR as propane‐1,3‐diol, from 0% to > 97% or 98.5%, respectively, at t = 24 h. In the control samples, there was no or only minor (9.8%) abiotic degradation of 3‐NOP to propane‐1,3‐diol. The FEEDAP Panel concluded that, under the anaerobic test conditions used for the rumen fluid incubations, there was a complete hydrolysis of the nitrate ester 3‐NOP within 24 h.
In a third in vitro trial, (3‐14C)‐3‐NOP was incubated in a concentration of 1 mg/L with sheep, goat and cow rumen fluids in the presence of feed for 16 h at 39°C under anaerobic conditions. The pH of the final mixture being between 6.9 and 7.2.23 Only a minor proportion of the radioactivity (≤ 5.5%) remained in the non‐dissolved fractions after separation and centrifugation of the incubated samples. In the liquid phases, 3‐NOP concentrations were decreased to 0.9%, 0.7% and 1.0% after 16 h for sheep, goat and cow, respectively (mean values, n = 6 incubations per species). The major metabolite produced was propane‐1,3‐diol representing 63.7%, 46.8% and 82.8% of the extractable radioactivity in the sheep, goat and cow rumen fluid incubated samples, respectively. From the remaining radioactivity, the major part was distributed in two polar peaks RI1 and RI3. The latter was identified by co‐chromatography as oxetane (1,3‐propylene oxide), the ratio of RI1 and RI3 depended on the work‐up procedure. The authors assumed that RI1 is either an intermediate that forms RI3 (oxetane) or is oxetane itself that coelutes by interaction with matrix material as RI1, since by re‐chromatography of RI1 this metabolite fraction was quantitatively transformed to RI3. In one series of control samples with autoclaved sheep, goat and cow rumen fluids, there was no loss of 3‐NOP due to the work‐up procedure or to abiotic degradation. An additional series of control samples (3‐14C)‐3‐NOP was conducted under aerobic conditions with sheep, goat and cow rumen fluids for 16 h at 39°C. 3‐NOP concentrations decreased to 39.4%, 27.9% and 31.9% for sheep, goat and cow, respectively (mean values, n = 2 per species). The only major metabolite found was propane‐1,3‐diol, representing 57.8%, 70.5% and 66.4% of the extractable radioactivity. Under these conditions, only traces of oxetane were detected. Overall, the FEEDAP Panel concluded that incubations of (3‐14C)‐3‐NOP with goat, sheep and cow rumen fluid showed no obvious differences between the three species investigated. Under anaerobic conditions, there was a complete hydrolysis of the nitrate ester 3‐NOP within 16 h, propane‐1,3‐diol and therefore inorganic nitrate being the major metabolites produced. The authors consider that the additional production of oxetane might be an artefact due to work‐up or storage of samples. Since oxetane has only been detected with high variation in one of the three in vitro trials and has not been found in the in vivo studies conducted in rat, goats and cows, the FEEDAP Panel considered unlikely that oxetane is formed in vivo.
In vivo studies
For 7 days, a lactating goat received (3‐14C)‐3‐NOP by gavage at daily doses of 4.34 mg/kg bw per day, corresponding to a concentration of 111.7 mg/kg DM if given in feed.■■■■■ From the total radioactivity applied, 1.9% was excreted via faeces, 3.5% via urine and 6.42% via milk while 4.98% remained in the total edible tissues and 1.14% in the intestinal tract content. Total radioactivity excreted amounted to 11.85% and total recovery at sacrifice was 17.97%. The FEEDAP Panel considered that the rest of the radioactivity may be transformed to volatile metabolites, most likely 14CO2. The level of radioactive residues in plasma was steadily increasing from 0.61 mg 3‐NOP‐eq/kg, measured 24 h after the first administration, to 3.72 mg 3‐NOP‐equivalents/kg at sacrifice. Plasma proteins were precipitated from plasma collected at sacrifice. Eighty‐five per cent of the plasma radioactivity remained non‐extractable in the precipitate, indicating that the radiolabel from (3‐14C)‐3‐NOP was incorporated into the proteins. A mean of 75.2% and 59.0% of the milk radioactivity was detected in the aqueous phase of milk at 0–8 h and 8–24 h after the sixth day of administration of (3‐14C)‐3‐NOP, respectively. The rest of the radioactivity was linked to the fat and protein of the milk in which it is assumed to be incorporated. No nitrate esters, i.e. 3‐NOP or 3‐nitrooxypropionic acid (NOPA), were detectable in milk with a limit of detection (LOD) of 0.82 and 0.43 mg 3‐NOP‐equivalent/kg for milk collected 0–8 h and 8–24 h post‐dose, respectively. The only radiolabelled metabolite present in the aqueous phase of milk was identified as lactose. The FEEDAP Panel concluded that 3‐NOP (and/or its gastrointestinal degradation products) is almost completely absorbed, is unlikely to accumulate, and, considering possible biochemical pathways, is extensively metabolised to building blocks for endogenous compounds (radioactivity associated with lactose, milk protein, milk fat, plasma protein) and primarily exhaled as CO2.
In a second experiment, a lactating goat was pre‐dosed on five days by gavage with non‐labelled 3‐NOP followed by administration on two days of (3‐14C)‐3‐NOP by gavage at daily doses of 3.28 mg/kg bw per day, corresponding to 101.8 mg/kg DM.■■■■■ From the radioactivity totally applied, 0.71% were excreted via faeces, 1.97% via urine and 4.48% via milk while 8.99% remained in the total edible tissues and 14.87% in the intestinal tract content. The total radioactivity excreted amounted to 7.18% and total recovery at sacrifice was 31.04%. The FEEDAP Panel considered that the rest of the radioactivity may be transformed to volatile metabolites, most likely 14CO2. The level of radioactive residues in plasma was rapidly increasing to 2.31 mg 3‐NOP‐equivalents/kg, measured 0.5 h after the first administration and amounted to 1.6 mg 3‐NOP‐equivalents/kg at sacrifice. In the aqueous phase of plasma, the percentage of radioactivity decreased from 95.6% 0.5 h post‐dose to 10.6% 24 h post‐dose. Concomitantly, the percentage of total plasma radioactivity associated with the plasma protein fraction increased from 4.4% 0.5 h post‐dose to 89.4% 24 h post‐dose. No nitrate esters, i.e. 3‐NOP or NOPA, were detectable in the aqueous phase of plasma (LOD ≤ 0.07 mg/kg). The plasma concentration of nitrite and nitrate measured at t = 0 was 2.2 μmol/L and increased to levels in the range of 9.0–68.4 μmol/L between 0.5 h and 4 h post‐dose. Twenty‐four hours post‐dose the concentration was at the background level (3.1 μmol/L) again. Four very polar metabolite fractions were characterised in plasma. The analyses of milk fractions revealed that 77.6–84.2% of the milk radioactivity remained in the aqueous phase with chromatograms showing one polar radioactive peak identified as radiolabelled lactose in the previous study. For the aqueous milk fraction, radioactive signal was not detectable in the region of the retention times for the nitrate esters, i.e. 3‐NOP and NOPA (LOD ≤ 0.21 mg/kg). The FEEDAP Panel considered that these results confirmed previous conclusions that 3‐NOP is extensively metabolised to building blocks for endogenous compounds and primarily exhaled as CO2. Based on the outcome of the plasma analyses authors conclude that 3‐NOP is hydrolysed prior or during uptake from the gastrointestinal tract and that the radioactivity most probably gets incorporated into plasma proteins.
For 5 days, a solution of 3‐NOP in water was administered to a lactating goat by gavage at a daily dose of 4 mg 3‐NOP/kg bw corresponding to 100 mg 3‐NOP/kg DM.26 Milk was collected before dosing and twice each day from intervals 0 to 8 h and 8 to 24 h after each treatment and the concentration of inorganic nitrate and nitrite was measured colourimetrically with the Griess assay (LLQ = 6.3 nmol nitrate + nitrite/g milk). The pre‐dose concentration and all values for the milking intervals 8–24 h after each dose were at or below the LLQ, whereas levels of nitrate and nitrite in the milking intervals 0–8 h were between 8.9 and 12.8 nmol/g. According to the authors, measured values were in the range of known and normal milk concentrations.
Four lactating cows received a formulation of 10% (3‐14C)‐3‐NOP, 40% propylene glycol and 50% SiO2 (the additive under evaluation) by capsules for 7 days at a daily dose level of 1.8 g (3‐14C)‐3‐NOP/animal (corresponding to 3.60 mg (3‐14C)‐3‐NOP/kg bw per day or a concentration of approximately 160 mg (3‐14C)‐3‐NOP/kg DM).■■■■■ The daily dose was divided into two doses administered every 12 h. From the radioactivity totally applied, a mean of 2.7% was excreted via faeces, 4% via urine and 16.82% via milk while 6.2% remained in the total tissues. The total radioactivity excreted amounted to 23.5% and total recovery at sacrifice was approximately 30%. The authors postulated that unextracted radioactivity in the liver, kidney and muscle was mostly incorporated into proteins since the radioactivity was liberated following treatment of the debris with protease. Glucose was identified as the major metabolite in the liver, representing > 97% of the extractable radioactivity. Furthermore, 14CO2 could be identified in the exhaled air collected in short intervals (10 min). In milk, approximately 70–80% of the radioactivity was present in the aqueous phase with lactose representing > 98% of the extractable radioactivity. 3‐NOP and NOPA were not found in milk above the LOQ of 8 μg 3‐NOP‐equivalents/kg nor in tissues above the LOQ of 21 μg 3‐NOP‐equivalents/kg. 3‐NOP was not detected in any of the matrices except for the urine of one animal (day 2–day 6) where it was present at only 0.1% of the dose. NOPA was not detected in any urine sample above the LOQ (≤ 0.1% of the dose). Plasma was the only matrix in which NOPA was detected. It was found in the samples of all four cows 1 h post‐first dose, 12 h post‐first dose and 1 h post last dose with concentrations ranging from 65 μg 3‐NOP‐equivalents/kg to 104 μg 3‐NOP‐equivalents/kg (1.3–9.4% TRR). From the identification of 14CO2 in exhaled air, the identification of lactose and glucose as metabolites and the association of radioactivity with proteins, the FEEDAP Panel concluded that 3‐NOP is extensively metabolised and incorporated into endogenous compounds.
Additional information is available from two feeding studies in beef cattle. In the first study 3‐NOP was administered at a daily dose of 2 g/animal (corresponding to 284 mg 3‐NOP/kg feed).28 Plasma samples were taken at different time points up to 180 min after feeding and exhibited a rapid increase of NOPA concentrations. The individual NOPA concentrations at the last sampling time point varied in the range of 22.3–207 ng/mL (control: < 5 ng/mL). In the second study (test levels: 0, 100 or 200 mg 3‐NOP/kg feed DM),■■■■■ due to rapid elimination, no NOPA could be detected in plasma samples 24 h after feeding (LLQ: < 5 ng/mL).
Conclusions on ADME in ruminants
Overall, based on the combined evidence provided by the available kinetic in vitro and in vivo studies the FEEDAP Panel considered that 3‐NOP and/or its degradation products (mainly 1,3‐propanediol) are almost completely absorbed and extensively metabolised by goats and cows. Besides inorganic nitrate, metabolites of 3‐NOP contribute to formation of endogenous compounds, such as lactose and glucose, and are transformed to CO2 which is exhaled. NOPA is another metabolite of 3‐NOP which has been identified in plasma of cows and which has not been detected in other matrices of cows or in samples taken from goats under the conditions of the above‐mentioned kinetic studies. 3‐NOP was not detected in any of the matrices analysed in the described in vivo studies (except for low urine levels in one of four cows).
3.2.1.3. Overall conclusions on ADME
Based on the results of the studies, the FEEDAP Panel concludes that:
The metabolism of 3‐NOP, either ingested by laboratory animals or escaping rumen fermentation, is qualitatively similar. 3‐NOP is metabolised extensively and rapidly to NOPA, 3‐HPA, nitrate/nitrite and CO2. The 3‐NOP carbon skeleton is used for the synthesis of endogenous compounds (carbohydrates, proteins and fatty acids).
In ruminants, 3‐NOP is mainly converted by ruminal fermentation into 1,3‐propanediol. This explains the absence of 3‐NOP in any of the matrices analysed in the in vivo studies (except for low urine levels in one of four cows) and the very limited amounts of NOPA detected in the plasma, milk and tissues; whereas NOPA occurs in higher amounts in laboratory animals.
3.2.2. Residue studies
In ruminants, 3‐NOP is hydrolysed in the gastrointestinal tract prior or during uptake, the remaining is rapidly and extensively metabolised to endogenous compounds. According to the design of an ADME study with 4 cows and a daily dose of 1.8 g radiolabelled 3‐NOP for 7 days (for details, see Section 3.2.1.2), 3‐NOP and NOPA were analysed in milk and tissues and found to be below the LOQ (21 and 8 μg 3‐NOP eq/kg in tissues and milk, respectively). The major 3‐NOP derived milk residue was lactose. Considering that 3‐NOP was not detected in milk and tissues and that other possible metabolites deriving from 3‐NOP (e.g. 1,3‐propanediol, inorganic NO3 −, lactose and CO2) are not considered of toxicological concern as being constituents or normally present in the body; only the possible presence of NOPA was further evaluated in milk and animal tissues.
Two studies (dose‐range finding study and tolerance study) conducted in dairy cows (for details see Section 3.2.4) reported data on NOPA residues in milk and tissues. NOPA was determined in both studies by an internally validated liquid chromatography tandem mass spectrometry (LC–MS/MS) analytical method. The LOQs were 1 μg/kg and 5 μg/kg for milk and tissues, respectively.
The dose‐range finding study showed some weaknesses: only few animals were used (16 cows in total), lack of confirmation of the target concentration of additive in the feed linked to a possible tendency of the additive to decrease over the time, limited ability of the additive to homogeneously distributed in feed.30 Another limitation consists in the unexpectedly high differences of NOPA concentration between morning and afternoon milking.31 The FEEDAP Panel was therefore not in a position to use the data of the dose‐range finding study for an assessment of consumer exposure to NOPA.
In the tolerance study,■■■■■ four groups of 20 cows each were fed diets containing 0, 80, 100 and 200 mg 3‐NOP/kg feed DM (corresponding to 0.8×, 1× and 2× the maximum proposed use level), respectively, for 56 days. All cows were milked twice daily, however, only morning milk was collected for further milk analysis. NOPA was determined 6 days before study start and then at different times during the experiment (SD 2, 8, 15, 22, 29, 36, 43, 50 and 56 days). Eight pre‐selected animals per group were slaughtered and samples of liver, kidneys, muscle and fat were taken for further NOPA analysis. From the 17 cows of the low use level group (80 mg 3‐NOP/kg feed DM), for which milk samples were analysed for NOPA, milk of 15 cows never exceeded 1 μg/kg, only two cows showed in a total of 5 samples concentrations between 1.11 and 2.51 μg/kg (median 1.74). One hundred and thirty‐one milk samples out of 136 were below 1 μg/kg. Milk NOPA appeared higher in the high use level group (100 mg 3‐NOP/kg feed DM) compared to the low use level group. From a total of 19 cows representing 152 samples, 136 samples never exceed the LOQ. Twelve cows were always below that value. Milk with NOPA above 1 μg/kg came from 7 cows (median 1.52).
The highest individual NOPA concentration within the high use level group was 3.66 μg/kg milk. This value was used for the worst‐case exposure calculation to NOPA residues in milk.
The contents of NOPA in tissues (liver, kidney, muscle, fat) were all below the LOQ (5 μg/kg), whatever the 3‐NOP level administered. This value was used for the worst‐case exposure calculation to NOPA residues in tissues.
3.2.3. Toxicological studies
3.2.3.1. Genotoxicity of the active substance 3‐NOP
Bacterial reverse mutation assays
In order to investigate the potential of 3‐NOP (purity ≥ 98%) and/or its metabolites to induce gene mutations in bacteria, two Ames tests were performed according to OECD TG 471 and following Good Laboratory Practice (GLP) in four strains of Salmonella Typhimurium (TA98, TA100, TA1535 and TA1537) and Escherichia coli WP2uvrA, in the presence or absence of metabolic activation.■■■■■ Based on the results of a dose‐range finding test, the substance was tested up to 5,000 μg/plate. Appropriate positive control chemicals and DMSO (as vehicle control) were evaluated concurrently. All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix. No precipitate and toxicity were observed in any experimental condition. No increase in the mean number of revertant colonies was observed at any tested concentration in any tester strains with or without S9‐mix.
An Ames microsuspension test (Micro‐Ames Test) was also performed to evaluate the potential of 3‐NOP (purity ≥ 98%) to induce gene mutations in bacteria using the Salmonella Typhimurium strains TA98, TA100, TA102, TA1535 and TA1537 in the presence and absence of metabolic activation.34 The substance was tested at a concentration range of 1.6 to 500 μg/plate, comparable to 16 to 5,000 μg/plate in the standard Ames test, since the sensitivity of the micro‐Ames test is increased by a factor of 10 compared to the standard test. Appropriate positive and negative control chemicals were used, and the results obtained confirmed the reliability of the test system. No significant increase in the mean number of revertant colonies was observed in any experimental condition.
In vitro micronucleus assays
The applicant has submitted four in vitro micronucleus tests using different cell lines.
An in vitro micronucleus assay was performed according to OECD TG 487 and following GLP to evaluate the potential of 3‐NOP (purity 99.4%) to induce chromosome damage in human peripheral blood lymphocytes in the absence and presence of metabolic activation.■■■■■ Based on the results of the dose‐range finding test, the compound was tested at 164, 512 and 1,211 μg/mL (corresponding to 10 mmol/L), applying a short treatment (3 + 24 h of recovery) with and without S9‐mix and a continuous treatment (24 h) without S9‐mix. The highest dose tested corresponds to the top dose recommended by OECD TG 487. Appropriate positive and negative control chemicals were used, and the results obtained confirmed that the experimental system was sensitive and valid. No cytotoxicity was observed in any experimental condition as well as no increase in the frequency of micronuclei.
An in vitro micronucleus assay was also carried out according to OECD TG 487 (non‐GLP compliant) to evaluate the potential of 3‐NOP (purity 97.4%) to induce chromosome damage in Chinese hamster V79 cells in the absence and presence of metabolic activation.36 Based on the results of the dose‐range finding test, the compound was tested up to 1,243 μg/mL in the absence of S9‐mix applying a continuous treatment (24 h) and up to 310 μg/mL in a short treatment (4 + 20 h recovery) with S9‐mix. Appropriate positive and negative control chemicals were used and the results obtained confirmed that the experimental system was sensitive and valid. No significant cytotoxicity was observed in any experimental condition. In the absence of metabolic activation, no increase in micronucleus frequency was observed. In the presence of metabolic activation, a statistically significant increase in micronuclei, dose‐related and above the historical vehicle control range was induced by treatment with the test item (p < 0.05). The Panel noted that the study is reported in a short version, non‐GLP.
An in vitro micronucleus assay was carried out to evaluate the potential of 3‐NOP (purity 98.1%) to induce chromosome damage in Chinese hamster V79 cells.■■■■■ The study was performed in accordance to OECD TG 487, with the exception that test article treatments were only performed in the presence of metabolic activation, and in compliance with GLP. Based on the results of a preliminary cytotoxicity test, five concentrations were selected for the analysis of micronucleus frequency after short treatment in the presence of metabolic activation (3 + 21 h of recovery) ranging from 300 to 600 μg/mL. Appropriate positive control chemicals were used and the results obtained confirmed that the experimental system was sensitive and valid. About 50% of cytotoxicity was observed at the two highest concentrations tested. A statistically significant increase in micronuclei, dose‐related and above the historical vehicle control range, was observed at 540, 570 and 600 μg/mL. To investigate the mechanism of micronuclei formation, i.e. clastogenic or aneugenic, the content of micronuclei was investigated by the CREST staining. The results obtained indicate a clastogenic activity of 3‐NOP that induced 79% micronuclei containing chromosome fragments at 540 μg/mL. Based on these data, the Panel concluded that 3‐NOP can induce chromosome damage through a clastogenic mechanism of action.
3‐NOP was tested in an in vitro micronucleus assay using duplicate cultures of human lymphoblastoid TK6 cells, both in the absence and presence of metabolic activation (S9 from β‐Naphthoflavone/Phenobarbital‐induced animals).■■■■■ The test article was formulated in purified water and the maximum concentration used was 1,220 μg/mL (equivalent to 10 mmol/L) in the experiments without S9, where no cytotoxicity (measured as replication index) was reported. In the presence of S9, the treatment was conducted up to 1,000 μg/mL, where 54% cytotoxicity was reported. Micronuclei were analysed in binucleated cells (cytochalasin‐B method). Three treatment schedules were applied: 3‐h treatment followed by 27‐h recovery with and without S9 and 27‐h treatment followed by 27‐h recovery only without S9. In cells treated with the test item for 3 + 27 h in the presence of S9, a statistically significant increase in micronucleated binucleated (MNBN) cells was observed at the intermediate concentration analysed (750 μg/mL inducing 29% cytotoxicity), although the values fell within the range of the historical vehicle control. Following 3+27‐h treatment without S9, a statistically significant increase in MNBN cells was observed at the high concentration analysed (1,220 μg/mL inducing 0% cytotoxicity). Both replicate cultures exhibited a MNBN cell value that marginally exceeded the normal range of the historical vehicle control. Following the extended treatment (27 + 27 h) without S9, frequencies of MNBN cells were significantly (p ≤ 0.05) higher than those observed in the concurrent vehicle control cultures for the highest two concentrations analysed with a statistically significant linear trend test. The historical control range was exceeded only in one of the two replicate cultures at the intermediate concentration. It is concluded that 3‐NOP induced a weak increase in micronuclei in cultured human lymphoblastoid TK6 cells. It should be noted that the treatment protocol applied for the extended treatment was not fully compliant with OECD TG 487, that recommends to treat the cells for 1.5–2 normal cell cycle lengths in the presence of cytochalasin‐B and to harvest at the end of the treatment period, while in this study cytochalasin‐B was added during an additional 27‐h recovery time after the treatment.
In vitro mammalian cell gene mutation test
To assess the mutagenic potential of 3‐NOP (purity 98.1%) in a mammalian cell system, the L5178Y TK +/− mouse lymphoma assay was performed in accordance with GLP and OECD TG 476 applying a short treatment (3 h) in the absence and presence of S9‐mix and a 24 h treatment in the absence of S9‐mix.■■■■■ Based on the results of the dose‐range finding test, the compound was tested up to 1,211 μg/mL (corresponding to 10 mmol/L). Precipitation of the test item was not observed at any concentration and experimental condition. Positive control chemicals induced statistically significant increases in mutation frequencies (MF), confirming the sensitivity of the assay and the efficacy of the S9‐mix, while MF in the negative controls were within the historical vehicle control ranges. No biologically significant increases in MF were observed at any concentration analysed in the presence and absence of S9‐mix and no statistically significant linear trends were observed.
Cell transformation assay in Syrian hamster embryo cells (SHE assay)
In order to evaluate the carcinogenic potential of 3‐NOP (purity 99%), an in vitro cell transformation assay was performed in Syrian hamster embryo cells according to OECD draft proposal (2012) and following GLP applying a continuous treatment time (7 days).40 Based on the results of the dose‐range finding test, the compound (purity 98%) was tested at six concentrations ranging from 500 to 2,500 μg/mL. The positive control induced a statistically significant increases in morphologically transformed colonies, while the negative controls were within the historical vehicle control range. Cytotoxicity was observed at the highest concentration. No significant increase in the frequency of morphologically transformed colonies was detected at any concentration analysed.
In vivo micronucleus test
A micronucleus test was performed in bone marrow cells of NMRI BR mice according to OECD TG 474 to evaluate the potential of 3‐NOP (purity 98.3%) to induce chromosomal damage.41 Animals were treated intraperitoneally at doses of 250, 500 and 1,000 mg/kg bw. The highest dose was selected in a preliminary toxicity study, showing mortality at 2,000 mg/kg bw. Mice from all dose groups were sacrificed 24 h after dosing, and mice from top dose and control groups were also sampled 48 h after treatment. Two thousand polychromatic erythrocytes (PCEs) were scored for each animal for the analysis of micronuclei. Positive and negative control values of micronucleus frequency were within the historical control ranges of the laboratory confirming the sensitivity of the assay. No cytotoxicity was observed in the bone marrow; thus, no evidence of target tissue exposure was provided. The frequency of micronuclei was comparable between treated and negative control groups.
A micronucleus test was also performed in bone marrow cells of Wistar WI (Han) rats according to OECD TG 474 to evaluate the potential of 3‐NOP (purity 99.4%) to induce chromosomal damage after oral administration.■■■■■ Animals were treated by gavage at 375, 750 and 1,500 mg/kg bw on the basis of the result of a dose‐range finding test showing severe symptoms and mortality at 2,000 mg/kg bw. Rats from all dose groups were killed 24 h after dosing, and animals from top dose and control groups were also sampled 48 h after treatment. Four thousand PCEs were scored for each animal for the analysis of micronuclei. Positive and negative control values of micronucleus frequency were within the historical control ranges of the laboratory confirming the sensitivity of the assay. No cytotoxicity was observed in the bone marrow. An increase in the mean frequency of PCEs was observed at the highest dose in males (where it was statistically significant) and females; however, the reported values were within the historical vehicle control data range. Therefore, not all the criteria for a clearly positive result indicated by OECD TG 474 were met.
3.2.3.2. Genotoxicity of the metabolite NOPA
Bacterial reverse mutation assay
The potential of NOPA to induce gene mutations was investigated using the plate incorporation test in the Salmonella Typhimurium strains TA1535, TA1537, TA98, TA100, and in the Escherichia coli strain WP2 uvrA. The assay was performed with and without liver microsomal activation.43 Each concentration, including the controls, was tested in triplicate. The test item was tested at the following concentrations: 3; 10; 33; 100; 333; 1,000; 2,500; and 5,000 μg/plate. The plates incubated with the test item showed normal background growth up to 5,000 μg/plate in all the experimental conditions used and no precipitation was observed. Substantial and dose‐dependent increases in revertant colony numbers were noted in strains TA1535 and TA100 both in the presence and absence of S9 mix. Positive and negative controls gave the expected responses. In conclusion, NOPA induced gene mutations by base pair substitutions in the genome of strains TA1535 and TA100 in the presence and absence of S9 mix.
In vitro micronucleus assay
To evaluate the potential of NOPA (purity 98.4%) to induce chromosome damage, an in vitro micronucleus test was performed in whole blood human lymphocytes according to OECD TG 487 and following GLP.44 Three concentrations of NOPA, ranging from 229 to 1,321 μg/mL (approx. 10 mmol/L), were selected for the analysis of micronuclei after a short treatment in the absence and presence of metabolic activation (4 + 20 h of recovery) and a continuous treatment without metabolic activation. The highest concentration tested corresponds to the top dose recommended by OECD TG 487 for the in vitro mammalian cell micronucleus test. Appropriate positive and negative control chemicals were used and the results obtained confirmed that the experimental system was sensitive and valid. No significant cytotoxicity was observed in any experimental condition. No significant increase in the frequency of micronuclei was induced by NOPA treatments. Based on these results, the Panel concluded that NOPA did not induce chromosomal damage under the experimental conditions applied in this study.
Transgenic rodent gene mutation assay
The effect of NOPA on the mutant frequency of the cII gene was analysed in liver and duodenum of male and female transgenic Fischer 344 Big Blue® rats in compliance with OECD TG 488.45 The dose levels were selected based on a 14‐day oral gavage dose‐range finding study previously performed.46 The test item was administered at the following dose levels: 0 (vehicle control), 150, 300 and 600 mg/kg bw per day (Groups 1, 2, 4 and 6, respectively), to six male rats per group and 0 (vehicle control), 250, 500 and 1,000 mg/kg bw/per day (Groups 1, 3, 5 and 7, respectively), to six female rats per group. Phosphate buffered saline, pH 7.4 was used as the vehicle. Test article and vehicle control formulations were administered once daily by oral gavage for 28 consecutive days, at a dose volume of 10 mL/kg. DNA isolated from previous positive control animals (F344 transgenic male rats orally exposed to N‐ethyl‐N‐nitrosourea) was used as a positive control. Neither mortality nor morbidity were observed, but clinical signs were reported in both male and female animals. Animals were killed on day 31 (females) or day 56 (males). At necropsy, the liver and duodenum were collected for mutant analysis. No increase in the mutant frequency of the cII gene in liver and duodenum of Big Blue® rats was observed. Negative and positive controls performed as expected, demonstrating the validity of the test system. Under the conditions of this study, NOPA at doses up to 600 mg/kg bw per day in males and 1,000 mg/kg bw per day in females did not induce cII mutants in liver and duodenum.
In vivo micronucleus assay in peripheral blood reticulocytes (combined with the Transgenic rodent gene mutation assay)
The animals treated with NOPA for the transgenic rodent gene mutation assay described above (Fischer 344 Big Blue® rats) were also evaluated for clastogenic and/or aneugenic activity in an in vivo micronucleus assay in peripheral blood reticulocytes in compliance with OECD TG 474.45 On days 4 and 29, peripheral blood was collected from animals for the detection of micronuclei using flow cytometry. No significant increases in the incidence of MN‐RETs were observed in the animals treated with the test article. A significant decrease in % reticulocytes was observed in male animals at day 29, indicating that the test article was cytotoxic. At both time points, the vehicle controls were within the historical 95% control limit range. No concurrent positive control was used, but positive control samples provided by the kit used for the assay were analysed and gave the appropriate positive responses in the assay. It is concluded that NOPA was negative for the induction of micronuclei in peripheral blood of rat in the experimental conditions used.
3.2.3.3. Conclusions on genotoxicity
For 3‐NOP, negative results were observed in three Ames tests, in one in vitro mammalian cell gene mutation assay, in one SHE assay, in one in vitro MN test performed on whole blood human lymphocytes and in one in vivo MN test performed in mice. However, in the latter, the FEEDAP Panel noted that there was no evidence of bone marrow exposure.
Positive results were observed in two in vitro MN tests performed in Chinese hamster V79 cells; in one of these studies, a clastogenic activity was demonstrated by the CREST analysis.
Equivocal results were observed in one in vitro MN test performed in TK6 cells and in one in vivo MN test performed in rats.
Based on the above, the FEEDAP Panel concludes that the genotoxicity potential of 3‐NOP cannot be ruled out.
The metabolite NOPA induced gene mutations in a bacterial reverse mutation assay, while no chromosomal damage was reported in an in vitro MN assay. A transgenic rodent gene (TGR) mutation assay conducted as in vivo follow‐up for the positive Ames test, showed negative results for the induction of gene mutations in liver and duodenum. Moreover, in an in vivo MN test combined with the TGR, no increase of micronuclei was observed in peripheral blood reticulocytes. Based on the data available, the FEEDAP Panel concludes that the metabolite NOPA does not raise concern for genotoxicity.
3.2.3.4. Subchronic toxicity studies
Rats
Based on the results of a 10‐day range finding study,47 groups of 10 male and 10 female Wistar Han rats were exposed by oral gavage to 3‐NOP at 0 (control), 0 (placebo), 20, 100 or 500 mg/kg bw per day, in a repeated dose toxicity study with a reproduction/developmental toxicity screening test following the relevant OECD TGs (OECD TG 407; 421 and 422).48 Males were exposed for 29 days, i.e. 2 weeks prior to mating, during mating, and up to necropsy. Females were exposed for 42–52 days, i.e. during 2 weeks prior to mating, during mating, during post‐coitum, and during the last 4 days of lactation. All females at 500 mg/kg bw showed evidence of mating but none of the females became pregnant, and no corpora lutea or implantation sites were found in any of these females. At 20 and 100 mg/kg, mating, fertility and conception indices, pre‐coital time and numbers of corpora lutea and implantation sites were normal. Treatment with 500 mg 3‐NOP/kg bw per day caused adverse effects in the testes and epididymides characterised by severe reduction of the spermatogenesis. The FEEDAP Panel considered 100 mg 3‐NOP/kg bw per day as the no observed adverse effect level (NOAEL) for males and females, based on the effects on spermatogenesis and on corpora lutea.
Wistar rats (15/sex per group) received 3‐NOP by oral gavage at dose levels of 0 (control), 50, 100 or 300 mg test item/kg bw per day, for 90 consecutive days.■■■■■ The study was conducted in compliance with OECD TG 408. An extra 5 animals/sex in the control and high‐dose group included as recovery groups which were necropsied after 13 weeks on control diet. Slight to severe decrease in spermatogenesis was observed in the testes (characterised by tubular atrophy of germ cells, presence of multinucleated giant cells and tubular vacuolation) of most males of the 300 mg/kg bw per day group. Sperm motility was reduced in most of the males at 300 mg/kg bw per day accompanied by decreased total sperm counts in testes and epididymides. No evidence of recovery was observed after 13 weeks. No treatment‐related effects were observed in females treated with up to 300 mg 3‐NOP/kg bw per day for 13 weeks. The FEEDAP Panel considered 100 mg 3‐NOP/kg bw per day as the NOAEL for males, based on the effects on spermatogenesis, and 300 mg/kg bw per day for females.
Mice
Based on the results of a 6‐day range finding study,■■■■■ 10 CByB6F1 Hybrid mice/sex per group were given 3‐NOP by oral gavage at dose levels of 0 (control), 100, 300 or 700 mg/kg bw per day for 28 days.■■■■■ The study was conducted under GLP conditions and in compliance with OECD TG 407. A toxicokinetic evaluation for metabolite NOPA showed that its plasma concentration increased rapidly (< 1 h) and was rapidly eliminated in both sexes (t1/2 ranged: 0.5–1.4 h in males and 0.7–1.2 h in females). Peak concentrations of NOPA increased dose proportionally. The NOPA AUC increased in a more than dose proportional manner on days 1 and 28 in both sexes. Exposure to NOPA did not change significantly upon repeated dosing, in terms of Cmax and AUC in males and females. At the highest dose (700 mg/kg bw per day), a lower systemic exposure in terms of AUC was noted after repeated administration in males and females than after single dose. No sex differences in exposure were observed during the study for NOPA. Animals at 700 mg/kg bw per day showed partly closed eyes, piloerection, irregular or slow breathing, decreased activity and hunched gait, rare episodes of cold to touch. These signs appeared 1 h after dosing and some of them persisted at 3 h or the following morning after dosing. There were no effects on body weight and body weight gains at all dose levels. A reduction of 6% in mean overall food consumption was observed at 700 mg/kg bw per day, when compared with the control group. Statistically significant dose‐related decrease of triglycerides was reported in animals at 300 and 700 mg/kg bw per day. Significant dose related changes of serum phosphorus and potassium were noted at 300 and 700 mg/kg bw per day. A statistically significant decrease of absolute and relative thymus weights was observed in males at 700 mg/kg bw per day, of 22% and 23%, respectively, but with no clear dose response. No treatment‐related effects were observed at gross pathology. Histopathological investigation showed an increased incidence of males at 700 mg/kg bw per day with mild inflammation of the eye striated muscle (4/10) and minimal to mild adrenal cortical vacuolation (4/10) when compared to control group (0/10). A NOAEL of 100 mg 3‐NOP/kg bw per day was identified, based on blood biochemical changes observed at 300 mg/kg bw per day.
Dogs
Based on the results of two 14‐day range finding studies,■■■■■ Beagle dogs were given 3‐NOP by oral gavage at dose levels of 0, 10, 30, 100 and 300 (2 × 150) for 90 days, according to OECD TG 409.■■■■■ The animals (4 males and 4 females each group) were exposed to the test item at 0, 10, 30, 100 and 300 (2 × 150) mg/kg bw per day, followed by a 4‐week recovery period. The animals in the control and high‐dose groups were dosed twice daily. The other groups were administered once per day. The high‐dose level was selected based on the previous dose‐range‐finding studies conducted on the same species/strain.■■■■■ One female dog treated with 300 mg 3‐NOP/kg bw per day was sacrificed after 3 weeks of dosing, following a sudden onset of severe clinical signs (including abnormal gait, lethargy, tremors and repetitive epileptic seizures). In this animal, NOPA plasma levels after dosing was high and very slowly decreased. The reason was unknown. According to the authors of the study, epilepsy is known to occur spontaneously in Beagle dogs. Overall, there were no 3‐NOP‐related changes in body weight, food consumption, haematology, clinical biochemistry, urinalysis, organ weights, sperm analysis, in ophthalmoscopic or ECG examinations, macroscopic examination or microscopic examination at any dose levels tested. NOAELs of 300 mg 3‐NOP/kg bw per day for males and 100 mg 3‐NOP/kg bw per day for females (based on the severe clinical signs observed in one female animal) were identified.
The FEEDAP Panel noted that the severe effects seen on testes and epididymides, affecting spermatogenesis, were observed in rats only. The FEEDAP Panel agreed on an overall NOAEL of 100 mg/kg bw per day for 3‐NOP, derived from subchronic toxicity studies conducted in rats, mice and dogs.
3.2.3.5. Chronic and carcinogenicity studies
Wistar rats (20 males and/or females per group) were orally administered with 3‐NOP at dose levels of 0, 25 (males only), 50 (males and females), 100 (males and females), 300 (males only) or 600 (females only) mg/kg bw per day by gavage for 52 weeks.■■■■■ The study was conducted under GLP conditions and in line with the OECD TG 452. The dose levels were based on the results of the 90‐day study conducted in rats where testicular toxicity was observed in males at 300 mg/kg bw per day but no adverse effects were seen in females (described in Section 3.2.3.4). The dose for females was therefore set at 600 mg/kg bw per day. Microscopic examination revealed impaired spermatogenesis characterised by severe atrophy (depletion of all germ cell types in almost all tubules, resulting in the presence of Sertoli cells only) in the testes (including the presence of giant cells) and reduced sperm and cellular debris in the lumen of the epididymides in all males of the 300 mg/kg bw per day group. Sperm motility and sperm counts were decreased, in all males of this group. About half of the females of the 600 mg/kg bw per day group showed minimal hepatocellular cytoplasmic eosinophilic inclusions, which is not accompanied by any indication of liver damage or changes in the liver parameters. Therefore, the authors considered these hepatocellular changes to be non‐adverse. The authors of the study considered 100 mg 3‐NOP/kg bw per day as the NOAEL for males and 600 mg 3‐NOP/kg bw per day for females. The FEEDAP Panel agrees with these conclusions.
Fifty Wistar rats per sex and group were administered with 3‐NOP at dose levels of 0, 25 (males only), 50 (males and females), 100 (males and females) and 300 (females only) mg/kg bw per day by oral gavage for 2 years.■■■■■ The study was conducted in line with the OECD TG 451. No accumulation in plasma was noted for NOPA in males and females after 13 and 52 weeks of dosing at all dose levels when compared to day 1. No sex differences were observed during the study regarding exposure to NOPA at 50 and 100 mg 3‐NOP/kg bw per day. In the liver of females treated with 300 mg 3‐NOP/kg bw per day, minimal to slight hepatocellular cytoplasmic eosinophilic inclusions were observed in 4 out of 49 animals. The present finding confirms the results observed in 50% of the females treated with 600 mg 3‐NOP/kg bw per day in the 52‐week study and supports the conclusion of the applicant that this phenomenon does not lead to any degenerative or proliferative changes in the liver, hence may be considered as non‐adverse. Treatment with the test substance resulted in an increase in the incidence of benign gastrointestinal mesenchymal tumours in the small intestines of females treated with 300 mg 3‐NOP/kg bw per day. In addition to these mesenchymal tumours, hyperplasia of a similar type of mesenchymal cell was observed in the small intestine muscle layer of one female of both the 50 and 300 mg/kg bw per day group. No gastrointestinal mesenchymal tumours or hyperplastic mesenchymal cells were noted in the small intestines of males. The applicant organised a Pathology Working Group (PWG) to evaluate the specific intestinal findings in this study. A Panel of five board‐certified pathologists examined tissue sections of small intestines of rats to characterise the findings originally diagnosed as gastrointestinal mesenchymal tumours or mesenchymal cell hyperplasia and to discuss the carcinogenicity potential of these findings. In females, the PWG noted three benign mesenchymal cell tumours in the 300 mg/kg bw per day group and one animal each in the 50 and 100 mg/kg bw per day group. In two additional females of the 300 mg/kg bw per day and two males of the 100 mg/kg bw per day group, mesenchymal cell hyperplasia was observed. The increased incidence of the benign mesenchymal tumours was not considered statistically significant. Regarding the significance of these finding in the small intestines as evidence of a carcinogenic potential of 3‐NOP, the PWG took into consideration that there were no similar findings in the gastrointestinal system of the 52‐week oral toxicity study nor any gastrointestinal tract lesions at higher doses and no positive genotoxicity following an extensive battery of studies. Based on the weight of evidence, the PWG concluded unanimously that 3‐NOP was not carcinogenic in Wistar rats. The FEEDAP Panel considered that, even if a statistical significance was not demonstrated, benign gastrointestinal mesenchymal tumours in the small intestines are rare findings and could not be considered as chance findings. Therefore, the NOAEL from this study is 100 mg 3‐NOP/kg bw per day.
3.2.3.6. Reproductive/developmental toxicity studies
The two‐generation reproduction toxicity study was carried out in Wistar rats by oral gavage at dose levels of 0, 25, 50 and 100 mg/kg bw per day (Groups 1–4; 24 males and females per dose level) and a fifth group of 10 females treated with 600 mg/kg bw per day for the first generation only, according to OECD TG 416.■■■■■
F0‐males and F0‐females were exposed to the test substance from 10 weeks prior to mating and exposure was continued until euthanasia. For 600 mg/kg bw per day F0‐females, this was done from 2 weeks prior to mating. F0‐females were allowed to produce and rear a litter until days 21–23 of lactation. On day 4 of lactation or shortly thereafter, litters were reduced in size to eight pups by random culling of F1‐pups. After weaning, one F1‐male and one F1‐female of each litter of Groups 1–4 were selected for mating with a pup of another litter of the same dose group to produce a F2‐generation. F1‐females were allowed to produce and rear a litter until days 21–23 of lactation. No toxicologically relevant effects were noted for both generations up to 100 mg/kg. At 600 mg/kg, three F0‐females showed clinical signs (lethargy, flat posture, uncoordinated movements and/or piloerection) for the first 1–2 days but recovered from then onwards, except for one female that showed piloerection and pale appearance during lactation. Additionally, there was an initial body weight loss noted for 5 out of 10 F0‐females at 600 mg/kg during the first week of treatment. The remaining parameters investigated at 600 mg/kg for one generation were unaffected by treatment. No reproduction or developmental toxicity was observed for both generations up to the highest dose level tested. Although spleen weights of the F2‐generation were statistically significantly lower in male pups at 25 mg/kg (absolute and relative to body weight), 50 mg/kg (absolute) and 100 mg/kg (absolute and relative to body weight) and in female pups at 50 mg/kg (absolute) and 100 mg/kg (absolute and relative to body weight), there were no test item‐related microscopic findings observed in the spleen of these pups. Therefore, the effects on spleen weight were considered by the authors of the study, as not adverse. The FEEDAP agrees with this consideration. A NOAEL of 100 mg/kg bw per day was identified for parental toxicity and of at least 100 mg/kg bw per day for reproduction and developmental toxicity.
A prenatal developmental toxicity study was carried out in Wistar rats (22 females per dose level) by oral gavage from days 6 to 20 post‐coitum at dose levels of 0, 100, 300 and 1,000 mg/kg bw per day according to OECD TG 414.■■■■■ Maternal toxicity was observed at 1,000 mg/kg. Several females showed lethargy, flat posture, hunched posture, uncoordinated movements and/or piloerection on one or more occasions. In addition, at 1,000 mg/kg, reduced body weights and body weight gain were observed throughout the treatment period, body weight gain corrected for uterine weight were reduced, and food consumption was decreased during the first week of treatment. No toxicologically relevant effects were observed for the lower doses. Fetuses were weighed, sexed and examined for external, visceral and skeletal malformations and developmental variations. No developmental toxicity was observed in any of the treated groups. A maternal NOAEL 300 mg/kg bw per day was identified based on clinical signs, reduced body weights and body weight gain observed at the highest dose level and the developmental NOAEL was considered as being at least 1,000 mg/kg bw per day.
Another prenatal developmental toxicity study similar in approach as the one on rats was carried out in New Zealand White rabbits (22 females per dose level) by oral gavage according to OECD TG 414.■■■■■ From days 6 to 29 post‐coitum rabbits were dosed with 0, 50, 150 and 450 mg/kg bw per day of 3‐NOP. No toxicologically relevant maternal toxicity was observed in any of the dose groups. The fetuses were weighed, sexed and examined for external, visceral and skeletal malformations and developmental variations. No developmental toxicity was observed in any of the 50, 150 and 450 mg/kg groups.
A NOAEL of at least 450 mg/kg bw per day for maternal and developmental toxicity was identified.
The FEEDAP Panel agreed on an overall NOAEL of 100 mg 3‐NOP/kg bw per day from subchronic toxicity studies in rats, mice and dogs, from a carcinogenicity study in rats and from two‐generation reproduction toxicity study conducted in rats. The most relevant effects were observed in testes of rats exposed to 3‐NOP at 300 mg/kg bw per day.
3.2.3.7. Mechanistic toxicity studies
Additional in vivo ■■■■■ (single dose or repeated doses), in vitro ■■■■■ and ex vivo ■■■■■ studies performed with 3‐NOP and its metabolites (e.g. NOPA and 3‐HPA), were performed to elucidate the potential mode of action on the testes and epididymides and interspecies differences in susceptibility. Overall, these studies showed that the parent compound itself (3‐NOP) is unlikely to contribute to the testicular toxicity due to its fast metabolism to NOPA. Based on the absence of any testicular/epididymal abnormality 10 days after sc administration of 3‐HPA, it may be concluded that this metabolite is not responsible for the testicular toxicity. NOPA can be considered as the ultimate toxicant. Ex vivo/in vitro cultures of seminiferous tubuli of testicular tissue obtained from rats, dogs and non‐human primates showed that Sertoli cells (the primary target cell) from dogs were not susceptible to NOPA and those of monkeys reacted differently (tendency to decrease in number in cells) as compared with rats (increase the number of cells). Furthermore, transcriptomics analysis indicated different reaction across the various species. The authors considered these results as an indication that the testicular toxicity induced by administration of 3‐NOP is rat specific. The FEEDAP Panel agreed with this conclusion.
3.2.3.8. Summary on ADME, residues and toxicological studies
The FEEDAP Panel performed an overall assessment of the available evidence taking into consideration the toxicological studies, including genotoxicity studies, as well as the specific metabolism of 3‐NOP and NOPA in the laboratory animals and in target species.
The FEEDAP Panel considered the following:
3‐NOP
Negative results for genotoxicity were observed in three Ames tests, in one in vitro mammalian cell gene mutation assay, in one SHE assay, in one in vitro MN test performed on whole blood human lymphocytes and in one in vivo micronucleus test performed in mice, but in the latter there was no evidence of bone marrow exposure. Positive results for genotoxicity were observed in two in vitro MN tests performed in Chinese hamster V79 cells, in one of these studies a clastogenic activity was demonstrated by CREST staining. Equivocal results for genotoxicity were observed in on in vitro MN test performed in TK6 cells and in one in vivo MN test performed in rats. Based on the above, the genotoxicity potential of 3‐NOP cannot be ruled out.
A 2‐year carcinogenicity study in rats reported an increase in rare mesenchymal intestinal tumours after 3‐NOP treatment (not statistically significant compared to the control group), which cannot be considered as a chance finding.
The FEEDAP Panel identified a lowest NOAEL of 100 mg 3‐NOP/kg bw per day from subchronic toxicity studies in rats, mice and dogs, from a carcinogenicity study in rats and from two‐generation reproduction toxicity study conducted in rats. The most relevant effects were observed in testes of rats exposed to 3‐NOP at 300 mg/kg bw per day.
Based on in vitro and in vivo studies, 3‐NOP is rapidly and is almost entirely metabolised by rumen bacteria to 1,3 propanediol, CO2, NO3 −, these being the main metabolites.
1,3‐Propanediol, as for 1,2‐propanediol which could be used as a feed material, is expected to be dehydrated and oxidised in vivo, giving further volatile fatty acids that are then absorbed by the rumen epithelium.
3‐NOP, following the interaction with the target enzyme methyl‐coenzyme M reductase, is partly reduced to nitrite (Duin et al., 2016), which in the rumen is rapidly converted to ammonia and incorporated into proteins.
Being a small molecule, 3‐NOP could diffuse through membranes like the rumen epithelium. As a result, the compound would quickly reach the liver through the portal system, where it could be denitrated and/or oxidised, escaping systemic circulation.
3‐NOP was not found in plasma of cows nor in milk or other edible tissues (< LOQ).
NOPA
Based on the data available, NOPA is not considered genotoxic. It is considered the ultimate toxicant and responsible for the testicular toxicity observed in rats only.
NOPA is the major metabolite in rodents but not in ruminants.
NOPA was detected in cows’ plasma at negligible concentration.
NOPA was not found in milk and edible tissues above the LOQ in an ADME study performed in four lactating cows.
From the tolerance study, the highest concentration of NOPA in milk was 3.66 μg/kg derived from a single animal. The concentration of NOPA in tissues was always below the LOQ.
Based on the above, the FEEDAP Panel concluded that potential systemic toxicity of the additive is unlikely in the target species as well as toxicity at site of contact level due to the rapid metabolism of 3‐NOP in the rumen. Consequently, the risk for genotoxicity in the target species is negligible. The metabolite NOPA is considered responsible for the adverse effects observed in rats at testes and epididymis level; however, it is not considered genotoxic. Residues of NOPA in milk and animal tissues are limited, not being this metabolite the predominant one in the target species.
3.2.4. Safety for the target species
3.2.4.1. Literature search
In support of the safety for the target species, the applicant provided the reports from a literature search. Two databases were used (Pubmed and Scifinder), the strings for the search and the exclusion criteria were provided. The literature review compiled 16 relevant articles reporting data from studies in which 3‐NOP was used as an additive in ruminant diets. However, the value of the data gathered from this literature review is limited (e.g. these studies were designed to determine the efficacy and the active substance was tested at low concentrations) and cannot be used to support the safety of the additive for ruminants.
3.2.4.2. Tolerance studies
Two studies were performed with lactating dairy cows receiving the additive under assessment at increasing concentrations in feed.
Firstly, a dose‐range finding study was performed involving 16 Holstein Friesian dairy cows (3–6 years old, 485–746 kg bw and between 92 and 124 days in milk (DIM) at the beginning of the experiment) that were distributed in four experimental groups (4 cows per group).■■■■■ The cows were fed a total mixed ration (TMR) composed of maize silage, lucerne hay, soya bean meal, dairy nuts/cake and minerals/vitamins premix. In each of the four experimental groups, the TMR was supplemented to provide doses of 0 (control), 1.6 (1×), 8 (5×) or 16 (10×) g 3‐NOP/day (corresponding to 0, 80, 400 or 800 mg/kg complete feed). The most sensitive endpoint was feed intake which was significantly (p < 0.05) reduced in group 10× compared to the control. Two cows had to be euthanised prematurely due to reduced feed intake and lethargy (one from group 5× and one from group 10×).
A tolerance study was carried out with 80 Holstein Friesian dairy cows (multi‐ and primiparous, 2–7 years of age, 480‐792 kg bw and between 53 and 141 DIM at the beginning of the study) allocated to four experimental groups (20 cows per group).■■■■■ Regarding the experimental design, cows were blocked according to milk yield recorded during the pre‐treatment period and then one cow per block was randomly assigned to one of the experimental groups. In each of the four experimental groups, the TMR was supplemented to achieve intended concentrations of 0 (control), 80 (0.8×), 100 (1×) or 200 (2×) mg 3‐NOP/kg feed DM. The cows were fed a TMR composed of maize silage, lucerne hay, soya bean meal, dairy cake and minerals/vitamins premix. The additive used was a preparation containing 3‐NOP at 10.9% (w/w) formulated with propylene glycol and adsorbed on silicon dioxide and was administered orally mixed in daily freshly prepared TMR. The experiment included a pre‐treatment period of 10 days (–10 to –1) in which all cows were fed the control diet, and then a treatment period of 56 days in which the corresponding experimental diets were offered ad libitum to each group. During weeks 1, 4 and 8 of the study, representative batches of each experimental diet were analysed for 3‐NOP concentration and homogeneity. As confirmed by analysis, the dietary concentrations were 0, 90.2, 106.8 and 220.4 mg 3‐NOP/kg feed DM in the control, 0.8×, 1× and 2× groups, respectively.
The measurements and observations recorded in each cow during the study were: feed intake and water consumption (daily), milk production (at each milking, twice daily), milk composition (protein, fat, lactose, urea and somatic cell count (SCC) in samples collected in the morning milking at days 1, 2, 8, 15, 22, 29, 36, 43, 50 and 56), body weight (weekly), faecal scoring (twice weekly), clinical health observations (daily), clinical veterinary assessments and examinations (clinical examinations by a veterinarian pre‐treatment and on study days 8, 29 and 54). Milk samples were also analysed for NO2/NO3 − content. Blood samples from all cows were collected prior to the start of the study (days –10 and –1) and on study days 5, 33 and 56 for haematology65 and biochemistry65 and coagulation67 and serum amyloid A analysis. Urine samples68 were collected at pre‐treatment and at days 5, 33, and 57. At the end of the trial (day 57), 32 pre‐selected cows (random selection, 8 per group) were killed for gross necropsy and histopathological examination. Selected organs69 were weighted and relative weight as percentage of bw was calculated. Additionally, samples were collected from a number of organs and viscera70 for histopathological evaluation. Milk, plasma and tissue samples (liver, kidney, loin muscle, perirenal and omental fat) were collected during the study for residue analysis.
Each cow was considered as the experimental unit. Data were subjected to ANCOVA with diet and day in treatment as factors, and baseline (value pre‐treatment) as covariate. Additionally, a test of non‐inferiority of each supplemented group compared with the control group was performed, using a pre‐defined non‐inferiority criterion of 80% (accepting up to ‐20% versus control group as non‐inferior). As for the blood biochemistry, a reference range was generated for each parameter using all data recorded from non‐supplemented animals (pre‐treatment, and then control cows at all samplings). The 2.5% and 97.5% percentiles obtained from the non‐treatment data were used as the reference range. This was considered as the ‘on‐study non‐treatment reference range’.
One animal in the control group was excluded during pre‐treatment period. Daily amounts of 3‐NOP ingested by each cow were calculated from feed intake and measured concentrations of 3‐NOP in feed, and were in accordance with intended doses, in spite of the wide variation among days within each animal. The mean daily 3‐NOP ingested was 0, 2.1, 2.6 and 5 g/day for groups control, 0.8×, 1× and 2×, respectively.
DM and water intake were significantly (p < 0.05) lower in the 2× than in the control cows, although the difference was not significant when the non‐inferiority test was applied (Table 1). No differences among groups were observed in final bw or in bw change, milk or milk protein yield. Milk fat yield and energy corrected milk yield were greater in the 2× than in the control group (p < 0.05). As for milk composition, protein and fat contents in milk were increased in the 2× compared with the control group, and there were no differences among groups in lactose, urea or SCC concentrations. Thus, although the DM intake in the cows of the 2× group was 4% less than that in the control group, this had no effects on bw change or milk yield, and energy corrected milk yield was increased by 7% in the 2× group compared with the control.
Table 1.
Feed intake, body weight change and milk production performance in the tolerance study
| mg 3‐NOP/kg feed DM | ||||
|---|---|---|---|---|
| Control | 90.2 (× 0.8) | 106.8 (× 1) | 220.4 (× 2) | |
| Dry matter intake (kg/day) | 22.7 | 22.9 | 22.8 | 21.8* |
| Water consumption (kg/day) | 96.1 | 95.9 | 94.6 | 87.0* |
| Body weight (kg) | 670 | 676 | 675 | 670 |
| Body weight change (kg) | 36.9 | 43.6 | 42.2 | 36.6 |
| Milk yield (kg/day) | 27.9 | 28.7 | 27.5 | 27.5 |
| Protein yield (kg/day) | 0.89 | 0.92 | 0.88 | 0.91 |
| Fat yield (kg/day) | 0.76 | 0.81 | 0.74 | 0.89* |
| Energy corrected milk (kg/day) | 23.0 | 24.3■ | 22.7 | 24.7* |
3‐NOP: 3‐nitrooxypropanol; DM: dry matter.
■p < 0.10, *p < 0.05, level of significance of the difference compared with the control group.
Faecal scores were consistent and showed little variation, both among different treatment groups and over the course of the study (range 3.04–3.42). Differences in urinalysis values were in general minor and negligible.
There were no significant (p > 0.10) differences among groups in most of the haematology and blood biochemistry parameters measured. Table 2 shows the values of those blood parameters for which significant differences among groups were detected.
Table 2.
Haematology, blood biochemistry and necropsy parameters in the tolerance study
| mg 3‐NOP/kg feed DM | ||||
|---|---|---|---|---|
| Control | 90.2 (× 0.8) | 106.8 (× 1) | 220.4 (× 2) | |
| Monocytes d5 (×109/L) | 0.54 | 0.48 | 0.46 | 0.43* |
| Monocytes d56 (×109/L) | 0.36 | 0.31 | 0.30■ | 0.30■ |
| Red blood cells (×1012/L) | 6.78 | 6.52* | 6.39* | 6.42* |
| Haematocrit (%) | 29 | 28* | 28* | 27* |
| Mean corpuscular haemoglobin (pg) | 16.2 | 16.5* | 16.5■ | 16 |
| Haemoglobin d56 (g/L) | 112 | 110 | 109 | 103* |
| Prothrombin time (s) | 28.9 | 27.3* | 27.7■ | 27.0* |
| Fibrinogen (g/L) | 0.85 | 0.88 | 0.93 | 1.00* |
| ALT (U/L) | 30 | 27* | 27* | 24* |
| LDH d56 (U/L) | 1267 | 1249 | 1245 | 1034* |
| Total cholesterol (mmol/L) | 4.50 | 4.26■ | 4.18* | 4.18* |
| Triglycerides (mmol/L) | 0.143 | 0.126■ | 0.130 | 0.124* |
| Albumin (g/L) | 36 | 36 | 36 | 35* |
| Ca (mmol/L) | 2.40 | 2.46■ | 2.47* | 2.40 |
| Amylase (U/L) | 34 | 34 | 34 | 31* |
| Ovary absolute size (g) | 35.2 | 31.9 | 29.7 | 25.8* |
| Ovary relative size (g per kg final bw) | 0.050 | 0.048 | 0.040 | 0.039■ |
3‐NOP: 3‐nitrooxypropanol; DM: dry matter; ALT: alanine transaminase; LDH: lactate dehydrogenase; bw: body weight.
■p < 0.10, *p < 0.05, level of significance of the difference compared with the control group.
Although statistically significant differences were detected, some of these differences seem to be of no clinical relevance such as, for instance, the decreases observed in ALT, LDH, amylase, cholesterol or triglycerides. Albumin was decreased to a minor extent, and no clinical relevance is expected. The changes in serum Ca were rather small and seemed to be not dose related.
The changes in coagulation parameters were also of a minor magnitude. The effects on monocytes were rather subtle (a slight decrease at 2× level (p < 0.10) at day 56) and difficult to interpret clinically, as a decrease in these cells becomes clinically meaningful when accompanied by changes in the white cell numbers. There were significant differences among groups in erythrocyte numbers, haematocrit, haemoglobin and MCH, but average values varied over a very narrow range and the magnitude of the differences can be regarded as small in most cases. It is worth mentioning that in all cases the values were within expected biological variability and within the reference ranges derived in the study.
There were no significant differences among groups in organ weights expressed either as absolute weight or relative to bw, except for the absolute and relative weights of ovaries that were smaller in the 2× group than in the control group. Histopathology was normal in most cases, and the histological findings were considered not related to 3‐NOP. For instance, the number of cows with signs of liver chronic inflammation was 2, 0, 3 and 5 (out of a total of 8 cows) in the control, 0.8×, 1× and 2× groups, respectively, but the differences were not significant statistically and were attributed to a possible infestation by Fasciola hepatica.
Given the statistical differences found in some of the endpoints, some uncertainty remains on the tolerance of 3‐NOP by dairy cows at the overdose (2×) level. Therefore, the FEEDAP Panel considers the additive as safe for dairy cows at the maximum recommended concentration in feed (100 mg 3‐NOP/kg DM). A margin of safety could not be established and consequently no extrapolation of the safe feed level to other animal species/categories is possible.
3.2.4.3. Conclusions on safety for the target species
The FEEDAP Panel considers that Bovaer® 10 is safe for dairy cows at the maximum recommended level in feed of 100 mg 3‐NOP/kg DM (corresponding to 88 mg/kg complete feed). A margin of safety could not be established. Consequently, the FEEDAP Panel is not in a position to conclude on a safe dietary concentration of Bovaer® 10 for other ruminant species/categories.
Systemic exposure or site of contact toxicity for the active substance 3‐NOP, for which genotoxicity and carcinogenicity have not been fully clarified in laboratory animals, is unlikely in the target species based on ADME data available (3‐NOP is quickly and nearly completely degraded in the rumen before entering the intestine).
3.2.5. Safety for the consumer
The FEEDAP Panel notes that the genotoxicity of 3‐NOP cannot be ruled out and that pre‐neoplastic lesions and tumours observed in the 2‐year carcinogenicity study with 3‐NOP are biologically relevant. However, the consumer is not exposed to 3‐NOP. Therefore, the above findings are not considered relevant for the safety for the consumer.
In addition, the metabolite NOPA, detected at very low concentration in milk, did not show genotoxic activity in a complete battery of in vitro and in vivo tests.
Overall, the FEEDAP Panel concludes that the use of Bovaer® 10 as feed additive does not raise concern for the safety of the consumer regarding genotoxicity. Consequently, a health‐based guidance value (acceptable daily intake, ADI) of 1 mg 3‐NOP/kg bw per day can be derived from the NOAEL (100 mg/kg bw per day) identified in repeated dose toxicity studies performed in laboratory animals exposed to 3‐NOP, applying an uncertainty factor of 100 to account inter‐ and intraspecies variability.
The qualitative metabolic similarity of 3‐NOP between the target animal and the laboratory animals has been demonstrated. NOPA is the main metabolite in rats and is considered to be responsible for the adverse effects seen in rodents. Therefore, the Panel considered that the NOAEL identified for 3‐NOP is considered valid also for NOPA and that the uncertainty factor of 100 is adequate to use the ADI derived for 3‐NOP from the rodent studies for assessing the safety for consumers exposed to NOPA from ruminant derived foodstuffs.
The exposure of the consumer to NOPA in foodstuffs has been calculated according to the methodology and principles described in the FEEDAP Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017).
For the exposure calculation to NOPA, the values corresponding to the LOQ (5 μg/kg) were used for all the mammalian tissues, and for milk the concentration of 3.66 μg/L (the highest individual value analysed).
Consumer exposure to NOPA for the different population categories is reported in Table 3.
Table 3.
Consumer exposure to NOPA resulting from the use of 3‐NOP in dairy cows
| Population class | Number of surveys | Maximum HRP* (μg/kg bw per day) |
|---|---|---|
| Infants | 6 | 0.4542 |
| Toddlers | 10 | 0.4674 |
| Other children | 18 | 0.5952 |
| Adolescents | 17 | 0.2260 |
| Adults | 17 | 0.1293 |
| Elderly | 14 | 0.1162 |
| Very elderly | 12 | 0.1266 |
3‐NOP: 3‐nitrooxypropanol; bw: body weight.
Highest reliable percentile.
The exposure calculation is considered a worst‐case scenario considering (i) the use of the highest individual NOPA milk concentration found among 19 cows during 56 days study duration which allows also to overlook the absence of PM milk data, and (ii) that no residue could be found in animal tissues and the LOQ was used instead.
The maximum HRP values for all population classes were at least three orders of magnitude below the health‐based guidance value (HBGV) of 1 mg/kg bw per day. The population category most exposed to NOPA was ‘other children’ with a maximum HRP of 0.6 μg/kg bw per day accounting for 0.06% of the established ADI (1 mg/kg bw per day).
3.2.5.1. Conclusions on safety for the consumer
The FEEDAP Panel concludes that the additive is safe for consumers exposed to products from dairy cows fed with Bovaer® 10 up to 100 mg 3‐NOP/kg feed DM.
3.2.6. Safety for user
3.2.6.1. Effects on the respiratory system
Due to the dusting potential of the additive (up to 390 mg/m3), the FEEDAP Panel considered that exposure through inhalation is likely.
An acute inhalation test in Wistar rats, performed according to OECD TG 403 was provided.■■■■■ No mortality occurred. Microscopic examination of the nasal cavity revealed that 3‐NOP‐related morphologic alterations were present in the acute phase (day 1) animals at 1 and 5 mg/L, and at a lower severity in a single female (day 15) of the main group at 5 mg/L. Based on the complete, or ongoing, recovery of the findings on day 15 compared to day 1, at both 1 and 5 mg/L the findings were considered acute mildly irritating, but non‐adverse. Methaemoglobin was observed at both doses. Nevertheless, it was considered not toxicologically relevant based on the absence of a response to the dose and low order of magnitude. For the other assessed parameters, 3‐NOP did not cause any relevant changes within the study.
The inhalation LC50 over 4 h and the no observed adverse effect concentration (NOAEC) of the 3‐NOP were considered to exceed 5 mg/L. Consequently, 3‐NOP is classified as class 5 of acute inhalation toxicity, according to the GHS (2019).72
Based on the findings observed on the microscopic examination of the nasal cavity, as well as of the methaemoglobin issue, the FEEDAP Panel concludes that 3‐NOP may be harmful, if inhaled.
3.2.6.2. Effects on the skin and eyes
The skin corrosion potential of 3‐NOP was tested in a valid study performed according to OECD TG 431, which showed that it is not corrosive to skin.73
The skin irritation potential of 3‐NOP was tested in a valid study performed according to OECD TG 439, which showed that it is a skin irritant.74
The eye irritation potential of 3‐NOP was tested in a valid study performed according to OECD TG 437, which showed that it is an eye irritant.■■■■■
In a valid skin sensitisation study following OECD TG 429, 3‐NOP did not show any skin sensitisation potential.■■■■■
3.2.6.3. Conclusions on safety for the user
The FEEDAP Panel concludes that the active substance 3‐NOP may be harmful if inhaled. It is irritant (but not corrosive) to skin, irritant to the eyes but it is not a skin sensitiser.
Furthermore, the FEEDAP Panel considered that as the genotoxicity of 3‐NOP is not completely elucidated, the exposure through inhalation of the additive may represent an additional risk for the user handling the additive.
3.2.7. Safety for the environment
The applicant provided an assessment on the safety of the additive for the environment indicating the physical chemical properties of the active substance; Phase I predicted environmental concentrations (PECs); information on fate and behaviour to refine the PECs■■■■■; a biodegradability study■■■■■; and ecotoxicity studies for freshwater and sediment (algal growth inhibition, acute toxicity in Daphnia magna, and acute toxicity in fish) in relation to Phase II.■■■■■
The FEEDAP Panel notes that the product is intensively metabolised and practically not excreted in the environment.80 This is derived from an ADME study in which the radio‐labelled active substance at a dose 50% above the maximum recommended dose was administered to complete feed of dairy cows.■■■■■ 3‐NOP is extensively metabolised into endogenous compounds as lactose, glucose, galactose and constituents of proteins and fatty acids and ultimately excreted as CO2. In a worst‐case scenario, ≤ 0.2% of 3‐NOP and NOPA may be found in total excreta. The extractable and soluble excreta and urine metabolites correspond to a reduced percentage (< 5%) and represent no safety concern for the environment.
3‐NOP, with a log KOW of 0.399, does not have the potential for bioaccumulation, and a risk for secondary poisoning is unlikely to occur.
The FEEDAP Panel concludes that the use of the additive up to 100 mg 3‐NOP/kg feed on DM basis in ruminants for milk production and reproduction, is of no concern for the environment.
3.3. Efficacy
The active substance of the additive Bovaer® (3‐NOP) is a structural analogue of methyl coenzyme M. Methanogen archaea produce CH4 from CO2 and H2 in a reaction catalysed by the enzyme methyl coenzyme M reductase. 3‐NOP saturates the enzyme and thus reduces the capacity of the archaea to form CH4 (Duin et al., 2016). This effect will cause an increase in metabolic H2 (not used for methanogenesis) and a shift in the fermentation pattern increasing (in theory based on fermentation stoichiometry) the molar proportion of propionate and butyrate and reducing that of acetate (Wang et al., 2014).
3.3.1. In vitro studies
Three in vitro studies were conducted to verify the mode of action of the additive in the reduction of the enteric methane (CH4). In the three studies, ruminal fermentation was simulated in vitro using a chemostat (so called RUSITEC, RUmen SImulation TEChnique) for the continuous culture of ruminal microorganisms.82 In all the studies, there was a control group (no addition of 3‐NOP) and one or more treated groups, in which 3‐NOP was added to the feed at different concentrations. The results consistently showed that the addition of 3‐NOP reduces methane output significantly. This was accompanied by changes in the fermentation end‐products, with a significant decrease of acetate. Propionate was not affected.
The FEEDAP Panel considered these studies as supporting evidence of the efficacy of the additive under assessment.
3.3.2. Data from literature
Two publications showing the effects of 3‐NOP on ruminal methanogenesis were submitted. In both publications, data from several studies were gathered and subject to pooled meta‐analysis. The study from which data were obtained was included in the model as a random effect. Studies were considered as short‐term, as methane was measured in respiratory chambers for a short period (few days).
The first meta‐analysis83 used a total of 12 in vivo studies from 10 scientific publications, with data from dairy cows, beef cattle and sheep, and a range of 3‐NOP inclusion rates in the daily ration of the animals from 0 to 280 mg/kg feed DM intake. It was concluded that enteric CH4 emissions decreased in response to the addition of 3‐NOP to the diet, showing a linear dose‐response relationship. When used at 100 mg 3‐NOP/kg DMI, the CH4 decreased by about 20%.
The second meta‐analysis84 evaluated data from 11 experiments and 38 treatments (9 publications) and considered the following factors: cattle type (beef or dairy), measurement techniques (GreenFeed® technique, sulfur hexafluoride tracer, respiration chambers), DM intake, body weight, 3‐NOP dose, roughage proportion and diet composition (crude protein and neutral detergent fibre contents). The effect of 3‐NOP decreasing CH4 emission from cattle was dose‐related and negatively associated with the fibre content of the daily ration.
Both studies also showed that methane was consistently reduced in response to the dietary inclusion of 3‐NOP.
The FEEDAP Panel considered the meta‐analyses provided as supporting evidence of the efficacy of the additive under assessment.
3.3.3. Long‐term studies in animals
Three long‐term studies with dairy cows evaluated the effect of 3‐NOP on enteric methane output. The experimental design and protocol were the same for the three studies, which were run at the same time (June to November 2018) in three different locations: Wageningen (The Netherlands),85 Reading (UK)86 and Pennsylvania (USA).87 Holstein Friesian dairy cows at different stages of lactation were used in the studies.
At the beginning of each study, the dairy cows were blocked by pairs based on their parity, DIM and milk production and then allocated to one of the two experimental groups (control group or 3‐NOP group). In the treated (3‐NOP) group, the additive was incorporated into a total mixed ration (TMR) fed to the cows to provide 60 mg 3‐NOP/kg DM. The additive (different batches being used in each study) was a preparation that contained 10.9% (w/w) of 3‐NOP with propylene glycol (˜ 40%), adsorbed on silicic acid, precipitated and dried. In the control group, the cows were fed the same diet but supplemented with a placebo (propylene glycol adsorbed on silicic acid, precipitated and dried) instead of the additive.
Studies 1 and 3 had a total duration of 19 weeks, study 2 had the duration of 20 weeks, all with 3 consecutive periods. During the first week, the cows became adapted to the system (GreenFeed®) used to measure the enteric methane production. This adaptation week was followed by a so‐called baseline period of 3 weeks (4 weeks in study 2), in which all cows from both groups were fed the control diet with no additive and all measurements (including methane production) were recorded. The main experimental period lasted 15 weeks (105 days), split into five intervals of 3 weeks each. During this period, all measurements were recorded and cows in each group received the corresponding diet (either control or supplemented with 3‐NOP).
In the three studies, all measurements were taken on each animal, so each cow was an experimental unit. Veterinary and clinical examinations were made regularly. Cows were weighed out at each milking in studies 1 and 3 or weekly in study 2. Milk production (kg/day) was recorded at each milking (twice daily), and milk composition (including fat, protein, lactose, total solids, casein, urea, fatty acid profile and somatic cell count) was determined weekly. Protein, fat and lactose yields (kg/day) and energy corrected milk (ECM) yield (kg/day) were calculated. Feed efficiency (as a function of total milk or of energy corrected milk yield) was calculated as kg milk per kg DM intake. Methane production was estimated from spot measurements taken using the GreenFeed® system (Huhtanen et al., 2019). Each time a cow entered into a feeder cabin attracted by a bait, the methane emission was measured and the time remaining in the cabin was recorded. Both measurements were linked to the individual cow entering the cabin (identified with an electronic ear tag). The method measures the methane emission only during the time the cow stayed in the cabin. It was therefore important to ensure that dairy cows entered into the feeder regularly during the day and stayed in the cabin for enough time. To obtain reliable estimates of methane emissions, recordings were taken for not less than 2 weeks. Accordingly, during the main experimental period, three‐week intervals were considered long enough to collect a substantial number of GreenFeed® visits per cow, and thus to obtain robust methane emission estimates. Then, the average daily emission for each 3‐week interval was estimated. Enteric methane emission was calculated per day (g CH4/day), per kg DM intake (so called methane yield in g CH4/kg DMI) or per kg energy corrected milk (so called methane emission intensity in g CH4/kg ECM). Carbon dioxide and hydrogen emissions were also determined.
The daily ration was composed of a partial mixed ration (PMR) and a bait used to attract animals into the GreenFeed® cabins. The PMR was a mix of roughages, concentrates and a complementary feed, was provided once daily (after the morning milking) and offered ad libitum. The bait was a highly palatable feed and did not contain any 3‐NOP. Daily feed intake was measured individually.
Along with the similar experimental design, duration and procedures used, the three studies differed only in the number of cows used and the diets fed to the animals, as shown in Table 4. The diets were common mixed rations for high‐yielding dairy cows.
Table 4.
Specific features of each long‐term study conducted with the additive
| Study | Total no of cows (in each group) | Concentration of additive in feed* mg 3‐NOP/kg DM intake | Diet composition |
|---|---|---|---|
| 1 WUR | 64 (32) | 54 | 70% forage (maize silage, grass silage) + 30% concentrate (wheat, maize, soybean oil, palm kernel, molasses) Chemical composition: 162 g CP and 397 g NDF/kg DM, 419 g DM/kg feed |
| 2 UK | 42 (21) | 57 | 60% forage (maize silage, alfalfa haylage, hay/straw) + 40% concentrate (wheat distiller, rapeseed meal, soya hulls, palm kernel, cane molasses, and premixes) Chemical composition: 166 g CP and 340 g NDF/kg DM, 376 g DM/kg feed |
| 3 PSU | 48 (24) | 61 | 70% forage (maize silage, grass silage) + 30% concentrate (maize, whole soybean meal, canola meal, cane molasses and premix) Chemical composition: 156 g CP and 295 g NDF/kg DM, 473 g DM/kg feed |
3‐NOP: 3‐nitrooxypropanol; DM: dry matter; CP: crude protein; NDF: neutral detergent fibre.
Based on the concentrations determined analytically (measured weekly) and the total DM intake recorded.
In all cases, data were averaged for each 3‐week interval (1–3, 4–6, 7–9, 10–12 and 13–15 weeks) and the mean values were used in the statistical analysis. Each study was laid out in a randomised complete block design with two treatment groups (control and 3‐NOP) and five repeated measures (the five measurement intervals for each cow during the main experimental period). For all variables (except the overall change in body weight), a mixed effects model with repeated measures was fitted. The average value of the variable for each cow recorded during the baseline was used as a covariate. The covariate structure was chosen based on information criteria. Statistical power and normality tests were performed in for all variables. Body weight gain was analysed using a two‐sample t‐test.
Results on methane emissions are shown in Table 5. In the three studies, the addition of 60 mg 3‐NOP per kg DM to dairy cow TMR was associated with a significant decrease (by between 20 and 35% compared with the control) in enteric methane emission per day, per kg DM intake (yield) and per kg EC milk yield (intensity). Concurrently, there was an increase in the emission of hydrogen. Within each study, the magnitude of the decrease in methane emission in the cows receiving 3‐NOP was similar over the 15 weeks of the main experimental period.
Table 5.
Effects of 3‐NOP dietary supplementation on methane emissions (average values over the main 15‐week experimental period, with baseline values below between brackets) in the three long‐term studies
| Study | mg 3‐NOP/kg TMR DM intake | g CH4/day | g CH4/kg DM intake | g CH4/kg EC milk | g H2/day |
|---|---|---|---|---|---|
| 1 WUR | 0 (control) | 423 (464) | 22.4 (25.0) | 17.1 (18.6) | ND |
| 54 | 332* (480) | 17.7* (25.5) | 12.9* (18.7) | ND | |
| 2 UK | 0 (control) | 449 (406) | 20.3 (16.9) | 12.3 (10.4) | 2.6 (2.7) |
| 57 | 292* (400) | 13.5* (16.7) | 8.4* (10.1) | 10.6* (2.4) | |
| 3 PSU | 0 (control) | 410 (369) | 16.7 (15.0) | 11.2 (10.0) | 0.5 (0.5) |
| 61 | 308* (403) | 12.1* (14.9) | 8.2* (9.7) | 2.6* (0.4) |
3‐NOP: 3‐nitrooxypropanol; DM: dry matter; TMR: total mixed ration; EC: energy corrected.
Significantly different from the control value at p < 0.01.
As shown in Tables 6 and 7, the effects of the additive on methane production did not induce relevant effects on the zootechnical performance of the animals. Daily EC milk yield was decreased in study 2 (Table 3) when cows were fed the 3‐NOP diet, as a result of a significant decrease in the milk fat content (Table 4). The effect of 3‐NOP on milk fat content was not considered consistent, because it was decreased in study 2, but increased in study 3. Feed efficiency was increased in cows fed the 3‐NOP diet in study 1.
Table 6.
Effects of 3‐NOP dietary supplementation on feed intake, initial and final body weight and milk production in the three long‐term studies
| Study | mg 3‐NOP/kg TMR DM intake | TMR DM intake (kg/day) | Initial BW (kg) | Final BW (kg) | kg milk/day | kg EC milk/day |
|---|---|---|---|---|---|---|
| 1 WUR | 0 (control) | 19.1 | 629 | 664 | 24.0 | 25.0 |
| 54 | 18.4 | 631 | 668 | 24.5 | 25.7 | |
| 2 UK | 0 (control) | 23.3 | 712 | 739 | 37.4 | 37.0 |
| 57 | 22.9 | 694 | 721 | 36.0 | 35.0* | |
| 3 PSU | 0 (control) | 25.3 | 596 | 640 | 38.8 | 37.4 |
| 61 | 25.7 | 608 | 648 | 38.2 | 37.5 |
3‐NOP: 3‐nitrooxypropanol; DM: dry matter; TMR: total mixed ration; bw: body weight; EC: energy corrected.
Significantly different from the control value at p < 0.1.
Table 7.
Effects of 3‐NOP dietary supplementation on milk composition and feed efficiency in the three long‐term studies
| Study | mg 3‐NOP/kg TMR DM intake | Milk fat (g/kg) | Milk protein (g/kg) | kg EC milk/kg DMI |
|---|---|---|---|---|
| 1 WUR | 0 (control) | 43.2 | 35.7 | 1.30 |
| 54 | 43.4 | 36.3* | 1.36* | |
| 2 UK | 0 (control) | 41.7 | 32.8 | 1.59 |
| 57 | 39.9* | 33.4 | 1.53 | |
| 3 PSU | 0 (control) | 38.3 | 31.0 | 1.49 |
| 61 | 40.2* | 31.5 | 1.47 |
3‐NOP: 3‐nitrooxypropanol; DM: dry matter; TMR: total mixed ration; DMI: days in milk; EC: energy corrected.
Significantly different from the control value at p < 0.1.
3.3.4. Conclusions on efficacy
The results of the three efficacy studies showed that 3‐NOP can reduce enteric methane production in dairy cows when added to mixed rations at 60 mg/kg DM feed (corresponding to 53 mg/kg complete feed) without effects on performance. The results of the in vitro tests and metanalyses of in vivo data would support this conclusion. Due to the mode of action of 3‐NOP to decrease methanogenesis and the similarity in rumen fermentation processes and microbial population, these results can be extrapolated to all other ruminants for milk production and reproduction.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation88 and Good Manufacturing Practice.
3.5. Conclusions
The FEEDAP Panel concludes that Bovaer® 10 is safe for dairy cows at the maximum recommended level in feed of 100 mg 3‐NOP/kg DM feed (corresponding to 88 mg/kg complete feed, DM 88%). A margin of safety could not be established. Consequently, the FEEDAP Panel is not in a position to conclude on a safe dietary concentration of the additive for other ruminant species/categories.
The use of Bovaer® 10 in animal nutrition under the conditions of use proposed is of no concern for consumer safety. The use of the additive is considered of no concern for the environment.
The FEEDAP Panel concludes that the active substance 3‐NOP may be harmful if inhaled. It is irritant (but not corrosive) to skin, irritant to the eyes but it is not a skin sensitiser. As the genotoxicity of 3‐NOP is not completely elucidated, the exposure through inhalation of the additive may represent an additional risk for the user handling the additive.
The additive is considered to be efficacious to reduce enteric methane production when given orally at the concentration of 60 mg 3‐NOP/kg DM feed (corresponding to 53 mg/kg complete feed, DM 88%) to dairy cows. This conclusion is extrapolated to all other ruminants for milk production and reproduction.
4. Documentation as provided to EFSA/Chronology
| Date | Event |
|---|---|
| 04/09/2019 | Dossier received by EFSA. Bovaer® 10 (3‐nitrooxypropanol) for dairy cows for milk production, cows for reproduction, dairy sheep for milk production, ewes for reproduction, dairy goats for milk production, goats for reproduction, other ruminants for milk production, other ruminants for reproduction. Submitted by DSM Nutritional Products Sp. z o.o. on behalf of DSM Nutritional Products Ltd. |
| 11/09/2019 | Reception mandate from the European Commission |
| 23/10/2019 | Application validated by EFSA – Start of the scientific assessment |
| 20/12/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: safety for the environment |
| 27/01/2020 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation of the additive and conditions of use |
| 15/02/2020 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 17/02/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 25/02/2020 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: safety for the consumer and toxicological studies |
| 31/03/2021 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 17/07/2021 | Comments received from Member States |
| 30/09/2021 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- 3‐NOP
3‐hydroxy‐propionic acid
- 3‐NOP
3‐nitrooxypropanol
- ADI
average daily intake
- ADME
absorption, distribution, metabolism and excretion
- ANS
EFSA Scientific Panel on Additives and Nutrient Sources added to Food
- ALT
alanine transaminase
- AUC
area under the curve
- BW
body weight
- CREST
antikinetochore antibody staining
- CP
crude protein
- CV
coefficient of variation
- DM
dry matter
- DIM
days in milk
- DMI
dry matter intake
- DMSO
dimethyl sulfoxide
- EC
energy corrected
- ECG
electrocardiogram
- ECM
energy corrected milk
- EURL
European Union Reference Laboratory
- FACS
fluorescence‐activated cell sorting
- GLP
good laboratory practice
- HPLC
high‐performance liquid chromatography
- HRP
highest reliable percentile
- IR
infrared analysis
- iv
intravenous
- LC/MS
liquid chromatography–mass spectrometry
- LDH
lactate dehydrogenase
- LLQ
lower limit of quantification
- LOD
limit of detection
- LOQ
limit of quantification
- log Kow
logarithm of octanol–water partition coefficient
- MCH
mean corpuscular haemoglobin
- MF
mutation frequencies
- MN
micronucleus (assay)
- MNBN
micronucleated binucleated
- MN‐RETs
micronucleated reticulocytes
- MS
mass spectrometry
- MTBE
methyl‐tert‐butyl‐ether
- NDF
neutral detergent fibre
- NMR
nuclear magnetic resonance
- NOAEC
no observed adverse effect concentration
- NOAEL
no observed adverse effect level
- NOPA
3‐nitrooxypropionic acid
- OECD
Organisation for Economic Co‐operation and Development
- PCEs
polychromatic erythrocytes
- PECs
predicted environmental concentrations
- PMR
partial mixed ration
- PPDN
1,3‐propane diol dinitrate
- PWG
pathology working group
- RH
relative humidity
- RUSITEC
RUmen SImulation TEChnique
- TMR
total mixed ration
- TR
total radioactivity
- TRR
total residual radioactivity
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of the Analysis for Bovaer® 10
1.
In the current application authorisation is sought under Article 4(1) for 3‐nitrooxypropanol (preparation of minimum of 10% of 3‐nitrooxypropanol) under the category/functional group 4(c) ‘zootechnical additives’/‘substances which favourably affect the environment’, according to Annex I of Regulation (EC) No 1831/2003. Specifically, the authorisation is sought for the use of the feed additive for dairy cows and cows for reproduction, dairy sheep and ewes for reproduction, dairy goats and goats for reproduction, other ruminants for milk production and reproduction.
The feed additive is a white powder preparation containing a minimum of 10% (w/w) of 3‐nitrooxypropanol as an active substance. The feed additive also contains propylene glycol and silicon dioxide as carriers.
The feed additive is intended to be incorporated into premixtures and feedingstuffs. The Applicant proposed levels of 3‐nitrooxypropanol ranging from 53 to 88 mg/kg complete feedingstuffs.
For the quantification of the 3‐nitrooxypropanol content in the feed additive, premixtures and feedingstuffs the Applicant proposed a single‐laboratory validated and further verified method based on reversed phase high performance liquid chromatography (HPLC) coupled to spectrophotometric (UV) detection.
The following performance characteristics were reported by the Applicant in the frame of the validation and verification studies for the quantification of 3‐nitrooxypropanol content:
-
–
in the feed additive: a relative standard deviation for repeatability (RSDr) ranging from 0.2% to 1.0%; a relative standard deviation for intermediate precision (RSDip) ranging from 0.3% to 1.0%; and a recovery rate (Rrec) ranging from 100% to 101%.
-
–
in premixtures (2,870–17,390 mg/kg): a RSDr ranging from 0.4% to 1.1%; a RSDip ranging from 0.8% to 1.5%; and a Rrec ranging from 100% to 101%.
-
–
in feedingstuffs (29–132 mg/kg): a RSDr ranging from 0.6% to 5.2%; a RSDip ranging from 1.0% to 5.2%; a Rrec ranging from 98% to 101%; and a limit of quantification (LOQ) ranging from 8 to 14 mg of 3‐nitrooxypropanol/kg feedingstuffs.
Based on the experimental evidence available the EURL recommends for the official control the above mentioned single‐laboratory validated and further verified reversed phase HPLC‐UV method for the quantification of 3‐nitrooxypropanol in the feed additive, premixtures and feedingstuffs.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005, as last amended by Regulation (EU) 2015/1761) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Fašmon Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Aquilina G, Bories G, Brantom PG, Gropp J, Svensson K, Tosti L, Anguita M, Galobart J, Manini P, Tarrès‐Call J and Pizzo F, 2021. Scientific Opinion on the safety and efficacy of a feed additive consisting of 3‐nitrooxypropanol (Bovaer® 10) for ruminants for milk production and reproduction (DSM Nutritional Products Ltd). EFSA Journal 2021;19(11):6905, 35 pp. 10.2903/j.efsa.2021.6905
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00592
Panel members: Vasileios Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The Panel wishes to thank the following for the support provided to this scientific output Antonio Finizio, Andreas Focks, Matteo Lorenzo Innocenti, Daniel Pagès Plaza, Ivana Teodorovic, Maria Vittoria Vettori.
Adopted: 30 September 2021
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
DSM Nutritional Products Ltd (represented in the EU by DSM Nutritional Products Sp. z o.o.), Wurmisweg 576, Kaiseraugst (Switzerland).
FEED dossier reference: FAD‐2019‐0057.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2019-0057-nitrooxypropanol.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical Dossier/Section II/Annex II_6.
■■■■■
■■■■■
■■■■■
Technical Dossier/Section II/Annex II_23.
Technical Dossier/Section II/Annex II_22.
■■■■■
■■■■■
■■■■■
■■■■■
Technical Dossier/Section II/Annex II_38.
Technical Dossier/Section III/Annex III_21.
Technical Dossier/Section III/Annex III_031.
Technical Dossier/Section III/Annex III_033.
Technical Dossier/Section III/Annex III_034.
■■■■■
Technical Dossier/Section III/Annex III_22‐23.
Technical Dossier/Section III/Annex III_024.
■■■■■
■■■■■
Technical Dossier/Section III/Annex III_027.
■■■■■
Technical Dossier/Section III/Annex III_124.
■■■■■
Reliability and accuracy of any study, in which a potential effect of a feed additive should be examined, depends on the certainty that the intended dose/concentration in feed was applied. Measures to ensure that are in feed analyses of the test item and as few as possible feed batches to reduce bias. In the present study (see Section 3.2.4.2), feed (TMR) was prepared twice daily per individual cow. Considering 90 study days, 180 feed batches had to be prepared per cow, resulting in 180 × 4 (cows per group) = 720 batches per treatment group. It is clear that this huge number of batches could not be analytically controlled and provides – at least theoretically – entrance for bias. During week 1, 6 and 12 representative batches from each of the three concentrations of the test item were analysed. For the three treatment groups (1×, 5×,10×), the analysed concentrations of the test item were 57, 32 and 53% of the target concentration in week 1, 107, 108 and 117% in week 6, and 79, 86 and 55% in week 12, respectively. However, the applicant calculated data for the actual daily dose to demonstrate that the target doses were reached (target 1.6 g vs actual 1.72 g/day and cow; target 8.0 g vs. actual 8.53 g/day and cow; target 16 g vs. actual 15.72 g/day and cow). The means show large ranges. 1.35–2.07 g/day for the 4 cows of the 1× group, 0.40–10.27 g/day for the 3 cows of 5× group, and 1.56–20.32 g/day for the 3 cows of the 10× group. It is unclear which feed concentration was taken for the weeks in which no feed samples were taken and analysed. These data are not considered sound. The study shows such serious flaws that it could not be considered for quantitative residue data in milk.
Rico DE, Marshall ER, Kaylegian KE, Dechow CD, 2014. Within‐milking variation in milk composition and fatty acid profile of Holstein dairy cows. Journal of Dairy Science, 97, 4259–4268.
■■■■■
■■■■■
Technical Dossier/Section III/Annex II_38.
■■■■■
Technical Dossier/Section III/Annex II_41.
■■■■■
■■■■■
■■■■■
Technical Dossier/Section III/Annex II_44.
Technical Dossier/Section III/Annex II_49.
■■■■■
Technical Dossier/Supplementary Information (March 2021)/Section III/Annex III_153.
Technical Dossier/Supplementary Information (March 2021)/Section III/Annex III_154.
Technical Dossier/Supplementary Information (March 2021)/Section III/Annex III_156.
Technical Dossier/Supplementary Information (March 2021)/Section III/Annex III_155.
Technical Dossier/Section III/Annex II_51‐52.
Technical Dossier/Section III/Annex II_53‐55.
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
Total white blood cell count; differential white blood cell count: neutrophils, lymphocytes, monocytes, eosinophils, basophils; large unclassified cells; red blood cell count; reticulocyte count (absolute and relative); haematocrit; mean corpuscular volume; mean corpuscular haemoglobin; mean corpuscular haemoglobin concentration; platelet count; haemoglobin.
Albumin; glucose; total protein; haptoglobin; globulin; albumin‐globulin ratio; alanine aminotransferase; aspartate aminotransferase; alkaline phosphatase; lactate dehydrogenase; glutamate dehydrogenase; sorbitol dehydrogenase; gamma glutamyl transferase; creatine phosphokinase; triglycerides; total cholesterol; phospholipids; creatinine; calcium; chloride; magnesium; phosphate; potassium; sodium; urea; amylase; total bilirubin.
Prothrombin time, activated partial thromboplastin time; fibrinogen.
Colour, protein, pH, ketones, bilirubin, and urobilinogen; crystals, casts, red blood cell, white blood cells and epithelial cell in sediment; Specific gravity.
Adrenal glands, heart, kidneys, liver, ovaries, spleen and thymus.
Adrenal glands, thyroid gland, heart, kidneys, liver, spleen, forestomachs (rumen, reticulum, omasum), abomasum, small intestine (duodenum, jejunum, ileum), large intestine (caecum, colon, rectum), pancreas, lungs, ovaries, oviducts, and thymus.
■■■■■
Technical Dossier/Section III/Annex III_113.
Technical Dossier/Section III/Annex III_114.
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
Technical dossier/Supplementary information February 2020/Bovaer 10 SIn answer EFSA ‐Annex 1, supplementary information.
■■■■■
Technical Dossier/Section IV/Annex IV_9‐11.
Technical Dossier/Section IV/Annex IV_12.
Technical Dossier/Section IV/Annex IV_13.
Technical Dossier/Section IV/Annex IV_14‐38.
Technical Dossier/Section IV/Annex IV_39‐57.
Technical Dossier/Section IV/Annex IV_58‐85.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Duin EC, Waner T, Shima S, Prakash D, Cronin B, Yáñez‐Ruiz D, Duval S, Rümbeli R, Stemmler RT, Thauer RK and Kindermann M, 2016. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3‐nitrooxypropanol. Proceedings of the National Academy of Sciences of the United States of America, 113, 6172–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHS , 2019. Globally harmonized system of classification and labelling of chemicals. Available online: https://unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev08/ST-SG-AC10-30-Rev8e.pdf
- Huhtanen P, Ramin M and Hristov AN, 2019. Enteric methane emission can be reliably measured by the GreenFeed monitoring unit. Livestock Science, 222, 31–40. [Google Scholar]
- Wang M, Sun XZ, Janssen PH, Tang SX and Tan ZL, 2014. Responses of methane production and fermentation pathways to the increased dissolved hydrogen concentration generated by eight substrates in in vitro ruminal cultures. Animal Feed Science and Technology, 194, 1–11. [Google Scholar]


